Abstract
The development of effective pharmacotherapy for obesity will benefit from a more complete understanding of the neural pathways and the neurochemical signals whose actions result in the reduction of the size of meals. This review examines the neural control of meal size and the integration of two principal sources of that control - satiation signals arising from the gastrointestinal tract and CNS leptin signaling. Four types of integrations that are central to the control of meal size are described and each involves the neurons of the nucleus tractus solitarius (NTS) in the dorsal hindbrain. Data discussed show that NTS neurons integrate information arising from: [1] ascending GI-derived vagal afferent projections, [2] descending neuropeptidergic projections from leptin-activated arcuate and paraventricular nucleus neurons, [3] leptin signaling in NTS neurons themselves and [4] melanocortinergic projections from NTS and hypothalamic POMC neurons to NTS neurons and via melanocortinergic modulation of vagal afferent nerve terminals.
The dramatic increase in the prevalence of obese and overweight individuals over the past two decades has intensified interest in obesity (and its co-morbidities - type II diabetes mellitus, cardiovascular disease, and certain cancers) and the need for additional research on its mediating mechanisms. The “obesity epidemic” is attributed to features of modern life - increased consumption of abundant and palatable energy-dense foods (1–2) and reduced physical activity (3) – that interact with an energy balance regulatory system that evolved in response to food scarcity (4–6). Despite the increased prevalence of obesity in adult and pediatric populations, and an appreciation of the enormous economic and personal costs of treating obesity and its co-morbidities, there are presently no pharmacological treatments that curtail excess feeding and yield meaningful sustained weight loss. The failure to identify effective drug treatments for obesity is, of course, unrelated to the significant scientific effort and economic investment by the government (see NIH Strategic Plan for Obesity Research) and by the pharmaceutical industry. In fact, by targeting the regulatory neural systems governing energy balance at a cellular level, basic science has succeeded in identifying critical roles for novel hormones (leptin, glucagon-like peptide-1, ghrelin), neuropeptides (melanocortin, melanin-concentrating hormone), and other neurochemicals (serotonin, dopamine, opioids and cannabinoids), as well as their respective receptors in energy balance regulation (7–16).
A likely explanation for the absence of effective drug treatments can be found in the multi-determined nature of an energy balance control system that evolved under pressures of food scarcity. Physiologic systems are confronted with a difficult problem – how to maintain supplies of the energy-rich molecules that are in constant demand to power the myriad cellular processes necessary for survival and reproduction. It appears that our energy balance regulatory systems “solved” the problem of chronic energy demand by evolving multiple and, in some instances, redundant sub-systems. A neuroendocrine system that employs multiple signals, receptors, and brain circuits that operate with a degree of redundancy would appear to be better able to ensure that adequate energy is available to power the demands of survival and reproduction than a CNS control system centered in a single brain site using fewer feedback signals. As food scarcity was the prevailing condition during our ancestral development and food abundance occurred only periodically, the regulatory systems of our Paleolithic ancestors performed well (4–6) - matching energy intake (feeding behavior) with energy expenditure in most individuals (3). Historically, obesity was uncommon under those conditions. But, in recent decades the food availability and energy expenditure demands of our environment have changed dramatically. The development of agribusiness with support from government provides abundant quantities of low-cost, energy-dense foods (17) that, given the bias of our regulatory system towards consumption during periods of food availability (18), appears to promote a state of chronic hyperphagia that drives positive energy balance, increased adiposity, and ultimately obesity in many individuals.
The problem at hand then is the need to develop effective and safe pharmacological treatments for obesity (radical surgical treatment is effective but has associated risks). This task leads us to an in-depth consideration of the brain mechanisms responsible for the inhibition of food intake in the non-obese state. Humans and many other mammals (including common laboratory rodent models) consume food in discrete episodes or meals. Distinguishable behavioral responses are controlled by various brain regions and neurochemical systems that impact on various aspects of meal taking, including the initiation, size, and frequency of meals. Given the aforementioned abundant food environment, the initiation of meals, for most of us, is not associated with hunger (homeostatic need, negative energy balance or the absence of gastrointestinal satiation signals). Rather, meal initiation for many people is based on appetite -the time of day, the constraints of our schedules, social circumstances, the hedonic allure of foods (referred to as reward or food reward), and learned associations (19–22). These contributory factors to meal initiation are considered non-homeostatic (not driven by energy deficit). While their contribution to the control of feeding and the obesity epidemic is clear, our current understanding of the neurons, signaling cascades, neural circuits and neurochemicals that mediate their biological influences on feeding is poorly developed. By contrast, the physiological underpinnings of the factors that control meal termination and thereby meal size (the amount of food consumed during a meal) has been more extensively studied.
G. P. Smith distinguishes between two categories of signals that contribute to the control of meal size (23). He uses the term direct controls of meal size, to describe signals arising from the alimentary canal in response to contact with ingested food or to the products of its digestion. Direct controls, more commonly referred to as satiation signals, include sensory signals arising from the interaction of ingested food with the gastrointestinal (GI) tract and include mechanical distension of the stomach, nutrient stimulation of the intestine, and intestinal hormones released in response to calorie-bearing nutrients (e.g., cholecystokinin [CCK], glucagon-like peptide 1 [GLP-1], peptide YY 3-36 [PYY3-36]) (24). It is well known that these satiation signals activate receptors on vagal afferent neurons whose cell bodies reside in the nodose ganglion and whose central processes terminate on neurons of specified subnuclear regions of the nucleus tractus solitarius (NTS) (25–28). NTS neurons project to many other neurons throughout the neuraxis including those in the pons (parabrachial nucleus, PBN), hypothalamus (lateral hypothalamus, LH; paraventricular nucleus, PVH), basal forebrain (bed nucleus of stria terminalis; central nucleus of the amygdala), thalamus, and agranular insular cortex. This projection system is referred to as the ascending central visceral afferent pathway (29–30).
Smith (23) and others [e.g. Berthoud (31); Woods (32)] agree that meal size is influenced by other types of signals including metabolic signals (fuel availability), such as circulating levels of nutrients (e.g. glucose, free fatty acids and amino acids). In addition, correlates of stored energy commonly referred to as adiposity signals (leptin, insulin), rhythmic signals (cyclic sex hormones such as estrogen, diurnal or circadian signals), and signals associated with conditioning, environmental temperature, or ecology contribute to meal size control. Smith parts company with others considering the control of meal size by his creation of a separate category for these signals and refers to them as indirect controls (96). The “indirect” designation is employed to contrast with “direct” and to define a specific relationship between the mechanism of effect for these signals and the neural processing of the satiation signals arising from direct contact of ingested food within the GI tract. Smith describes the intake inhibitory impact of these signals as indirect because he views their action as modulating or amplifying the neural processing of direct (a.k.a. satiation) signals as opposed to their having an independent behavioral action of their own. This idea is empirically generative as it suggests experiments that may better define the neural pathways and signaling cascades that control the termination of feeding behavior and perhaps by so doing, these notions may inform the selection of new drugs to more effectively treat obesity. The balance of this article explores the neural pathways and the neurochemical signals that mediate the food intake inhibitory actions of satiation signals and the interaction between adiposity signals and satiation signals.
The Neural Circuits Mediating the Meal Size Effects of Satiation Signals
The idea that hypothalamic neurons play a critical role in the neural control of feeding and in meal size control specifically, is one that has been with us for quite some time having been established by pioneering work early in the 20th century. The analysis of the functional connection between human hyperphagia and obesity and the contribution of pituitary and/or hypothalamic tumors led a number of investigators to conclude that hypothalamic neurons contribute to the physiological control of feeding [the history of human data on this point is reviewed in (33)]. Complementary data – hyperphagia and obesity – arising from targeted bilateral lesions of medial hypothalamic neurons in rodent models supported this conclusion (34–35). A different behavioral syndrome – aphagia followed by hypophagia (anorexia) - was observed following targeted bilateral destruction of neurons in a different part of the hypothalamus – the lateral area (36–37). These studies, and others employing chemical and electrical stimulation of hypothalamic neurons established a clear connection between different populations of hypothalamic neurons, receptors for classic neurotransmitters and the neural circuits that control meal termination and initiation (38–40). I refer to this period of experimentation on the neural control of food intake as the “old neurobiology of energy balance.” The development of molecular biological techniques and their application to understanding the biological basis of the obesity of genetically obese mice (the obese, diabetic and yellow mice) led to the current modern or “new neurobiology of energy balance.” As stated, the focus on hypothalamic neurons as the major player in food intake control was already rooted and for this and other reasons, the spotlight has remained on this part of the brain.
The powerful molecular and genetic techniques employed in a variety of investigations made within the past two decades have greatly added to our knowledge of the neural control circuitry meditating food intake. Individual neuron phenotypes have been described as have intracellular signaling cascades and projection paths of relevance to defining control circuits and two key discoveries were made. Most notable was the finding that the obesity and hyperphagia of the obese (ob/ob) mouse was explained by the absence of a functional form of a peptide, leptin, made by white adipocytes (16). This single discovery transformed our view of the function of white adipose tissue from one of simple energy storage to the view that adipose tissue is endocrine in nature. In addition, the discovery of leptin ligand and its functional receptor completely revolutionized our thinking about the systems neuroscience of energy balance control. Simply put, the finding that injecting ob/ob mice with the functional form of leptin reversed their hyperphagia and thereby “cured” their obesity (16) was interpreted, in conjunction with other findings, to indicate that the leptin acting on its receptors (LepR aka ObRb) in the brain contributes significantly to the control of feeding (41–42). These findings led researchers to focus on CNS sites of leptin signaling in their search for insights into the neural control of energy balance and meal size. LepR-bearing neurons were described in many brain regions but those in the arcuate hypothalamic nucleus (ARC) received the greatest attention. A second major discovery was the central melanocortin system, its functional connection to leptin signaling, and its association with two types of ARC neurons. This discovery rests on a constellation of findings (43). One identified a subpopulation of LepR-expressing ARC neurons that expressed proopiomelanocortin (POMC), the precursor of α-MSH, an agonist of the melanocortin-3 and -4 receptors (MC3-R and MC4-R). A second finding identified a separate set of LepR-expressing ARC neurons that express agouti-related peptide (AgRP), an endogenous antagonist of the MC3-R and MC4-Rs. Exogenous delivery of these MC3/4-R ligands was observed to have potent and bidirectional effects on food intake and overall energy balance regulation. Taken together with the functional effects of CNS leptin signaling, the discovery of a central melanocortin system dramatically altered the systems neuroscience of energy balance and promoted a laser-like focus on ARC neurons that has guided a majority of subsequent experimentation.
The arcuate perspective, depicted to varying degrees in the widely viewed cartoon diagrams of M. W. Schwartz and colleagues (7) and M. Cowley and colleagues (44), highlights the role of arcuate neurons in the energy balance responses to leptin by emphasizing the downstream mediation of the melanocortin neuropeptides, as well as roles for the neuropeptide Y (NPY) and cocaine and amphetamine related transcript (CART) neuropeptides that are co-expressed respectively with AgRP and POMC. These depictions also highlight the prominent position of axon projections from ARC neurons to other regions of the brain with relevance to feeding behavioral control, including the hypothalamic paraventricular nucleus (PVH) and the NTS in the dorsal medulla. The arcuate perspective has accommodated new data that shed light on the roles of particular cell signaling cascades in leptin-responsive ARC neurons such as adenosine monophosphate kinase (AMPK), 44/42 mitogen-activated protein kinase (44/42MAPK; previous referred to as ERK1/2), phosphoinositide-3 kinase (PI3K), suppressor of cytokine signaling 3 (SOCS3), and signal transducer and activator of transcription 3 (STAT3) e.g., (45–49). These discoveries and related concepts were generative and led to a large number of investigations that yielded important details of a circuit diagram for the neural control of food intake. With time however, it has become clear that the arcuate perspective, while important, is incomplete as it does not: [1] account for responses to relevant signals and integrations performed by other hypothalamic, as well as extra-hypothalamic neurons whose contribution to food intake control has been gradually emerging, and [2] directly connect the output of ARC neurons with feeding behavior effector circuitry that include the hindbrain neurons and local hindbrain projections that control the ingestive consummatory behaviors (licking, swallowing and chewing) that generate or terminate food intake and thereby determine meal size (50–52). Clearly, it will take additional time and effort to more fully define the circuit diagram (e.g., key integrating neurons, intracellular signaling cascades contributing to the integrations, neurochemical mediators, relevant afferent signals and behavioral effector pathways) for the control of meal size.
Much of the balance of this review grapples with some of the unresolved issues in the neural control of meal size and focuses on a different neural substrate - NTS neurons in the caudal brainstem – with a focus on the relevance of leptin signaling, satiation signal processing and their common influence on the excitability and output of these neurons. Also considered are the NTS signaling cascades (e.g., AMPK, 44/42MAPK) and the downstream neurochemical mediation of meal size effects of leptin, e.g., melanocortin and oxytocin, and the neurochemical mediators of the meal size effects of satiation signals vagally transmitted to NTS neurons.
Caudal Brainstem Circuits are Sufficient for Meal Size Control
As stated earlier, vagal afferent signals are first processed centrally by the neurons of the NTS; these neurons project to and influence other neurons of the ascending visceral afferent pathway that include hypothalamic and other basal forebrain neurons. Despite the direct neural connection between the vagal afferent processing NTS neurons and the pre-oral motor neurons of the parvocellular and intermediate reticular formation (PCRt, IRt) that contribute to determining whether the oral motor response elements of ingestion or those of rejection are elicited (52–53), the perspective of the field has been to consider the hypothalamus to caudal brainstem communication and hypothalamic neuronal processing of GI-initiated satiation signals as a requirement for the control of meal size.
This view is challenged by experiments that eliminate hypothalamic-caudal brainstem communication and thereby the contribution of hypothalamic processing, integration and efferent commands (descending projections from hypothalamic neurons) by the complete surgical transection of the brain at the level of the diencephalic-mesencephalic border. When such experiments show that exposure to the same stimuli drives comparable responses from supracollicular decerebrate and neurologically intact control rats, it is concluded that – hypothalamic processing is not a neural requirement and that neural circuits within the caudal brainstem are sufficient for mediating the observed response[s] (54). Rodent licking is a highly stereotyped and rhythmic response; rats licking liquid diet emit on average 6 licks/sec. Licking functions to transport food intraorally into position to trigger swallowing. In response to oral infusion of liquid diet (at a rate matched to the voluntary ingestion rate) the pattern of taste-driven, oral motor responses and the frequency and patterning of rhythmic licking and swallowing observed in chronically maintained decerebrate rats and intact brain control rats are comparable (54–55). Further support for the conclusion that the caudal brainstem is sufficient for oral sensory-motor integration is found in human clinical data that show that oral motor responses elicited by oral application of taste chemicals are identical in intact and anencephalic human neonates (e.g., brains not developed beyond the midbrain) (56). A similar approach was taken to address whether the isolated caudal brainstem is sufficient for the control of meal size. Those experiments added another variable – GI satiation signal[s] – and examined whether in the presence of signals of this type the meal size (amount of an orally infused liquid diet that is ingested) of intact control and decerebrate rats is comparably affected relative to a control condition. The GI stimuli examined, in separate experiments, are intragastric infusion of liquid diet, intraperitoneal (ip) injection of the duodenal peptide cholecystokinin (CCK) or ip injection of exendin-4 (Ex-4), and a stable ligand of the glucagon-like-peptide-1 receptor (GLP-1R). Intragastric nutrient infusion stimulates a broad range of satiation signals that include gastric distension, intestinal nutrient signaling and the endogenous secretion of CCK, GLP-1 and other intestinal hormones and neurotransmitters. Each GI stimulus treatment examined reduced the meal size of intact and decerebrate rats comparably (57–59).
