Abstract
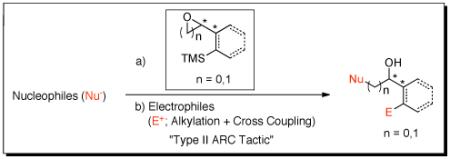 Union of Type II Anion Relay Chemistry (ARC) with Pd-induced Cross Coupling Reactions (CCR) has been achieved, in conjunction with the design, synthesis, and evaluation of a new class of bifunctional linchpins, comprising a series of vinyl silanes bearing β- or γ-electrophilic sites. The synthetic tactic permits both alkylation and Pd-mediated CCR of the anions derived via 1,4-silyl C(sp2)→O Brook Rearrangements.
Union of Type II Anion Relay Chemistry (ARC) with Pd-induced Cross Coupling Reactions (CCR) has been achieved, in conjunction with the design, synthesis, and evaluation of a new class of bifunctional linchpins, comprising a series of vinyl silanes bearing β- or γ-electrophilic sites. The synthetic tactic permits both alkylation and Pd-mediated CCR of the anions derived via 1,4-silyl C(sp2)→O Brook Rearrangements.
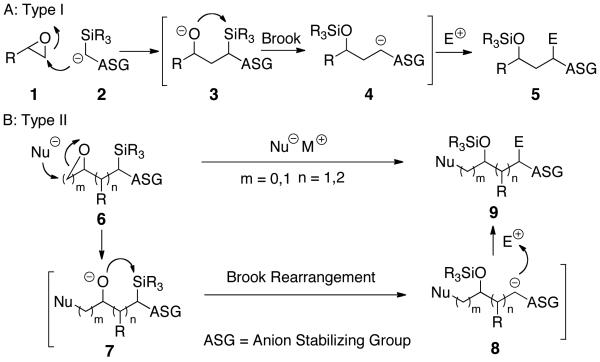
Type I and II Anion Relay Chemistry (ARC),1 exploiting Brook Rearrangements (Scheme 1A and 1B),2 comprises a powerful linchpin tactic for the rapid assembly of high levels of molecular complexity, as demanded by natural product total synthesis. Extension of the Type II ARC process (Scheme 1B) to include transition metal promoted Cross-Coupling Reactions (CCR), as the culminating event in the Type II ARC process (cf. 8→9) would, in general, greatly extend the scope of this evolving synthetic tactic. Recently, we recorded a single example employing ortho-TMS benzaldehyde 10 as linchpin that demonstrated the feasibility of uniting Anion Relay Chemistry with Pd-mediated cross coupling (Scheme 2).3 The reaction sequence involved treatment of 10 with n-BuLi followed in turn by addition of CuI and HMPA to induce 1,4-silyl C(sp2)→O migration, vinyl bromide with a catalytic amount of Pd(PPh3)4 for the CCR, and TBAF to remove the TMS group; tricomponent adduct 10a was produced in 56% yield.
Scheme 1.
Type I and II Anion Relay Chemistry (ARC)
Scheme 2.
Type II Anion Relay Chemistry (ARC) and Cross-Coupling Reactions (CCR).
Convinced that this “one-flask” multicomponent protocol would hold considerable potential, in general, we initiated a program to unite Anion Relay Chemistry with the cross coupling tactic. We quickly recognized, as reported by Takeda et al.4 for ortho-TMS benzyl alcohol, that the use of CuI, and in our case a mixture of HMPA and THF (1:1), is required to trigger the 1,4-Brook rearrangement. Towards this end, addition of n-BuLi to 10, followed by CuI/HMPA:THF induced silyl migration. Subsequent addition of diverse vinyl and aryl halides in the presence of 3 mol % Pd(PPh3)4 in THF at room temperature then, led to a series of cross coupled adducts (10a-10h) with yields ranging from 50-67% (Table 1).
Table 1.
