Abstract
Glucose is the primary fuel for the vast majority of cells, and animals have evolved essential cellular, autonomic, endocrine, and behavioral measures to counteract both hypo- and hyperglycemia. A central component of these counterregulatory mechanisms is the ability of specific sensory elements to detect changes in blood glucose and then use that information to produce appropriate counterregulatory responses. Here we focus on the organization of the neural systems that are engaged by glucosensing mechanisms when blood glucose concentrations fall to levels that pose a physiological threat. We employ a classic sensori-motor integrative schema to describe the peripheral, hindbrain, and hypothalamic components that make up counterregulatory mechanisms in the brain. We propose that models previously developed to describe how the forebrain modulates autonomic reflex loops in the hindbrain offer a reasoned framework for explaining how counterregulatory neural mechanisms in the hypothalamus and hindbrain are structured.
1) INTRODUCTION
True neuronal glucosensation was first defined over fifty five years ago by Mayer [71] as the ability of certain neurons to transduce fluctuations in available glucose into electrical or neural mechanisms that could regulate food intake. It has been known for about 40 years that some neurons possess this ability [84]. More recently, the nature and location of brain glucosensing mechanisms has received a great deal of attention. For the most part this is because of the desire to understand how the brain controls metabolism in both health and disease, particularly with regard to diabetes and obesity. Consequently, elucidating the role played by brain glucosensing mechanisms in generating pathological counterregulatory responses has been particularly prominent [64,70].
Plasma glucose concentrations are normally maintained between 3.0–5.6mM, but can vary between 2mM and 10mM or higher in pathological conditions. Within the brain however, cerebrospinal fluid is buffered to the extent that the range within which glucose concentrations vary is much lower and narrower (0.5–2.5mM). Many electrophysiological studies have now clearly shown that some neurons possess specialized mechanisms that allow them to act as glucosensors and alter their firing rates with fluctuating ambient glucose concentrations. The terms glucose-excited (GE) and glucose-inhibited (GI) have been introduced to describe neurons that increase or decrease their firing rate as local glucose concentrations rise [64,102].
GE and GI neurons are found at various location in the brain, but attention is most closely focused on those in the hindbrain and hypothalamus. However, important glucosensing elements are also found in the hepatic portal/mesenteric vein (PMV), gut, carotid body, and oral cavity. Those in the PMV encode information about blood glucose fluxes just before it enters the liver from the gut. This information is conveyed to the hindbrain by way of the vagus nerve and spinal cord [38,79,118]. These widely distributed glucosensors therefore collectively constitute important entry points into the brain for information about circulating glucose. The brain then assimilates this information with other neural procesess to influence the behavioral, autonomic, and neuroendocrine motor functions that regulate metabolism.
This review will focus on the organization of the neural networks engaged by glucosensing elements in the brain and PMV. Our goal is to provide a neuroanatomical framework for considering how information derived from glucosensory mechanisms is distributed within the brain to control counterregulation. Although a basic outline of these networks was proposed twenty five years ago by Oomura [86], detailed knowledge of their functional organization has remained sparse despite the large amounts of detailed information now available on the connectional organization of regions containing glucosensing neurons (see [92,108,127] for reviews). This connectional information provides a rich framework for both developing and constraining models for how the brain uses information from circulating glucose to control metabolism.
Before discussing the functional organization of these networks in more detail, it is worth briefly considering the motor actions they engage.
Counterregulation
Counterregulatory responses to hypoglycemia consists of a coordinated group of endocrine and behavioral events [28]. The effects of these responses are temporally distributed in a way that not only restores euglycemia, but also provides an adaptive mechanism to deal with a state—or potential state—of negative energy balance. Primary responses consist of a reduction in plasma insulin concentrations, and increases in plasma epinephrine (and nor-epinephrine) and glucagon. These events are essentially reactive, and will rapidly increase plasma glucose by decreasing glucose uptake into cells and increasing hepatic glucose production. Secondary responses include a rapid increases n plasma glucocorticoid, and growth hormone concentrations. The downstream effect of glucocorticoid and growth hormone are more adaptive than reactive responses because they provide the animal with the ability to modify metabolic processes over longer periods of time. Finally, hypoglycemia can also stimulate feeding behavior thereby increasing the availability of fuels within the body. These counterregulatory responses collectively provide the animal with the ability to maintain euglycemia in the face of severe glycemic challenges.
Although hypoglycemia is an important complication in the management of type 1 diabetes, under normal circumstances animals may never encounter the depth of hypoglycemia required to activate all these responses. In this respect a full counterregulatory response is analogous to the increased plasma angiotensin II that follows hemorrhage or severe hypovolemia. Full angiotensin II release may never be fully engaged in an animal’s lifetime, but like counterregulation, the capacity remains a critical and potentially life saving mechanism.
2) CIRCULATING GLUCOSE AS A INTEROSENSORY STIMULUS
A Basic Model For Sensory-Motor Integration
The basic principles of sensory-motor integration form a cornerstone of modern neuroscience. Thus, for any particular modality, sensory information is first transduced by specific receptor elements, which then engage dedicated projections (‘labeled lines’) to take the encoded information in two directions. First, into networks located proximal to motor neurons to control their activity in a reflex manner; or second, more deeply into the brain where information eventually reaches the cortex and is perceived as a sensation. Complex mechanisms will also integrate ascending sensory signals with the output from other neural processes (arousal state, circadian clock signals, reward, learning and memory, etc.) to generate complex and coordinated motor actions (eg. [127] for review). This basic arrangement—first advocated by Cajal and Sherrington—has since been clarified by literally thousands of studies.
Circulating glucose as a humerosensory stimulus
Although this schema was developed to explain the sensory-motor integration that controlled striated musculature (ie. behavior), the principles upon which it is based have also been used to consider the organization of systems controlling autonomic and neuroendocrine motor outputs, at least in terms of their primary components [67,92,99,120,125,127]. In this way sympathetic, parasympathetic, and neuroendocrine motor neurons each have premotor control networks that manage simple output patterns (eg. pulsatile hormone release, cardiovascular reflexes, counterregulatory responses, etc.). But the networks responsible for individual hormone release patterns are also modulated by more widely derived influences. This integration allows complex and coordinated autonomic and neuroendocrine output patterns that are often linked with appropriate adaptive behaviors.
If we consider glucose as an interosensory signal that engages specific neural networks in the brain to influence behavioral, autonomic, and neuroendocrine motor function, then this general scheme offers us a framework for considering how these networks might be arranged.
Basic Organization of a Network for Glucosensory-Motor Integration
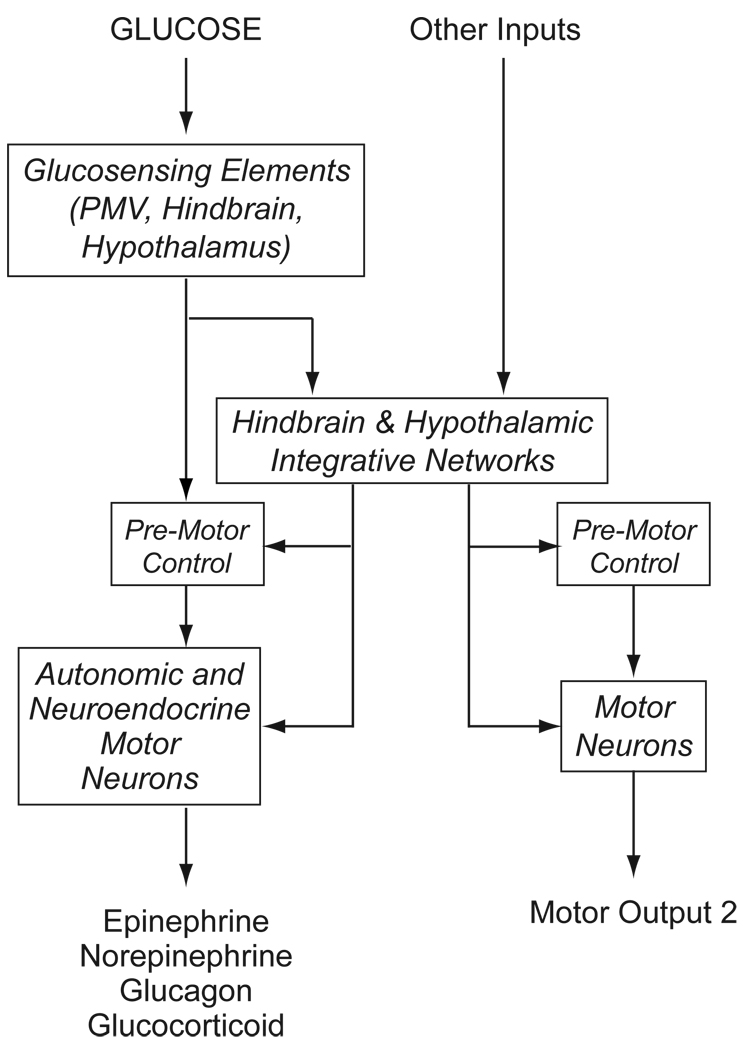
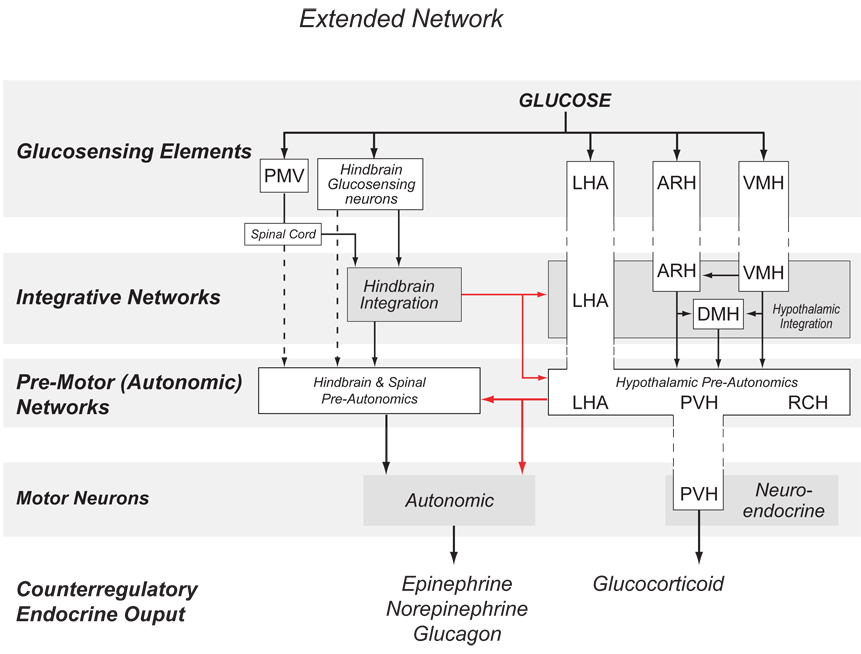
Four main components make up the network (Fig. 1):
- Glucosensing elements responsible for detecting glucose fluxes and transducing them into altered neuronal firing rates.
- These are located in the PMV, hindbrain, and hypothalamus. Other possible locations in the brain have not been explored in any detail.
- Downstream integrative networks that receive direct projections from glucosensing elements.
- Motor neurons responsible for controlling autonomic and neuroendocrine output.
- These are located in the hypothalamus, hindbrain, and in autonomic ganglia. Their output controls and/or modulates effector cells in the adrenal medulla (chromaffin cells), anterior pituitary (corticotopes and somatotropes), and pancreatic islets (α- and β-cells)
Figure 1.
A schematic diagram to illustrate how glucose, acting as an interosensory stimulus engages glucosensing elements that transduce information into neural signals that control hypoglycemic counterregulation. Consistent with classic sensorimotor integration, these signals use neural pathways to engage either premotor control networks to drive autonomic and neuroendocrine motor neurons in a reflex manner; or more complex integrative networks in the hypothalamus and hindbrain. These integrative networks allow glucosensation to be integrated with other information that can modulate counterregulatory responses, as well as providing a way for glucosensation to influence other motor process, such as those that control reproductive behavior.
