Abstract
Atopic dermatitis is a common, complex disease that frequently follows a chronic, relapsing course and impacts the quality of life of patients and families in a significant manner. New insights into the pathophysiology of AD point to an important role of structural abnormalities in the epidermis combined with immune dysregulation. Patients with AD have a unique predisposition to colonization or infection by a number of microbial organisms, most notably Staphylococcus aureus and herpes simpex virus. A multi-pronged approach directed at healing or protecting the skin barrier and addressing the immune dysregulation is necessary to improve the likelihood of successful outcomes.
Keywords: Atopic dermatitis, filaggrin, epidermal barrier, Staphylococcus aureus, Herpes simplex virus, superantigens, T regulatory cells, eczema herpeticum, antimicrobial peptides, probiotics
Atopic dermatitis (AD), a complex chronic relapsing inflammatory skin disorder continues to be an important disease worldwide.1,2 Lifetime prevalence in school aged children in the United States has been reported to be up to 17%.3 Similarly high prevalence rates have also been observed in a number of other countries.4 Most recently, data on eczema symptoms from over a million children in 97 countries showed that AD is a major problem in developing, as well as developed countries.5 Atopy remains an important association with an incidence of approximately 80% in infants with AD recently reported in Australia and the United Kingdom.6 Importantly, a high percentage of children with AD (~66%) develop asthma and/or allergies, typically by 3 years of age.7 Patients with severe or persistent disease and their families experience significant impairment in their quality of life, which can contribute to poor outcomes with prescribed treatment.8 In addition, AD places a heavy economic burden not only on patients and their families, but also on society as a whole.9,10 This review will highlight some of the recent studies that provide insights into the pathophysiology of the AD with emphasis on the unique role that microbial organisms play in this disease and implications for therapy.
EPIDERMAL ABNORMALITIES AND IMMUNE DYSREGULATION IN AD: RECONCILING THE OUTSIDE-IN AND INSIDE-OUT HYPOTHESES
The pathophysiology of AD remains incompletely understood, although gene-environment interactions in genetically predisposed individuals (see article by Barnes and colleagues in this issue) play a central role.11,12 A number of critical systemic and skin immune abnormalities, including increased serum IgE and sensitization to allergens, elevated Th2-type cytokine expression in acute lesions, increased numbers of T cells expressing cutaneous lymphocyte-associated antigen (CLA) (the homing receptor for the skin), increased expression of FcεRI on both Langerhans cells and inflammatory dendritic epidermal cells, as well as decreased expression of antimicrobial peptides.13,14 A growing number of studies have shown a highly significant association between abnormalities in the epidermal barrier and risk of developing early-onset, severe, persistent AD.15-17 Of note, these may be due to both mutations of genes encoding proteins such as filaggrin,14 as well as modulation of epidermal protein levels by Th2-type cytokines.18,19 While some researchers have argued that the primary defect in AD resides in the epidermal barrier,20 it is important to recognize that a significant number of patients with AD do not have any of the known FLG mutations, and conversely, approximately 40% of individuals with FLG -null alleles do not develop AD.21 In addition, while the natural history of patients with AD and FLG mutations has not been fully elucidated, many of these patients outgrow their disease or go into an extended remission, although they tend to have a more prolonged course than those without FLG mutations.22 A critical link between the barrier defect in AD patients with FLG mutations and Th2 polarization could be explained in part by enhanced allergen penetration through the damaged epidermis accompanied by increased production of thymic stromal lymphopoietin (TSLP) by keratinocytes leading to a Th2-type milieu.23 Importantly, patients with FLG gene mutations are at increased risk for developing asthma, but only in the context of having AD, pointing to the importance of allergic sensitization through a damaged skin barrier.24 Conversely, AD patients with more polarized Th2-type disease with allergies and asthma and increased biomarkers including serum IgE, TSLP and cutaneous T cell-attracting chemokine were also more likely to have severe skin disease complicated by eczema herpeticum (EH), Staphylococcus (S.) aureus or molluscum infections.25 In addition, patients with FLG mutations have been found to have an increased risk for EH, a serious complication of AD.26 It is worth noting that the epidermal differentiation complex, a candidate gene region for AD localized on chromosome 1q21 includes a number of other genes encoding structural proteins of epidermal cornification, including S100A proteins, small proline-rich region proteins and late envelope proteins.27 Using a proteomics approach, Howell et al28 identified S100/A11 as a target in Th2 cytokine-mediated inhibition of filaggrin and the antimicrobial peptide, HBD-3, expression in AD skin, pointing to immune dysregulation effecting both epidermal barrier integrity and innate immune response. Still, the relationship of skin barrier and immune abnormalities to the increased susceptibility to microbial colonization and infections remains to be fully elucidated.29 Of interest, emerging observations that topical calcineurin inhibitors can in part correct the barrier defect in AD and that gentamicin can restore the production of functional filaggrin chains provides further evidence of the complex relationship of the epidermal barrier and the immune system.30 While future studies may shed further light on unique AD phenotypes and a more individualized approach to care, at present, a comprehensive multi-pronged treatment approach addressing both the epidermal barrier and immune dysregulation is most likely to result in disease control for our patients.31
AD: IS PREVENTION POSSIBLE?
A recent review addressed the subject of therapeutic attempts to shift the presumed Th2 response early in life to a Th1 response through the administration of probiotics either to pregnant women and subsequently to at risk newborns to prevent AD or even to treat established AD.30 While one meta-analysis suggested a modest role for probiotics in children with moderately severe disease in reducing the Scoring of Atopic Dermatitis Severity Index score,32 another found that current evidence is more convincing for efficacy of probiotics in prevention, rather than treatment of pediatric AD.33 In contrast, a study designed to replicate an earlier one that showed beneficial effects of probiotics in AD found that supplementation with Lactobacillus GG during pregnancy and early infancy neither reduced the incidence of AD nor altered the severity of AD in affected children but was associated with an increased rate of recurrent episodes of wheezing bronchitis.34 Furthermore, a recent Cochrane review concluded that probiotics are not an effective treatment for eczema in children and that probiotic treatment carries a small risk of adverse events.35 Salfeld and Kopp recently reviewed this subject, pointing to flaws in methodology of some of the analyses and heterogeneity of treatment protocols.36 They concluded that selection of the most beneficial probiotic strain(s), use of probiotics with or without prebiotics, timing of supplementation, along with optimal dose and delivery remain to be determined. Thus, probiotics for the prevention of AD remain investigational and, at the present time, cannot be recommended for primary prevention.
AD: MOVING BEYOND THE TH1/TH2 PARADIGM
A role for new subsets of T cells in AD is being increasingly appreciated. Naturally occurring CD4+CD25+ FoxP3 expressing T regulatory (Treg) cells with normal immunosuppressive activity appear to be expanded in the peripheral blood of patients with AD.37 However, after stimulation by the superantigen (SAg), staphylococcal enterotoxin B (SEB), Treg cells lose their immunosuppressive activity, suggesting a novel mechanism by which SAgs could augment T-cell activation in patients with AD.38 Hijen, et al39 recently confirmed increased numbers of Treg cells found in the peripheral blood of patients with AD and hypothesized that this was due to strong inflammatory signals with Treg cell suppressive activity subverted by proinflammatory mediators. Verhagen et al40 showed CD4+CD25+FoxP3+ Treg cells were not found in lesional AD skin or in atopy patch test sites of AD patients. Thus, a dysregulation of disease causing effector T cells is observed in AD lesions in association with an impaired CD4+CD25+FoxP3+ Treg cell infiltration.
