INTRODUCTION
Dr. Charles H. Mayo stated in 1922 “It is unfortunate that so few appreciate from what small causes diseases come.” Today, with advances in technology, the burgeoning field of nanotechnology brings new meaning to Dr. Mayo’s words. Manufactured and natural materials of nanometer scale (Figure 1) provide the medical community with new products for diagnostics,1 drug delivery,2 and biomaterials for restorative and reconstructive surgery.3 A key requirement for these new products is that the nanoparticle itself does not initiate detrimental or pathogenic processes in healthy tissue. This prerequisite is an important consideration in the field of nanotechnology, as nano-materials can have unique effects on biological systems, depending on many factors including their original composition and size. Indeed, materials on a nano level often have different characteristics and effects than the same material at a macro level.4 Nano-sized lipid/protein and protein/mineral complexes are present in the blood and come from diverse sources. For instance, activated mammalian cells as well as bacteria release biologically active vesicles which are in the same size range as manufactured nanoparticles.5 These and other biologically derived nanoparticles (NPs) require further investigation as potential participants in disease processes. This brief review will focus on the contribution of biologically derived NPs to soft tissue calcification.
Figure 1.
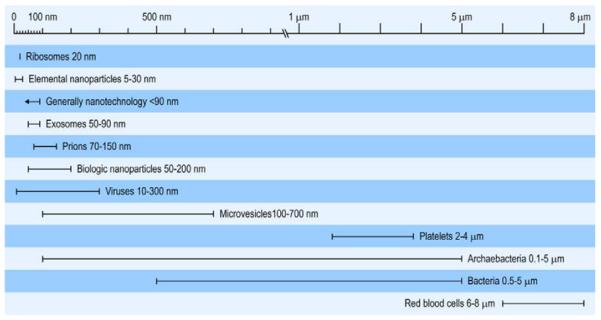
Comparisons of sizes of elemental and biological material considered as nanoparticles, microparticles and microvesicles with classical bacteria and blood elements.
HISTORICAL ASPECTS OF CALCIFYING NANOPARTICLES AS PATHOGENS
Calcium carbonate spheres ranging from 30 nm to 300 nm in size were observed in samples from hot springs in Viterbo, Italy by geologist Dr. Robert Folk and co-workers. Because their structure was similar to bacteria, and because of their nanometer size, they were referred to as “nan(n)obacteria.”6 Similar structures isolated from bovine serum propagate and calcify under cell culture conditions, further suggesting that these particles were biological and perhaps a new type of life form.7 Related structures were observed on a Martian meteorite shortly thereafter.8 Similar nanobacteria-like particles, or NPs have been identified in and isolated from diverse geologic sources, mammalian blood,7, 9, 10 and a variety of diseased tissues including calcified arteries,11 atherosclerotic plaques,12 calcified aortic valves,13 kidney stones,14,15 gall stones,16 ovarian cancer,17 prostate stones,18 and breast implants.19
These NPs have been grouped together not due to confirmed chemical or biological analysis, but rather due to their common morphology (Figure 2). Indeed, a definitive lack of biochemical criteria for identification and classification of biologically derived NPs continues to cause confusion and debate within the field. An association between chronic infection and cardiovascular and renal disease and contracture of breast implants20-25 provided some support for the original description of NPs as “nan(n)obacteria.” However, the inability to reliably and reproducibly sequence nucleic acids from NP isolates has raised controversy regarding their classification as a life form (NCBI nucleotide gene bank, accession #X98418).26, 27 Furthermore, evidence also supports the hypothesis that biologically derived NPs are only protein/mineral complexes that form a chemical film in culture conditions.9, 27
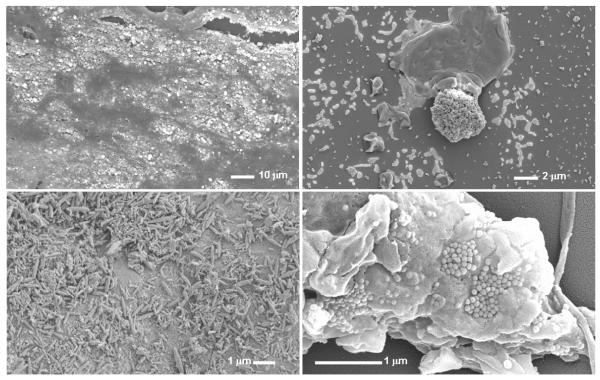
Figure 2.
