Abstract
Objectives
This review examines the burden and patterns of disease in systemic lupus erythematosus (SLE) and the influence and interactions of gender, ethnicity, age, and psychosocial attributes with respect to disease progression, focusing on issues relevant to clinical practice and research.
Methods
PubMed literature search complemented by review of bibliographies listed in identified articles.
Results
An increased risk among reproductive age women is clearly seen in African Americans in the United States. However, in other populations, a different pattern is generally seen, with the highest age-specific incidence rates occurring in women after age 40 years. The disease is 2 to 4 times more frequent, and more severe, among nonwhite populations around the world and tends to be more severe in men and in pediatric and late-onset lupus. SLE patients now experience a higher than 90% survival rate at 5 years. The less favorable survival experience of ethnic minorities is possibly related to socioeconomic status rather than to ethnicity per se, and adequate social support has been shown to be a protective factor, in general, in SLE patients. Discordance between physician and patient ratings of disease activity may affect quality of care.
Conclusions
Our understanding of ways to improve outcomes in SLE patients could benefit from patient-oriented research focusing on many dimensions of disease burden. Promising research initiatives include the inclusion of community-based patients in longitudinal studies, use of self-assessment tools for rating disease damage and activity, and a focus on self-perceived disease activity and treatment compliance.
Keywords: systemic lupus erythematosus, epidemiology, mortality, disease activity, quality of life, ethnicity, socioeconomic status
Systemic lupus erythematosus (SLE or lupus) is a complex and severe rheumatic disease with exceedingly diverse clinical manifestations. Overall improvements in medical care including the availability of antibiotics, antihypertensives, and renal replacement therapy coupled with the judicious use of glucocorticoid, antimalarial, and immunosuppressive drugs have led to improved survival of SLE patients in the past 50 years (1). Despite the improvements in care, patients often suffer long-term morbidity that can adversely affect their quality of life and their ability to work, resulting in substantial direct and indirect costs.
Ethnicity, a broader construct than is implied by the term “race,” encompasses genetic, geographic, cultural, social, and other characteristics shared within a population (2-4). Not surprisingly, the phenotypic expression of lupus varies between individuals of different ethnic groups. A growing body of research has sought to characterize the influence of socioeconomic (5) factors and ethnicity on the incidence, activity, and progression of the disease. Less attention has been paid to the influence of psychosocial factors on disease progression (Fig. 1). In this article, we review relevant research findings pertaining to these dimensions of SLE, focusing on studies conducted in the past 30 years. We also highlight current data gaps and discuss recommendations for future research.
Figure 1.

A model of the factors affecting the course and outcome of SLE (modified from (5)).
Methods
This review is based on publications found through searches of the MEDLINE database for relevant articles using the combination search terms of lupus and (epidemiology, incidence, prevalence, mortality, gender, ethnicity, damage, quality of life). References within these selected reports were also reviewed. We included epidemiological studies of incidence or prevalence that spanned the years 1975 to 2000, and mortality studies of cohorts assembled in 1975 or later. The mortality studies were limited to inception or near-inception cohorts (that is, cohorts of patients who entered the study within 5 years of diagnosis).
Results
Incidence and Prevalence of SLE Around the World
Incidence rates of SLE range from approximately 1 to 10 per 100,000 person-years (Table 1) (6-24) and prevalence rates generally range from 20 to 70 per 100,000 (Table 2) (6,8-10,12-18,21-35). In reviewing the available studies, it is important to note that epidemiological studies of SLE may differ by sampling and recruitment methodologies used. The classification criteria initially developed by the American Rheumatism Association, now the American College of Rheumatology (ACR) in 1971 (36) were revised in 1982 (37) and updated in 1997 (38). Early studies, particularly those conducted in hospital settings, failed to capture cases of mild SLE. Studies relying on self-report of an SLE diagnosis, without review of symptom history or medical records, have reported higher prevalence rates (39,40). Another issue with respect to studies that rely on the medical system to ascertain patients is the potential contribution of undiagnosed disease to the total burden within a population. A community survey in Birmingham (United Kingdom), combined with antinuclear antibody testing and clinical assessment of “positive” respondents, reported a prevalence of diagnosed SLE in women ages 18 to 65 years of 54 per 100,000; with the addition of the cases found during the screening, this estimate rose to 200 per 100,000 (41).
Table 1.
Incidence of Systemic Lupus Erythematosus in Adults, by Location, in Studies Spanning 1975 to 2000
| Author (Reference), Country (Area), Study Perioda | Total Rate per 100,000 per yearb (n) | Female Rate per 100,000 per yearb (n) |
|---|---|---|
| Americas | ||
| Uramoto (6), United States (MN), 1980 to 1992 | 5.6 (48) | 9.4 (42) |
| McCarty (7), United States (PA), 1985 to 1990 | 2.4 (191) | |
| African Americans 9.2 (45) | ||
| Whites 3.5 (129) | ||
| Naleway (8), United States (WI), 1991 to 2001 | 5.1 (44) | 8.2 (36) |
| Peschken (9), Canada (Manitoba), 1980 to 1996 | First Nations ∼ 3.5 (49) | — |
| Whites ∼ 1.2 (177) | ||
| Nossent (10), Curaçao 1980 to 1989 | Afro-Caribbean 4.6 (68) | 7.9 (60) |
| Vilar (11), Brazil, 2000 | 8.7 (43) | 14.1 (38) |
| Europe | ||
| Stahl-Hallengren (12,13), Sweden, 1981 to 1991 | 1981-86 4.5 (38) | 1981-86 5.4 (32) |
| 1987-91 4.5 (41) | — | |
| Voss (14), Denmark, 1980 to 1994c | 1980-84 1.0 (—) | — |
| 1985-89 1.1 (—) | — | |
| 1990-94 2.5 (—) | — | |
| Nossent (15), Norway, 1978 to 1996 | 2.9 (83) | 5.1 (73) |
| Johnson (16), United Kingdom (Birmingham), 1991 | Total 3.8 (33) | Total 6.8 (31) |
| — | Afro-Caribbean 22.8 (6) | |
| — | Asian 29.2 (8) | |
| — | Whites 4.5 (17) | |
| Hopkinson (17,18), United Kingdom (Nottingham), 1989 to 1990 | Total 4.0 (23) | Total 6.5 (19) |
| Afro-Caribbean 31.9 (3) | — | |
| Whites 3.4 (19) | — | |
| Nightingale (19), United Kingdom, 1992 to 1998 | 3.0 (390) | 5.3 (349) |
| Somers (20), United Kingdom, 1990 to 1999 | 4.7 (1638) | 7.9 (1374) |
| Gudmundsson (21), Iceland, 1975 to 1984 | 3.3 (76) | 5.8 (67) |
| López (22), Spain, 1998 to 2002 | 2.2 (116) | 3.6 (102) |
| Alamanos (23), Greece, 1982 to 2001 | 1.9 (178) | — |
| Oceania | ||
| Anstey (24), Australia, 1986 to 1990 | Aboriginal 11 (13) | — |
Abbreviations: MN = Minnesota, PA = Pennsylvania, WI = Wisconsin.
Nightingale (19) and Somers (20) used the United Kingdom General Practitioner Research Database; Gudmundsson (21) used hospital records, and all other studies used various type medical records for case ascertainment.
Age-adjusted rates provided when available; group specific estimates provided when based on 2 or more cases. —, data not reported.
Voss (14) included a total of 107 patients, but the number per time period was not provided.
Table 2.