In addition, IL-17 secreting Th17 cells in AD have been investigated in several studies. IL-17 was first shown to be preferentially elevated in acute vs chronic AD lesions.41 In a murine epicutaneous antigen challenge model analogous to human AD, IL-17 expression was induced not only in the skin, but also in the airways.42 Using a murine model of filaggrin deficiency to examine whether this abnormality predisposes to skin inflammation and epicutaneous sensitization with protein antigen, this group also showed that filaggrin-deficient mice exhibited Th17-dominated skin inflammation and eczematous changes with increased expression of IL-17 in the epidermis and increased antigen-specific IgE in their serum.43 In an atopy patch test model, IL-17 secretion was shown to be enhanced by SEB.44 Induced IL-17 upregulated the antimicrobial peptide HBD-2 in human keratinocytes in vivo, although co-expressed IL-4/IL-13 partially inhibited this effect. Thus, while IL-17 secreting T cells appear to infiltrate acute AD lesions and IL-17 secretion can be triggered by SAgs, subsequent ineffective IL-17-dependent upregulation of HBD-2 in patients with AD may result from partial inhibition by the Th2 cytokine milieu. A study in IL-4 and IL-13 knockout mice supports a role for IL-4 as a Th2 cytokine that downregulates the IL-17 response in epicutaneously sensitized mice.45 This in turn could be one reason why AD patients remain colonized by S. aureus. Other investigators have also found that compared to psoriasis, Th17 cells appear to have a diminished role in AD skin and that the associated reduced expression of innate defense genes may contribute to the increased skin infections in AD.46
THE ITCH-SCRATCH CYCLE IN AD
Pruritus is a major symptom of AD and impacts quality of life of patients in a significant manner. The clinical observation that pruritus in AD patients is often not relieved by antihistamines suggests that mediators other than histamine such as cytokines and neuropeptides may be involved.47 IL-31, a cytokine that is increased in AD skin lesions48 has been implicated in the development of chronic dermatitis in transgenic mice that overexpress IL-31 through induction of severe pruritus.49 Proposed sources of IL-31 include skin-infiltrating CLA+ T cells and peripheral blood CD45RO CLA+ T cells.50 Recent data provide evidence that, irrespective of the atopic phenotype, serum IL-31 levels correlate with disease activity in AD.51 Importantly, staphylococcal superantigens have been shown to rapidly induce IL-31 mRNA expression in the skin of atopic individuals in vivo and in PBMCs in vitro, suggesting that chronic colonization and superinfection by S. aureus can contribute to pruritus and inflammatory changes in AD.48 Given that pruritus in AD is often resistant to antihistamines, IL-31 represents a potential target for antipruritic and anti-inflammatory measures in the treatment of AD. Gutzmer et al52 recently reported that the H4 histamine receptor is upregulated on TH2-type cells, suggesting a role of the H4R in a TH2 milieu. Of note, they showed that H4R stimulation led to upregulation of IL-31 and stimulation of PBMCs with H4R ligand plus SEB resulted in higher IL-31 mRNA levels. These observations suggest a link between histamine and induction of pruritus, especially in AD and that the H4R represents a potential therapeutic target.
Another mechanism for chronic infl ammation in AD driven by immune dysregulation is an autoimmune model. The itch-scratch cycle can lead to damage of epidermal keratinocytes and release of intra-cellular antigens, which in a subset of AD patients could lead to a chronic autoreactiv e form of AD even without exposure to allergens. 53 Alrichter et al54 recently found that sera from 28% of AD patients showed IgE autoreactivity directed against epiderma l or epithelial cell line-derived proteins including cytoplasmic and cell membrane-associated moieties. This autoreactivity in AD patients was significantly correlated with the se verity of the disease, defined by the total serum IgE levels and by clinical scoring indexes.
AD AND MICROBES
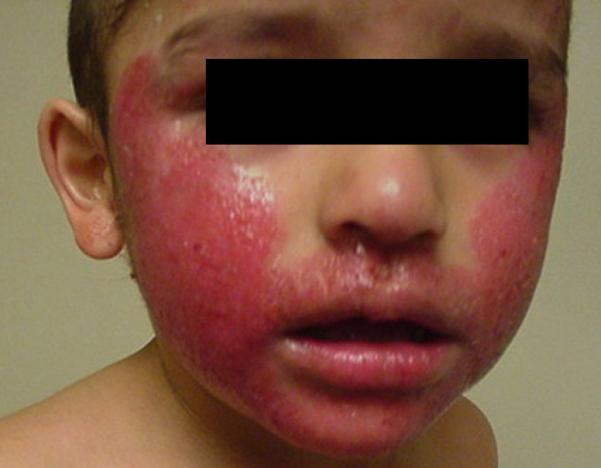
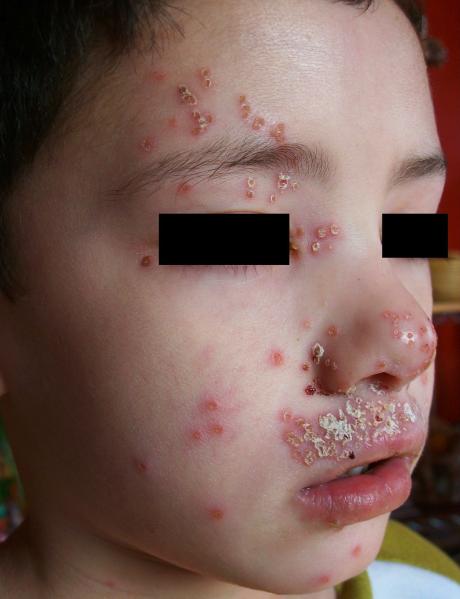
Patients with AD have a unique propensity to be colonized or infected by a number Rof microbial organisms (Fig 1a and 1b). Dysregulation of the adaptive immune response Cwith elevated total and specific IgE levels has been associated with disease severity and infectious complications.2,55,56 More recently, the contribution of innate immune system abnormalities, including reduction in antimicrobial peptides, diminished recruitment of cells such as neutrophils to the skin and toll-like receptor defects57 and epidermal barrier abnormalities12 in microbial colonization or infection in AD were the subjects of comprehensive reviews. As one example of the relationship between innate and adaptive immune responses and infectious complications of AD, both mobilization of HBD-3 and killing of S. aureus by keratinocytes from AD patients were shown to be significantly inhibited by the Th2 cytokines IL-4 and IL-13, while neutralization of these cytokines significantly improved these activities.58 In another example, serum IgE levels in AD patients with herpes simplex virus (HSV) infections were found to be inversely correlated with cathelicidin LL-37 expression.59 How aberrations in adaptive and innate immune responses and barrier abnormalities all interact in AD remains to be fully investigated. Further insights may come from studies of immunodeficiency patients with an AD-type cutaneous phenotype. Of interest, a recent study looking at a group of patients with poorly characterized combined immunodeficiency whose clinical presentation included recurrent cutaneous infections with S. aureus or HSV and who also had elevated serum IgE and eczematous rash found homozygous or compound heterozygous deletions and point mutations in the gene encoding the dedicator of cytokinesis 8 protein (DOCK8) leading to absence of DOCK8 protein in lymphocytes.60
Figure 1a.
Child with atopic dermatitis superinfected with toxin secreting Staphylococcus aureus.
Fig 1b.
Child with atopic dermatitis with course complicated by eczema herpeticum.
S. aureus can be cultured from 90% of skin lesions and importantly can colonize normal appearing skin in AD.61 Patients with more severe disease have been shown to have higher levels of S. aureus in their home environments.62 S. aureus can exacerbate or contribute to persistent skin inflammation in AD by secreting toxins with superantigenic properties, resulting in marked activation of T cells and other immune cells. Application of SEB to the skin can induce eczematous changes accompanied by infiltration of T cells selectively expanded in response to the SAg.63 In addition, AD patients can make specific IgE antibodies directed against the toxins found on their skin with basophils from these patients releasing histamine on exposure to the relevant toxin.64,65 This suggests that SAgs can induce mast cell degranulation after penetrating the epidermal barrier and contribute to pruritus and acute inflammatory events along with participating in chronic skin inflammation. S. aureus isolates from patients with steroid-resistant AD have been shown to produce increased numbers of SAgs compared with isolates from controls.66 Thus, SAgs may offer a selective advantage for colonization of patients.
