Abstract
Pathological gambling has many similarities to pharmacological addiction. Notably, both pathological gambling and drug addiction are characterized by aberrations in hypothalamic-pituitary-adrenal (HPA) axis responding. As well, there are indications that gender differences may play a role in these processes. Whether gender and/or HPA response are associated with pathological gambling was of interest. Recreational and pathological gamblers (15 men and 6 women per group) had the HPA factor, cortisol, assessed in saliva collected before and after watching a video of their preferred mode of gambling (slot machines, horse race betting, scratch-off tickets, blackjack, video poker, craps, sports betting, online casino games, or lottery tickets), and a video of neutral stimuli (a rollercoaster ride). Basal levels of salivary cortisol did not significantly differ among recreational and pathological gamblers. However, recreational gamblers demonstrated significantly increased salivary cortisol levels after the gambling and rollercoaster videos, whereas pathological gamblers demonstrated no salivary cortisol increase in response to either video stimulus. There was also a non-significant trend for women to have a greater cortisol response to video stimuli compared to men. These data suggest that pathological gambling is associated with hypoactive HPA response to gambling stimuli, similar to chronic drug exposure, and gender may contribute to this effect.
Keywords: Addiction, Gender Differences, Hypothalamic-Pituitary-Adrenal Axis, Pathological Gambling, Problem Gambling, Recreational Gambling
1. Introduction
Pathological gambling can be viewed as a process similar to other addictions [1]. In support, gambling and substance abuse are often co-morbid [2]. Engaging in gambling is rewarding and is associated with activation of substrates and brain regions that are associated with natural or drug-induced reward [3–6]. Moreover, therapeutics that are effective in treating substance addictions are also efficacious in treating pathological gambling [7–9]. Despite intriguing similarities in the nosology, epidemiology, etiology, treatment, face, construct and/or predictive validity between pathological gambling and addiction, pathological gambling is complex, and the neurobiological substrates need to be better understood.
Engaging in gambling instantiates many of the same physiological processes that are associated with drugs of abuse. First, there are autonomic effects of gambling. We and others have demonstrated that non-pathological gambling is associated with increases in heart rate [10–14]. As well, autonomic stimulation is observed with engagement in drug-seeking and drug-taking. Autonomic arousal is typically accompanied by neuroendocrine arousal as well.
Under normative conditions, engaging in psychologically- or physiologically-arousing behavior activates the hypothalamic-pituitary-adrenal (HPA) stress axis. Stress/HPA responses are characterized by release of neuropeptide from the hypothalamus and pituitary which acts at the adrenal glands. In response, adrenals produce the glucocorticoid, cortisol, which can travel throughout circulation to the brain to act in hypothalamus to downregulate its own production and act at other central targets to promote the physiological and psychological effects that are associated with stress. Recreational gambling can increase concentrations of cortisol in circulation but among problem and/or pathological gamblers the HPA stress responses may be aberrant. A similar process is observed among alcoholics and users of cocaine, wherein the initial exposure to the drug results in a large HPA response; however, as exposure becomes chronic, HPA responding is dampened. This dampening of HPA response may play a role in the addiction cycle.
In rodents, administration of glucocorticoid is rewarding [15,16] and in people, cortisol administration or stress has been demonstrated to promote cocaine craving [17,18]. Notably, attenuated HPA responding, as is the case during times of chronic stress, is associated with increased drug usage [19,20]. As such, HPA activation may be a formative part of the reward and/or addiction process and this may be particularly relevant for gambling.
Similar to pharmacological addiction, there is some evidence that suggests there are gender differences in problem and pathological gambling. In particular, men may have a higher prevalence for engaging in pathological gambling or using drugs of abuse, however, women may demonstrate more severe pathology once exposure has occurred [20–22]. While some studies have been conducted to assess the role that HPA factors, such as cortisol, may play in pathological addiction, findings are not consistent and have largely not accounted for differences in gender.