Caudal brainstem mediation of the meal size effects of glucagon-like peptide-1
GLP-1, released from ‘L’-type enteroendocrine cells in the distal small intestine in response to nutrient stimulation, acts on peripheral GLP-1R (9) The feeding and gastric emptying inhibition triggered by peripheral injection of the GLP-1R agonist Ex-4 is blocked by vagotomy or by vagal C-fiber de-afferentation with capsaicin treatment (60–61) suggesting that peripherally administered GLP-1R agonists do not produce their functional effects via direct activation of central GLP-1Rs but rather activate central circuits through vagal transmission (62–66). Logically then, a role for neural processing from any of several structures in the ascending visceral afferent pathway (defined above) contributes to the mediation of the meal size and gastric emptying responses. A determination of which of these implicated structures is necessary for GLP-1R-mediated response production had not been made. Despite this limitation however, it is often asserted that hypothalamic/forebrain processing is critical for mediating the effects of peripheral GLP-1R stimulation (67–72). Figure 1 shows that decerebrate rats lacking all neural communication between the caudal brainstem and hypothalamus display the same dose-related suppression of intake and gastric emptying rate for the peripherally applied GLP-1R agonist as that observed in neurologically intact control rats. These results show that NTS and/or PBN processing of vagal afferent signals and their connections to caudal brainstem interneurons and oral motor response circuitry is sufficient to mediate the meal size effects of peripheral GLP-1R activation. Thus, the neuronal activation of PVH, ARC and other hypothalamic/forebrain neurons by peripheral GLP-1R treatment observed in intact rats (67–72), while of potential importance to mediating response production in intact-brain rats, is not required for the suppression of intake and gastric emptying rate observed in decerebrate rats.
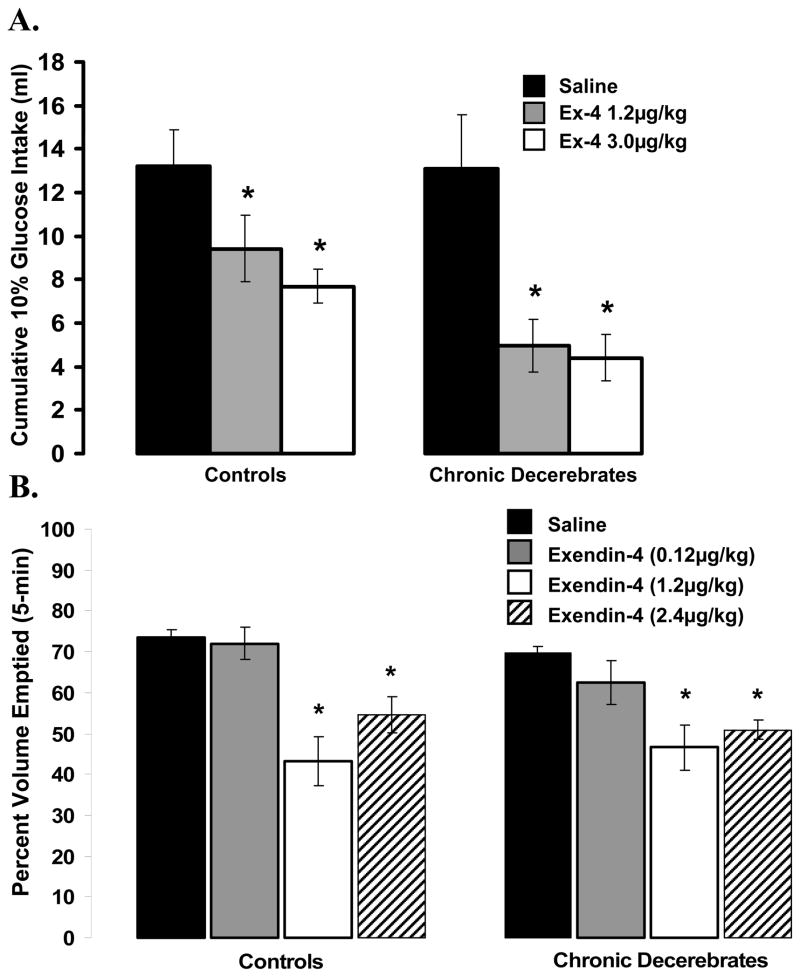
Figure 1.
[Top] Intraoral glucose (10%) intake for chronic decerebrate (CD) and control rats did not differ as a function of the neurological condition of the rat. Intraperitoneal administration of the GLP-1R agonist exendin-4 [Ex-4] at 1.2 and 3.0 μg/kg suppressed intake significantly in both control and CD rats, compared with respective vehicle intakes. The suppression of intake by peripheral Ex-4 was not statistically different between control and CD rats, indicating that forebrain processing and forebrain-caudal brainstem communication is not necessary for the intake suppression triggered by peripheral GLP-1 receptor stimulation. *, P < 0.05 from respective vehicle.
[Bottom] For control and CD rats, ip administration of Ex-4 (1.2 and 2.4 μg/kg) significantly suppressed 5 min gastric emptying of 0.9% saline, compared with vehicle in similar fashions. The gastric-emptying rates for the vehicle condition for control and CD rats did not statistically differ.
Collectively, the comparable meal size reducing effects observed for intact control and decerebrate rats in response to the satiation signals examined support the conclusion that caudal brainstem neurons integrate food-related GI and oral afferent signals and engage local, caudal brainstem effector circuitry to control meal size. These data do not logically discount a possible role of hypothalamic neurons (or other forebrain neurons for that matter) in the processing of direct (satiation) and/or indirect (e.g. adiposity) signals and the influence of their axonal projections via descending mono- or poly-synaptic projections to caudal brainstem effector circuits. Data of relevance to a hypothalamic contribution to meal size control will be discussed later in this review.
A Population of NTS Neurons Responsive to GI Satiation Signals and Leptin May Provide an Explanation for Leptin’s Suppressive Effects of Meal Size
NTS neurons process satiation signals and project to oral motor control neurons in the parvocellular and intermediate reticular formation
The data from decerebrate experiments provide a rationale for focusing attention on the contribution of NTS neurons to a more anatomically distributed model of the neural control of meal size than that offered by the arcuate perspective. The NTS is a made up of a number of cytoarchitectonically defined subnuclei that are located in the dorsal medial medulla, ventral to the area postrema and the 4th ventricle and dorsal to the autonomic and somatic efferent systems of the dorsal motor nucleus of the vagus nerve, PCRt, IRt, and hypoglossal nucleus. Neurons within the medial, intermediate, central, dorsomedial, gelatinosus, and commissural NTS subnuclei are activated by satiation signals arising from GI stimulation (28, 73–79). Different NTS subnuclei within rostro-caudally arrayed NTS divisions process oral chemical (taste) and GI afferent signals; there is no evidence that there is convergence of taste and satiation signal processing within a given population of NTS neurons (80). These anatomically disparate NTS neurons that process taste and GI signals do, however, converge onto common target neurons in the subjacent IRt and in the PBN. Distinct NTS neurons processing taste or hepatic afferents project to common neurons in the caudal dorsomedial region of the PBN. Combined taste and hepatic stimulation produces additive effects on the neurophysiological response of these PBN neurons (80–81). The output of taste processing NTS neurons and hepatic sensory processing neurons also converge onto common neurons of the IRt. Both IRt and PCRt neurons appear to be of direct relevance to meal size control as: [1] results from parenchymal infusion of GABA agonists or glutamate antagonists into these and other neurons just medial to this region in the PCRt reveal that these neurons are critical in determining whether the oral motor response elements of ingestion (e.g, licking and swallowing) or those of rejection (e.g., gaping) are elicited and; [2] the taste-processing rostral NTS neurons that project to PCRt also project to IRt thereby similarly implicating these IRt neurons in ingestive consummatory behavior production (50–51, 54).
Leptin has meal size effects that are mediated by an interaction with the neural processing of GI satiation signals in medial NTS neurons
Only very recently has attention been directed to evaluating the contribution of other, extra-ARC and even extra-hypothalamic leptin receptors (82–86). Results make clear that LepR-bearing ventromedial hypothalamic nucleus (VMN) and ventral tegmental area (VTA) neurons, like those of ARC, contribute to leptin’s effect on food intake (83–84). The mechanism underlying the feeding effect resulting from altered leptin signaling in VTA or VMN, however, is as yet undefined.
For a variety of reasons our attention was drawn to leptin signaling in NTS neurons for an explanation of leptin’s effects on feeding. First, NTS neurons express LepR and intra-NTS parenchymal delivery of leptin, at doses that are ineffective when delivered to the adjacent 4th ventricle (intracerebroventricular, icv), reduces food intake and body weight (87). Second, behavioral analyses show that the intake reduction triggered by leptin treatment (either systemic or icv) is achieved by reductions in meal size but does not result from alteration in meal number or frequency (88–90). Complementing this perspective are data showing that Koletsky rats that lack leptin receptor signaling take larger-sized meals (91). These data are consistent with the view that a meal size mechanism accounts for the food intake reducing effects of central leptin signaling. Of great interest, the same meal size mechanism accounts for the intake suppressive effects of satiation signaling (24, 92). Third, Smith’s hypothesis - that adipose signals like leptin should be considered indirect controls of meal size that induce their behavioral effect by modulating the neural processing of satiation signals (23) – finds support in the results of a study by Schwartz and Moran (76). These authors measured the effect of different levels of gastric distention on the neurophysiological response of medial NTS (mNTS) neurons that receive afferent terminals of vagal mechanosensory neurons. They determined the function describing the effect of different levels of gastric distention on the neurophysiological response of mNTS neurons under control conditions and showed that leptin delivered to the forebrain ventricle (icv) amplified the neurophysiological effect of gastric distention. Under leptin, the dose-response function was dramatically left-shifted compared to its position under vehicle conditions. Thus, in response to CNS leptin delivery, reduced levels of gastric distention produced greater neurophysiological response. These are very interesting data and are complemented by other findings showing that forebrain icv delivery of leptin potentiates the inhibitory effects of GI stimulation on intake (76, 93–95). From a systems neuroscience perspective a logical follow-up question is: What is the relevant CNS site (or sites) of leptin signaling that mediates the amplified response observed in these experiments?
While ARC LepR expressing neurons are the assumed target of forebrain ventricular (lateral or 3rd icv) leptin injection, this mode of delivery leaves unresolved which of the LepR-bearing neurons stimulated by exogenous leptin mediate the observed effects. Ligand could bind to LepR in various hypothalamic nuclei but could also access more caudal receptor-bearing sites due to the caudal flow of CSF (96). As LepR are expressed in NTS, and as functional effects of leptin are obtained from NTS leptin delivery (87), we hypothesized that the amplification of mNTS neurophysiological response of gastric distention and the intake inhibitory effects of GI stimulation in response to forebrain ventricle leptin delivery was mediated by leptin signaling in the mNTS neurons themselves. We performed a series of experiments to explore this and related questions.
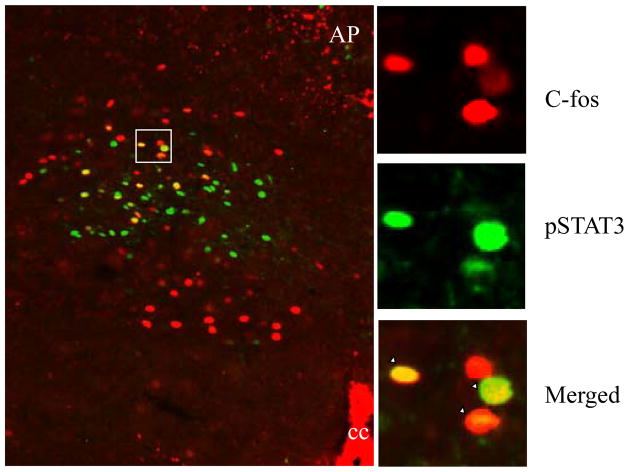
In collaboration with Christian Bjorbaek’s laboratory, we undertook a detailed anatomical analysis of the NTS subnuclear expression of LepR using ip leptin-induced phosphorylated STAT3 (pSTAT3) immunoreactivity (IR) and showed that pSTAT3-IR was limited to a portion of a single NTS subnucleus, neurons of the mNTS, restricted to the level of the area postrema (28). We were struck by this expression pattern, as mNTS neurons were also known to process GI vagal afferent signals arising from ingested food acting on the stomach and intestine (73, 76, 78). Therefore, we examined whether leptin signaling (ip leptin-induced pSTAT3-IR) and neuronal activation [measured by Fos-like immunoreactivity (Fos-LI)] following physiological levels of gastric distension occurred in the same mNTS neurons. Figure 2 uses double-fluorescent immunohistochemistry (IHC) for pSTAT3 (green cells) and for distension induced Fos-LI (red cells) to reveal that 39% of leptin-responsive mNTS neurons also respond to gastric balloon distension (yellow cells in merged image).
Figure 2.
A significant proportion of leptin-responsive cells respond to gastric distension. A representative merged microphotograph of double IHC (P-STAT3 and c-Fos) from gastric distension combined with leptin-treated rats is shown on the left. On the right are shown high magnifications (top, P-STAT3 green fluorescence IHC; middle, c-Fos red fluorescence IHC; bottom, merged microphotograph from the double IHC) of the area marked on the left. Examples of double-labeled cells are shown in yellow. cc, Central canal. Scale bars, 200 μm.
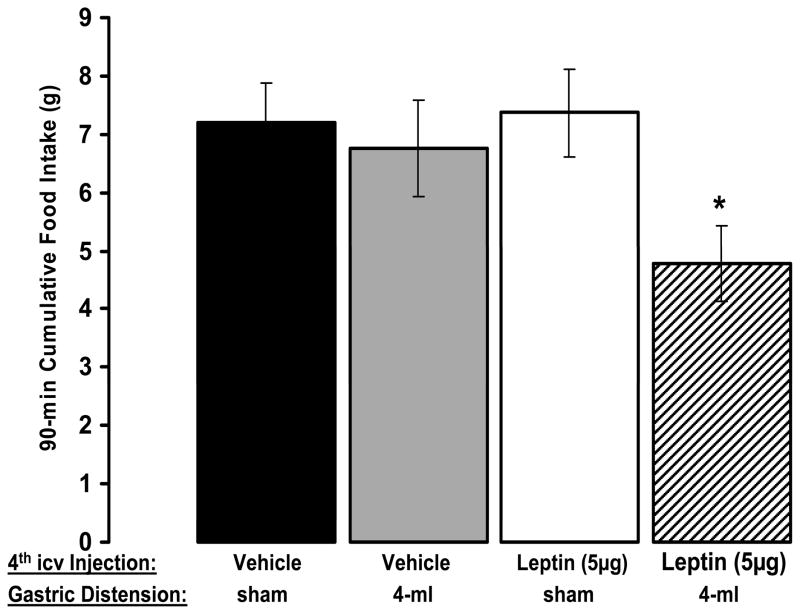
This experiment identified a population of neurons driven by both leptin and by GI signals. Next, a paradigm was developed to examine whether hindbrain leptin delivery amplifies the intake inhibitory effects of gastric distension as had been shown with forebrain icv leptin injection. Dose-response studies determined a 4th-icv leptin dose and a volume of gastric distension level that were sub-threshold for intake suppression. Figure 3 illustrates that in combination these sub-threshold treatments significantly suppress food intake when compared to the effects of vehicle treatment, or to the presentation of leptin or distension alone. Combined with the IHC findings, these results show that: [1] NTS leptin-responsive neurons are located exclusively in the mNTS at the level of the area postrema, a key vagal afferent projection zone of the GI system; [2] a significant proportion of leptin-responsive neurons in the mNTS are activated by gastric distension; and [3] hindbrain leptin delivery is sufficient to potentiate the intake-suppressive effects of an otherwise ineffective volume of gastric distension.
Figure 3.
Leptin delivered to the hindbrain is sufficient to amplify the intake-suppressive effects of an otherwise ineffective volume of gastric distension. Cumulative chow intake was not affected by either 4-ml gastric distension or icv leptin treatment alone at 30, 60, or 90 min. However, when combined, gastric distension and leptin significantly suppressed cumulative intakes at 60 and 90 min compared with results from the vehicle/sham-distension condition. *, P < 0.05.
Intestinal nutrient stimulation, like gastric distention, drives the neurophysiologic response of neurons in the mNTS and elicit vagally mediated satiation signals (97). In a follow-up study, a concentration (kcal/ml)-effect curve for duodenally infused Intralipid (triglyceride emulsion) on intake suppression was determined. The combination of an intake-subthreshold concentration (0.33kcal/ml) of this intra-duodenal infusate and a behaviorally ineffective 4th icv leptin dose resulted in a significant suppression of intake (Hayes and Grill, unpublished). Overall, these findings show that hindbrain leptin signaling potentiates the intake suppressive effects of both gastric and intestinally derived satiation signaling that activate mNTS neurons. These findings are consistent with Smith’s view of the relationship between indirect (leptin) and direct (GI satiation) control of meal size and define mNTS neurons as a site of such signal integration.