Other products of S. aureus likely contribute to disease in AD. Recently, children with impetiginized AD were found to have elevated levels of lipoteichoic acid (LTA) that correlated with lesional EASI scores and S. aureus CFU.67 The amounts of LTA in the skin lesions were sufficient to exert biological effects on various cell types in vitro, as well as epidermal cytokine gene expression when skin was exposed to LTA ex vivo. This study provides a further mechanism by which S. aureus can exacerbate AD.
Methicillin-resistant S. aureus (MRSA) has emerged as an important pathogen that has rapidly evolved from a cause of nosocomial to community-acquired infections (see review by Schlievert, et al in this issue).68 MRSA invariably produce SAgs. Wang et al69 identified a novel class of secreted S. aureus peptides, phenol-soluble modulins (PSMs), that contribute to the enhanced virulence of community acquired (CA)-MRSA. These peptides appear to target neutrophils, recruiting, activating and then lysing these cells. They also showed that the PSMs are under the regulatory control of the accessory gene regulator (agr) quorum-sensing system, which controls the expression of a number of staphylococcal virulence factors. Patients with AD may be particularly susceptible to colonization and infection by MRSA as well as to the acute and chronic consequences of the SAgs that these organisms produce, as they are frequently treated, often for extended courses with anti-staphylococcal antibiotics.70 However, the question of whether AD patients serve as an important reservoir of MRSA in the community has not been thoroughly investigated.71 Understanding the underlying mechanisms for infection and colonization by S. aureus of the skin of patients with AD including differences between methicillin sensitive versus MRSA is critical for developing more effective treatment strategies for this serious public health problem.
The clinical course in patients with AD can also be complicated by both localized and disseminated cutaneous viral infections, most often caused by HSV, human papilloma or molluscum viruses.72 Eczema herpeticum (EH) is a potentially life-threatening disseminated HSV-1 or less commonly HSV-2 infection that occurs in 10–20% of patients with AD.73 Risk factors for EH include early onset of AD, severe and untreated AD, head and neck dermatitis, previous EH or HSV infections, elevated total serum IgE with higher level of specific sensitizations, especially against M. sympodialis.73 Recent studies from the NIH/NIAID Atopic Dermatitis and Vaccinia Network (ADVN) showed that compared to AD patients without a history of EH, AD patients with EH have a more severe Th2-polarized disease with greater allergen sensitization including to staphylococcal toxins and are also more likely to develop cutaneous infections with S. aureus.26 In addition, AD patients of both European and African ancestry with the R501X mutation in the gene encoding filaggrin have been found to have an even greater risk for EH, suggesting that a defective skin barrier can also contribute to this serious complication.27 Importantly, patients with AD are also at risk for potentially life-threatening complications from both smallpox infection and from vaccinia virus (eczema vaccinatum) used to prevent smallpox.75,76 When skin biopsies from AD subjects are inoculated with vaccinia virus, there is increased viral replication compared to healthy controls.77 In addition, levels of the antimicrobial cathelicidin LL-37 are low while expression of IL-4 and IL-13 is elevated in AD skin and antibodies against these Th2 cytokines inhibit vaccinia growth and enhance production of LL-37. Of clinical importance, such studies may provide insights into identifying individuals who are at greatest risk for complications from immunization with vaccinia virus.
BACTERIAL COLONIZATION AND INFECTION IN AD: TO TREAT OR NOT TO TREAT (WITH ANTIBIOTICS)
It is important to recognize that S. aureus is a common commensal organism in humans and can be cultured from non-lesional skin in a significant number of patients with AD (see review by Schlievert et al in this issue).68 Colonization by toxin-secreting S. aureus can contribute to pruritus and persistent inflammation.64 In addition, colonization is a risk factor for infection.79 Nevertheless, a number of factors contribute to difficulties in implementing successful strategies to clear colonization. S. aureus can be found in the house dust of most AD patients and patients with more severe disease have higher levels of S. aureus in their home environment.62 Patients treated with antibiotics quickly become re-colonized, often with the same toxin-secreting organisms.70 Family members often serve as the source of rapid re-colonization.79 Of note, in the recent study by Huang et al80, even after 3 months of twice weekly dilute bleach bath therapy and monthly 5 day courses of nasal mupirocin, patients were still colonized by S. aureus. Furthermore, the authors of a Cochrane Database systematic review failed to find clear evidence of benefit for antimicrobial interventions in patients with AD. However, they point out that the studies were small and poorly reported and may not have shown the anticipated benefit.81
Given the complex pathophysiology of AD, a multipronged approach directed at healing or protecting the skin barrier and addressing the immune dysregulation will improve the likelihood of successful outcomes. This includes proper skin hydration, identification and elimination of flare factors such as irritants, allergens, infectious agents and emotional stressors, addressing the itch-scratch cycle as well as pharmacologic therapy (reviewed in depth in reference 31). In theory, use of topical inhibitors of proteases should be an effective treatment strategy in AD. To date, however, several trials with these agents have yielded disappointing results.31 An important concept with therapeutic implications is the recognition that normal-appearing skin in AD is not immunologically normal.82 Furthermore, increased binding of S. aureus to AD skin is related to underlying skin inflammation61 and anti-inflammatory treatment with topical steroids and calcineurin inhibitors reduces S. aureus colonization.83 One approach to patients whose eczema tends to relapse in the same location is that of proactive therapy. After a period of stabilization, topical steroids84,85 or calcineurin inhibitors86-88 are applied to areas of previously involved, but normal appearing skin, rather than waiting for eczema to flare. Importantly, proactive therapy is an attempt to control residual disease since even normal-appearing skin in AD may be colonized by S. aureus and is characterized by immunological abnormalities, not the application of an active drug to non-affected skin.89 Other approaches include silver-impregnated clothing, which has been shown to reduce staphylococcal colonization, improve clinical parameters and reduce topical steroid use in AD.90,91 High-dose intravenous immunoglobulin (IVIG) could have immunomodulatory effects in AD and in addition, IVIG could interact directly with microbes or toxins involved in the pathogenesis of AD. IVIG has been shown to contain high concentrations of staphylococcal toxin-specific antibodies that inhibit the in vitro activation of T cells by staphylococcal toxins.92 Treatment of severe refractory AD with IVIG has yielded conflicting results. Studies have not been controlled and have involved small numbers of patients.93 Children appear to have a better response than adults. However, controlled studies are needed to answer the question of efficacy in a more definitive manner. New strategies may evolve from an important ongoing project looking at skin microbial flora with molecular rather than traditional microbiological tools.94
Abscesses associated with MRSA generally respond to drainage and most CA-MRSA isolates are susceptible to trimethoprim-sulfamethizole or tetracycline, although obtaining cultures and sensitivities is important. Rifampin has been used in combination with other antibiotics but should never be used alone to treat staphylococcal infection. Other options include vancomycin, fluoroquinolones, daptomycin, newer-generation carbapenems, and linezolid.95 In a study of children with culture-proven CA-MRSA, treatment with incision and drainage without adjunctive antibiotic therapy was effective management of skin and soft-tissue abscesses with a diameter of less than 5 cm in immunocompetent children.96 Extensive practical information on isolation of MRSA patients and other practical issues can be found on the Center for Disease Control and Prevention’s website (www.cdc.gov/ncidod/dhqp/ar_mrsa.html).