Prevalence of Systemic Lupus Erythematosus in Adults, by Location, in Studies Spanning 1975 to 2000
| Author (Reference), Country (Area), Study Perioda | Total Rate per 100,000b (n) | Female Rate per 100,000b (n) |
|---|---|---|
| Americas | ||
| Uramoto (6), United States (MN), 1992 | 130 (—) | — |
| Maskarinec (25), United States (HI), 1989 | Total 42 (454) | Total 74 (401) |
| Non-whites 78 (315) | ||
| Whites 71 (86) | ||
| Chakravarty (26), United States (CA, PA), 2000 | California 108 (—) | Total 184 (—) |
| African American 406 (—) | ||
| Hispanic 139 (—) | ||
| Asian, Pacific Island 93 (—) | ||
| Whites 164 (—) | ||
| Pennsylvania 150 (—) | Total 253 (—) | |
| African American 694 | ||
| Hispanic 245 (—) | ||
| Asian, Pacific Island 103 (—) | ||
| Whites 203 (—) | ||
| Naleway (8), United States (WI), 2001 | 79 (64) | 132 (54) |
| Boyer (27), United States (AK), 1991 | 112 (9) | 166 (8) |
| Peschken (9), Canada (Manitoba), 1996 | Total 22 (257) | — |
| First Nations 42 (49) | — | |
| Whites 21 (177) | — | |
| Nossent (10), Curaçao, 1989 | Afro-Caribbean 48 (69) | 84 (63) |
| Europe | ||
| Stahl-Hallengren (12,13), Sweden, 1986, 1991 | 1986 42 (44) | — |
| 1991 68 (41) | — | |
| Voss (14), Denmark, 1994 | 22 (104) | 38 (93) |
| Nossent (15), Norway, 1995 | 50 (89) | 89 (79) |
| Hochberg (28), United Kingdom, 1982 | 7 (20) | 13 (20) |
| Johnson (16), United Kingdom (Birmingham), 1991 | Total 28 (242) | Total 50 (227) |
| Afro-Caribbean 112 (50) | Afro-Caribbean 197 (48) | |
| Asian (Indian) 47 (36) | Asian (Indian) 97 (35) | |
| Whites 21 (155) | Whites 36 (143) | |
| Hopkinson (17,18), United Kingdom (Nottingham), 1990 | Total 25 (147) | Total 45 (136) |
| Afro-Caribbean 207 (21) | — | |
| Asian (Indian) 49 (7) | — | |
| Asian (Chinese) 93 (2) | — | |
| Whites 20 (117) | — | |
| Samanta (29), 1989, United Kingdom (Leicester) | Total 26 (50) | Total - |
| Asian 64 (19) | Asian (Indian) 73 (13) | |
| Whites 20 (31) | Whites 32 (26) | |
| Molokhia (30), United Kingdom (London), ages 15 to 64, 1999 | — | Afro Caribbean 177 (72) |
| — | West African 110 (20) | |
| — | Whites 35 (66) | |
| Gourley (31), Ireland, 1993 | 25 (415) | — |
| Gudmundsson (21), Iceland, 1984 | 36 (86) | 62 (77) |
| Lopez (22), Spain, 2002 | 34 (367) | 58 (324) |
| Alamanos (23), Greece, 2001 | 38 (193) | 67 (170) |
| Middle East | ||
| Al-Arfaj (32), Saudi Arabia, 1992 | 19 (2) | 37 (2) |
| Oceania | ||
| Bossingham (33), Australia, 1996 to 1998 | Total 45 (108) | — |
| Aboriginal 93 (26) | — | |
| Segasothy (34), Australia, 1999 | Aboriginal 74 (18) | — |
| Whites 19 (6) | — | |
| Anstey (24), Australia, 1991 | Aboriginal 52 (13) | Aboriginal 100 (13) |
| Hart (35), New Zealand, 1980 | Total 18 (136) | — |
| Polynesians 51 (34) | — | |
| Whites 15 (96) | — |
Abbreviations: AK = Alaska, CA = California, HI = Hawaii, MN = Minnesota, PA = Pennsylvania, WI = Wisconsin.
Chakravarty (26) used state hospitalization databases, adjusted for estimate of proportion of SLE patients hospitalized annually. Hochberg (28) used the United Kingdom General Practitioner Research Database. Gudmundsson (21) used hospital records. Al-Arfaj (32) used a survey with follow-up examination, and all other studies used various type medical records for case ascertainment.
Age-adjusted rates provided when available; group-specific estimates provided when based on 2 or more cases. —, data not reported.
A recent study from Olmstead County, Minnesota analyzed SLE incidence rates in 2 periods (6). The age- and sex-adjusted incidence rate was higher in the latter period (1.5 and 5.6 per 100,000 person-years, respectively, in 1950-1979 and 1980-1992). Similar increases were seen in an incidence study in 1980 to 1984, 1985 to 1989, and 1990 to 1994 in Denmark (14). Although some of this increase is likely to reflect an actual increase in disease occurrence, a more accurate ascertainment of cases and the inclusion of milder cases of SLE because of the wider availability and use of antinuclear antibody testing are also likely to have contributed to these changes. There are few members of ethnic minority groups in these study populations; thus, it is difficult to generalize from these results to the experience in other groups or locations.
The strongest risk factor for SLE is clearly gender: SLE is much more frequent among women than men. In most studies, 90% or more of patients are women, and thus as expected, in studies in which gender-specific rates are given, the incidence and prevalence rates for men are approximately 1/10th those in women. Rates for women and for the total population are shown in Table 1. The rates for the total population are essentially a weighted average of the rates for men and the rates for women. Thus if the rates are 10 times higher in women compared with men, and if there is approximately an equal proportion of males and females in the general population, a weighted average of these rates (ie, the rates for the total population) will be approximately 50% lower than the rates in women. As an example, consider a population in which the incidence rate in men is 1 per 100,000 person-years, and the incidence rate in women is 10 per 100,000 person-years. The average of these 2 rates (assuming equal proportions of men and women in the population) is 5.5 per 100,000 person-years, or approximately half the rate seen in women. This pattern can be seen in Table 1, with total population rates ranging from about 30% to 70% lower than the rates in women.
SLE has been described in 6 continents (Europe, North America, South America, Africa, Asia, and Australia). The disease is rare in Africa but common in African descendants around the world; however, some degree of under-ascertainment is likely to occur in Africa per se (42). Incidence and prevalence rates in people of African or of Asian background are approximately 2 to 3 times higher than in white populations (7,16,17,26,29,43). The disease is also more common among Aboriginal than non-Aboriginal Australians (33,34), and in some First Nations or Native American groups in Canada (9) and the United States (27).
Childhood incidence and prevalence rates of SLE are considerably lower than adult rates. The annual incidence rate of SLE in children (<16 years) was less than 1 per 100,000 persons in studies from Europe and North America (reviewed in (44)). In Taiwan, the prevalence of childhood SLE was estimated as 6.3 per 100,000 (45).
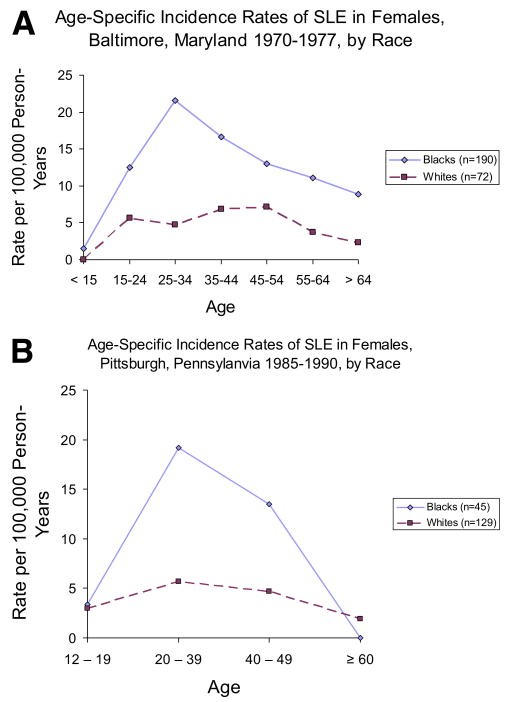
SLE is often described as a disease that most often strikes reproductive-age women. This pattern is clearly seen in data for black women in the United States (Fig. 2). The studies shown in Figure 2 (7,43) also clearly show the increased risk seen among African Americans compared with whites. However, in other populations, a different pattern is generally seen among women, with the highest age-specific incidence rates after age 40 years, as seen in a large study from the United Kingdom (20) as well as in smaller studies from the Sweden and Iceland (12,21) (Fig. 3). Although there are no incident data for Hispanics in the United States or Latin America, the published data suggest they also develop lupus earlier in life (46,47).
Figure 2.
Age-specific incidence rates for SLE in females, from 2 studies from the United States with data for African Americans and whites. (A) is from Hochberg (43) and (B) is from McCarty and coworkers (7). (Color version of figure is available online.)
Figure 3.
Age-specific incidence rates for SLE in females in the United Kingdom (20) (calculated by interpolation from graph), Sweden (12), and Iceland (21). (Color version of figure is available online.)
Mortality Risk in SLE Patients
Considered in years past a fatal disorder, patients with SLE now live years if not decades after diagnosis. The 5-year survival rate among 99 patients seen at Johns Hopkins University from 1949 to 1953 was 50% (48). In contrast, since the mid-1970s, most studies in Europe, the United States, Canada, and Latin America have demonstrated 5-year survival rates among newly diagnosed patients of over 90%, and 15 to 20 years survival rates of around 80% (Table 3) (5,6,8-10,12,21,24,47,49-58). Identification of milder cases, or cases earlier in the disease process, made possible by improvements in diagnostic testing, may contribute to the observed improvements in survival.
Table 3.