Hindbrain AMPK Signaling Contributes to the Intake Suppressive Effect of Leptin and May Mediate the Integration of Leptin and Satiation Signaling in NTS neurons
Having identified a population of neurons that responds to both leptin and GI-triggered satiation signals and shown that leptin signaling in these neurons amplifies the intake inhibitory effects of gastric distention and of intestinal nutrient stimulation we are proceeding in several directions to further develop support for the hypothesis mNTS neurons contribute to the neural control of food intake.
The potentiation of the intake inhibitory effects of GI satiation signals by leptin is likely mediated by a signaling pathway in mNTS neurons that is common to the intracellular signaling cascades of leptin and GI satiation signals. Adenosine monophosphate-activated protein kinase (AMPK) is described as a fuel sensing enzyme as its activity is regulated by the cellular AMP/ATP ratio and by upstream kinases (45, 98). CNS AMPK activity is viewed as an important element in energy status assessment and as a mediator of the anorectic effects of leptin. As such, AMPK activity in CNS nuclei has become a major focus of interest in the neuroscience of energy balance regulation (48, 98–99). To date, studies have focused on the functional effects of AMPK activity in neurons of the ARC and PVH hypothalamic nuclei. Hypothalamic AMPK activity is increased by food deprivation (48, 98, 100) and by the energy reducing effects of 2-deoxy-D-glucose or insulin treatment (99, 101–102). In the ARC and PVH, the elevation in AMPK activity induced by food deprivation is inhibited by treatment with leptin, or by the effects of refeeding (48, 98). Furthermore, it appears that the inhibition of hypothalamic AMPK activity by leptin is necessary for leptin’s intake and body weight-suppressive effects, as the elevation of constitutive AMPK activity blocks the anorectic effects of hypothalamic leptin treatment (48). Refeeding has many sequelae and it is not clear which contribute the effect on AMPK activity. Direct GI vagal afferent signals, triggered by the presence of ingested food within the GI tract following a period of deprivation may be among “refeeding” sequelae that contribute to the inhibition of CNS AMPK activity.
AMPK is expressed in brain regions other than the hypothalamus, including those also implicated in the neural control of energy balance, but the contribution of extra-hypothalamic CNS AMPK activity to energy balance control has not attracted attention (103). While a role for AMPK activity in NTS neurons in the control of food intake is unexplored, it is well known that treatments that reduce energy availability in these neurons trigger behavioral, as well as endocrine and autonomic responses (96, 104–110). We performed a series of experiments to examine the following set of hypotheses: [1] energy status alters AMPK activity in NTS-enriched hindbrain (dorsal medial medulla tissue block) lysates, [2] inhibition of NTS AMPK activity affects food intake and body weight gain, and [3] hindbrain AMPK activity mediates the intake reducing effect of hindbrain leptin treatment (111).
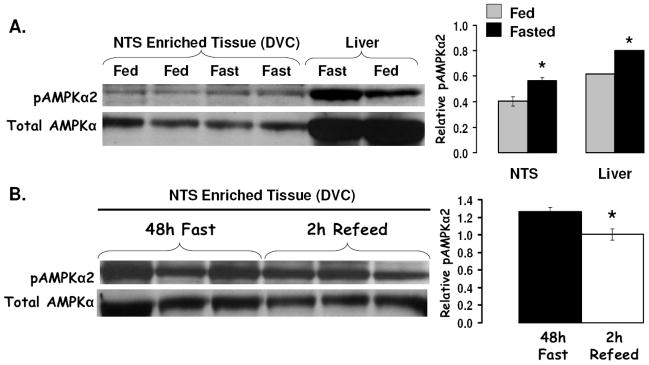
Figure 4a shows that food deprivation significantly increased pAMPK (pAMPK-Thr 172) levels in both NTS-enriched and in liver lysates. A 2h period of refeeding (food access following a 48h food deprivation) reduced pAMPK levels in NTS-enriched hindbrain lysates relative to values from 48h food deprived rats (Figure 4b). AMPK activity assays provided complementary data and confirmed that food deprivation (48h) significantly increased AMPK activity in NTS-enriched and in hypothalamus-enriched lysates similarly compared to AMPK activity levels in ad libitum fed rats. Pharmacological inhibition of hindbrain AMPK activity with 4th icv application of a selective inhibitor of AMPK, compound C, suppressed food intake and body weight gain. Figure 5 shows that direct parenchymal injection of compound C (with a dose that was ineffective when delivered to the ventricle) to the mNTS at the level of the AP reduces food intake and body weight. Application of the same dose of compound C to the mNTS 1mm rostral at the level of the 4th ventricle was without effect on food intake and body weight gain. To determine whether the intake suppressive effects of hindbrain leptin delivery depended on its alteration of hindbrain AMPK activity, a pharmacological strategy was used. First, the effects of a dose of leptin that suppressed food intake was examined and shown to significantly decrease pAMPK levels in the NTS-enriched hindbrain tissue (Figure 6b). The hindbrain ventricular dose of leptin did not affect hypothalamic pAMPK levels, confirming the site specific effects of 4th icv injection (Figure 6c). Next, in a complementary behavior study, a dose of the AMPK promoter AICAR (shown to be without effect on food intake) was administered to the hindbrain with leptin to examine whether hindbrain leptin’s intake suppressive effects were mediated by the inhibition of AMPK activity in the dorsomedial medulla. Figure 6a shows that AICAR pretreatment completely blocked the intake suppressive effect of leptin but had no effect of its own at the dose tested. This pattern of results is consistent with the hypotheses (enumerated above) that motivated these experiments.
Figure 4.
A) Compared to rats fed ad libitum, 24h food deprivation increased pAMPKα levels in NTS-enriched tissue (dorsovagal complex, DVC). These data indicate that pAMPKα levels in DVC and liver tissues are similarly responsive to energy status, with food deprivation increasing pAMPKα in both the liver (control) and NTS by approximately 25%. (B) Elevated pAMPKα levels in NTS-enriched tissue of 48h food deprived rats is reduced following a 2h reefed. Representative immunoblots for total AMPK and pAMPKα are shown. * = P< 0.05.
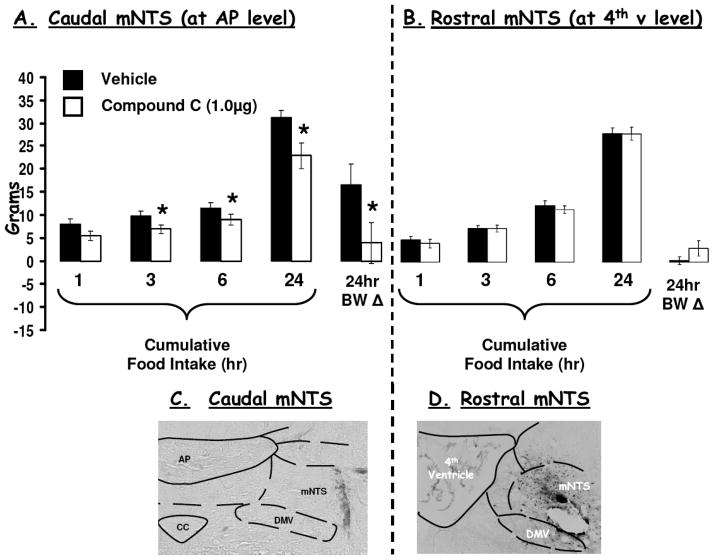
Figure 5.
(A) Decreasing AMPK activity with Compound C 9a dose that was ineffective delivered to the ventricle) injected into the caudal mNTS, at the AP level, significantly suppressed food intake at 3h, 6h and 24h, as well as 24h body weight gain compared to intakes and body weight following vehicle injections. (B) By contrast, when compound C was delivered to the rostral mNTS (at the 4th ventricle level) neither food intake nor 24h body weight gain was affected. * = P<0.05 from respective vehicle intakes and body weights.
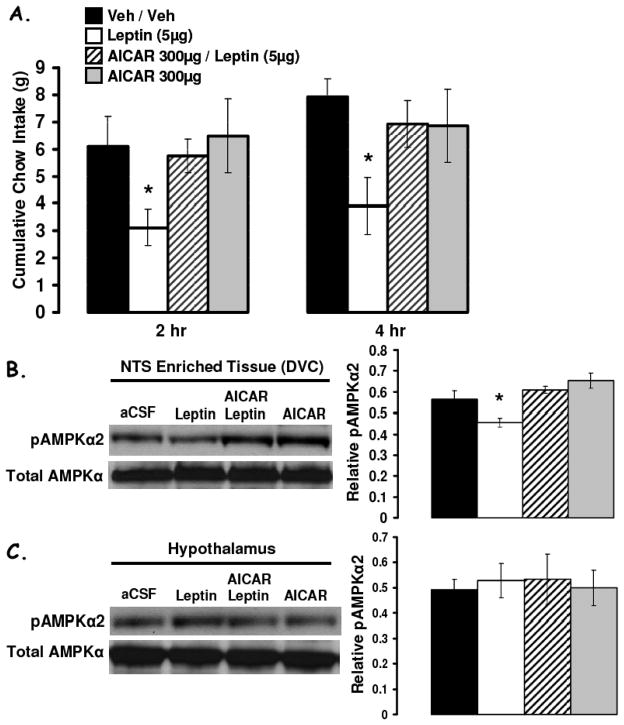
Figure 6.
(A) Increasing hindbrain AMPK activity (4th icv AICAR) reversed the suppression of cumulative food intake at 2h and 4h following 4th icv leptin administration. (B) In ad libitum fed rats, 4th icv administration of leptin (5μg) suppressed pAMPKα levels in NTS-enriched tissue (DVC) 2h after 4th icv administration compared to control injections. This suppression in pAMPKα levels in the DVC was reversed by 4th icv administration of AICAR, at a dose that was without effect on its own. (C) Conversely, no alterations in pAMPKα levels were observed in hypothalamic lysates by 4th icv administration of leptin, AICAR, or their combination, thus indicating a hindbrain site of action for the 4th icv administered compounds.* = P<0.05 from respective vehicle intakes.
One of the key conclusions from these experiments is that the food intake inhibitory effect of hindbrain leptin signaling is mediated at least in part, by AMPK activity. This result is consistent with that of Minokoshi et al (48) who show that the intake suppression that follows hypothalamic leptin signaling is AMPK mediated. These results add to the literature that have begun to consider a link between brain pAMPK activity and the control of energy balance and, in addition, broaden the neuroanatomical basis for this contribution. Other signaling pathways downstream of LepR activation including 44/42 MAPK, PI3K, SOCS3, and STAT3 are also under investigation as contributors to leptin’s control of energy balance (46–47, 49, 98, 112).
Endogenous Leptin Signaling in the NTS Contributes to the Meal Size Effects of CCK and to the Control of Baseline Food Intake
The NTS leptin data reviewed thus far – that targeted exogenous delivery of leptin to a population of LepR-expressing mNTS neurons reduces food intake, reduces pAMPK activity, and amplifies the meal size reducing effects of satiation signals – suggests that leptin signaling in this brain region is relevant to intake and meal size suppressive action(s) triggered by leptin. To probe the contribution of endogenous leptin signaling in neurons of the NTS and adjacent AP to the meal size effects of satiation signals and to the control of food intake more broadly, we employed RNA interference (RNAi) technology to knock down leptin receptor expression in the mNTS and adjacent AP. Ralph DiLeone’s group (83) were the first to employ RNAi-mediated chronic LepR knockdown in a rat model to evaluate the functional contribution of LepR signaling in VTA neurons. Through bilateral stereotaxic delivery of adeno-associated virus (AAV) mediated incorporation of sh-LepR mRNA or scrambled control sh-RNA these authors showed that RNAi-mediated knockdown of LepR in the VTA parenchyma yielded a feeding phenotype - increased intake of chow and high-fat diets. Here, we applied that strategy to assess the endogenous function of the LepR-bearing NTS neurons in collaboration with the DiLeone and Bence labs (113).
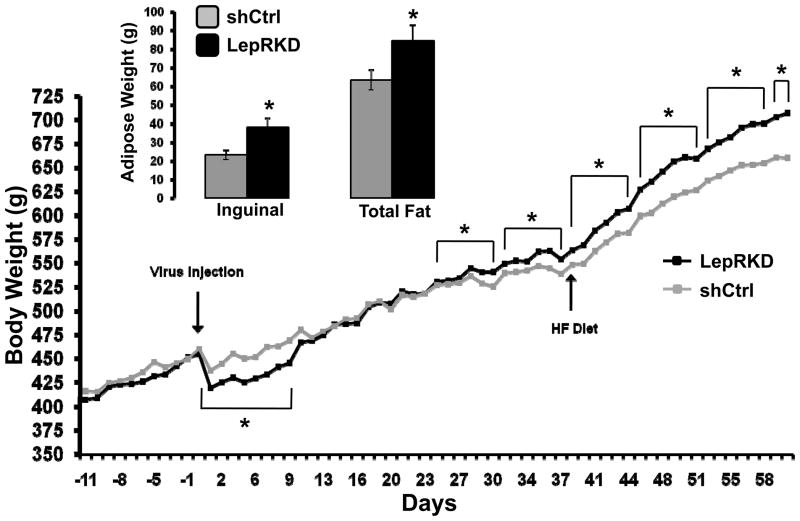
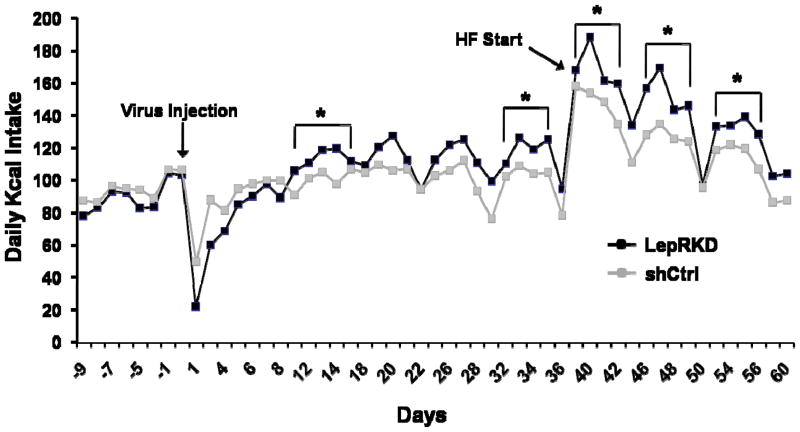
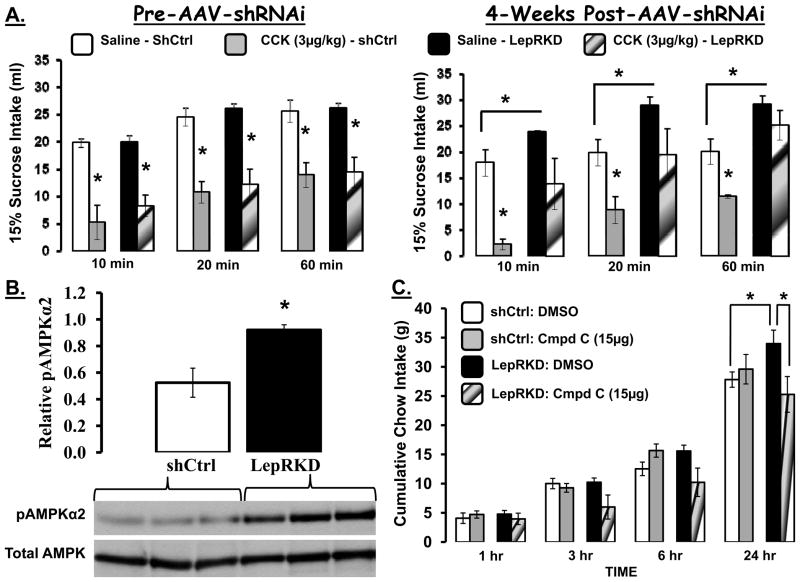
At the completion of these experiments micropunches of the AAV-shLepR-infected mNTS/AP tissue [identified by visualization of enhanced green fluorescent protein (EGFP; co-expressed by the AAV)] were taken; representative qPCR analysis revealed an approximate 40% decrease in LepR mRNA in mNTS/AP neurons infected with AAV-shLepR compared with neurons infected with AAV- shCtrl. During maintenance on chow diet beginning 3.5 weeks post-viral delivery average weekly body weight for mNTS LepRKD rats was significantly greater than that of shCtrl rats (Figure 7). This increased body weight gain for the LepRKD rats persisted when rats were switch to HF diet (60% Kcal from fat) maintenance. Analysis of white adipose tissue (WAT) depots showed that the WAT mass was significantly larger in mNTS/AP LepRKD rats compared to shCtrl rats and that an increase in inguinal fat mass alone accounted for the overall increase in total WAT mass (Figure 7 inset). Contributions to the observed body weight arising from alterations in food intake and or energy expenditure effect were evaluated. Analysis of core temperature and spontaneous activity for mNTS-directed AAV-LepRKD and –shCtrl rats revealed no group differences in either energy expenditure parameter following viral delivery compared to within-subject pre-viral delivery baseline values. By contrast, as seen in Figure 8, beginning two weeks after viral delivery daily and averaged weekly energy intakes of the LepR-KD rats were significantly greater than the food intake of sh-Ctrl rats maintained on standard chow or on high-fat diet.
