Abstract
Human immunodeficiency virus-associated nephropathy (HIVAN) is a leading cause of end-stage renal disease in African Americans. The HIV-1 virus infects podocytes, cells integral to formation of the glomerular filtration barrier, often leading to focal segmental glomerulosclerosis. HIVAN is typically a complication of late-stage HIV infection, associated with low CD4 cell counts and elevated serum HIV RNA levels. Highly active antiretroviral therapy (HAART) is partially protective and has altered the natural history of HIV-associated kidney disease. Nonetheless, HIVAN remains an important public health concern among HIV-infected African Americans. Although polymorphisms in the MYH9 gene on chromosome 22 are strongly associated with HIVAN, as well as with idiopathic focal segmental glomerulosclerosis and global glomerulosclerosis (historically labeled "hypertensive nephrosclerosis"), the majority of HIV-infected patients who are genetically at risk from MYH9 do not appear to develop severe kidney disease. Therefore, we postulate that additional environmental exposures and/or inherited factors are necessary to initiate human HIVAN. Gene-environment interactions have also been proposed as necessary for initiation of HIVAN in murine models. It is important that these novel risk factors be identified, as prevention of environmental exposures and targeting of additional gene products may reduce the risk for HIVAN, even among those harboring two risk alleles in MYH9.
Keywords: African Americans, FSGS, HIV-associated nephropathy, kidney disease, MYH9
Introduction
HIVAN was initially described as “acquired immunodeficiency syndrome (AIDS) Nephropathy” in 1984.1 HIVAN is a common cause of CKD, observed primarily in African Americans (AA).2–6 Pathologically, HIVAN is characterized by the collapsing variant of focal segmental glomerulosclerosis (FSGS), with microcystic tubular dilation, tubulointerstitial nephritis, and tubuloreticular inclusions.7,8 HIVAN can be a rapidly progressive form of FSGS and is characterized by podocyte proliferation. Patients with pathologically-proven HIVAN demonstrate poorer renal survival relative to HIV-infected individuals with other etiologies of CKD.3,9,10 HIV infection and diabetes appear to convey similar risks for kidney disease in AA.11
Epidemiology of HIV-associated nephropathy
The U.S. Renal Data System (USRDS) reported that 0.97% of the 375,152 incident patients initiating chronic dialysis between January 1992 and June 1997 had HIVAN, and 87% were AA.6 Four thousand new cases of ESRD were attributed to HIV in the U.S. in 2005,12 making HIVAN the third leading cause of ESRD in AA between the ages of 20–64 years. The incidence of HIVAN peaked in the U.S. during the mid-1990s13 and declined by 50% in the 1998–2001 time period, relative to 1995–1997, in association with the widespread use of HAART.2 Although the incidence of HIVAN decreased during the HAART era, its prevalence is now increasing due to the aging of patients as a result of improved survival among those with HIV infection.14 The true prevalence of HIVAN remains unknown, since the diagnosis requires viral testing and renal histologic analysis; more cases likely exist than are reported. In addition, non-HIVAN causes of kidney disease are often seen in HIV-infected patients, some of which result from HAART.15 Table 1 summarizes the published incidence and prevalence rates of HIVAN among AA HIV-infected subjects. The prevalence of HIVAN in HIV-infected AA ranges from 3.5% for proteinuria to 12% in postmortem studies.16,17 A report in South Africans revealed higher frequencies of HIV immune complex kidney disease (HIVICK), as well as membranous, mesangial hyperplasia, and IgA nephropathy, relative to AA.18
Table 1.
Incidence and prevalence of HIVAN among HIV-infected African Americans. Abbreviations: HIVAN, HIV-associated nephropathy; TDCCJI, Texas Department of Criminal Justice Inmates.