Pd-Mediated Cross-Coupling Reactions via Type II ARC
 | ||
|---|---|---|
| entry | electrophile | R |
| 1 |  |
 10a: (67%) |
| 2 |  |
 10b: (55%) |
| 3 |  |
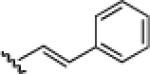 10c: (65%) |
| 4 | 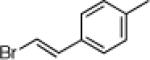 |
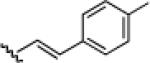 10d: (57%) |
| 5 | 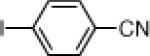 |
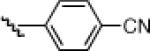 10e: (60%) |
| 6 | 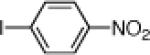 |
10f: (58%) |
| 7 | 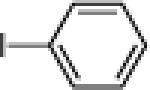 |
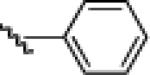 10g: (58%) |
| 8 |  |
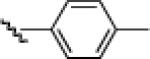 10h: (50%) |
Conditions: 1.2 equiv n-BuLi, 1.2 equiv CuI.
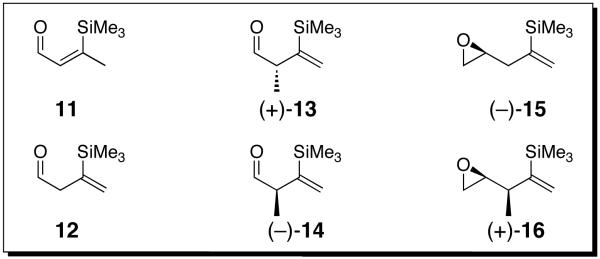
Having established the initial scope of the combined ARC-II/Pd-mediated CCR protocol, we turned to the design, synthesis and evaluation of a new class of bifunctional vinyl silanes, with electrophilic sites β or γ to the silane (Figure 1), first to explore their utility as linchpins for the Type II ARC tactic and then as linchpins in the combined ARC-II/Pd-mediated CCR process.
Figure 1.
Vinyl Silane Bifunctional Linchpins
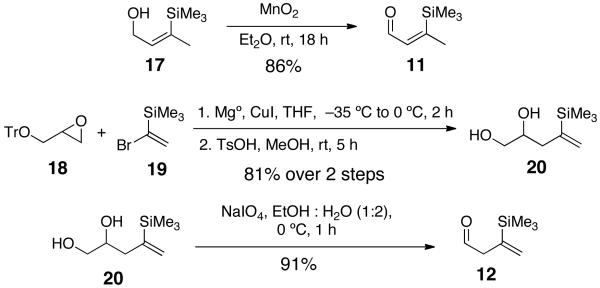
Linchpin 11 was readily available via oxidation of known alcohol 17,5 while 12 was prepared from epoxide 186 and commercial vinyl bromide 19 (Scheme 3).
Scheme 3.
Preparation of Linchpins 11 and 12
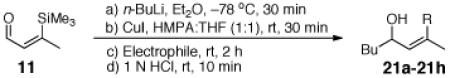
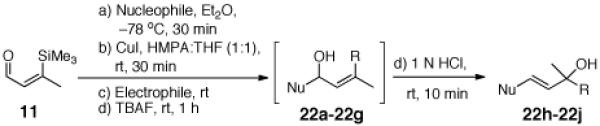
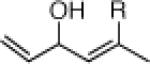
To explore the ARC tactic with 11, we selected conditions that proved effective with 10.3 As illustrated in Table 2, addition of n-BuLi in Et2O, followed by CuI (1.2 equiv) in a mixture of HMPA/THF (1:1), and then a variety of carbon- and heteroatom-based electrophiles furnished adducts 21a-21d in 63-68% yield. Under these conditions, the 1,4 silyl migration proceeded rapidly (ca. 30 min). Equally important, palladium-mediated cross coupling reactions, initiated via the ARC Type II process, proved feasible. For example, addition of 3 mol % Pd(PPh3)4 and a series of vinyl and aryl halides after the HMPA/THF induced Brook rearrangement furnished cross coupled adducts 21e-22h in 52-61% yield. Other common nucleophiles proved effective as initiators of both the Type II ARC and the combined ARC-II/Pd-mediated CCR tactics (Table 3). Use of TBAF to remove the TMS group proved critical to avoid partial allylic rearrangements of 22a-22f; use of 1 N HCl led to facile allylic rearrangement (cf. 22h-22j). Neither allylic rearrangement nor cross-coupling was observed upon use of anion derived from dithiane, the latter due to catalyst poisoning by the dithiane.7
Table 2.
