Abstract
In vertebrates, class 3 semaphorins (SEMA3) control axon behaviour by binding to neuronal cell surface receptors composed of a ligand binding subunit termed neuropilin (NRP) and a signal transduction subunit of the A-type plexin family (PLXNA). We have determined the requirement for SEMA3/NRP/PLXN signalling in the development of the facial nerve, which contains axons from two motor neuron populations, branchiomotor and visceromotor neurons. Loss of either SEMA3A/NRP1 or SEMA3F/NRP2 caused defasciculation and ectopic projection of facial branchiomotor axons. In contrast, facial visceromotor axons selectively required SEMA3A/NRP1. Thus, the greater superficial petrosal nerve was defasciculated, formed ectopic projections and failed to branch in its target area when either SEMA3A or NRP1 were lost. To examine which A-type plexin conveyed SEMA3/neuropilin signals during facial nerve development, we combined an expression analysis with loss of function studies. Even though all four A-type plexins were expressed in embryonic motor neurons, PLXNA1 and PLXNA2 were not essential for facial nerve development. In contrast, loss of PLXNA4 phenocopied the defects of SEMA3A and NRP1 mutants, and loss of PLXNA3 phenocopied the defects of SEMA3F and NRP2 mutants. The combined loss of PLXNA3 and PLXNA4 impaired facial branchiomotor axon guidance more severely than loss of either plexin alone, suggesting that both pathways normally cooperate; in contrast, loss of both plexins did not impair facial visceromotor defects any worse than loss of PLXNA4. We conclude that PLXNA3 and PLXNA4 synergise to pattern the facial nerve, whereby both are required in branchiomotor neurons, but only PLXNA4 is essential for visceromotor neurons.
Keywords: facial nerve, facial branchiomotor neuron, facial visceromotor neuron, greater superficial petrosal nerve, axon guidance, neuropilin, plexin, semaphorin, SEMA3A, SEMA3F
INTRODUCTION
The brainstem of the adult vertebrate brain contains several different types of motor neurons that control important physiological processes, for example feeding, speech and eye movement. These motor neurons can be classified into subtypes according to their synaptic targets and the projection pattern of their axons as they leave the brain. The axons of branchiomotor neurons innervate muscles within the branchial arches, whilst the axons of visceromotor neurons innervate parasympathetic ganglia and smooth muscles. The somatic motor neurons, on the other hand, innervate muscles derived from paraxial or prechordal mesoderm. As they leave the hindbrain, the axons of visceromotor and branchiomotor neurons converge on shared dorsal exit points, whereas the axons of most somatic motor neurons exit ventrally. The anatomical organisation and function of these brainstem nerves is set up during embryonic development, when hindbrain motor neurons develop in lineage-restricted compartments termed rhombomeres and express selective subsets of transcription factors to control the responsiveness of their axons to environmental guidance cues (reviewed by Cordes, 2001). Thus, axon migration within the hindbrain is governed by anteroposterior and dorsoventral cues that guide axons to defined exit points, whilst axon migration outside the hindbrain is controlled by a combination of repulsive cues that surround the nerve path and attractive target-derived cues. In combination, these patterning mechanisms ensure that the axons of hindbrain motor neurons are wired appropriately to perform their adult functions. The identification of genes that control axonal patterning of the cranial nerves during embryogenesis therefore enhances our understanding of congenital abnormalities such as Duane syndrome and congenital facial nerve palsy, and in the future may also contribute to improved diagnosis of these conditions (reviewed by Traboulsi, 2007). In addition, the brainstem motor neurons present particularly good model systems to study the molecular mechanisms of axon guidance, as the anatomically well-defined arrangement of their cell bodies within the rhombomeres of the developing brain facilitates the identification of axon guidance receptors that are used by specific cranial motor nerves (Fig.1B; see, for example, Auclair et al., 1996; Jacob and Guthrie, 2000; Lumsden and Keynes, 1989; Studer et al., 1996).
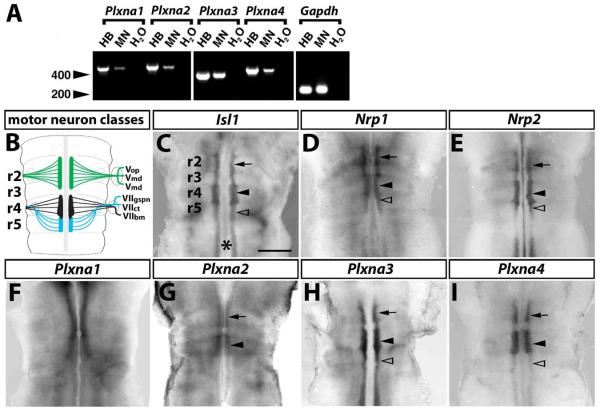
Fig. 1. A-type plexin expression in motor neurons.
(A) RT-PCR analysis of mouse hindbrain tissue (HB), primary rat embryonic motor neurons (MN) and a negative control (H2O) for Plxna1-a4 and a Gapdh control; molecular weight marker: 200 and 400 bp (arrowheads). (B) Motor neuron populations in the mouse hindbrain at 10.5 dpc; r2-derived neurons are shown in green, r4-derived neurons in black and r5-derived neurons in blue; abbreviations: VOP, VMX, VMD - ophthalmic, maxillary and mandibular branches of the Vth cranial (trigeminal) nerve; VIIGSPN, VIICT, VIIBM - greater superficial petrosal nerve, chorda tympani and branchiomotor nerve of the VIIth cranial (facial) nerve. (C-I) Wholemount in situ hybridisation of 10.5 dpc mouse hindbrains. (C) A probe specific for the motor neuron marker Isl1 reveals the nascent motor neuron column on each side of the midline (asterisk); focal thickenings at the level of r2 and r4 contain the trigeminal (arrow) and facial branchiomotor neurons (black arrowhead), respectively. Facial visceromotor neurons are born in r5 (clear arrowhead); r5 also contains caudally migrating facial branchiomotor neurons. (D,E) Hindbrain motor neurons express Nrp1 (D) and Nrp2 (E). Plxna1 is expressed near the midline in all rhombomeres anterior to r5 (F); the expression patterns of Plxna2 (G), Plxna3 (H) and Plxna4 (I) are consistent with a role in trigeminal (arrow) and facial (arrowhead) branchiomotor neurons; Plxna3 (H) and Plxna4 (I) are also expressed in posterior hindbrain motor neurons, including those in r5 (clear arrowhead). Scale bar (C-I): 500 μm.
The facial nerve contains axons from both branchiomotor and visceromotor neurons, which are born in two different rhombomeres (r) of the developing hindbrain. Whilst facial branchiomotor neurons are born in r4, facial visceromotor neurons are born in r5 (Fig. 1B,C; Auclair et al., 1996; Jacob and Guthrie, 2000; Lumsden and Keynes, 1989; Studer et al., 1996). Having left the hindbrain through a shared exit point in r4, their axons pass through the geniculate ganglion complex and then segregate again to innervate specific targets in the head and neck. The facial branchiomotor neurons (FBM) innervate the muscles of the second branchial arch, whereas the facial visceromotor neurons innervate the submandibular ganglion as the chorda tympani (CT) and the sphenopalatine ganglion as the greater superficial petrosal nerve (GSPN) (Fig. 1B).
