Abstract
Gonadal steroids are among the many factors that influence food intake and body weight in mammals. Hormonal effects on these processes are particularly striking in female rats, which show large increases in food intake and body weight after ovariectomy. A key role of estradiol in the control of food intake and energy balance in humans is evidenced by the fact that the incidence of obesity increases greatly after menopause [1]. The actions of estradiol on neural systems that regulate eating may also account in part for sex differences in food intake and eating disorders, which occur much more frequently in young women [65]. This paper presents a minireview of research examining the changes in feeding that occur during the ovarian cycle, the effects of estradiol withdrawal and replacement on food intake and body weight, and the neurobiological mechanisms by which estradiol influences feeding behavior. A model of hormone action on food intake that emerges from this research views estradiol as an indirect control of eating and meal size, producing changes in feeding behavior by modulating the central processing of both satiating and orexigenic peptides that represent direct controls of eating. Some of the shortcomings of the model and directions for future research are discussed.
1. Introduction
The goal of this review is not to provide a comprehensive analysis of the research on the control of ingestive behavior by ovarian hormones. Instead, the goal is to focus on the ability of estradiol to influence feeding via interactions with peptidergic systems known to be involved in the control of food intake; cholecystokinin (CCK), neuropeptide Y (NPY), and ghrelin, and how that research does or does not fit with Smith's theoretical model of the direct and indirect controls of meal size. Thus, the pages that follow are not intended to reflect all the work in this area of ingestive behavior, but to show progress and shortcomings in our understanding of how estradiol interacts with the neurobiological controls of food intake. Readers interested in the effects of estradiol on other signaling molecules that affect feeding are referred to the work of Mystkowski and Schwartz [50], Rivera and Eckel [55], and Eckel et al. [31], while readers interested in the effects of estradiol on energy balance and the availability of metabolic fuels are referred to the work of Schneider [60].
2. Changes in food intake during ovarian cycles
In rats, the release of estradiol and progesterone from the ovaries occurs cyclically, with a period of 4-5 days and, along with neuroendocrine events in the hypothalamus and anterior pituitary, comprises the estrous cycle. Apart from changes in female sexual behavior seen during the estrous cycle, food intake also fluctuates in response to these ovarian rhythms. Female rats show cyclic changes in eating during its 4- to 5-day estrous cycle, with reduced food intake occurring during the night of proestrus, following the rise of estradiol secretion that begins during diestrus and continues into the afternoon of proestrus [8, 11, 72]. Cyclic changes in food intake have also been reported to occur in mammals that have long ovarian cycles accompanied by a prolonged luteal phase such as the sheep [70], guinea pig [22], and rhesus monkey, with reduced food intake occurring around the time of ovulation in these animals [21]. Similar data have also been obtained in human females. In an analysis of 19 separate studies addressing the relationship between ovarian hormones and food intake, Buffenstein et al. [10] reported a mean decrease of 250 kcal per day during the periovulatory phase of the menstrual cycle, with some studies finding a decrease of more than 600 kcal per day. In rats, the decrease in food intake that occurs during proestrus is accomplished by a decrease in meal size without a compensatory increase in meal frequency [8, 35]. Comparable analyses of changes in meal size and number across ovarian cycles in the other species cited above have not, to the best of my knowledge, been conducted.
3. Effects of ovariectomy and estradiol replacement
The research described above suggests that food intake and meal size are significantly reduced at a periovulatory point in estrous and menstrual cycles following the rise of estradiol secretion. Experiments examining the effects of ovariectomy and hormone replacement have provided direct evidence that estradiol is the hormone responsible for the changes in feeding behavior seen during the ovarian cycle. Ovariectomy of adult rats causes a significant increase in food intake and meal size, and a concomitant increase in body weight [2, 8, 40, 77]. In rhesus females, bilateral removal of the ovaries also causes hyperphagia that persists for approximately 3 weeks, along with increased weight gain [66]. The prevalence of obesity also increases in postmenopausal women compared with age-matched controls [67], and estrogen replacement therapy (ERT) has been shown to blunt the increases in body weight and adiposity [71]. Thus, human and animal studies demonstrate that withdrawal of estradiol via ovariectomy or menopause leads to increases in body weight and fat accumulation. Whether the effects of ERT on body weight gain in postmenopausal women results from a decrease in food intake (as has been shown in rhesus females), a direct action on adipose tissue, or both remains unclear. Treating ovariectomized rats and guinea pigs with physiological doses of estradiol decreases food intake and body weight [2, 69, 77, 22]. Similar effects of estradiol replacement on food intake have also been reported in ovariectomized rhesus monkeys [23]. Progesterone treatment alone has no significant effects on feeding behavior in ovariectomized rodents or rhesus females, although pharmacological doses of progesterone have been shown to antagonize the effects of estradiol on food intake [34, 77, 24]. In ovariectomized rats, estradiol decreases eating by causing a decrease in meal size [2, 8]. Although it is likely that estradiol decreases food intake in ovariectomized guinea pigs and rhesus monkeys by influencing meal size, experimental tests of this hypothesis have not been conducted. The fact that the inhibitory effect of estradiol on food intake appears to result from changes in meal size suggests that estradiol may influence feeding by advancing the onset of satiety, an idea that will be developed in subsequent sections.