Figure 1 uses the classic sensory-motor integration model to organize these components into a neural network that hypoglycemia can engage to generate counterregulatory responses. Its structure has a number of important functional implications.
FIrst, it provides an opportunity for ‘reflex’ release of counterregulatory hormones. The classic definition of a reflex action involves direct engagement of pre-motor/motor networks by the output from sensory transduction elements with little or no integrative processing. This implies that glucose is able to influence counterregulatory processes more or less directly, and requires that the pre-motor or motor neurons themselves express the cellular mechanisms for glucosensing or receive direct projections from glucosensing neurons. Currently it is unclear whether this happens, primarily because the precise location and phenotype of most glucosensing neurons is unknown. However, recent reports have identified glucosensing neurons in the dorsal motor nucleus of the vagus (DMX) [5], which houses pre-ganglionic parasympathetic neurons that can influence islet function. Furthermore, the existence of hindbrain glucoreceptors, together with the fact that glucosensing information from the PMV important for sympathoadrenal responses is relayed by spinal pathways [38] that most likely terminate in the the NTS, raise the possibility of hindbrain ‘reflex loops’ for counterregulatory responses that require little or no input from the forebrain.
In terms of the motor elements themselves, there is no evidence that glucose acts directly on chromaffin cells to increase epinephrine secretion, or that CRH neurons in the paraventricular nucleus of the hypothalamus (PVH) are themselves glucosensing. Although glucose obviously has direct effects on α- and β-islet cells to control glucagon and insulin release, these actions are neurally modulated but not neurally mediated [eg. 62].
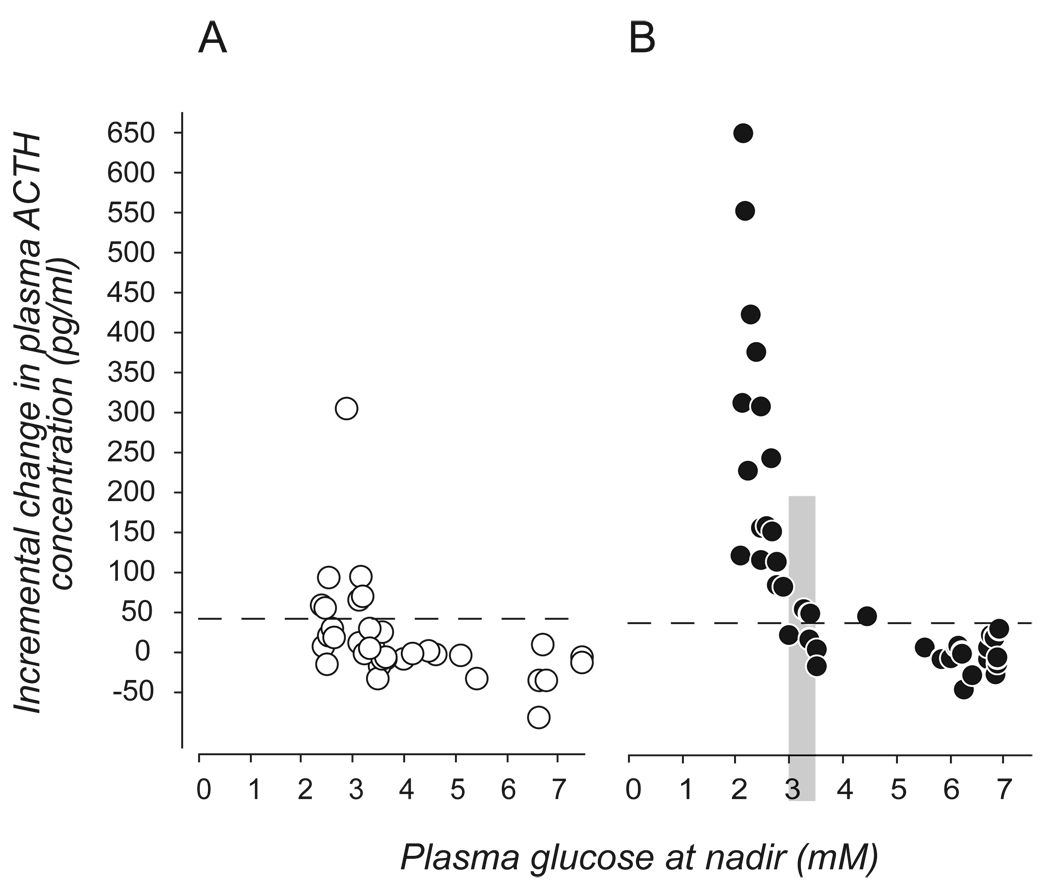
Second, projections from glucosensing elements into the integrative networks of the hindbrain and hypothalamus provide a way for information about circulating glucose to be incorporated with inputs from other regions in the brain for example, circadian influences. Thus, projections from the suprachiasmatic nucleus (SCH) can, by way of the sub-paraventricular zone and dorsomedial nucleus (DMH), be integrated with glucosensing information from the hindbrain to affect the magnitude of ACTH and glucocorticoid responses to hypoglycemia across the day (Fig. 2 [45,52]).
Figure 2.
The magnitude of the ACTH response to hypoglycemia varies across the day. The two panels show the incremental change in rat plasma ACTH concentrations 20mins after an intravenous injection of insulin given 2h after lights on (A, open circles) or 1h before lights off (B, closed circles). Each symbol represents the response of a single animal. Dashed horizontal lines represent 2 SD above the mean increment of the saline-injected controls at that time. The vertical shaded bar in B) denotes the range of glucose concentrations containing the glycemic threshold. (Data from [45]).
Third, the arrangement shown in Figure 1 provides a way for changes in blood glucose to influence a range of non-counterregulatory functions such as reproduction, by engaging separate pre-motor and motor networks (Motor Output 2 in Fig, 1) [99].
Finally, we should note that the role of any one particular brain region is likely to be more complex than is apparent from this simple model, and that the overall contributions of a particular anatomically defined region may span more than one of these hierarchical levels. For example, parts of the LHA contain glucosensing neurons, pre-autonomic neurons, and also contribute to the integrative networks (Fig 3). Similarly, some neurons in the VMH and ARH are glucosensing, while other VMH and ARH neurons probably act at the integrative network level.
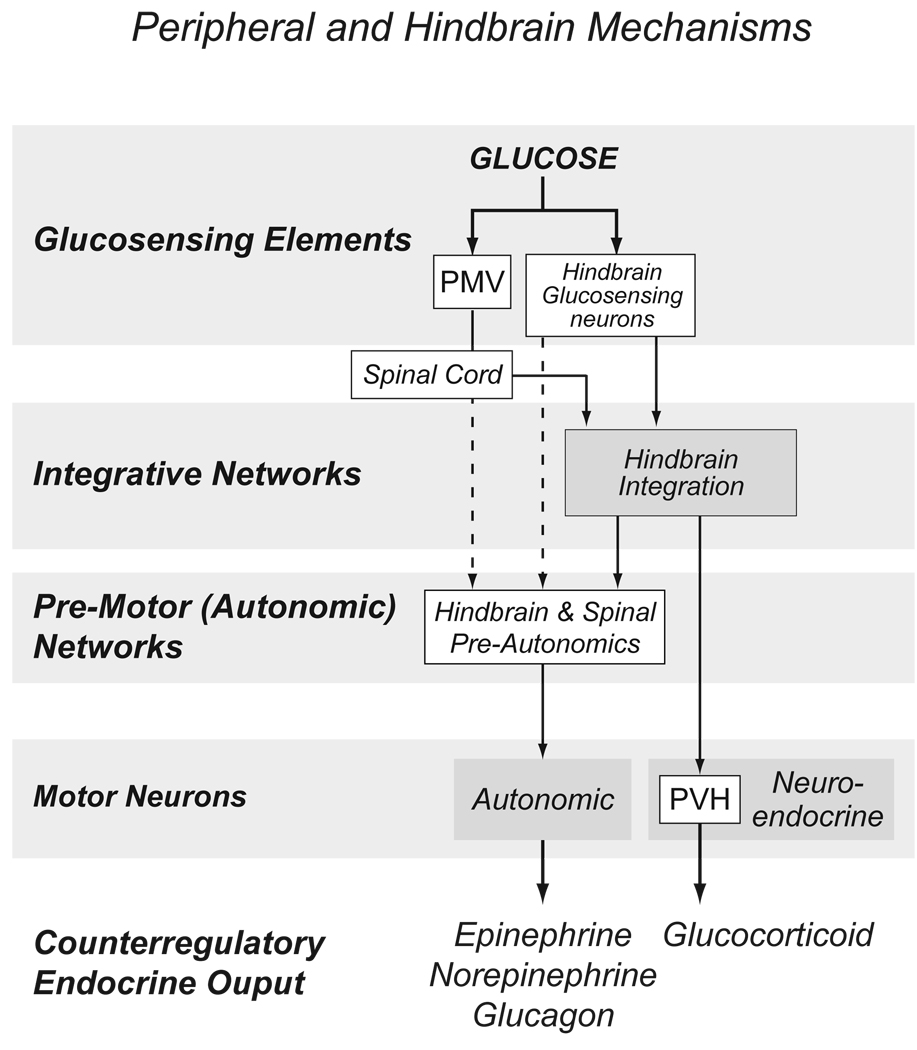
Figure 3.
An expansion of the model shown in Figure 1 to illustrate the organization of hindbrain and peripheral components involved with counterregulation. Solid lines represent known projections; dotted lines represent postulated connections. Abbreviations: PMV, hepatic portal/mesenteric vein; PVH, paraventricular nucleus of the hypothalamus.
The next three sections provides a more detailed description of the components and their projections at each level of Figure 1.
3) DISTRIBUTED NETWORK OF GLUCOSENSORS
Electrophysiology is currently the most rigorous method for identifying glucosensing neurons, but it has limited ability to map the precise locations of large numbers of neurons. The complex and perhaps multiple cellular mechanisms that underlie glucosensation [64,70] have made it difficult to find specific neuroanatomical markers for glucosensing neurons. It therefore remains unclear exactly where most glucosensing neurons are located in relation to precise neuroanatomical borders. However, there is consensus from many studies that important glucosensors for various counterregulatory responses are found in at least three locations: the hepatic portal-mesenteric veins (PMV), the hindbrain, and the hypothalamus (Fig. 1).
Hepatic Portal/Mesenteric Vein
The presence of glucose sensors in portal hepatis was first proposed by Rusek [90] based on the ability of portal glucose infusions to suppress hypoglycemia-induced hunger. It was subsequently shown that the sympathoadrenal response to hypoglycemia could be severely diminished by normalizing blood glucose levels in the portal vein alone [34,48]. Further, denervating the portal and mesenteric veins via topical phenol [49] or capsaicin [39] is equally effective in reducing counterregulatory responses to hypoglycemia. The portal-mesenteric glucose sensors appear particularly important when hypoglycemia develops slowly (as is the case in most clinical situations), accounting for 90%–95% of the sympathoadrenal response [91].
It has been known for some time that certain neurons in the CNS will respond to elevations in portal vein glucose levels. The majority of identifiable glucose sensitive neurons in the LHA [100] and NTS [1] also respond to portal vein glucose infusions. More recently it has been shown that hypoglycemia-induced FOS activation in the hindbrain is largely eliminated by chemical denervation of the PMV [12]. However, the route used to transmit portal-mesenteric glucosensation to the CNS remains somewhat controversial. Niijima [77,78] reported an inverse correlation between afferent discharges in the hepatic branch of the vagus and portal glucose concentrations. These observations have led to the widely held belief that glucose sensing in the portal vein is mediated by vagal afferents [79,118]. However, evidence from several studies casts doubt on this conclusion. Acute interruption of vagal transmission failed to impact upon the counterregulatory response to insulin-induced hypoglycemia [22,50]. Furthermore, chronic sectioning of the hepatic vagus or subdiaphragmatic vagus (bilateral) maintains normal sympathoadrenal responses to hypoglycemia [38]. In contrast, animals undergoing celiac-superior mesenteric ganglionectomy demonstrate a profound reduction in their catecholamine response to hypoglycemia [38]. As the celiac ganglion is located caudal to the site of the bilateral subdiaphragmatic vagotomy, these results indicate a spinal origin for those glucose-sensitive afferents innervating the portal vein. The portal vasculature is richly innervated by afferent fibers of spinal origin [6,7] and the majority of CGRP and substance P containing sensory neurons innervating the portal vasculature are spinal in origin [43]. Thus, the portal vasculature appears to be innervated by two sets of glucose-sensing afferents, i.e. vagal and spinal. Since the vagal afferents have been implicated in feeding behavior [107], spinal and vagal afferents may serve opposing functions analogous to those proposed for the glucose-excited and glucose-inhibited neurons of the CNS [64].