Scanning electron micrographs (SEMS) of a calcification from a carotid arterial plaque (upper panels) and a calcified capsule removed from a silicon breast implant (lower panels). Left panels are SEMs of the tissue prior to isolation of the NPs. Right panels are SEMs of NPs cultured from the respective tissue homogenates. X-ray microanalysis of the bright areas in the upper left panel indicates the presence of calcium and phosphate. Bar indicates scale.
CALCIFYING NANOPARTICLES AND BIOFILM
Microbial biofilms are a source of chronic infection in clinical settings,28 often forming on implanted devices and ultimately leading to a loss of device function as well as significant patient morbidity and even mortality.25, 29-32 In culture, NPs derived from bovine blood and human saliva, calcified arteries, and kidney stones can each form films in vitro that contain hydroxyapatite and organic particles.7, 11, 26 Therefore, NP biofilms are a cause for concern, not only for their calcifying potential, but also for their potential to serve as a nidus for crystal formation and tissue damage. Formation of biologically-derived NP biofilm is reduced by inhibitors of aerobic metabolism and antibiotics, suggesting that biofilm formation by these entities requires enzymatic activity.15, 33 Recent unpublished work from our laboratory indicates that both enzymatic and physical-chemical interactions are required for NP biofilm formation since tetracycline, an antibiotic that acts by blocking ribosomal protein synthesis but also has calcium chelating activity, is more effective in reducing NP biofilm than gentamicin which also acts to block bacterial ribosomal function, but lacks significant calcium chelating properties. These studies highlight the potential utility of this in vitro NP model system to identify factors that mediate formation of biofilm on implantable devices.
Acute and chronic (sub-clinical) infection have each been associated with soft tissue calcification.23-25, 34, 35 When activated, certain bacteria and mammalian cells release membrane vesicles which can range in size from 100nm - 700nm (Figure 1).36 In mammalian systems, these microvesicles (also called microparticles) attach to and activate other cells, transfer material from cell to cell, and may be thrombogenic and markers of early disease processes in asymptomatic patients.5, 37 It is possible that bacterial-derived nano/microvesicles might be the source of the short length DNA sequences that have been amplified or isolated from calcifications including atherosclerotic plaques, renal stones, and breast implants. This would explain why complete, genome-length sequences have not yet been isolated from NP isolates propagated in vitro.26, 27 The hypothesis that bacterial microvesicles can contribute to the development of calcified biofilms, and could represent an unappreciated source of surgical infection and potential post operative complication, remains to be tested. Indeed, recent evidence points to an even greater variety of commensal microbes on human skin than previously thought.38
DO CALCIFYING NANOPARTICLES CAUSE DISEASE?
Although nano-sized particles can be identified within soft tissue calcifications, the question remains whether or not they are the result or cause of disease processes, or perhaps both. Following Koch’s postulates to determine the causative nature of pathogens,39 investigators have taken cultured NPs and injected them into naïve animals. When NPs derived from gall stones and kidney stones are injected directly into the organ of interest in healthy animals, calcifications resembling the original pathology developed in the target organ.16, 40 In addition, arterial occlusions with calcification were observed in endothelium-denuded carotid arteries of rabbits inoculated intravenously with arterial-derived or kidney stone-derived NPs.41,42 These occlusions and intimal hyperplasia differed from that observed in animals that had been inoculated with either saline, lipopolysaccharide (LPS) used to simulate bacterial induced inflammation, or hydroxyapatite (HA) crystals, as a control for the calcific shell of the NPs. Arterial remodeling in rabbits inoculated with HA crystals exposed to cell culture conditions resembled that of rabbits inoculated with bovine-derived NPs. In these groups, the injured arteries exhibited increased hyperplasia and discontinuity of the media and internal elastic lamina.42 These experiments demonstrate three important points: 1) biologically derived NPs precipitated disease processes in healthy tissue; 2) the nature of the pathology varies with the origin/chemical composition of the NPs and the target tissue; and 3) biologically derived NPs circulate and may exacerbate pathological processes in tissue at sites distant from their point of origin.
CONCLUSIONS AND FUTURE DIRECTIONS
Technological advances have provided means to identify the presence and composition of nano- and micro-sized particles and vesicles in human blood and tissue. Emerging experimental evidence suggests that these particles can contribute to pathological processes. However, validating causation may require an alternative set of criteria than those originally outlined by Koch for bacterial causes of disease, since biologic NPs may not be a life form, yet still cause disease. An alternative set of criteria, described by Hill for environmental factors,43 may be useful in identifying associations of NPs with pathologies. An ongoing challenge for investigators is to identify the heterogenous biochemical properties of nano- and micro-particles in the blood of humans in health and disease and to rigorously test their acute and chronic pathogenic properties, as well as those of NPs being developed for diagnostic and therapeutic purposes.