4. Central effects of estradiol
Several lines of evidence indicate that the effects of estradiol on food intake are mediated by its actions on estrogen receptors within the brain. In the early 1970's, Wade and Zucker [75] were the first to report that direct stimulation of the ventromedial hypothalamus (VMH) by estradiol influenced feeding behavior in female rats. They found that central implants of undiluted estradiol benzoate (EB) in the VMH decreased food intake in ovariectomized rats during a 3-day period of hormonal stimulation. Implants of EB in the preoptic area (POA) had no significant effects on feeding [75]. Those findings were replicated by other investigators [5, 42] and incorporated into a set point model of food intake which hypothesized that estradiol's effects on feeding behavior were secondary to the reduction of a body weight set point via the actions of estradiol in the VMH [42, 76]. Data from our lab and from other investigators indicate that the effects of estradiol on food intake may be mediated by its actions on estrogen receptors in hypothalamic regions other than the VMH, and that estrogenic effects on feeding aren't necessarily secondary to a lowering of a body weight set point. In these experiments, estradiol implants in the paraventricular nucleus of the hypothalamus (PVN), a brain region involved in the control of feeding behavior [7, 46] decreased food intake in ovariectomized rats and guinea pigs [12, 53, 14]. Direct placement of estradiol in other brain regions (posterior hypothalamus, POA, VMH) had no significant effects on food intake [12]. The fact that PVN implants of estradiol suppressed eating in the absence of changes in lipoprotein lipase activity in adipose tissue suggest that peripheral metabolic changes are not responsible for the changes in feeding seen after estrogenic stimulation of the PVN [53]. In addition, the findings that estradiol implants in the PVN can inhibit food intake without inducing significant levels of lordosis behavior indicates that steroid spread from the PVN to the VMH did not underlie the observed effects on feeding [12]. Although the results of these studies suggest that the PVN is an important site of action for estrogenic effects on feeding, other experiments have failed to replicate these findings [25, 41]. More recent findings suggest that the effects of estradiol on food intake are not limited to the hypothalamus but also involve actions on estrogen receptors in the hindbrain. In this experiment, surgical placement of an estradiol-containing haemostatic cloth onto the surface of the hindbrain (over the caudal region of the nucleus of the solitary tract) in ovariectomized rats decreased food intake 72 hours after hormone application [78]. These authors also reported that hindbrain placement of estradiol increased CCK-induced Fos activity in the caudal nucleus of the solitary tract (NTS). It is possible that the effects of estradiol on feeding involve actions in an estrogen-sensitive, PVN-hindbrain pathway that participates in the neural control of food intake and autonomic functions [43, 59, 68]. Neuroanatomical evidence in support of this hypothesis comes from research showing that estrogen-sensitive PVN neurons in the parvocelluar portion of this brain region form reciprocal connections with neurons in the dorsal vagal complex of the medulla (NTS, dorsal motor nucleus of the vagus) [19]. It will be important for future studies to further explore the central sites at which estradiol acts to inhibit food intake.
The central effects of estradiol on feeding behavior depend upon its ability to bind to and activate estrogen receptors (ER) in the brain regions described above. However, the subtype of the receptor responsible for estrogenic effects on eating (ERα, ERβ) is still not clear. In one study, intracerebroventricular (icv) infusions of anti-sense oligodeoxynucleotides for ERβ but not ERα blocked the effects of systemic estradiol on food intake and body weight, suggesting that central ERβ receptors are involved in the anorectic effects of estradiol in ovariectomized rats [47]. In ovariectomized mice with null mutations of ERα(αERKO), systemic estradiol treatment did not produce the expected decrease in food intake, suggesting that perhaps in mice activation of ERα is responsible for the effects of estradiol on feeding [36]. To extend this latter observation to the female rat, investigators have examined the effects of selective ER agonists on food intake and body weight. In these studies, systemic injections of the ERα agonist PPT produced both a chronic [56] and an acute [58] suppression of food intake and body weight in ovariectomized rats. These investigators also found that peripheral administration of the ERβ agonist DPN had no significant effects on feeding or body weight. The fact that PPT mimicked the effects of estradiol on food intake by producing a decrease in nocturnal meal size [58] provides additional support for the idea that estradiol's actions in ovariectomized rats involve the activation of ERα However, the hypothesis that activation of ERα is necessary for the effects of estradiol on feeding behavior is not consistent with the results of experiments showing that direct placement of estradiol in the PVN [9, 14, 53] or VMH [5, 42, 75] reduces food intake in ovariectomized rats and guinea pigs. Although the PVN contains estrogen-sensitive neurons, the subtype of the estrogen receptor found in the PVN is ERβ not ERα [61]. In addition, selective silencing of ERα expression in the VMH did not attenuate the effects of systemic estradiol treatment on feeding behavior in ovariectomized rats [49]. It will be important for future studies to further explore the involvement of these subtypes of the ER by utilizing icv infusions of ER agonists in specific brain regions and/or RNA silencing of ER expression to gain a better understanding of the involvement of these ER subtypes in the anorectic action of estradiol.