EFFECTS OF TOPICAL THERAPY ON SKIN BARRIER IN AD
A recent study looking at children with AD versus children with other atopic diseases and non-atopic controls confirmed that skin barrier function as assessed by transepidermal water loss (TEWL) is intrinsically compromised in children with AD, but not in children with other allergic conditions. 97 In addition, the authors showed that TEWL was higher in white than in African American children with AD and that the magnitude of skin barrier dysfunction correlated with disease severity. While TEWL might be a useful biomarker in AD, racial and pigmentation differences will need to be considered. More recently, Jensen et al 98 looked at TEWL, as well as several other parameters of epidermal barrier including stratum corneum hydration and dye penetration and showed improvement in all parameters when AD patients were treated with both a topical steroid (betamethasone valerate 0.1% cream) and a topical calcineurin inhibitor (pimecrolimus 1% cream) applied to paired lesions of the upper extremities. Electron microscopic evaluation of barrier structure showed prevalently ordered stratum corneum lipid layers and regular lamellar body extrusion in the calcineurin inhibitor treated skin but inconsistent extracellular lipid bilayers and only partially filled lamellar bodies in the steroid treated skin. Both treatments normalized epidermal differentiation and reduced epidermal hyperproliferation. Betamethasone valerate was superior in reducing clinical symptoms and epidermal proliferation, but twice daily use over the 3-week period of the study led to epidermal thinning. The authors concluded that because pimecrolimus improved the epidermal barrier and did not cause atrophy, it might be more suitable for long-term treatment of AD. However, the fact that the topical steroid was more effective in reducing clinical symptoms and inflammation supports the use of topical steroids for acute intervention of AD flares.99
DEALING WITH VIRAL AND FUNGAL COMPLICATIONS
It is important for clinicians to be aware of the possibility of HSV complicating AD, especially EH. Vesicular lesions are umbilicated, tend to occur in crops and often become hemorrhagic and crusted. Lesions may coalesce into large, denuded areas. HSV may be misdiagnosed as impetigo, although herpetic lesions can become superinfected.100 The presence of punched-out erosions, vesicles, and/or infected skin lesions that fail to respond to oral antibiotics should prompt a search for HSV utilizing PCR, viral culture or Giemsa-stained Tzanck smear of cells scraped from the base of a freshly unroofed vesicle. Treatment may be with oral acyclovir or other antivirals such as valacyclovir for less severe infections or intravenous acyclov ir for widely disseminated disease or toxic-appearing patients. 72 Ophthalmology consultation should be obtained for patients with periocular or suspected eye involvement. Lumbar puncture should be considered if meningitis is suspected, but the presence of infected lesions over the lumbar areas precludes this procedure. Antiviral prophylaxis may be necessary for patients with recurrent herpetic outbreaks.
Fungi may also play a role in chronic inflammation of AD. IgE antibodies against M. sympodialis are found in AD patients, most frequently in patients with a head and neck distribution of dermatitis.2 However, even patients with IgE antibodies to M. sympodialis often respond better to topical steroids than to topical antifungal therapy and systemic antifungal therapy may benefit AD patients through anti-inflammatory properties.
NOVEL DIRECTIONS IN THERAPY
Vitamin D deficiency is being increasi ngly recognized in the US population and may play a role in allergic illnesses.101 Importantly, vitamin D may play an important role in regulation of antimicrobial peptides in keratinocytes.102 A trial with oral vitamin D in patients with AD supports this hypothesis.103 In addition, in one small pediatric study, children with AD treated with oral vitamin D in a randomized, controlled trial showed improvement in the Investigator Global Assessment score in four of five subjects treated with vitamin D versus one of six on placebo.104 Similar changes favoring vitamin D therapy were also seen in the EASI score. Larger ADVN sponsored trials with oral vitamin D are currently in progress.
The emergence of CA-MRSA has created an urgency to develop novel strategies to combat this microbial organism. The best studied and probably most important virulence regulator in staphylococci is the accessory gene regulator agr.105 Of note, strong expression of agr appears to be an important characteristic of CA-MRSA strains.106 Agr signals through an exported autoinducing peptide (AIP), and administration of an inhibiting AIP together with an infectious strain leads to a significant reduction in S. aureus infectivity in a mouse subcutaneous infection model.107 In a novel approach, Park et al 108 reported on the use of anti-AIP antibodies to inhibit agr function, demonstrating that antibodies designed against the AIP of one S. aureus agr subgroup specifically prevent agr expression and S. aureus disease in an animal model of abscess formation. These antibodies also provided protection when used for passive immunization, i.e., when administered prior to infection. While these findings provide cautious optimism in describing a successful strategy versus this key staphylococcal virulence regulator, it is worth noting that inhibition of the agr regulator in staphylococcal organisms results in upregulation of other virulence factors that are under opposite regulation by agr.109 An important consideration in devising new therapeutic strategies against S. aureus is recognizing that protective immunity to staphylococcal infections does not appear to exist to any significant degree, partly due to the fact that our immune system is in constant contact with staphylococcal antigens and many strains are commensal organisms. In addition, S. aureus produces protein A to help it evade acquired host defense. While several attempts to develop protective vaccines have met with failure in clinical trials (e.g. StaphVax), promising results based on using a combination of systematically selected antigens have been reported.105 These combinatory vaccines target microbial surface components recognizing adhesive matrix molecules (MSCRAMMs), a family of bacterial proteins that bind to human extracellular matrix components. Stranger-Jones, et al110 developed a vaccine based on a combination of antigens that provided complete protection from lethal doses of S. aureus in a murine challenge model. Importantly, MSCRAMM vaccines have been shown to prevent colonization.111 Whether this will result in decreased infection rates remains to be determined. Another potential antigenic target for vaccine development against S. aureus is teichoic acid, which has been implicated in nasal colonization and biofilm formation. 112 Of note, a recent study in children with impetiginized AD found significant levels of lipoteichoic acid in infected lesions that were able to induce epidermal cytokine gene expression ex vivo.67 A new conjugated vaccine (PentaStaph, Nabi Biopharmaceuticals, Rockville, MD) that includes both α-toxin and Panton-Valentine leukocidin is currently in clinical trials.
A novel approach in dealing with staphylococcal toxins involves engineering binding affinity agents capable of neutralizing these SAgs. Soluble forms of the engineered Vβ proteins produced in E. coli were shown to be effective inhibitors of SEB-mediated T-cell activation and completely neutralized the lethal activity of SEB in animal models. 113 More recently, the same group was able to express Vβ domains in tandem as a single-chain protein and neutralized the clinically important SAgs SEB and TSST-1 with a single agent demonstrating the feasibility of engineering a broader spectrum antagonist capable of neutralizing multiple toxins.114
NEW ANTIVIRAL STRATEGIES
Given the post-9/11 concerns about use of smallpox and other agents as weapons of bioterrorism and the unique susceptibility of AD patients to serious adverse effects from vaccination with vaccinia virus (VV), novel approaches in protecting or treating a vulnerable population are urgently needed.75,76 With this goal, the NIH established the ADVN. The increased propensity of AD patients toward eczema vaccinatum may be related to a deficiency of antimicrobial peptides.77,115 Although cathelicidins and HBD-3 exhibit potent antiviral activity against VV 116, their use as anti-VV agents is limited due to rapid degradation by endogenous tissue proteases. Ceragenins are synthetic antimicrobial compounds designed to mimic the structure and function of endogenous antimicrobial peptides.117 They have been shown to disrupt bacterial membranes without damaging mammalian cell membranes.118 Of note, due to their synthetic nature, ceragenins are not subject to human protease degradation and therefore have a longer tissue half-life. Recently, investigators at National Jewish Health and Brigham Young University showed that one candidate compound (CSA-13) exhibits potent antiviral activity against VV via direct antiviral effects and by stimulating the expression of endogenous antimicrobial peptides with known antiviral activity.119 They also demonstrated that topical application of CSA-13 was able to reduce satellite lesion formation, suggesting that treatment with CSA-13 may be an intervention for patients with disseminated VV skin infection.