To begin to elucidate the role HPA function and/or gender may play in pathological gambling, we compared salivary cortisol levels of men and women that were recreational or pathological gamblers. Saliva samples were collected at baseline, following a video that depicted engagement in gambling, and after a video that depicted a neutral, stimulating scene (a rollercoaster ride). We anticipated that pathological gamblers would have an attenuated HPA response following exposure to gambling cues compared to recreational gamblers. It is notable that brief exposure to drug cues can readily reinstate craving for an addiction among human addicts and can reinstate a previously extinguished addiction among animals. As well, we anticipated that gender differences in HPA response may influence arousal to gambling cues.
2. Materials and Methods
These methods were pre-approved by the Internal Review Board at The University at Albany-SUNY for use with human subjects and are in compliance with the Declaration of Helsinki.
2.1. Participants
Forty-two adults from the Albany, NY area were recruited through advertisement in the newspaper, local flyers, and flyers in a treatment center hosting a local chapter of Gamblers Anonymous. Twenty-one individuals were active pathological gamblers and 21 were non-pathological, recreational gamblers who never met criteria for problem or pathological gambling (15 men and 6 women per group). Participants were at least 18 years of age, and did not meet criteria for a psychotic disorder, dementia, or current alcohol or substance abuse. Demographic data, including age, education, and preferred mode of gambling were collected to reveal no significant differences between groups.
2.2. Measures
2.2.1. The NORC DSM-IV Screen for Gambling Problems (NODS)
The National Opinion Research Center (NORC) has developed a screening measure for gambling problems based on the DSM-IV-TR [23] criteria for pathological gambling. The NODS is a 17-item measure that yields a score between 0 and 10 and loads on the 10 DSM-IV criteria for pathological gambling. A score of 5 or greater indicates the presence of pathological gambling. The NODS has established psychometric properties, including strong test-retest reliability and internal consistency [24]. In the current study, all participants considered pathological gamblers attained a score of ≥ 5 while recreational gamblers all received a score of < 5.
2.2.2. South Oaks Gambling Screen (SOGS)
The SOGS [25] is a 20-item gambling screen that yields a score between 0 and 20, with a score of 3 or 4 indicating a possible problem gambler, and a score of 5 or higher indicating a probable pathological gambler [25]. The SOGS is widely used in clinical and epidemiological studies and has well-established psychometric properties, including high internal reliability (Cronbach’s α =.97) and adequate 1-month test-retest reliability (r =.61 to 1.0 [25]). In the present investigation, all pathological gamblers attained scores of ≥ 5 whereas recreational gambling control scores were all ≤ 2.
2.2.4. Salivary Cortisol
Saliva was collected using “Salimetrics Oral Swabs” (Salimetrics, LLC, State College, PA) per manufacturer’s instruction. Briefly, participants were instructed to place the oral swab between their lower gum and cheek for 2 minutes or until saturated [26]. Three saliva samples were collected from each participant. Samples were stored at −20 °C until later analysis via commercially-available cortisol enzyme immunoassay kits (Salimetrics, LLC, State College, PA) [27–30]. Briefly, samples were slowly thawed on ice and centrifuged at 1500 × g for 15 min. Samples were visually-inspected for presence of blood contaminants (albeit, none were found) then pipetted into microtitre plates pre-coated with antibodies to cortisol followed by cortisol bound to horseradish peroxidase. Sample pH is detected via a colorimetric reaction within wells since pH ≤ 3.5 or ≥ 9 can impair ELISA efficiency [31]. In the present investigation, pH was not beyond normal range for any of the samples measured. Tetramethylbenzidine was added to each well and optical density (450 nm) was determined via an ELX800 universal microplate plate reader (BioTek Instruments, Inc., Winooski, VT). All samples, including standard curve (0 – 3 μg/dl) and unknowns, were run in duplicate. Wells containing known high and low cortisol concentrations were utilized to correct for multiple plate comparisons. Intra- and inter-plate co-efficient of variances were both 0.01.