| Reference # |
Location | Study Type | Definition of HIVAN |
HIVAN Prevalence |
HIVAN Incidence |
|---|---|---|---|---|---|
| 15 | Galveston, TX |
Cross-sectional | Biopsy or clinical | 3.5% (10/282) | |
| 16 | TDCCJI, Tx |
Cross-sectional, Post-mortem |
Autopsy | 12% (25/209) | |
| 2 | Baltimore, MD |
Longitudinal | Biopsy or clinical | 10.1/1000 person-years |
|
| 9 | Baltimore, MD |
Longitudinal | Biopsy or clinical | 1% per year |
HIVAN is most often a complication of the late stages of HIV infection, associated with low CD4 cell counts and elevated serum HIV RNA levels.2,3,9,10,19–21 However, HIVAN can develop in patients with undetectable viral loads and high CD4 cell counts.3,22 Prior history of a low CD4 cell count (ie, nadir CD4 cell count <200 cells/mm3) is a risk factor for HIVAN,9 and there is a shorter time to initiation of renal replacement therapy in those with lower CD4 cell counts.3 Prior to the availability of HAART, the prognosis for patients with HIVAN was dismal, and survival was measured in weeks to months.23 There are several lines of evidence supporting a beneficial role of HIV treatment in improving survival and preserving kidney function, including the negative correlation between CD4 cell count and HIVAN, the decline in the incidence of HIVAN during the HAART era, and case reports of resolution of renal failure after initiation of HAART.23–27 Nevertheless, HAART is only partially protective, and many patients with HIVAN ultimately progress to ESRD.2,23,28 CD4 cell count restoration using HAART does not correlate with improved renal outcomes,10,23 and 65% of patients who progress to ESRD after a diagnosis of HIVAN have had undetectable HIV RNA levels for median durations approaching two years.23
Pathogenesis of HIVAN
Although HIV-1 viral mRNA and DNA have been detected in renal glomerular and tubular epithelial cells, the mode of HIV-1 viral entry into kidney cells has not been determined.29 Renal epithelial cells lack both CD4 and CD4 co-receptors CXCR4 and CCR5, used by HIV-1 for cellular entry.30 However, expression of CXCR5, an HIV-2 co-receptor, has been demonstrated on podocytes.31 A "renal reservoir" of HIV-1 has been described during primary viral infection.32 Renal biopsies have revealed restoration of the architecture of the renal tubules and resolution of podocyte hypertrophy and glomerular collapse three months after the initiation of antiretroviral therapy. The number of podocytes expressing viral mRNA was unchanged. Similarly, Bruggeman and others observed replication of HIV in renal cells of individuals with HIVAN despite treatment with antiretroviral agents and undetectable serum viral loads.29
Local replication of HIV-1 in renal tubular cells has been confirmed.33 DNA extracted from renal tubular cells in two patients with HIVAN was used to amplify HIV-1 V3-loop or gp120-envelope sequences. Sequences were compared to the corresponding sequences in monocytes obtained from these same individuals. Sequences obtained from kidney tissue formed tissue-specific subclusters, suggesting local (renal) replication of HIV-1.33
Normally, podocytes are post-mitotic and do not proliferate. In HIVAN, podocytes appear to de-differentiate and proliferate. Proliferation of de-differentiated podocytes has been proposed as a common mechanism in the development of FSGS and HIVAN.34 Renal biopsy specimens from individuals with FSGS and HIVAN reveal a lack of expression of mature podocyte markers, such as WT-1 (Wilms Tumor-1), CALLA (common acute lymphoblastic leukemia angtigen), C3b receptor, GLEPP-1 (glomerular epithelial protein-1), podocalyxin, and synaptopodin.35 While expression of these markers is lost in the collapsed glomeruli of individuals with HIVAN and collapsing FSGS, expression is conserved in other proteinuric renal diseases, such as minimal change disease (MCD) and membranous glomerulonephritis. In addition, staining for the cell cycle protein Ki-67, an indicator of cell proliferation, was observed in renal tissue from HIVAN, but absent in MCD and membranous glomerulonephritis. Similarly, Winston and colleagues reported that Ki-67 staining was present in podocytes and tubular epithelial cells in HIVAN prior to antiretroviral therapy and disappeared during therapy.32
Expression of HIV in the kidney appears to be necessary for the development of HIVAN, as evidenced by cross-transplantation experiments. Renal transplants were performed between HIV-1 transgenic mice and normal litter mates. The normal kidneys did not develop evidence of HIVAN, while the transgenic kidneys developed nephropathy. This experiment suggests that HIVAN resulted directly from HIV-1 gene expression in the kidney rather than as a result of exposure to circulating virus or other circulating factors.36 Using transgenes encoding the HIV-1 gene products vpr, vif, tat, rev, nef and vpu, Zhong and others observed that transgenic mice expressing HIV-1 selectively in podocytes developed proteinuria and collapsing FSGS with microcystic tubular dilation consistent with HIVAN.37
Of the nine genes encoded by HIV-1, nef and vpr have been implicated in HIVAN. By creating HIV-transgenic mice with mutations in vpr, nef, or both sequences, Dickie and associates found that proteinuria and focal glomerulosclerosis only developed in mice with an intact vpr gene, while mice with mutated nef had less severe disease.38 The findings show a fundamental role for vpr in induction of HIVAN and are consistent with other studies revealing interactions between nef and vpr.39 Expression of vpr in cultured renal tubule epithelial cells impairs cytokinesis, leading to cell enlargement and multinucleation.37 Genetic backgrounds are important in the susceptibility to podocyte injury. In the experiments by Zuo and colleagues and Zhong and others, the transgenic lines developed podocyte injury on FVB/N genetic backgrounds, but not on C57BL/6 backgrounds. 37,39