Key Take Home Messages for Clinicians
Clinicians caring for patients with AD need to understand the important relationship between skin barrier abnormalities and immune dysregulation in this common, but complex disease. They need to appreciate the strong association between FGN mutations and early-onset, persistent severe AD and the association with allergic sensitization and asthma. Furthermore, it is important to recognize the unique propensity for patients with AD to be colonized or infected by microbes, especially toxin-secreting S. aureus, including MRSA, as well as HSV. Management needs to be directed at basic skin care including repair and protection of the skin barrier with proper hydration and topical therapy, which includes both moisturizers and anti-inflammatory medications. Of note, these measures reduce microbial colonization and decrease the need for specific antimicrobial therapy even in patients colonized by MRSA. In patients, suspected of having an infectious complication of AD, obtaining culture and sensitivities from patients with difficult-to-treat AD can help identify resistant organisms and direct antimicrobial therapy if needed. In addition, culture or PCR can identify HSV, which is often missed, especially if superinfected by S.aureus. Identification and avoidance of irritants and proven allergens can further decrease skin inflammation and lessen the need for medications. Breaking the itch-scratch cycle and addressing sleep disturbance together with education are critical components of successful management of AD. Proactive treatment with topical steroids or calcineurin inhibitors in patients with recurrent AD involves application of medication 2–3 times weekly to previously involved, but normal appearing skin, recognizing that normal-appearing skin in AD is not immunologically normal and is often colonized by S. aureus. While this approach has been shown to decrease flares over extended periods of time, it would currently be considered off-label therapy. Eczema herpeticum is potentially a medical emergency and patients may require intravenous antiviral therapy as well as assessment for ocular involvement. With the reintroduction of smallpox vaccination, clinicians should also be vigilant of the possibility of eczema vaccinatum in any AD patient who has had recent close contacts with another individual who has been immunized with this live viral vaccine.
Acknowledgments
Funding source: This work was supported by NIH/NIAID contracts N01 AI 40029 and 40030 as well as AR41256
Abbreviations used
- agr
Accessory gene regulator
- AD
Atopic dermatitis
- ADVN
Atopic Dermatitis and Vaccinia Network
- AIP
Autoinducible peptide
- CFU
Colony forming units
- CA
Community acquired
- CLA
Cutaneous lymphocyte-associated antigen
- DOCK8
Dedicator of cytokinesis 8 protein
- EASI
Eczema Area and Severity Index
- EH
Eczema herpeticum
- FGN
Filaggrin gene
- HSV
Herpes simplex virus
- HBD
Human beta defensin
- IL
Interleukin
- IVIG
Intravenous immunoglobulin
- LTA
Lipoteichoic acid
- M
Malassezia
- MRSA
Methicillin resistant Staphylococcus aureus
- MSCRAMMs
Microbial surface components recognizing adhesive matrix molecules
- NIAID
National Institute of Allergy and Infectious Diseases
- PBMC
Peripheral blood mononuclear cells
- PSM
Phenol-soluble modulin
- PCR
Polymerase chain reaction
- S
Staphylococcus
- SEB
Staphylococcal enterotoxin B
- SAg
Superantigen
- Th
T helper
- TEWL
Transepidermal water loss
- TSLP
Thymic stromal lymphopoietin
- VV
Vaccinia virus
Footnotes
What do we know?
- Atopic dermatitis is a global health problem, strongly associated with asthma and allergic sensitization.
- Compared to atopic dermatitis patients without FGN mutations, those patients with atopic dermatitis who have mutations in the FGN gene have disease that is earlier in onset, more severe and more persistent and more likely to be associated with asthma and allergic sensitization.
- Most patients with atopic dermatitis are colonized by toxin-secreting S. aureus, even on normal-appearing skin.
- Patients with atopic dermatitis with FLG mutations have been found to have an increased risk for eczema herpeticum.
- Topical anti-inflammatory measures can reduce S. aureus colonization.
What is still unknown?
- The exact relationship between barrier dysfunction and immune dysregulation in atopic dermatitis.
- What other skin barrier proteins besides filaggrin are essential for normal barrier function?
- A full understanding of why patients with atopic dermatitis compared to other inflammatory dermatoses have more problems with microbial colonization and infection.
- Specific biomarkers for atopic dermatitis and unique phenotypes.
- Optimal individualized therapy.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Leung DYM, Boguniewicz M, Howell M, Nomura I, Hamid QA. New insights into atopic dermatitis. J Clin Invest. 2004;113:651–7. doi: 10.1172/JCI21060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Boguniewicz M, Schmid-Grendelmeier P, Leung DYM. Clinical Pearls: Atopic dermatitis. J Allergy Clin Immunol. 2006;118:40–3. doi: 10.1016/j.jaci.2006.04.044. [DOI] [PubMed] [Google Scholar]
- 3.Laughter D, Istvan JA, Tofte SJ, Hanifin JM. The prevalence of atopic dermatitis in Oregon schoolchildren. J Am Acad Dermatol. 2000;43:649–5. doi: 10.1067/mjd.2000.107773. [DOI] [PubMed] [Google Scholar]
- 4.Williams H, Stewart A, von Mutius E, Cookson W, Anderson HR, International Study of Asthma and Allergies in Childhood (ISAAC) Phase One and Three Study Groups Is eczema really on the increase worldwide? J Allergy Clin Immunol. 2008;121:947–54. doi: 10.1016/j.jaci.2007.11.004. [DOI] [PubMed] [Google Scholar]
- 5.Odhiambo JA, Williams HC, Clayton TO, Robertson CF, Asher MI, ISAAC Phase Three Study Group Global variations in prevalence of eczema symptoms in children from ISAAC Phase Three. J Allergy Clin Immunol. 2009 doi: 10.1016/j.jaci.2009.10.009. in press. [DOI] [PubMed] [Google Scholar]
- 6.De Benedictis FM, Franceschini F, Hill D, Naspitz C, Simons FER, Wahn U, et al. The allergic sensitization in infants with atopic eczema from different countries. Allergy. 2009;64:295–303. doi: 10.1111/j.1398-9995.2008.01779.x. [DOI] [PubMed] [Google Scholar]
- 7.Kapoor R, Menon C, Hoffstad O, Bilker W, Leclerc P, Margolis DJ. The prevalence of atopic triad in children with physician-confirmed atopic dermatitis. J Am Acad Dermatol. 2008;58:68–3. doi: 10.1016/j.jaad.2007.06.041. [DOI] [PubMed] [Google Scholar]