2.3. Procedure
Following completion of demographic data as well as NODS and SOGS questionnaires, a baseline sample of saliva was collected. Participants watched three videos that were each 2 minutes long and complete with full audio. Videos were films of actors engaged in casino gambling and other gambling scenes. Prior to watching each video there was a 2 minute relaxation period during which participants listened to relaxing music. First, participants watched two gambling scenarios, one that depicted a winning gambling scenario, and one that depicted a losing gambling scenario in their preferred mode of gambling (slot machines, horses at off-track betting, scratch-off tickets, blackjack, video poker, craps, sports betting, online casino games, and lottery tickets). The order of presentation among the winning and losing scenarios was counterbalanced across subjects and conditions (order of presentation did not account for variation in salivary cortisol levels). After viewing the two gambling scenarios, a second saliva sample was collected. Participants then watched a non-gambling related exciting scenario depicting a rollercoaster ride. Each salivary collection was 20 minutes apart from the last. Following each video, participants completed a Likert-type rating scale (0–10) wherein 0 indicated videos were “not at all exciting” and 10 indicated videos were extremely exciting per previously reported methods [13]. Following the third saliva collection, participants were debriefed and compensated with an honorarium of $35 in gift certificates. All measures were assessed between 1500 and 1800 h in order to minimize diurnal cortisol variation as a potential confound.
2.4. Statistical Analyses
Two-way Analyses of Variance (ANOVAs) with gender (male, female) and gambling condition (recreational, pathological) were utilized to determine whether there were differences in age or level of education. Chi-square analysis was utilized to assess whether there were differences in preferred mode of gambling among participants and multivariate ANOVAs were utilized to assess differences in subjective excitement in response to videos followed by pairwise comparison t-tests with alpha corrected for multiple comparisons. Differences in salivary cortisol concentrations were elucidated using repeated measures ANOVAs with gender, gambling condition, and time of sampling (baseline, post-gambling video, post-rollercoaster video). To determine group differences, significant effects of repeated measures ANOVAs were followed up with one-way ANOVAs with alpha level corrected for multiple comparisons. Alpha level for significance was p < 0.05 and trends towards significance were reported when p < 0.10.
3. Results
Both groups of gamblers found the video stimuli exciting. On a scale from 0 (not at all exciting) to 10 (extremely exciting) the recreational gamblers rated (mean ± SEM) the winning gambling scenario 5.4 ± 0.5, the losing gambling scenario 3.9 ± 0.5, and the rollercoaster ride 5.5 ± 0.6. The pathological gamblers gave the winning, losing, and rollercoaster videos ratings of 6.1 ± 0.6, 3.3 ± 0.7, and 5.9 ± 0.5, respectively. While there were no differences between recreational and pathological gamblers [Hotellings, Trace 0.06, F(3,39) = 0.75, p = 0.53], both groups found the winning scenario and rollercoaster video significantly more exciting (p < 0.05).