5. Estradiol, CCK and satiety
Another approach we and other investigators have utilized to study the effects of estradiol on food intake has been to evaluate the ability of estradiol to enhance satiety signals that arise during the course of a meal. This research has focused on the interactions between estradiol and cholecystokinin (CCK). CCK is a peptide hormone released by the small intestine during a meal where it acts on CCK1 receptors in the gut whose stimulation activates afferent fibers of the vagus nerve. These vagal fibers stimulate interconnected neural structures in the brain (e.g., NTS, parabrachial nucleus, PVN) resulting in the cessation of food intake accomplished primarily by decreased meal size [62]. Work done in our lab and by other researchers has shown that peripheral treatment with estradiol increases the suppressive effects of intraperitoneal (ip) injections of CCK on food intake in female rats [3, 13, 37, 48]. This effect of estradiol on CCK-induced satiety is not an additive effect of the individual agents. In our hands, combined treatment of estradiol and CCK (5.0 μg/kg) suppressed intake during a 60 minute feeding test by 36%, whereas treatment with estradiol or CCK alone produced a suppression of food intake of 9% and 12%, respectively [12]. Similar findings have also been obtained in intact, cycling female rats, as ip injections of CCK (2.5 μg/kg) reliably suppressed one hour food intake during late diestrus-II (when estradiol levels are high) but not during diestrus-I (when estradiol levels are low) [27]. In addition, administration of devazepide, a CCK1 receptor antagonist, attenuated the anorectic effects of estradiol in ovariectomized rats [16]. In this experiment, peripheral treatment with devazepide (.1 mg/kg) significantly increased intake during a 30 minute feeding test in animals treated with estradiol. Devazepide had no significant effects on food intake in untreated, ovariectomized females [16]. Similar effects of CCK receptor blockade on meal patterns in intact, cycling female rats have also been obtained. Specifically, devazepide (.1 mg/kg) increased nocturnal food intake and meal size during proestrus but had no significant effects on feeding when animals were tested during diestrus [29]. Data from our lab and others indicate that the potentiation of CCK's effects on food intake by estradiol may involve estrogenic actions in the brain. For example, estradiol implants in the PVN enhanced the satiety action of peripherally administered CCK, whereas cannulae placed in other brain regions (e.g., VMH, lateral ventricles) had no significant effects on CCK-induced satiety [15]. Estradiol has also been shown to increase CCK-induced c-Fos immunoreactivity in both the NTS and the PVN [30]. In addition, estradiol fails to enhance the satiety action of CCK in female ERαKO mice [36]. These findings are consistent with the idea that the anorectic effects of estradiol stem in part from the ability of estradiol to potentiate the satiety action of CCK by activating estrogen receptors (ERα? ERβ?) in hypothalamic and hindbrain sites that process the vagally mediated signal initiated by the actions of CCK in the abdomen.
6. Interactions with orexigenic peptides
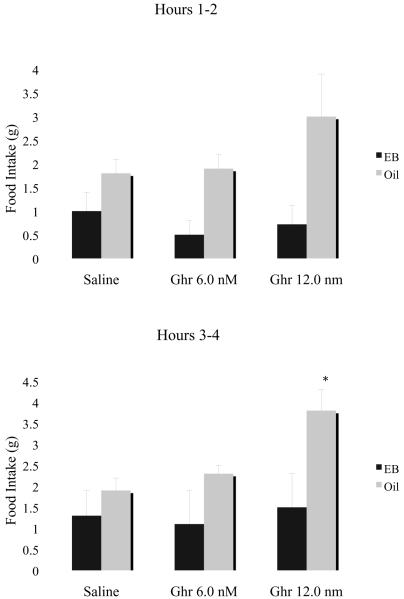
Although the effects of estradiol on food intake appear to be mediated in part by interactions with CCK systems that participate in the control of meal size, the observation that CCK antagonists do not completely reverse the anorectic action of estradiol indicates that CCK is not the only factor involved in mediating estrogenic effects on feeding [29]. More recent work in our lab and by other investigators has focused on the ability of estradiol to attenuate the orexigenic effects of ghrelin and NPY. Discovered in 1999 as an endogenous ligand for the growth hormone secretagogue receptor [44], ghrelin is a peptide produced by the stomach that is best known for its effects on hunger [20]. Fasting increases ghrelin levels in rodents and humans whereas eating suppresses ghrelin secretion [6]. Administration of ghrelin also increases eating in animals and humans [44, 74], and peripheral or central administration of antibodies to ghrelin inhibit food intake in rats [51, 4]. To investigate the role of ghrelin in the decrease in food intake produced by estradiol, we treated ovariectomized rats with EB (5.0 μg per animal, n = 5) or the sesame oil vehicle (n = 4) for 2 days. Seventy-two hours after the onset of EB and oil treatments, animals were given ip injections of rat ghrelin (6.0 or 12.0 nM/animal, Bachem, Temecula, CA) or the saline vehicle in a repeated measures design during the diurnal period. Food intake (NOYES Precision Pellets) was measured during the 4 hour period following ghrelin or saline treatment using computer controlled food dispensers present in the home cage (Med Associates, St. Albans, VT). The procedure was repeated each week until all animals received ip injections of saline and both doses of ghrelin. As shown in Figure 1, compared to the saline condition, ghrelin (12.0 nM) increased cumulative food intake in the oil group but not in EB-treated females (t [3] = 5.12, p < 0.01). These findings are consistent with a previous report showing that male rats and untreated, ovariectomized females are more responsive to the orexigenic effects of ghrelin (given peripherally or centrally) than intact or EB-treated ovariectomized females [17]. In these studies, estradiol appeared to attenuate ghrelin's effect on feeding in ovariectomized animals by increasing the latency to eat in feeding tests conducted during the first 2 hours of the nocturnal period. Significant effects of ghrelin on meal size were not observed in EB- or oil-treated females [17]. In our hands, ip injections of ghrelin increased food intake and meal frequency, but not meal size, in female rats during the first 2 hours of nocturnal feeding during diestrus but not during proestrus [18]. Further work is needed to clarify the mechanism by which estradiol reduces the acute effects of ghrelin on feeding behavior.
Figure 1.
Effects of ghrelin or saline on food intake in ovariectomized rats treated with estradiol benzoate (EB) or oil. Data represent mean ±SE of cumulative intake during the 4-hour period following ghrelin or saline treatment. * Significantly different from saline condition (P < 0.05).
Taken together, the results of these experiments suggest that the effects of estradiol on feeding behavior may also involve an attenuation of orexigenic signals, possibly by modulating the effects of the peripheral ghrelin signal on hypothalamic neuropeptides involved in the control of food intake (e.g., NPY). Consistent with this hypothesis, estradiol has been shown to attenuate the orexigenic effects of icv infusions of NPY and the release of NPY in the PVN of ovariectomized rats [57, 9].
