Abstract
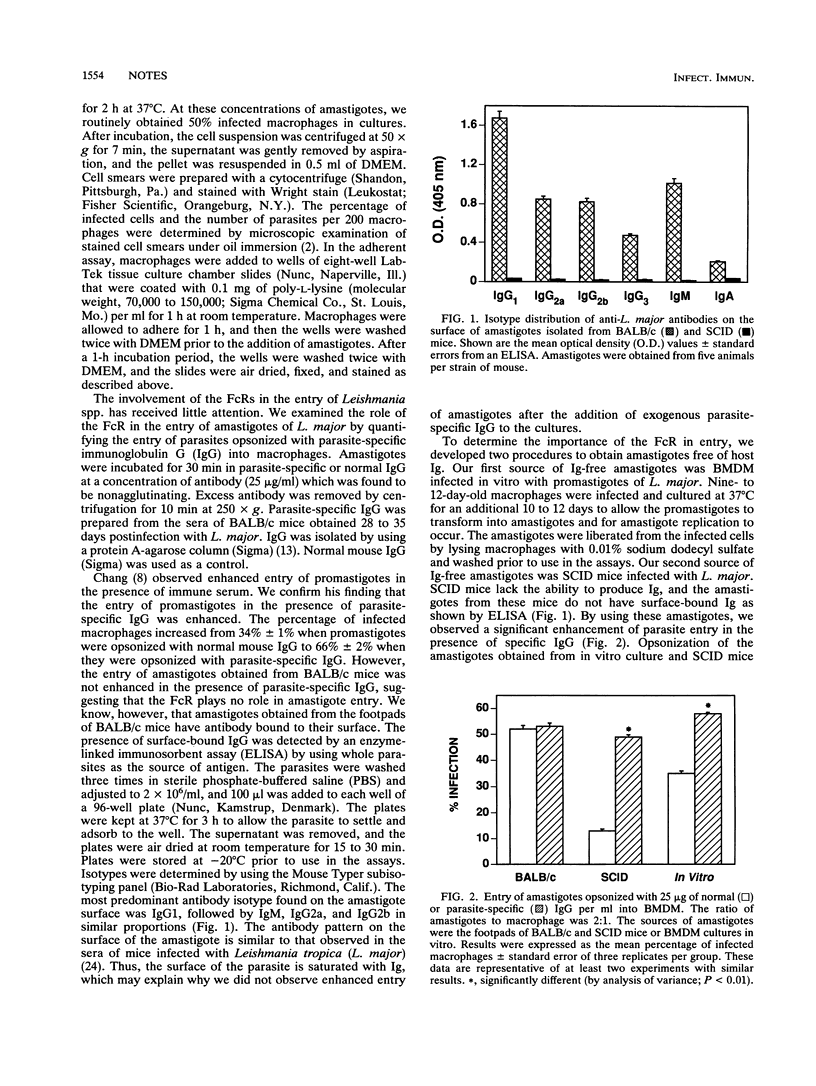
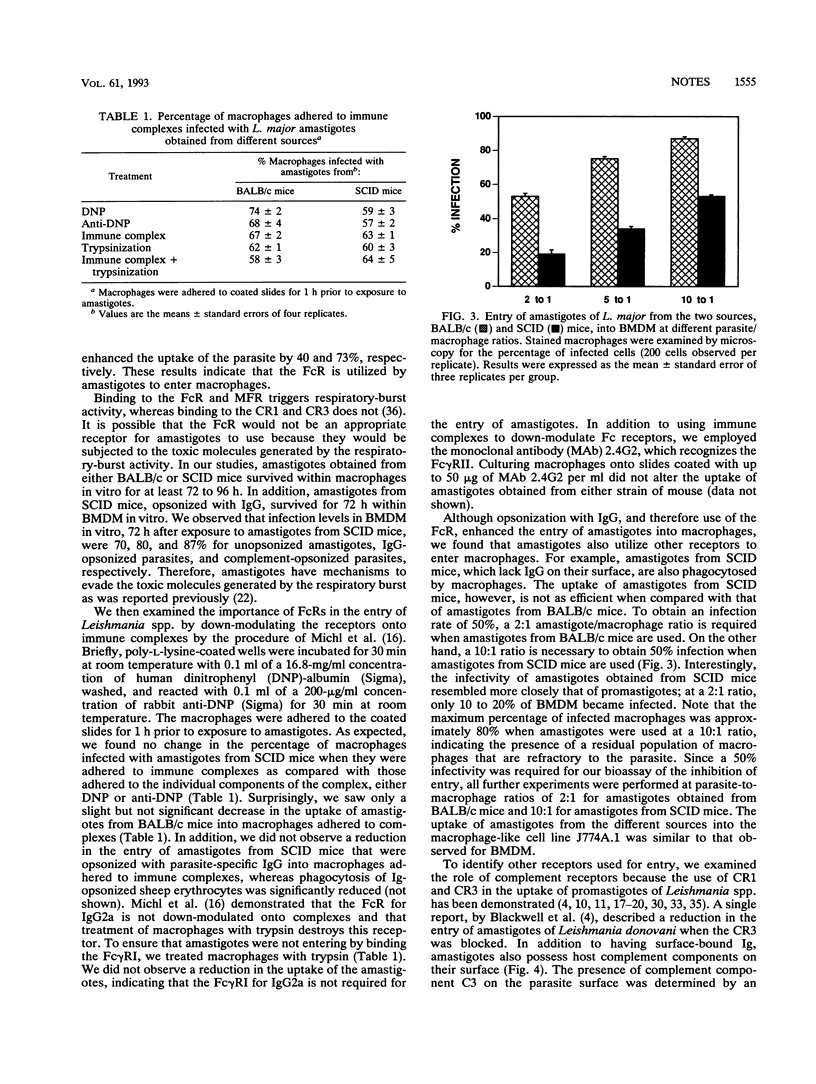
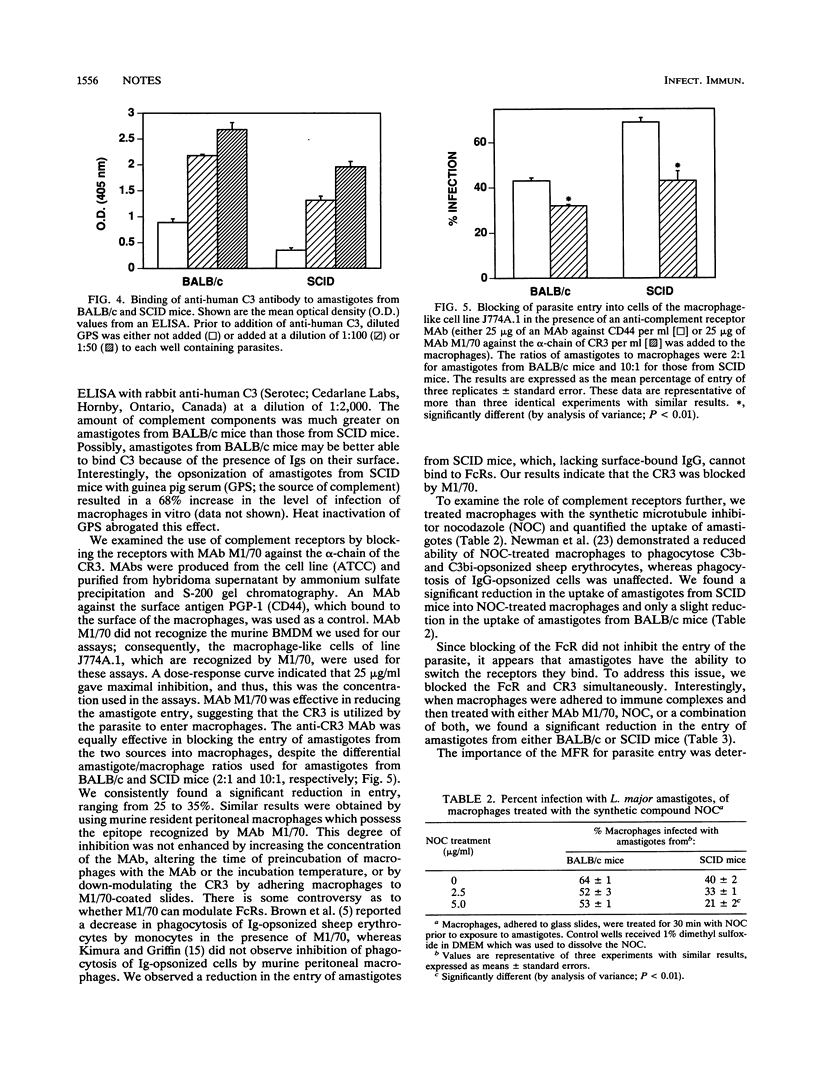
We investigated the mechanisms of entry of amastigotes of Leishmania major from two different sources into macrophages by comparing their use of the Fc receptor (FcR), complement receptor type 3 (CR3), and mannose-fucose receptor (MFR). Amastigotes were obtained from BALB/c mice and SCID mice. FcR involvement was examined by opsonizing L. major with parasite-specific immunoglobulin G (IgG). Antiparasite IgG did not alter the uptake of amastigotes from BALB/c mice since these amastigotes had antibody bound to their surface: IgG1 was the most predominant antibody, followed by IgG2b, IgM, and IgG2a. However, opsonization with antiparasite IgG enhanced the entry of amastigotes that lacked antibody on their surface, namely, amastigotes obtained from SCID mice or from macrophages infected in vitro. These results indicate that the FcR is important for amastigote entry into macrophages. Down-modulation of FcRs onto immune complexes, however, did not reduce the entry of amastigotes containing surface-bound IgG into macrophages. Monoclonal antibodies against the CR3 inhibited the entry of amastigotes from either BALB/c or SCID mice into J774A.1 macrophage-like cells. Simultaneous blocking of FcR and CR3 further increased the inhibition of phagocytosis. Treatment of macrophages with soluble mannan or down-modulating the MFR onto mannan-coated coverslips had no effect on the entry of amastigotes from BALB/c or SCID mice. Thus, the MFR does not appear to be used by amastigotes of L. major. We show that ingestion of amastigotes appears to occur primarily through the FcR and CR3; however, additional receptors may also participate in the uptake of amastigotes.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Basu N., Sett R., Das P. K. Down-regulation of mannose receptors on macrophages after infection with Leishmania donovani. Biochem J. 1991 Jul 15;277(Pt 2):451–456. doi: 10.1042/bj2770451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belosevic M., Davis C. E., Meltzer M. S., Nacy C. A. Regulation of activated macrophage antimicrobial activities. Identification of lymphokines that cooperate with IFN-gamma for induction of resistance to infection. J Immunol. 1988 Aug 1;141(3):890–896. [PubMed] [Google Scholar]