Three-Component Coupling of Linchpin 11 with Various Electrophiles
 | ||
|---|---|---|
| entry | electrophile | R |
| 1 |
 21a: (65%) |
|
| 2 | 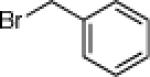 |
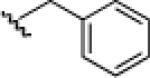 21b: (66%) |
| 3 | PhS-SPh |
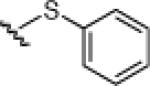 21c: (68%) |
| 4 | Bu3SnCl |
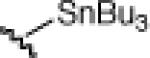 21d: (63%) |
| 5 | 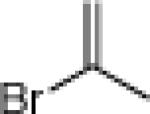 |
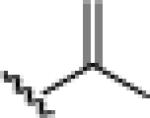 21e: (53%)a |
| 6 | 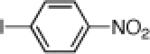 |
21f: (52%)a |
| 7 | 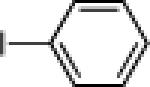 |
 21g: (54%)a |
| 8 |  |
 21h: (61%)a |
Conditions: 1.2 equiv n-BuLi, 1.2 equiv CuI.
3 mol % Pd(PPh3)4, THF, rt, 6 h.
Table 3.
Three-Component Coupling of Linchpin 11 with Various Nucleophiles and CCR with Phenyl Iodide
 | |||
|---|---|---|---|
| entry | nucleophile | product | |
| 1 |  |
 22a: (62%)b,c 22b: (45%)b,d |
22h: (64%)a |
| 2 | 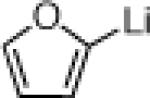 |
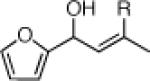 22c: (65%)b,c 22d: (50%)b,d |
 22i: (56%)a |
| 3 |  |
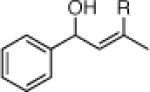 22e: (66%)b,c 22f: (51%)b,d |
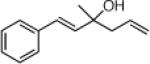 22j: (61%)a |
| 4 | 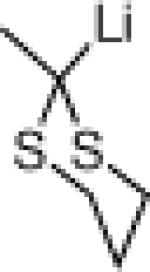 |
 22g: (58%)a,c,e |
NR |
Conditions:
1 N HCl, rt, 10 min.
TBAF, rt, 1 h.
R = Allyl, Allyl bromide, rt, 2 h.
R = Ph, 3 mol % Pd(PPh3)4, Phenyl iodide THF, rt, 6 h.
No cross-coupling product was obtained due to catalyst poisoning by dithiane.
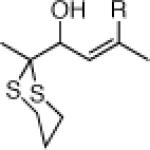
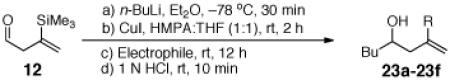
Encouraged by the viability of the Type II ARC process employing 11, we turned next to 12 as the bifunctional linchpin (Table 4). Initially, silyl migration proved problematic, furnishing only trace amounts of the desired product when employing the conditions which proved effective at triggering silyl migration with 11. However, when two equivalents of both n-BuLi as the nucleophile and CuI were employed in a mixture of HMPA and THF (1:1), complete 1,4-silyl C(sp2)→O migration occurred albeit more slowly over the course of 2 h. Addition of a series of electrophiles (2.0 equiv) furnished alkylation adducts 23a-23d (Table 4, Entries 1-4) in ca. 50% yield, while modest yields of cross-coupling products 23e-23f were obtained upon addition of 3 mol % Pd(PPh3)4 followed by aryl iodides (Entries 5-6).
Table 4.