7. Estradiol as an indirect control of food intake
What theoretical model of feeding behavior best explains how estradiol acts to influence eating? Earlier models characterized the effects of estradiol on food intake in terms of hormonal modulation of long-term controls on feeding [52, 76], or in terms of a hormone-induced lowering of a body weight set point [76]. A more compelling and experimentally testable model, articulated by Smith [64] and Eckel [28], explains the effects of estradiol on feeding in terms of a theoretical framework of direct and indirect controls of meal size. These ideas conceptualize the various stimuli and conditions that change eating as affecting one of two systems: (1) a direct sensory control system responsible for encoding, transmitting, and processing sensory stimuli that accompany ingestion (e.g., the chemical and mechanical receptors that run from the tongue to the end of the small intestine plus their afferent connections) and (2) indirect control systems that do not have direct sensory contact with food stimuli but instead encode, transmit, and process other stimuli that exert effects on eating and meal size (e.g., foraging experience, circadian rhythms, adiposity levels, gonadal hormones, etc.). This extensive flow of sensory information that occurs during a meal is supplemented by postingestive afferent input arising from vagal afferent fibers and spinal visceral afferents. This pattern of sensory activity provides the brain with feedback about ingested food that is processed by neural networks involved in the control of food intake. Viewed in this way, meal size is determined by the relative strength and central interactions of the positive and negative sensory feedback, produced by food, that ultimately act on the central network for feeding which controls the rate and duration of eating [64]. The indirect controls are not directly affected by food stimuli that activate the receptors mentioned above, and have a duration of action that lasts beyond a single meal. In the theoretical system outlined by Smith, indirect controls influence meal size by modulating some features of the direct control system (e.g., central processing of the sensory input, changing metabolic or endocrine responses to food stimuli, changing the number or sensitivity of receptors, etc.). Viewing estradiol as an indirect control of eating and meal size is consistent with some of the existing data on estradiol and feeding described in this paper [2, 8]. The research done in our lab and by others on estradiol-CCK interactions discussed in earlier sections [3, 13, 38, 48] is also consistent with this conceptual framework because it identifies a direct control of meal size (e.g., CCK) that is modulated by estradiol.
8. Shortcomings of the model and directions for future research
According to Smith's original model, the neurology of the direct and indirect controls of meal size was clearly delineated; the hindbrain is responsible for mediating the effects of direct controls of eating whereas the indirect controls require the forebrain for their effects on behavior to be produced [63]. Much of the support for this idea of the neurology of eating comes from the work of Grill and Norgren on the controls of food intake in the chronic decerebrate rat [39]. These studies revealed that the brainstem has the ability to respond to the positive and negative feedback signals from food stimuli to control meal size and food intake in animals with no reciprocal fibers connecting the hindbrain and forebrain. The fact that the chronic decerebrate rat does not increase eating after food deprivation and does not acquire conditioned taste aversions suggests that these (and other) indirect controls of eating require the forebrain. This model has proven to be heuristic during the ten plus years since its publication, but it is becoming clear that the strict interpretation of the neurology of the direct and indirect controls needs to be modified. Defining a neuropeptide or steroid as a direct or indirect control of eating based in part on their site of action in the brain becomes a tautology; indirect controls require the forebrain therefore a chemical that acts in the forebrain to affect feeding is an indirect control. In the years since the publication of Smith's theory, it has been shown that molecules conceptualized as direct controls of eating (e.g., ghrelin) can influence food intake by acting in the hindbrain [32, 33] and the hypothalamus [45, 51]. Recent findings indicate that estradiol, typically viewed as an indirect control of eating [16, 28, 64], can inhibit food intake and enhance CCK-induced fos expression in the NTS when applied directly to the hindbrain, with no apparent activation of forebrain sites upstream [73]. These findings suggest that the neurology of direct and indirect controls of eating breaks down when one attempts to classify molecules according to that conceptual framework. Relaxing the neurological criteria for these physiological controls of feeding would still make the model useful and experimentally testable, as indirect controls of eating would still require the identification of a direct control that mediates its effect (e.g., estradiol as an indirect control modifying the actions of CCK, ghrelin, and NPY).
Despite the progress in understanding how estradiol interacts with the neurobiological controls of food intake to influence ingestive behavior, a number of questions remain. To the best of my knowledge, the ability of estradiol to inhibit food intake in the chronic decerebrate rat has not been examined. Should estradiol affect feeding in these animals, then this would lend additional support to the hypothesis that the actions of estradiol in the hindbrain can suppress food intake in the absence of hormonal activation of hypothalamic sites. As discussed in previous sections, the results of experiments in our lab and by other investigators indicate that estradiol attenuates the acute effects of ghrelin on food intake, and that this effect of estradiol on the orexigenic action of ghrelin is not mediated by a change in meal size. This represents a unique effect of estradiol on feeding behavior, as numerous studies have demonstrated that estradiol inhibits food intake via a decrease in meal size that's brought about by the enhancement of the satiety action of CCK [see 16 and 28 for a review]. It will be important for future research to elucidate the mechanism and site of action for this estradiol-ghrelin interaction. In addition, although several lines of evidence implicate ERα as the subunit of the estrogen receptor responsible for hormonal effects on food intake [56, 58, but see 47], experiments utilizing RNA silencing of ERα and ERβ subunit expression in specific brain regions can provide additional information on the brain site and estrogen receptor subunit at which estradiol acts to influence feeding behavior. Finally, the involvement of estrogen receptors located on neural membranes (mER) in the anorectic action of estradiol is an area that warrants further investigation. The fact that STX, an ER antagonist that does not bind to ERα or ERβ but activates mER, reduced weight gain in ovariectomized guinea pigs is intriguing, and suggests that mER may also play a role in the inhibitory effects of estradiol on food intake and body weight [54]. Whether STX can also influence feeding behavior in ovariectomized animals remains to be determined. Gaining a better understanding of the roles that these various subtypes of the ER play in the control of food intake by estradiol may also provide useful information on the factors that lead to increased obesity after menopause and contribute to sex differences in eating disorders, which occur more frequently in young women [65].