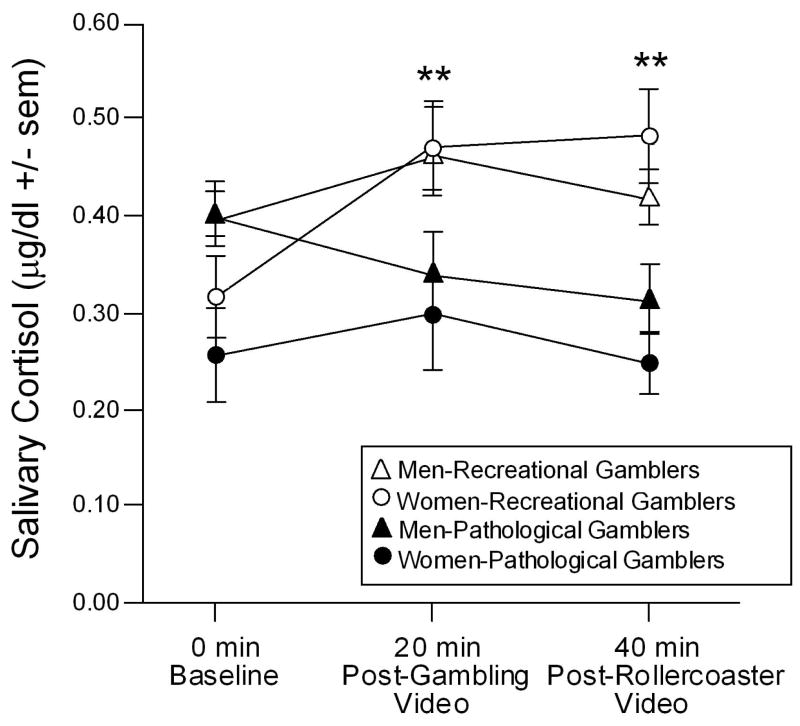
Differences in cortisol response were also observed between groups. As shown in figure 1, there was a significant interaction between the time of saliva sampling and pathological gambling condition [F(2,76) = 4.29, p < 0.05]. Recreational gamblers had significantly greater concentrations of salivary cortisol following the gambling or rollercoaster videos than at baseline, whereas pathological gamblers did not demonstrate significant changes in salivary cortisol following either video. There was also a tendency for time of sampling to interact with gender [F(2,76) = 2.58, p < 0.10]. This was due to men having greater salivary cortisol concentrations at baseline compared to women, but commensurate salivary cortisol following the videos.
Figure 1. Cortisol response to gambling stimuli.
Depicts salivary cortisol concentrations (mean ± standard error of the mean) among recreational and pathological gamblers (15 men and 6 women per group) at baseline, after watching a video depicting gambling cues, and after watching a video of a rollercoaster ride. ** indicates a significant interaction wherein recreational gamblers are different from pathological gamblers at all timepoints except baseline, p < 0.05.
4. Discussion
The hypothesis that HPA responding would be attenuated among pathological, compared to recreational, gamblers was upheld. As well, there were apparent effects for gender to influence HPA responding among gamblers, albeit, these did not reach statistical significance. These findings support and extend our understanding of HPA characterization in pathological gambling in several important ways.
In the present investigation, pathological gamblers demonstrated an attenuated HPA response to gambling and arousing stimuli compared to recreational gamblers. Similarly, functional magnetic resonance imaging studies have reported that compulsive slot machine gamblers demonstrate attenuated activation of ventral striatum and ventromedial prefrontal cortex in response to gambling compared to non-compulsive gambling controls [6]. These brain regions are known to be important for reward and engagement in motivated behavior. In support, patients with lesions to the ventromedial region of the prefrontal cortex are noted for impulsive decision-making that often yields small gains that are wiped out through significant losses [3]. HPA function may be important for activity in these regions given that glucocorticoid secretion is associated with enhancement of mesolimbic dopamine release, an important neurotransmitter for reward [32–34]. Others have found that cerebrospinal concentrations of dopamine are decreased among pathological gamblers compared to healthy controls [35]. As well, opiate antagonists have demonstrated efficacy in treating pathological gambling [7–9] and central opiate administration to mesolimbic brain regions enhances dopaminergic activity among rodents [36,37]. These data suggest that HPA hyporesponsivity may be an important factor in the pathology of gambling addiction. However, it is notable that we have found no basal differences in salivary cortisol concentrations between pathological and recreational gamblers. These findings are consistent with previous investigations demonstrating a dysregulation of basal catecholamine levels in brain [38,39], but normative circulating cortisol [40]. Thus, dampened arousal of the HPA axis is associated with pathological gambling and may play a role in maintenance of this behavior.