The transmembrane protein neuropilin 1 (NRP1) is essential for the patterning of the facial nerve in the mouse, as it binds SEMA3A to guide facial branchiomotor axons in the second branchial arch and the vascular endothelial growth factor isoform VEGF164 to control the position of facial branchiomotor neuron cell bodies within the hindbrain (Kitsukawa et al., 1997; Schwarz et al., 2004; Taniguchi et al., 1997). Mouse embryos lacking NRP1 or SEMA3A also show defasciculation of other cranial nerves, including the trigeminal, glossopharyngeal and vagus nerves (Kitsukawa et al., 1997; Taniguchi et al., 1997). Neuropilin 2 (NRP2) binds a different subset of class 3 semaphorins, and its principal ligand during axon guidance is SEMA3F (Chen et al., 1997). Loss of NRP2 or SEMA3F causes partial defasciculation of the facial branchiomotor and ophthalmic trigeminal nerves and severe defasciculation of the oculomotor nerve; in addition, the trochlear nerve fails to project to its target in these mutants (Chen et al., 2000; Giger et al., 2000; Sahay et al., 2003).
Neither NRP1 nor NRP2 are able to convey semaphorin signals on their own (Feiner et al., 1997). Rather, they recruit a member of the plexin family to control cytoskeletal behaviour in neurons (Rohm et al., 2000; Tamagnone et al., 1999). The neuropilins can associate with one of four different A-type plexins (PLXNA) in vitro (Rohm et al., 2000; Suto et al., 2003; Takahashi et al., 1999; Takahashi and Strittmatter, 2001; Tamagnone et al., 1999). However, targeted mouse mutations demonstrated plexin selectivity during semaphorin/neuropilin signalling in vivo. Thus, knockout studies did not confirm a direct role for PLXNA1 in sensory nerve axon guidance (Takegahara et al., 2006), even though truncated PLXNA1 protein blocks SEMA3A-induced growth cone turning in cultured sensory neurons (for example Rohm et al., 2000; Takahashi et al., 1999). Instead, specific combinations of the other three A-type plexins have been implicated in different axon guidance pathways both in vitro and in vivo (Bagri et al., 2003; Cheng et al., 2001; Palaisa and Granato, 2007; Suto et al., 2005; Tanaka et al., 2007; Waimey et al., 2008; Yaron et al., 2005). For example, PLXNA4 and, to a lesser extent, PLXNA3 are involved in the patterning of SEMA3A-responsive sensory and sympathetic axons, whilst PLXNA3, but not PLXNA4, is essential for the guidance of SEMA3F-responsive trochlear axons (Cheng et al., 2001; Suto et al., 2005; Yaron et al., 2005). In the mouse, PLXNA4 also cooperates with PLXNA2 to control the projection of hippocampal mossy fibres, but this mechanism depends on the transmembrane semaphorin SEMA6A, rather than a secreted semaphorin (Suto et al., 2007). SEMA6A/PLXNA2 signalling also controls the migration of cerebellar granule cells (Renaud et al., 2008). In the chick, PLXNA1, PLXNA2 and PLXNA4 are all expressed in spinal motor neurons (Mauti et al., 2006), but the role of the A-type plexins in the development of hindbrain motor neurons has not yet been investigated. This report therefore provides the first systematic analysis of axon guidance pathways controlling facial nerve patterning. In particular, we demonstrate that axonal patterning of facial branchiomotor and visceromotor neurons is differentially regulated by a specific combination of class 3 semaphorins and their neuropilin/plexin receptors. Our observations support the previously proposed concept of selective ligand/receptor pairings in semaphorin pathways. Moreover, they significantly advance our knowledge of facial nerve development, which is affected in several types of congenital human craniofacial syndromes.
EXPERIMENTAL PROCEDURES
Animals
To obtain mouse embryos of defined gestational ages, mice were mated in the evening, and the morning of vaginal plug formation was counted as 0.5 days post coitum (dpc). Mice lacking SEMA3A, SEMA3F, PLXNA1, PLXNA2, PLXNA3 or PLXNA4 have previously been described (Cheng et al., 2001; Giger et al., 2000; Gu et al., 2003; Suto et al., 2005; Takegahara et al., 2006; Taniguchi et al., 1997; Yaron et al., 2005). Genotyping protocols can be supplied on request.
RT-PCR analysis of hindbrain tissue and isolated motor neurons
12.5 dpc mouse hindbrains were dissected in ice-cold PBS. Primary motor neurons were purified from dissected embryonic rat neural tubes (14.5 dpc, corresponding to 13.5 dpc in the mouse (Henderson et al., 1995). RNA was extracted with Tri Reagent (Helena BioSciences) and subjected to RT-PCR using Superscript II (Invitrogen) for cDNA synthesis. For PCR amplification, the following oligonucleotide pairs were used: Nrp1 5′-gttgctgtgcgccacgctcgcccttg-3′ and 5′-ctgaagaggagcggatccggccaggag-3′ (431 bp); Gapdh 5′-gctgagtatgtcgtggagtc-3′ and 5′-ttggtggtgcaggatgcatt-3′ (192 bp); Plxna1 5′-tctagattctggtggaccttgcaaac-3′ and 5′-tctagaaggtgagtggcaatggatg (488 bp); Plxna2 5′-tccactctgagaatcgtgac-3′ and 5′-gagctcatagtccagcattg-3′ (530 bp); Plxna3 5′-gtgaacaagctgctcctcatag-3′ and 5′-gtgtctgaagggatcttgatc-3′ (358 bp); Plxna4 5′-tgtacacctcaaagcttgtg-3′ and 5′-gtccctgtcttctgtgaaga-3′ (467 bp).
Immunolabelling
Mouse embryos were fixed in ice-cold Dent's fixative (four parts methanol, 1 part dimethylsulfoxide) and incubated in Dent's fixative containing 10% hydrogen peroxide for 2 hours to quench endogenous peroxidase activity. Samples were washed several times in TBS (10mM Tris-HCl pH8.0 and 150mM NaCl), incubated for 30 min in blocking solution (normal goat serum containing 20% DMSO and 0.12% thimerosal; Sigma) and incubated over night in blocking solution that contained rabbit anti neurofilament antibodies (Chemicon). After several washes in TBS, samples were incubated over night in blocking solution containing horseradish peroxidase-conjugated goat anti rabbit IgG (DAKO). After washing, peroxidase activity was detected with diaminobenzidine and hydrogen peroxide (SigmaFast; Sigma). Stained samples were fixed in formaldehyde and then dehydrated in methanol. Samples were then cleared in a solution containing 2 parts benzyl benzoate and 1 part benzyl alcohol. Images were recorded using the MZ16 microscope (Leica) equipped with a ProgResC14 digital camera (Jenoptiks). In some experiments, the neurofilament antibody was detected with Alexa488-conjugated goat anti rabbit IgG (Molecular Probes) and images were then recorded using an Olympus SZX16 fluorescent stereomicroscope equipped with an OCRA-HR digital camera (Hamamatsu Photonics). Images were processed using Openlab 2.2 software (Improvision Ltd.) and Adobe Photoshop 7.0 (Adobe Systems, Inc.).
In situ hybridisation
For in situ hybridisation, embryos were fixed over night in 4% formaldehyde in phosphate buffered saline (PBS), washed in PBS, dehydrated in methanol and stored at −20°C. In situ hybridization was performed according to a previously published method (Riddle et al., 1993) using digoxigenin-labelled riboprobes transcribed from plasmids containing the following cDNAs: Sema3a and Sema3f (gift from M. Tessier-Lavigne), Isl1 (gifts of T. Jessell, Columbia University, New York), Nrp1 (gift from M. Fruttiger, University College London) and Phox2b (gift from C. Goridis, INSERM, Marseille, France). Riboprobes for A-type plexins were transcribed from expressed sequence tag (EST) IMAGE clones (Invitrogen), clone IDs: 1853022 (Plxna1), 5364824 (Plxna2), 401253 (Plxna3) and 4316766 (Plxna4). Images were acquired as described above.