Nef is also involved in the pathogenesis of HIVAN. Using podocytes infected with constructs containing premature stop codons in HIV-1 env, vif, vpr, vpu, nef, or rev genes, podocyte proliferation and anchorage-independent growth were seen in all of the constructs except those containing the nef deletion.40 HIV-1 nef has been shown to result in proliferation of podocytes and decreased expression of synaptopodin and CALLA.41 Expression of nef in podocytes resulted in loss of staining for synaptopodin and WT-1 and expression of the proliferation markers Ki-67 and phosphor-Stat3.42 Infection with nef also triggers pathways involved in inflammation, altering the actin cytoskeleton. Infection of podocytes with nef resulted in increased Src kinase activity and phosphorylation of Stat3, a signaling pathway necessary for cell proliferation. Nef induced MAPK1,2 (mitogen-activated protein kinase-1,2) phosphorylation in podocytes both in vitro and in vivo.43 Lu and others demonstrated that infection with HIV-1 nef resulted in changes in the actin cytoskeleton and podocyte effacement in vitro. Actin stress fibers form as a result of activation of Rho and lamellipodia form as a result of activation of Rac. Nef was found to inhibit RhoA activity and increase activity of Rac1 via Src-induced phosphorylation of Diaphenous interacting protein, p190RhoGAP and vav2.44
Genetic factors in human HIVAN
The association of HIVAN with AA ethnicity was recognized in multiple early studies.2–4,6,45 Among HIV-infected patients, AA had a 31-fold higher risk of ESRD compared to European Americans (EA).14 The association with African ancestry has also been reported in Europe and Africa.19,20,46–48
African Americans have disproportionate risks of several common etiologies of CKD, including FSGS and the distinct collapsing form of FSGS as seen in HIVAN. While these findings suggested a genetic basis, many attributed this risk to ethnic differences in socioeconomic status and lifestyle. In the late 1990’s, we demonstrated marked familial aggregation of HIVAN in AA,28 and these patients often had close relatives with ESRD lacking HIV infection. Kidney failure in these relatives was typically attributed to essential hypertension, diabetes, or idiopathic FSGS.49 The clustering of disparate forms of ESRD in AA families suggested the existence of overarching “renal failure susceptibility genes,” genes independent of the systemic processes of HIV infection, hyperglycemia, or hypertension.50
It has since been shown that polymorphisms in the MYH9 gene are strongly associated with HIVAN in AA, as well as with idiopathic FSGS and clinically diagnosed “hypertension-associated” ESRD (global glomerulosclerosis).51–53 MYH9 encodes non-muscle myosin heavy chain IIA and is expressed in the glomerular podocyte. The population attributable risk for HIVAN in AA related to MYH9 is 79%, with an odds ratio of 5.51 Population attritutable risk reflects the percentage of disease that would disappear if the risk allele were replaced by neutral alleles. In this case, 79% of HIVAN in AA could be prevented if European-derived or non-African derived MYH9 variants were inherited. Sixty percent of AA in the general population possessed one MYH9 risk allele compared to 4% of EA, and 35% of AA are homozygous for MYH9 risk alleles compared to only 1% of EA. Ethnic differences in MYH9 genotype distributions seem to account for the excess risk of non-diabetic ESRD observed in AA.51;52
The realization that approximately 4–5% of AA individuals inheriting two copies of MYH9 risk variants ultimately developed FSGS (personal communication, Dr. Jeffrey Kopp) demonstrated that additional factors or “second hits” must be involved in the initiation of MYH9-associated nephropathy. Since approximately 20% of AA MYH9 risk homozygotes with HIV-1 infection develop HIVAN (personal communication, Dr. Jeffrey Kopp), a fourfold increase over rates seen in the absence of HIV infection, we postulate that HIV-1 is one environmental co-factor. Additional co-factors could include non-HIV-1 environmental exposures (gene-environment interactions) or could result from gene-gene interactions (particularly between MYH9 and other podocyte-specific gene polymorphisms). The proposed second hit model for initiation of HIVAN is not limited to this disease, but is also expected in the full spectrum of "MYH9-associated nephropathies" (FSGS and global glomerulosclerosis), since MYH9 is thought to be an overarching "renal failure susceptibility gene" independent of HIV infection.
Murine models of HIVAN suggest the existence of podocyte-specific gene polymorphisms that, with MYH9, may produce gene-gene interactions. Linkage analysis of HIVAN in mice reveal susceptibility loci located on chromosomes 3 (HIVAN1) and 13 (HIVAN2).54,55 Expression quantitative trait locus (eQTL) analysis of podocyte genes in an HIV-1 transgenic mouse model is a potentially powerful tool for detecting HIVAN genetic susceptibility in the murine model, with further implications for human disease. Transcript levels of the podocyte gene nephrosis 2 homologue (Nphs2) were shown to be heritable and controlled by an ancestral cis-eQTL, inducing threefold variation in gene expression. Downstream changes in other podocyte genes were induced by Nphs2 expression. Nphs2 expression is further controlled by two trans-eQTLs localizing to nephropathy susceptibility loci (HIVAN1 and HIVAN2). This report demonstrated that transcript levels of Nphs2 and other podocyte-expressed genes are networked. The genetic response to HIV-1 infection (a gene-environment interaction) appeared to alter this network and produce HIVAN. This would appear relevant to the human disease, where the presence of MYH9 risk alleles and HIV-1 infection do not universally initiate HIVAN.