There was a non-significant tendency for gender to influence salivary cortisol wherein men had higher levels than did women, and male controls demonstrated less of an increase in response to arousing stimuli than did females. Others have demonstrated that basal salivary cortisol concentrations can be greater among adult men compared to women, however, the reverse can also be true depending on many factors including age, health, and menstrual cycle [41,42]. It is a more common finding that women demonstrate greater lability in cortisol response to physiological arousal with a much larger peak in salivary cortisol than do men [43,44]. It is notable that, among the pathological gambling group (but not controls), men demonstrated greater lability in salivary cortisol than did women. In response to the gambling stimuli, pathological gambling men demonstrated a strong attenuation of HPA activation compared to recreational gambling men. Conversely, pathological gambling women demonstrated a similar, but markedly blunted, response to the gambling stimuli than did recreational gambling women. Although only a trend towards a significant interaction between gender and gambling pathology was observed in the present study, it is an intriguing avenue for future investigations with access to larger sample sizes.
The present investigation did have some limitations. Given the current sample size, cue exposure was conducted within subjects. As such, the arousing neutral stimulus scenario (rollercoaster video) was preceded by the gambling stimuli for all participants. Past investigations have found that cortisol is elevated in response to gambling in a casino and remains elevated [12], albeit, these findings are likely also associated with the casino-experience as well as the gambling experience. In the present study, we cannot determine the extent to which exposure to the gambling stimuli may have influenced exposure to the neutral arousing stimuli. Future investigations may aim to use a fully between-subjects design or collect saliva samples before and after exposure to arousing stimuli. As well, this design would parse out potential differences in HPA responding to either a gambling win or a gambling loss (which could not be determined in the counterbalanced design utilized in the present investigation). However, we did observe significant differences in subjective measures of arousal depending on whether participants viewed a winning or losing scenario. As such, differences in response to winning versus losing gambling stimuli among recreational and pathological gambling men and women remains an area of future interest.
There have now been several investigations assessing the neuroendocrine profile of problem and pathological gambling, however, findings are not consistent. Generally, dysregulation of the HPA axis is reported among pathological compared to recreational gamblers, however, gender differences are largely understudied in this phenomenon. Notably, more recent evidence suggests that endogenous diurnal variation in cortisol may be dysregulated. Specifically, problem or pathological gamblers may have a greater cortisol rise following waking than do recreational gambling controls [45]. While this effect was attenuated as cortisol levels declined over the next five hours, the degree to which differences in HPA reactivity accounts for aspects of gambling pathology remains of interest. The present investigation suggests that pathological gambling is accompanied by a hypo-arousal of the HPA axis in response to gambling cues and that gender differences may contribute to this response. As well, investigating neureoendocrine factors that may be specific to subtypes of pathological gambling may further elucidate neurobiological targets that underlie gambling addiction.