Acknowledgements
This manuscript is adapted from a lecture given at the Conversations in the Capital District on Hormones held at the University at Albany, SUNY, on October 23-24, 2008. Portions of the research described in this paper were supported by NIH Grant HD053382.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].American College of Obstetricians and Gynecologists Body mass index and insulin resistance. Obstet Gynecol. 2004;104:5s–10. doi: 10.1097/01.AOG.0000138805.07080.5e. [DOI] [PubMed] [Google Scholar]
- [2].Asarian L, Geary N. Cyclic estradiol treatment normalizes body weight and restores physiological patterns of spontaneous feeding and sexual receptivity in ovariectomized rats. Horm Behav. 2002;42:1–12. doi: 10.1006/hbeh.2002.1835. [DOI] [PubMed] [Google Scholar]
- [3].Asarian L, Geary N. Cyclic estradiol treatment phasically potentiates endogenous cholecystokinin's satiating action in ovariectomized rats. Peptides. 1999;20:445–50. doi: 10.1016/s0196-9781(99)00024-8. [DOI] [PubMed] [Google Scholar]
- [4].Bagnasco M, Tulipano G, Melis MR, Argiolas A, Cocchi D, Muller EE. Endogenous ghrelin is an orexigenic peptide acting in the arcuate nucleus in response to fasting. Regul Pept. 2003;111:161–67. doi: 10.1016/s0167-0115(02)00283-5. [DOI] [PubMed] [Google Scholar]
- [5].Beatty WW, O'Briant DA, Vilberg TR. Suppression of feeding by hypothalamic implants of estradiol in male and female rats. Bull Psychon Soc. 1974;3:273–279. [Google Scholar]
- [6].Beck B, Musse N, Stricker-Kongrad A. Ghrelin, macronutrient intake and dietary prefrences in long-evans rats. Biochem Biophys Res Comm. 2002;292:1031–35. doi: 10.1006/bbrc.2002.6737. [DOI] [PubMed] [Google Scholar]
- [7].Berthoud HR. Multiple neural systems controlling food intake and body weight. Neurosci Biobehav Rev. 2002;26:393–428. doi: 10.1016/s0149-7634(02)00014-3. [DOI] [PubMed] [Google Scholar]
- [8].Blaustein JD, Wade GN. Ovarian influences on meal patterns of female rats. Physiol Behav. 1976;17:201–8. doi: 10.1016/0031-9384(76)90064-0. [DOI] [PubMed] [Google Scholar]
- [9].Bonavera JJ, Dube MG, Kalra PS, Kalra SP. Anorectic effects of estrogen may be mediated by decreased neuropeptide Y release in the hypothalamic paraventricular nucleus. Endocrinology. 1994;134:2367–2370. doi: 10.1210/endo.134.6.8194462. [DOI] [PubMed] [Google Scholar]
- [10].Buffenstein R, Poppitt SD, McDevitt RM, Prentice AM. Physiol Behav. 1995;58:1067–1077. doi: 10.1016/0031-9384(95)02003-9. [DOI] [PubMed] [Google Scholar]
- [11].Butcher RL, Collins WE, Fugo NW. Plasma concentrations of LH, FSH, prolactin, progesterone, and estradiol-17β throughout the 4-day estrous cycle of the rat. Endocrinology. 1974;94:1074–8. doi: 10.1210/endo-94-6-1704. [DOI] [PubMed] [Google Scholar]
- [12].Butera PC, Beikirch RJ. Central implants of dilute estradiol: independent effects on ingestive and reproductive behaviors of ovariectomized rats. Brain Res. 1989;491:266–73. doi: 10.1016/0006-8993(89)90062-0. [DOI] [PubMed] [Google Scholar]
- [13].Butera PC, Bradway DM, Cataldo NJ. Modulation of the satiety effect of cholecystokinin by estradiol. Physiol Behav. 1993;53:1235–38. doi: 10.1016/0031-9384(93)90387-u. [DOI] [PubMed] [Google Scholar]
- [14].Butera PC, Czaja JA. Intracranial estradiol in ovariectomized guinea pigs: effects on ingestive behaviors and body weight. Brain Res. 1984;322:41–48. doi: 10.1016/0006-8993(84)91178-8. [DOI] [PubMed] [Google Scholar]
- [15].Butera PC, Xiong M, Davis RJ, Platania SP. Central implants of dilute estradiol enhance the satiety effect of CCK-8. Behav Neurosci. 1996;110:823–30. doi: 10.1037//0735-7044.110.4.823. [DOI] [PubMed] [Google Scholar]
- [16].Butera PC. CNS steroids and the control of feeding. In: Stone TW, editor. CNS neurotransmitters and neuromodulators: neuroactive steroids. CRC Press; New York: 1996. pp. 211–235. [Google Scholar]
- [17].Clegg DJ, Brown LM, Zigman JM, Kemp CJ, Strader AD, Benoit SC, Woods SC, Mangiaracina M, Geary N. Estradiol-dependent decrease in the orexigenic potency of ghrelin in female rats. Diabetes. 2007;56:1051–1058. doi: 10.2337/db06-0015. [DOI] [PubMed] [Google Scholar]
- [18].Clough SJ. Unpublished Honors Thesis. Department of Biochemistry, Niagara University; 2009. Effects of ghrelin on spontaneous food intake during the rat ovarian cycle. [Google Scholar]
- [19].Corodimas KP, Morrell JI. Estradiol-concentrating forebrain and midbrain neurons project directly to the medulla. J Comp Neurol. 1990;291:609–20. doi: 10.1002/cne.902910408. [DOI] [PubMed] [Google Scholar]