- Bermudez L. E., Young L. S., Enkel H. Interaction of Mycobacterium avium complex with human macrophages: roles of membrane receptors and serum proteins. Infect Immun. 1991 May;59(5):1697–1702. doi: 10.1128/iai.59.5.1697-1702.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackwell J. M., Ezekowitz R. A., Roberts M. B., Channon J. Y., Sim R. B., Gordon S. Macrophage complement and lectin-like receptors bind Leishmania in the absence of serum. J Exp Med. 1985 Jul 1;162(1):324–331. doi: 10.1084/jem.162.1.324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown E. J., Bohnsack J. F., Gresham H. D. Mechanism of inhibition of immunoglobulin G-mediated phagocytosis by monoclonal antibodies that recognize the Mac-1 antigen. J Clin Invest. 1988 Feb;81(2):365–375. doi: 10.1172/JCI113328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bullock W. E., Wright S. D. Role of the adherence-promoting receptors, CR3, LFA-1, and p150,95, in binding of Histoplasma capsulatum by human macrophages. J Exp Med. 1987 Jan 1;165(1):195–210. doi: 10.1084/jem.165.1.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakraborty P., Das P. K. Role of mannose/N-acetylglucosamine receptors in blood clearance and cellular attachment of Leishmania donovani. Mol Biochem Parasitol. 1988 Feb;28(1):55–62. doi: 10.1016/0166-6851(88)90180-6. [DOI] [PubMed] [Google Scholar]
- Chang K. P. Leishmania donovani-macrophage binding mediated by surface glycoproteins/antigens: characterization in vitro by a radioisotopic assay. Mol Biochem Parasitol. 1981 Nov;4(1-2):67–76. doi: 10.1016/0166-6851(81)90030-x. [DOI] [PubMed] [Google Scholar]
- Channon J. Y., Roberts M. B., Blackwell J. M. A study of the differential respiratory burst activity elicited by promastigotes and amastigotes of Leishmania donovani in murine resident peritoneal macrophages. Immunology. 1984 Oct;53(2):345–355. [PMC free article] [PubMed] [Google Scholar]
- Cooper A., Rosen H., Blackwell J. M. Monoclonal antibodies that recognize distinct epitopes of the macrophage type three complement receptor differ in their ability to inhibit binding of Leishmania promastigotes harvested at different phases of their growth cycle. Immunology. 1988 Dec;65(4):511–514. [PMC free article] [PubMed] [Google Scholar]