Hindbrain
The hindbrain houses important glucosensing elements [4,68], and although the precise location and nature of all these neurons is not known, the area postrema [40], the nucleus of the solitary tract (NTS) and the DMX all contain neurons that behave as classic glucosensors [5,73].
Hypothalamus
Neurons whose firing rates are altered after changes in ambient glucose concentrations were first identified in the hypothalamus [3,63–65]. Since then two hypothalamic locations have received most attention with regard to glucosensation.
Lateral Hypothalamic Area
The lateral hypothalamus (LHA) is large heterogeneously organized region that extends the entire length of the hypothalamus. It has long been associated with metabolic regulation. First, as a region that is important for feeding behavior [2]; as a site of glucosensing neurons [83,84]; and then with the discovery of orexin, as a region that can integrate metabolic and arousal state regulation [80]. Unfortunately, detailed information about the precise location of LHA glucosensing neurons is lacking, making it difficult to correlate this information with other functional and connectional aspects of the LHA.
Ventromedial Hypothalamus
Since the original identification of glucosensing neurons in the hypothalamus, much evidence has shown that neurons in the ventromedial hypothalamus—which includes the ventromedial (VMH), arcuate (ARH), and tuberal (TU) nuclei—play a critical regulatory role in developing sympathoadrenal and glucagon responses to hypoglycemia [eg. 10,13,14,24,25,102]. Although Ono reported glucoresponsive neurons recorded in vitro were located “near the center of the VMH”, (they illustrate examples in what appears to be the dorsomedial part of the VMH [82]), the exact locations of most glucosensing neurons in the ventromedial hypothalamus have not been mapped in any detail.
At this point it is worth making a comment about nomenclature. The abbreviation ‘VMH’ is sometimes used—often without further critical definition—to denote the ‘ventromedial hypothalamus’. This is a region encompassing a number of hypothalamic nuclei. Others use ‘VMH’ for the ‘ventromedial nucleus of the hypothalamus’, which is a well-defined and discrete cell group. VMN is often used to denote the ventromedial nucleus of the hypothalamus. Because the ‘ventromedial hypothalamus’ and ‘ventromedial nucleus of the hypothalamus’ are overlapping but different regions, using the same acronym for both has led to a great deal of confusion (see also [59]). We will use ‘VMH’ to denote the ‘ventromedial nucleus of the hypothalamus’, and will spell out the term ‘ventromedial hypothalamus’ in full when we refer to that region. Our usage derives from the neuroanatomical convention used by both Swanson [109] and Paxinos & Watson [87].
4) PRE-MOTOR CONTROL
Counterregulatory responses are regulated by sets of pre-motor neurons that provide direct projections to the appropriate neuroendocrine motor and preganglionic neurons in the hypothalamus, hindbrain, and spinal cord. Although some of these pre-motor neurons were first identified using conventional single neuron tracing techniques, the transneuronal transport of viral particles has proved the most revealing technique for those neurons that provide pre-motor and integrative projections into specific peripheral targets. In this way, injections of virus into the pancreas [18,51] or adrenal gland [55–57,106] have shown which regions in the hindbrain and hypothalamus contain pre-motor and integrative projection neurons (Fig. 3 & Fig. 4). These studies have shown that many of the same locations in the hindbrain and hypothalamus constitute the pre-autonomic network for regulating adrenal and pancreatic function. Although some degree of topographic distribution has been noted for those neurons within hindbrain and hypothalamic pre-motor networks associated with particular functions [20,92,132], further studies are needed to clarify exactly which specific neurons are responsible for particular counterregulatory functions, and where they are located.
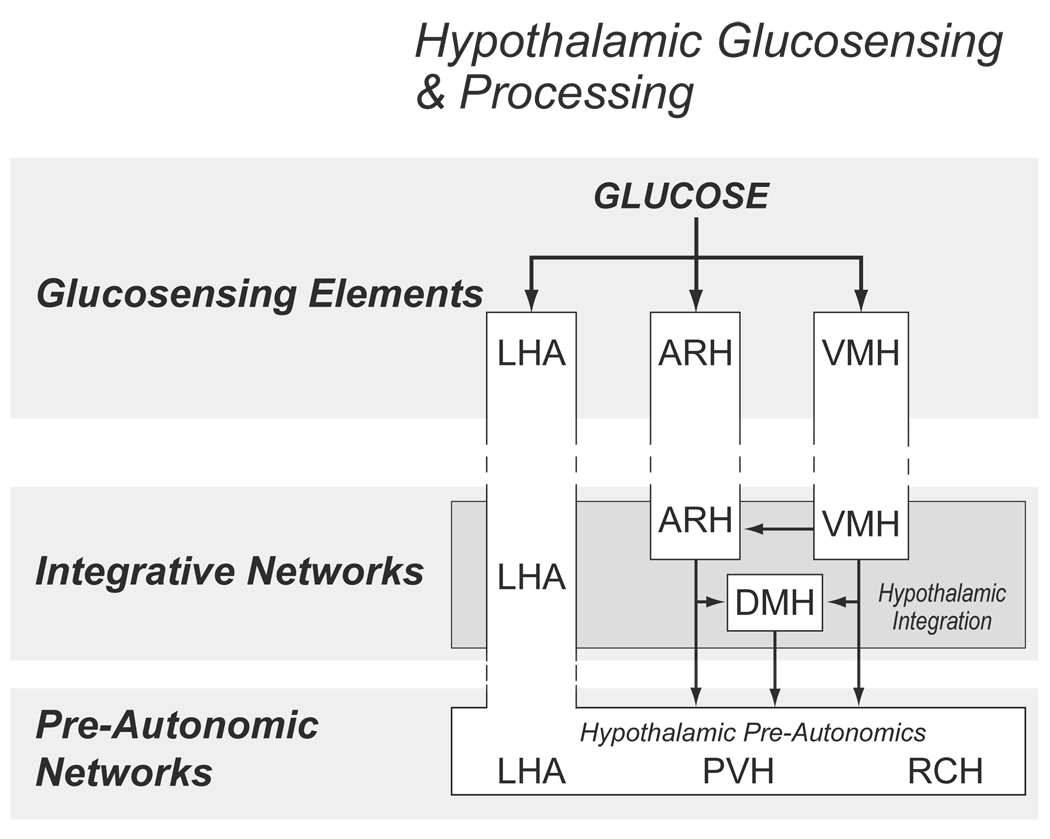
Figure 4.
An expansion of the model shown in Figure 1 to illustrate the organization of hypothalamic components involved with counterregulation. Solid lines represent known projections. Abbreviations: ARH, arcuate nucles; DMH, dorsomedial nucleus; LHA, lateral hypothalamic area; PVH, paraventricular nucleus of the hypothalamus; RCH, retrochiasmatic area; VMH, ventromedial nucleus.
Hindbrain
Injections of pseudorabies virus into the pancreas or adrenal gland have identified a limited number of hindbrain regions that provide pre-motor regulation for counterregulatory function (Fig. 3) [18–20,51,57,106]. These are the NTS, locus coeruleus, the A5 catecholamine cell group, rostral ventrolateral medulla, lateral paragigantocellular reticular nucleus, and medullary raphe nuclei. Interestingly, some of these regions contribute only to pre-autonomic parasympathetic regulation of the pancreas (the NTS, locus coeruleus), while others contributed to both parasympathetic and sympathetic control networks (the A5 catecholamine cell group, rostral ventrolateral medulla, lateral paragigantocellular reticular nucleus) [18.51].
Hypothalamus
We have known for many years that the hypothalamus contains sets of neurons that project to the hindbrain and spinal cord (eg. [94,111]) and can potentially regulate autonomic function in general, and counterregulation in particular [76]. Like the hindbrain, injections of viruses into the adrenal gland and pancreas have revealed pre-autonomic hypothalamic neurons in some detail [8–20,51,57,106]. Thus the PVH, LHA and retrochiasmatic area (RCH) in particular contribute to hypothalamic pre-autonomic control of these tissues, thereby providing the most direct way for the hypothalamus to engage the autonomic motor components of counterregulation (Fig. 4).
Paraventricular Nucleus
The PVH is a major hypothalamic region for counterregulation. It contains two functional parts; sets of parvicellular and magnocellular neuroendocrine neurons that project to the posterior pituitary or the median eminence, and a set of parvicellular neurons that project caudally to the hindbrain and spinal cord [111]. Some of these PVH neurons are key players for two different aspects of counterregulation.
First, the fact that injections of NPY, NE, or opiate agonists into the PVH, each of which increase plasma epinephrine and glucose concentrations [60,63] show that some PVH neurons with descending projections can regulate plasma glucose concentrations. The descending PVH projections responsible for regulating the adrenal medulla and pancreas have been revealed by virus injections in these organs [8–20,51,57,106]. Labeled neurons are found in the lateral, dorsal, and ventral parvicellular parts of the PVH. It should be noted that these neurons are quite distinct, both functionally and neuroanatomically, from medial parvicellular corticotrophin-releasing hormone (CRH) and TRH neuroendocrine neurons, which are never labeled by virus injections into the peripheral organs [51].
Second, CRH neuroendocrine motor neurons in the medial parvicellular (mp) part of the PVH drive the release of ACTH and glucocorticoid. Glucocorticoids are important metabolic hormones for the later stages of the counterregulatory response [28]. Hypoglycemia is a potent stimulus for ACTH release and like other components of counterregulation, is triggered when plasma glucose concentrations reach a distinct threshold [28]; in the rat, this is approximately 3.25mM (Fig. 2 [45]). The key glucosensing elements for ACTH release are located in the hindbrain rather than the hypothalamus [4,89]. These elements then engage catecholaminergic projections that densely innervate the PVH [89].
The PVH receives sets of projections from other hypothalamic regions that are key for metabolic regulation. In particular, the ARH [17,97,], LHA [97,128], and DMH [97,115] all provide strong projections into the PVH that likely play important modulatory roles for shaping counterregulatory responses.
Finally, it is worth noting that despite the presence of various genes and proteins linked to glucosensation [68,88], clear electrophysiological evidence of glucosensing neurons in the PVH is lacking.
Lateral Hypothalamus
The first studies to examine descending hypothalamic projections with sensitive neuroanatomical tracing techniques identified the LHA as an important source of projections to the hindbrain and spinal cord (eg. [94]). These findings have now been confirmed many times. More recently, virus injections into the pancreas or adrenal gland have shown which LHA neurons might control counterregulatory function [18–20,51,55,56]. However, their precise locations have yet to be mapped onto fine grained maps that will allow comparison with other data.
Given the extremely complex nature of the LHA, a complete picture of all LHA projections is still lacking. However, some general observations are apparent. First, the LHA provides complex ascending and descending projections. Many of these projections contain orexin or MCH, including some that are labeled after virus injections in the adrenal gland [56]. Other peptidergic LHA neurons, including those expressing neurotensin and in some circumstances CRH, also provide descending projections [23,54]. Second, the LHA has more local and often bidirectional projections with other hypothalamic regions concerned with counterregulation including the VMH [21], ARH [16,17], and PVH [97,128]. These projections provide a substrate for complex regulatory actions on counterregulatory function within the hypothalamus.