- [20].Cummings D, Purnell J, Frayo R, Schmidova K, Wisse B, Weigle D. A preprandial rise in plasma ghrelin levels suggests a role in meal initiation in humans. Diabetes. 2001;50:1714–19. doi: 10.2337/diabetes.50.8.1714. [DOI] [PubMed] [Google Scholar]
- [21].Czaja JA. Food rejection by female rhesus monkeys during the menstrual cycle and early pregnancy. Physiol Behav. 1975;4:579–87. doi: 10.1016/0031-9384(75)90185-7. (1975) [DOI] [PubMed] [Google Scholar]
- [22].Czaja JA, Butera PC, McCaffrey TA. Independent effects of estradiol on water and food intake. Behav Neurosci. 1983;97:210–19. doi: 10.1037//0735-7044.97.2.210. [DOI] [PubMed] [Google Scholar]
- [23].Czaja JA, Goy RW. Ovarian hormones and food intake in female guinea pigs and rhesus monkeys. Horm Behav. 1975;6:329–36. doi: 10.1016/0018-506x(75)90003-3. [DOI] [PubMed] [Google Scholar]
- [24].Czaja JA. Ovarian influences on primate food intake: assessment of progesterone actions. Physiol Behav. 1978;21:923–31. doi: 10.1016/0031-9384(78)90167-1. [DOI] [PubMed] [Google Scholar]
- [25].Dagnault A, Richard D. Lesions of the hypothalamic paraventricular nuclei do not prevent the effect of estradiol on energy and fat balance. Am J Physiol. 1994;267:E32–E38. doi: 10.1152/ajpendo.1994.267.1.E32. [DOI] [PubMed] [Google Scholar]
- [26].Dalvit-McPhillips S. The effect of the human menstrual cycle on nutrient intake. Physiol Behav. 1983;31:209–12. doi: 10.1016/0031-9384(83)90120-8. [DOI] [PubMed] [Google Scholar]
- [27].Dulawa SC, Vanderweele DA. Cholecystokinin and estradiol synergistically potentiate satiety in rats. Peptides. 1994;15:913–918. doi: 10.1016/0196-9781(94)90050-7. [DOI] [PubMed] [Google Scholar]
- [28].Eckel LA. Estradiol: a rhythmic, inhibitory, indirect control of meal size. Physiol Behav. 2004;82:35–41. doi: 10.1016/j.physbeh.2004.04.023. [DOI] [PubMed] [Google Scholar]
- [29].Eckel LA, Geary N. Endogenous cholecystokinin's satiating action increases during estrus in female rats. Peptides. 1999;20:451–56. doi: 10.1016/s0196-9781(99)00025-x. [DOI] [PubMed] [Google Scholar]
- [30].Eckel LA, Houpt TA, Geary N. Estradiol treatment increases cholecystokinin-induced c-Fos expression in the brains of ovariectomized rats. Am J Physiol. 2002;283:R1378–85. doi: 10.1152/ajpregu.00300.2002. [DOI] [PubMed] [Google Scholar]
- [31].Eckel LA, Langhans WL, Kahler A, Campfield LA, Smith FJ, Geary N. Chronic administration of OB protein decreases food intake by selectively reducing meal size in female rats. Am J Physiol. 1998;44:R186–R198. doi: 10.1152/ajpregu.1998.275.1.R186. [DOI] [PubMed] [Google Scholar]
- [32].Faulconbridge LF, Cummings DE, Kaplan JM, Grill HJ. Hyperphagic effects of brainstem ghrelin administration. Diabetes. 2003;52:2260–2265. doi: 10.2337/diabetes.52.9.2260. [DOI] [PubMed] [Google Scholar]
- [33].Faulconbridge LF, Grill HJ, Kaplan JM, Daniels D. Caudal brainstem delivery of ghrelin induces fos expression in the nucleus of the solitary tract, but not in the arcuate or paraventricular nucleus of the hypothalamus. Brain Res. 2008;1218:151–157. doi: 10.1016/j.brainres.2008.04.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Galetti F, Klopper A. The effect of progesterone on the quantity and distribution of body fat in the female rat. Acta Endocrinol (Copenhagen) 1964;46:379–85. doi: 10.1530/acta.0.0460379. [DOI] [PubMed] [Google Scholar]
- [35].Geary N, Asarian L. Cyclic estradiol treatment normalizes body weight and meal size in ovariectomized rats. Physiol Behav. 1999;67:141–7. doi: 10.1016/s0031-9384(99)00060-8. [DOI] [PubMed] [Google Scholar]
- [36].Geary N, Asarian L, Korach KS, Pfaff DW, Ogawa N. Deficits in E2-dependent control of feeding, weight gain, and cholecystokinin satiation in ER-alpha null mice. Endocrinology. 2001;142:4751–4757. doi: 10.1210/endo.142.11.8504. [DOI] [PubMed] [Google Scholar]
- [37].Geary N, Trace D, McEwen B, Smith GP. Cyclic estradiol replacement increases the satiety effect of CCK-8 in ovariectomized rats. Physiol Behav. 1994;56:281–289. doi: 10.1016/0031-9384(94)90196-1. [DOI] [PubMed] [Google Scholar]
- [38].Geary N, Trace D, McEwen B, Smith GP. Cyclic estradiol replacement increases the satiety effect of CCK-8 in ovariectomized rats. Physiol Behav. 1994;56:281–289. doi: 10.1016/0031-9384(94)90196-1. [DOI] [PubMed] [Google Scholar]
- [39].Grill HJ, Norgren R. Neurological tests and behavioral deficits in chronic thalamic and chronic decerebrate rats. Brain Res. 1978;143:299–308. doi: 10.1016/0006-8993(78)90570-x. [DOI] [PubMed] [Google Scholar]