Survival Probabilities for Systemic Lupus Erythematosus Patients in Cohorts of Newly Diagnosed or Recently Diagnosed Patients Constituted Since 1975
| Survival % by years Follow-up | ||||||
|---|---|---|---|---|---|---|
| Author (Reference), Country (Area), Study Perioda |
N Total |
N Deaths |
5 | 10 | 15 | 20 |
| Americas | ||||||
| Pistiner (49), United States (CA), 1980 to 1989 | 195 | — | 97 | 93 | — | — |
| Kasitanon (50), United States (MD), 1987 to 2004 | 1378 | 118 | 95 | 91 | 85 | 78 |
| African-Americans | 543 | 69 | — | — | — | 71 |
| Whites | 767 | 47 | — | — | — | 84 |
| Uramoto (6) United States (MN), 1980 to 1992 | 48 | — | 95 | 72 | — | — |
| Naleway (8), United States (WI), 1991 to 2001 | 44 | 8 | 88 | 76 | — | — |
| Alarcón (5,51) United States (AL, TX), 1994 to 2006 | ||||||
| African-American | 221 | 32 | 90 | — | — | — |
| Hispanic | 117 | 17 | 87 | — | — | — |
| Whites | 176 | 13 | 94 | — | — | — |
| Campbell (52), United States (NC, SC), 1995 to 2001 | 265 | 32 | 90 | — | — | — |
| Peschken (9), Canada (Manitoba), 1980 to 1996 | ||||||
| First Nations | 49 | — | 95 | 82 | 75 | — |
| Whites | 177 | — | 98 | 95 | 92 | — |
| Nossent (132) Curaçao, 1980 to 1990 | 68 | 25 | 60 | 46 | — | — |
| Pons-Estel (47), Latin America, 1997 to 2003b | 1214 | 34 | 95 | — | — | — |
| Europe | ||||||
| Gudmundsson (21), Iceland, 1975 to 1988 | 76 | 17 | 84 | 78 | — | — |
| Stahl-Hallengren (12), Sweden, 1981 to 1996 | 81 | 17 | 93 | 83 | — | — |
| Alamanos (53), Greece, 1981 to 2001 | 185 | 21 | 96 | 87 | — | — |
| Manger (54), Germany, 1985 to 1999 | 338 | 35 | 97 | 90 | — | — |
| Asia | ||||||
| Wang (55), Taiwan (pediatric), 1980 to 1990 | 52 | 32 | 60 | 44 | — | — |
| 1991 to 2001 | 101 | 18 | 85 | 78 | — | — |
| Mok (56), Hong Kong, 1992 to 1999 | 182 | 9 | 93 | — | — | — |
| Kasitanon (57), Thailand, 1986 to 2000b | 349 | 52 | 84 | 75 | — | — |
| Murali (58), India, 1981 to 1993 | 98 | 23 | 77 | 60 | — | — |
| Oceania | ||||||
| Anstey (24), Australia, 1984 to 1991 | 22 | 9 | 60 | — | — | — |
Abbreviations: AL = Alabama, MN = Minnesota, NC = North Carolina, PA = Pennsylvania, SC = South Carolina, TX = Texas, WI = Wisconsin.
Group-specific estimates provided when based on 2 or more deaths. Data for Kasitanon (50) (survival rates for total population), Uramoto (6), and Peschken (9) estimated from graphs. —, data not reported. Alarcón (51) included patients diagnosed up to 5 years before study entry; median time from diagnosis to study was 18 months. Campbell (52) included patients diagnosed up to 4 years before study entry; median time from diagnosis to study was 13 months. Kasitanon (50) accounted for difference between diagnosis and study entry using delay entry into the risk sets in calculating survival in hazards models. All other studies were inception cohorts (entering all patients at time of diagnosis).
Pons-Estel (47) presented 4-year survival probability.
Because of the relatively high mortality rate in the first few years after disease diagnosis (59,60), survival rates are likely to be lower in inception cohorts than in noninception cohorts (ie, survivor cohorts or left-censored data). Improved survival over time has been noted in analyses of period-specific survival rates since 1980 within adult patients in Canada (61), California (49), Minnesota (6), Sweden (62), and pediatric patients in Taiwan (55). Although the improvement in survival in SLE has been greater than that observed in the general population (63), life expectancy in SLE patients is still below that of comparable demographic groups (6,64,65). Of note, the improvement in survival over time has become evident when examining all causes of death together, but not when examining those deaths attributed to cardiovascular disease (62). It is also important to note that contemporary survival rates in many parts of Asia have yet to reach those of Europe and North America (Table 3), suggesting the importance of economic development and socioeconomic factors in the prognosis of SLE.
Because only about 10% of SLE patients are male, relatively large studies are needed to characterize potential gender-related differences in mortality risk. Most large (>150 patients) inception (49,52-54) and noninception (66-69) cohort studies have reported a lower survival rate among men compared with women; an exception is the study by Reveille and coworkers (70), in which mortality risks for men and women were similar.
Virtually all studies that examine mortality rates for specific ethnic groups (9,49-51,70-73) reported higher mortality risks among the black, Hispanic, and First Nations groups compared with white (or majority) populations. This differential risk by ethnicity was not seen, however, in several of the studies that adjusted for socioeconomic status (eg, by considering income or insurance status or education level) (49,51,71,72,74). This in an important consideration in the interpretation of the role of ethnicity in mortality, given that ethnicity reflects socioeconomic variables and psychological factors that could directly affect mortality risk and that may be amenable to interventions.
Clinical Expression and Progression of Disease
Results pertaining to differences in clinical expression of SLE between men and women are somewhat variable, which may reflect the imprecision often seen in the small samples of men that are included in many of these studies. Larger studies have indicated an increased renal involvement in men compared with women (69,75-79). In the LUMINA (LUpus in MInorities, NAture versus Nurture) study, men accrued damage early in their disease course, predisposing them to accrue more damage subsequently (78,80).
Minority patients by and large have more abrupt disease onset, more severe clinical manifestations, and higher degrees of disease activity compared with white SLE patients (80,81). Regardless of their age and gender, Hispanic, African American, and Asian SLE patients tend to have more hematological, serosal, neurological, and renal manifestations (29,46,47,75,82-84). These groups also accrue more damage over time (85) and at a faster pace (86) than white SLE patients and develop specific damage items more often (renal and integument) (85,87,88). Differences observed among ethnic groups early in the course of the disease probably reflect the genetic component of ethnicity, while differences observed later in its course may be more indicative of the nongenetic component of ethnicity such as socioeconomic status and access to medical care (2-5,88).
Pediatric lupus (before age 16 years) often presents with major organ system involvement including renal (46,89-92) and neuropsychiatric (55,92-94) disease. Furthermore, the transition from pediatric to adult care may be quite difficult for these patients. Independence and responsibility are highly desirable but they should be balanced with adequate social support and the discussion of issues particularly pertinent to this age group such as safe sexual practices and the prevention of other risky behaviors. Late-onset lupus (age 50 years and above) tends to have a more insidious onset (95,96), less major organ system involvement (97), and lower degrees of disease activity (98,99), yet these patients tend to have a poor outcome, in terms of both damage accrual (85,100) and mortality (51,96,101,102). Other factors associated with age (comorbidities) probably explain these findings (103).
The development of clinical instruments for the assessment of disease activity (104) and disease damage (105) has facilitated research on SLE progression. Recent studies have reported differences in the way patients and physicians perceive the impact of SLE. In terms of disease activity, for example, patients tend to score subjective manifestations, which overall indicate how well they feel, whereas physicians tend to score objective manifestations, particularly laboratory parameters (106-108). Disease activity scores using visual analog scales are often discordant between patients and physicians, with 28% differing by ±2.5 cm in 1 study (107), and 58% differing by ±1.0 cm in another study (106). This difference may have implications for disease management. For example, if a physician scores higher than the patient, some level of noncompliance is likely to occur; in the opposite situation, patients may request treatment beyond what physicians think is necessary. In terms of damage, patients may also put more weight on manifestations that impact on their appearance, body image, or self-esteem (eg, extensive skin scarring) than in features that are less tangible or symptomatic (proteinuria or diminished renal function, for example) (109).
Within the social context, social support has been identified as a modulating factor in disease activity, damage accrual, and even functioning (80,85,110-114). Karlson and coworkers, for example, in a cross-sectional study of 200 SLE patients from 5 centers, found that lack of social support was associated with poorer physical and mental functioning (114), whereas social support was negatively associated with disease activity in the LUMINA study (80). There may be important variation in these effects among ethnic groups, however. Social support was associated with better mental health outcomes in black and in white SLE patients in the 5-center study described previously, but some of the other positive effects of social support were only seen among whites or patients in the higher socioeconomic status groups (42). In addition, lower levels of social support and higher degrees of helplessness and of abnormal coping styles have been consistently shown in Texan-Hispanics and to a lesser extent in African Americans, than in Caucasians and Puerto Rican-Hispanics from the LUMINA cohort. Patients from these minority ethnic groups tend, overall, to experience worse disease outcomes (5,46). Adequate social support acts as a protective factor or buffer, making it possible for patients and their families to navigate the health and social systems, utilize them, and benefit from them. This is the case not only in patients with SLE but also in patients with other chronic diseases (115-118). Moreover, interventions aimed at improving social support have been shown to have some benefits, albeit modest, in SLE (119). Other socioeconomic parameters such as education, marital status, occupation, and income contribute to the overall social context in which the disease occurs and progresses.
