Abstract
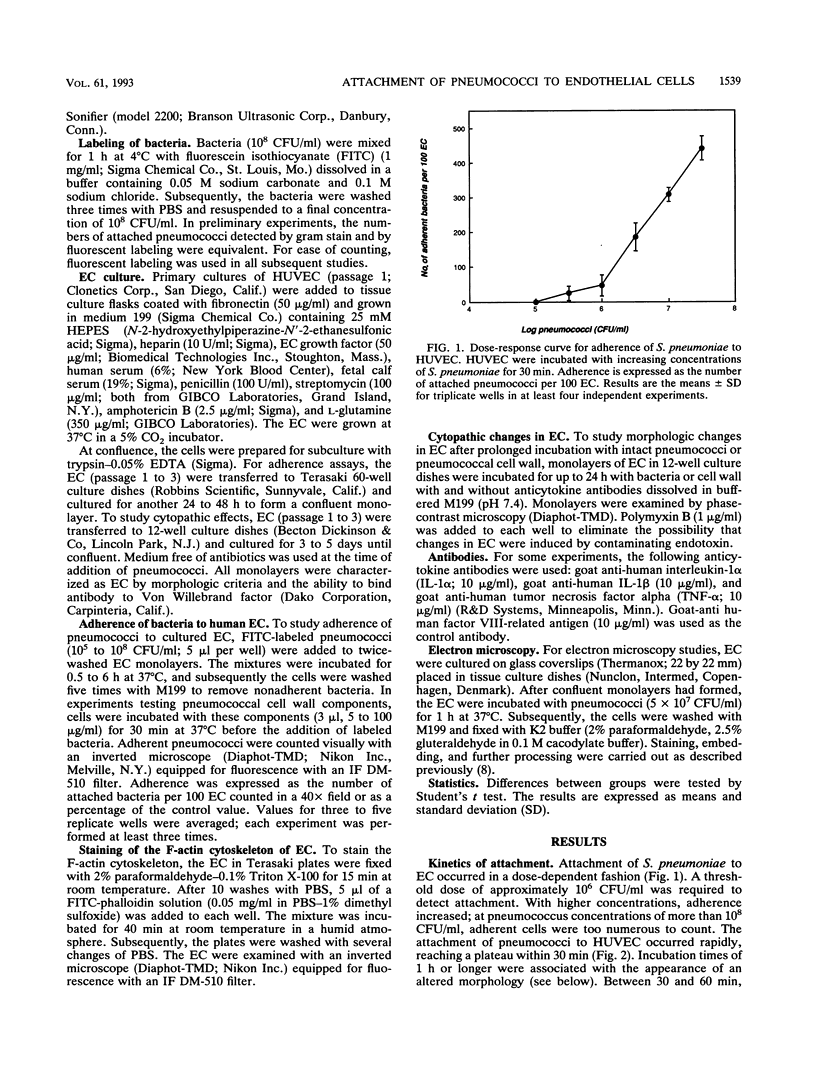
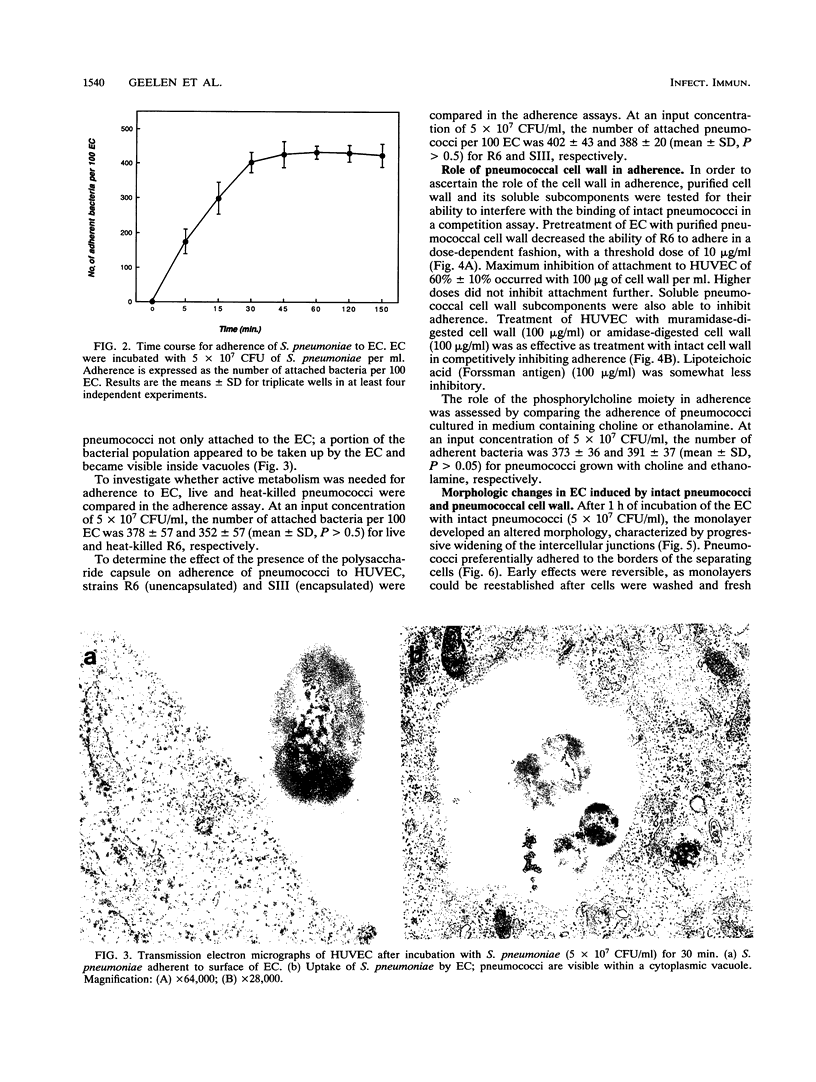
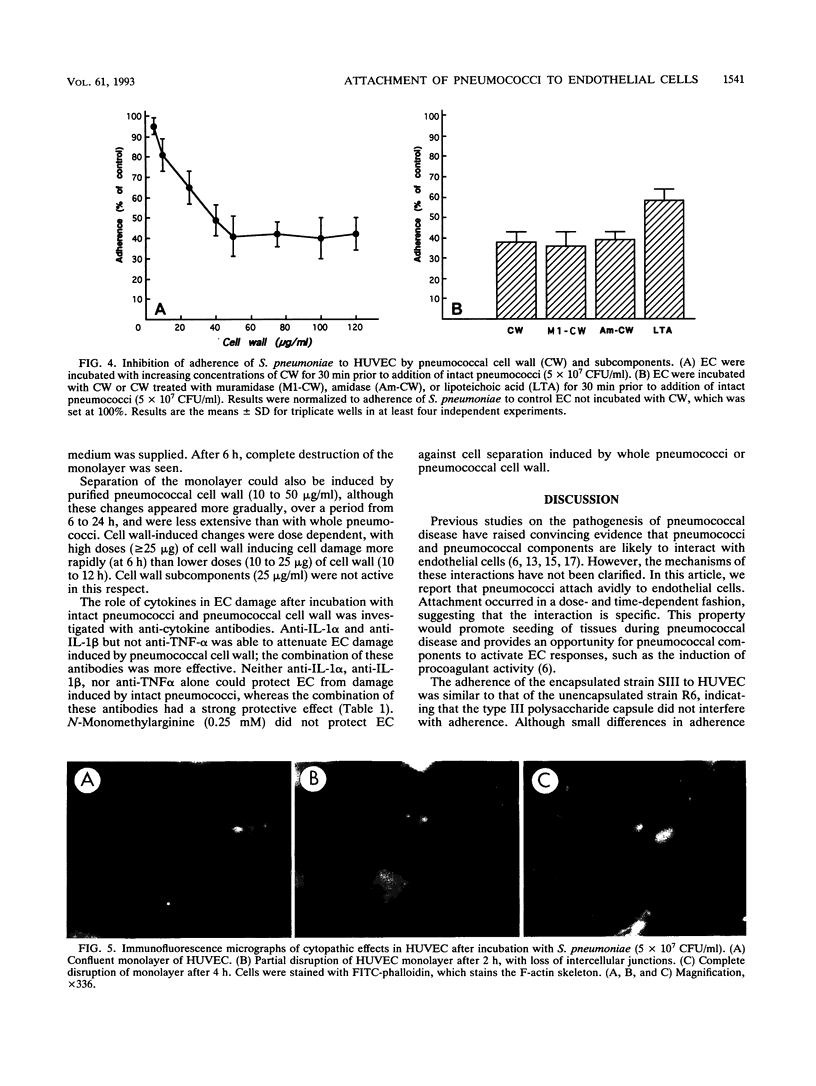
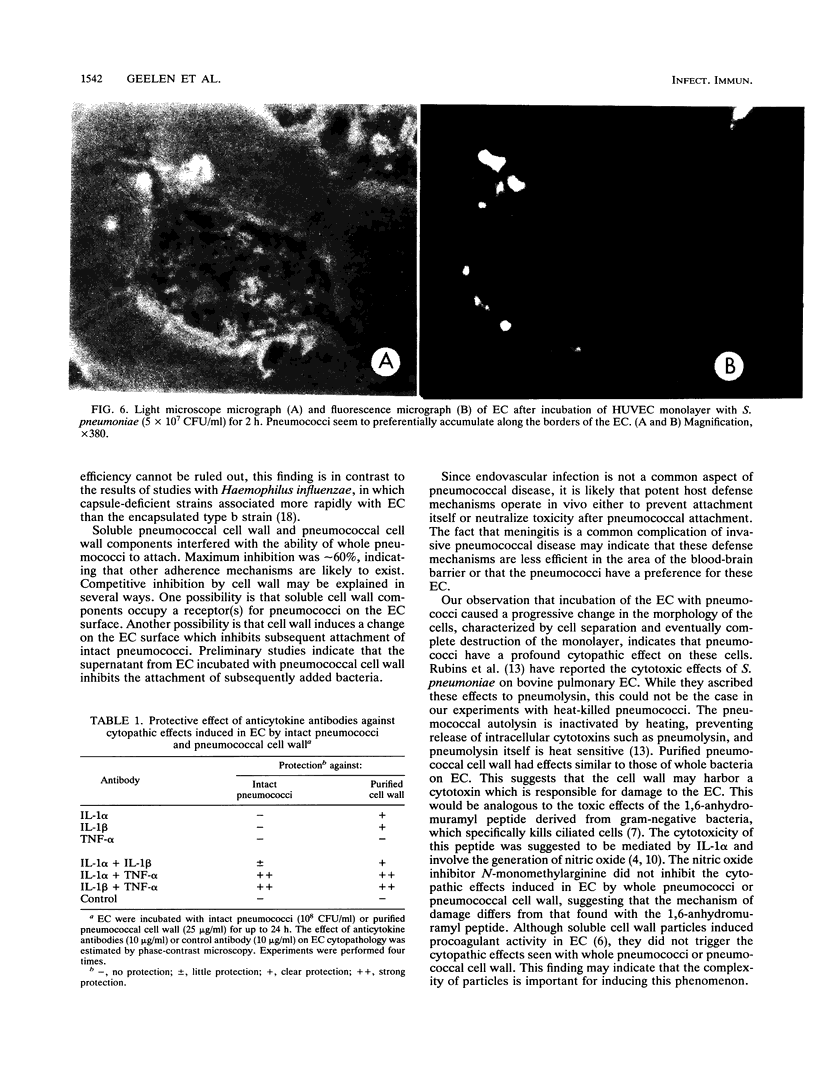
Streptococcus pneumoniae interacts with vascular endothelial cells during the course of bacteremia. In this study, we characterized the initial attachment of pneumococci to human endothelial cells (EC) and the response of the endothelium to this interaction. Pneumococci adhered to EC in a dose-dependent fashion. Attachment was rapid, with the majority of bacteria attached by 30 min. No difference was found between the attachment of unencapsulated (R6) and encapsulated (SIII) strains. Purified pneumococcal cell wall components competitively inhibited attachment of R6 by a maximum of 60% in a dose-dependent manner. Following attachment of pneumococci or exposure of EC to pneumococcal cell wall, pronounced changes in EC morphology ensued, resulting in striking separation of the cells of the monolayer and, eventually, destruction of the cells. The cytopathic effects of the cell wall were inhibited by antibodies to interleukin-1 but not to tumor necrosis factor. Both antibodies were required to neutralize the cytopathology caused by intact pneumococci. We conclude that pneumococci attach rapidly to human EC and that the cell wall is important in this interaction. Intact pneumococci and pneumococcal cell wall induce profound morphologic changes in human EC, leading to loss of barrier integrity. These cytopathic effects are likely to be cytokine mediated.