At this point it is worth noting that attempting to assign discrete LHA loci to particular functions has proved extremely difficult primarily because of our poor understanding of how the many LHA cell groups are organized. Consequently there is often no clear anatomical framework for considering different sets of experimental data. This difficulty, together with the fact that the medial forebrain bundle—the most complex fiber tract in the rodent brain—runs through the LHA, has greatly complicated the interpretation of experiments aimed at manipulating LHA function, particularly with lesions or injections. To help clarify LHA structure, Swanson and his colleagues have proposed that it is comprised of at least 9 different regions, some of which can be further subdivided [109,113]. Although many of these regions are seemingly poorly differentiated from each other, the nature of their connections provides a basis for more explicit parcellation (see [113]). Continually refining our understanding of LHA structure and connectional organization should provide a better framework for understanding how this large and complex region contributes to metabolic regulation.
Retrochiasmatic Area
The retrochiasmatic area is a located in the ventral part of the hypothalamus between the suprachiasmatic nucleus rostrally, and the ARH caudally. Like the LHA, the RCH contains retrogradely labeled neurons after injections of tracer in the spinal cord and hindbrain [35], and virus injections into the pancreas or adrenal gland. Little is currently known about the specific role of RCH neurons in counterregulatory function.
5) INTEGRATIVE NEURAL NETWORKS ENGAGED BY GLUCOSENSORS
Having discussed the sensory and pre-motor components of glucosensing networks, we now ask how sensory and motor components might be linked to form a functional network.
HINDBRAIN
Hindbrain neurons function as glucosensors, as recipients of the neural pathways engaged by glucosensors in the PMV, and as components of important premotor networks (Fig. 3). But the organizational details for how they integrate glucosensory information with other hindbrain systems to regulate counterregulatory responses remains unclear, primarily because the chemical phenotypes and connections of the all neurons directly involved with counterregulation are mostly uncharacterized. The potential for other hindbrain regions such as the parabrachial nucleus or the A2/C2 catecholaminergic neurons in the NTS to influence counterregulation is clear from their strong bidirectional projections with hypothalamic and hindbrain regions implicated in adrenal or pancreatic function. However, without sensitive, specific, and rapid markers of neuronal activation to identify neurons that respond specifically to hypoglycemia (see 129]), it remains difficult to determine which hindbrain neurons are intimately involved with integrating glucosensory information, and how they are connectionally and functionally organized.
HYPOTHALAMUS
Dorsomedial Nucleus
The DMH has long been linked to metabolic control (eg. [9]), including counterregulatory ACTH responses to hypoglycemia [37]. This influence is mediated, at least in part, by its strong projections to the PVH. The DMH also likely plays a pivotal role in mediating time-of-day effects on metabolic processes [53,95], as exemplified by the differential ACTH responses to hypoglycemia across the day (Fig. 2).
Projections of the Dorsomedial Nucleus
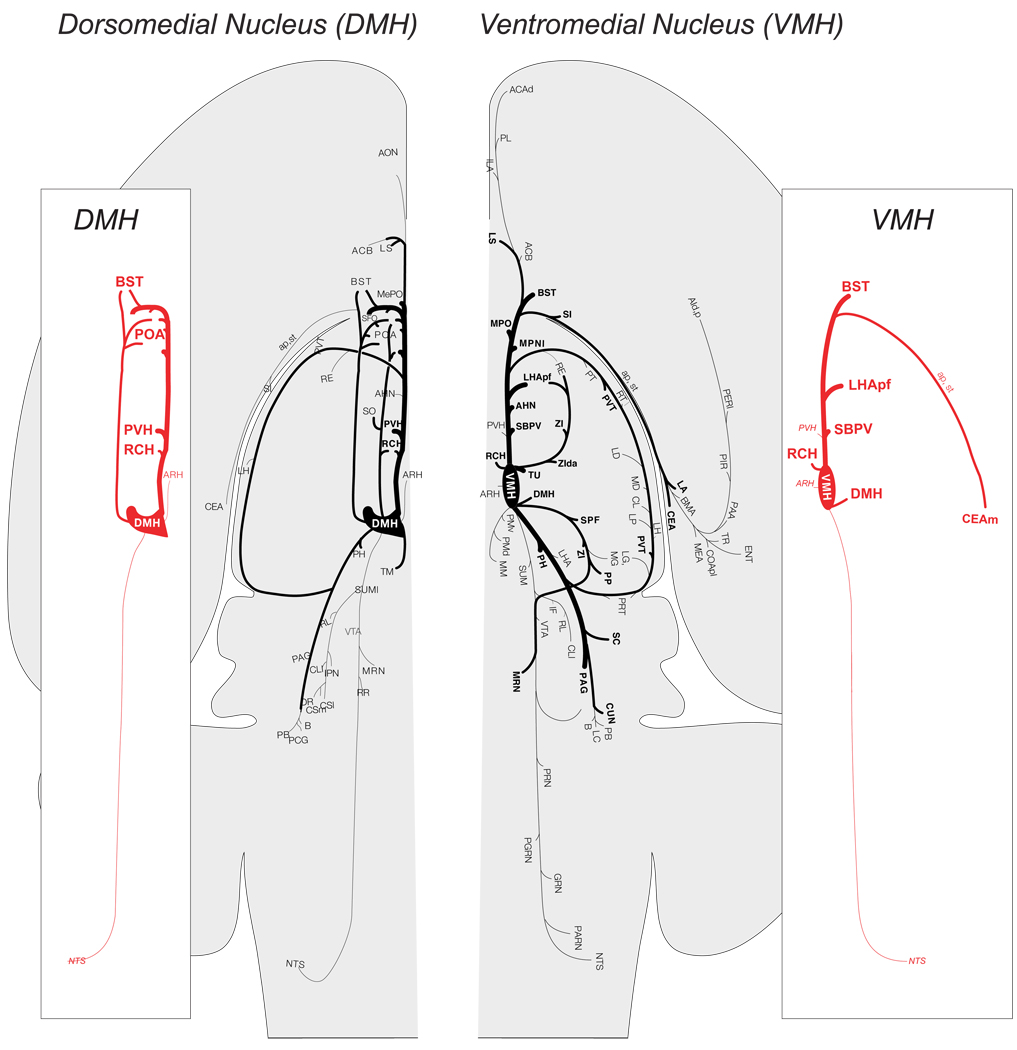
Figure 5 shows that DMH projections are generally confined to the forebrain; few substantial projections are seen further caudal than the periaqueductal gray [115,116]. This pattern supported the idea that the DMH acts as a visceromotor coordinator or pattern generator [117]. DMH neurons are labeled by virus injections into the adrenal, but the survival time required for a significant labeling suggests that labeling is derived primarily from projections to the PVH [18,55]. These findings are consistent with a DMH contribution to an integrative network that influences the output patterns of hypothalamic pre-autonomic neurons (Fig. 4), particularly in the PVH, and RCH [117].
Figure 5.
The efferent projections of the dorsomedial nucleus (DMH) and ventromedial nucleus (VMH) as revealed by the anterograde tracer Phaseolus vulgaris leucoagglutinin (PHAL) and then plotted onto a flat map of the rat brain [109]. The thickness of the lines represent the relative strengths of each projection. The side panels emphasize those projections from each nucleus to those regions that are most closely associated with metabolic regulation. Note the paucity of projections from each nucleus to those regions of the hindbrain important for autonomic function. (Adapted from [21,115]). Abbreviations in the side panels : ap, ansa peduncularis; ARH, arcuate nucleus hypothalamus; BST, bed nuclei stria terminalis; CEAm, central nucleus amygdala, medial part; DMH, dorsomedial nucleus hypothalamus; LHApf, lateral hypothalamic area, perifornical part; NTS, nucleus ofthe solitary tract; POA, preoptic area; PVH, paraventricular nucleus hypothalamus; RCH, retrochiasmatic area; SBPV, subparavenrticular zone; st, stria terminalis; VMH, ventromedial nucleus hypothalamus. Please refer to [21,115] for other abbreviations.
Ventromedial Nucleus of the Hypothalamus (VMH)
The VMH is most closely associated with three distinct functions: female sexual behavior, territoriality, and metabolism, making it a good candidate component in the hypothalamic integrative network for metabolic regulation. However, the precise role of the VMH in metabolic regulation has been vigorously debated for many years (eg. [44,59]) primarily because of the apparent discrepancy between functional and neuroanatomical data. A solid body of studies demonstrates an important role for the VMH in glucosensing and counterregulation [10,13,24,25,102,119]. Yet the neuroanatomical substrates of these effects remain frustratingly elusive, and reconciling these many and diverse studies has proved difficult.
The structure of the VMH is quite complex. In keeping with its diverse functions, the ventrolateral part of the VMH shows very strong expression of both gonadal steroid receptors, while the dorsomedial part preferentially expresses the short form of the leptin receptor and exhibits increased Fos protein in response to peripheral leptin injections [36].
Unlike neurons of the PVH and ARH whose dendrites tend to remain within the confines of the nuclear borders, VMH dendrites extend far out into the adjacent neuropil and often into the neighboring DMH, LHA, and ARH [72,114,121,122]. This structural complexity of the VMH, together with its proximity to the ARH, complicates the interpretation of experiments involving lesions or the injection of pharmacological or pathway tracing agents.
Projections of the Ventromedial Nucleus
Fine-grained anterograde tracing studies show that the three different parts of the VMH have rather distinct projection patterns [21]. But the VMH as a whole has a large and complex output. It is directed primarily towards targets in the diencephalon, telencephalon, and periaqueductal gray. (Fig. 5) Critically, tract tracing studies have consistently failed to identify direct connections between any part of the VMH and those regions in the spinal cord and hindbrain most closely associated with autonomic motor regulation [21,12,93,94,96,111]. Indeed, like the DMH, any VMH projections found further caudal than the periaqueductal gray are remarkably sparse (Fig. 5; [21]). Furthermore, injections of viral particles that migrate across synapses in a retrograde direction to label second-order projections have consistently failed to label significant numbers of VMH neurons when injections are made into the liver, pancreas, or adrenal gland [18,51,55]. These results clearly show that the VMH is not part of the immediate pre-motor hypothalamic regulatory network for counterregulation.
Given the lack of direct efferent projections to the hindbrain and spinal cord, how does the VMH regulate counterregulatory function? The most parsimonious explanation is that VMH must first engage pre-autonomic hypothalamic elements in the LHA, PVH, and RCH (Fig. 5 & Fig. 6).
Figure 6.
An expansion of the model shown in Figure 1 to illustrate interactions between the expanded network of peripheral, hindbrain and hypothalamic components involved with hypoglycemic counterregulation. Solid lines represent known projections. Connections between the hypothalamus and hindbrain are shown in red. Abbreviations: as Fig 3 and Fig 4.
The dorsomedial part of the VMH projects quite strongly to the more rostral parts of the perifornical LHA. Although some fibers are found lateral to the fornix most are located medially. No study has yet attempted to match the projection pattern of VMH neurons with the location of the pre-autonomic neurons in the LHA, and it remains unclear whether VMH fibers project specifically to those parts of the LHA containing pre-autonomic neurons.
The PVH also receives projections from the VMH, but these are much more sparse than those from the ARH, DMH and LHA. The more robust VMH projections into the PVH appear to target its anterior parvicellular part rather than its more caudal regions [21,97]. The PVHap is functionally poorly understood, but it does contain a few neurons that project to the dorsal vagal complex and spinal cord [46,96,111] as well as a much more substantial population of neuroendocrine neurons [101,111]. It is not known whether VMH projections to this part of the PVH constitute a conduit by which glucosensing information is directed to the hindbrain and spinal cord.
The VMH provides a strong input to the subparaventricular zone [21,36,124,126], which is a major component of the system used by the suprachiasmatic nucleus to input circadian information into hypothalamic regulatory systems [95,124]. VMH projections to the subparaventricular zone offer one way for glucosensing and circadian information to interact and modulate inputs to the PVH that affect counterregulatory responses (eg. Fig. 2).
Neuroanatomical data therefore show that the VMH has projections to regions that can engage pre-autonomic and neuroendocrine motor networks, but it remains unclear whether the parts of the LHA and PVH that receive VMH projections contain significant numbers of pre-autonomic neurons [21]. However, Buijs and colleagues [18] did report retrogradely labeled virus in the VMH and ARH 5 days after injections of pseudorabies virus into the pancreas, which they interpreted as constituting third-order neurons in the regulatory network. On this evidence at least VMH neurons are able to access pre-autonomic neurons that regulate pancreatic function, but the precise locations where this interaction occurs remain unknown.