DiI labelling of facial visceromotor neurons
11.0 dpc wild type embryos were fixed over night in 4% formaldehyde in PBS. DiI crystals (Invitrogen) were then placed into small incisions of dorsal r5 hindbrain tissue. DiI-labelled embryos were incubated in PBS with 0.02% sodium azide at 37°C in the dark for 9 days. The lower jaw was removed for photography. Images were recorded with the SZX16 dissecting microscope as described above.
RESULTS
Expression pattern of A-type plexins during hindbrain development
To determine which plexins are expressed in developing motor neurons, we initially analysed rat embryo primary motor neurons by RT-PCR and detected expression of all 4 A-type plexins (Fig. 1A). We then compared the expression pattern of these plexins to that of the motor neuron marker Isl1 (Fig. 1C) and the plexin co-receptors Nrp1 (Fig. 1D) and Nrp2 (Fig. 1E) in mouse hindbrains at 10.5 dpc, when cranial motor neurons extend axons through their hindbrain exit points into the periphery. Plxna1 was expressed near the midline in all rhombomeres anterior to r5, but its pattern was not consistent with specific expression in facial branchiomotor or visceromotor neurons (compare Fig. 1C with F). In contrast, the patterns of Plxna2 (Fig. 1G), Plxna3 (Fig. 1H) and Plxna4 (Fig. 1I) were consistent with expression by trigeminal and facial branchiomotor neurons; in addition, Plxna3 and Plxna4 were also expressed in the r5 motor column, where facial visceromotor neurons originate, and in posterior hindbrain motor neurons (Fig. 1H,I). In summary, the expression patterns suggested that all four plexins play a role in hindbrain development, with PLXNA3 and PLXNA4 being the best candidate mediators of facial motor neuron patterning.
PLXNA3 and PLXNA4 convey semaphorin signals to guide facial branchiomotor axons
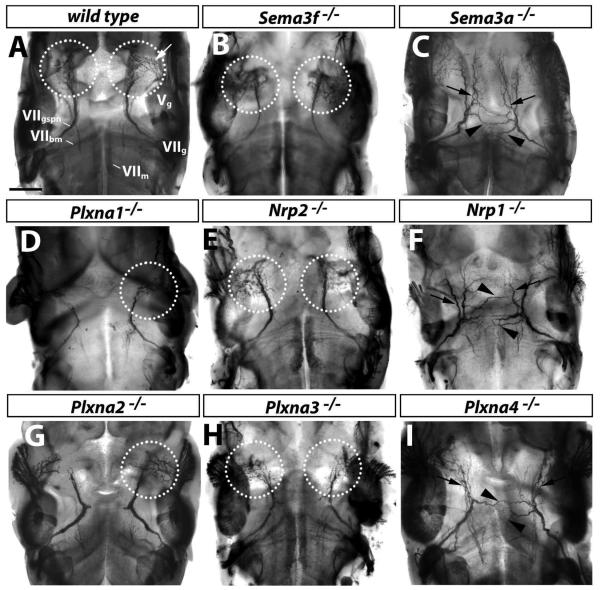
To determine the functional requirement for the A-type plexins in facial nerve development, we examined axonal patterning in the corresponding loss of function mouse mutants. We found that loss of PLXNA1 or PLXNA2 did not obviously impair axon guidance or fasciculation of the facial or trigeminal nerves (compare Fig. 2A with D, G; 3/3 cases). In contrast, loss of PLXNA3 or PLXNA4 caused defasciculation of the facial branchiomotor nerve (white arrowheads in Fig. 2H,I; 3/3 and 4/4 cases, respectively). Loss of either plexin also caused defasciculation of the ophthalmic branch of the trigeminal nerve (white arrows in Fig. 2H,I; see Cheng et al., 2001; Yaron et al. 2005); however, the severity was variable between mutants of each genotype (data not shown). Interestingly, the maxillary and mandibular branches of the trigeminal nerve were defasciculated in Plxna4-null mutants (compare Fig. 2A with black arrows and arrowheads in 2I), but were not obviously affected in Plxna3-null mutants (compare Fig. 2A with H).
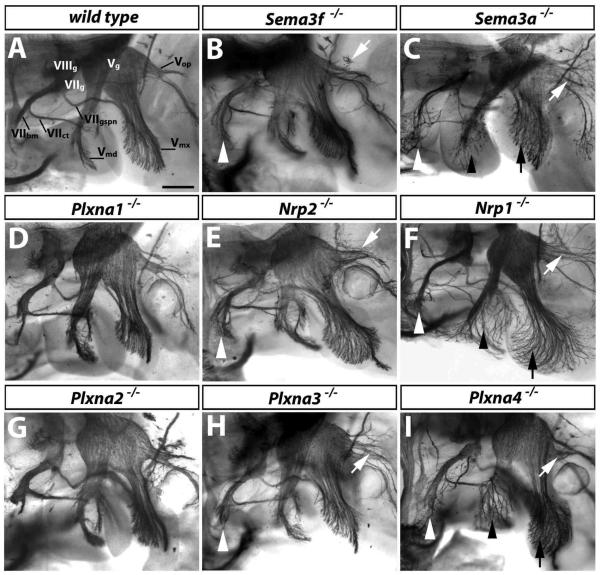
Fig. 2. PLXNA3 and PLXNA4 convey semaphorin signals during axon guidance of facial branchiomotor neurons.
Wholemount neurofilament staining of 11.5 dpc wild type (A) and mutant embryos (B-I); a lateral view of the cranial nerves V (trigeminal) and VII (facial) is shown. The facial nerve segregates into 3 branches to form the facial branchiomotor nerve (VIIbm), the chorda tympani (VIIct) and the greater superficial petrosal nerve (VIIgspn). The trigeminal nerve is also organised into 3 main branches: the mandibular (Vmd), maxillary (Vmx), and ophthalmic (Vop) nerves. The facial (VIIg), vestibuloacoustic (VIIIg) and trigeminal (Vg) ganglia are indicated. Loss of PLXNA1 (D) or PLXNA2 (G) did not impair axon guidance of these nerves. Loss of SEMA3F (B), NRP2 (E) or PLXNA3 (H) caused defects in the VIIbm (white arrowhead) and Vop (white arrows). Loss of SEMA3A (C), NRP1 (F) or PLXNA4 (I) also caused VIIbm and Vop defects, with VIIbm defects being more severe than in mutants lacking SEMA3F, NRP2 or PLXNA3. Note that the Vop defect was mild in the particular Plxna4-null mutant shown, but was stronger in other cases. Loss of SEMA3A, NRP1 or PLXNA4 also caused prominent defasciculation of the mandibular (black arrowheads) and maxillary (black arrows) nerves (C, F, I). Scale bar: 250 μm.
Consistent with the idea that PLXNA3 preferentially associates with NRP2 to form the SEMA3F receptor (Yaron et al., 2005), mutants lacking SEMA3F or NRP2 were similar to mutants lacking PLXNA3, displaying defasciculation of the facial branchiomotor and ophthalmic trigeminal branch, but not maxillary or mandibular trigeminal branches (compare Fig. 2H with B,E). On the other hand, mutants lacking NRP1 or SEMA3A were more similar to those lacking PLXNA4, with defasciculation of the facial branchiomotor nerve and the ophthalmic trigeminal branch, as well as the mandibular and maxillary trigeminal branches (compare Fig. 2F and C with I).