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andersson B., Beachey E. H., Tomasz A., Tuomanen E., Svanborg-Edén C. A sandwich adhesion on Streptococcus pneumoniae attaching to human oropharyngeal epithelial cells in vitro. Microb Pathog. 1988 Apr;4(4):267–278. doi: 10.1016/0882-4010(88)90087-3. [DOI] [PubMed] [Google Scholar]
- Andersson B., Dahmén J., Frejd T., Leffler H., Magnusson G., Noori G., Edén C. S. Identification of an active disaccharide unit of a glycoconjugate receptor for pneumococci attaching to human pharyngeal epithelial cells. J Exp Med. 1983 Aug 1;158(2):559–570. doi: 10.1084/jem.158.2.559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burman L. A., Norrby R., Trollfors B. Invasive pneumococcal infections: incidence, predisposing factors, and prognosis. Rev Infect Dis. 1985 Mar-Apr;7(2):133–142. doi: 10.1093/clinids/7.2.133. [DOI] [PubMed] [Google Scholar]
- Cookson B. T., Cho H. L., Herwaldt L. A., Goldman W. E. Biological activities and chemical composition of purified tracheal cytotoxin of Bordetella pertussis. Infect Immun. 1989 Jul;57(7):2223–2229. doi: 10.1128/iai.57.7.2223-2229.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer H., Tomasz A. Peptidoglycan cross-linking and teichoic acid attachment in Streptococcus pneumoniae. J Bacteriol. 