Arcuate Nucleus
For many years attention focused on the ARH as controller of neuroendocrine function because of its location within the “hypophysiotrophic area“ [114]. A role in metabolic control began to emerge when it was noted that neonatally applied monosodium glutamate lesioned the ARH and produced obese animals [81]. But the findings showing that NPY was potent orexigen [27,66,104], that NPY neurons were found in the ARH, and that ARH Npy expression was elevated by streptozotocin [130] or food deprivation [15], finally shifted interest towards the metabolic regulatory functions of the ARH. Since then the discovery of leptin, insulin, and ghrelin receptors on ARH NPY/AgRP and POMC neurons [8,26,69,131], and the characterization of ARH glucosensing neurons [123] have reinforced the idea that the ARH is a key region by which circulating metabolic signals can access control networks in the brain [61].
Projections of the Arcuate Nucleus
The location and small size of the ARH has made it very difficult to trace its efferent projections using conventional tracing techniques. However, the discovery that ARH NPY neurons are the only source of agouti-related protein (AGRP) in the brain offered a way to delineate ARH projections using immunocytochemistry, or at least those derived from NPY/AGRP neurons. Another important cell type in the ARH are neurons containing Pomc-derived peptides, particularly α-MSH. The use of α-MSH immunocytochemistry has also helped delineate ARH projections. However, the fact that POMC neurons are also found in the hindbrain complicates interpretation of ARH projections, at least to this part of the brain.
Using AGRP or α-MSH immunocytochemistry ARH projections were seen in the telencephalon, hypothalamus, with some descending projections reported to the periaqueductal gray and parabrachial nucleus [17,47] that confirm an earlier report using retrograde tracing [Moga et al, 1990]. Importantly however, few AGRP fibers were reported in the caudlal hindbrain and spinal cord.
Within the hypothalamus, ARH neurons have strong projections to the LHA and PVH [16,97] thereby providing a direct link with pre-autonomic control networks. NPY/AGRP neurons provide a dense input to medial parvicellular parts of the PVH [42] that house neuroendocrine neurons projecting to the median eminence. However, in the rat at least, the majority of the NPY input to neuroendocrine CRH neurons does not originate in the ARH but from epinephrine-containing neurons in the hindbrain [42]. This input, along with norepinephrine-containing projections, is critical for ACTH and glucocorticoid responses to glucoprivation and hypoglycemia [58,89]. AGRP fibers are also found in part of the DMH [17,47] and within the ventromedial hypothalamus where they circumscribe but do not enter the VMH [17].
6) THE EXTENDED NETWORK AND INTERACTIONS BETWEEN COMPONENTS
Up to this point we have been discussing the various glucoregulatory components in relative isolation. And to some extent, this is how have they have been viewed by the field in the past. But it is very unlikely that this how the brain regulates metabolism. The various components are connected, often with a degree of complexity that can provide greater flexibility for counterregulation than can be achieved by the hindbrain or hypothalamus alone. For example, the hindbrain contains all the requisite components for it to generate counterregulatory responses without any forebrain intervention (Fig. 1 & Fig. 3). This notion is consistent with the findings that chronic decerebrate rats show a robust sympathoadrenal response to pharmacologic glucoprivic challenges [33]. However, it remains to be determined how these animals cope with more physiological glycemic challenges.
How are these interactions mediated? We have already described descending projections from the hypothalamus and hindbrain. But a more complete network (Fig. 6) must also incorporate projections from the hindbrain to the hypothalamus. These connections together allow the exchange of information in both directions and likely provide for greater adaptability in counterregulation. It should be noted that Figure 6 omits telencephalic projections into the extended network that are revealed using peripheral virus injections [18,51]. Whether they contribute to counterregulation is unknown.
Hindbrain to hypothalamic projections are many and varied. They contain a variety of neurochemicals, the best characterized of which are those containing catecholamines. These projections offer a good model for considering the functional implications of the wider interactions between the different components in the hindbrain and hypothalamus.
It has been known for about 45 years that hindbrain CAergic neurons provide a rich innervation of the hypothalamus [41]. Catecholaminergic neurons release norepinephrine (NE) or epinephrine (E) at their terminals, and may colocalize NPY [98], galanin [65], and/or possibly glutamate [105]. Virtually all hypothalamic regions receive some degree of catecholaminergic innervation, but the PVH receives a particularly dense input [110]. PVH inputs originate from a number of different hindbrain cell groups, and each provides a somewhat different distribution to PVH subdivisions [29]. In particular, neurons in A2/C2 (medial NTS) regions innervate parvicellular parts of the PVH including its lateral region that in turn, sends projections back to pre-autonomic control regions in the hindbrain and spinal cord [112].
Parts of the LHA and DMH also receive significant catecholaminergic inputs [110]. However, their density is not as robust as that innervating the PVH. Of particular importance for considering counterregulatory mechanisms is the fact that, when examined with neuroanatomical methods, the ARH and VMH themselves are virtually devoid of catecholaminergic innervation [75,110] whereas their immediate surrounds are more heavily innervated—particularly on the dorsomedial and ventromedial side of the VMH, and in the tuberal nucleus [110].
The peri-VMH region contain VMH dendrites that extend well beyond the nucleus itself [72,114], which may provide the structural substrate for the direct catecholaminergic-ventromedial hypothalamic interactions revealed by Beverly and coworkers. These studies highlight the complex interactions between the catecholaminergic innervation of the ventromedial hypothalamus and its local glucosensing mechanisms [10,11,30–32]. Thus, insulin-induced hypoglycemia robustly increases NE release in the ventromedial hypothalamus and the PVH [11]. Interestingly, maintenance of local glucose concentrations in the ventromedial hypothalamus blocks both sympathoadrenal responses to rapid insulin-induced hypoglycemia and local NE release [32]. As well as reinforcing the role that hypothalamic glucosensors play in developing counterregulatory responses, these studies reveal potentially critical interactions between glucosensing mechanisms and the catecholaminergic innervation of the ventromedial hypothalamus. But the fact that maintaining local extracellular glucose also abolishes NE release [32] leaves unresolved questions about the specific role of local catecholaminergic innervation, and whether hypothalamic glucosensing is indeed self-sufficient for activating counterregulatory responses.
7) CONCLUSION
Mayer’s fundamental idea that some neurons act as glucosensors [71] has spurred a great deal of research into the way the brain uses the interosensory information derived from metabolic processes to generate and modulate adaptive responses. In particular, we now have a good idea about the cellular and molecular mechanisms that underlie glucosensation. We also know that glucosensing neurons in the PMV, hypothalamus, and hindbrain are important for counterregulation. But the finding that glucosensors in the PMV are required for sympathoadrenal responses to slow- but not fast-onset hypoglycemia [91], and that ACTH responses to hypoglycemia require hindbrain but not hypothalamic glucosensation [58,89], both illustrate the fact the glucosensors in each of these locations provide opportunities for differential and nuanced processing rather than simple redundancy. These findings pose the question about the particular function each set of glucosensors contributes to counterregulation in the varied circumstances in which it is invoked.
One way to think about the likely distinct hypothalamic and hindbrain contributions to counterregulation is to consider how the brain organizes other adaptive autonomic and behavioral responses. Here the forebrain provides crucial and often powerful modulatory influences onto the ‘reflex loops’ that exist in the hindbrain [67,92,103,127]. The fact that the hindbrain can, or at least has the potential to initiate counterregulatory responses without any input from the forebrain [33], supports the notion that counterregulation also has a hierarchical, or at least multi-layered organization. Currently we do not adequately understand the nature and detailed arrangement of the components illustrated in Figure 6 to be able to make a determination about whether this hypothesis is correct. However, it is anticipated that future studies will provide the functional and neuroanatomical data to refine and expand the network models we have discussed here.
Acknowledgments
Work from the authors’ laboratories is supported by NS029728 from NINDS, NIH and grant 1-2008-710 from the Juvenile Diabetes Research Foundation (AGW); and DK062471 from NIDDK, NIH and grant 1-2007-605 the JDRF (CMD).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Adachi A, Shimizu N, Oomura Y, Kobáshi M. Convergence of hepatoportal glucose-sensitive afferent signals to glucose-sensitive units within the nucleus of the solitary tract. Neurosci. Letters. 1984;46:215–218. doi: 10.1016/0304-3940(84)90444-0. [DOI] [PubMed] [Google Scholar]
- 2.Anand BK, Brobeck JR. Hypothalamic control of food intake in rats and cats. Yale J. Biol. Med. 1951;24:123–140. [PMC free article] [PubMed] [Google Scholar]
- 3.Anand BK, Chhina GS, Sharma KN, Dua S, Singh B. Activity of single neurons in the hypothalamic feeding centers: effect of glucose. Am. J. Physiol. 1964;207:1146–1154. doi: 10.1152/ajplegacy.1964.207.5.1146. [DOI] [PubMed] [Google Scholar]
- 4.Andrew SF, Dinh TT, Ritter S. Localized glucoprivation of hindbrain sites elicits corticosterone and glucagon secretion. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2007;292:R1792–R1798. doi: 10.1152/ajpregu.00777.2006. [DOI] [PubMed] [Google Scholar]