ACKNOWLEDGEMENTS
This work was supported by grants from the NIH HL88988; the Fetzer Foundation; Division of Surgical Research, Mayo Clinic; the Mayo Foundation and the College of Medicine, Mayo Clinic. The authors gratefully acknowledge our collaborators in the Department of Surgery, Drs. Nho V. Tran and George Pisimisis, technical assistance of Mr. Larry Hunter and study coordinator, Ms. Teresa Zais.
Sources of Financial Support: This work was supported by grants from the NIH HL88988; the Fetzer Foundation; Division of Surgical Research, Mayo Clinic; the Mayo Foundation and the College of Medicine, Mayo Clinic.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Liu J, Levine AL, Mattoon JS, Yamaguchi M, Lee RJ, Pan X, et al. Nanoparticles as image enhancing agents for ultrasonography. Phys Med Biol. 2006 May 7;51(9):2179–89. doi: 10.1088/0031-9155/51/9/004. [DOI] [PubMed] [Google Scholar]
- 2.Peters D, Kastantin M, Kotamraju VR, Karmali PP, Gujraty K, Tirrell M, et al. Targeting atherosclerosis by using modular, multifunctional micelles. PNAS. 2009;106:9815–9. doi: 10.1073/pnas.0903369106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang G, Siggers K, Zhang S, Jiang H, Xu Z, Zernicke RF, et al. Preparation of BMP-2 containing bovine serum albumin (BSA) nanoparticles stabilied by polymer coating. Pharm Res. 2008;25:2896–909. doi: 10.1007/s11095-008-9692-2. [DOI] [PubMed] [Google Scholar]
- 4.Nel A, Xia T, Madler L, Li N. Toxic potential of materials at the nanolevel. Science. 2006;311:622–7. doi: 10.1126/science.1114397. [DOI] [PubMed] [Google Scholar]
- 5.Piccin A, Murphy WG, Smith OP. Circulating microparticles: pathophysiology and clinical implications. Blood Reviews. 2007;21:157–71. doi: 10.1016/j.blre.2006.09.001. [DOI] [PubMed] [Google Scholar]
- 6.Folk RL. Bacteria and nannobacteria revealed in hardgrounds, calcite cements, native sulfur, sulfide minerals, and travertines; Geological Society of America Annual Meeting, Abstracts with Programs; 1992.p. 104. [Google Scholar]
- 7.Kajander EO, Kuronen I, Akerman KK, Pelttari A, Cifticioglu N. Nanobacteria from blood, the smallest culturable autonomously replicating agent on Earth. SPIE. 1997;3111:420–8. [Google Scholar]
- 8.McKay DS, Gibson EK, Jr., Thomas-Keprta KL, Vali H, Romanek CS, Clemett SJ, et al. Search for past life on Mars: Possible relic biogenic activity in Martian meteorite ALH84001. Science. 1996;273:924–30. doi: 10.1126/science.273.5277.924. [DOI] [PubMed] [Google Scholar]
- 9.Raoult D, Drancourt M, Azza S, Nappex C, Guieu R, Rolain J, et al. Nanobacteria Are Mineralo Fetuin Complexes. PLoS Pathogens. 2008;4(2):e41. doi: 10.1371/journal.ppat.0040041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Martel J, Ding-E Young J. Purported nanobacteria in human blood as calcium carbonate nanoparticles. PNAS. 2008 doi: 10.1073/pnas.0711744105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Miller VM, Rodgers G, Charlesworth JA, Kirkland B, Severson SR, Rasmussen TE, et al. Evidence of nanobacterial-like structures in human calcified arteries and cardiac valves. Am J Physiol: Heart Circ Physiol. 2004;287:H1115–H24. doi: 10.1152/ajpheart.00075.2004. [DOI] [PubMed] [Google Scholar]
- 12.Folk RL. Nannobacteria in the natural environment and in medicine. Alpe Adria Microbiol J. 1998;7:87–95. [Google Scholar]
- 13.Bratos-Perez MA, Sanchez PL, Garcia de Cruz S, Villacorta E, Palacios IF, Fernandez-Fernandez JM, et al. Association between self-replicating calcifying nanoparticles and aortic stenosis: a possible link to valve calcification. Eur Heart J. 2008;29:371–6. doi: 10.1093/eurheartj/ehm592. [DOI] [PubMed] [Google Scholar]