- Da Silva R. P., Hall B. F., Joiner K. A., Sacks D. L. CR1, the C3b receptor, mediates binding of infective Leishmania major metacyclic promastigotes to human macrophages. J Immunol. 1989 Jul 15;143(2):617–622. [PubMed] [Google Scholar]
- Ezekowitz R. A., Williams D. J., Koziel H., Armstrong M. Y., Warner A., Richards F. F., Rose R. M. Uptake of Pneumocystis carinii mediated by the macrophage mannose receptor. Nature. 1991 May 9;351(6322):155–158. doi: 10.1038/351155a0. [DOI] [PubMed] [Google Scholar]
- Jackson P. R., Pappas M. G., Hansen B. D. Fluorogenic substrate detection of viable intracellular and extracellular pathogenic protozoa. Science. 1985 Jan 25;227(4685):435–438. doi: 10.1126/science.2578226. [DOI] [PubMed] [Google Scholar]
- Kimura M., Griffin F. M., Jr C3bi/CR3 is a main ligand-receptor interaction in attachment and phagocytosis of C3-coated particles by mouse peritoneal macrophages. Scand J Immunol. 1992 Aug;36(2):183–191. doi: 10.1111/j.1365-3083.1992.tb03090.x. [DOI] [PubMed] [Google Scholar]
- Michl J., Pieczonka M. M., Unkeless J. C., Silverstein S. C. Effects of immobilized immune complexes on Fc- and complement-receptor function in resident and thioglycollate-elicited mouse peritoneal macrophages. J Exp Med. 1979 Sep 19;150(3):607–621. doi: 10.1084/jem.150.3.607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosser D. M., Edelson P. J. Activation of the alternative complement pathway by Leishmania promastigotes: parasite lysis and attachment to macrophages. J Immunol. 1984 Mar;132(3):1501–1505. [PubMed] [Google Scholar]
- Mosser D. M., Edelson P. J. The mouse macrophage receptor for C3bi (CR3) is a major mechanism in the phagocytosis of Leishmania promastigotes. J Immunol. 1985 Oct;135(4):2785–2789. [PubMed] [Google Scholar]
- Mosser D. M., Edelson P. J. The third component of complement (C3) is responsible for the intracellular survival of Leishmania major. 1987 May 28-Jun 3Nature. 327(6120):329–331. doi: 10.1038/327329b0. [DOI] [PubMed] [Google Scholar]