1985 Jul;163(1):46–54. doi: 10.1128/jb.163.1.46-54.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geelen S., Bhattacharyya C., Tuomanen E. Induction of procoagulant activity on human endothelial cells by Streptococcus pneumoniae. Infect Immun. 1992 Oct;60(10):4179–4183. doi: 10.1128/iai.60.10.4179-4183.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan G., Britton W. J., Hancock G. E., Theuvenet W. J., Smith K. A., Job C. K., Roche P. W., Molloy A., Burkhardt R., Barker J. The systemic influence of recombinant interleukin 2 on the manifestations of lepromatous leprosy. J Exp Med. 1991 Apr 1;173(4):993–1006. doi: 10.1084/jem.173.4.993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krivan H. C., Roberts D. D., Ginsburg V. Many pulmonary pathogenic bacteria bind specifically to the carbohydrate sequence GalNAc beta 1-4Gal found in some glycolipids. Proc Natl Acad Sci U S A. 1988 Aug;85(16):6157–6161. doi: 10.1073/pnas.85.16.6157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powderly W. G., Stanley S. L., Jr, Medoff G. Pneumococcal endocarditis: report of a series and review of the literature. Rev Infect Dis. 1986 Sep-Oct;8(5):786–791. doi: 10.1093/clinids/8.5.786. [DOI] [PubMed] [Google Scholar]
- Riesenfeld-Orn I., Wolpe S., Garcia-Bustos J. F., Hoffmann M. K., Tuomanen E. Production of interleukin-1 but not tumor necrosis factor by human monocytes stimulated with pneumococcal cell surface components. Infect Immun. 