Novel environmental factors potentially involved in MYH9-associated HIVAN
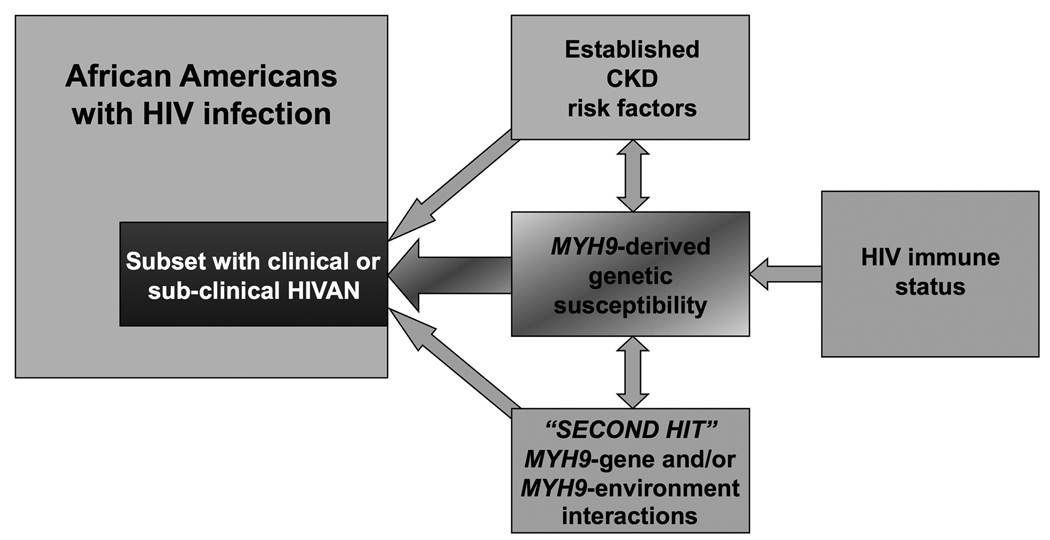
HIVAN illustrates that both host genetic factors and environmental exposures are jointly required for the initiation of kidney disease, with evidence of the role of MYH9 genotype and of infection of renal cells by HIV-1. As stated above, we believe that factors other than HIV infection may be necessary for the initiation of HIVAN in MYH9 homozygotes (Figure 1). It also remains possible that MYH9-induced abnormalities in platelet function contribute to glomerular injury.56
Figure 1.
Potential co-factors involved in MYH9-associated HIVAN. Abbreviations: HIV, human immunodeficiency virus; HIVAN, HIV-associated nephropathy.
As HIV is a lymphotropic virus and causes immunodeficiency, other lymphotropic viruses, such as human herpes virus (HHV)-6, cytomegalovirus (CMV), and human T-cell lymphotropic virus-I (HTLV-I) and HTLV-II have the potential to contribute to MYH9-associated FSGS, in the presence or absence of HIV-1 infection.57–59 After primary infection, HHVs maintain latent persistence, typically punctuated by sporadic episodes of symptomatic lytic activation. After kidney transplantation, renal biopsies often reveal positive polymerase chain reaction for CMV and HHV-6.60 Immunosuppression facilitates reactivation of these viruses, and HIV-infected patients are at high risk. In addition, CMV and HTLV-I/II share transmission routes with HIV and are likely more prevalent among those with HIV infection. It is possible that exposure to non-HIV lymphotropic viruses could interact with MYH9 genotype to initiate MYH9-nephropathy.
Viruses infecting the nephro-urinary system could also potentially act as second hits in the development of HIVAN, especially in patients with advanced immunodeficiency. BK and JC polyoma viruses infect the urologic system and are associated with nephropathy.61 It is thought that viremia during primary JC and BK polyomavirus infections results in seeding of the kidney, establishing clinically latent infection. Approximately 30–50% of normal individuals have detectable BK or JC virus sequences in renal tissue obtained at surgery or autopsy, and PCR has demonstrated BK and JC viruria in asymptomatic non-immunocompromised adults.62 BK nephropathy has been reported in transplanted and HIV-infected patients.61,63 These findings suggest that BK and JC polyomavirus could be infections (or co-infections with HIV) that might initiate MYH9-associated nephropathy. Further research is needed in order to assess the role of these or other novel HIVAN risk factors among genetically at-risk individuals. Table 2 lists viral infections that we postulate could initiate MYH9-associated nephropathy or HIVAN and provides data on the prevalence of positive serology, viremia, and viruria in immunocompetent subjects. No data on the prevalence of these viruses in HIV-infected patients are available.
Table 2.
Viral infections potentially associated with MYH9-associated HIV-associated nephropathy. Abbreviations: HHV, human herpes virus; CMV, cytomegalovirus; HTLV, human T-cell lymphotropic virus; PCR, polymerase chain reaction.
| Virus | % seroprevalence in U.S. population |
% detectable blood PCR, non-immunocompromised |
% detectable urine PCR, non-immunocompromised |
|---|---|---|---|
| HHV-6 A/B | 60%–100 | 30% (type B) | --- |
| CMV | 37%–70 | 1%–43% | |
| HTLV-I | 2%–15 | --- | --- |
| HTLV-II | 2%–15 | --- | --- |
| JC polyoma | 60%–80 | 0.9% | 38%–72% |
| BK polyoma | 60%–80 | 8.2% | 14%–43% |
Hepatitis C (HCV) and hepatitis B (HBV) virus share transmission routes with HIV and could potentially be involved in the pathogenesis MYH9-associated nephropathy. While epidemiological analyses show a link between HCV seropositivity and risk for ESRD,64 a role for HCV in the pathogenesis of HIVAN is unknown. Likewise, HBV-co-infection (HBV surface antigen positivity) is reportedly present more often than in the general HIV population,3 but involvement in HIVAN has not been established.