Figure 7.
Cumulative body weight of chow-maintained LepRKD and shCtrl rats pre- and post-mNTS/AP directed AAV delivery. * = P< 0.05 for bracketed weekly averages. Inset graph shows inguinal and total WAT mass for LepRKD and shCtrl rats. * = P<0.05.
Figure 8.
Daily food intake (Kcal) of rats with AAV-RNAi mediated knockdown of NTS leptin receptors (bilateral injection of sh-LepR – Lep KD) or rats that received NTS control sh-RNA injections (sh Ctrl). Beginning two weeks after bilateral intraparenchymal NTS injections daily food intake and averaged weekly energy intakes of the LepKD rats were significantly greater than the food intake of sh Ctrl rats maintained during standard chow or high-fat diet maintenance.
To explore the hypothesis that the observed increase in food intake following reduced NTS leptin signaling results from reduced sensitivity to the intake suppressive effects of GI satiation signals, we probed the intake suppressive effect of ip CCK injection on the intake of a preferred liquid food (15% sucrose) in these groups of rats weekly over the multi-week course of the experiment. Figure 9A shows that sucrose intake for the vehicle (saline) ip injection condition was identical for both groups of rats at baseline (prior to NTS AAV-RNAi injections). At baseline, ip CCK (3μg/kg) yielded a short latency and marked suppression of intake that was equivalent for both groups. Four weeks following AAV delivery, during the period of chow diet maintenance two effects emerged: [1] NTS-LepR-KD rats showed a reduced sensitivity to the intake suppressive effects of CCK - no significant suppression of sucrose intake observed in sh-LepR mRNA rats compared to NTS-sh-Ctrl treated rats and [2] sucrose consumption for the vehicle-injection condition was greater in LepR-KD rats compared to rats with intact NTS leptin signaling (sh-Ctrl). The reduced sensitivity to the intake suppressive action of the satiation signal CCK, and the overall increase in energy intake observed when leptin receptor signaling was knocked down for NTS/AP neurons (only one of many LepR-expressing nuclei) provide significant and direct support for a role of endogenous NTS leptin signaling in the in the normal control of food intake (113).
Figure 9.
A. Sucrose intake (15%) for rats injected ip with saline or with CCK. On left - sucrose intake of both groups during the week of virus injection is comparable under saline; CCK suppresses intake of both groups comparably. On right- 4 weeks after virus injection Lep KD group ingest more sucrose under saline injection conditions than controls and CCK fails to suppress the intake of the Lep KD group compared with the sh Ctrl group. B. LepRKD rats showed increased pAMPKα2 levels in mNTS/AP micropunched tissue compared to shCtrl rats under ad libitum fed conditions. Representative immunoblots for total AMPK and pAMPKα2 are shown. Relative pAMPKα2 = the ratio of pAMPKα2 to total AMPK. * = P< 0.05. C. Cumulative chow intake for LepRKD and shCtrl rats following 4th icv delivery of compound C (15μg) or vehicle (DMSO). * = P< 0.05.
To examine whether NTS/AP LepR knock down altered basal hindbrain AMPK activity (ad libitum chow fed rats) and that such a change could contribute to the observed hyperphagia immunoblot analyses of phosphorylation of AMPKα2 (pAMPKα2) and total AMPKα levels from tissue lysates of mNTS and AP were compared in the two groups seven weeks post-viral delivery. Figure 9B shows significant elevations of basal pAMPKα2 in rats with LepR knockdown in mNTS/AP neurons compared to shCtrl rats; total AMPKα levels were equivalent. To determine whether this basal elevation in mNTS/AP pAMPKα2 levels in mNTS-directed AAV-LepRKD rats may have contributed to the chronic hyperphagia of these rats, the feeding effects produced by pharmacological inhibition of hindbrain AMPK activity by 4th icv delivery of compound C was tested. Figure 9C shows that daily (24h) chow intake for mNTS/AP LepRKD rats was significantly greater than that of shCtrl rats following 4th icv vehicle administration confirming the hyperphagia discussed above. Fourth icv administration of compound C, at a dose subthreshold for effect in shCtrl rats, significantly suppressed 24h food intake in LepRKD rats to a level equal to the intake of shCtrl rats following 4th icv vehicle administration. We conclude that endogenous NTS LepR signaling is required for food intake and body weight control through a mechanism involving AMPK activity and NTS LepR interactions with GI satiation signal processing
ARC Leptin Signaling Results in Changes in Satiation Signal Sensitivity that may also Contribute to Leptin’s Effect on Meal Size
As mentioned above, other LepR-bearing nuclei appear to also contribute to leptin’s effect on feeding. Rats with leptin receptor knockdown targeted to the VTA (83), and mice with leptin receptor knockout restricted to the steroidogenic factor-1-positive neurons of VMN (85) are hyperphagic under certain conditions. Thus far, however, there is no indication that an alteration in satiation signal processing contributes to the observed hyperphagia in the VTA and VMN models. By contrast, a case for a connection between ARC leptin signaling and satiation signal processing has been made by Morton and colleagues (91). These authors show that the hyperphagia that drives the obesity of Koletsky (fak/fak) rats that lack leptin receptor signaling is characterized by [1] increases in meal size but not in meal frequency in comparison with FA/FA lean control rats and [2] reduced sensitivity to the intake inhibitory effect of a dose of CCK in fak/fak rats relative to lean FA/FA control rats.
This pattern of results is consistent with the perspective pursued above – that leptin signaling increases sensitivity to the intake inhibitory effects of GI generated satiation signals. The data raise the question – whether it is the absence of CNS leptin signaling globally or in specified neurons that contributes to the observed hyperphagia, increased meal size and insensitivity to CCK. The pattern of data from the Koletsky (fak/fak) rat is consistent with the data just reviewed above that highlight a role for NTS LepR signaling using a method that leaves leptin signaling intact in all brain areas other than the NTS. Morton and colleagues (91) employed a different strategy to examine the role for ARC region leptin receptor signaling in the meal size and CCK sensitivity response of fak/fak rats. These authors restored (rescued) leptin signaling in fak/fak rats in a region of the medial hypothalamus that contains neurons that normally (in FA/FA control rats) express LepR and presumably in others that do not. In response to bilateral injections of an adenovirus expressing either LepR (Ad-LepR) or a reporter gene (Ad-lacZ) into the ARC region of fak/fak rats, several phenotypic changes were observed.
In the Ad-LepR fak/fak rats compared with the Ad-lacZ group of fak/fak rats: [1] food intake and meal size significantly declined, [2] CCK sensitivity was enhanced and [3] CCK driven Fos-LI was increased in the NTS and AP. The authors conclude that the observed effects are mediated by hypothalamic leptin signaling via descending hypothalamic neuropeptide projections to NTS neurons. These descending projections were postulated to modulate the hindbrain circuits that control the intake-suppressive response to CCK and control for meal size. The specific neuropeptides proposed to mediate the effects of these descending projections - melanocortin and oxytocin - are discussed later in this review. Taken together with the data reviewed for NTS leptin signaling, these findings expand the neural perspective on the meal size action of leptin to include two anatomically distinct sites of leptin signaling – NTS and ARC neurons.
Caudal Brainstem Melanocortin Receptors Contribute to the Meal Size Effect of CCK
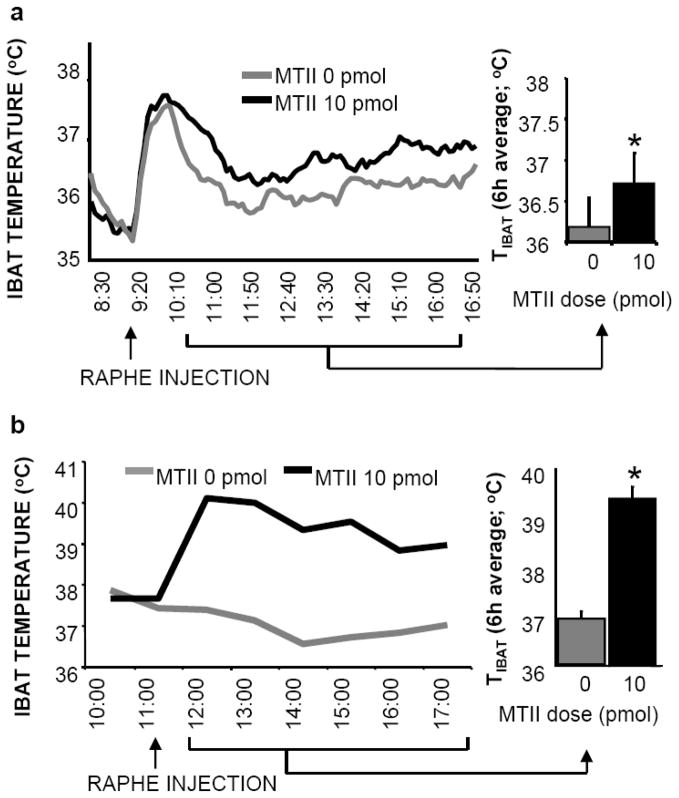
The action of melanocortin ligands on their CNS receptors (MC3/4-Rs) provides another critical component of the CNS control of energy balance; effects on food intake as well as energy expenditure are observed with MC3/4R ligand treatments. Polymorphisms and mutations of the POMC gene, as well as mutations of MC4-R are associated with human obesity (114–116). The induction of similar mutations in mice also results in hyperphagia, reduced energy expenditure, and an obese phenotype (117–118). While MC3/4-Rs are anatomically distributed throughout the neuraxis and NTS POMC neurons provide a source of endogenous agonist, the field is consistent in its emphasis on the role of ARC POMC neurons and hypothalamic MC3/4-Rs (particularly those in PVH) in mediating the CNS control of food intake and energy expenditure (119–120). MC4-Rs are expressed on neurons of the PBN, rostral ventrolateral medulla, and raphe pallidus; MC4-R expression in NTS is the densest in the rodent brain (121–122). We show that direct NTS injection of ventricle subthreshold, picomolar doses of MC3/4-R agonist (MTII) or antagonist (SHU-9119) reduces or stimulates food intake, respectively (123–124). To more fully define the functional contribution of caudal brainstem MC-Rs it is useful to expand the range of responses examined to include effects on feeding as well as on energy expenditure and sympathetic output. Direct MTII injection into PBN, rostral ventrolateral medulla and raphe pallidus suppresses food intake and in other studies, low-dose agonist delivery is shown to increase energetic and sympathetic responses (124–125). A comparison of the MTII-elicited energetic and sympathetic responses to hindbrain parenchymal delivery in supracollicular decerebrate and pair-fed intact control rats reveals that local caudal brainstem neural processing is sufficient for the MC4-R driven responses and that hypothalamic-forebrain neural processing is not required (125). Figure 10 shows that direct medullary raphe injection of MTII triggers brown adipose tissue thermogenesis in decerebrate and pair-fed intact control rats.
Figure 10.
10 pmol MTII stimulation of medullary raphe MC4-Rs produces hyperthermia in both intact rats (a) and chronic decerebrate rats (b). Line graphs represent across-rat average parameter measurements through the 8-h recording period. The bracketed time period on the line graph x-axis indicates the periods used in the histograms. Histograms in represent 6-h means + SEM. *, P < 0.05.
Other investigators have addressed the role of caudal brainstem MC-Rs in the intake inhibitory response to CCK and in the control of meal size more broadly. Fan and colleagues (126) performed a number of studies that collectively argue for the involvement of caudal brainstem MC4-R and NTS POMC neurons in the intake suppressive effect of satiation signals. They show that systemic administration of the duodenal peptide, CCK, activates (Fos-LI) NTS POMC-EGFP neurons (126–127). Consistent with this result are data showing that electrical activation of the solitary tract evokes excitatory postsynaptic currents (EPSCs) in NTS POMC-EGFP neurons (127). CCK treatment is effective in mice that are null for the MC3-R but fails to reduce food intake in MC-4 knockout mice (126). A complementary study contrasts the efficacy of 3rd icv or 4th icv delivery of SHU-9119. When delivered to the 3rd ventricle SHU-9119 does not alter the intake suppressive effect of systemic CCK but when delivered to the 4th ventricle SHU-9119 reverses the intake suppressive effect of CCK. These results help distinguish roles for hypothalamic-forebrain versus caudal brainstem MC-Rs (126). Berthoud’s lab has also examined the link between caudal brainstem MC-Rs and the intake inhibitory effect of satiation signals (128). These investigators reasoned that if melanocortin receptors contribute to the mediation of the intake effects of satiation signals, then pharmacological manipulations of caudal brainstem MC-Rs should yield effects on meal size but not on meal frequency (129–130). They found that the hyperphagia induced by a dose of the antagonist SHU-9119 applied to the 4th ventricle was characterized by increases in meal size and had no effect on meal frequency or meal duration; comparable results in the opposing direction were obtained for the intake reducing effect of 4th icv delivery of the agonist (MTII). Collectively, these findings make a strong case for caudal brainstem MC4-R mediation of the intake inhibitory effects of CCK and of the control of meal size more generally.
Recent data from Wan et al (131) add a new and potentially important element to the circuit diagram mediating the meal size effects of caudal brainstem MC4-Rs. Their study reveals that MC4-Rs are also expressed on the vagal afferent terminals (pre-synaptic) that project to NTS neurons in addition to NTS neurons themselves (post-synaptic). Reverse transcription-PCR analyses show that MC4-Rs, but not MC3-Rs, are expressed in the cell bodies of vagal afferents in the rat nodose ganglia. Whole-cell recordings from rat caudal brainstem slices show that thirty-two percent of the NTS neurons examined responded to bath application of MTII or α-MSH. Twenty-four percent of the NTS neurons increased and 8% decreased in the frequency, but not the amplitude, of spontaneous EPSCs. Support for the function of MC4-R expressed on vagal afferent terminals relative to those expressed on the NTS neurons comes from a surgical vagal deafferentation study that showed a reduction (from 24% to 9%) in the percentage of NTS neurons that responded to MTII with an increase in EPSC frequency compared with responses slices taken from rats with intact vagal afferents. These authors conclude that MC4-R signaling involves pre-synaptic enhancement of glutamate synaptic transmission and suggest that this mechanism, rather than postsynaptic activation of NTS neurons, may account for the melanocortinergic-induced decrease of food intake via enhancement of vagal afferent satiation signals from the GI tract (26). It will be important for future studies to distinguish between the contributions of the vagal afferent terminal pre-synaptic and NTS post-synaptic MC-4R signaling to food intake control.
Caudal Brainstem Melanocortin Receptors Contribute to the Intake Inhibitory Effect of Hindbrain Leptin Signaling
Data reviewed here support the conclusion that meal size control arises from leptin receptor signaling in NTS and in ARC and from caudal brainstem MC4-R signaling. MC-Rs are hypothesized to be downstream mediators of the effects of hypothalamic leptin signaling (120, 132–134). Support for this hypothesis comes from experiments that show that: leptin signaling in these neurons increases POMC gene expression (135–136) and pretreatment with MC-R antagonist attenuates the anorexic effects, as well as some of the sympathetically-mediated energetic effects of 3rd icv leptin delivery (120, 133). To examine whether caudal brainstem MC-Rs contribute to the mediation of energy balance effects of hindbrain-delivered leptin, two types of experiments were undertaken (132). The first sought to determine whether caudal brainstem application of leptin triggered a profile of energy balance effects that were similar to effects observed with 4th icv or caudal brainstem parenchymal injection of MTII. Experiments showed that 4th icv and intra-NTS injection of leptin or of MTII triggered similar intake and body weight effects (suppression) and energetic-sympathetic responses (hyperthermia and tachycardia) (87, 132, 137). The same pharmacological strategy – leptin receptor stimulation combined with MC3/4-R antagonist pretreatment - that yielded data (120, 134) providing key support for MC-R mediation of hypothalamic leptin signaling was applied in a 4th icv ligand delivery experiment. Figure 11 shows that SHU-9119, delivered at a dose lacking effect on any measured parameter, reversed the intake and body weight suppressive effects of 4th icv application of leptin and also reversed the hyperthermia triggered by leptin (132). These data are consistent with the hypothesis that caudal brainstem MC-Rs are part of the downstream mediation of hindbrain leptin-induced energy balance effects. The data parallel the relationship between hypothalamic leptin signaling and endogenous activation of MC-Rs.