- 8.Beattie PE, Lewis-Jones MS. A comparative study of impairment of Quality Of Life (QOL) in children with skin disease and children with other chronic childhood diseases. Br J Dermatol. 2006;155:145–5. doi: 10.1111/j.1365-2133.2006.07185.x. [DOI] [PubMed] [Google Scholar]
- 9.Boguniewicz M, Abramovits W, Paller A, Whitaker-Worth DL, Prendergast M, Cheng JW, et al. A multiple-domain framework of clinical, economic, and patient-reported outcomes for evaluating benefits of intervention in atopic dermatitis. J Drugs Dermatol. 2007;6:416–3. [PubMed] [Google Scholar]
- 10.Mancini AJ, Kaulback K, Chamlin SL. The socioeconomic impact of atopic dermatitis in the United States: A systematic review. Pediat Dermatol. 2008;25:1–6. doi: 10.1111/j.1525-1470.2007.00572.x. [DOI] [PubMed] [Google Scholar]
- 11.Barnes KC. An update on the genetics of atopic dermatitis: Scratching the surface in 2009. J Allergy Clin Immunol. 2010;125:xx–xx. doi: 10.1016/j.jaci.2009.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cork MJ, Danby SG, Vasilopoulos Y, Hadgraft J, Lane ME, Moustafa M, et al. Epidermal barrier dysfunction in atopic dermatitis. J Invest Dermatol. 2009;129:1892–1908. doi: 10.1038/jid.2009.133. [DOI] [PubMed] [Google Scholar]
- 13.Boguniewicz M, Leung DYM. Atopic dermatitis. J Allergy Clin Immunol. 2006;117:S475–80. doi: 10.1016/j.jaci.2005.10.018. [DOI] [PubMed] [Google Scholar]
- 14.Ong PY, Ohtake T, Brandt C, Strickland I, Boguniewicz M, Ganz T, et al. Endogenous antimicrobial peptides and skin infections in atopic dermatitis. N Engl J Med. 2002;347:1151–60. doi: 10.1056/NEJMoa021481. [DOI] [PubMed] [Google Scholar]
- 15.Palmer CNA, Irvine AD, Terron-Kwiatkowski A, Zhao Y, Liao H, Lee SP, et al. Common loss-of-function variants of the epidermal barrier protein filaggrin are a major predisposing factor for atopic dermatitis. Nat Genet. 2006;38:441–6. doi: 10.1038/ng1767. [DOI] [PubMed] [Google Scholar]
- 16.Weidinger S, Illig T, Baurecht H, Irvine AD, Rodriguez E, Diaz-Lacava A, et al. Loss-of-function variations within the filaggrin gene predispose for atopic dermatitis with allergic sensitizations. J Allergy Clin Immunol. 2006;118:214–9. doi: 10.1016/j.jaci.2006.05.004. [DOI] [PubMed] [Google Scholar]
- 17.Rodriguez E, Baurecht H, Herberich E, Wagenpfeil S, Brown SJ, Cordell HJ, et al. Meta-analysis of filaggrin polymorphisms in eczema and asthma: robust risk factors in atopic disease. J Allergy Clin Immunol. 2009;123:1361–70.e7. doi: 10.1016/j.jaci.2009.03.036. [DOI] [PubMed] [Google Scholar]
- 18.Howell MD, Kim BE, Gao P, Grant AV, Boguniewicz M, Debenedetto A, et al. Cytokine modulation of AD filaggrin skin expression. J Allergy Clin Immunol. 2007;120:150–5. doi: 10.1016/j.jaci.2007.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kim BE, Leung DY, Boguniewicz M, Howell MD. Loricrin and involucrin expression is down-regulated by Th2 cytokines through STAT-6. Clin Immunol. 2008;126:332–7. doi: 10.1016/j.clim.2007.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Elias PM, Hatano Y, Williams ML. Basis for the barrier abnormality in atopic dermatitis: outside-inside-outside pathogenic mechanisms. J Allergy Clin Immunol. 2008;121:1337–43. doi: 10.1016/j.jaci.2008.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.O’Regan GM, Sandilands A, McLean WH, Irvine AD. Filaggrin in atopic dermatitis. J Allergy Clin Immunol. 2008;122:689–93. doi: 10.1016/j.jaci.2008.08.002. [DOI] [PubMed] [Google Scholar]
- 22.Henderson J, Northstone K, Lee SP, Liao H, Zhao Y, Pembrey M, et al. The burden of disease associated with filaggrin mutations: a population-based, longitudinal birth cohort study. J Allergy Clin Immunol. 2008;121:872–7. doi: 10.1016/j.jaci.2008.01.026. [DOI] [PubMed] [Google Scholar]
- 23.Gao P, Leung DY, Rafaels NM, Hand T, Boguniewicz M, Hata TR, Schneider L, Hanifin JM, Gallo RL, Gao L, Yang M, Beaty TH, Beck LA, Barnes KC. Genetic variants in TSLP and its receptor, IL7R, contribute to an increased risk for atopic dermatitis and eczema herpeticum in two American populations. J Allergy Clin Immunol. 2009;123:S70. manuscript currently under review. [Google Scholar]
- 24.McLean WH, Palmer CN, Henderson J, Kabesch M, Weidinger S, Irvine AD. Filaggrin variants confer susceptibility to asthma. J Allergy Clin Immunol. 2008;121:1294–6. doi: 10.1016/j.jaci.2008.02.039. [DOI] [PubMed] [Google Scholar]
- 25.Beck LA, Boguniewicz M, Hata T, Schneider LC, Hanifin J, Gallo R, et al. Phenotype of atopic dermatitis subjects with a history of eczema herpeticum. J Allergy Clin Immunol. 2009;124:260–9. doi: 10.1016/j.jaci.2009.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gao PS, Rafaels NM, Hand T, Murray T, Boguniewicz M, Hata T, et al. Filaggrin mutations that confer risk of atopic dermatitis confer greater risk for eczema herpeticum. J Allergy Clin Immunol. 2009;124:507–13. e1–7. doi: 10.1016/j.jaci.2009.07.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Morar N, Willis-Owen SA, Moffatt MF, Cookson WO. The genetics of atopic dermatitis. J Allergy Clin Immunol. 2006;118:24–34. doi: 10.1016/j.jaci.2006.03.037. [DOI] [PubMed] [Google Scholar]
- 28.Howell MD, Fairchild HR, Kim BE, Bin L, Boguniewicz M, Redzic JS, et al. Th2 cytokines act on S100/A11 to downregulate keratinocyte differentiation. J Invest Dermatol. 2008;128:2248–58. doi: 10.1038/jid.2008.74. [DOI] [PubMed] [Google Scholar]
- 29.Leung DYL. Our evolving understanding of the functional role of filaggrin in atopic dermatitis. J Allergy Clin Immunol. 2009;124:494–5. doi: 10.1016/j.jaci.2009.07.041. [DOI] [PubMed] [Google Scholar]
- 30.Jung T, Stingl G. Atopic dermatitis: Therapeutic concepts evolving from new pathophysiologic insights. J Allergy Clin Immunol. 2008;122:1074–81. doi: 10.1016/j.jaci.2008.09.042. [DOI] [PubMed] [Google Scholar]
- 31.Boguniewicz M, Nicol NH, Kelsay K, Leung DYM. A multidisciplinary approach to evaluation and treatment of atopic dermatitis. Semin Cut Med Surg. 2008;27:115–27. doi: 10.1016/j.sder.2008.05.001. [DOI] [PubMed] [Google Scholar]
- 32.Michail SK, Stolfi A, Johnson T, Onady GM. Efficacy of probiotics in the treatment of pediatric atopic dermatitis: a meta-analysis of randomized controlled trials. Ann Allergy Asthma Immunol. 2008;101:508–16. doi: 10.1016/S1081-1206(10)60290-6. [DOI] [PubMed] [Google Scholar]