Conclusions
HIVAN is a common cause of ESRD in AA. HIV-1 infects podocytes comprising the glomerular filtration barrier and causes FSGS. HIVAN is most often a complication of late stages of HIV infection, and HAART is partially protective. While polymorphisms in the MYH9 gene are strongly associated with HIVAN in AA, not all HIV-infected patients who are at risk based upon their MYH9 alleles develop HIVAN. Since the majority of HIV-infected MYH9-risk homozygotes do not develop nephropathy, additional environmental and/or inherited factors are clearly required. Based on the results of murine transgenic models of HIVAN, we postulate that MYH9-podocyte-specific gene interactions may increase susceptibility to human HIVAN. Also, based on the immune effects of HIV-1 we postulate that other lymphotropic and/or nephropathic viral infections (gene-environment interactions) may increase the risk for developing MYH9-associated HIVAN. Further research is urgently needed to detect these novel HIVAN risk factors and develop methods to block the development of HIVAN among genetically at-risk individuals. It is now clear that HIVAN lies within the broad spectrum of "MYH9-associated nephropathy," kidney diseases including idiopathic FSGS and global glomerulosclerosis in non-HIV-infected individuals.
Acknowledgment
This work was supported in part by NIH grants RO1 DK 070941 (BIF) and RO1DK084149 (BIF).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors report no conflicts of interest in this work.
REFERENCES
- 1.Rao TK, Filippone EJ, Nicastri AD, et al. Associated focal and segmental glomerulosclerosis in the acquired immunodeficiency syndrome. N Engl J Med. 1984;310:669–673. doi: 10.1056/NEJM198403153101101. [DOI] [PubMed] [Google Scholar]
- 2.Lucas GM, Eustace JA, Sozio S, Mentari EK, Appiah KA, Moore RD. Highly active antiretroviral therapy and the incidence of HIV-1-associated nephropathy: a 12-year cohort study. AIDS. 2004;18:541–546. doi: 10.1097/00002030-200402200-00022. [DOI] [PubMed] [Google Scholar]
- 3.Szczech LA, Gupta SK, Habash R, et al. The clinical epidemiology and course of the spectrum of renal diseases associated with HIV infection. Kidney Int. 2004;66:1145–1152. doi: 10.1111/j.1523-1755.2004.00865.x. [DOI] [PubMed] [Google Scholar]
- 4.Carbone L, D'Agati V, Cheng JT, Appel GB. Course and prognosis of human immunodeficiency virus-associated nephropathy. Am J Med. 1989;87:389–395. doi: 10.1016/s0002-9343(89)80819-8. [DOI] [PubMed] [Google Scholar]
- 5.Cantor ES, Kimmel PL, Bosch JP. Effect of race on expression of acquired immunodeficiency syndrome-associated nephropathy. Arch Intern Med. 1991;151:125–128. [PubMed] [Google Scholar]
- 6.Abbott KC, Hypolite I, Welch PG, Agodoa LY. Human immunodeficiency virus/acquired immunodeficiency syndrome-associated nephropathy at end-stage renal disease in the United States: patient characteristics and survival in the pre highly active antiretroviral therapy era. J Nephrol. 2001;14:377–383. [PubMed] [Google Scholar]
- 7.D'Agati V, Suh JI, Carbone L, Cheng JT, Appel G. Pathology of HIV-associated nephropathy: a detailed morphologic and comparative study. Kidney Int. 1989;35:1358–1370. doi: 10.1038/ki.1989.135. [DOI] [PubMed] [Google Scholar]
- 8.Winston JA, Bruggeman LA, Ross MD, et al. Nephropathy and establishment of a renal reservoir of HIV type 1 during primary infection. N Engl J Med. 2001;344:1979–1984. doi: 10.1056/NEJM200106283442604. [DOI] [PubMed] [Google Scholar]
- 9.Lucas GM, Lau B, Atta MG, Fine DM, Keruly J, Moore RD. Chronic kidney disease incidence, and progression to end-stage renal disease, in HIV-infected individuals: a tale of two races. J Infect Dis. 2008;197:1548–1557. doi: 10.1086/587994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Berliner AR, Fine DM, Lucas GM, et al. Observations on a cohort of HIV-infected patients undergoing native renal biopsy. Am J Nephrol. 2008;28:478–486. doi: 10.1159/000112851. [DOI] [PubMed] [Google Scholar]