Figure 11.
Hindbrain melanocortin receptors mediate hindbrain leptin induced a) hypophagia, b) body weight loss and c) hyperthermia. The histograms provide average values ± SEM. *, P < 0.05.
There is general agreement that the anatomical basis of the linkage between hypothalamic leptin signaling and endogenous melanocortin agonist release occurs via ARC-POMC neurons that express LepR, as leptin signaling in these neurons results in increased POMC gene expression (120, 133–136). Controversy, however, surrounds the question of whether NTS-POMC neurons are also leptin signaling neurons. Contributing to the controversy are differences between the ARC and the NTS subpopulations of POMC expressing neurons. Techniques that are used routinely to identify ARC-POMC neurons – immunohistochemistry for POMC products or in situ hybridization for POMC mRNA expression – cannot be used to unambiguously identify NTS POMC neurons (138). For this reason, other techniques are used to provide an alternate strategy or proxy for identifying NTS-POMC expressing neurons. In an experiment employing one line of POMC-EGFP mice [EGFP cassette introduced by standard techniques into the 5′ untranslated region of exon 2 of a mouse Pomc genomic clone containing 13 kb of 5′ and 2 kb of 3′ flanking sequences (44)] - systemic leptin treatment resulted in pSTAT3 IR in the NTS POMC-EGFP neurons that were located in the majority caudal to the area postrema (137). While this result is consistent with the hypothesis that NTS POMC neurons express LepR and are leptin signaling neurons, results from a similar experimental approach were different. The identical approach was applied to POMC-EGFP mice derived using a different strategy - [POMC-CRE mice (138) and Z/EG-EGFP reporter mice were crossed to produce POMC-EGFP mice] --- leptin treatment in this line of mice resulted in pSTAT3 IR expression in the medial NTS at the level of the AP, as described above, but POMC-EGFP NTS neurons that were located more caudally in these mice and no PSTAT3 IR was expressed in these NTS POMC-EGFP neurons (139). The conflicting data clearly require resolution. Whether or not NTS-POMC neurons are leptin signaling, the energy balance effects triggered by NTS leptin signaling (reviewed above) clearly involve endogenous melanocortin signaling via caudal brainstem MC-Rs; the anatomical details of this arrangement await additional study.
There are two potential sources of the endogenous α-MSH ligand for NTS MC3/4-R – ARC-POMC and NTS-POMC neurons – and either or both could contribute to control of meal size and to the intake inhibitory effects of CCK and leptin that are mediated by caudal brainstem MC-Rs. Unfortunately, there are very few studies that directly address this issue at the appropriate anatomical level required for characterization of the projection path of the α-MSH fibers originating in ARC-POMC and NTS-POMC neurons; such results are required to determine whether or not each population projects local and more distally (139–140).
A recent study by Zheng et al (141) provides a variety of data of relevance to this important question. In a previous study, Zheng et al (129) used retrograde tracer techniques to show that ARC neurons immunoreactive for α-MSH project to the vicinity of NTS neurons. Unfortunately, this technique did not provide sufficient detail of termination sites. Therefore, the recent study used a technique designed to unambiguously define the termination of these projections. An adenoviral transfer vector containing the cytomegalovirus promoter upstream of a transcription-blocking cassette followed by sequences encoding farnesylated EGFP (EGFPf) was used to generate Ad-iZ/EGFPf, which mediates the expression of EGFPf only when transduced into cre-expressing cells. When Ad-iZ/EGFPf was stereotaxically delivered into the brains of Pomccre mice, EGFPf was expressed only in neuroanatomically-restricted POMC-neurons (thereby avoiding the possible inclusion of fibers originating from non-POMC neuron at the same site). Unilateral injection of this tracer resulted in GFP-positive axon profiles in the NTS and other areas of the caudal brainstem. IHC staining for α-MSH was also used and showed that many of these GFP-positive axon profiles also expressed α-MSH. Importantly, the double-labeled axon profiles unambiguously demonstrate the presence of α-MSH fibers terminating in NTS that originate in ARC-POMC neurons.
To determine the proportion of NTS terminating α-MSH fibers that arise from ARC-POMC neurons versus NTS-POMC neurons, α-MSH IR in the NTS and vicinity of intact rats were compared with that seen in decerebrate rats. The authors reasoned that supracollicular transection would result in the complete degeneration of axonal projections originating from ARC-POMC neurons and that any remaining α-MSH axonal profiles would originate in NTS-POMC neurons. In intact control rats, very fine axon profiles and a moderate number of relatively large caliber axon profiles were present throughout the NTS. By contrast, in decerebrate rats most of the larger caliber fibers were missing and only the fine immunoreactivity remained in this region. Quantitative analysis of three rostro-caudal levels of the NTS showed significant reduction in the number of α-MSH-IR fibers. From this analysis it appears that approximately 70% of the α-MSH fibers projecting to NTS neurons arise from ARC-POMC neurons and that some 30% arise locally from NTS-POMC neurons. From this result we can conclude that NTS neurons are supplied with endogenous MC-R agonist by ARC-POMC and by NTS-POMC neurons. Other experiments are required to determine the agonist source for other MC4-R bearing nuclei.
Oxytocinergic projections from hypothalamus to NTS may also contribute to the meal size effects of leptin treatment and vagal afferent signaling
The data just reviewed provide an anatomical link between hypothalamic leptin signaling and the activation of hindbrain MC-Rs via descending melanocortinergic projections. These data suggest a putative mechanism to account for the enhanced sensitivity to CCK observed in the experiments of Morton et al (91). The link between hypothalamic leptin signaling and hindbrain control of meal size and sensitivity to satiation signals is not restricted to a role for melanocortin signaling and has been hypothesized to involve descending oxytocinergic projections to the NTS arising in neurons of the parvocellular region of paraventricular (pPVH) hypothalamus that receive projections from ARC-POMC neurons (142–143). Support for this hypothesis comes from a variety of data including several reports from Blevins and colleagues (142–143). These authors show close anatomical proximity between CCK-activated (Fos-LI) neurons in medial and gelatinosus subnuclei of the NTS and oxytocin-containing fibers projecting from the pPVH (143). Further, injections of fluorescent cholera toxin-B into the NTS labeled a subset of pPVH oxytocin positive neurons that expressed Fos-LI in response to 3rd icv leptin. Complementing these data, the intake inhibitory effect of 3rd icv leptin delivery was shown to be attenuated by icv pretreatment with an oxytocin receptor antagonist, [d-(CH2)5,Tyr(Me)2,Orn8]-vasotocin (OVT)]. In a separate experiment, the authors showed that OVT treatment significantly reduced the number of Fos-LI mNTS neurons that were activated by leptin treatment. These authors conclude that the release of oxytocin from a descending projection pathway pPVH to NTS contributes, at least in part, to the meal size reducing effects of hypothalamic leptin signaling.
Recent data from Peters and colleagues (144) provide anatomical support for the hypothesis of Blevins et al. and, importantly, a cellular mechanism for the effect of oxytocin on NTS neurons that may be of relevance to the control of meal size and to the integration of leptin and satiation signals (144). Cell bodies of NTS neurons that fired action potentials in response to electrical stimulation of the vagus nerve were found within the NTS at the level of the area postrema. Half of these labeled neurons received close appositions from oxytocin- IR terminals on their cell bodies and dendrites. NTS neurons in caudal brainstem slices were neurophysiologically identified by their solitary tract stimulation-induced glutamatergic EPSCs. Oxytocin application increased the frequency, but not amplitude, of miniature EPSCs in approximately half of the NTS neurons examined. Analysis of the solitary tract-evoked EPSCs indicated that oxytocin selectively increased the release of glutamate from the vagal afferents terminating on the NTS neurons. The findings of Peters and colleagues (144) suggest that oxytocin released from pPVH axons acts on a subset of medial NTS neurons to enhance visceral afferent transmission via pre-synaptic and post-synaptic mechanisms. Of interest is the similarity in the cellular mechanisms highlighted by these experiments and in those of Wan et al (131) that focus on melanocortinergic influences on NTS neurons.
Vagal Afferent Neurons are Responsive to Leptin and Provide Another Site of Integration for Leptin and GI Satiation Signaling
Data reviewed above focus on two anatomically distributed sites of leptin signaling that provide a substrate for the amplification of the neural processing of GI satiation signals and the resulting reduction of meal size driven by leptin. Recent discoveries of: [1] a GI source of leptin and [2] LepR expression in vagal afferent neurons brings attention to a third site of leptin-GI signal integration of potential direct relevance to the neural control of meal size. Bado and colleagues (145) showed that leptin is synthesized in chief cells and some mucosal endocrine cells in the gastric mucosa and importantly that this gastric-derived leptin is secreted in response to food intake and CCK secretion. The physiological relevance of the prandially released gastric leptin is underscored by data showing that the cell bodies of vagal afferent neurons (located in the nodose ganglion) express LepR and that leptin delivery to the celiac artery (supplying blood to the GI tract), that is without effect on systemic leptin level, activates vagal afferents and reduces food intake underscore (146–150). Recent experiments of Peters and colleagues (150–152) evaluated the hypothesis that gastric leptin acts as a satiation signal by activating LepR-expressing terminals of vagal afferent neurons in a paracrine-like fashion (145, 149).
These authors infused low doses of leptin into the celiac artery, which perfuses the stomach and upper small intestines, and found that these infusions reduced liquid meal (sucrose) intake when compared with results from matched jugular vein infusions of leptin. To verify the vagal afferent mediation of the intake reducing effect for celiac arterial leptin infusion, subdiaphragmatic vagotomy and systemic capsaicin pretreatment were performed and for each, the leptin-induced intake suppression was abolished. In addition, co-infusion of leptin in the celiac artery together with ip CCK administration resulted in a synergistic suppression of sucrose intake compared with exposure to either ligand individually. The synergistic effects of leptin and CCK on intake could result from a synergistic effect on vagal afferent discharge frequency as was reported for the conjoint effects of CCK and gastric distention (76). To examine this idea changes in cytosolic calcium levels induced by leptin and CCK were measured in cultured nodose ganglion neurons labeled with a retrograde neuronal tracer injected into either the stomach or the duodenum. Peters and colleagues (150) found that leptin significantly augmented vagal afferent neurophysiological responses to low doses of CCK. Also of interest, for some vagal afferents the addition of leptin to the medium revealed a response to low-dose CCK that was absent without leptin, further suggesting an interaction of leptin and CCK on vagal afferent neurons. Thus, both CCK and leptin activated vagal afferent neurons labeled from the stomach and intestine. All of the leptin-sensitive neurons labeled from the stomach also responded to CCK.
These data support the view that afferent vagal neurons themselves are a site of leptin-satiation signal integration of direct relevance to the control of meal size. From this perspective NTS neurons receive already integrated leptin-GI-derived satiation signaling from vagal afferents to which additional processing involving brain leptin and neuropeptide signaling is performed. The synergistic interaction of leptin and CCK seen in food intake and in neurophysiologic response of vagal afferents is analogous to the amplification of the effects of CCK and gastric distention by systemic and brain-directed leptin discussed above.
Conclusions: Integrations Performed by NTS Neurons are Central to Meal Size Control
This review examines the neural control of meal size and the integration of two principal sources of that control - GI satiation signals and leptin signaling. Four types of integrations are considered and each involves NTS neurons. Data discussed show that NTS neurons integrate information arising from: [1] ascending GI-derived vagal afferent projections and [2] descending neuropeptidergic projections from leptin-activated medial hypothalamic neurons. Two additional sources of information that are also processed by NTS neurons are also highlighted in this review: [1] direct NTS leptin signaling and [2] melanocortin signaling in NTS neurons and in their driven by ligand supplied by NTS POMC neurons and ARC POMC neurons.
Perspective
The history of research on the neural control of food intake reveals a strong tendency to emphasize the contribution of a single brain region, often a specific set of medial hypothalamic neurons, and to avoid simultaneous consideration of the contributions of other brain regions to the control of meal size specifically and food intake more generally. This tendency is, of course, not unique to this field and likely derives from the reductionist strategies scientists employ. Our methods, no matter how much more sophisticated they become, are applied in a way that is designed to highlight the contribution of a given set of “targeted neurons” If eliminating (or enhancing) the function of the targeted neurons results in a statistically meaningful feeding phenotype, then we as scientists are likely to emphasize their role in our discussions of neural control. There are times that we choose to, or are urged by our reviewers to consider the role of brain regions not directly studied in our experiments. Nonetheless, it is rare to find papers that consider the contribution of more than one site to the neural mediation of meal size control (82, 153–156). While clearly less common and more difficult, it will be useful to more frequently apply a constructionist perspective that leads to a systems approach for explaining the neural control of food intake.
With respect to the energy balance effects of CNS leptin signaling there appears to be a developing awareness that a systems approach including anatomically distributed elements will provide a more complete explanation of function than that achieved by focusing on a single group of targeted neurons or “center” (82–85). This review has emphasized data that support contributions from mNTS neurons, ARC neurons, and vagal afferent neurons in explaining the meal size effects of leptin signaling. Other investigators are developing data that argue for a yet broader anatomical view of CNS leptin signaling that will likely include roles for neurons of VTA, LH, VMN and perhaps even from other LepR expressing brain regions such as hippocampus (not yet well explored) as they contribute, in yet undefined ways, to the overall effect of leptin on food intake. It remains to be determined whether leptin signaling from these sites is also relevant to meal size control or if they contribute to other food intake functions that may include food-reward or the associative control of feeding.
We are entering a time marked by a growing appreciation that brain regions other than ARC and NTS contribute to the neural control of food intake (153–154, 156–159). Contributions of yet other brain regions such as the central nucleus of the amygdala, bed nucleus of the stria terminalis, nucleus accumbens, ventral and dorsal striatum, and prefrontal cortex have begun to be explored. Investigators are already orienting towards experiments aimed at examining whether neurons in those structures contribute to some aspects of the control of meal initiation such as food reward, associative control of feeding, timing of feeding, cognitive control, temporal control, and social control of feeding. Experiments on animal models as well as on human subjects targeting this broader array of structures are underway. Application of a systems approach will be very useful for constructing a more adequate neural model for food intake control than that provided by the arcuate model. Typically, that model has looked no further than a few groups of neurons to “explain” a host of effects on food intake. In addition, adherents of the explanatory power of the arcuate model have unfortunately limited themselves to a consideration of feeding effects driven by homeostatic need and avoided a consideration of the neural basis of meal initiation systems that collectively contribute to explain why humans surrounded by abundant quantities of tasty, energy dense foods chronically overeat. The development of a more complete neural and neurochemical circuit diagram for the control of food intake will surely extend beyond the structures highlighted by the focus on a limited number of signals and neural circuits such as those targeted for consideration in this review and will likely include roles for other signals such as serotonin, opiates, dopamine, and CGRP – to name a few (86, 160–164). Further development of a systems approach is more likely to provide insights that will lead to the development of effective pharmacotherapies for limiting the hyperphagia that drives the pathophysiology of obesity that is of such pressing concern for improving human health.