- Mosser D. M., Springer T. A., Diamond M. S. Leishmania promastigotes require opsonic complement to bind to the human leukocyte integrin Mac-1 (CD11b/CD18). J Cell Biol. 1992 Jan;116(2):511–520. doi: 10.1083/jcb.116.2.511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosser D. M., Vlassara H., Edelson P. J., Cerami A. Leishmania promastigotes are recognized by the macrophage receptor for advanced glycosylation endproducts. J Exp Med. 1987 Jan 1;165(1):140–145. doi: 10.1084/jem.165.1.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray H. W. Cell-mediated immune response in experimental visceral leishmaniasis. II. Oxygen-dependent killing of intracellular Leishmania donovani amastigotes. J Immunol. 1982 Jul;129(1):351–357. [PubMed] [Google Scholar]
- Newman S. L., Mikus L. K., Tucci M. A. Differential requirements for cellular cytoskeleton in human macrophage complement receptor- and Fc receptor-mediated phagocytosis. J Immunol. 1991 Feb 1;146(3):967–974. [PubMed] [Google Scholar]
- Olobo J. O., Handman E., Curtis J. M., Mitchell G. F. Antibodies to Leishmania tropica promastigotes during infection in mice of various genotypes. Aust J Exp Biol Med Sci. 1980 Dec;58(6):595–601. doi: 10.1038/icb.1980.61. [DOI] [PubMed] [Google Scholar]
- Palatnik C. B., Borojevic R., Previato J. O., Mendonça-Previato L. Inhibition of Leishmania donovani promastigote internalization into murine macrophages by chemically defined parasite glycoconjugate ligands. Infect Immun. 1989 Mar;57(3):754–763. doi: 10.1128/iai.57.3.754-763.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payne N. R., Horwitz M. A. Phagocytosis of Legionella pneumophila is mediated by human monocyte complement receptors. J Exp Med. 1987 Nov 1;166(5):1377–1389. doi: 10.1084/jem.166.5.1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizvi F. S., Ouaissi M. A., Marty B., Santoro F., Capron A. The major surface protein of Leishmania promastigotes is a fibronectin-like molecule. Eur J Immunol. 1988 Mar;18(3):473–476. doi: 10.1002/eji.1830180323. [DOI] [PubMed] [Google Scholar]
- Russell D. G., Talamas-Rohana P., Zelechowski J. Antibodies raised against synthetic peptides from the Arg-Gly-Asp-containing region of the Leishmania surface protein gp63 cross-react with human C3 and interfere with gp63-mediated binding to macrophages. Infect Immun. 1989 Feb;57(2):630–632. doi: 10.1128/iai.57.2.630-632.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell D. G., Wilhelm H. The involvement of the major surface glycoprotein (gp63) of Leishmania promastigotes in attachment to macrophages. J Immunol. 1986 Apr 1;136(7):2613–2620. [PubMed] [Google Scholar]