- 33.Lee J, Seto D, Bielory L. Meta-analysis of clinical trials of probiotics for prevention and treatment of pediatric atopic dermatitis. J Allergy Clin Immunol. 2008;121:116–21. doi: 10.1016/j.jaci.2007.10.043. [DOI] [PubMed] [Google Scholar]
- 34.Kopp MV, Hennemuth I, Heinzmann A, Urbanek R. Randomized, double-blind, placebo-controlled trial of probiotics for primary prevention: no clinical effects of Lactobacillus GG supplementation. Pediatrics. 2008;121:e850–6. doi: 10.1542/peds.2007-1492. [DOI] [PubMed] [Google Scholar]
- 35.Boyle RJ, Bath-Hextall FJ, Leonardi-Bee J, Murrell DF, Tang ML. Probiotics for treating eczema. Cochrane Database Syst Rev. 2008 Oct 8;(4) doi: 10.1002/14651858.CD006135.pub2. CD006135. [DOI] [PubMed] [Google Scholar]
- 36.Salfeld P, Kopp MV. Probiotics cannot be generally recommended for primary prevention of atopic dermatitis. J Allergy Clin Immunol. 2009;124:170. doi: 10.1016/j.jaci.2009.02.038. [DOI] [PubMed] [Google Scholar]
- 37.Ou LS, Goleva E, Hall C, Leung DY. T regulatory cells in atopic dermatitis and subversion of their activity by superantigens. J Allergy Clin Immunol. 2004;113:756–63. doi: 10.1016/j.jaci.2004.01.772. [DOI] [PubMed] [Google Scholar]
- 38.Cardona ID, Goleva E, Ou LS, Leung DY. Staphylococcal enterotoxin B inhibits regulatory T cells by inducing glucocorticoid-induced TNF receptor-related protein ligand on monocytes. J Allergy Clin Immunol. 2006;117:688–95. doi: 10.1016/j.jaci.2005.11.037. [DOI] [PubMed] [Google Scholar]
- 39.Hijen DJ, Haeck I, van Kraats AA, Nijhuis E, de Bruin-Weller MS, Bruijnzeel-Koomen CA, et al. Cyclosporin A reduces CD4+CD25+ regulatory T-cell numbers in patients with atopic dermatitis. J Allergy Clin Immunol. 2009;124:856–8. doi: 10.1016/j.jaci.2009.07.056. [DOI] [PubMed] [Google Scholar]
- 40.Verhagen J, Akdis M, Traidl-Hoffmann C, Schmid-Grendelmeier P, Hijnen D, Knol EF, et al. Absence of T-regulatory cell expression and function in atopic dermatitis skin. J Allergy Clin Immunol. 2006;117:176–83. doi: 10.1016/j.jaci.2005.10.040. [DOI] [PubMed] [Google Scholar]
- 41.Toda M, Leung DY, Molet S, Boguniewicz M, Taha R, Christodoulopoulos P, et al. Polarized in vivo expression of IL-11 and IL-17 between acute and chronic skin lesions. J Allergy Clin Immunol. 2003;111:875–81. doi: 10.1067/mai.2003.1414. [DOI] [PubMed] [Google Scholar]
- 42.He R, Oyoshi MK, Jin H, Geha RS. Epicutaneous antigen exposure induces a Th17 response that drives airway inflammation after inhalation challenge. Proc Natl Acad Sci USA. 2007;104:15817–22. doi: 10.1073/pnas.0706942104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Oyoshi Michiko K., Murphy George F., Geha Raif S. Filaggrin-deficient mice exhibit TH17-dominated skin inflammation and permissiveness to epicutaneous sensitization with protein antigen. J Allergy Clin Immunol. 2009;124:485–93. doi: 10.1016/j.jaci.2009.05.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Eyerich K, Pennino D, Scarponi C, Foerster S, Nasorri F, Behrendt H, et al. IL-17 in atopic eczema: Linking allergen-specific adaptive and microbial-triggered innate immune response. J Allergy Clin Immunol. 2009;123:59–66. doi: 10.1016/j.jaci.2008.10.031. [DOI] [PubMed] [Google Scholar]
- 45.He R, Kim HY, Yoon J, Oyoshi MK, MacGinnitie A, Goya S, et al. Exaggerated IL-17 response to epicutaneous sensitization mediates airway inflammation in the absence of IL-4 and IL-13. J Allergy Clin Immunol. 2009;124:761–70. doi: 10.1016/j.jaci.2009.07.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Guttman-Yassky E, Lowes MA, Fuentes-Duculan J, Zaba LC, Cardinale I, Nograles KE, et al. Low expression of the IL-23/Th17 pathway in atopic dermatitis compared to psoriasis. J Immunol. 2008;181:7420–7. doi: 10.4049/jimmunol.181.10.7420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sun YG, Zhao ZQ, Meng XL, Yin J, Liu XY, Chen ZF. Cellular basis of itch sensation. Science. 2009 Aug 6; doi: 10.1126/science.1174868. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sonkoly E, Muller A, Lauerma AI, Pivarcsi A, Soto H, Kemeny L, et al. IL-31: a new link between T cells and pruritus in atopic skin inflammation. J Allergy Clin Immunol. 2006;117:411–7. doi: 10.1016/j.jaci.2005.10.033. [DOI] [PubMed] [Google Scholar]
- 49.Dillon SR, Sprecher C, Hammond A, Bilsborough J, Rosenfeld-Franklin M, Presnell SR, et al. Interleukin 31, a cytokine produced by activated T cells, induces dermatitis in mice. Nat Immunol. 2004;5:752–60. doi: 10.1038/ni1084. [DOI] [PubMed] [Google Scholar]
- 50.Bilsborough J, Leung DY, Maurer M, Howell M, Boguniewicz M, Yao L, et al. IL-31 is associated with cutaneous lymphocyte antigen-positive skin homing T cells in patients with atopic dermatitis. J Allergy Clin Immunol. 2006;117:418–25. doi: 10.1016/j.jaci.2005.10.046. [DOI] [PubMed] [Google Scholar]
- 51.Raap U, Wichmann K, Bruder M, Stander S, Wedi B, Kapp A, et al. Correlation of IL-31 serum levels with severity of atopic dermatitis inflammation in AD. J Allergy Clin Immunol. 2008;122:421–3. doi: 10.1016/j.jaci.2008.05.047. [DOI] [PubMed] [Google Scholar]
- 52.Gutzmer R, Mommert S, Gschwandtner M, Zwingmann K, Stark H, Werfel T. The histamine H4 receptor is functionally expressed on TH2 cells. J Allergy Clin Immunol. 2009;123:619–25. doi: 10.1016/j.jaci.2008.12.1110. [DOI] [PubMed] [Google Scholar]
- 53.Bieber T. Atopic dermatitis. N Engl J Med. 2008;358:1483–94. doi: 10.1056/NEJMra074081. [DOI] [PubMed] [Google Scholar]
- 54.Altrichter S, Kriehuber E, Moser J, Valenta R, Kopp T, Stingl G. Serum IgE autoantibodies target keratinocytes in patients with atopic dermatitis. J Invest Dermatol. 2008;128:2232–9. doi: 10.1038/jid.2008.80. [DOI] [PubMed] [Google Scholar]
- 55.Salt BH, Boguniewicz M, Leung DY. Severe refractory atopic dermatitis in adults is highly atopic. J Allergy Clin Immunol. 2007;119:508–9. doi: 10.1016/j.jaci.2006.11.006. [DOI] [PubMed] [Google Scholar]
- 56.Bunikowski R, Mielke M, Skarabis H, Herz U, Bergmann RL, Wahn U, et al. Prevalence and role of serum IgE antibodies to the Staphylococcus aureus-derived superantigens SEA and SEB in children with atopic dermatitis. J Allergy Clin Immunol. 1999;103(1 Pt 1):119–24. doi: 10.1016/s0091-6749(99)70535-x. [DOI] [PubMed] [Google Scholar]
- 57.De Benedetto A, Agnihothri R, McGirt LY, Bankova LG, Beck LA. Atopic dermatitis: A disease caused by innate immune defects? J Invest Dermatol. 2009;129:14–30. doi: 10.1038/jid.2008.259. [DOI] [PubMed] [Google Scholar]