Semaphorin expression during axon guidance of facial visceromotor neurons
To examine if semaphorin-signalling through NRP/PLXN complexes also guides the visceromotor component of the facial nerve, we first characterised the axonal path of the greater superficial petrosal nerve (GSPN). Between 10.5 and 12.5 dpc, GSPN axons extend from the hindbrain through the facioacoustic ganglion and then anteriorly, towards the site where the sphenopalatine ganglion forms (compare Fig. 3A,C with F). Anterograde DiI injection into the r5-derived hindbrain region, which gives rise to the facial visceromotor neurons, confirmed that these axons were indeed extended by facial visceromotor neurons (compare Fig. 3A with B).
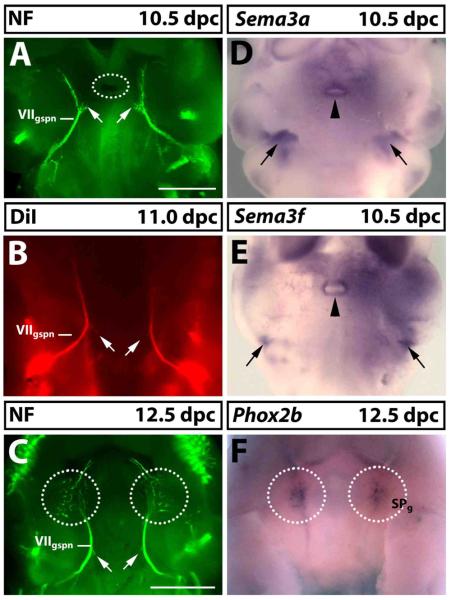
Fig. 3. Semaphorin expression during facial visceromotor axon guidance.
(A-C) Wholemount visualisation of the GSPN nerve (VIIgspn) by neurofilament immunofluorescence (A,C) or anterograde DiI labelling (B) at 10.5 (A), 11.0 (B) or 12.5 dpc (C); ventral view, anterior up. The lower jaw including their trigeminal nerve branches was removed to afford better visualisation of the VIIgspn. At 10.5 dpc, VIIgspn axons extended from the main nerve trunk (arrows in A) towards Rathke's pouch (circled in A), but these axons had retracted by 11.0 dpc (arrows in B) and 12.5 dpc (arrows in C). (D,E) Wholemount in situ hybridisation at 10.5 dpc for Sema3a (D) and Sema3f (E) and at 12.5 dpc for the sphenopalatine ganglion (SPg) marker Phox2b (F). The VIIgspn encounters two areas of high semaphorin expression as it extends towards its target site, one in the second pharyngeal arch (arrows in D,E) and one surrounding Rathke's pouch (arrowheads in D,E). At 12.5 dpc, VIIgspn axons arborated in the area where the SPg had formed (compare circled areas in C with F). Scale bars: (A,B,D,E) and (C,F) 500 μm.
Using Phox2b as a marker, it was previously reported that sphenopalatine ganglion neurons differentiate from 11.5 dpc onwards and begin to form a ganglion at 12.0 dpc (Enomoto et al., 2001). Consistent with this observation, we identified the anlagen of the sphenopalatine ganglion at 12.5 dpc in wholemounts (circled in Fig. 3F) and found that the ganglia were well established by 13.5 dpc (see below, Fig. 5A). By 12.5 dpc, the GSPN nerve had arborated in the area where the ganglion formed (compare Fig. 3C with F), presumably to establish contact with neurons in the sphenopalatine ganglion. Axon arborisation was present in most wild type embryos at 11.5 dpc (Fig. 4A). In 11.0 dpc embryos, GSPN axons had reached the target field, but had not yet arborised (Fig. 3B). Taken together, these observations suggest that synaptic contact between presynaptic and postsynaptic sphenopalatine neurons is initiated between 11.25 and 11.5 dpc.
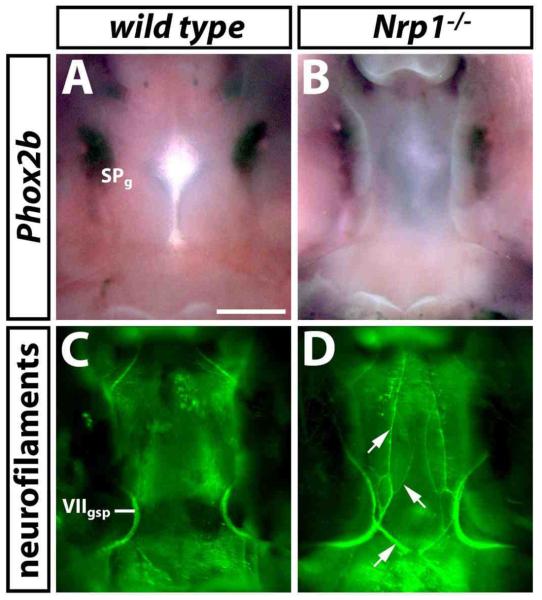
Fig. 5. Facial visceromotor axon guidance defects in the absence of SEMA3A/NRP1 signalling are not due to loss of the target ganglion.
(A,B) Wholemount in situ hybridisation with a probe specific for Phox2b identifies the sphenopalatine ganglion (SPg) in wild type (A) and NRP1 null mutant (B) littermates at 13.5 dpc (ventral view). (C,D) Wholemount neurofilament staining of the developing palate of the same samples after in situ hybridisation shows an abnormal trajectory of the VIIgspn in the NRP1 null mutant embryo (aberrant axons indicated with arrows in D). Note that the alkaline phosphatase reaction product, which was deposited during in situ hybridisation, partially obscured the VIIgspn. Scale bar (A-D): 500 μm.
Fig. 4. SEMA3A signals through NRP1 and PLXNA4 to guide the axons of facial visceromotor neurons.
(A) Wholemount neurofilament staining of wild type mouse embryos at 11.5 dpc reveals migrating facial branchiomotor neurons (VIIm), and their axons within the hindbrain, as well as the facioacoustic (VIIg) and trigeminal ganglia (Vg) and the greater superficial petrosal nerve (VIIgspn) in the periphery. VIIgspn axons arborate in the area where the sphenopalatine ganglion forms (circled); the white arrow denotes trigeminal axons that innervate the sphenopalatine ganglion. The lower jaw was removed to afford better visualisation of VIIgspn axons. (B-I) Corresponding wholemount neurofilament staining of loss of function mutants. Loss of SEMA3F (B) or NRP2 (E) did not affect VIIgspn axons. Similarly, loss of PLXNA1 (D), PLXNA2 (G) or PLXNA3 (H) did not impair their guidance. In contrast, loss of SEMA3A (C), NRP1 (F) or PLXNA4 (I) caused defasciculation (arrows) and midline crossing (arrowheads) of VIIgspn axons; moreover, there was no arborisation in the area where the sphenopalatine ganglion forms (compare A with C,F,I). Scale bar: 250 μm.
Sema3a and Sema3f were both expressed in regions surrounding the path of GSPN axons. Firstly, they were expressed bilaterally in an area flanking the axons as they extended towards the midline of the stomadeum (black arrows in Fig. 3D,E). This expression pattern was consistent with their general role as repellent cues that channel growing axons into fascicles. Secondly, both semaphorins were expressed around the opening of Rathke's pouch, which is located between the paired GSPN branches in the roof of the stomadeum (black arrowheads in Fig. 3D,E). This expression pattern was consistent with a role in repelling axons from the midline region; in agreement with this idea, short axon branches, which extended from each nerve towards the midline at 10.5 dpc, had retracted by 12.5 dpc (compare white arrows in Fig. 3A with C).