- Russell D. G., Wright S. D. Complement receptor type 3 (CR3) binds to an Arg-Gly-Asp-containing region of the major surface glycoprotein, gp63, of Leishmania promastigotes. J Exp Med. 1988 Jul 1;168(1):279–292. doi: 10.1084/jem.168.1.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlesinger L. S., Bellinger-Kawahara C. G., Payne N. R., Horwitz M. A. Phagocytosis of Mycobacterium tuberculosis is mediated by human monocyte complement receptors and complement component C3. J Immunol. 1990 Apr 1;144(7):2771–2780. [PubMed] [Google Scholar]
- Schlesinger L. S., Horwitz M. A. Phagocytosis of Mycobacterium leprae by human monocyte-derived macrophages is mediated by complement receptors CR1 (CD35), CR3 (CD11b/CD18), and CR4 (CD11c/CD18) and IFN-gamma activation inhibits complement receptor function and phagocytosis of this bacterium. J Immunol. 1991 Sep 15;147(6):1983–1994. [PubMed] [Google Scholar]
- Talamás-Rohana P., Wright S. D., Lennartz M. R., Russell D. G. Lipophosphoglycan from Leishmania mexicana promastigotes binds to members of the CR3, p150,95 and LFA-1 family of leukocyte integrins. J Immunol. 1990 Jun 15;144(12):4817–4824. [PubMed] [Google Scholar]
- Wilson M. E., Pearson R. D. Evidence that Leishmania donovani utilizes a mannose receptor on human mononuclear phagocytes to establish intracellular parasitism. J Immunol. 1986 Jun 15;136(12):4681–4688. [PubMed] [Google Scholar]
- Wilson M. E., Pearson R. D. Roles of CR3 and mannose receptors in the attachment and ingestion of Leishmania donovani by human mononuclear phagocytes. Infect Immun. 1988 Feb;56(2):363–369. doi: 10.1128/iai.56.2.363-369.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright S. D., Silverstein S. C. Receptors for C3b and C3bi promote phagocytosis but not the release of toxic oxygen from human phagocytes. J Exp Med. 1983 Dec 1;158(6):2016–2023. doi: 10.1084/jem.158.6.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyler D. J., Sypek J. P., McDonald J. A. In vitro parasite-monocyte interactions in human leishmaniasis: possible role of fibronectin in parasite attachment. Infect Immun. 1985 Aug;49(2):305–311. doi: 10.1128/iai.49.2.305-311.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]