Acknowledgments
This research was supported by The University at Albany-SUNY Faculty Research Award Program funding to Cheryl Frye and Edelgard Wulfert and an R21 MH064568 from the National Institute of Mental Health to Edelgard Wulfert.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Tamminga CA, Nestler EJ. Pathological gambling: Focusing on the addiction, not the activity. Am J Psychiatry. 2006;163:180–181. doi: 10.1176/appi.ajp.163.2.180. [DOI] [PubMed] [Google Scholar]
- 2.Lesieur HR, Blume SB, Zoppa RM. Alcoholism, drug abuse, and gambling. Alcohol Clin Exp Res. 1986;10:33–38. doi: 10.1111/j.1530-0277.1986.tb05610.x. [DOI] [PubMed] [Google Scholar]
- 3.Bechara A. Neurobiology of decision-making: risk and reward. Semin Clin Neuropsychiatry. 2001;6:205–216. doi: 10.1053/scnp.2001.22927. [DOI] [PubMed] [Google Scholar]
- 4.Kalenscher T, Ohmann T, Güntürkün O. The neuroscience of impulsive and self-controlled decisions. Int J Psychophysiol. 2006;62:203–211. doi: 10.1016/j.ijpsycho.2006.05.010. [DOI] [PubMed] [Google Scholar]
- 5.Goodman A. Neurobiology of addiction. An integrative review. Biochem Pharmacol. 2008;75:266–322. doi: 10.1016/j.bcp.2007.07.030. [DOI] [PubMed] [Google Scholar]
- 6.Reuter J, Raedler T, Rose M, Hand I, Gläscher J, Büchel C. Pathological gambling is linked to reduced activation of the mesolimbic reward system. Nat Neurosci. 2005;8:147–148. doi: 10.1038/nn1378. [DOI] [PubMed] [Google Scholar]
- 7.Kim SW, Grant JE, Adson DE, Shin YC. Double-blind naltrexone and placebo comparison study in the treatment of pathological gambling. Biol Psychiatry. 2001;49:914–921. doi: 10.1016/s0006-3223(01)01079-4. [DOI] [PubMed] [Google Scholar]
- 8.Grant JE, Kim SW, Hollander E, Potenza MN. Predicting response to opiate antagonists and placebo in the treatment of pathological gambling. Psychopharmacology. 2008;200:521–527. doi: 10.1007/s00213-008-1235-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Grant JE, Potenza MN, Hollander E, Cunningham-Williams R, Nurminen T, Smits G, Kallio A. Multicenter investigation of the opioid antagonist nalmefene in the treatment of pathological gambling. Am J Psychiatry. 2006;163:303–312. doi: 10.1176/appi.ajp.163.2.303. [DOI] [PubMed] [Google Scholar]
- 10.Coventry KR, Constable B. Physiological arousal & sensation-seeking in female fruit machine gamblers. Addiction. 1999;94:425–430. doi: 10.1046/j.1360-0443.1999.94342512.x. [DOI] [PubMed] [Google Scholar]
- 11.Coventry KR, Hudson J. Gender differences and physiological arousal the role of winning in fruit machine gamblers. Addiction. 2001;96:871–879. doi: 10.1046/j.1360-0443.2001.9668718.x. [DOI] [PubMed] [Google Scholar]
- 12.Meyer G, Hauffa BP, Schedlowski M, Pawlak C, Stadler MA, Exton MS. Casino gambling increase heart rate and salivary cortisol in regular gamblers. Biol Psychiatry. 2000;48:948–953. doi: 10.1016/s0006-3223(00)00888-x. [DOI] [PubMed] [Google Scholar]
- 13.Wulfert E, Roland BD, Hartley J, Wang N, Franco C. Heart rate arousal and excitement in gambling: Winners versus losers. Psychol Addict Behav. 2005;19:311–316. doi: 10.1037/0893-164X.19.3.311. [DOI] [PubMed] [Google Scholar]
- 14.Wulfert E, Franco C, Williams K, Roland BD, Maxson JH. The role of money in the excitement of gambling. Psychol Addict Behav. 2008;22:380–390. doi: 10.1037/0893-164X.22.3.380. [DOI] [PubMed] [Google Scholar]
- 15.Deroche V, Piazza PV, Deminiere JM, Le Moal M, Simon H. Rats orally self-administer corticosterone. Brain Res. 1993;622:315–320. doi: 10.1016/0006-8993(93)90837-d. [DOI] [PubMed] [Google Scholar]