1989 Jul;57(7):1890–1893. doi: 10.1128/iai.57.7.1890-1893.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubins J. B., Duane P. G., Charboneau D., Janoff E. N. Toxicity of pneumolysin to pulmonary endothelial cells in vitro. Infect Immun. 1992 May;60(5):1740–1746. doi: 10.1128/iai.60.5.1740-1746.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saukkonen K., Sande S., Cioffe C., Wolpe S., Sherry B., Cerami A., Tuomanen E. The role of cytokines in the generation of inflammation and tissue damage in experimental gram-positive meningitis. J Exp Med. 1990 Feb 1;171(2):439–448. doi: 10.1084/jem.171.2.439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlech W. F., 3rd, Ward J. I., Band J. D., Hightower A., Fraser D. W., Broome C. V. Bacterial meningitis in the United States, 1978 through 1981. The National Bacterial Meningitis Surveillance Study. JAMA. 1985 Mar 22;253(12):1749–1754. [PubMed] [Google Scholar]
- Tuomanen E., Liu H., Hengstler B., Zak O., Tomasz A. The induction of meningeal inflammation by components of the pneumococcal cell wall. J Infect Dis. 1985 May;151(5):859–868. doi: 10.1093/infdis/151.5.859. [DOI] [PubMed] [Google Scholar]
- Virji M., Kayhty H., Ferguson D. J., Alexandrescu C., Moxon E. R. Interactions of Haemophilus influenzae with cultured human endothelial cells. Microb Pathog. 1991 Mar;10(3):231–245. doi: 10.1016/0882-4010(91)90057-h. [DOI] [PubMed] [Google Scholar]