- 5.Balfour RH, Hansen AM, Trapp S. Neuronal responses to transient hypoglycaemia in the dorsal vagal complex of the rat brainstem. J. Physiol. 2006;570:469–484. doi: 10.1113/jphysiol.2005.098822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barja F, Mathison R. Adrenergic and peptidergic (substance P and vasoactive intestinal peptide) innervation of the rat portal vein. Blood Vessels. 1982;19:263–272. doi: 10.1159/000158392. [DOI] [PubMed] [Google Scholar]
- 7.Barja F, Mathison R. Sensory innervation of the rat portal vein and the hepatic artery. J. Auton. Nerv. Syst. 1984;10:117–125. doi: 10.1016/0165-1838(84)90050-x. [DOI] [PubMed] [Google Scholar]
- 8.Baskin DG, Breininger JF, Schwartz MW. Leptin receptor mRNA identifies a subpopulation of neuropeptide Y neurons activated by fasting in rat hypothalamus. Diabetes. 1999;48:828–833. doi: 10.2337/diabetes.48.4.828. [DOI] [PubMed] [Google Scholar]
- 9.Bellinger LL, Bernardis LL. The dorsomedial hypothalamic nucleus and its role in ingestive behavior and body weight regulation: lessons learned from lesioning studies. Physiol. Behav. 2002;76:431–442. doi: 10.1016/s0031-9384(02)00756-4. [DOI] [PubMed] [Google Scholar]
- 10.Beverly JL, de Vries MG, Beverly MF, Arseneau LM. Norepinephrine mediates glucoprivic-induced increase in GABA in the ventromedial hypothalamus of rats. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2000;279:R990–R996. doi: 10.1152/ajpregu.2000.279.3.R990. [DOI] [PubMed] [Google Scholar]
- 11.Beverly JL, de Vries MG, Bouman SD, Arseneau LM. Noradrenergic and GABAergic systems in the medial hypothalamus are activated during hypoglycemia. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2001;280:R563–R569. doi: 10.1152/ajpregu.2001.280.2.R563. [DOI] [PubMed] [Google Scholar]
- 12.Bohland MA, Khan AM, Watts AG, Donovan CM. Hypoglycemic Activation of Select Hindbrain Neurons is Mediated by Portal-Mesenteric Vein Glucosensors. Diabetes. 2005;54:A27. doi: 10.2337/db13-1600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Borg MA, Sherwin RS, Borg WP, Tamborlane WV, Shulman GI. Local ventromedial hypothalamus glucose perfusion blocks counterregulation during systemic hypoglycemia in awake rats. J. Clin. Invest. 1997;99:361–365. doi: 10.1172/JCI119165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Borg WP, During MJ, Sherwin RS, Borg MA, Brines ML, Shulman GI. Ventromedial hypothalamic lesions in rats suppress counterregulatory responses to hypoglycemia. J. Clin. Invest. 1994;93:1677–1682. doi: 10.1172/JCI117150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brady LS, Smith MA, Gold PW, Herkenham M. Altered expression of hypothalamic neuropeptide mRNAs in food-restricted and food-deprived rats. Neuroendocrinology. 1990;52:441–447. doi: 10.1159/000125626. [DOI] [PubMed] [Google Scholar]
- 16.Broberger C, de Lecea L, Sutcliffe JG, Hökfelt T. Hypocretin/orexin- and melanin-concentrating hormone-expressing cells form distinct populations in the rodent lateral hypothalamus: relationship to the neuropeptide Y and agouti gene-related protein systems. J. Comp. Neurol. 1998;402:460–474. [PubMed] [Google Scholar]
- 17.Broberger C, Johansen J, Johansson C, Schalling M, Hökfelt T. The neuropeptide Y/agouti gene-related protein (AGRP) brain circuitry in normal, anorectic, and monosodium glutamate-treated mice. Proc. Natl. Acad. Sci. USA. 1998;95:15043–15048. doi: 10.1073/pnas.95.25.15043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Buijs RM, Chun SJ, Niijima A, Romijn HJ, Nagai K. Parasympathetic and sympathetic control of the pancreas: a role for the suprachiasmatic nucleus and other hypothalamic centers that are involved in the regulation of food intake. J. Comp. Neurol. 2001;431:405–423. doi: 10.1002/1096-9861(20010319)431:4<405::aid-cne1079>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 19.Buijs RM, Wortel J, Van Heerikhuize JJ, Feenstra MG, Ter Horst GJ, Romijn HJ, Kalsbeek A. Anatomical and functional demonstration of a multisynaptic suprachiasmatic nucleus adrenal (cortex) pathway. Eur. J. Neurosci. 1999;11:1535–1544. doi: 10.1046/j.1460-9568.1999.00575.x. [DOI] [PubMed] [Google Scholar]
- 20.Buijs RM, la Fleur SE, Wortel J, Van Heyningen C, Zuiddam L, Mettenleiter TC, Kalsbeek A, Nagai K, Niijima A. The suprachiasmatic nucleus balances sympathetic and parasympathetic output to peripheral organs through separate preautonomic neurons. J. Comp. Neurol. 2003;464:36–48. doi: 10.1002/cne.10765. [DOI] [PubMed] [Google Scholar]
- 21.Canteras NS, Simerly RB, Swanson LW. Organization of projections from the ventromedial nucleus of the hypothalamus: a Phaseolus vulgaris-leucoagglutinin study in the rat. J. Comp. Neurol. 1994;348:41–79. doi: 10.1002/cne.903480103. [DOI] [PubMed] [Google Scholar]
- 22.Cardin S, Jackson P, Edgerton D, Neal D, Coffey C, Cherrington A. Effect of vagal cooling on the counterregulatory response to hypoglycemia induced by a low dose of insulin in the concious dog. Diabetes. 2001;50:558–564. doi: 10.2337/diabetes.50.3.558. [DOI] [PubMed] [Google Scholar]
- 23.Cechetto DF, Saper CB. Neurochemical organization of the hypothalamic projection to the spinal cord in the rat. J. Comp. Neurol. 1988;272:579–604. doi: 10.1002/cne.902720410. [DOI] [PubMed] [Google Scholar]
- 24.Chan O, Zhu W, Ding Y, McCrimmon RJ, Sherwin RS. Blockade of GABA(A) receptors in the ventromedial hypothalamus further stimulates glucagon and sympathoadrenal but not the hypothalamo-pituitary-adrenal response to hypoglycemia. Diabetes. 2006;55:1080–1087. doi: 10.2337/diabetes.55.04.06.db05-0958. [DOI] [PubMed] [Google Scholar]
- 25.Chan O, Cheng H, Herzog R, Czyzyk D, Zhu W, Wang A, McCrimmon RJ, Seashore MR, Sherwin RS. Increased GABAergic tone in the ventromedial hypothalamus contributes to suppression of counterregulatory responses after antecedent hypoglycemia. Diabetes. 2008;57:1363–1370. doi: 10.2337/db07-1559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cheung CC, Clifton DK, Steiner RA. Proopiomelanocortin neurons are direct targets for leptin in the hypothalamus. Endocrinol. 1997;138:4489–4492. doi: 10.1210/endo.138.10.5570. [DOI] [PubMed] [Google Scholar]
- 27.Clark JT, Kalra PS, Crowley WR, Kalra SP. Neuropeptide Y and human pancreatic polypeptide stimulate feeding behavior in rats. Endocrinology. 1984;115:427–429. doi: 10.1210/endo-115-1-427. [DOI] [PubMed] [Google Scholar]
- 28.Cryer PE. Hierarchy of physiological responses to hypoglycemia: relevance to clinical hypoglycemia in type I (insulin dependent) diabetes mellitus. Horm. Metab. Res. 1997;29:92–96. doi: 10.1055/s-2007-978997. [DOI] [PubMed] [Google Scholar]
- 29.Cunningham ET, Jr, Sawchenko PE. Anatomical specificity of noradrenergic inputs to the paraventricular and supraoptic nuclei of the rat hypothalamus. J. Comp. Neurol. 1988;274:60–76. doi: 10.1002/cne.902740107. [DOI] [PubMed] [Google Scholar]
- 30.de Vries MG, Arseneau LM, Lawson MA, Beverly JL. Extracellular glucose in rat ventromedial hypothalamus during acute and recurrent hypoglycemia. Diabetes. 2003;52:2767–2773. doi: 10.2337/diabetes.52.11.2767. [DOI] [PubMed] [Google Scholar]
- 31.de Vries MG, Lawson MA, Beverly JL. Dissociation of hypothalamic noradrenergic activity and sympathoadrenal responses to recurrent hypoglycemia. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2004;286:R910–R915. doi: 10.1152/ajpregu.00254.2002. [DOI] [PubMed] [Google Scholar]
- 32.de Vries MG, Lawson MA, Beverly JL. Hypoglycemia-induced noradrenergic activation in the VMH is a result of decreased ambient glucose. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2005;289:R977–R981. doi: 10.1152/ajpregu.00403.2005. [DOI] [PubMed] [Google Scholar]
- 33.DiRocco RJ, Grill HJ. The forebrain is not essential for sympathoadrenal hyperglycemic response to glucoprivation. Science. 1979;204:1112–1114. doi: 10.1126/science.451558. [DOI] [PubMed] [Google Scholar]
- 34.Donovan C, Hamilton-Wessler M, Halter J, Bergman R. Primacy of liver glucosensors in the sympathetic response to progressive hypoglycemia. Proc. Natl. Acad. ScI. 1994;91:2863–2867. doi: 10.1073/pnas.91.7.2863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Elias CF, Lee C, Kelly J, Aschkenasi C, Ahima RS, Couceyro PR, Kuhar MJ, Saper CB, Elmquist JK. Leptin activates hypothalamic CART neurons projecting to the spinal cord. Neuron. 1998;21:1375–1385. doi: 10.1016/s0896-6273(00)80656-x. [DOI] [PubMed] [Google Scholar]
- 36.Elmquist JK, Ahima RS, Elias CF, Flier JS, Saper CB. Leptin activates distinct projections from the dorsomedial and ventromedial hypothalamic nuclei. Proc. Natl. Acad. Sci. USA. 1998;95:741–746. doi: 10.1073/pnas.95.2.741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Evans SB, Wilkinson CW, Gronbeck P, Bennett JL, Zavosh A, Taborsky GJ, Jr, Figlewicz DP. Inactivation of the DMH selectively inhibits the ACTH and corticosterone responses to hypoglycemia. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2004;286:R123–R128. doi: 10.1152/ajpregu.00328.2003. [DOI] [PubMed] [Google Scholar]
- 38.Fujita S, Donovan CM. Celiac-superior mesenteric ganglionectomy, but not vagotomy, suppresses the sympathoadrenal response to insulin-induced hypoglycemia. Diabetes. 2005;54:3258–3264. doi: 10.2337/diabetes.54.11.3258. [DOI] [PubMed] [Google Scholar]
- 39.Fujita S, Bohland MA, Sanchez-Watts G, Watts AG, Donovan CM. Hypoglycemic detection at the portal vein is mediated by capsaicin-sensitive primary sensory neurons. Amer. J. of Physiol. Endocrinol. & Metabol. 2007;293:E96–E101. doi: 10.1152/ajpendo.00415.2006. [DOI] [PubMed] [Google Scholar]
- 40.Funahashi M, Adachi A. Glucose-responsive neurons exist within the area postrema of the rat: in vitro study on the isolated slice preparation. Brain Res. Bull. 1993;32:531–535. doi: 10.1016/0361-9230(93)90303-s. [DOI] [PubMed] [Google Scholar]
- 41.Fuxe K. Evidence for the existence of monoamine neurons in the central nervous system. iv. distribution of monoamine nerve terminals in the central nervous system. Acta Physiol. Scand. Suppl. 1965;247:37–85. [PubMed] [Google Scholar]
- 42.Füzesi T, Wittmann G, Liposits Z, Lechan RM, Fekete C. Contribution of noradrenergic and adrenergic cell groups of the brainstem and agouti-related protein-synthesizing neurons of the arcuate nucleus to neuropeptide-Y innervation of corticotropin-releasing hormone neurons in hypothalamic paraventricular nucleus of the rat. Endocrinology. 2007;148:5442–5450. doi: 10.1210/en.2007-0732. [DOI] [PubMed] [Google Scholar]
- 43.Goehler L, Sternini C. Calcitonin gene-related peptide innervation of the rat hepatobiliary system. Peptides. 1996;17:209–217. doi: 10.1016/0196-9781(95)02126-4. [DOI] [PubMed] [Google Scholar]
- 44.Gold RM. Hypothalamic obesity: the myth of the ventromedial nucleus. Science. 1973;182:488–490. doi: 10.1126/science.182.4111.488. [DOI] [PubMed] [Google Scholar]