- 11.Choi AI, Rodriguez RA, Bacchetti P, Bertenthal D, Hernandez GT, O'Hare AM. Racial differences in end-stage renal disease rates in HIV infection versus diabetes. J Am Soc Nephrol. 2007;18:2968–2974. doi: 10.1681/ASN.2007040402. [DOI] [PubMed] [Google Scholar]
- 12.United States Renal Data System. Bethesda, MD: 2005. USRDS 2005 Annual Data Report; Atlas of End-Stage Renal Disease in the United States, National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases. [Google Scholar]
- 13.Ross MJ, Klotman PE. Recent progress in HIV-associated nephropathy. J Am Soc Nephrol. 2002;13:2997–3004. doi: 10.1097/01.asn.0000040750.40907.99. [DOI] [PubMed] [Google Scholar]
- 14.Lucas GM, Mehta SH, Atta MG, et al. End-stage renal disease and chronic kidney disease in a cohort of African-American HIV-infected and at-risk HIV-seronegative participants followed between 1988 and 2004. AIDS. 2007;21:2435–2443. doi: 10.1097/QAD.0b013e32827038ad. [DOI] [PubMed] [Google Scholar]
- 15.Cohen SD, Kimmel PL. Renal biopsy is necessary for the diagnosis of HIV-associated renal diseases. Nat Clin Pract Neph. 2009;5:22–23. doi: 10.1038/ncpneph0990. [DOI] [PubMed] [Google Scholar]
- 16.Ahuja TS, Borucki M, Funtanilla M, Shahinian V, Hollander M, Rajaraman S. Is the prevalence of HIV-associated nephropathy decreasing? Am J Nephrol. 1999;19:655–659. doi: 10.1159/000013537. [DOI] [PubMed] [Google Scholar]
- 17.Shahinian V, Rajaraman S, Borucki M, Grady J, Hollander WM, Ahuja TS. Prevalence of HIV-associated nephropathy in autopsies of HIV-infected patients. Am J Kidney Dis. 2000;35:884–888. doi: 10.1016/s0272-6386(00)70259-9. [DOI] [PubMed] [Google Scholar]
- 18.Gerntholtz TE, Goetsch SJ, Katz I. HIV-related nephropathy: a South African perspective. Kidney Int. 2006;69:1885–1891. doi: 10.1038/sj.ki.5000351. [DOI] [PubMed] [Google Scholar]
- 19.Williams DI, Williams DJ, Williams IG, Unwin RJ, Griffiths MH, Miller RF. Presentation, pathology, and outcome of HIV associated renal disease in a specialist centre for HIV/AIDS. Sex Transm Infect. 1998;74:179–184. doi: 10.1136/sti.74.3.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Laradi A, Mallet A, Beaufils H, Allouache M, Martinez F. HIV-associated nephropathy: outcome and prognosis factors. Groupe d' Etudes Nephrologiques d'Ile de France. J Am Soc Nephrol. 1998;9:2327–2335. doi: 10.1681/ASN.V9122327. [DOI] [PubMed] [Google Scholar]
- 21.Winston JA, Klotman ME, Klotman PE. HIV-associated nephropathy is a late, not early, manifestation of HIV-1 infection. Kidney Int. 1999;55:1036–1040. doi: 10.1046/j.1523-1755.1999.0550031036.x. [DOI] [PubMed] [Google Scholar]
- 22.Izzedine H, Wirden M, Launay-Vacher V. Viral load and HIV-associated nephropathy. N Engl J Med. 2005;353:1072–1074. doi: 10.1056/NEJMc051607. [DOI] [PubMed] [Google Scholar]
- 23.Post FA, Campbell LJ, Hamzah L, et al. Predictors of renal outcome in HIV-associated nephropathy. Clin Infect Dis. 2008;46:1282–1289. doi: 10.1086/529385. [DOI] [PubMed] [Google Scholar]
- 24.Atta MG, Gallant JE, Rahman MH, et al. Antiretroviral therapy in the treatment of HIV-associated nephropathy. Nephrol Dial Transplant. 2006;21:2809–2813. doi: 10.1093/ndt/gfl337. [DOI] [PubMed] [Google Scholar]
- 25.Wali RK, Drachenberg CI, Papadimitriou JC, Keay S, Ramos E. HIV-1-associated nephropathy and response to highly-active antiretroviral therapy. Lancet. 1998;352:783–784. doi: 10.1016/S0140-6736(98)24037-2. [DOI] [PubMed] [Google Scholar]
- 26.Scialla JJ, Atta MG, Fine DM. Relapse of HIV-associated nephropathy after discontinuing highly active antiretroviral therapy. AIDS. 2007;21:263–264. doi: 10.1097/QAD.0b013e3280119592. [DOI] [PubMed] [Google Scholar]
- 27.Kirchner JT. Resolution of renal failure after initiation of HAART 3 cases and a discussion of the literature. AIDS Read. 2002;12:103–102. [PubMed] [Google Scholar]
- 28.Freedman BI, Soucie JM, Stone SM, Pegram S. Familial clustering of end-stage renal disease in blacks with HIV-associated nephropathy. Am J Kidney Dis. 1999;34(2):254–258. doi: 10.1016/s0272-6386(99)70352-5. [DOI] [PubMed] [Google Scholar]