Acculturation is the exchange of cultural features when two cultures come in contact with each other for the first time. Changes in both cultures may occur but the 2 cultures remain distinct. In the case of Hispanics moving into the United States, a higher degree of acculturation indicates that more features of the American culture had been acquired. The term is applied principally to the minority culture (immigrants) adopting features of the majority culture (host country). Discrimination and acculturation are two aspects of the social context that have been less well explored as to their impact on disease activity, damage, and functioning. These two constructs have been explored in the LUMINA patients. Neither inadequate acculturation by Texan-Hispanic patients into the mainstream American culture nor self-perceived discrimination by patients regardless of their ethnic category were found to have a direct impact on disease activity, damage accrual, or the patients' self-reported levels of functioning (118,120,121).
Health-Related Quality of Life
The level of self-reported physical and mental functioning or self-reported health-related quality of life is another important dimension to consider in SLE. Most studies have used the 36-item short form (SF-36) of the Medical Outcomes Survey (122) and have shown that patients with SLE function at levels below those of the general population (123-126). Health-related quality of life appears to be mediated not only by specific disease manifestations, by the degree of disease activity and of damage accrued, but also by the patient's level of helplessness and ability to cope with the disease. Two recent studies using shorter forms of this instrument (127,128) reported a significant reduction of overall self-perceived levels of well-being in LUMINA patients (129), or a reduction in physical function score in the Carolina Lupus Study patients compared with a general population comparison group (52). In the LUMINA study, these measures were affected not only by disease-related parameters but by social support, helplessness, and abnormal illness-related behaviors (129).
Discussion
We have reviewed the published literature related to the incidence and prevalence rates of SLE as well as the factors affecting the expression and the intermediate and long-term outcomes of the disease, including mortality risk, organ damage, and health-related quality of life. The disease is overall more frequent among minority population groups around the world and the outcome of the disease is less favorable in these populations. The role of socioeconomic factors in the course and outcome of the disease is noteworthy, and given the association of poor socioeconomic and minority status, the role of both in the final outcome of the disease may be difficult to disentangle. SLE at the extremes of life (pediatric and late-onset) may be particularly severe.
Significant advances have been made in the past 2 decades in understanding the genetic predisposition to disease occurrence and the underlying pathophysiological mechanisms accounting for the inflammatory process and the irreversible organ damage that may ensue over time. In addition, methodological developments have enabled a standardized ascertainment of disease activity, damage, function, and quality of life in the patient affected with lupus. Basic research continues to play an invaluable role in understanding the disease and its course, and this knowledge will hopefully translate to better therapeutic options for those affected with SLE. Despite these advances, significant gaps in our knowledge remain, and other important areas of research need to be addressed by those working in the field.
Several specific recommendations can be made based on our assessment of limitations and gaps in our understanding of the epidemiology and progression of SLE. For example, little research exists pertaining to the incidence or prevalence of SLE in many areas (particularly outside of Europe and North America) or among some groups within the United States (eg, Hispanics and Asian Americans). Data from countries that are undergoing a transition to industrial economies would be useful in determining the impact of these changes on disease risk. The different age patterns in incidence rate among women from different ethnic, socioeconomic, or geographic areas raise important questions for clinicians and researchers (eg, does the higher rates in older women in some areas reflect an increased diagnosis of milder disease?).
Research designs that allow for the separate examination of socioeconomic factors and ethnicity in studies of disease severity and progression could provide insights that would lead to the development of more focused and productive interventions. In terms of disease progression, studies based on the previously constituted cohorts may not be inclusive enough of the less severe cases of lupus managed by community rather than by academic rheumatologists. It is important to design studies that include community-based patients in longitudinal observational studies. Studies from disease registries may be useful for incidence or prevalence data if a specific population base or catchment can be defined and, can be useful for study of long-term outcomes if systematic follow-up is achieved. One issue with respect to mortality studies is the source of data relating to SLE-related mortality. Reliance on cause of death data obtained from death certificates is likely to lead to significant under-ascertainment of the impact of SLE (130). This is likely to be less of an issue in clinic-based cohort studies that are actively following patients than in population studies relying on death certificate to ascertain SLE-related mortality.
The development of self-assessment tools for the ascertainment of disease activity (131) and damage (132) may help facilitate these study designs. It is also important to determine the impact of lupus in other important outcomes not assessed with currently available instruments. Generic instruments such as the SF-36 or the SF-6D do not address specific domains that are quite relevant for those affected with the disease. Some of the outcomes that warrant more attention include body image and the perception of self-esteem. In addition, the relationship between self-perceived disease activity and compliance with treatment needs to be further explored. As part of the education and empowering process, lupus patients need to understand the “hidden” manifestations of the disease and appreciate their possible detrimental consequences, so compliance with treatment regimens will improve.
The agenda for the next generation of lupus researchers, while quite vast, promises to improve our understanding of the disease. Coupled with laboratory research, clinical- and population-based research will translate into better outcomes for patients afflicted with this disease.
Acknowledgments
This article is based on presentations and discussions that took place during the workshop on “Lupus and the Environment: Disease Development, Progression and Flare,” which was held in Washington, DC, September, 2005. The concept for this focused workshop was produced by the Federal Interagency Working Group on Women's Health and the Environment and support was provided by the U.S. Department of Health and Human Services' Office of Women's Health, The National Institute of Environmental Health Sciences, and the Lupus Foundation of America. The views expressed are those of the authors and do not necessarily reflect the views or policies of the U.S. EPA, the National Institute of Environmental Health Sciences, or the Office of Women's Health of the Department of Health and Human Services.
References
- 1.Trager J, Ward MM. Mortality and causes of death in systemic lupus erythematosus. Curr Opin Rheumatol. 2001;13:345–51. doi: 10.1097/00002281-200109000-00002. [DOI] [PubMed] [Google Scholar]
- 2.Alarcón GS. Of ethnicity, race and lupus. Lupus. 2001;10:594–6. doi: 10.1191/096120301682430159. [DOI] [PubMed] [Google Scholar]
- 3.Huth EJ. Identifying ethnicity in medical papers. Ann Intern Med. 1995;122:619–21. doi: 10.7326/0003-4819-122-8-199504150-00012. [DOI] [PubMed] [Google Scholar]