- 45.Gorton LM, Khan AM, Bohland MA, Sanchez-Watts G, Donovan CM, Watts AG. A role for the forebrain in mediating time-of-day differences in glucocorticoid counterregulatory responses to hypoglycemia. Endocrinol. 2007;148:6026–6039. doi: 10.1210/en.2007-0194. [DOI] [PubMed] [Google Scholar]
- 46.Hallbeck M, Larhammar D, Blomqvist A. Neuropeptide expression in rat paraventricular hypothalamic neurons that project to the spinal cord. J. Comp. Neurol. 2001;433:222–238. doi: 10.1002/cne.1137. [DOI] [PubMed] [Google Scholar]
- 47.Haskell-Luevano C, Chen P, Li C, Chang K, Smith MS, Cameron JL, Cone RD. Characterization of the neuroanatomical distribution of agouti-related protein immunoreactivity in the rhesus monkey and the rat. Endocrinol. 1999;140:1408–1415. doi: 10.1210/endo.140.3.6544. [DOI] [PubMed] [Google Scholar]
- 48.Hevener A, Bergman R, Donovan C. Novel glucosensor for hypoglycemic detention localized to the portal vein. Diabetes. 1997;46:1521–1525. doi: 10.2337/diab.46.9.1521. [DOI] [PubMed] [Google Scholar]
- 49.Hevener A, Bergman R, Donovan C. Portal vein afferents are critical for hypoglycemic detection. Diabetes. 2000;49:8–12. doi: 10.2337/diabetes.49.1.8. [DOI] [PubMed] [Google Scholar]
- 50.Jackson P, Cardin S, Coffey C, Neal D, Allen E, Penaloza A, Snead W, Cherrington A. The effect of hepatic denervation on the counter-regulatory response to insulin-induced hypoglycemia in the dog. Am. J. Physiol. 2000;279:E1249–E1257. doi: 10.1152/ajpendo.2000.279.6.E1249. [DOI] [PubMed] [Google Scholar]
- 51.Jansen AS, Hoffman JL, Loewy AD. CNS sites involved in sympathetic and parasympathetic control of the pancreas: a viral tracing study. Brain Res. 1997;766:29–38. doi: 10.1016/s0006-8993(97)00532-5. [DOI] [PubMed] [Google Scholar]
- 52.Kalsbeek A, Ruiter M, la Fleur SE, Van Heijningen C, Buijs RM. The diurnal modulation of hormonal responses in the rat varies with different stimuli. J. Neuroendocrinol. 2003;15:1144–1155. doi: 10.1111/j.1365-2826.2003.01112.x. [DOI] [PubMed] [Google Scholar]
- 53.Kalsbeek A, Foppen E, Schalij I, Van Heijningen C, van der Vliet J, Fliers E, Buijs RM. Circadian control of the daily plasma glucose rhythm: an interplay of GABA and glutamate. PLoS One. 2008;3:e3194. doi: 10.1371/journal.pone.0003194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kelly AB, Watts AG. The region of the pontine parabrachial nucleus is a major target of dehydration-sensitive CRH neurons in the rat lateral hypothalamic area. J. Comp. Neurol. 1998;394:48–63. doi: 10.1002/(sici)1096-9861(19980427)394:1<48::aid-cne5>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 55.Kerman IA, Akil H, Watson SJ. Rostral elements of sympatho-motor circuitry: a virally mediated transsynaptic tracing study. J. Neurosci. 2006;26:3423–3433. doi: 10.1523/JNEUROSCI.5283-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kerman IA, Bernard R, Rosenthal D, Beals J, Akil H, Watson SJ. Distinct populations of presympathetic-premotor neurons express orexin or melanin-concentrating hormone in the rat lateral hypothalamus. J. Comp. Neurol. 2007;505:586–601. doi: 10.1002/cne.21511. [DOI] [PubMed] [Google Scholar]
- 57.Kerman IA, Enquist LW, Watson SJ, Yates BJ. Brainstem substrates of sympatho-motor circuitry identified using trans-synaptic tracing with pseudorabies virus recombinants. J. Neurosci. 2003;23:4657–4666. doi: 10.1523/JNEUROSCI.23-11-04657.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Khan AM, Rapp KL, Ponzio TA, Sanchez-Watts G, Watts AG. Stimulus-, circuit- and intracellular-level determinants of MAP kinase and CREB activation in parvicellular hypothalamic paraventricular neurons 865.23. Society for Neuroscience Meeting Planner. 2008 Online. [Google Scholar]
- 59.King BM. The rise, fall, and resurrection of the ventromedial hypothalamus in the regulation of feeding behavior and body weight. Physiol. Behav. 2006;87:221–244. doi: 10.1016/j.physbeh.2005.10.007. [DOI] [PubMed] [Google Scholar]
- 60.Kiritsy-Roy JA, Appel NM, Bobbitt FG, Van Loon GR. Effects of mu-opioid receptor stimulation in the hypothalamic paraventricular nucleus on basal and stress-induced catecholamine secretion and cardiovascular responses. J. Pharmacol. Exp. Ther. 1986;239:814–822. [PubMed] [Google Scholar]
- 61.Könner AC, Janoschek R, Plum L, Jordan SD, Rother E, Ma X, Xu C, Enriori P, Hampel B, Barsh GS, Kahn CR, Cowley MA, Ashcroft FM, Brüning JC. Insulin action in AgRP-expressing neurons is required for suppression of hepatic glucose production. Cell Metab. 2007;5:438–449. doi: 10.1016/j.cmet.2007.05.004. [DOI] [PubMed] [Google Scholar]
- 62.Kurose T, Seino Y, Nishi S, Tsuji K, Taminato T, Tsuda K, Imura H. Mechanism of sympathetic neural regulation of insulin, somatostatin, and glucagon secretion. Am. J. Physiol. 1990;258:E220–E227. doi: 10.1152/ajpendo.1990.258.1.E220. [DOI] [PubMed] [Google Scholar]
- 63.Leibowitz SF, Sladek C, Spencer L, Tempel D. Neuropeptide Y, epinephrine and norepinephrine in the paraventricular nucleus: stimulation of feeding and the release of corticosterone, vasopressin and glucose. Brain Res. Bull. 1988;21:905–912. doi: 10.1016/0361-9230(88)90025-1. [DOI] [PubMed] [Google Scholar]
- 64.Levin BE, Routh VH, Kang L, Sanders NM, Dunn-Meynell AA. Neuronal glucosensing: What do we know after 50 years. Diabetes. 2004;53:2521–2528. doi: 10.2337/diabetes.53.10.2521. [DOI] [PubMed] [Google Scholar]
- 65.Levin MC, Sawchenko PE, Howe PR, Bloom SR, Polak JM. Organization of galanin-immunoreactive inputs to the paraventricular nucleus with special reference to their relationship to catecholaminergic afferents. J. Comp. Neurol. 1987;261:562–582. doi: 10.1002/cne.902610408. [DOI] [PubMed] [Google Scholar]
- 66.Levine AS, Morley JE. Neuropeptide Y: a potent inducer of consummatory behavior in rats. Peptides. 1984;5:1025–1029. doi: 10.1016/0196-9781(84)90165-7. [DOI] [PubMed] [Google Scholar]
- 67.Loewy AD. Forebrain nuclei involved in autonomic control. Prog. Brain Res. 1991;87:253–268. doi: 10.1016/s0079-6123(08)63055-1. [DOI] [PubMed] [Google Scholar]
- 68.Lynch RM, Tompkins LS, Brooks HL, Dunn-Meynell AA, Levin BE. Localization of glucokinase gene expression in the rat brain. Diabetes. 2000;49:693–700. doi: 10.2337/diabetes.49.5.693. [DOI] [PubMed] [Google Scholar]
- 69.Marks JL, Porte D, Jr, Stahl WL, Baskin DG. Localization of insulin receptor mRNA in rat brain by in situ hybridization. Endocrinol. 1990;127:3234–3236. doi: 10.1210/endo-127-6-3234. [DOI] [PubMed] [Google Scholar]
- 70.Marty N, Dallaporta M, Thorens B. Brain glucose sensing, counterregulation, and energy homeostasis. Physiol. 2007;22:241–251. doi: 10.1152/physiol.00010.2007. [DOI] [PubMed] [Google Scholar]
- 71.Mayer J. Glucostatic mechanism of regulation of food intake. N. Engl. J. Med. 1953;249:13–16. doi: 10.1056/NEJM195307022490104. [DOI] [PubMed] [Google Scholar]
- 72.Millhouse OE. The organization of the ventromedial hypothalamic nucleus. Brain Res. 1973;55:71–87. [PubMed] [Google Scholar]
- 73.Mizuno Y, Oomura Y. Glucose responding neurons in the nucleus tractus solitarius of the rat: in vitro study. Brain Res. 1984;307:109–116. doi: 10.1016/0006-8993(84)90466-9. [DOI] [PubMed] [Google Scholar]
- 74.Moga MM, Saper CB, Gray TS. Neuropeptide organization of the hypothalamic projection to the parabrachial nucleus in the rat. J. Comp. Neurol. 1990;295:662–682. doi: 10.1002/cne.902950409. [DOI] [PubMed] [Google Scholar]
- 75.Moore RY, Bloom FE. Central catecholamine neuron systems: anatomy and physiology of the norepinephrine and epinephrine systems. Annu. Rev. Neurosci. 1979;2:13–68. doi: 10.1146/annurev.ne.02.030179.000553. [DOI] [PubMed] [Google Scholar]
- 76.Motawei K, Pyner S, Ranson RN, Kamel M, Coote JH. Terminals of paraventricular spinal neurones are closely associated with adrenal medullary sympathetic preganglionic neurones: immunocytochemical evidence for vasopressin as a possible neurotransmitter in this pathway. Exp. Brain Res. 1999;126:68–76. doi: 10.1007/s002210050717. [DOI] [PubMed] [Google Scholar]
- 77.Niijima A. Afferent impulse discharges from glucoreceptors in the liver of the guinea pig. Annu. N. Y. Acad. Sci. 1969;157:690–700. doi: 10.1111/j.1749-6632.1969.tb12914.x. [DOI] [PubMed] [Google Scholar]
- 78.Niijima A. Glucose sensitive afferent nerve fibers in the liver and regulation of blood glucose. Brain Res. Bull. 1980;5:175–179. [Google Scholar]
- 79.Niijima A. Nervous regulation of metabolism. Prog. Neurobiol. 1989;33:135–147. doi: 10.1016/0301-0082(89)90037-3. [DOI] [PubMed] [Google Scholar]
- 80.Ohno K, Sakurai T. Orexin neuronal circuitry: role in the regulation of sleep and wakefulness. Front. Neuroendocrinol. 2008;29:70–87. doi: 10.1016/j.yfrne.2007.08.001. [DOI] [PubMed] [Google Scholar]
- 81.Olney JW. Brain lesions, obesity, and other disturbances in mice treated with monosodium glutamate. Science. 1969;164:719–721. doi: 10.1126/science.164.3880.719. [DOI] [PubMed] [Google Scholar]
- 82.Ono T, Nishino H, Fukuda M, Sasaki K, Muramoto K, Oomura Y. Glucoresponsive neurons in rat ventromedial hypothalamic tissue slices in vitro. Brain Res. 1982;232:494–499. doi: 10.1016/0006-8993(82)90295-5. [DOI] [PubMed] [Google Scholar]
- 83.Oomura Y, Kimura K, Ooyama H, Maeno T, Iki M, Kuniyoshi M. Reciprocal activities of the ventromedial and lateral hypothalamic areas of cats. Science. 1964;143:484–485. doi: 10.1126/science.143.3605.484. [DOI] [PubMed] [Google Scholar]
- 84.Oomura Y, Ono T, Ooyama H, Wayner MJ. Glucose and osmosensitive neurones of the rat hypothalamus. Nature. 1969;222:282–284. doi: 10.1038/222282a0. [DOI] [PubMed] [Google Scholar]
- 85.Oomura Y, Ooyama H, Sugimori M, Nakamura T, Yamada Y. Glucose inhibition of the glucose-sensitive neurone in the rat lateral hypothalamus. Nature. 1974;247:284–286. doi: 10.1038/247284a0. [DOI] [PubMed] [Google Scholar]
- 86.Oomura Y, Yoshimatsu H. Neural network of glucose monitoring system. J. Auton. Nerv. Syst. 1984;10:359–372. doi: 10.1016/0165-1838(84)90033-x. [DOI] [PubMed] [Google Scholar]
- 87.Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. 7th Edition. Elsevier; 2007. [DOI] [PubMed] [Google Scholar]
- 88.Ren X, Zhou L, Terwilliger R, Newton SS, de Araujo IE. Sweet taste signaling functions as a hypothalamic glucose sensor. Front Integr. Neurosci. 2009;3:12. doi: 10.3389/neuro.07.012.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ritter S, Watts AG, Dinh TT, Sanchez-Watts G, Pedrow C. Immunotoxin lesion of hypothalamically projecting norepinephrine and epinephrine neurons differentially affects circadian and stressor-stimulated corticosterone secretion. Endocrinology. 2003;144:1357–1367. doi: 10.1210/en.2002-221076. [DOI] [PubMed] [Google Scholar]