- 29.Bruggeman LA, Ross MD, Tanji N, et al. Renal epithelium is a previously unrecognized site of HIV-1 infection. J Am Soc Nephrol. 2000;11:2079–2087. doi: 10.1681/ASN.V11112079. [DOI] [PubMed] [Google Scholar]
- 30.Eitner F, Cui Y, Hudkins KL, et al. Chemokine receptor CCR5 and CXCR4 expression in HIV-associated kidney disease. J Am Soc Nephrol. 2000;11:856–867. doi: 10.1681/ASN.V115856. [DOI] [PubMed] [Google Scholar]
- 31.Huber TB, Reinhardt HC, Exner M, et al. Expression of functional CCR and CXCR chemokine receptors in podocytes. J Immunol. 2002;168:6244–6252. doi: 10.4049/jimmunol.168.12.6244. [DOI] [PubMed] [Google Scholar]
- 32.Winston JA, Bruggeman LA, Ross MD, et al. Nephropathy and establishment of a renal reservoir of HIV type 1 during primary infection. N Engl J Med. 2001;344:1979–1984. doi: 10.1056/NEJM200106283442604. [DOI] [PubMed] [Google Scholar]
- 33.Marras D, Bruggeman LA, Gao F, et al. Replication and compartmentalization of HIV-1 in kidney epithelium of patients with HIV-associated nephropathy. Nat Med. 2002;8:522–526. doi: 10.1038/nm0502-522. [DOI] [PubMed] [Google Scholar]
- 34.D'Agati VD. Podocyte injury in focal segmental glomerulosclerosis: Lessons from animal models (a play in five acts) Kidney Int. 2008;73:399–406. doi: 10.1038/sj.ki.5002655. [DOI] [PubMed] [Google Scholar]
- 35.Barisoni L, Kriz W, Mundel P, D'Agati V. The dysregulated podocyte phenotype: a novel concept in the pathogenesis of collapsing idiopathic focal segmental glomerulosclerosis and HIV-associated nephropathy. J Am Soc Nephrol. 1999;10:51–61. doi: 10.1681/ASN.V10151. [DOI] [PubMed] [Google Scholar]
- 36.Bruggeman LA, Dikman S, Meng C, Quaggin SE, Coffman TM, Klotman PE. Nephropathy in human immunodeficiency virus-1 transgenic mice is due to renal transgene expression. J Clin Invest. 1997;100:84–92. doi: 10.1172/JCI119525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhong J, Zuo Y, Ma J, et al. Expression of HIV-1 genes in podocytes alone can lead to the full spectrum of HIV-1-associated nephropathy. Kidney Int. 2005;68:1048–1060. doi: 10.1111/j.1523-1755.2005.00497.x. [DOI] [PubMed] [Google Scholar]
- 38.Dickie P, Roberts A, Uwiera R, Witmer J, Sharma K, Kopp JB. Focal glomerulosclerosis in proviral and c-fms transgenic mice links Vpr expression to HIV-associated nephropathy. Virology. 2004;322:69–81. doi: 10.1016/j.virol.2004.01.026. [DOI] [PubMed] [Google Scholar]
- 39.Zuo Y, Matsusaka T, Zhong J, et al. HIV-1 genes vpr and nef synergistically damage podocytes, leading to glomerulosclerosis. J Am Soc Nephrol. 2006;17:2832–2843. doi: 10.1681/ASN.2005080878. [DOI] [PubMed] [Google Scholar]
- 40.Husain M, Gusella GL, Klotman ME, et al. HIV-1 Nef induces proliferation and anchorage-independent growth in podocytes. J Am Soc Nephrol. 2002;13:1806–1815. doi: 10.1097/01.asn.0000019642.55998.69. [DOI] [PubMed] [Google Scholar]
- 41.Sunamoto M, Husain M, He JC, Schwartz EJ, Klotman PE. Critical role for Nef in HIV-1-induced podocyte dedifferentiation. Kidney Int. 2003;64:1695–1701. doi: 10.1046/j.1523-1755.2003.00283.x. [DOI] [PubMed] [Google Scholar]
- 42.Husain M, D'Agati VD, He JC, Klotman ME, Klotman PE. HIV-1 Nef induces dedifferentiation of podocytes in vivo: a characteristic feature of HIVAN. AIDS. 2005;19:1975–1980. doi: 10.1097/01.aids.0000191918.42110.27. [DOI] [PubMed] [Google Scholar]
- 43.He JC, Husain M, Sunamoto M, et al. Nef stimulates proliferation of glomerular podocytes through activation of Src-dependent Stat3 and MAPK1,2 pathways. J Clin Invest. 2004;114:643–651. doi: 10.1172/JCI21004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lu TC, He JC, Wang ZH, et al. HIV-1 Nef disrupts the podocyte actin cytoskeleton by interacting with diaphanous interacting protein. J Biol Chem. 2008;283:8173–8182. doi: 10.1074/jbc.M708920200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cantor ES, Kimmel PL, Bosch JP. Effect of race on expression of acquired immunodeficiency syndrome-associated nephropathy. Arch Intern Med. 1991;151:125–128. [PubMed] [Google Scholar]