- 4.Senior PA, Bhopal R. Ethnicity as a variable in epidemiological research. BMJ. 1994;309:327–30. doi: 10.1136/bmj.309.6950.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fernandez M, Alarcón GS, Calvo-Alen J, Andrade R, McGwin G, Jr, Vila LM, et al. A multiethnic, multicenter cohort of patients with systemic lupus erythematosus (SLE) as a model for the study of ethnic disparities in SLE. Arthritis Rheum. 2007;57:576–84. doi: 10.1002/art.22672. [DOI] [PubMed] [Google Scholar]
- 6.Uramoto KM, Michet CJ, Jr, Thumboo J, Sunku J, O'Fallon WM, Gabriel SE. Trends in the incidence and mortality of systemic lupus erythematosus, 1950-1992. Arthritis Rheum. 1999;42:46–50. doi: 10.1002/1529-0131(199901)42:1<46::AID-ANR6>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 7.McCarty DJ, Manzi S, Medsger TA, Jr, Ramsey-Goldman R, LaPorte RE, Kwoh CK. Incidence of systemic lupus erythematosus. Race and gender differences. Arthritis Rheum. 1995;38:1260–70. doi: 10.1002/art.1780380914. [DOI] [PubMed] [Google Scholar]
- 8.Naleway AL, Davis ME, Greenlee RT, Wilson DA, McCarty DJ. Epidemiology of systemic lupus erythematosus in rural Wisconsin. Lupus. 2005;14:862–6. doi: 10.1191/0961203305lu2182xx. [DOI] [PubMed] [Google Scholar]
- 9.Peschken CA, Esdaile JM. Systemic lupus erythematosus in North American Indians: a population based study. J Rheumatol. 2000;27:1884–91. [PubMed] [Google Scholar]
- 10.Nossent JC. Systemic lupus erythematosus on the Caribbean island of Curacao: an epidemiological investigation. Ann Rheum Dis. 1992;51:1197–201. doi: 10.1136/ard.51.11.1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vilar MJ, Sato EI. Estimating the incidence of systemic lupus erythematosus in a tropical region (Natal, Brazil) Lupus. 2002;11:528–32. doi: 10.1191/0961203302lu244xx. [DOI] [PubMed] [Google Scholar]
- 12.Stahl-Hallengren C, Jonsen A, Nived O, Sturfelt G. Incidence studies of systemic lupus erythematosus in Southern Sweden: increasing age, decreasing frequency of renal manifestations and good prognosis. J Rheumatol. 2000;27:685–91. [PubMed] [Google Scholar]
- 13.Jonsson H, Nived O, Sturfelt G, Silman A. Estimating the incidence of systemic lupus erythematosus in a defined population using multiple sources of retrieval. Br J Rheumatol. 1990;29:185–8. doi: 10.1093/rheumatology/29.3.185. [DOI] [PubMed] [Google Scholar]
- 14.Voss A, Green A, Junker P. Systemic lupus erythematosus in Denmark: clinical and epidemiological characterization of a county-based cohort. Scand J Rheumatol. 1998;27:98–105. doi: 10.1080/030097498440958. [DOI] [PubMed] [Google Scholar]
- 15.Nossent HC. Systemic lupus erythematosus in the Arctic region of Norway. J Rheumatol. 2001;28:539–46. [PubMed] [Google Scholar]
- 16.Johnson AE, Gordon C, Palmer RG, Bacon PA. The prevalence and incidence of systemic lupus erythematosus in Birmingham, England. Relationship to ethnicity and country of birth. Arthritis Rheum. 1995;38:551–8. doi: 10.1002/art.1780380415. [DOI] [PubMed] [Google Scholar]
- 17.Hopkinson ND, Doherty M, Powell RJ. The prevalence and incidence of systemic lupus erythematosus in Nottingham, UK, 1989-1990. Br J Rheumatol. 1993;32:110–5. doi: 10.1093/rheumatology/32.2.110. [DOI] [PubMed] [Google Scholar]
- 18.Hopkinson ND, Doherty M, Powell RJ. Clinical features and race-specific incidence/prevalence rates of systemic lupus erythematosus in a geographically complete cohort of patients. Ann Rheum Dis. 1994;53:675–80. doi: 10.1136/ard.53.10.675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nightingale AL, Farmer RD, de Vries CS. Incidence of clinically diagnosed systemic lupus erythematosus 1992-1998 using the UK General Practice Research Database. Pharmacoepidemiol Drug Saf. 2006;15:656–61. doi: 10.1002/pds.1199. [DOI] [PubMed] [Google Scholar]
- 20.Somers EC, Thomas SL, Smeeth L, Schoonen WM, Hall AJ. Incidence of systemic lupus erythematosus in the United Kingdom, 1990-1999. Arthritis Rheum. 2007;57:612–8. doi: 10.1002/art.22683. [DOI] [PubMed] [Google Scholar]
- 21.Gudmundsson S, Steinsson K. Systemic lupus erythematosus in Iceland 1975 through 1984. A nationwide epidemiological study in an unselected population. J Rheumatol. 1990;17:1162–7. [PubMed] [Google Scholar]
- 22.Lopez P, Mozo L, Gutierrez C, Suarez A. Epidemiology of systemic lupus erythematosus in a northern Spanish population: gender and age influence on immunological features. Lupus. 2003;12:860–5. doi: 10.1191/0961203303lu469xx. [DOI] [PubMed] [Google Scholar]
- 23.Alamanos Y, Voulgari PV, Siozos C, Katsimpri P, Tsintzos S, Dimou G, et al. Epidemiology of systemic lupus erythematosus in northwest Greece 1982-2001. J Rheumatol. 2003;30:731–5. [PubMed] [Google Scholar]
- 24.Anstey NM, Bastian I, Dunckley H, Currie BJ. Systemic lupus erythematosus in Australian aborigines: high prevalence, morbidity and mortality. Aust NZ J Med. 1993;23:646–51. doi: 10.1111/j.1445-5994.1993.tb04720.x. [DOI] [PubMed] [Google Scholar]
- 25.Maskarinec G, Katz AR. Prevalence of systemic lupus erythematosus in Hawaii: is there a difference between ethnic groups? Hawaii Med J. 1995;54:406–9. [PubMed] [Google Scholar]
- 26.Chakravarty EF, Bush TM, Manzi S, Clarke AE, Ward MM. Prevalence of adult systemic lupus erythematosus in California and Pennsylvania in 2000: estimates obtained using hospitalization data. Arthritis Rheum. 2007;56:2092–4. doi: 10.1002/art.22641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Boyer GS, Templin DW, Lanier AP. Rheumatic diseases in Alaskan Indians of the southeast coast: high prevalence of rheumatoid arthritis and systemic lupus erythematosus. J Rheumatol. 1991;18:1477–84. [PubMed] [Google Scholar]
- 28.Hochberg MC. Prevalence of systemic lupus erythematosus in England and Wales, 1981-2. Ann Rheum Dis. 1987;46:664–6. doi: 10.1136/ard.46.9.664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Samanta A, Feehally J, Roy S, Nichol FE, Sheldon PJ, Walls J. High prevalence of systemic disease and mortality in Asian subjects with systemic lupus erythematosus. Ann Rheum Dis. 1991;50:490–2. doi: 10.1136/ard.50.7.490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Molokhia M, McKeigue P. Risk for rheumatic disease in relation to ethnicity and admixture. Arthritis Res. 2000;2:115–25. doi: 10.1186/ar76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gourley IS, Patterson CC, Bell AL. The prevalence of systemic lupus erythematosus in Northern Ireland. Lupus. 1997;6:399–403. doi: 10.1177/096120339700600410. [DOI] [PubMed] [Google Scholar]
- 32.Al-Arfaj AS, Al-Balla SR, Al-Dalaan AN, Al-Saleh SS, Bahabri SA, Mousa MM, et al. Prevalence of systemic lupus erythematosus in central Saudi Arabia. Saudi Med J. 2002;23:87–9. [PubMed] [Google Scholar]
- 33.Bossingham D. Systemic lupus erythematosus in the far north of Queensland. Lupus. 2003;12:327–31. doi: 10.1191/0961203303lu381xx. [DOI] [PubMed] [Google Scholar]
- 34.Segasothy M, Phillips PA. Systemic lupus erythematosus in Aborigines and Caucasians in central Australia: a comparative study. Lupus. 2001;10:439–44. doi: 10.1191/096120301678646191. [DOI] [PubMed] [Google Scholar]
- 35.Hart HH, Grigor RR, Caughey DE. Ethnic difference in the prevalence of systemic lupus erythematosus. Ann Rheum Dis. 1983;42:529–32. doi: 10.1136/ard.42.5.529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cohen AS, Reynolds WE, Franklin EC, Kulka JP, Ropes MW, Shulman L, et al. Preliminary criteria for the classification of systemic lupus erythematosus. Bull Rheum Dis. 1971;21:643–8. [Google Scholar]
- 37.Tan EM, Cohen AS, Fries JF, Masi AT, McShane DJ, Rothfield NF, et al. The 1982 revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. 1982;25:1271–7. doi: 10.1002/art.1780251101. [DOI] [PubMed] [Google Scholar]
- 38.Hochberg MC. Updating the American College of Rheumatology revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. 1997;40:1725. doi: 10.1002/art.1780400928. [DOI] [PubMed] [Google Scholar]
- 39.Hochberg MC, Perlmutter DL, Medsger TA, Steen V, Weisman MH, White B, et al. Prevalence of self-reported physician-diagnosed systemic lupus erythematosus in the USA. Lupus. 1995;4:454–6. doi: 10.1177/096120339500400606. [DOI] [PubMed] [Google Scholar]