PLXNA4 conveys SEMA3A/NRP1 signals to guide facial visceromotor neurons
To test the hypothesis that NRP/PLXN signalling was essential for the guidance of facial visceromotor axons, we examined the behaviour of GSPN axons in loss of function mutants at 11.5 dpc by wholemount neurofilament staining (Fig. 4). Whilst axons formed normal projections in 5/5 Nrp2-null and 3/3 Sema3F-null mutants (compare Fig. 4A with B,E), we observed striking axon guidance errors in 5/5 Nrp1-null and 5/5 Sema3a-null mutants (Fig. 4C,F). Specifically, there was defasciculation (arrows in Fig. 4C,F) and midline crossing of ectopic axons (arrowheads in Fig. 4C,F) near Rathke's pouch, consistent with the idea that Sema3a normally provides repulsive cues for GSPN axons (see Fig. 3D). Moreover, there was no arborisation in the area where the sphenopalatine ganglion forms; rather, some axons appeared to extend beyond the target area (compare Fig. 4A with Fig. 4C,F).
We next examined which plexin transduced SEMA3A/NRP1 signals in facial visceromotor neurons. In PLXNA1 and PLXNA2 mutants, only the GSPN branch in the faster developing left side of the embryo had begun to arborate in the target area at 11.5 dpc, but axon pathfinding appeared intact (Fig. 4 D,G); this observation is consistent with an overall developmental delay rather than a specific defect in axon guidance. In agreement with the idea that SEMA3F and NRP2 were not required for axon guidance of the GSPN, loss of PLXNA3 did not impair the pathfinding of the GSPN nerve (Fig. 4H). Instead, the defects of SEMA3A/NRP1 mutants were phenocopied by mutants lacking PLXNA4 (compare areas indicated by arrows and arrowheads in Fig. 4C,F with I). Taken together, the observations described in Figs. 3 and 4 suggest that SEMA3A signals through NRP1/PLXNA4 to guide visceromotor axons towards the site of sphenopalatine ganglion formation, preventing midline crossing and promoting target innervation.
Defects in visceromotor axon guidance occur in the presence of the sphenopalatine ganglion
It has been proposed that the sphenopalatine ganglion is essential for the pathfinding of GSPN axons from the facial ganglion to the target area (Jacob et al., 2000). We therefore asked if these pathfinding errors were secondary to loss of the sphenopalatine ganglion. This was also an important question, as the sphenopalatine ganglion is formed from r4-derived neural crest cells, and a subpopulation of r4-derived neural crest cells that contributes to sensory gangliogenesis migrates abnormally in Sema3a- and Nrp1-null mutants (Schwarz et al., 2008). We found that the paired sphenopalatine ganglia, even though mildly misshapen, were present in 4/4 Nrp1-null mutant embryos at 13.5 dpc (compare Fig. 5A with B). Nevertheless, the axonal trajectory of the GSPN was grossly abnormal in all those cases (compare Fig. 5C with D). We conclude that the pathfinding errors of visceromotor axons in mutants lacking SEMA3A, NRP1 or PLXNA4 are not due to loss of the target ganglion.
PLXNA3 and PLXNA4 cooperate during facial nerve development
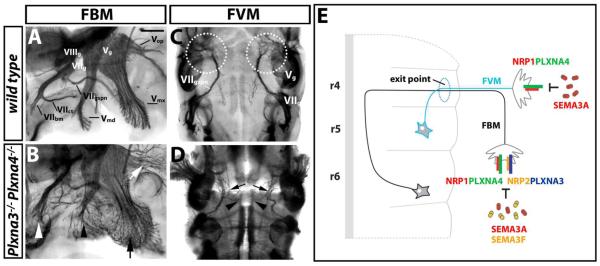
The observations described above suggest that PLXNA3 and PLXNA4 independently transmit semaphorin signals to guide facial and trigeminal axons. Consistent with this idea, the facial branchiomotor nerve and all trigeminal nerve branches were more severely mispatterned in double knockouts than in single mutants (compare Fig. 6B with 4H,I; 6/6 cases). On the other hand, the facial visceromotor defects of double knockouts were not more severe than those of single Plxna4 mutants (compare Fig. 6D with 4I; 4/4 cases). This observation is in agreement with the finding that PLXNA3 is not required for the patterning of this facial nerve branch (see Fig. 4). Therefore, PLXNA3 and PLXNA4 synergise to pattern the facial nerve, whereby both are required in branchiomotor neurons, but only PLXNA4 is essential in visceromotor neurons.
Fig. 6. PLXNA3 and PLXNA4 cooperation during facial branchiomotor, but not visceromotor axon guidance.
(A-D) Wholemount neurofilament staining of wild type (A,C) and Plxna3/Plxna4 double null mutants (B,D) at 11.5 dpc reveals the vestibuloacoustic (VIIIg), facial (VIIg) and trigeminal ganglia (Vg) as well as the three main facial and trigeminal nerve branches. (A,B) A lateral view of the head in a wild type shows the three facial branches, the branchiomotor nerve (VIIbm), the chorda tympani (VIIct) and the greater superficial petrosal nerve (VIIgspn), as well the mandibular (Vmd), maxillary (Vmx), and ophthalmic (Vop) branches of the trigeminal nerve. (B) The combined loss of PLXNA3 and PLXNA4 disrupted the patterning of the facial branchiomotor nerve (white arrowhead) as well as the mandibular (black arrowhead), maxillary (black arrow) and ophthalmic (white arrow) trigeminal nerve branches more severely than loss of either plexin alone (compare B with Fig. 2H,I). (C) A ventral view shows that VIIgspn axons normally arborate in the area where the sphenopalatine ganglion forms (circled). (D) The combined loss of PLXNA3 and PLXNA4 caused defasciculation (arrows) and midline crossing (arrowheads) of VIIgspn axons, and there was no arborisation in the area where the sphenopalatine ganglion forms; however, defects were not worse than those caused by loss of PLXNA4 alone (compare D with Fig. 4I). Scale bar (A-D) 250 μm. (E) Working model for facial nerve patterning: SEMA3A and SEMA3F cooperate to pattern facial branchiomotor neuron (FBM) axons by binding to NRP1/PLXNA4 and NRP2/PLXNA3, respectively; SEMA3A also patterns facial visceromotor neuron (FVM) axons by binding to NRP1/PLXNA4 complexes.
DISCUSSION
Essential roles for class 3 semaphorins and their receptors in facial branchiomotor axon guidance
We have previously shown that SEMA3A signalling through NRP1 patterns the axons of facial branchiomotor neurons in the second branchial arch, affecting their pathfinding and fasciculation (Schwarz et al., 2004). We now show that SEMA3F and NRP2 also contribute to the patterning of facial branchiomotor neurons, because loss of either molecule results in similar axonal defects as loss of SEMA3A or NRP1 (Fig. 2). Our observation that one type of neuron can respond to two different semaphorins is consistent with the previous finding that hippocampal neurons respond two both SEMA3A and SEMA3F under physiological circumstances (Chedotal et al., 1998). Two different plexins were essential to transduce SEMA3A/NRP1 and SEMA3F/NRP2 signals, PLXNA3 and PLXNA4 (Fig. 2). These findings are consistent with previous observations in other model systems of axon guidance, which showed that PLXNA3 and PLXNA4 preferentially mediate SEMA3F and SEMA3A signals, respectively (Yaron et al., 2005).
PLXNA1 and PLXNA2 were also expressed in the hindbrain at the time of motor neuron differentiation, but their loss did not impair fasciculation or path finding of facial branchiomotor axons. We did, however, observe a general delay in development in both types of null-mutants, which manifested itself in delayed GSPN and trigeminal nerve branching in the area where the sphenopalatine ganglion forms (Fig. 4). Because this delay was not seen in Nrp1- or Nrp2-null mutants, the delay did not reflect a cell-autonomous defect in the neuropilin-mediated guidance of motor axons. Instead, it is likely an indirect consequence of an overall developmental delay or a defect in the nerve environment. Consistent with the latter possibility, PLXNA1 has been implicated as a SEMA6A-receptor in boundary cap patterning at the interface between central and peripheral nervous system to assist the ordered exit of axons form the neural tube (Mauti et al., 2007). It would also be interesting to examine if PLXNA1 and PLXNA2 play a role in SEMA3A-guided patterning events for which PLXNA3 and PLXNA4 are dispensable, for example the guidance of trunk neural crest cells (Waimey et al., 2008).