- 16.Piazza PV, Deroche V, Deminiere JM, Maccari S, Le Moal M, Simon H. Corticosterone in the range of stress-induced levels possesses reinforcing properties: implications for sensation-seeking behaviors. Proc Natl Acad Sci U S A. 1993;90:11738–11742. doi: 10.1073/pnas.90.24.11738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Elman I, Lukas SE, Karlsgodt KH, Gasic GP, Breiter HC. Acute cortisol administration triggers craving in individuals with cocaine dependence. Psychopharmacol Bull. 2003;37:84–89. [PubMed] [Google Scholar]
- 18.Sinha R, Fuse T, Aubin LR, O’Malley SS. Psychological stress, drug-related cues and cocaine craving. Psychopharmacology. 2000;152:140–148. doi: 10.1007/s002130000499. [DOI] [PubMed] [Google Scholar]
- 19.Gold PW, Chrousos GP. Organization of the stress system and its dysregulation in melancholic and atypical depression: High vs low CRH/NE states. Mol Psychiatry. 2002;7:254–75. doi: 10.1038/sj.mp.4001032. [DOI] [PubMed] [Google Scholar]
- 20.Wilson DH, Gilliland J, Ross NA, Derevensky J, Gupta R. Video lottery terminal access and gambling among high school students in Montréal. Can J Public Health. 2006;97:202–206. doi: 10.1007/BF03405585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Blanco C, Hasin DS, Petry N, Stinson FS, Grant BF. Sex differences in subclinical and DSM-IV pathological gambling: results from the National Epidemiologic Survey on Alcohol and Related Conditions. Psychol Med. 2006;36:943–953. doi: 10.1017/S0033291706007410. [DOI] [PubMed] [Google Scholar]
- 22.Westermeyer J, Boedicker AE. Course, severity, and treatment of substance abuse among women versus men. Am J Drug Alcohol Abuse. 2000;26:523–535. doi: 10.1081/ada-100101893. [DOI] [PubMed] [Google Scholar]
- 23.American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4. Washington, DC: American Psychiatric Association; 2000. (text revision) [Google Scholar]
- 24.National Opinion Research Center. Gambling impact and behavior study: Final report to the National Gambling Impact Study Commission. Chicago, IL: University of Chicago Press; 1999. [Google Scholar]
- 25.Lesieur HR, Blume SB. The South Oaks Gambling Screen (SOGS): a new instrument for the identification of pathological gamblers. Am J Psychiatry. 1987;144:1184–8. doi: 10.1176/ajp.144.9.1184. [DOI] [PubMed] [Google Scholar]
- 26.Granger DA, Kivlighan KT, Fortunato C, Harmon AG, Hibel LC, Schwartz EB, Whembolua GL. Integration of salivary biomarkers into developmental and behaviorally-oriented research: problems and solutions for collecting specimens. Physiol Behav. 2007;92:583–590. doi: 10.1016/j.physbeh.2007.05.004. [DOI] [PubMed] [Google Scholar]
- 27.Brown GL, McGarvey EL, Shirtcliff EA, Keller A, Granger DA, Flavin K. Salivary cortisol, dehydroepiandrosterone, and testosterone interrelationships in healthy young males: a pilot study with implications for studies of aggressive behavior. Psychiatry Res. 2008;159:67–76. doi: 10.1016/j.psychres.2007.06.012. [DOI] [PubMed] [Google Scholar]
- 28.Gordis EB, Granger DA, Susman EJ, Trickett PK. Salivary α-amylase-cortisol asymmetry in maltreated youth. Horm Behav. 2008;53:96–103. doi: 10.1016/j.yhbeh.2007.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gordis EB, Granger DA, Susman EJ, Trickett PK. Asymmetry between salivary cortisol and α-amylase reactivity to stress: relation to aggressive behavior in adolescents. Psychoneuroendocrinology. 2006;31:976–987. doi: 10.1016/j.psyneuen.2006.05.010. [DOI] [PubMed] [Google Scholar]