Three-Component Coupling of Linchpin 12 with Various Electrophiles
 | ||
|---|---|---|
| entry | electrophile | R |
| 1 |
 23a: (49%) |
|
| 2 | 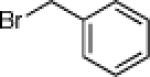 |
 23b: (42%) |
| 3 | PhS-SPh |
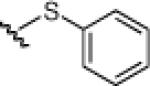 23c: (52%) |
| 4 | 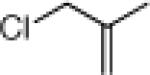 |
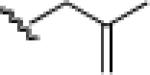 23d: (51%) |
| 5 | 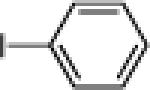 |
 23e: (45%)a |
| 6 |  |
 23f: (44%)a |
Conditions: 2.0 equiv n-BuLi, 2.0 equiv CuI.
3 mol % Pd(PPh3)4, THF, rt, 12 h.
The variable time course between 11 and 12 for 1,4-silyl group migration is understandable in terms of the mechanism of the Brook rearrangement.8 In the case of linchpin 12, the 1,4-silyl C(sp2)→O migration is slow, due to a combination of the distance between the silicon and oxygen atoms and the multiple bond rotations required to arrive at the cyclic transition state.9
From the perspective of complex molecule synthesis, a drawback of employing α-unsubstituted linchpin aldehydes 11 and 12 entails the lack of stereochemical control upon initial nucleophilic addition and the lack of the ubiquitous methyl substituents found in polyketides. We therefore turned to α-substituted linchpins (+)-13 and (−)-14.10 We reasoned that addition of alkyllithiums to (+)-13 and (−)-14 would produce, with good diastereoselectivity, the corresponding syn alkoxides. Confirmation of this scenario would further increase the utility of both the Type II ARC and ARC-II/Pd-induced CCR tactics, not only for natural product total synthesis, but also polyketide diversity synthesis.11
Pleasingly, addition of n-BuLi to either (+)-13 or (−)-14 (Table 5), followed in turn by silyl migration induced by CuI in a mixture of HMPA and THF (1:1) and reaction with a series of electrophiles furnished multicomponent adducts 24a-25h in yields ranging from 56 to 75% (Table 5).12 Effective cross coupling unions also occurred after 1,4-Brook rearrangement, upon addition of 3 mol % Pd(PPh3)4, followed by vinyl or aryl halides. Importantly, no epimerization of the α-methyl substituent was observed during this process.
Table 5.
Three-Component Coupling of Linchpin (+)-13 or (−)-14 with Various Electrophiles
 | |||
|---|---|---|---|
| entry | electrophile | R | yield |
| 1 |  |
24a: (65%) 25a: (68%) | |
| 2 |  |
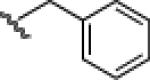 |
24b: (66%) 25b: (68%) |
| 3 | PhS-SPh | 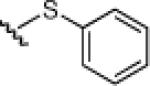 |
24c: (56%) 25c: (57%) |
| 4 | Bu3SnCl | 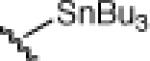 |
24d: (66%) 25d: (67%) |
| 5 | 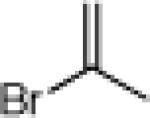 |
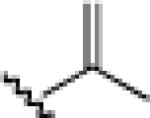 |
24e: (70%)a 25e: (69%)a |
| 6 | 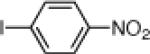 |
24f: (62%)a 25f: (60%)a | |
| 7 | 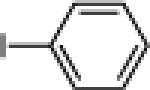 |
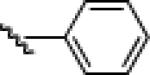 |
24g: (71%)a 25g: (72%)a |
| 8 | 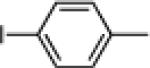 |
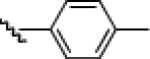 |
24h: (75%)a 25h: (74%)a |
Conditions: 2.0 equiv n-BuLi, 2.0 equiv CuI.
3 mol % Pd(PPh3)4, THF, rt, 12 h.