Acknowledgments
I sincerely thank Dr. Matthew Hayes for his critical reading of the manuscript and the useful suggestions he provided during its development. I am grateful to the colleagues and collaborators without whom the work discussed involving our laboratory would not have been possible – my sincere thanks to Karolina Skibicka, Matt Hayes, Kendra Bence, Christian Bjorbaek, Lihong Huo, Ralph DiLeone, Hans-Rudi Berthoud, Lisa Maeng, and Theresa Leichner. This work was supported by NIH-DK 21397.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Rolls BJ, Drewnowski A, Ledikwe JH. Changing the energy density of the diet as a strategy for weight management. J Am Diet Assoc. 2005;105:S98–103. doi: 10.1016/j.jada.2005.02.033. [DOI] [PubMed] [Google Scholar]
- 2.Bjorntorp P. Thrifty genes and human obesity. Are we chasing ghosts? Lancet. 2001;358:1006–1008. doi: 10.1016/S0140-6736(01)06110-4. [DOI] [PubMed] [Google Scholar]
- 3.Hayes M, Chustek M, Heshka S, Wang Z, Pietrobelli A, Heymsfield SB. Low physical activity levels of modern Homo sapiens among free-ranging mammals. Int J Obes (Lond) 2005;29:151–156. doi: 10.1038/sj.ijo.0802842. [DOI] [PubMed] [Google Scholar]
- 4.Leonard WR. Food for thought. Dietary change was a driving force in human evolution. Sci Am. 2002;287:106–115. [PubMed] [Google Scholar]
- 5.Leonard WR, Robertson ML. Nutritional requirements and human evolution: a bioenergetics model. Am J Hum Biol. 1992;4:179–195. doi: 10.1002/ajhb.1310040204. [DOI] [PubMed] [Google Scholar]
- 6.Sorensen MV, Leonard WR. Neandertal energetics and foraging efficiency. J Hum Evol. 2001;40:483–495. doi: 10.1006/jhev.2001.0472. [DOI] [PubMed] [Google Scholar]
- 7.Barsh GS, Schwartz MW. Genetic approaches to studying energy balance: perception and integration. Nat Rev Genet. 2002;3:589–600. doi: 10.1038/nrg862. [DOI] [PubMed] [Google Scholar]
- 8.Ahima RS, Saper CB, Flier JS, Elmquist JK. Leptin regulation of neuroendocrine systems. Front Neuroendocrinol. 2000;21:263–307. doi: 10.1006/frne.2000.0197. [DOI] [PubMed] [Google Scholar]
- 9.Holst JJ. The physiology of glucagon-like peptide 1. Physiol Rev. 2007;87:1409–1439. doi: 10.1152/physrev.00034.2006. [DOI] [PubMed] [Google Scholar]
- 10.Ellacott KL, Cone RD. The role of the central melanocortin system in the regulation of food intake and energy homeostasis: lessons from mouse models. Philos Trans R Soc Lond B Biol Sci. 2006;361:1265–1274. doi: 10.1098/rstb.2006.1861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pissios P, Bradley RL, Maratos-Flier E. Expanding the scales: The multiple roles of MCH in regulating energy balance and other biological functions. Endocr Rev. 2006;27:606–620. doi: 10.1210/er.2006-0021. [DOI] [PubMed] [Google Scholar]
- 12.Elmquist JK, Maratos-Flier E, Saper CB, Flier JS. Unraveling the central nervous system pathways underlying responses to leptin. Nat Neurosci. 1998;1:445–450. doi: 10.1038/2164. [DOI] [PubMed] [Google Scholar]
- 13.Tschop M, Smiley DL, Heiman ML. Ghrelin induces adiposity in rodents. Nature. 2000;407:908–913. doi: 10.1038/35038090. [DOI] [PubMed] [Google Scholar]
- 14.Small CJ, Bloom SR. Gut hormones and the control of appetite. Trends Endocrinol Metab. 2004;15:259–263. doi: 10.1016/j.tem.2004.06.002. [DOI] [PubMed] [Google Scholar]
- 15.Tecott LH, Sun LM, Akana SF, Strack AM, Lowenstein DH, Dallman MF, Julius D. Eating disorder and epilepsy in mice lacking 5-HT2c serotonin receptors. Nature. 1995;374:542–546. doi: 10.1038/374542a0. [DOI] [PubMed] [Google Scholar]
- 16.Zhang Y, Proenca R, Maffei M, Barone M, Leopold L, Friedman JM. Positional cloning of the mouse obese gene and its human homologue. Nature. 1994;372:425–432. doi: 10.1038/372425a0. [DOI] [PubMed] [Google Scholar]
- 17.Pollan M. The Omnivore’s Dilemma: A Natural History of Four Meals. New York: Penguin; 2006. [Google Scholar]
- 18.Schwartz MW, Woods SC, Seeley RJ, Barsh GS, Baskin DG, Leibel RL. Is the energy homeostasis system inherently biased toward weight gain? Diabetes. 2003;52:232–238. doi: 10.2337/diabetes.52.2.232. [DOI] [PubMed] [Google Scholar]
- 19.Woods SC. The eating paradox: how we tolerate food. Psychol Rev. 1991;98:488–505. doi: 10.1037/0033-295x.98.4.488. [DOI] [PubMed] [Google Scholar]
- 20.Strubbe JH, Woods SC. The timing of meals. Psychol Rev. 2004;111:128–141. doi: 10.1037/0033-295X.111.1.128. [DOI] [PubMed] [Google Scholar]
- 21.Lowe MR, Levine AS. Eating motives and the controversy over dieting: eating less than needed versus less than wanted. Obes Res. 2005;13:797–806. doi: 10.1038/oby.2005.90. [DOI] [PubMed] [Google Scholar]
- 22.Woods SC, D’Alessio DA. Central control of body weight and appetite. J Clin Endocrinol Metab. 2008;93:S37–50. doi: 10.1210/jc.2008-1630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Smith GP. The direct and indirect controls of meal size. Neurosci Biobehav Rev. 1996;20:41–46. doi: 10.1016/0149-7634(95)00038-g. [DOI] [PubMed] [Google Scholar]
- 24.Moran TH. Gut peptide signaling in the controls of food intake. Obesity (Silver Spring) 2006;14(Suppl 5):250S–253S. doi: 10.1038/oby.2006.318. [DOI] [PubMed] [Google Scholar]
- 25.Powley TL. Vagal circuitry mediating cephalic-phase responses to food. Appetite. 2000;34:184–188. doi: 10.1006/appe.1999.0279. [DOI] [PubMed] [Google Scholar]
- 26.Ritter RC. Gastrointestinal mechanisms of satiation for food. Physiol Behav. 2004;81:249–273. doi: 10.1016/j.physbeh.2004.02.012. [DOI] [PubMed] [Google Scholar]
- 27.Berthoud HR. The caudal brainstem and the control of food intake and energy balance. New York: Plenum; 2004. [Google Scholar]
- 28.Huo L, Maeng L, Bjorbaek C, Grill HJ. Leptin and the control of food intake: neurons in the nucleus of the solitary tract are activated by both gastric distension and leptin. Endocrinology. 2007;148:2189–2197. doi: 10.1210/en.2006-1572. [DOI] [PubMed] [Google Scholar]
- 29.Lundy RFJ, Norgren R. Gustatory system. In: Paxinos G, editor. The Rat Nervous System. San Diego, CA: Elsevier Academic Press; 2004. pp. 891–921. [Google Scholar]
- 30.Saper CB. Central Autonomic System. In: Paxinos G, editor. The Rat Nervous System. San Diego, CA: Elsevier Academic Press; 2004. pp. 761–796. [Google Scholar]
- 31.Berthoud HR, Sutton GM, Townsend RL, Patterson LM, Zheng H. Brainstem mechanisms integrating gut-derived satiety signals and descending forebrain information in the control of meal size. Physiol Behav. 2006;89:517–524. doi: 10.1016/j.physbeh.2006.08.018. [DOI] [PubMed] [Google Scholar]
- 32.Woods SC, Schwartz MW, Baskin DG, Seeley RJ. Food intake and the regulation of body weight. Annu Rev Psychol. 2000;51:255–277. doi: 10.1146/annurev.psych.51.1.255. [DOI] [PubMed] [Google Scholar]
- 33.Bray GA, Gallagher TF., Jr Manifestations of hypothalamic obesity in man: a comprehensive investigation of eight patients and a reveiw of the literature. Medicine (Baltimore) 1975;54:301–330. doi: 10.1097/00005792-197507000-00002. [DOI] [PubMed] [Google Scholar]
- 34.Hetherington AW, Ranson SW. Hypothalamic lesions and adiposity in the rat. Anat Rec. 1940;78:149–172. [Google Scholar]
- 35.Mu JY, Yin TH, Hamilton CL, Brobeck JR. Variability of body fat in hyperphagic rats. Yale J Biol Med. 1968;41:133–142. [PMC free article] [PubMed] [Google Scholar]
- 36.Teitelbaum P, Epstein AN. The lateral hypothalamic syndrome: recovery of feeding and drinking after lateral hypothalamic lesions. Psychol Rev. 1962;69:74–90. doi: 10.1037/h0039285. [DOI] [PubMed] [Google Scholar]
- 37.Anand BK, Brobeck JR. Hypothalamic control of food intake in rats and cats. Yale J Biol Med. 1951;24:123–140. [PMC free article] [PubMed] [Google Scholar]
- 38.Stellar E. The physiology of motivation. Psychol Rev. 1954;61:5–22. doi: 10.1037/h0060347. [DOI] [PubMed] [Google Scholar]
- 39.Coons EE, Levak M, Miller NE. Lateral hypothalamus: learning of food-seeking response motivated by electrical stimulation. Science. 1965;150:1320–1321. doi: 10.1126/science.150.3701.1320. [DOI] [PubMed] [Google Scholar]
- 40.Miller NE. Chemical Coding of Behavior in the Brain. Science. 1965;148:328–338. doi: 10.1126/science.148.3668.328. [DOI] [PubMed] [Google Scholar]
- 41.de Luca C, Kowalski TJ, Zhang Y, Elmquist JK, Lee C, Kilimann MW, Ludwig T, Liu SM, Chua SC., Jr Complete rescue of obesity, diabetes, and infertility in db/db mice by neuron-specific LEPR-B transgenes. J Clin Invest. 2005;115:3484–3493. doi: 10.1172/JCI24059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cohen P, Zhao C, Cai X, Montez JM, Rohani SC, Feinstein P, Mombaerts P, Friedman JM. Selective deletion of leptin receptor in neurons leads to obesity. J Clin Invest. 2001;108:1113–1121. doi: 10.1172/JCI13914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cone RD. Studies on the physiological functions of the melanocortin system. Endocr Rev. 2006;27:736–749. doi: 10.1210/er.2006-0034. [DOI] [PubMed] [Google Scholar]
- 44.Cowley MA, Smart JL, Rubinstein M, Cerdan MG, Diano S, Horvath TL, Cone RD, Low MJ. Leptin activates anorexigenic POMC neurons through a neural network in the arcuate nucleus. Nature. 2001;411:480–484. doi: 10.1038/35078085. [DOI] [PubMed] [Google Scholar]
- 45.Kahn BB, Alquier T, Carling D, Hardie DG. AMP-activated protein kinase: ancient energy gauge provides clues to modern understanding of metabolism. Cell Metab. 2005;1:15–25. doi: 10.1016/j.cmet.2004.12.003. [DOI] [PubMed] [Google Scholar]
- 46.Bates SH, Kulkarni RN, Seifert M, Myers MG., Jr Roles for leptin receptor/STAT3-dependent and -independent signals in the regulation of glucose homeostasis. Cell Metab. 2005;1:169–178. doi: 10.1016/j.cmet.2005.02.001. [DOI] [PubMed] [Google Scholar]
- 47.Niswender KD, Morton GJ, Stearns WH, Rhodes CJ, Myers MG, Jr, Schwartz MW. Intracellular signalling. Key enzyme in leptin-induced anorexia. Nature. 2001;413:794–795. doi: 10.1038/35101657. [DOI] [PubMed] [Google Scholar]
- 48.Minokoshi Y, Alquier T, Furukawa N, Kim YB, Lee A, Xue B, Mu J, Foufelle F, Ferre P, Birnbaum MJ, Stuck BJ, Kahn BB. AMP-kinase regulates food intake by responding to hormonal and nutrient signals in the hypothalamus. Nature. 2004;428:569–574. doi: 10.1038/nature02440. [DOI] [PubMed] [Google Scholar]
- 49.Bjorbaek C, Elmquist JK, Frantz JD, Shoelson SE, Flier JS. Identification of SOCS-3 as a potential mediator of central leptin resistance. Mol Cell. 1998;1:619–625. doi: 10.1016/s1097-2765(00)80062-3. [DOI] [PubMed] [Google Scholar]
- 50.Chen Z, Travers JB. Inactivation of amino acid receptors in medullary reticular formation modulates and suppresses ingestion and rejection responses in the awake rat. Am J Physiol Regul Integr Comp Physiol. 2003;285:R68–83. doi: 10.1152/ajpregu.00054.2003. [DOI] [PubMed] [Google Scholar]
- 51.Chen Z, Travers SP, Travers JB. Muscimol infusions in the brain stem reticular formation reversibly block ingestion in the awake rat. Am J Physiol Regul Integr Comp Physiol. 2001;280:R1085–1094. doi: 10.1152/ajpregu.2001.280.4.R1085. [DOI] [PubMed] [Google Scholar]
- 52.Nasse J, Terman D, Venugopal S, Hermann G, Rogers R, Travers JB. Local circuit input to the medullary reticular formation from the rostral nucleus of the solitary tract. Am J Physiol Regul Integr Comp Physiol. 2008;295:R1391–1408. doi: 10.1152/ajpregu.90457.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Travers SP, Travers JB. Taste-evoked Fos expression in nitrergic neurons in the nucleus of the solitary tract and reticular formation of the rat. J Comp Neurol. 2007;500:746–760. doi: 10.1002/cne.21213. [DOI] [PubMed] [Google Scholar]
- 54.Grill HJ, Norgren R. The taste reactivity test. II. Mimetic responses to gustatory stimuli in chronic thalamic and chronic decerebrate rats. Brain Res. 1978;143:281–297. doi: 10.1016/0006-8993(78)90569-3. [DOI] [PubMed] [Google Scholar]
- 55.Kaplan JM, Grill HJ. Swallowing during ongoing fluid ingestion in the rat. Brain Res. 1989;499:63–80. doi: 10.1016/0006-8993(89)91135-9. [DOI] [PubMed] [Google Scholar]
- 56.Steiner JE. The gustofacial response: observation on normal and anencephalic newborn infants. Symp Oral Sens Percept. 1973:254–278. [PubMed] [Google Scholar]
- 57.Hayes MR, Skibicka KP, Grill HJ. Caudal brainstem processing is sufficient for behavioral, sympathetic, and parasympathetic responses driven by peripheral and hindbrain glucagon-like-peptide-1 receptor stimulation. Endocrinology. 2008;149:4059–4068. doi: 10.1210/en.2007-1743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Seeley RJ, Grill HJ, Kaplan JM. Neurological dissociation of gastrointestinal and metabolic contributions to meal size control. Behav Neurosci. 1994;108:347–352. [PubMed] [Google Scholar]
- 59.Grill HJ, Smith GP. Cholecystokinin decreases sucrose intake in chronic decerebrate rats. Am J Physiol. 1988;254:R853–856. doi: 10.1152/ajpregu.1988.254.6.R853. [DOI] [PubMed] [Google Scholar]
- 60.Talsania T, Anini Y, Siu S, Drucker DJ, Brubaker PL. Peripheral exendin-4 and peptide YY(3-36) synergistically reduce food intake through different mechanisms in mice. Endocrinology. 2005;146:3748–3756. doi: 10.1210/en.2005-0473. [DOI] [PubMed] [Google Scholar]
- 61.Imeryuz N, Yegen BC, Bozkurt A, Coskun T, Villanueva-Penacarrillo ML, Ulusoy NB. Glucagon-like peptide-1 inhibits gastric emptying via vagal afferent-mediated central mechanisms. Am J Physiol. 1997;273:G920–927. doi: 10.1152/ajpgi.1997.273.4.G920. [DOI] [PubMed] [Google Scholar]
- 62.Osaka T, Endo M, Yamakawa M, Inoue S. Energy expenditure by intravenous administration of glucagon-like peptide-1 mediated by the lower brainstem and sympathoadrenal system. Peptides. 2005;26:1623–1631. doi: 10.1016/j.peptides.2005.02.016. [DOI] [PubMed] [Google Scholar]