In many model systems, SEMA3A acts as an environmental repellent for growing axons (for example, (Luo et al., 1993; Messersmith et al., 1995). In addition, SEMA3A expression by motor neurons in the chick was previously shown to desensitise axons as they approach their target field (Moret et al., 2007). SEMA3A and SEMA3F are both expressed in the second pharyngeal arch from 8.5 dpc onwards, prior to the extension of motor axons out of the hindbrain (Schwarz et al., 2008). This observation is consistent with a model in which axon patterning is achieved by repulsive guidance cues in the environment. At subsequent developmental stages, SEMA3A is also expressed by the facial branchiomotor neurons themselves (Schwarz et al, 2004). Therefore, both paracrine and autocrine SEMA signalling through NRP/PLXN complexes may contribute to axon patterning of facial branchiomotor neurons, but these possibilities cannot be addressed with existing mouse models.
Target-independent guidance of facial visceromotor axons by SEMA3A signalling through NRP1/PLXNA4 receptors
In the vertebrate trunk, preganglionic and postganglionic sympathetic axons pathfind in the absence of their target, suggesting that guidance cues for sympathetic axons are derived from sources other than the target ganglia (for example, Guidry and Landis, 1995; Yip, 1987). Whilst parasympathetic neurons share transcription factors with sympathetic neurons to control their differentiation and have similar trophic requirements (reviewed in Jacob et al., 2000), it is not clear if parasympathetic axons use guidance mechanisms that are similar to those of sympathetic axons.
A previous study using a loss of function mutant for the transcription factor Phox2a raised the possibility that facial visceromotor, unlike sympathetic axons, use a target-dependent mechanism of axon guidance, in which the sphenopalatine ganglion plays an essential role (Jacob et al., 2000). Specifically, the sphenopalatine ganglion was absent in these mutants, and this was accompanied by loss of the GSPN. However, these studies were complicated by the fact that Phox2a is not only essential for the formation of the sphenopalatine ganglion, but also for the formation of the geniculate ganglion (Jacob and Guthrie, 2000; Morin et al., 1997), which provides an intermediate target for facial visceromotor neurons en route from the hindbrain to the sphenopalatine ganglion. Moreover, Phox2a is expressed by all hindbrain motor neurons (Tiveron et al., 1996), including facial visceromotor neurons (Jacob et al., 2000). This observation raises the possibility that Phox2a contributes to visceromotor patterning in additional mechanisms that do not involve signals from the sphenopalatine ganglion. For example, the geniculate ganglion may be critical for pathfinding of facial visceromotor axons. Alternatively, Phox2a might control the expression of receptors in facial visceromotor neurons that are critical for nerve survival after axons have reached their target ganglion. This possibility was not addressed in the previous study, as the analysis of the GSPN was performed at 13.5 dpc, well after the axons of this nerve had reached the sphenopalatine ganglion in wild type mice and were likely to have made synaptic contact (see Figs. 3, 4).
We now demonstrate that facial visceromotor axons home in on their target area between 10.5 and 11.5 dpc, before the sphenopalatine ganglion is formed at 12.5 dpc (Fig. 3, 4). Moreover, we show that loss of SEMA3A signalling through NRP1/PLXNA4 impairs axon pathfinding at 11.5 dpc, i. e. before the sphenopalatine ganglion has formed (Figs. 4, 5). Finally, pathfinding errors in Nrp1-null mutants cannot be explained by loss of the geniculate ganglion, as it forms in embryos lacking NRP1 (Schwarz et al., 2008). Taken together, our observations suggest that preganglionic parasympathetic axons use target-independent mechanisms of axon guidance to approach their target field, and that the parasympathetic ganglia are more likely to play a role during subsequent stages of visceromotor axon patterning, perhaps by inducing axon arborisation or stimulating survival pathways.
Differential use of plexins during axon guidance of facial nerve subdivisions
All A-type plexins have been implicated as signal transducers for neuropilin-bound semaphorin signals during axon guidance in vitro. Initially, truncated PLXNA1 protein was shown to block SEMA3A-induced growth cone turning in cultured sensory neurons (for example (Rohm et al., 2000; Takahashi et al., 1999). However, subsequent knockout studies in mice did not confirm a direct role for PLXNA1 in sensory nerve axon guidance (Takegahara et al., 2006). Rather, PLXNA4 was found to be the main physiological co-receptor for NRP1 in several different SEMA3A-mediated axon patterning events, such as sensory nerve fasciculation and sympathetic axon guidance (Cheng et al., 2001; Suto et al., 2005; Yaron et al., 2005). We found that all four A-type plexins were expressed in primary embryonic motor neurons, and that facial motor neurons in situ expressed PLXNA2, PLXNA3 and PLXNA4 (Fig. 1). However, despite its expression in the motor column at r5-level, PLXNA2 was not essential for facial branchiomotor or visceromotor guidance. This observation may indicate that PLXNA2 expression in this domain has no functional significance, or that it plays a role in another type of r5-derived motor neuron, which forms the abducens nerve. On the other hand, PLXNA3 and PLXNA4 were essential for facial nerve development, as both contributed to the patterning of facial branchiomotor axons (Fig. 2); moreover, PLXNA4, but not PLXNA3, controlled facial visceromotor axon guidance (Fig. 4). Accordingly, compound mutants lacking both PLXNA3 and PLXNA4 showed more severe defects in facial branchiomotor neuron patterning, whilst facial visceromotor axon guidance was not affected any worse in these mutants relative to single Plxna4-null mutants (compare with 2H,I and 4 H,I with Fig. 6B,D).
It has previously been proposed that PLXNA4 preferentially interacts with NRP1 to form the main SEMA3A receptor, whilst PLXNA3 associates with NRP2 to form the main SEMA3F receptor (Yaron et al., 2005). We have made several observations that support this concept. Firstly, cranial nerve defects in mice lacking SEMA3A or NRP1 are more similar to those of mice lacking PLXNA4, whereas defects in mice lacking SEMA3F or NRP2 are more similar to those of mice lacking PLXNA3 (Fig. 2). Secondly, facial visceromotor neurons respond to SEMA3A and require NRP1 and PLXNA4, but not NRP2 or PLXNA3 for their guidance (Fig. 5). Finally, mutants lacking both PLXNA3 and PLXNA4 show a worse phenotype than single mutants with respect to facial branchiomotor axon guidance, which depends on both SEMA3A and SEMA3F, whereas they do not show a worse phenotype with respect to facial visceromotor neuron patterning, which relies on SEMA3A, but not SEMA3F (Fig. 2, 4, 6). We therefore conclude that SEMA3A/NRP1/PLXNA4 and SEMA3F/NRP2/PLXNA3 pathways co-operate during facial nerve development (see model in Fig. 6E).
Whilst the Plxna4-null facial nerve phenotype was phenocopied in Sema3a-or Nrp1-null mutants (Figs. 2, 4), SEMA3A/NRP1 may signal through PLXNA3 in other axon types in the mouse (Cheng et al., 2001). Moreover, in zebrafish, SEMA3A1 signals through PLXNA3 to control fasciculation and target selection of VIIth nerve motor axons (Tanaka et al., 2007). Given the essential role of PLXNA3 in mediating semaphorin signals in zebrafish and mouse, it is surprising that the chick genome lack a PLXNA3 homolog (Mauti et al., 2006). It therefore appears that PLXNA3 and PLXNA4 have undergone species-dependent specialisation to mediate selective axon guidance events, which is exemplified by the requirement for both plexins during distinct aspects of facial nerve development in the mouse (see model in Fig. 6E).