- 14.Ciftcioglu N, Bjorklund M, Kuorikoski K, Bergstrom K, Kajander EO. Nanobacteria: An infectious cause for kidney stone formation. Kidney Intl. 1999;56:1893–8. doi: 10.1046/j.1523-1755.1999.00755.x. [DOI] [PubMed] [Google Scholar]
- 15.Kumar V, Farell G, Yu S, Harrington S, Fitzpatrick L, Rzewuska E, et al. Cell biology of IP pathologic renal calcification: contribution of crystal transcytosis, cell-mediated R calcification, and nanoparticles. J Investig Med. 2006 Nov;54(7):412–24. doi: 10.2310/6650.2006.06021. [DOI] [PubMed] [Google Scholar]
- 16.Wang L, Shen W, Wen J, An X, Cao L, Wang B. An animal model of black pigment gallstones caused by nanobacteria. Digestive Dis Sci. 2006;51:1126–32. doi: 10.1007/s10620-006-8019-6. [DOI] [PubMed] [Google Scholar]
- 17.Sedivy R, Battistutti WB. Nanobacteria promote crystallization of psammoma bodies in ovarian cancer. APMIS. 2003;111:951–4. doi: 10.1034/j.1600-0463.2003.1111006.x. [DOI] [PubMed] [Google Scholar]
- 18.Shoskes DA, Thomas KD, Gomez E. Anti-nanobacterial therapy for men with chronic prostatitis/chronic pelvic pain syndrome and prostatic stones: Preliminary experience. J Urol. 2005;173:474–7. doi: 10.1097/01.ju.0000150062.60633.b2. [DOI] [PubMed] [Google Scholar]
- 19.Deva A, Chang L. Bacterial Biofilms: A Cause for Accelerated Capsular contracture? Aesthetic Surgery Journal. 1999;16(MarchApril):130–3. [Google Scholar]
- 20.Beck JD, Offenbacher S. The association between periodontal diseases and E cardiovascular diseases: A state-of-the-science review. Ann Periodontol. 2001;6:9–15. doi: 10.1902/annals.2001.6.1.9. [DOI] [PubMed] [Google Scholar]
- 21.Epstein SE, Zhu J, Burnett MS, Zhou YF, Vercellotti GM, Hajjar D. Infection and atherosclerosis: Potential roles of pathogen burden and molecular mimicry. Arterioscler Thromb Vasc Biol. 2000;20:1417–20. doi: 10.1161/01.atv.20.6.1417. [DOI] [PubMed] [Google Scholar]
- 22.Prasad A, Zhu J, Halcox JPJ, Waclawiw MA, Epstein SE, Quyyumi AA. Predisposition to atherosclerosis by infections. Role of endothelial dysfunction. Circulation. 2002;106:184–90. doi: 10.1161/01.cir.0000021125.83697.21. [DOI] [PubMed] [Google Scholar]
- 23.Muhlestein JB, Anderson JL. Chronic infection and coronary artery disease. Cardiol Clin. 2003;21(3):333–62. doi: 10.1016/s0733-8651(03)00054-7. [DOI] [PubMed] [Google Scholar]
- 24.Pajkos A, Deva AK, Vickery K, Cope C, Chang L, Cossart YE. Detection of subclinical infection in significant breast implant capsules. Plast Reconstr Surg. 2003 Apr 15;111(5):1605–11. doi: 10.1097/01.PRS.0000054768.14922.44. [DOI] [PubMed] [Google Scholar]
- 25.Del Pozo JL, Tran NV, Petty PM, Johnson CH, Walsh MF, Bite U, et al. Pilot Study of Association of Bacteria on Breast Implants with Capsular Contracture. J Clin Microbiol. 2009;47:1333–7. doi: 10.1128/JCM.00096-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cisar JO, Xu D-Q, Thompson J, Swaim W, Hu L, Kopecko DJ. An alternative interpretation of nanobacteria-induced biomineralization. Proc Natl Acad Sci U.S.A. 2000;97:11511–5. doi: 10.1073/pnas.97.21.11511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Young JD, Martel J, Young L, Wu CY, Young A, Young D. Putative nanobacteria represent physiological remnants and culture by-products of normal calcium homeostasis. PLoS ONE. 2009;4(2):e4417. doi: 10.1371/journal.pone.0004417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Costerton JW, Stewart PS, Greenberg EP. Bacterial biofilms: a common cause of persistent infections. Science. 1999 May 21;284(5418):1318–22. doi: 10.1126/science.284.5418.1318. [DOI] [PubMed] [Google Scholar]