- 40.Ward MM. Prevalence of physician-diagnosed systemic lupus erythematosus in the United States: results from the third national health and nutrition examination survey. J Womens Health (Larchmt) 2004;13:713–8. doi: 10.1089/jwh.2004.13.713. [DOI] [PubMed] [Google Scholar]
- 41.Johnson AE, Gordon C, Hobbs FD, Bacon PA. Undiagnosed systemic lupus erythematosus in the community. Lancet. 1996;347:367–9. doi: 10.1016/s0140-6736(96)90539-5. [DOI] [PubMed] [Google Scholar]
- 42.Bae SC, Fraser P, Liang MH. The epidemiology of systemic lupus erythematosus in populations of African ancestry: a critical review of the “prevalence gradient hypothesis”. Arthritis Rheum. 1998;41:2091–9. doi: 10.1002/1529-0131(199812)41:12<2091::AID-ART2>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 43.Hochberg MC. The incidence of systemic lupus erythematosus in Baltimore, Maryland, 1970-1977. Arthritis Rheum. 1985;28:80–6. doi: 10.1002/art.1780280113. [DOI] [PubMed] [Google Scholar]
- 44.Huemer C, Huemer M, Dorner T, Falger J, Schacherl H, Ber-necker M, et al. Incidence of pediatric rheumatic diseases in a regional population in Austria. J Rheumatol. 2001;28:2116–9. [PubMed] [Google Scholar]
- 45.Huang JL, Yao TC, See LC. Prevalence of pediatric systemic lupus erythematosus and juvenile chronic arthritis in a Chinese population: a nation-wide prospective population-based study in Taiwan. Clin Exp Rheumatol. 2004;22:776–80. [PubMed] [Google Scholar]
- 46.Alarcón GS, Friedman AW, Straaton KV, Moulds JM, Lisse J, Bastian HM, et al. Systemic lupus erythematosus in three ethnic groups: III. A comparison of characteristics early in the natural history of the LUMINA cohort. LUpus in MInority populations: NAture vs Nurture Lupus. 1999;8:197–209. doi: 10.1191/096120399678847704. [DOI] [PubMed] [Google Scholar]
- 47.Pons-Estel BA, Catoggio LJ, Cardiel MH, Soriano ER, Gentiletti S, Villa AR, et al. The GLADEL multinational Latin American prospective inception cohort of 1,214 patients with systemic lupus erythematosus: ethnic and disease heterogeneity among “Hispanics”. Medicine (Baltimore) 2004;83:1–17. doi: 10.1097/01.md.0000104742.42401.e2. [DOI] [PubMed] [Google Scholar]
- 48.Merrell M, Shulman LE. Determination of prognosis in chronic disease, illustrated by systemic lupus erythematosus. J Chronic Dis. 1955;1:12–32. doi: 10.1016/0021-9681(55)90018-7. [DOI] [PubMed] [Google Scholar]
- 49.Pistiner M, Wallace DJ, Nessim S, Metzger AL, Klinenberg JR. Lupus erythematosus in the 1980s: a survey of 570 patients. Semin Arthritis Rheum. 1991;21:55–64. doi: 10.1016/0049-0172(91)90057-7. [DOI] [PubMed] [Google Scholar]
- 50.Kasitanon N, Magder LS, Petri M. Predictors of survival in systemic lupus erythematosus. Medicine (Baltimore) 2006;85:147–56. doi: 10.1097/01.md.0000224709.70133.f7. [DOI] [PubMed] [Google Scholar]
- 51.Alarcón GS, McGwin G, Jr, Bastian HM, Roseman J, Lisse J, Fessler BJ, et al. Systemic lupus erythematosus in three ethnic groups. VII [correction of VIII]. Predictors of early mortality in the LUMINA cohort. LUMINA Study Group. Arthritis Rheum. 2001;45:191–202. doi: 10.1002/1529-0131(200104)45:2<191::AID-ANR173>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 52.Campbell R, Jr, Cooper GS, Gilkeson GS. Two aspects of the clinical and humanistic burden of systemic lupus erythematosus: mortality risk and quality of life early in the course of disease. Arthritis Rheum. 2008;59:458–64. doi: 10.1002/art.23539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Alamanos Y, Voulgari PV, Papassava M, Tsamandouraki K, Drosos AA. Survival and mortality rates of systemic lupus erythematosus patients in northwest Greece. Study of a 21-year incidence cohort. Rheumatology (Oxford) 2003;42:1122–3. doi: 10.1093/rheumatology/keg291. [DOI] [PubMed] [Google Scholar]
- 54.Manger K, Manger B, Repp R, Geisselbrecht M, Geiger A, Pfahlberg A, et al. Definition of risk factors for death, end stage renal disease, and thromboembolic events in a monocentric cohort of 338 patients with systemic lupus erythematosus. Ann Rheum Dis. 2002;61:1065–70. doi: 10.1136/ard.61.12.1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wang LC, Yang YH, Lu MY, Chiang BL. Retrospective analysis of mortality and morbidity of pediatric systemic lupus erythematosus in the past two decades. J Microbiol Immunol Infect. 2003;36:203–8. [PubMed] [Google Scholar]
- 56.Mok CC, Lee KW, Ho CT, Lau CS, Wong RW. A prospective study of survival and prognostic indicators of systemic lupus erythematosus in a southern Chinese population. Rheumatology (Oxford) 2000;39:399–406. doi: 10.1093/rheumatology/39.4.399. [DOI] [PubMed] [Google Scholar]
- 57.Kasitanon N, Louthrenoo W, Sukitawut W, Vichainun R. Causes of death and prognostic factors in Thai patients with systemic lupus erythematosus. Asian Pac J Allergy Immunol. 2002;20:85–91. [PubMed] [Google Scholar]
- 58.Murali R, Jeyaseelan L, Rajaratnam S, John L, Ganesh A. Systemic lupus erythematosus in Indian patients: prognosis, survival and life expectancy. Natl Med J India. 1997;10:159–64. [PubMed] [Google Scholar]
- 59.Hochberg MC. Mortality from systemic lupus erythematosus in England and Wales, 1974-1983. Br J Rheumatol. 1987;26:437–41. doi: 10.1093/rheumatology/26.6.437. [DOI] [PubMed] [Google Scholar]
- 60.Urowitz MB, Bookman AA, Koehler BE, Gordon DA, Smythe HA, Ogryzlo MA. The bimodal mortality pattern of systemic lupus erythematosus. Am J Med. 1976;60:221–5. doi: 10.1016/0002-9343(76)90431-9. [DOI] [PubMed] [Google Scholar]
- 61.Urowitz MB, Gladman DD, Abu-Shakra M, Farewell VT. Mortality studies in systemic lupus erythematosus. Results from a single center. III. Improved survival over 24 years. J Rheumatol. 1997;24:1061–5. [PubMed] [Google Scholar]
- 62.Bjornadal L, Yin L, Granath F, Klareskog L, Ekbom A. Cardiovascular disease a hazard despite improved prognosis in patients with systemic lupus erythematosus: results from a Swedish population based study 1964-95. J Rheumatol. 2004;31:713–9. [PubMed] [Google Scholar]
- 63.Abu-Shakra M, Gladman DD, Urowitz MB. Mortality studies in SLE: how far can we improve survival of patients with SLE. Autoimmun Rev. 2004;3:418–20. doi: 10.1016/j.autrev.2004.07.003. [DOI] [PubMed] [Google Scholar]
- 64.Moss KE, Ioannou Y, Sultan SM, Haq I, Isenberg DA. Outcome of a cohort of 300 patients with systemic lupus erythematosus attending a dedicated clinic for over two decades. Ann Rheum Dis. 2002;61:409–13. doi: 10.1136/ard.61.5.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Jacobsen S, Petersen J, Ullman S, Junker P, Voss A, Rasmussen JM, et al. Mortality and causes of death of 513 Danish patients with systemic lupus erythematosus. Scand J Rheumatol. 1999;28:75–80. doi: 10.1080/030097499442522. [DOI] [PubMed] [Google Scholar]
- 66.Bellomio V, Spindler A, Lucero E, Berman A, Santana M, Moreno C, et al. Systemic lupus erythematosus: mortality and survival in Argentina. A multicenter study. Lupus. 2000;9:377–81. [PubMed] [Google Scholar]
- 67.Swaak AJ, Nossent JC, Bronsveld W, Van Rooyen A, Nieuwenhuys EJ, Theuns L, et al. Systemic lupus erythematosus. I. Outcome and survival: Dutch experience with 110 patients studied prospectively. Ann Rheum Dis. 1989;48:447–54. doi: 10.1136/ard.48.6.447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Blanco FJ, Gomez-Reino JJ, de la Mata J, Corrales A, Rodriguez-Valverde V, Rosas JC, et al. Survival analysis of 306 European Spanish patients with systemic lupus erythematosus. Lupus. 1998;7:159–63. doi: 10.1191/096120398678919930. [DOI] [PubMed] [Google Scholar]
- 69.Ward MM, Studenski S. Systemic lupus erythematosus in men: a multivariate analysis of gender differences in clinical manifestations. J Rheumatol. 1990;17:220–4. [PubMed] [Google Scholar]
- 70.Reveille JD, Bartolucci A, Alarcón GS. Prognosis in systemic lupus erythematosus. Negative impact of increasing age at onset, black race, and thrombocytopenia, as well as causes of death. Arthritis Rheum. 1990;33:37–48. doi: 10.1002/art.1780330105. [DOI] [PubMed] [Google Scholar]
- 71.Ward MM, Pyun E, Studenski S. Long-term survival in systemic lupus erythematosus. Patient characteristics associated with poorer outcomes. Arthritis Rheum. 1995;38:274–83. doi: 10.1002/art.1780380218. [DOI] [PubMed] [Google Scholar]