- 30.Granger DA, Blair C, Willoughby M, Kivlighan KT, Hibel LC, Fortunato CK, Wiegand LE Family Life Project Investigators. Individual differences in salivary cortisol and alpha-amylase in mothers and their infants: relation to tobacco smoke exposure. Dev Psychobiol. 2007;49:692–701. doi: 10.1002/dev.20247. [DOI] [PubMed] [Google Scholar]
- 31.Schwartz EB, Granger DA, Susman EJ, Gunnar MR, Laird B. Assessing salivary cortisol in studies of child development. Child Dev. 1998;69:1503–1513. [PubMed] [Google Scholar]
- 32.Barrot M, Abrous DN, Marinelli M, Rougé-Pont F, Le Moal M, Piazza PV. Influence of glucocorticoids on dopaminergic transmission in the rat dorsolateral striatum. Eur J Neurosci. 2001;13:812–818. doi: 10.1046/j.1460-9568.2001.01434.x. [DOI] [PubMed] [Google Scholar]
- 33.Piazza PV, Le Moal M. The role of stress in drug self-administration. Trends Pharmacol Sci. 1998;19:67–74. doi: 10.1016/s0165-6147(97)01115-2. [DOI] [PubMed] [Google Scholar]
- 34.Rougé-Pont F, Deroche V, Le Moal M, Piazza PV. Individual differences in stress-induced dopamine release in the nucleus accumbens are influenced by corticosterone. Eur J Neurosci. 1998;10:3903–3907. doi: 10.1046/j.1460-9568.1998.00438.x. [DOI] [PubMed] [Google Scholar]
- 35.Bergh C, Eklund T, Södersten P, Nordin C. Altered dopamine function in pathological gambling. Psychol Med. 1997;27:473–475. doi: 10.1017/s0033291796003789. [DOI] [PubMed] [Google Scholar]
- 36.Broekkamp CL, Phillips AG, Cools AR. Stimulant effects of enkephalin microinjection into the dopaminergic A10 area. Nature. 1979;278:560–562. doi: 10.1038/278560a0. [DOI] [PubMed] [Google Scholar]
- 37.Matthews RT, German DC. Electrophysiological evidence for excitation of rat ventral tegmental area dopamine neurons by morphine. Neuroscience. 1984;11:617–625. doi: 10.1016/0306-4522(84)90048-4. [DOI] [PubMed] [Google Scholar]
- 38.Roy A, Adinoff B, Roehrich L, Lamparski D, Custer R, Lorenz V, Barbaccia M, Guidotti A, Costa E, Linnoila M. Pathological gambling. A psychobiological study. Arch Gen Psychiatry. 1988;45:369–373. doi: 10.1001/archpsyc.1988.01800280085011. [DOI] [PubMed] [Google Scholar]
- 39.Roy A, De Jong J, Linnoila M. Extraversion in pathological gamblers. Correlates with indexes of noradrenergic function. Arch Gen Psychiatry. 1989;46:679–681. doi: 10.1001/archpsyc.1989.01810080009001. [DOI] [PubMed] [Google Scholar]
- 40.Ramirez LF, McCormick RA, Lowy MT. Plasma cortisol and depression in pathological gamblers. Br J Psychiatry. 1988;153:684–6. doi: 10.1192/bjp.153.5.684. [DOI] [PubMed] [Google Scholar]
- 41.Aardal E, Holm AC. Cortisol in saliva--reference ranges and relation to cortisol in serum. Eur J Clin Chem Clin Biochem. 1995;33:927–932. doi: 10.1515/cclm.1995.33.12.927. [DOI] [PubMed] [Google Scholar]
- 42.Kajantie E, Phillips DI. The effects of sex and hormonal status on the physiological response to acute psychosocial stress. Psychoneuroendocrinology. 2006;31:151–178. doi: 10.1016/j.psyneuen.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 43.Kumsta R, Entringer S, Hellhammer DH, Wüst S. Cortisol and ACTH responses to psychosocial stress are modulated by corticosteroid binding globulin levels. Psychoneuroendocrinology. 2007;32:1153–1157. doi: 10.1016/j.psyneuen.2007.08.007. [DOI] [PubMed] [Google Scholar]
- 44.Weekes NY, Lewis RS, Goto SG, Garrison-Jakel J, Patel F, Lupien S. The effect of an environmental stressor on gender differences on the awakening cortisol response. Psychoneuroendocrinology. 2008;33:766–772. doi: 10.1016/j.psyneuen.2008.03.003. [DOI] [PubMed] [Google Scholar]
- 45.Wohl MJ, Matheson K, Young MM, Anisman H. Cortisol rise following awakening among problem gamblers: dissociation from comorbid symptoms of depression and impulsivity. J Gambl Stud. 2008;24:79–90. doi: 10.1007/s10899-007-9080-6. [DOI] [PubMed] [Google Scholar]