- 29.Braxton EE, Jr., Ehrlich GD, Hall-Stoodley L, Stoodley P, Veeh R, Fux C, et al. Role of biofilms in neurosurgical device-related infections. Neurosurg Rev. 2005 Oct;28(4):249–55. doi: 10.1007/s10143-005-0403-8. [DOI] [PubMed] [Google Scholar]
- 30.Garcia-Lechuz J, Bouza E. Treatment recommendations and strategies for the management of bone and joint infections. Expert Opinion on Pharmacotherapy. 2009;10:35–55. doi: 10.1517/14656560802611766. [DOI] [PubMed] [Google Scholar]
- 31.Norowski PAJ, Bumgardner JD. Biomaterial and antibiotic strategies for periimplantitis: a review. Journal of Biomedical Materials Research. 2009;88:530–43. doi: 10.1002/jbm.b.31152. [DOI] [PubMed] [Google Scholar]
- 32.Beathard GA, Urbanes A. Infection associated with tunneled hemodialysis catheters. Seminars in Dialysis. 2008;21:528–38. doi: 10.1111/j.1525-139X.2008.00497.x. [DOI] [PubMed] [Google Scholar]
- 33.Bjorklund M, Ciftcioglu N, Kajander EO. Extraordinary survival of nanobacteria C under extreme conditions. SPIE. 1998;3441:123–9. [Google Scholar]
- 34.Bobryshev YV, Lord RS, Tran D. Chlamydia pneumoniae in foci of “early” calcification of the tunica media in arteriosclerotic arteries: an incidental presence? Am J Physiol Heart Circ Physiol. 2006;290:H1510–H9. doi: 10.1152/ajpheart.01055.2005. [DOI] [PubMed] [Google Scholar]
- 35.Mattila KJ, Valle MS, Nieminen MS, Valtone VV, Hietaniemi KL. Dental infections and coronary atherosclerosis. Atherosclerosis. 1993;103(2):205–11. doi: 10.1016/0021-9150(93)90263-t. [DOI] [PubMed] [Google Scholar]
- 36.Spiewak R, Dutkiewicz J. In vitro study of pro-inflammatory and anti-tumour properties of microvesicles from bacterial cell wall of Pantoea agglomerans. Ann Agric Environ Med. 2008 Jun;15(1):153–61. [PubMed] [Google Scholar]
- 37.Jayachandran M, Litwiller RD, Owen WG, Heit JA, Behrenbeck TR, Mulvagh SL, et al. Characterization of Blood Borne Microparticles as Markers of Premature Coronary Calcification in Recently Menopausal Women. Am J Physiol Heart Circ Physiol. 2008;295:931–8. doi: 10.1152/ajpheart.00193.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Grice EA, Kong HH, Renaud G, Young AC, Program NCS, Bouffard GG, et al. A diversity profile of the human skin microbiota. Genome Res. 2008;18:1043–50. doi: 10.1101/gr.075549.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Grimes DJ. Koch’s postulates - then and now. Microbe. 2006;1:223–8. [Google Scholar]
- 40.Shiekh FA, Khullar M, Singh SK. Lithogenesis: Induction of renal calcifications by nanobacteria. Urol Res. 2006 doi: 10.1007/s00240-005-0034-0. DOI 10.1007/s00240-005-0034-0. [DOI] [PubMed] [Google Scholar]
- 41.Schwartz MA-K, Lieske JC, Kumar V, Farell-Baril G, Miller VM. Human-derived nanoparticles and vascular responses to injury in rabbit carotid arteries: proof of principle. International Journal of Nanomedicine. 2008;3:243–8. doi: 10.2147/ijn.s2473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schwartz MK, Lieske JC, Hunter LW, Miller VM. Systemic Injection of Planktonic Forms of Mammalian-derived Nanoparticles Alters Arterial Response to Injury in Rabbits. AJP Heart & Circ. 2009;296:1434–41. doi: 10.1152/ajpheart.00993.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hill AB. The Environment and Disease: Association or Causation? Proceedings of the Royal Society of Medicine. 1965 May;58:295–300. [PMC free article] [PubMed] [Google Scholar]