- 90.Russek M. Participation of hepatic glucoreceptors in the control of intake of food. Nature. 1963;197:79–80. doi: 10.1038/197079b0. [DOI] [PubMed] [Google Scholar]
- 91.Saberi M, Bohland M, Donovan CM. The locus for hypoglycemic detection shifts with the rate of fall in glycemia: the role of portal-superior mesenteric vein glucose sensing. Diabetes. 2008;57:1380–1386. doi: 10.2337/db07-1528. [DOI] [PubMed] [Google Scholar]
- 92.Saper CB. The central autonomic nervous system: conscious visceral perception and autonomic pattern generation. Annu. Rev. Neurosci. 2002;25:433–469. doi: 10.1146/annurev.neuro.25.032502.111311. [DOI] [PubMed] [Google Scholar]
- 93.Saper CB, Swanson LW, Cowan WM. The efferent connections of the ventromedial nucleus of the hypothalamus of the rat. J. Comp. Neurol. 1976;169:409–442. doi: 10.1002/cne.901690403. [DOI] [PubMed] [Google Scholar]
- 94.Saper CB, Loewy AD, Swanson LW, Cowan WM. Direct hypothalamo-autonomic connections. Brain Res. 1976;117:305–312. doi: 10.1016/0006-8993(76)90738-1. [DOI] [PubMed] [Google Scholar]
- 95.Saper CB, Lu J, Chou TC, Gooley J. The hypothalamic integrator for circadian rhythms. Trends Neurosci. 2005;28:152–157. doi: 10.1016/j.tins.2004.12.009. [DOI] [PubMed] [Google Scholar]
- 96.Sawchenko PE, Swanson LW. Immunohistochemical identification of neurons in the paraventricular nucleus of the hypothalamus that project to the medulla or to the spinal cord in the rat. J. Comp. Neurol. 1982;205:260–272. doi: 10.1002/cne.902050306. [DOI] [PubMed] [Google Scholar]
- 97.Sawchenko PE, Swanson LW. The organization of forebrain afferents to the paraventricular and supraoptic nuclei of the rat. J. Comp. Neurol. 1983;218:121–144. doi: 10.1002/cne.902180202. [DOI] [PubMed] [Google Scholar]
- 98.Sawchenko PE, Swanson LW, Grzanna R, Howe PR, Bloom SR, Polak JM. Colocalization of neuropeptide Y immunoreactivity in brainstem catecholaminergic neurons that project to the paraventricular nucleus of the hypothalamus. J. Comp. Neurol. 1985;241:138–153. doi: 10.1002/cne.902410203. [DOI] [PubMed] [Google Scholar]
- 99.Schneider JE, Watts AG. Energy Partitioning, Ingestive Behavior and Reproductive Success. In: Pfaff DW, editor. Hormones, Brain and Behavior. 2nd Edition. Vol 1. Academic Press; 2009. pp. 205–258. [Google Scholar]
- 100.Shimizu N, Oomura Y, Novin D, Grijalva C, Cooper P. Functional correlation between lateral hypothalamic glucose-sensitive neurons and hepatic portal glucosesensitive units in rats. Brain Res. 1985;265:49–54. doi: 10.1016/0006-8993(83)91332-x. [DOI] [PubMed] [Google Scholar]
- 101.Simmons DM, Swanson LW. Comparison of the spatial distribution of seven types of neuroendocrine neurons in the rat paraventricular nucleus: toward a global 3D model. J. Comp. Neurol. 2009;516:423–441. doi: 10.1002/cne.22126. [DOI] [PubMed] [Google Scholar]
- 102.Song Z, Levin BE, McArdle JJ, Bakhos N, Routh VH. Convergence of pre- and postsynaptic influences on glucosensing neurons in the ventromedial hypothalamic nucleus. Diabetes. 2001;50:2673–2681. doi: 10.2337/diabetes.50.12.2673. [DOI] [PubMed] [Google Scholar]
- 103.Spyer KM. Annual review prize lecture. Central nervous mechanisms contributing to cardiovascular control. J. Physiol. 1994;474:1–19. doi: 10.1113/jphysiol.1994.sp019997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Stanley BG, Leibowitz SF. Neuropeptide Y: stimulation of feeding and drinking by injection into the paraventricular nucleus. Life Sci. 1984;35:635–642. doi: 10.1016/0024-3205(84)90032-8. [DOI] [PubMed] [Google Scholar]
- 105.Stornetta RL, Sevigny CP, Guyenet PG. Vesicular glutamate transporter DNPI/VGLUT2 mRNA is present in C1 and several other groups of brainstem catecholaminergic neurons. J. Comp. Neurol. 2002;444:191–206. doi: 10.1002/cne.10141. [DOI] [PubMed] [Google Scholar]
- 106.Strack AM, Sawyer WB, Platt KB, Loewy AD. CNS cell groups regulating the sympathetic outflow to adrenal gland as revealed by transneuronal cell body labeling with pseudorabies virus. Brain Res. 1989;491:274–296. doi: 10.1016/0006-8993(89)90063-2. [DOI] [PubMed] [Google Scholar]
- 107.Stricker E, McCann M. Visceral factors in the control of food intake. Brain Res. Bull. 1985;14:687–692. doi: 10.1016/0361-9230(85)90119-4. [DOI] [PubMed] [Google Scholar]
- 108.Swanson LW. Cerebral hemisphere regulation of motivated behavior. Brain Res. 2000;886:113–164. doi: 10.1016/s0006-8993(00)02905-x. [DOI] [PubMed] [Google Scholar]
- 109.Swanson LW. Models and Schematics. 3rd ed. Amsterdam: Elsevier; 2004. Brain Maps: Structure of the Rat Brain. A Laboratory Guide with Printed and Electronic Templates for Data. [Google Scholar]
- 110.Swanson LW, Hartman BK. The central adrenergic system. An immunofluorescence study of the location of cell bodies and their efferent connections in the rat utilizing dopamine-beta-hydroxylase as a marker. J. Comp. Neurol. 1975;163:467–505. doi: 10.1002/cne.901630406. [DOI] [PubMed] [Google Scholar]
- 111.Swanson LW, Kuypers HG. The paraventricular nucleus of the hypothalamus: cytoarchitectonic subdivisions and organization of projections to the pituitary, dorsal vagal complex, and spinal cord as demonstrated by retrograde fluorescence double-labeling methods. J. Comp. Neurol. 1980;194:555–570. doi: 10.1002/cne.901940306. [DOI] [PubMed] [Google Scholar]
- 112.Swanson LW, Sawchenko PE. Hypothalamic integration: organization of the paraventricular and supraoptic nuclei. Annu. Rev. Neurosci. 1983;6:269–324. doi: 10.1146/annurev.ne.06.030183.001413. [DOI] [PubMed] [Google Scholar]
- 113.Swanson LW, Sanchez-Watts G, Watts AG. Comparison of melanin-concentrating hormone and hypocretin/orexin mRNA expression patterns in a new parcelating scheme of the lateral hypothalamic zone. Neurosci. Letters. 2005;387:80–84. doi: 10.1016/j.neulet.2005.06.066. [DOI] [PubMed] [Google Scholar]
- 114.Szentágothai J, Flerko B, Mess B, Halasz B. Hypothalamic control of the anterior pituitary; an experimental-morphological study. 3rd Edn. Budapest: Akadémiai Kiadó; 1968. [Google Scholar]
- 115.Thompson RH, Canteras NS, Swanson LW. Organization of projections from the dorsomedial nucleus of the hypothalamus: a PHA-L study in the rat. J. Comp. Neurol. 1996;376:143–173. doi: 10.1002/(SICI)1096-9861(19961202)376:1<143::AID-CNE9>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 116.Thompson RH, Swanson LW. Organization of inputs to the dorsomedial nucleus of the hypothalamus: a reexamination with Fluorogold and PHAL in the rat. Brain Res. Brain Res. Rev. 1998;27:89–118. doi: 10.1016/s0165-0173(98)00010-1. [DOI] [PubMed] [Google Scholar]
- 117.Thompson RH, Swanson LW. Structural characterization of a hypothalamic visceromotor pattern generator network. Brain Res. Brain Res. Rev. 2003;41:153–202. doi: 10.1016/s0165-0173(02)00232-1. [DOI] [PubMed] [Google Scholar]
- 118.Thorens, Larsen PJ. Gut-derived signaling molecules and vagal afferents in the control of glucose and energy homeostasis. Curr. Opin. Clin. Nutrition & Metab. Care. 2004;7:471–478. doi: 10.1097/01.mco.0000134368.91900.84. [DOI] [PubMed] [Google Scholar]
- 119.Tong Q, Ye C, McCrimmon RJ, Dhillon H, Choi B, Kramer MD, Yu J, Yang Z, Christiansen LM, Lee CE, Choi CS, Zigman JM, Shulman GI, Sherwin RS, Elmquist JK, Lowell BB. Synaptic glutamate release by ventromedial hypothalamic neurons is part of the neurocircuitry that prevents hypoglycemia. Cell Metab. 2007;5:383–393. doi: 10.1016/j.cmet.2007.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Ulrich-Lai YM, Herman JP. Neural regulation of endocrine and autonomic stress responses. Nat. Rev. Neurosci. 2009;10:397–409. doi: 10.1038/nrn2647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.van den Pol AN. The magnocellular and parvocellular paraventricular nucleus of rat: intrinsic organization. J. Comp. Neurol. 1982;206:317–345. doi: 10.1002/cne.902060402. [DOI] [PubMed] [Google Scholar]
- 122.van den Pol AN, Cassidy JR. The hypothalamic arcuate nucleus of rat--a quantitative Golgi analysis. J. Comp. Neurol. 1982;204:65–98. doi: 10.1002/cne.902040108. [DOI] [PubMed] [Google Scholar]
- 123.Wang R, Liu X, Hentges ST, Dunn-Meynell AA, Levin BE, Wang W, Routh VH. The regulation of glucose-excited neurons in the hypothalamic arcuate nucleus by glucose and feeding-relevant peptides. Diabetes. 2004;53:1959–1965. doi: 10.2337/diabetes.53.8.1959. [DOI] [PubMed] [Google Scholar]
- 124.Watts AG. The efferent projections of the suprachiasmatic nucleus. Anatomical insights into the control of circadian rhythms. In: Klein D, Moore RY, Reppert SM, editors. The Suprachiasmatic Nucleus: The Mind's Clock. Oxford University Press; 1991. pp. 77–104. [Google Scholar]
- 125.Watts AG. Glucocorticoid regulation of peptide genes in neuroendocrine CRH neurons: a complexity beyond negative feedback. Front. Neuroendocrinol. 2005;26:109–130. doi: 10.1016/j.yfrne.2005.09.001. [DOI] [PubMed] [Google Scholar]
- 126.Watts AG, Swanson LW. Efferent projections of the suprachiasmatic nucleus: II. Studies using retrograde transport of fluorescent dyes and simultaneous peptide immunohistochemistry in the rat. J. Comp. Neurol. 1987;258:230–252. doi: 10.1002/cne.902580205. [DOI] [PubMed] [Google Scholar]
- 127.Watts AG, Swanson LW. Anatomy of Motivational Systems. In: Pashler H, Gallistell R, editors. ‘Stevens’ Handbook of Experimental Psychology, Vol. 3, Learning, Motivation, and Emotion. 3rd Edition. John Wiley & Sons; 2002. pp. 563–632. [Google Scholar]
- 128.Watts AG, Sanchez-Watts G, Kelly AB. Distinct patterns of neuropeptide gene expression in the lateral hypothalamic area and arcuate nucleus are associated with dehydration induced anorexia. J. Neurosci. 1999;19:6111–6121. doi: 10.1523/JNEUROSCI.19-14-06111.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Watts AG, Khan AM, Sanchez-Watts G, Salter D, Neuner CM. Activation in neural networks controlling ingestive behaviors: what does it mean, and how do we map and measure it? Physiol. Behav. 2006;89:501–510. doi: 10.1016/j.physbeh.2006.05.025. [DOI] [PubMed] [Google Scholar]
- 130.White JD, Olchovsky D, Kershaw M, Berelowitz M. Increased hypothalamic content of preproneuropeptide-Y messenger ribonucleic acid in streptozotocin-diabetic rats. Endocrinol. 1990;126:765–772. doi: 10.1210/endo-126-2-765. [DOI] [PubMed] [Google Scholar]
- 131.Willesen MG, Kristensen P, Rømer J. Co-localization of growth hormone secretagogue receptor and NPY mRNA in the arcuate nucleus of the rat. Neuroendocrinol. 1999;70:306–316. doi: 10.1159/000054491. [DOI] [PubMed] [Google Scholar]
- 132.Zhang YH, Lu J, Elmquist JK, Saper CB. Lipopolysaccharide activates specific populations of hypothalamic and brainstem neurons that project to the spinal cord. J. Neurosci. 2000;20:6578–6586. doi: 10.1523/JNEUROSCI.20-17-06578.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]