- 72.Ginzler EM, Diamond HS, Weiner M, Schlesinger M, Fries JF, Wasner C, et al. A multicenter study of outcome in systemic lupus erythematosus. I. Entry variables as predictors of prognosis. Arthritis Rheum. 1982;25:601–11. doi: 10.1002/art.1780250601. [DOI] [PubMed] [Google Scholar]
- 73.Trends in deaths from systemic lupus erythematosus—United States, 1979-1998. MMWR Morb Mortal Wkly Rep. 2002;51:371–4. [PubMed] [Google Scholar]
- 74.Duran S, Apte M, Alarcón GS. Poverty, not ethnicity, accounts for the differential mortality rates among lupus patients of various ethnic groups. J Natl Med Assoc. 2007;99:1196–8. [PMC free article] [PubMed] [Google Scholar]
- 75.Cooper GS, Parks CG, Treadwell EL, St Clair EW, Gilkeson GS, Cohen PL, et al. Differences by race, sex and age in the clinical and immunologic features of recently diagnosed systemic lupus erythematosus patients in the southeastern United States. Lupus. 2002;11:161–7. doi: 10.1191/0961203302lu161oa. [DOI] [PubMed] [Google Scholar]
- 76.Molina JF, Drenkard C, Molina J, Cardiel MH, Uribe O, Anaya JM, et al. Systemic lupus erythematosus in males. A study of 107 Latin American patients. Medicine (Baltimore) 1996;75:124–30. doi: 10.1097/00005792-199605000-00002. [DOI] [PubMed] [Google Scholar]
- 77.Cervera R, Khamashta MA, Font J, Sebastiani GD, Gil A, Lavilla P, et al. Morbidity and mortality in systemic lupus erythematosus during a 5-year period. A multicenter prospective study of 1,000 patients. European Working Party on Systemic Lupus Erythematosus. Medicine (Baltimore) 1999;78:167–75. doi: 10.1097/00005792-199905000-00003. [DOI] [PubMed] [Google Scholar]
- 78.Andrade RM, Alarcón GS, Fernandez M, Apte M, Vila LM, Reveille JD. Accelerated damage accrual among men with systemic lupus erythematosus: XLIV. Results from a multiethnic US cohort. Arthritis Rheum. 2007;56:622–30. doi: 10.1002/art.22375. [DOI] [PubMed] [Google Scholar]
- 79.Garcia MA, Marcos JC, Marcos AI, Pons-Estel BA, Wojdyla D, Arturi A, et al. Male systemic lupus erythematosus in a Latin-American inception cohort of 1214 patients. Lupus. 2005;14:938–46. doi: 10.1191/0961203305lu2245oa. [DOI] [PubMed] [Google Scholar]
- 80.Alarcón GS, Calvo-Alen J, McGwin G, Uribe AG, Toloza SM, Roseman J, et al. Systemic lupus erythematosus in a multiethnic cohort: LUMINA XXXV. Predictive factors of high disease activity over time. Ann Rheum Dis. 2006;65:1168–74. doi: 10.1136/ard.200X.046896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Alarcón GS, Roseman J, Bartolucci AA, Friedman AW, Moulds JM, Goel N, et al. Systemic lupus erythematosus in three ethnic groups: II. Features predictive of disease activity early in its course. LUMINA Study Group Lupus in minority populations, nature versus nurture. Arthritis Rheum. 1998;41:1173–80. doi: 10.1002/1529-0131(199807)41:7<1173::AID-ART5>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 82.Ward MM, Studenski S. Clinical manifestations of systemic lupus erythematosus. Identification of racial and socioeconomic influences. Arch Intern Med. 1990;150:849–53. [PubMed] [Google Scholar]
- 83.Alarcón GS, McGwin G, Jr, Petri M, Reveille JD, Ramsey-Goldman R, Kimberly RP. Baseline characteristics of a multiethnic lupus cohort: PROFILE. Lupus. 2002;11:95–101. doi: 10.1191/0961203302lu155oa. [DOI] [PubMed] [Google Scholar]
- 84.Bastian HM, Roseman JM, McGwin G, Jr, Alarcón GS, Friedman AW, Fessler BJ, et al. Systemic lupus erythematosus in three ethnic groups. XII. Risk factors for lupus nephritis after diagnosis. Lupus. 2002;11:152–60. doi: 10.1191/0961203302lu158oa. [DOI] [PubMed] [Google Scholar]
- 85.Alarcón GS, McGwin G, Jr, Bartolucci AA, Roseman J, Lisse J, Fessler BJ, et al. Systemic lupus erythematosus in three ethnic groups. IX. Differences in damage accrual. Arthritis Rheum. 2001;44:2797–806. doi: 10.1002/1529-0131(200112)44:12<2797::aid-art467>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 86.Toloza SM, Uribe AG, McGwin G, Jr, Alarcón GS, Fessler BJ, Bastian HM, et al. Systemic lupus erythematosus in a multiethnic US cohort (LUMINA). XXIII. Baseline predictors of vascular events. Arthritis Rheum. 2004;50:3947–57. doi: 10.1002/art.20622. [DOI] [PubMed] [Google Scholar]
- 87.Rivest C, Lew RA, Welsing PM, Sangha O, Wright EA, Roberts WN, et al. Association between clinical factors, socioeconomic status, and organ damage in recent onset systemic lupus erythematosus. J Rheumatol. 2000;27:680–4. [PubMed] [Google Scholar]
- 88.Cooper GS, Treadwell EL, St Clair EW, Gilkeson GS, Dooley MA. Sociodemographic associations with early disease damage in patients with systemic lupus erythematosus. Arthritis Rheum. 2007;57:993–9. doi: 10.1002/art.22894. [DOI] [PubMed] [Google Scholar]
- 89.Font J, Cervera R, Espinosa G, Pallares L, Ramos-Casals M, Jimenez S, et al. Systemic lupus erythematosus (SLE) in childhood: analysis of clinical and immunological findings in 34 patients and comparison with SLE characteristics in adults. Ann Rheum Dis. 1998;57:456–9. doi: 10.1136/ard.57.8.456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Bakr A. Epidemiology treatment and outcome of childhood systemic lupus erythematosus in Egypt. Pediatr Nephrol. 2005;20:1081–6. doi: 10.1007/s00467-005-1900-2. [DOI] [PubMed] [Google Scholar]
- 91.Lehman TJ, McCurdy DK, Bernstein BH, King KK, Hanson V. Systemic lupus erythematosus in the first decade of life. Pediatrics. 1989;83:235–9. [PubMed] [Google Scholar]
- 92.Carreno L, Lopez-Longo FJ, Monteagudo I, Rodriguez-Mahou M, Bascones M, Gonzalez CM, et al. Immunological and clinical differences between juvenile and adult onset of systemic lupus erythematosus. Lupus. 1999;8:287–92. doi: 10.1191/096120399678847786. [DOI] [PubMed] [Google Scholar]
- 93.Sibbitt WL, Jr, Brandt JR, Johnson CR, Maldonado ME, Patel SR, Ford CC, et al. The incidence and prevalence of neuropsychiatric syndromes in pediatric onset systemic lupus erythematosus. J Rheumatol. 2002;29:1536–42. [PubMed] [Google Scholar]
- 94.Quintero-Del-Rio AI, Van M. Neurologic symptoms in children with systemic lupus erythematosus. J Child Neurol. 2000;15:803–7. doi: 10.1177/088307380001501207. [DOI] [PubMed] [Google Scholar]
- 95.Mak SK, Lam EK, Wong AK. Clinical profile of patients with late-onset SLE: not a benign subgroup. Lupus. 1998;7:23–8. doi: 10.1191/096120398678919723. [DOI] [PubMed] [Google Scholar]
- 96.Pu SJ, Luo SF, Wu YJ, Cheng HS, Ho HH. The clinical features and prognosis of lupus with disease onset at age 65 and older. Lupus. 2000;9:96–100. doi: 10.1191/096120300678828109. [DOI] [PubMed] [Google Scholar]
- 97.Ho CT, Mok CC, Lau CS, Wong RW. Late onset systemic lupus erythematosus in southern Chinese. Ann Rheum Dis. 1998;57:437–40. doi: 10.1136/ard.57.7.437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Formiga F, Moga I, Pac M, Mitjavila F, Rivera A, Pujol R. Mild presentation of systemic lupus erythematosus in elderly patients assessed by SLEDAI. SLE Disease Activity Index. Lupus. 1999;8:462–5. doi: 10.1177/096120339900800609. [DOI] [PubMed] [Google Scholar]
- 99.Ward MM, Polisson RP. A meta-analysis of the clinical manifestations of older-onset systemic lupus erythematosus. Arthritis Rheum. 1989;32:1226–32. doi: 10.1002/anr.1780321007. [DOI] [PubMed] [Google Scholar]
- 100.Maddison P, Farewell V, Isenberg D, Aranow C, Bae SC, Barr S, et al. The rate and pattern of organ damage in late onset systemic lupus erythematosus. J Rheumatol. 2002;29:913–7. [PubMed] [Google Scholar]
- 101.Costallat LT, Coimbra AM. Systemic lupus erythematosus: clinical and laboratory aspects related to age at disease onset. Clin Exp Rheumatol. 1994;12:603–7. [PubMed] [Google Scholar]
- 102.Boddaert J, Huong DL, Amoura Z, Wechsler B, Godeau P, Piette JC. Late-onset systemic lupus erythematosus: a personal series of 47 patients and pooled analysis of 714 cases in the literature. Medicine (Baltimore) 2004;83:348–59. doi: 10.1097/01.md.0000147737.57861.7c. [DOI] [PubMed] [Google Scholar]
- 103.Bertoli AM, Alarcón GS, Calvo-Alen J, Fernandez M, Vila LM, Reveille JD. Systemic lupus erythematosus in a multiethnic US cohort. XXXIII. Clinical [corrected] features, course, and outcome in patients with late-onset disease. Arthritis Rheum. 2006;54:1580–7. doi: 10.1002/art.21765. [DOI] [PubMed] [Google Scholar]