- [40].Holt H, Keeton RW, Vennesland B. The effect of gonadectomy on body structure and body weight in albino rats. Am J Physiol. 1936;114:515–22. [Google Scholar]
- [41].Hrupka BJ, Smith GP, Geary N. Hypothalamic implants of dilute estradiol fail to reduce feeding in ovariectomized rats. Physiol Behav. 2002;77:233–241. doi: 10.1016/s0031-9384(02)00857-0. [DOI] [PubMed] [Google Scholar]
- [42].Jankowiak R, Stern JJ. Food intake and body weight modifications following medial hypothalamic hormone implants in female rats. Physiol Behav. 1974;12:875–881. doi: 10.1016/0031-9384(74)90025-0. [DOI] [PubMed] [Google Scholar]
- [43].Kirchgessner AL, Sclafani A. PVN-hindbrain pathway involved in the hypothalamic hyperphagia-obesity syndrome. Physiol Behav. 1988;42:517–28. doi: 10.1016/0031-9384(88)90153-9. [DOI] [PubMed] [Google Scholar]
- [44].Kojima M, Hosoda H, Date Y, Nakazato M, Matauo H, Kangawa K. Ghrelin is a growth-hormone-releasing acylated peptide from stomach. Nature. 1999;402:656–60. doi: 10.1038/45230. [DOI] [PubMed] [Google Scholar]
- [45].Lawrence CB, Snape AC, Baudoin FM, Luckman SM. Acute central ghrelin and GH secretagogues induce feeding and activate brain appetite centers. Endocrinology. 2002;143:155162. doi: 10.1210/endo.143.1.8561. [DOI] [PubMed] [Google Scholar]
- [46].Leibowitz SF, Wortley KE. Hypothalamic control of energy balance: different peptides different functions. Peptides. 2004;25:473–504. doi: 10.1016/j.peptides.2004.02.006. [DOI] [PubMed] [Google Scholar]
- [47].Liang YQ, Akishita M, Kim S, Ako J, Hashimoto M, Iijima K, Ohike Y, Watanabe T, Sudoh N, Toba K, Yoshizumi M, Ouchi Y. Estrogen receptor βis involved in the anorectic action of estrogen. Int J Obesity. 2002;26:1103–1109. doi: 10.1038/sj.ijo.0802054. [DOI] [PubMed] [Google Scholar]
- [48].Linden A, Uvnas-Moberg K, Forsberg G, Bednar I, Sodersten P. Involvement of cholecystokin in food intake: III. Oestradiol poetentiates the inhibitory effect of cholecystokinin ocatapeptide on food intake in ovariectomized rats. J Neuroendocr. 1990;2:797–801. doi: 10.1111/j.1365-2826.1990.tb00643.x. [DOI] [PubMed] [Google Scholar]
- [49].Musatov S, Chen W, Pfaff DW, Mobbs CV, Yang XJ, Clegg DJ, Kaplitt MG, Ogawa S. Silencing of estrogen receptor-αin the ventromedial nucleus of the hypothalamus leads to metabolic syndrome. Proc Natl Acad Sci USA. 2007;104:25012506. doi: 10.1073/pnas.0610787104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Mystkowski P, Schwartz MW. Gonadal steroids and energy homeostasis in the leptin era. Nutrition. 2000;16:937–946. doi: 10.1016/s0899-9007(00)00458-5. [DOI] [PubMed] [Google Scholar]
- [51].Nakazato M, Murakami N, Date Y, Kojiima M, Matsuo H, Kangawa K, Matsukura S. A role for ghrelin in the central regulation of feeding. Nature. 2001;409:194–98. doi: 10.1038/35051587. [DOI] [PubMed] [Google Scholar]
- [52].Nance DM. The developmental and neural determinants of the effects of estrogen on feeding behavior in the rat: a theoretical perspective. Neurosci Biobehav Rev. 1983;7:189–207. doi: 10.1016/0149-7634(83)90015-5. [DOI] [PubMed] [Google Scholar]
- [53].Palmer K, Gray JM. Central vs. peripheral effects of estrogen on food intake and lipoprotein lipase activity in ovariectomized rats. Physiol Behav. 1986;32:187–89. doi: 10.1016/0031-9384(86)90404-x. [DOI] [PubMed] [Google Scholar]
- [54].Qiu J, Bosch MA, Tobias SC, Krust A, Graham SM, Murphy SJ, Korach KS, Chambon P, Scanlan TS, Ronnekleiv OK, Kelly MJ. A G-protein coupled estrogen receptor is involved in hypothalamic control of energy homeostasis. J Neurosci. 2006;26:5649–5655. doi: 10.1523/JNEUROSCI.0327-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Rivera HM, Eckel LA. The anorectic effect of fenfluramine is increased by estradiol treatment in ovariectomized rats. Physiol Behav. 2005;86:331–337. doi: 10.1016/j.physbeh.2005.08.004. [DOI] [PubMed] [Google Scholar]
- [56].Roesch DM. Effects of selective estrogen receptor agonists on food intake and body weight gain in rats. Physiol Behav. 2006;87:39–44. doi: 10.1016/j.physbeh.2005.08.035. [DOI] [PubMed] [Google Scholar]
- [57].Santollo J, Eckel LA. Estradiol decreases the orexigenic effect of neuropeptide Y, but not agouti-related protein, in ovariectomized rats. Physiol Behav. 2008;191:173–77. doi: 10.1016/j.bbr.2008.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Santollo J, Wiley MD, Eckel LA. Acute activation of ERα decreases food intake, meal size, and body weight in ovariectomized rats. Am J Physiol. 2007;293:R2194–R2201. doi: 10.1152/ajpregu.00385.2007. [DOI] [PubMed] [Google Scholar]