As with linchpin 11, other nucleophiles derived from furan, phenyl bromide and 2-methyl-1,3-dithiane prove to be effective at initiating the Type II ARC tactic to furnish, in a single flask, both three-component alkylation and cross-coupled adducts 26a-27e (Table 6).
Table 6.
Three-Component Coupling of Linchpin (+)-13 or (−)-14 with Various Nucleophiles and CCR with Phenyl iodide
 | |||
|---|---|---|---|
| entry | nucleophile | product | |
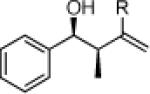
| 1 | 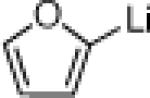 |
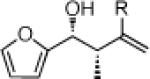 26a: (58%)a 26b: (58%)b |
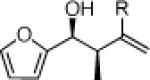 27a: (59%)a 27b: (55%)b |
| 2 | 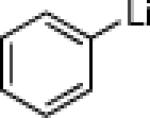 |
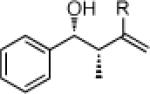 26c: (63%)a 26d: (59%)b |
 27c: (61%)a 27d: (57%)b |
| 3 | 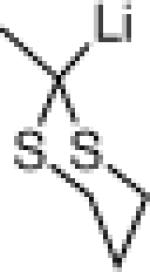 |
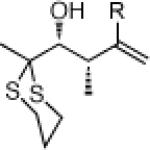 26e: (53%)a |
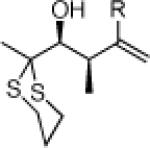 27e: (50%)a |
Conditions:
R = Allyl, Allyl bromide, rt, 12 h.
R = Ph, 3 mol % Pd(PPh3)4, Phenyl iodide, THF, rt, 12 h.
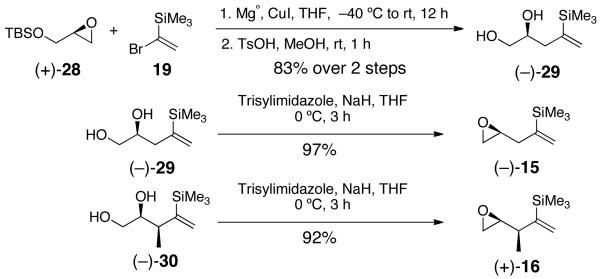
We next explored the possibility of extending the Type II ARC and ARC-II/Pd-induced CCR tactics to epoxide-based linchpins possessing an electrophilic site γ to the vinyl silane. This design led to linchpins (−)-15 and (+)-16, constructed as illustrated in Scheme 4.13,14
Scheme 4.
Preparation of Linchpins (−)-15 and (+)-16
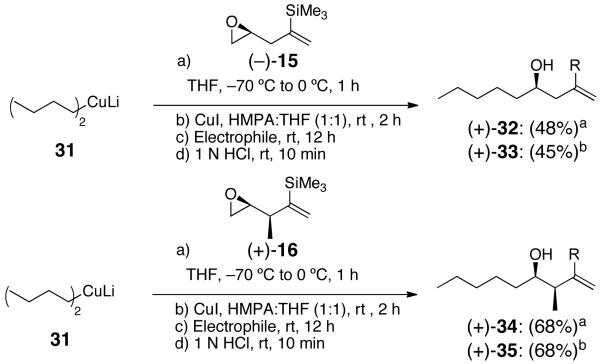
With the four-carbon bifunctional linchpins in hand, we employed lithium dibutylcuprate 31, known both to add to epoxides15 and to initiate 1,4-silyl C(sp3)→ O migration in Anion Relay Chemistry.1b Capture of allyl bromide after Brook rearrangement led respectively to adducts (+)-32 and (+)-34 (Scheme 5). Cross coupling reactions also proceeded upon addition of 3 mol % Pd(PPh3)4, followed by reaction with phenyl iodide to furnish (+)-33 and (+)-35, respectively.
Scheme 5.