- 104.Liang MH, Socher SA, Larson MG, Schur PH. Reliability and validity of six systems for the clinical assessment of disease activity in systemic lupus erythematosus. Arthritis Rheum. 1989;32:1107–18. doi: 10.1002/anr.1780320909. [DOI] [PubMed] [Google Scholar]
- 105.Gladman DD, Urowitz MB, Goldsmith CH, Fortin P, Ginzler E, Gordon C, et al. The reliability of the Systemic Lupus International Collaborating Clinics/American College of Rheumatology Damage Index in patients with systemic lupus erythematosus. Arthritis Rheum. 1997;40:809–13. doi: 10.1002/art.1780400506. [DOI] [PubMed] [Google Scholar]
- 106.Alarcón GS, McGwin G, Jr, Brooks K, Roseman JM, Fessler BJ, Sanchez ML, et al. Systemic lupus erythematosus in three ethnic groups. XI. Sources of discrepancy in perception of disease activity: a comparison of physician and patient visual analog scale scores. Arthritis Rheum. 2002;47:408–13. doi: 10.1002/art.10512. [DOI] [PubMed] [Google Scholar]
- 107.Yen JC, Neville C, Fortin PR. Discordance between patients and their physicians in the assessment of lupus disease activity: relevance for clinical trials. Lupus. 1999;8:660–70. doi: 10.1191/096120399680411362. [DOI] [PubMed] [Google Scholar]
- 108.Yen JC, Abrahamowicz M, Dobkin PL, Clarke AE, Battista RN, Fortin PR. Determinants of discordance between patients and physicians in their assessment of lupus disease activity. J Rheumatol. 2003;30:1967–76. [PubMed] [Google Scholar]
- 109.Ferraz LB, de Almeida FA, Vasconcellos MR, Ferraz MB. Alopecia impairs the quality of life of patients with lupus erythematosus. Arch Dermatol. 2006;142:110. doi: 10.1001/archderm.142.1.110-a. [DOI] [PubMed] [Google Scholar]
- 110.Alarcón GS, McGwin G, Jr, Sanchez ML, Bastian HM, Fessler BJ, Friedman AW, et al. Systemic lupus erythematosus in three ethnic groups. XIV. Poverty, wealth, and their influence on disease activity. Arthritis Rheum. 2004;51:73–7. doi: 10.1002/art.20085. [DOI] [PubMed] [Google Scholar]
- 111.Jump RL, Robinson ME, Armstrong AE, Barnes EV, Kilbourn KM, Richards HB. Fatigue in systemic lupus erythematosus: contributions of disease activity, pain, depression, and perceived social support. J Rheumatol. 2005;32:1699–705. [PubMed] [Google Scholar]
- 112.Kozora E, Ellison MC, Waxmonsky JA, Wamboldt FS, Patterson TL. Major life stress, coping styles, and social support in relation to psychological distress in patients with systemic lupus erythematosus. Lupus. 2005;14:363–72. doi: 10.1191/0961203305lu2094oa. [DOI] [PubMed] [Google Scholar]
- 113.McCracken LM, Semenchuk EM, Goetsch VL. Cross-sectional and longitudinal analyses of coping responses and health status in persons with systemic lupus erythematosus. Behav Med. 1995;20:179–87. doi: 10.1080/08964289.1995.9933735. [DOI] [PubMed] [Google Scholar]
- 114.Karlson EW, Daltroy LH, Lew RA, Wright EA, Partridge AJ, Fossel AH, et al. The relationship of socioeconomic status, race, and modifiable risk factors to outcomes in patients with systemic lupus erythematosus. Arthritis Rheum. 1997;40:47–56. doi: 10.1002/art.1780400108. [DOI] [PubMed] [Google Scholar]
- 115.Lett HS, Blumenthal JA, Babyak MA, Catellier DJ, Carney RM, Berkman LF, et al. Social support and prognosis in patients at increased psychosocial risk recovering from myocardial infarction. Health Psychol. 2007;26:418–27. doi: 10.1037/0278-6133.26.4.418. [DOI] [PubMed] [Google Scholar]
- 116.Shah S, Cook DG. Inequalities in the treatment and control of hypertension: age, social isolation and lifestyle are more important than economic circumstances. J Hypertens. 2001;19:1333–40. doi: 10.1097/00004872-200107000-00020. [DOI] [PubMed] [Google Scholar]
- 117.Penninx BW, van Tilburg T, Boeke AJ, Deeg DJ, Kriegsman DM, van Eijk JT. Effects of social support and personal coping resources on depressive symptoms: different for various chronic diseases? Health Psychol. 1998;17:551–8. doi: 10.1037//0278-6133.17.6.551. [DOI] [PubMed] [Google Scholar]
- 118.Lee SJ, Detels R, Rotheram-Borus MJ, Duan N, Lord L. Depression and social support among HIV-affected adolescents. AIDS Patient Care STDS. 2007;21:409–17. doi: 10.1089/apc.2006.0066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Karlson EW, Liang MH, Eaton H, Huang J, Fitzgerald L, Rogers MP, et al. A randomized clinical trial of a psychoeducational intervention to improve outcomes in systemic lupus erythematosus. Arthritis Rheum. 2004;50:1832–41. doi: 10.1002/art.20279. [DOI] [PubMed] [Google Scholar]
- 120.Alarcón GS, Rodriguez JL, Benavides G, Jr, Brooks K, Kurusz H, Reveille JD. Systemic lupus erythematosus in three ethnic groups. V. Acculturation, health-related attitudes and behaviors, and disease activity in Hispanic patients from the LUMINA cohort LUMINA Study Group Lupus in Minority Populations, Nature versus Nurture. Arthritis Care Res. 1999;12:267–76. doi: 10.1002/1529-0131(199908)12:4<267::aid-art5>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 121.Sanchez ML, Bastian HM, Fessler BJ, Reveille JD, Vila LM, Alarcón GS. Perception of discrimination oin SLE patients from a multiethnic cohort. Arthritis Rheum. 2004;2004:S241. [Google Scholar]
- 122.McHorney CA, Ware JE, Jr, Rogers W, Raczek AE, Lu JF. The validity and relative precision of MOS short- and long-form health status scales and Dartmouth COOP charts. Results from the Medical Outcomes Study. Med Care. 1992;30:MS253–65. doi: 10.1097/00005650-199205001-00025. [DOI] [PubMed] [Google Scholar]
- 123.Alarcón GS, McGwin G, Jr, Uribe A, Friedman AW, Roseman JM, Fessler BJ, et al. Systemic lupus erythematosus in a multiethnic lupus cohort (LUMINA). XVII. Predictors of self-reported health-related quality of life early in the disease course. Arthritis Rheum. 2004;51:465–74. doi: 10.1002/art.20409. [DOI] [PubMed] [Google Scholar]
- 124.Rinaldi S, Doria A, Salaffi F, Ermani M, Iaccarino L, Ghirardello A, et al. Health-related quality of life in Italian patients with systemic lupus erythematosus. I. Relationship between physical and mental dimension and impact of age. Rheumatology (Oxford) 2004;43:1574–9. doi: 10.1093/rheumatology/keh397. [DOI] [PubMed] [Google Scholar]
- 125.Thumboo J, Chan SP, Machin D, Soh CH, Feng PH, Boey ML, et al. Measuring health-related quality of life in Singapore: normal values for the English and Chinese SF-36 Health Survey. Ann Acad Med Singapore. 2002;31:366–74. [PubMed] [Google Scholar]
- 126.Thumboo J, Fong KY, Ng TP, Leong KH, Feng PH, Thio ST, et al. Validation of the MOS SF-36 for quality of life assessment of patients with systemic lupus erythematosus in Singapore. J Rheumatol. 1999;26:97–102. [PubMed] [Google Scholar]
- 127.Brazier J, Roberts J, Tsuchiya A, Busschbach J. A comparison of the EQ-5D and SF-6D across seven patient groups. Health Econ. 2004;13:873–84. doi: 10.1002/hec.866. [DOI] [PubMed] [Google Scholar]
- 128.Ware JE, Kosinsk M, Dewey JE, Gandek B. How to score and interpret single-item health status measures: a manual for users of the SF-8TM Health Survey. Lincoln, RI: QualityMetric Incorporated; 2001. [Google Scholar]
- 129.Sanchez ML, McGwin G, Jr, Duran S, Fernandez M, Apte M, Vila LM, et al. Factors associated with self-reported health-related quality of life (HRQOL) in patients from a US multiethnic SLE cohort. Lupus. 2007;16:195–6. [Google Scholar]
- 130.Calvo-Alen J, Alarcón GS, Campbell R, Jr, Fernandez M, Reveille JD, Cooper GS. Lack of recording of systemic lupus erythematosus in the death certificates of lupus patients. Rheumatology (Oxford) 2005;44:1186–9. doi: 10.1093/rheumatology/keh717. [DOI] [PubMed] [Google Scholar]
- 131.Karlson EW, Daltroy LH, Rivest C, Ramsey-Goldman R, Wright EA, Partridge AJ, et al. Validation of a Systemic Lupus Activity Questionnaire (SLAQ) for population studies. Lupus. 2003;12:280–6. doi: 10.1191/0961203303lu332oa. [DOI] [PubMed] [Google Scholar]
- 132.Sanchez ML, Costenbader KH, Karlson EW, Alarcón GS, Wolfe F. Is it possible to ascertain damage in lupus utilizing a self-reported nquestionnaire? Preliminary data on the Lupus Damage Index Questionnaire (LDIQ) Arthritis Rheum. 2008 in press. [Google Scholar]