- [59].Sawchenko PE, Swanson LW. Immunohistochemical identification of neurons in the paraventricular nucleus of the hypothalamus that project to the medulla or to the spinal cord in the rat. J Comp Neurol. 1982;205:260–72. doi: 10.1002/cne.902050306. [DOI] [PubMed] [Google Scholar]
- [60].Schneider JE. Energy balance and reproduction. Physiol Behav. 2005;81:289–317. doi: 10.1016/j.physbeh.2004.02.007. [DOI] [PubMed] [Google Scholar]
- [61].Shughrue PJ, Lane MV, Merchenthaler I. Compartive distribution of estrogen recepor-α and-βmRNA in the rat central nervous system. J Comp Neurol. 1997;388:507–525. doi: 10.1002/(sici)1096-9861(19971201)388:4<507::aid-cne1>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- [62].Smith GP, Gibbs J. The development and proof of the CCK hypothesis of satiety. In: Dourish CT, Cooper SJ, Iversen SD, Ivesen LL, editors. Multiple cholecystokinin receptors in the CNS. Oxford Univ Press; Oxford: 1992. pp. 166–82. [Google Scholar]
- [63].Smith GP. The controls of eating: A shift from nutritional homeostasis to behavioral neuroscience. Nutrition. 2000;16:814–20. doi: 10.1016/s0899-9007(00)00457-3. [DOI] [PubMed] [Google Scholar]
- [64].Smith GP. The direct and indirect control of meal size. Neurosci Biobehav Rev. 1996;20:41–6. doi: 10.1016/0149-7634(95)00038-g. [DOI] [PubMed] [Google Scholar]
- [65].Sodersten P, Bergh C. Anorexia nervosa: towards a neurobiologically based therapy. Eur J Pharmacol. 2003;480:67–74. doi: 10.1016/j.ejphar.2003.08.093. [DOI] [PubMed] [Google Scholar]
- [66].Sullivan E, Daniels A, Cameron J. Ovariectomy leads to rapid changes in food intake, body weight and metabolic regulation in female rhesus monkeys. Soc Neurosci Abstracts. 2003;803:19. [Google Scholar]
- [67].Svendsen OL, Hassager C, Christiansen C. Age and menopause-associated variations in body composition and fat distribution in healthy women as measured by dual-energy X-ray absorptiometry. Metabolism. 1995;44:369–373. doi: 10.1016/0026-0495(95)90168-x. [DOI] [PubMed] [Google Scholar]
- [68].Swanson LW, Kuypers HGJM. The paraventricular nucleus of the hypothalamus: cytoarchitectonic subdivisions and the organization of projections to the pituitary, dorsal vagal complex and spinal cord as demonstrated by retrograde fluorescence double labeling methods. J Comp Neurol. 1980;194:555–70. doi: 10.1002/cne.901940306. [DOI] [PubMed] [Google Scholar]
- [69].Tarttelin MF, Gorski RA. The effects of ovarian steroids on food and water intake and body weight in the female rat. Acta Endocrinol (Copenhagen) 1973;72:551–57. doi: 10.1530/acta.0.0720551. [DOI] [PubMed] [Google Scholar]
- [70].Tarttelin MF. Cyclical variations in food and water intake in ewes. J Physiol. 1968;195:29. [PubMed] [Google Scholar]
- [71].Tchernof A, Poehlman ET, Despres JP. Body fat distribution, the menopause transition, and hormone replacement therapy. Diabetes Metab. 2000;26:12–20. [PubMed] [Google Scholar]
- [72].ter Haar MB. Circadian and estrual rhythms in food intake in the rat. Horm Behav. 1972;3:213–20. doi: 10.1016/0018-506x(72)90034-7. [DOI] [PubMed] [Google Scholar]
- [73].Thammacharoen S, Lutz TA, Geary N, Asarian L. Hindbrain administration of estradiol inhibits feeding and activates estrogen receptor-α-expressing cells in the nucleus tractus solitarius of ovariectomized rats. Endocrinology. 2008;149:1609–1617. doi: 10.1210/en.2007-0340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].van der Leley AJ, Tschop M, Heiman ML, Ghigo E. Biological, physiological, pathophysiological, and pharmacological aspects of ghrelin. Endocrine Rev. 2004;25:426–57. doi: 10.1210/er.2002-0029. [DOI] [PubMed] [Google Scholar]
- [75].Wade GN, Zucker I. Modulation of food intake and locomotor activity in female rats by diencephalic hormone implants. J Comp Physiol Psychol. 1970;72:328–38. doi: 10.1037/h0029461. [DOI] [PubMed] [Google Scholar]
- [76].Wade GN. Sex hormones, regulatory behaviors, and body weight. In: Rosenblatt J, Hinde R, Shaw E, Beer C, editors. Advances in the study of behavior. Vol 6. Academic Press; New York: 1976. pp. 201–279. [Google Scholar]
- [77].Wade GN. Some effects of ovarian hormones on food intake and body weight in female rats. J Comp Physiol Psychol. 1975;88:183–91. doi: 10.1037/h0076186. [DOI] [PubMed] [Google Scholar]