Acknowledgements
We thank Drs Masahiko Taniguchi, David D. Ginty and Alex L. Kolodkin for providing mouse strains, Joaquim Vieira for help with genotyping and the staff of the Biological Resources Unit at the UCL Institute of Ophthalmology for help with mouse husbandry. This research was funded by a Career Development Award from the Medical Research Council to C.R. (G120/727).
References
- Auclair F, Valdes N, Marchand R. Rhombomere-specific origin of branchial and visceral motoneurons of the facial nerve in the rat embryo. J Comp Neurol. 1996;369:451–61. doi: 10.1002/(SICI)1096-9861(19960603)369:3<451::AID-CNE9>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- Bagri A, Cheng HJ, Yaron A, Pleasure SJ, Tessier-Lavigne M. Stereotyped pruning of long hippocampal axon branches triggered by retraction inducers of the semaphorin family. Cell. 2003;113:285–99. doi: 10.1016/s0092-8674(03)00267-8. [DOI] [PubMed] [Google Scholar]
- Chedotal A, Del Rio JA, Ruiz M, He Z, Borrell V, de Castro F, Ezan F, Goodman CS, Tessier-Lavigne M, Sotelo C, Soriano E. Semaphorins III and IV repel hippocampal axons via two distinct receptors. Development. 1998;125:4313–23. doi: 10.1242/dev.125.21.4313. [DOI] [PubMed] [Google Scholar]
- Chen H, Bagri A, Zupicich JA, Zou Y, Stoeckli E, Pleasure SJ, Lowenstein DH, Skarnes WC, Chedotal A, Tessier-Lavigne M. Neuropilin-2 regulates the development of selective cranial and sensory nerves and hippocampal mossy fiber projections. Neuron. 2000;25:43–56. doi: 10.1016/s0896-6273(00)80870-3. [DOI] [PubMed] [Google Scholar]
- Chen H, Chedotal A, He Z, Goodman CS, Tessier-Lavigne M. Neuropilin-2, a novel member of the neuropilin family, is a high affinity receptor for the semaphorins Sema E and Sema IV but not Sema III. Neuron. 1997;19:547–59. doi: 10.1016/s0896-6273(00)80371-2. [DOI] [PubMed] [Google Scholar]
- Cheng HJ, Bagri A, Yaron A, Stein E, Pleasure SJ, Tessier-Lavigne M. Plexin-A3 mediates semaphorin signaling and regulates the development of hippocampal axonal projections. Neuron. 2001;32:249–63. doi: 10.1016/s0896-6273(01)00478-0. [DOI] [PubMed] [Google Scholar]
- Cordes SP. Molecular genetics of cranial nerve development in mouse. Nat Rev Neurosci. 2001;2:611–23. doi: 10.1038/35090039. [DOI] [PubMed] [Google Scholar]
- Enomoto H, Crawford PA, Gorodinsky A, Heuckeroth RO, Johnson EM, Jr., Milbrandt J. RET signaling is essential for migration, axonal growth and axon guidance of developing sympathetic neurons. Development. 2001;128:3963–74. doi: 10.1242/dev.128.20.3963. [DOI] [PubMed] [Google Scholar]
- Feiner L, Koppel AM, Kobayashi H, Raper JA. Secreted chick semaphorins bind recombinant neuropilin with similar affinities but bind different subsets of neurons in situ. Neuron. 1997;19:539–45. doi: 10.1016/s0896-6273(00)80370-0. [DOI] [PubMed] [Google Scholar]
- Giger RJ, Cloutier JF, Sahay A, Prinjha RK, Levengood DV, Moore SE, Pickering S, Simmons D, Rastan S, Walsh FS, Kolodkin AL, Ginty DD, Geppert M. Neuropilin-2 is required in vivo for selective axon guidance responses to secreted semaphorins. Neuron. 2000;25:29–41. doi: 10.1016/s0896-6273(00)80869-7. [DOI] [PubMed] [Google Scholar]
- Gu C, Rodriguez ER, Reimert DV, Shu T, Fritzsch B, Richards LJ, Kolodkin AL, Ginty DD. Neuropilin-1 conveys semaphorin and VEGF signaling during neural and cardiovascular development. Dev Cell. 2003;5:45–57. doi: 10.1016/s1534-5807(03)00169-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guidry G, Landis SC. Sympathetic axons pathfind successfully in the absence of target. J Neurosci. 1995;15:7565–74. doi: 10.1523/JNEUROSCI.15-11-07565.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacob J, Guthrie S. Facial visceral motor neurons display specific rhombomere origin and axon pathfinding behavior in the chick. J Neurosci. 2000;20:7664–71. doi: 10.1523/JNEUROSCI.20-20-07664.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacob J, Tiveron MC, Brunet JF, Guthrie S. Role of the target in the pathfinding of facial visceral motor axons. Mol Cell Neurosci. 2000;16:14–26. doi: 10.1006/mcne.2000.0855. [DOI] [PubMed] [Google Scholar]
- Kitsukawa T, Shimizu M, Sanbo M, Hirata T, Taniguchi M, Bekku Y, Yagi T, Fujisawa H. Neuropilin-semaphorin III/D-mediated chemorepulsive signals play a crucial role in peripheral nerve projection in mice. Neuron. 1997;19:995–1005. doi: 10.1016/s0896-6273(00)80392-x. [DOI] [PubMed] [Google Scholar]
- Lumsden A, Keynes R. Segmental patterns of neuronal development in the chick hindbrain. Nature. 1989;337:424–8. doi: 10.1038/337424a0. [DOI] [PubMed] [Google Scholar]
- Luo Y, Raible D, Raper JA. Collapsin: a protein in brain that induces the collapse and paralysis of neuronal growth cones. Cell. 1993;75:217–27. doi: 10.1016/0092-8674(93)80064-l. [DOI] [PubMed] [Google Scholar]
- Mauti O, Domanitskaya E, Andermatt I, Sadhu R, Stoeckli ET. Semaphorin6A acts as a gate keeper between the central and the peripheral nervous system. Neural Develop. 2007;2:28. doi: 10.1186/1749-8104-2-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mauti O, Sadhu R, Gemayel J, Gesemann M, Stoeckli ET. Expression patterns of plexins and neuropilins are consistent with cooperative and separate functions during neural development. BMC Dev Biol. 2006;6:32. doi: 10.1186/1471-213X-6-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messersmith EK, Leonardo ED, Shatz CJ, Tessier-Lavigne M, Goodman CS, Kolodkin AL. Semaphorin III can function as a selective chemorepellent to pattern sensory projections in the spinal cord. Neuron. 1995;14:949–59. doi: 10.1016/0896-6273(95)90333-x. [DOI] [PubMed] [Google Scholar]
- Moret F, Renaudot C, Bozon M, Castellani V. Semaphorin and neuropilin co-expression in motoneurons sets axon sensitivity to environmental semaphorin sources during motor axon pathfinding. Development. 2007;134:4491–501. doi: 10.1242/dev.011452. [DOI] [PubMed] [Google Scholar]
- Morin X, Cremer H, Hirsch MR, Kapur RP, Goridis C, Brunet JF. Defects in sensory and autonomic ganglia and absence of locus coeruleus in mice deficient for the homeobox gene Phox2a. Neuron. 1997;18:411–23. doi: 10.1016/s0896-6273(00)81242-8. [DOI] [PubMed] [Google Scholar]