- 63.Yamamoto H, Lee CE, Marcus JN, Williams TD, Overton JM, Lopez ME, Hollenberg AN, Baggio L, Saper CB, Drucker DJ, Elmquist JK. Glucagon-like peptide-1 receptor stimulation increases blood pressure and heart rate and activates autonomic regulatory neurons. J Clin Invest. 2002;110:43–52. doi: 10.1172/JCI15595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Barragan JM, Eng J, Rodriguez R, Blazquez E. Neural contribution to the effect of glucagon-like peptide-1-(7-36) amide on arterial blood pressure in rats. Am J Physiol. 1999;277:E784–791. doi: 10.1152/ajpendo.1999.277.5.E784. [DOI] [PubMed] [Google Scholar]
- 65.Barragan JM, Rodriguez RE, Eng J, Blazquez E. Interactions of exendin-(9-39) with the effects of glucagon-like peptide-1-(7-36) amide and of exendin-4 on arterial blood pressure and heart rate in rats. Regul Pept. 1996;67:63–68. doi: 10.1016/s0167-0115(96)00113-9. [DOI] [PubMed] [Google Scholar]
- 66.Mack CM, Moore CX, Jodka CM, Bhavsar S, Wilson JK, Hoyt JA, Roan JL, Vu C, Laugero KD, Parkes DG, Young AA. Antiobesity action of peripheral exenatide (exendin-4) in rodents: effects on food intake, body weight, metabolic status and side-effect measures. Int J Obes (Lond) 2006;30:1332–1340. doi: 10.1038/sj.ijo.0803284. [DOI] [PubMed] [Google Scholar]
- 67.Abbott CR, Monteiro M, Small CJ, Sajedi A, Smith KL, Parkinson JR, Ghatei MA, Bloom SR. The inhibitory effects of peripheral administration of peptide YY(3-36) and glucagon-like peptide-1 on food intake are attenuated by ablation of the vagal-brainstem-hypothalamic pathway. Brain Res. 2005;1044:127–131. doi: 10.1016/j.brainres.2005.03.011. [DOI] [PubMed] [Google Scholar]
- 68.Kinzig KP, D’Alessio DA, Seeley RJ. The diverse roles of specific GLP-1 receptors in the control of food intake and the response to visceral illness. J Neurosci. 2002;22:10470–10476. doi: 10.1523/JNEUROSCI.22-23-10470.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Schick RR, Zimmermann JP, vorm Walde T, Schusdziarra V. Peptides that regulate food intake: glucagon-like peptide 1-(7-36) amide acts at lateral and medial hypothalamic sites to suppress feeding in rats. Am J Physiol Regul Integr Comp Physiol. 2003;284:R1427–1435. doi: 10.1152/ajpregu.00479.2002. [DOI] [PubMed] [Google Scholar]
- 70.Chaudhri OB, Parkinson JR, Kuo YT, Druce MR, Herlihy AH, Bell JD, Dhillo WS, Stanley SA, Ghatei MA, Bloom SR. Differential hypothalamic neuronal activation following peripheral injection of GLP-1 and oxyntomodulin in mice detected by manganese-enhanced magnetic resonance imaging. Biochem Biophys Res Commun. 2006;350:298–306. doi: 10.1016/j.bbrc.2006.09.033. [DOI] [PubMed] [Google Scholar]
- 71.Kastin AJ, Akerstrom V. Entry of exendin-4 into brain is rapid but may be limited at high doses. Int J Obes Relat Metab Disord. 2003;27:313–318. doi: 10.1038/sj.ijo.0802206. [DOI] [PubMed] [Google Scholar]
- 72.Ma X, Bruning J, Ashcroft FM. Glucagon-like peptide 1 stimulates hypothalamic proopiomelanocortin neurons. J Neurosci. 2007;27:7125–7129. doi: 10.1523/JNEUROSCI.1025-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 73.Willing AE, Berthoud HR. Gastric distension-induced c-fos expression in catecholaminergic neurons of rat dorsal vagal complex. Am J Physiol. 1997;272:R59–67. doi: 10.1152/ajpregu.1997.272.1.R59. [DOI] [PubMed] [Google Scholar]
- 74.Fraser KA, Raizada E, Davison JS. Oral-pharyngeal-esophageal and gastric cues contribute to meal-induced c-fos expression. Am J Physiol. 1995;268:R223–230. doi: 10.1152/ajpregu.1995.268.1.R223. [DOI] [PubMed] [Google Scholar]
- 75.Emond MH, Weingarten HP. Fos-like immunoreactivity in vagal and hypoglossal nuclei in different feeding states: a quantitative study. Physiol Behav. 1995;58:459–465. doi: 10.1016/0031-9384(95)00069-u. [DOI] [PubMed] [Google Scholar]
- 76.Schwartz GJ, Moran TH. Leptin and neuropeptide Y have opposing modulatory effects on nucleus of the solitary tract neurophysiological responses to gastric loads: implications for the control of food intake. Endocrinology. 2002;143:3779–3784. doi: 10.1210/en.2002-220352. [DOI] [PubMed] [Google Scholar]
- 77.Mazda T, Yamamoto H, Fujimura M, Fujimiya M. Gastric distention-induced release of serotonin stimulates c-fos expression in specific brain nuclei via 5-HT3 receptors in conscious rats. Am J Physiol Gastrointest Liver Physiol. 2003;287:G228–235. doi: 10.1152/ajpgi.00373.2003. [DOI] [PubMed] [Google Scholar]
- 78.Vrang N, Phifer CB, Corkern MM, Berthoud HR. Gastric distension induces c-Fos in medullary GLP-1/2-containing neurons. Am J Physiol Regul Integr Comp Physiol. 2003;285:R470–478. doi: 10.1152/ajpregu.00732.2002. [DOI] [PubMed] [Google Scholar]
- 79.Zheng H, Kelly L, Patterson LM, Berthoud HR. Effect of brain stem NMDA-receptor blockade by MK-801 on behavioral and fos responses to vagal satiety signals. Am J Physiol. 1999;277:R1104–1111. doi: 10.1152/ajpregu.1999.277.4.R1104. [DOI] [PubMed] [Google Scholar]
- 80.Hermann GE, Kohlerman NJ, Rogers RC. Hepatic-vagal and gustatory afferent interactions in the brainstem of the rat. J Auton Nerv Syst. 1983;9:477–495. doi: 10.1016/0165-1838(83)90008-5. [DOI] [PubMed] [Google Scholar]
- 81.Hermann GE, Rogers RC. Convergence of vagal and gustatory afferent input within the parabrachial nucleus of the rat. J Auton Nerv Syst. 1985;13:1–17. doi: 10.1016/0165-1838(85)90002-5. [DOI] [PubMed] [Google Scholar]
- 82.Myers MG, Jr, Munzberg H, Leinninger GM, Leshan RL. The geometry of leptin action in the brain: more complicated than a simple ARC. Cell Metab. 2009;9:117–123. doi: 10.1016/j.cmet.2008.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Hommel JD, Trinko R, Sears RM, Georgescu D, Liu ZW, Gao XB, Thurmon JJ, Marinelli M, DiLeone RJ. Leptin receptor signaling in midbrain dopamine neurons regulates feeding. Neuron. 2006;51:801–810. doi: 10.1016/j.neuron.2006.08.023. [DOI] [PubMed] [Google Scholar]
- 84.Fulton S, Pissios P, Manchon RP, Stiles L, Frank L, Pothos EN, Maratos-Flier E, Flier JS. Leptin regulation of the mesoaccumbens dopamine pathway. Neuron. 2006;51:811–822. doi: 10.1016/j.neuron.2006.09.006. [DOI] [PubMed] [Google Scholar]
- 85.Dhillon H, Zigman JM, Ye C, Lee CE, McGovern RA, Tang V, Kenny CD, Christiansen LM, White RD, Edelstein EA, Coppari R, Balthasar N, Cowley MA, Chua S, Jr, Elmquist JK, Lowell BB. Leptin directly activates SF1 neurons in the VMH, and this action by leptin is required for normal body-weight homeostasis. Neuron. 2006;49:191–203. doi: 10.1016/j.neuron.2005.12.021. [DOI] [PubMed] [Google Scholar]
- 86.Leinninger GM, Jo YH, Leshan RL, Louis GW, Yang H, Barrera JG, Wilson H, Opland DM, Faouzi MA, Gong Y, Jones JC, Rhodes CJ, Chua S, Jr, Diano S, Horvath TL, Seeley RJ, Becker JB, Munzberg H, Myers MG., Jr Leptin acts via leptin receptor-expressing lateral hypothalamic neurons to modulate the mesolimbic dopamine system and suppress feeding. Cell Metab. 2009;10:89–98. doi: 10.1016/j.cmet.2009.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Grill HJ, Schwartz MW, Kaplan JM, Foxhall JS, Breininger J, Baskin DG. Evidence that the caudal brainstem is a target for the inhibitory effect of leptin on food intake. Endocrinology. 2002;143:239–246. doi: 10.1210/endo.143.1.8589. [DOI] [PubMed] [Google Scholar]
- 88.Eckel LA, Langhans W, Kahler A, Campfield LA, Smith FJ, Geary N. Chronic administration of OB protein decreases food intake by selectively reducing meal size in female rats. Am J Physiol. 1998;275:R186–193. doi: 10.1152/ajpregu.1998.275.1.R186. [DOI] [PubMed] [Google Scholar]
- 89.Kahler A, Geary N, Eckel LA, Campfield LA, Smith FJ, Langhans W. Chronic administration of OB protein decreases food intake by selectively reducing meal size in male rats. Am J Physiol. 1998;275:R180–185. doi: 10.1152/ajpregu.1998.275.1.R180. [DOI] [PubMed] [Google Scholar]
- 90.Flynn MC, Scott TR, Pritchard TC, Plata-Salaman CR. Mode of action of OB protein (leptin) on feeding. Am J Physiol. 1998;275:R174–179. doi: 10.1152/ajpregu.1998.275.1.R174. [DOI] [PubMed] [Google Scholar]
- 91.Morton GJ, Blevins JE, Williams DL, Niswender KD, Gelling RW, Rhodes CJ, Baskin DG, Schwartz MW. Leptin action in the forebrain regulates the hindbrain response to satiety signals. J Clin Invest. 2005;115:703–710. doi: 10.1172/JCI200522081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Scott KA, Moran TH. The GLP-1 agonist exendin-4 reduces food intake in nonhuman primates through changes in meal size. Am J Physiol Regul Integr Comp Physiol. 2007;293:R983–987. doi: 10.1152/ajpregu.00323.2007. [DOI] [PubMed] [Google Scholar]
- 93.Emond M, Schwartz GJ, Ladenheim EE, Moran TH. Central leptin modulates behavioral and neural responsivity to CCK. Am J Physiol. 1999;276:R1545–1549. doi: 10.1152/ajpregu.1999.276.5.R1545. [DOI] [PubMed] [Google Scholar]
- 94.Barrachina MD, Martinez V, Wang L, Wei JY, Tache Y. Synergistic interaction between leptin and cholecystokinin to reduce short-term food intake in lean mice. Proc Natl Acad Sci U S A. 1997;94:10455–10460. doi: 10.1073/pnas.94.19.10455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Wang L, Martinez V, Barrachina MD, Tache Y. Fos expression in the brain induced by peripheral injection of CCK or leptin plus CCK in fasted lean mice. Brain Res. 1998;791:157–166. doi: 10.1016/s0006-8993(98)00091-2. [DOI] [PubMed] [Google Scholar]
- 96.Ritter RC, Slusser PG, Stone S. Glucoreceptors controlling feeding and blood glucose: location in the hindbrain. Science. 1981;213:451–452. doi: 10.1126/science.6264602. [DOI] [PubMed] [Google Scholar]
- 97.Zhang X, Fogel R, Renehan WE. Relationships between the morphology and function of gastric- and intestine-sensitive neurons in the nucleus of the solitary tract. J Comp Neurol. 1995;363:37–52. doi: 10.1002/cne.903630105. [DOI] [PubMed] [Google Scholar]
- 98.Xue B, Kahn BB. AMPK integrates nutrient and hormonal signals to regulate food intake and energy balance through effects in the hypothalamus and peripheral tissues. J Physiol. 2006;574:73–83. doi: 10.1113/jphysiol.2006.113217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Kim MS, Lee KU. Role of hypothalamic 5′-AMP-activated protein kinase in the regulation of food intake and energy homeostasis. J Mol Med. 2005;83:514–520. doi: 10.1007/s00109-005-0659-z. [DOI] [PubMed] [Google Scholar]
- 100.da Silva Xavier G, Leclerc I, Varadi A, Tsuboi T, Moule SK, Rutter GA. Role for AMP-activated protein kinase in glucose-stimulated insulin secretion and preproinsulin gene expression. Biochem J. 2003;371:761–774. doi: 10.1042/BJ20021812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Kim MS, Park JY, Namkoong C, Jang PG, Ryu JW, Song HS, Yun JY, Namgoong IS, Ha J, Park IS, Lee IK, Viollet B, Youn JH, Lee HK, Lee KU. Anti-obesity effects of alpha-lipoic acid mediated by suppression of hypothalamic AMP-activated protein kinase. Nat Med. 2004;10:727–733. doi: 10.1038/nm1061. [DOI] [PubMed] [Google Scholar]
- 102.Han SM, Namkoong C, Jang PG, Park IS, Hong SW, Katakami H, Chun S, Kim SW, Park JY, Lee KU, Kim MS. Hypothalamic AMP-activated protein kinase mediates counter-regulatory responses to hypoglycaemia in rats. Diabetologia. 2005;48:2170–2178. doi: 10.1007/s00125-005-1913-1. [DOI] [PubMed] [Google Scholar]
- 103.Culmsee C, Monnig J, Kemp BE, Mattson MP. AMP-activated protein kinase is highly expressed in neurons in the developing rat brain and promotes neuronal survival following glucose deprivation. J Mol Neurosci. 2001;17:45–58. doi: 10.1385/JMN:17:1:45. [DOI] [PubMed] [Google Scholar]
- 104.Ritter S, Dinh TT. 2-Mercaptoacetate and 2-deoxy-D-glucose induce Fos-like immunoreactivity in rat brain. Brain Res. 1994;641:111–120. doi: 10.1016/0006-8993(94)91822-8. [DOI] [PubMed] [Google Scholar]
- 105.Calingasan NY, Ritter S. Hypothalamic paraventricular nucleus lesions do not abolish glucoprivic or lipoprivic feeding. Brain Res. 1992;595:25–31. doi: 10.1016/0006-8993(92)91448-n. [DOI] [PubMed] [Google Scholar]
- 106.Ritter S, Taylor JS. Vagal sensory neurons are required for lipoprivic but not glucoprivic feeding in rats. Am J Physiol. 1990;258:R1395–1401. doi: 10.1152/ajpregu.1990.258.6.R1395. [DOI] [PubMed] [Google Scholar]
- 107.Jean A. The nucleus tractus solitarius: neuroanatomic, neurochemical and functional aspects. Arch Int Physiol Biochim Biophys. 1991;99:A3–52. doi: 10.3109/13813459109145916. [DOI] [PubMed] [Google Scholar]
- 108.Dallaporta M, Himmi T, Perrin J, Orsini JC. Solitary tract nucleus sensitivity to moderate changes in glucose level. Neuroreport. 1999;10:2657–2660. doi: 10.1097/00001756-199908200-00040. [DOI] [PubMed] [Google Scholar]
- 109.Ritter S, Dinh TT, Li AJ. Hindbrain catecholamine neurons control multiple glucoregulatory responses. Physiol Behav. 2006;89:490–500. doi: 10.1016/j.physbeh.2006.05.036. [DOI] [PubMed] [Google Scholar]
- 110.Ritter S, Dinh TT, Zhang Y. Localization of hindbrain glucoreceptive sites controlling food intake and blood glucose. Brain Res. 2000;856:37–47. doi: 10.1016/s0006-8993(99)02327-6. [DOI] [PubMed] [Google Scholar]
- 111.Hayes MR, Skibicka KP, Bence KK, Grill HJ. Dorsal hindbrain AMP-Kinase as an intracellular mediator of energy balance. Endocrinology. 2008 doi: 10.1210/en.2008-1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Rahmouni K, Sigmund CD, Haynes WG, Mark AL. Hypothalamic ERK mediates the anorectic and thermogenic sympathetic effects of leptin. Diabetes. 2009;58:536–542. doi: 10.2337/db08-0822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Hayes MR, Skibicka KP, Leichner TM, Guarnieri DJ, DiLeone RJ, Bence KK, Grill HJ. Endogenous leptin signaling in the caudal dorsal vagal complex is required for energy balance regulation. Cell Metab. 2009 doi: 10.1016/j.cmet.2009.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Comuzzie AG, Hixson JE, Almasy L, Mitchell BD, Mahaney MC, Dyer TD, Stern MP, MacCluer JW, Blangero J. A major quantitative trait locus determining serum leptin levels and fat mass is located on human chromosome 2. Nat Genet. 1997;15:273–276. doi: 10.1038/ng0397-273. [DOI] [PubMed] [Google Scholar]