- 46.Nochy D, Glotz D, Dosquet P, et al. Renal disease associated with HIV infection: a multicentric study of 60 patients from Paris hospitals. Nephrol Dial Transplant. 1993;8:11–19. doi: 10.1093/oxfordjournals.ndt.a092263. [DOI] [PubMed] [Google Scholar]
- 47.Nochy D, Glotz D, Dosquet P, et al. Renal lesions associated with human immunodeficiency virus infection: North American vs. European experience. Adv Nephrol Necker Hosp. 1993;22:269–286. [PubMed] [Google Scholar]
- 48.Cohen SD, Kimmel PL. HIV-associated renal diseases in Africa a desperate need for additional study. Nephrol Dial Transplant. 2007;22:2116–2119. doi: 10.1093/ndt/gfm263. [DOI] [PubMed] [Google Scholar]
- 49.Freedman BI, Spray BJ, Tuttle AB, Buckalew VM., Jr The familial risk of end-stage renal disease in African Americans. Am J Kidney Dis. 1993;21:387–393. doi: 10.1016/s0272-6386(12)80266-6. [DOI] [PubMed] [Google Scholar]
- 50.Freedman BI. Suseptibility genes for hypertension and renal failure. J Am Soc Nephrol. 2003;14:S192–S194. doi: 10.1097/01.asn.0000070075.89996.4a. [DOI] [PubMed] [Google Scholar]
- 51.Kopp JB, Smith MW, Nelson GW, et al. MYH9 is a major-effect risk gene for focal segmental glomerulosclerosis. Nat Genet. 2008;40:1175–1184. doi: 10.1038/ng.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kao WH, Klag MJ, Meoni LA, et al. MYH9 is associated with nondiabetic end-stage renal disease in African Americans. Nat Genet. 2008;40:1185–1192. doi: 10.1038/ng.232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Freedman BI, Hicks PJ, Bostrom MA, et al. Polymorphisms in the non-muscle myosin heavy chain 9 gene (MYH9) are strongly associated with end-stage renal disease historically attributed to hypertension in African Americans. Kidney Int. 2009 doi: 10.1038/ki.2008.701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gharavi AG, Ahmad T, Wong RD, et al. Mapping a locus for susceptibility to HIV-1-associated nephropathy to mouse chromosome 3. Proc Natl Acad Sci U S A. 2004;101:2488–2493. doi: 10.1073/pnas.0308649100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Papeta N, Chan KT, Prakash S, et al. Susceptibility loci for murine HIV-associated nephropathy encode trans-regulators of podocyte gene expression. J Clin Invest. 2009;119:1178–1188. doi: 10.1172/JCI37131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Pecci A, Malara A, Badalucco S, et al. Megakaryocytes of patients with MYH9-related thrombocytopenia present an altered proplatelet formation. Thromb Haemost. 2009;102:90–96. doi: 10.1160/TH09-01-0068. [DOI] [PubMed] [Google Scholar]
- 57.Straus SE. Philadelphia: Elsevier; 2005. Human herpesvirus-6 and -7, in Principles and practice of infectious diseases; pp. 1821–1825. [Google Scholar]
- 58.Crumpacker C, Wadhwa S. Philadelphia: Elsevier; 2005. Cytomegalovirus, in Principles and practice of infectious diseases; pp. 1786–1800. [Google Scholar]
- 59.Blattner W, Charurat M. Philadelphia: Elsevier; 2005. Human T-cell lymphotropic virus types I and II in Principles and practice of infectious diseases; pp. 2098–2118. [Google Scholar]
- 60.Sebekova K, Feber J, Carpenter B, et al. Tissue viral DNA is associated with chronic allograft nephropathy. Pediatr Transplant. 2005;9:598–603. doi: 10.1111/j.1399-3046.2005.00347.x. [DOI] [PubMed] [Google Scholar]
- 61.Demeter LM. Philadelphia: Elsevier; 2005. JC, BK, and other polyomaviruses: progressive multifocal leukoencephalopathy, in Principles and practice of infectious diseases, chap 141. [Google Scholar]
- 62.Zhong S, Zheng HY, Suzuki M, et al. Age-related urinary excretion of BK polyomavirus by nonimmunocompromised individuals. J Clin Microbiol. 2007;45:193–198. doi: 10.1128/JCM.01645-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Crum-Cianflone N, Quigley M, Utz G, Hale B. BK virus-associated renal failure among HIV patients. AIDS. 2007;21:1501–1502. doi: 10.1097/QAD.0b013e32823647d4. [DOI] [PubMed] [Google Scholar]
- 64.Tsui JI, Vittinghoff E, Shlipak MG, et al. Association of hepatitis C seropositivity with increased risk for developing end-stage renal disease. Arch Intern Med. 2007;167:1271–1276. doi: 10.1001/archinte.167.12.1271. [DOI] [PubMed] [Google Scholar]