- Palaisa KA, Granato M. Analysis of zebrafish sidetracked mutants reveals a novel role for Plexin A3 in intraspinal motor axon guidance. Development. 2007;134:3251–7. doi: 10.1242/dev.007112. [DOI] [PubMed] [Google Scholar]
- Renaud J, Kerjan G, Sumita I, Zagar Y, Georget V, Kim D, Fouquet C, Suda K, Sanbo M, Suto F, Ackerman SL, Mitchell KJ, Fujisawa H, Chedotal A. Plexin-A2 and its ligand, Sema6A, control nucleus-centrosome coupling in migrating granule cells. Nat Neurosci. 2008;11:440–9. doi: 10.1038/nn2064. [DOI] [PubMed] [Google Scholar]
- Riddle RD, Johnson RL, Laufer E, Tabin C. Sonic hedgehog mediates the polarizing activity of the ZPA. Cell. 1993;75:1401–16. doi: 10.1016/0092-8674(93)90626-2. [DOI] [PubMed] [Google Scholar]
- Rohm B, Ottemeyer A, Lohrum M, Puschel AW. Plexin/neuropilin complexes mediate repulsion by the axonal guidance signal semaphorin 3A. Mech Dev. 2000;93:95–104. doi: 10.1016/s0925-4773(00)00269-0. [DOI] [PubMed] [Google Scholar]
- Sahay A, Molliver ME, Ginty DD, Kolodkin AL. Semaphorin 3F is critical for development of limbic system circuitry and is required in neurons for selective CNS axon guidance events. J Neurosci. 2003;23:6671–80. doi: 10.1523/JNEUROSCI.23-17-06671.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz Q, Gu C, Fujisawa H, Sabelko K, Gertsenstein M, Nagy A, Taniguchi M, Kolodkin AL, Ginty DD, Shima DT, Ruhrberg C. Vascular endothelial growth factor controls neuronal migration and cooperates with Sema3A to pattern distinct compartments of the facial nerve. Genes Dev. 2004;18:2822–34. doi: 10.1101/gad.322904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz Q, Vieira JM, Howard B, Eickholt BJ, Ruhrberg C. Neuropilin 1 and 2 control cranial gangliogenesis and axon guidance through neural crest cells. Development. 2008;135:1605–13. doi: 10.1242/dev.015412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Studer M, Lumsden A, Ariza-McNaughton L, Bradley A, Krumlauf R. Altered segmental identity and abnormal migration of motor neurons in mice lacking Hoxb-1. Nature. 1996;384:630–4. doi: 10.1038/384630a0. [DOI] [PubMed] [Google Scholar]
- Suto F, Ito K, Uemura M, Shimizu M, Shinkawa Y, Sanbo M, Shinoda T, Tsuboi M, Takashima S, Yagi T, Fujisawa H. Plexin-a4 mediates axon-repulsive activities of both secreted and transmembrane semaphorins and plays roles in nerve fiber guidance. J Neurosci. 2005;25:3628–37. doi: 10.1523/JNEUROSCI.4480-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suto F, Murakami Y, Nakamura F, Goshima Y, Fujisawa H. Identification and characterization of a novel mouse plexin, plexin-A4. Mech Dev. 2003;120:385–96. doi: 10.1016/s0925-4773(02)00421-5. [DOI] [PubMed] [Google Scholar]
- Suto F, Tsuboi M, Kamiya H, Mizuno H, Kiyama Y, Komai S, Shimizu M, Sanbo M, Yagi T, Hiromi Y, Chedotal A, Mitchell KJ, Manabe T, Fujisawa H. Interactions between plexin-A2, plexin-A4, and semaphorin 6A control lamina-restricted projection of hippocampal mossy fibers. Neuron. 2007;53:535–47. doi: 10.1016/j.neuron.2007.01.028. [DOI] [PubMed] [Google Scholar]
- Takahashi T, Fournier A, Nakamura F, Wang LH, Murakami Y, Kalb RG, Fujisawa H, Strittmatter SM. Plexin-neuropilin-1 complexes form functional semaphorin-3A receptors. Cell. 1999;99:59–69. doi: 10.1016/s0092-8674(00)80062-8. [DOI] [PubMed] [Google Scholar]
- Takahashi T, Strittmatter SM. Plexina1 autoinhibition by the plexin sema domain. Neuron. 2001;29:429–39. doi: 10.1016/s0896-6273(01)00216-1. [DOI] [PubMed] [Google Scholar]
- Takegahara N, Takamatsu H, Toyofuku T, Tsujimura T, Okuno T, Yukawa K, Mizui M, Yamamoto M, Prasad DV, Suzuki K, Ishii M, Terai K, Moriya M, Nakatsuji Y, Sakoda S, Sato S, Akira S, Takeda K, Inui M, Takai T, Ikawa M, Okabe M, Kumanogoh A, Kikutani H. Plexin-A1 and its interaction with DAP12 in immune responses and bone homeostasis. Nat Cell Biol. 2006;8:615–22. doi: 10.1038/ncb1416. [DOI] [PubMed] [Google Scholar]
- Tamagnone L, Artigiani S, Chen H, He Z, Ming GI, Song H, Chedotal A, Winberg ML, Goodman CS, Poo M, Tessier-Lavigne M, Comoglio PM. Plexins are a large family of receptors for transmembrane, secreted, and GPI-anchored semaphorins in vertebrates. Cell. 1999;99:71–80. doi: 10.1016/s0092-8674(00)80063-x. [DOI] [PubMed] [Google Scholar]
- Tanaka H, Maeda R, Shoji W, Wada H, Masai I, Shiraki T, Kobayashi M, Nakayama R, Okamoto H. Novel mutations affecting axon guidance in zebrafish and a role for plexin signalling in the guidance of trigeminal and facial nerve axons. Development. 2007;134:3259–69. doi: 10.1242/dev.004267. [DOI] [PubMed] [Google Scholar]
- Taniguchi M, Yuasa S, Fujisawa H, Naruse I, Saga S, Mishina M, Yagi T. Disruption of semaphorin III/D gene causes severe abnormality in peripheral nerve projection. Neuron. 1997;19:519–30. doi: 10.1016/s0896-6273(00)80368-2. [DOI] [PubMed] [Google Scholar]
- Tiveron MC, Hirsch MR, Brunet JF. The expression pattern of the transcription factor Phox2 delineates synaptic pathways of the autonomic nervous system. J Neurosci. 1996;16:7649–60. doi: 10.1523/JNEUROSCI.16-23-07649.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traboulsi EI. Congenital cranial dysinnervation disorders and more. J Aapos. 2007;11:215–7. doi: 10.1016/j.jaapos.2007.04.007. [DOI] [PubMed] [Google Scholar]
- Waimey KE, Huang PH, Chen M, Cheng HJ. Plexin-A3 and plexin-A4 restrict the migration of sympathetic neurons but not their neural crest precursors. Dev Biol. 2008;315:448–58. doi: 10.1016/j.ydbio.2008.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaron A, Huang PH, Cheng HJ, Tessier-Lavigne M. Differential requirement for Plexin-A3 and -A4 in mediating responses of sensory and sympathetic neurons to distinct class 3 Semaphorins. Neuron. 2005;45:513–23. doi: 10.1016/j.neuron.2005.01.013. [DOI] [PubMed] [Google Scholar]
- Yip JW. Target cues are not required for the guidance of sympathetic preganglionic axons. Brain Res. 1987;429:155–9. doi: 10.1016/0165-3806(87)90149-0. [DOI] [PubMed] [Google Scholar]