- 115.Hinney A, Schmidt A, Nottebom K, Heibult O, Becker I, Ziegler A, Gerber G, Sina M, Gorg T, Mayer H, Siegfried W, Fichter M, Remschmidt H, Hebebrand J. Several mutations in the melanocortin-4 receptor gene including a nonsense and a frameshift mutation associated with dominantly inherited obesity in humans. J Clin Endocrinol Metab. 1999;84:1483–1486. doi: 10.1210/jcem.84.4.5728. [DOI] [PubMed] [Google Scholar]
- 116.Hixson JE, Almasy L, Cole S, Birnbaum S, Mitchell BD, Mahaney MC, Stern MP, MacCluer JW, Blangero J, Comuzzie AG. Normal variation in leptin levels in associated with polymorphisms in the proopiomelanocortin gene, POMC. J Clin Endocrinol Metab. 1999;84:3187–3191. doi: 10.1210/jcem.84.9.5951. [DOI] [PubMed] [Google Scholar]
- 117.Huszar D, Lynch CA, Fairchild-Huntress V, Dunmore JH, Fang Q, Berkemeier LR, Gu W, Kesterson RA, Boston BA, Cone RD, Smith FJ, Campfield LA, Burn P, Lee F. Targeted disruption of the melanocortin-4 receptor results in obesity in mice. Cell. 1997;88:131–141. doi: 10.1016/s0092-8674(00)81865-6. [DOI] [PubMed] [Google Scholar]
- 118.Yaswen L, Diehl N, Brennan MB, Hochgeschwender U. Obesity in the mouse model of pro-opiomelanocortin deficiency responds to peripheral melanocortin. Nat Med. 1999;5:1066–1070. doi: 10.1038/12506. [DOI] [PubMed] [Google Scholar]
- 119.Butler AA, Marks DL, Fan W, Kuhn CM, Bartolome M, Cone RD. Melanocortin-4 receptor is required for acute homeostatic responses to increased dietary fat. Nat Neurosci. 2001;4:605–611. doi: 10.1038/88423. [DOI] [PubMed] [Google Scholar]
- 120.Seeley RJ, Yagaloff KA, Fisher SL, Burn P, Thiele TE, van Dijk G, Baskin DG, Schwartz MW. Melanocortin receptors in leptin effects. Nature. 1997;390:349. doi: 10.1038/37016. [DOI] [PubMed] [Google Scholar]
- 121.Mountjoy KG, Mortrud MT, Low MJ, Simerly RB, Cone RD. Localization of the melanocortin-4 receptor (MC4-R) in neuroendocrine and autonomic control circuits in the brain. Mol Endocrinol. 1994;8:1298–1308. doi: 10.1210/mend.8.10.7854347. [DOI] [PubMed] [Google Scholar]
- 122.Kishi T, Aschkenasi CJ, Lee CE, Mountjoy KG, Saper CB, Elmquist JK. Expression of melanocortin 4 receptor mRNA in the central nervous system of the rat. J Comp Neurol. 2003;457:213–235. doi: 10.1002/cne.10454. [DOI] [PubMed] [Google Scholar]
- 123.Williams DL, Kaplan JM, Grill HJ. The role of the dorsal vagal complex and the vagus nerve in feeding effects of melanocortin-3/4 receptor stimulation. Endocrinology. 2000;141:1332–1337. doi: 10.1210/endo.141.4.7410. [DOI] [PubMed] [Google Scholar]
- 124.Skibicka KP, Grill HJ. Hypothalamic and hindbrain nuclei contribute to the anorexic, thermogenic and cardiovascular action of melanocortins. Endocrinology. 2009 doi: 10.1210/en.2009-0804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Skibicka KP, Grill HJ. Energetic responses are triggered by caudal brainstem melanocortin receptor stimulation and mediated by local sympathetic effector circuits. Endocrinology. 2008;149:3605–3616. doi: 10.1210/en.2007-1754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Fan W, Ellacott KL, Halatchev IG, Takahashi K, Yu P, Cone RD. Cholecystokinin-mediated suppression of feeding involves the brainstem melanocortin system. Nat Neurosci. 2004;7:335–336. doi: 10.1038/nn1214. [DOI] [PubMed] [Google Scholar]
- 127.Appleyard SM, Bailey TW, Doyle MW, Jin YH, Smart JL, Low MJ, Andresen MC. Proopiomelanocortin neurons in nucleus tractus solitarius are activated by visceral afferents: regulation by cholecystokinin and opioids. J Neurosci. 2005;25:3578–3585. doi: 10.1523/JNEUROSCI.4177-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Babic T, Townsend RL, Patterson LM, Sutton GM, Zheng H, Berthoud HR. Phenotype of neurons in the nucleus of the solitary tract that express CCK-induced activation of the ERK signaling pathway. Am J Physiol Regul Integr Comp Physiol. 2009;296:R845–854. doi: 10.1152/ajpregu.90531.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Zheng H, Patterson LM, Phifer CB, Berthoud HR. Brain stem melanocortinergic modulation of meal size and identification of hypothalamic POMC projections. Am J Physiol Regul Integr Comp Physiol. 2005;289:R247–258. doi: 10.1152/ajpregu.00869.2004. [DOI] [PubMed] [Google Scholar]
- 130.Sutton GM, Duos B, Patterson LM, Berthoud HR. Melanocortinergic modulation of cholecystokinin-induced suppression of feeding through extracellular signal-regulated kinase signaling in rat solitary nucleus. Endocrinology. 2005;146:3739–3747. doi: 10.1210/en.2005-0562. [DOI] [PubMed] [Google Scholar]
- 131.Wan S, Browning KN, Coleman FH, Sutton G, Zheng H, Butler A, Berthoud HR, Travagli RA. Presynaptic melanocortin-4 receptors on vagal afferent fibers modulate the excitability of rat nucleus tractus solitarius neurons. J Neurosci. 2008;28:4957–4966. doi: 10.1523/JNEUROSCI.5398-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Skibicka KP, Grill HJ. Hindbrain leptin stimulation induces anorexia and hyperthermia mediated by hindbrain melanocortin receptors. Endocrinology. 2009;150:1705–1711. doi: 10.1210/en.2008-1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Haynes WG, Morgan DA, Djalali A, Sivitz WI, Mark AL. Interactions between the melanocortin system and leptin in control of sympathetic nerve traffic. Hypertension. 1999;33:542–547. doi: 10.1161/01.hyp.33.1.542. [DOI] [PubMed] [Google Scholar]
- 134.Satoh N, Ogawa Y, Katsuura G, Numata Y, Masuzaki H, Yoshimasa Y, Nakao K. Satiety effect and sympathetic activation of leptin are mediated by hypothalamic melanocortin system. Neurosci Lett. 1998;249:107–110. doi: 10.1016/s0304-3940(98)00401-7. [DOI] [PubMed] [Google Scholar]
- 135.Schwartz MW, Seeley RJ, Woods SC, Weigle DS, Campfield LA, Burn P, Baskin DG. Leptin increases hypothalamic pro-opiomelanocortin mRNA expression in the rostral arcuate nucleus. Diabetes. 1997;46:2119–2123. doi: 10.2337/diab.46.12.2119. [DOI] [PubMed] [Google Scholar]
- 136.Elmquist JK. Hypothalamic pathways underlying the endocrine, autonomic, and behavioral effects of leptin. Int J Obes Relat Metab Disord. 2001;25(Suppl 5):S78–82. doi: 10.1038/sj.ijo.0801918. [DOI] [PubMed] [Google Scholar]
- 137.Williams DL, Bowers RR, Bartness TJ, Kaplan JM, Grill HJ. Brainstem melanocortin 3/4 receptor stimulation increases uncoupling protein gene expression in brown fat. Endocrinology. 2003;144:4692–4697. doi: 10.1210/en.2003-0440. [DOI] [PubMed] [Google Scholar]
- 138.Bronstein DM, Schafer MK, Watson SJ, Akil H. Evidence that beta-endorphin is synthesized in cells in the nucleus tractus solitarius: detection of POMC mRNA. Brain Res. 1992;587:269–275. doi: 10.1016/0006-8993(92)91007-2. [DOI] [PubMed] [Google Scholar]
- 139.Joseph SA, Pilcher WH, Bennett-Clarke C. Immunocytochemical localization of ACTH perikarya in nucleus tractus solitarius: evidence for a second opiocortin neuronal system. Neurosci Lett. 1983;38:221–225. doi: 10.1016/0304-3940(83)90372-5. [DOI] [PubMed] [Google Scholar]
- 140.Jacobowitz DM, O’Donohue TL. alpha-Melanocyte stimulating hormone: immunohistochemical identification and mapping in neurons of rat brain. Proc Natl Acad Sci U S A. 1978;75:6300–6304. doi: 10.1073/pnas.75.12.6300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Zheng H, Patterson LM, Rhodes C, Louis GW, Skibicka KP, Grill HJ, Myers MG, HRB Control of food intake by brainstem projections of arcuate POMC neurons. Am J Physiol 2009 [Google Scholar]
- 142.Blevins JE, Schwartz MW, Baskin DG. Evidence that paraventricular nucleus oxytocin neurons link hypothalamic leptin action to caudal brain stem nuclei controlling meal size. Am J Physiol Regul Integr Comp Physiol. 2004;287:R87–96. doi: 10.1152/ajpregu.00604.2003. [DOI] [PubMed] [Google Scholar]
- 143.Blevins JE, Eakin TJ, Murphy JA, Schwartz MW, Baskin DG. Oxytocin innervation of caudal brainstem nuclei activated by cholecystokinin. Brain Res. 2003;993:30–41. doi: 10.1016/j.brainres.2003.08.036. [DOI] [PubMed] [Google Scholar]
- 144.Peters JH, McDougall SJ, Kellett DO, Jordan D, Llewellyn-Smith IJ, Andresen MC. Oxytocin enhances cranial visceral afferent synaptic transmission to the solitary tract nucleus. J Neurosci. 2008;28:11731–11740. doi: 10.1523/JNEUROSCI.3419-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Bado A, Levasseur S, Attoub S, Kermorgant S, Laigneau JP, Bortoluzzi MN, Moizo L, Lehy T, Guerre-Millo M, Le Marchand-Brustel Y, Lewin MJ. The stomach is a source of leptin. Nature. 1998;394:790–793. doi: 10.1038/29547. [DOI] [PubMed] [Google Scholar]
- 146.Burdyga G, Spiller D, Morris R, Lal S, Thompson DG, Saeed S, Dimaline R, Varro A, Dockray GJ. Expression of the leptin receptor in rat and human nodose ganglion neurones. Neuroscience. 2002;109:339–347. doi: 10.1016/s0306-4522(01)00474-2. [DOI] [PubMed] [Google Scholar]
- 147.Buyse M, Ovesjo ML, Goiot H, Guilmeau S, Peranzi G, Moizo L, Walker F, Lewin MJ, Meister B, Bado A. Expression and regulation of leptin receptor proteins in afferent and efferent neurons of the vagus nerve. Eur J Neurosci. 2001;14:64–72. doi: 10.1046/j.0953-816x.2001.01628.x. [DOI] [PubMed] [Google Scholar]
- 148.Peiser C, Springer J, Groneberg DA, McGregor GP, Fischer A, Lang RE. Leptin receptor expression in nodose ganglion cells projecting to the rat gastric fundus. Neurosci Lett. 2002;320:41–44. doi: 10.1016/s0304-3940(02)00023-x. [DOI] [PubMed] [Google Scholar]
- 149.Wang YH, Tache Y, Sheibel AB, Go VL, Wei JY. Two types of leptin-responsive gastric vagal afferent terminals: an in vitro single-unit study in rats. Am J Physiol. 1997;273:R833–837. doi: 10.1152/ajpregu.1997.273.2.R833. [DOI] [PubMed] [Google Scholar]
- 150.Peters JH, Karpiel AB, Ritter RC, Simasko SM. Cooperative activation of cultured vagal afferent neurons by leptin and cholecystokinin. Endocrinology. 2004;145:3652–3657. doi: 10.1210/en.2004-0221. [DOI] [PubMed] [Google Scholar]
- 151.Peters JH, McKay BM, Simasko SM, Ritter RC. Leptin-induced satiation mediated by abdominal vagal afferents. Am J Physiol Regul Integr Comp Physiol. 2005;288:R879–884. doi: 10.1152/ajpregu.00716.2004. [DOI] [PubMed] [Google Scholar]
- 152.Peters JH, Ritter RC, Simasko SM. Leptin and CCK selectively activate vagal afferent neurons innervating the stomach and duodenum. Am J Physiol Regul Integr Comp Physiol. 2005 doi: 10.1152/ajpregu.00811.2005. [DOI] [PubMed] [Google Scholar]
- 153.Grill HJ, Kaplan JM. The neuroanatomical axis for control of energy balance. Front Neuroendocrinol. 2002;23:2–40. doi: 10.1006/frne.2001.0224. [DOI] [PubMed] [Google Scholar]
- 154.Watts AG. Neuropeptides and the integration of motor responses to dehydration. Annu Rev Neurosci. 2001;24:357–384. doi: 10.1146/annurev.neuro.24.1.357. [DOI] [PubMed] [Google Scholar]
- 155.Berthoud HR. Multiple neural systems controlling food intake and body weight. Neurosci Biobehav Rev. 2002;26:393–428. doi: 10.1016/s0149-7634(02)00014-3. [DOI] [PubMed] [Google Scholar]
- 156.Berthoud HR, Morrison C. The brain, appetite, and obesity. Annu Rev Psychol. 2008;59:55–92. doi: 10.1146/annurev.psych.59.103006.093551. [DOI] [PubMed] [Google Scholar]
- 157.Grill HJ, Skibicka KP, Hayes MR. Imaging obesity: fMRI, food reward, and feeding. Cell Metab. 2007;6:423–425. doi: 10.1016/j.cmet.2007.11.007. [DOI] [PubMed] [Google Scholar]
- 158.Batterham RL, ffytche DH, Rosenthal JM, Zelaya FO, Barker GJ, Withers DJ, Williams SC. PYY modulation of cortical and hypothalamic brain areas predicts feeding behaviour in humans. Nature. 2007;450:106–109. doi: 10.1038/nature06212. [DOI] [PubMed] [Google Scholar]
- 159.Farooqi IS, Bullmore E, Keogh J, Gillard J, O’Rahilly S, Fletcher PC. Leptin regulates striatal regions and human eating behavior. Science. 2007;317:1355. doi: 10.1126/science.1144599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Roth JD, Roland BL, Cole RL, Trevaskis JL, Weyer C, Koda JE, Anderson CM, Parkes DG, Baron AD. Leptin responsiveness restored by amylin agonism in diet-induced obesity: evidence from nonclinical and clinical studies. Proc Natl Acad Sci U S A. 2008;105:7257–7262. doi: 10.1073/pnas.0706473105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Heisler LK, Jobst EE, Sutton GM, Zhou L, Borok E, Thornton-Jones Z, Liu HY, Zigman JM, Balthasar N, Kishi T, Lee CE, Aschkenasi CJ, Zhang CY, Yu J, Boss O, Mountjoy KG, Clifton PG, Lowell BB, Friedman JM, Horvath T, Butler AA, Elmquist JK, Cowley MA. Serotonin reciprocally regulates melanocortin neurons to modulate food intake. Neuron. 2006;51:239–249. doi: 10.1016/j.neuron.2006.06.004. [DOI] [PubMed] [Google Scholar]
- 162.Chen M, Wang J, Dickerson KE, Kelleher J, Xie T, Gupta D, Lai EW, Pacak K, Gavrilova O, Weinstein LS. Central nervous system imprinting of the G protein G(s)alpha and its role in metabolic regulation. Cell Metab. 2009;9:548–555. doi: 10.1016/j.cmet.2009.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Yadav VK, Oury F, Suda N, Liu ZW, Gao XB, Confavreux C, Klemenhagen KC, Tanaka KF, Gingrich JA, Guo XE, Tecott LH, Mann JJ, Hen R, Horvath TL, Karsenty G. A serotonin-dependent mechanism explains the leptin regulation of bone mass, appetite, and energy expenditure. Cell. 2009;138:976–989. doi: 10.1016/j.cell.2009.06.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Kotz CM, Glass MJ, Levine AS, Billington CJ. Regional effect of naltrexone in the nucleus of the solitary tract in blockade of NPY-induced feeding. Am J Physiol Regul Integr Comp Physiol. 2000;278:R499–503. doi: 10.1152/ajpregu.2000.278.2.R499. [DOI] [PubMed] [Google Scholar]