Three-Component Coupling of Linchpins (−)-15 or (+)-16 with Various Electrophiles
In summary, the union of Type II Anion Relay Chemistry with Pd-mediated Cross Coupling has been achieved, thereby greatly expanding the scope of this multicomponent “one-flask” linchpin protocol. Equally important, a new class of three and four carbon, bifunctional linchpins comprising aryl and vinyl silanes bearing β- or γ-electrophilic sites, have been designed, synthesized and demonstrated to be competent in both Type II ARC and combined ARC-II/Pd-induced CCR processes. Studies to improve the efficiency of this tactic continue in our laboratory.
Supplementary Material
Acknowledgment
Support was provide by the National Institutes of Health (GM-29028 and GM-081253). We gratefully acknowledge Cephalon, Inc. for a Dr. Horst Witzel Fellowship awarded to Won-Suk Kim.
Footnotes
Supporting Information Available: Procedures and characterization data are available free of charge via the Internet: http://pubs.acs.org.
References
- 1.For reviews see: Smith AB, III, Adams CM. Acc. Chem. Res. 37:365. doi: 10.1021/ar030245r. Smith AB, III, Wuest WM. Chem. Commun. 2008:5883. doi: 10.1039/b810394a. Moser WH. Tetrahedron. 2001;57:2065.
- 2.Brook AG. Acc. Chem. Res. 1974;7:77. [Google Scholar]
- 3.Smith AB, III, Kim W-S, Wuest WM. Angew. Chem., Int. Ed. 2008;47:7082. doi: 10.1002/anie.200802301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Taguchi H, Takami K, Tsubouchi A, Takeda T. Tetrahedron Lett. 2004;45:429. [Google Scholar]
- 5.Han S, Kass RS. Tetrahderon Lett. 1997;38:7503. [Google Scholar]
- 6.Kamal A, Ramesh GBK, Krishnaji T, Ramu R. Tetrahderon: Asymmetry. 2006;17:1281. [Google Scholar]
- 7.Main CA, Petersson HM, Rahman SS, Hartley RC. Tetrahderon. 2008;64:901. [Google Scholar]
- 8.(a) Jiang X, Bailey WF. Organometallics. 1995;14:5704. [Google Scholar]; (b) Kawashima T, Naganuma K, Okazaki R. Organometallics. 1998;17:367. [Google Scholar]; (c) Naganuma K, Kawashima T, Okazaki R. Chem. Lett. 1999:1139. [Google Scholar]
- 9.The results on silyl migration are in accord with Takeda and coworkers. Tsubouchi A, Itoh M, Onishi K, Takeda T. Synthesis. 2004;9:1504.
- 10.Sato and coworkers demonstrated (+)-13 and (−)-14 underwent Grignard reactions with high syn diastereoselectivity, the result of a Cram product-like transition state.10b,10e Sato F, Kusakabe M, Kobayashi Y. J. Chem. Soc., Chem. Commun. 1984:1130. Sato F, Takeda Y, Uchiyama H, Kobayashi Y. J. Chem. Soc., Chem. Commun. 1984:1132. Kobayashi Y, Kitano Y, Sato F. J. Chem. Soc., Chem. Commun. 1984:1329. Sato F, Kusakabe M, Kato Y. J. Chem. Soc., Chem. Commun. 1984:1331. Samaddar AK, Chiba T, Kobayashi Y, Sato F. J. Chem. Soc., Chem. Commun. 1985:329.
- 11.Schreiber SL. Science. 2000;287:1964. doi: 10.1126/science.287.5460.1964. [DOI] [PubMed] [Google Scholar]
- 12.Absolute configuration was confirmed by Mosher ester analysis.
- 13.(a) Hicks DR, Fraser-Reid B. Synthesis. 1974:203. [Google Scholar]; (b) Cink RD, Forsyth CJ. J. Org. Chem. 1995;60:8122. [Google Scholar]
- 14.Kobayashi Y, Kitano Y, Takeda Y, Sato F. Tetrahedron. 1986;42:2937. [Google Scholar]
- 15.Herr RW, Wieland DM, Johnson CR. J. Am. Chem. Soc. 1970;92:3813. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.