- 58.Kisich KO, Carspecken CW, Fiéve S, Boguniewicz M, Leung DY. Defective killing of Staphylococcus aureus in atopic dermatitis is associated with reduced mobilization of human beta-defensin-3. J Allergy Clin Immunol. 2008;122:62–8. doi: 10.1016/j.jaci.2008.04.022. [DOI] [PubMed] [Google Scholar]
- 59.Howell MD, Wollenberg A, Gallo RL, Flaig M, Streib JE, Wong C, et al. Cathelicidin deficiency predisposes to eczema herpeticum. J Allergy Clin Immunol. 2006;117:836–41. doi: 10.1016/j.jaci.2005.12.1345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zhang Q, Davis JC, Lamborn IT, Freeman AF, Jing H, Favreau AJ, et al. Combined immunodeficiency associated with DOCK8 mutation. N Engl J Med. 2009 Sep 23; doi: 10.1056/NEJMoa0905506. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Cho SH, Strickland I, Boguniewicz M, Leung DY. Fibronectin and fibrinogen contribute to the enhanced binding of Staphylococcus aureus to atopic skin. J Allergy Clin Immunol. 2001;108:269–74. doi: 10.1067/mai.2001.117455. [DOI] [PubMed] [Google Scholar]
- 62.Leung AD, Schiltz AM, Hall CF, Liu AH. Severe atopic dermatitis is associated with a high burden of environmental Staphylococcus aureus. Clin Exp Allergy. 2008;38:789–93. doi: 10.1111/j.1365-2222.2008.02964.x. [DOI] [PubMed] [Google Scholar]
- 63.Skov L, Olsen JV, Giorno R, Schlievert PM, Baadsgaard O, Leung DY. Application of Staphylococcal enterotoxin B on normal and atopic skin induces up-regulation of T cells by a superantigen-mediated mechanism. J Allergy Clin Immunol. 2000;105:820–6. doi: 10.1067/mai.2000.105524. [DOI] [PubMed] [Google Scholar]
- 64.Leung DY, Harbeck R, Bina P, Reiser RF, Yang E, Norris DA, et al. Presence of IgE antibodies to staphylococcal exotoxins on the skin of patients with atopic dermatitis. Evidence for a new group of allergens. J Clin Invest. 1993;92:1374–80. doi: 10.1172/JCI116711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bunikowski R, Mielke ME, Skarabis H, Worm M, Anagnostopoulos I, Kolde G, et al. Evidence for a disease-promoting effect of Staphylococcus aureus-derived exotoxins in atopic dermatitis. J Allergy Clin Immunol. 2000;105:814–9. doi: 10.1067/mai.2000.105528. [DOI] [PubMed] [Google Scholar]
- 66.Schlievert PM, Case LC, Strandberg KL, Abrams BB, Leung DY. Superantigen profile of Staphylococcus aureus isolates from patients with steroid-resistant atopic dermatitis. Clin Infect Dis. 2008;46:1562–7. doi: 10.1086/586746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Travers JB, Kozman A, Mousdicas N, Saha C, Landis M, Al-Hassani M, et al. Infected atopic dermatitis lesions contain pharmacologic amounts of lipoteichoic acid. J Allergy Clin Immunol. doi: 10.1016/j.jaci.2009.09.052. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Schlievert PM, Strandberg KL, Lin Y-C, Peterson ML, Leung DY. Secreted virulence factor comparison between methicillin-resistant and methicillin-sensitive Staphylococcus aureus associated with human infections. J Allergy Clin Immunol. 2010;125:xx–xx. doi: 10.1016/j.jaci.2009.10.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wang R, Braughton KR, Kretschmer D, Bach TH, Queck SY, Li M, et al. Identification of novel cytolytic peptides as key virulence determinants for community-associated MRSA. Nat Med. 2007;13:1510–4. doi: 10.1038/nm1656. [DOI] [PubMed] [Google Scholar]
- 70.Boguniewicz M, Sampson H, Leung SB, Harbeck R, Leung DY. Effects of cefuroxime axetil on Staphylococcus aureus colonization and superantigen production in atopic dermatitis. J Allergy Clin Immunol. 2001;108:651–2. doi: 10.1067/mai.2001.118598. [DOI] [PubMed] [Google Scholar]
- 71.Chung H-J, et al. Epidemiologic characteristics of methicillin-resistant Staphylococcus aureus isolated from eczematous lesion of atopic dermatitis children. J Clin Microbiol. 2008;46:991–5. doi: 10.1128/JCM.00698-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wollenberg A, Wetzel S, Burgdorf WH, Haas J. Viral infections in atopic dermatitis: pathogenic aspects and clinical management. J Allergy Clin Immunol. 2003;112:667–74. doi: 10.1016/j.jaci.2003.07.001. [DOI] [PubMed] [Google Scholar]
- 73.Peng WM, Jenneck C, Bussmann C, Bogdanow M, Hart J, Leung DY, et al. Risk factors of atopic dermatitis patients for eczema herpeticum. J Invest Dermatol. 2007;127:1261–3. doi: 10.1038/sj.jid.5700657. [DOI] [PubMed] [Google Scholar]
- 74.Wollenberg A, Zoch C, Wetzel S, Plewig G, Przybilla B. Predisposing factors and clinical features of eczema herpeticum: a retrospective analysis of 100 cases. J Am Acad Dermatol. 2003;49:198–205. doi: 10.1067/s0190-9622(03)00896-x. [DOI] [PubMed] [Google Scholar]
- 75.Engler R, Kenner J, Leung DY. Smallpox vaccination: Risk considerations for patients with atopic dermatitis. J Allergy Clin Immunol. 2002;110:357–65. doi: 10.1067/mai.2002.128052. [DOI] [PubMed] [Google Scholar]
- 76.Vora S, Damon I, Fulginiti V, Weber SG, Kahana M, Stein SL, et al. Severe eczema vaccinatum in a household contact of a smallpox vaccinee. Clin Infect Dis. 2008;46:1555–61. doi: 10.1086/587668. [DOI] [PubMed] [Google Scholar]
- 77.Howell MD, Gallo RL, Boguniewicz M, Jones JF, Wong C, Streib JE, et al. Cytokine milieu of atopic dermatitis skin subverts the innate immune response to vaccinia virus. Immunity. 2006;24:341–8. doi: 10.1016/j.immuni.2006.02.006. [DOI] [PubMed] [Google Scholar]
- 78.von Eiff C, Becker K, Machka K, Stammer H, Peters G, Study Group Nasal carriage as a source of Staphylococcus aureus bacteremia. N Engl J Med. 2001;344:11–6. doi: 10.1056/NEJM200101043440102. [DOI] [PubMed] [Google Scholar]
- 79.Bonness S, Szekat C, Novak N, Bierbaum G. Pulsed-field gel electrophoresis of Staphylococcus aureus isolates from atopic patients revealing presence of similar strains in isolates from children and their parents. J Clin Microbiol. 2008;46:456–61. doi: 10.1128/JCM.01734-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Huang JT, Abrams M, Tlougan B, Rademaker A, Paller AS. Treatment of Staphylococcus aureus colonization in atopic dermatitis decreases disease severity. Pediatrics. 2009;123:e808–14. doi: 10.1542/peds.2008-2217. [DOI] [PubMed] [Google Scholar]
- 81.Birnie AJ, Bath-Hextall FJ, Ravenscroft JC, Williams HC. Interventions to reduce Staphylococcus aureus in the management of atopic eczema. Cochrane Database Syst Rev. 2008 Jul 16;(3) doi: 10.1002/14651858.CD003871.pub2. CD003871. [DOI] [PubMed] [Google Scholar]
- 82.Hamid Q, Boguniewicz M, Leung DY. Differential in situ cytokine gene expression in acute versus chronic atopic dermatitis. J Clin Invest. 1994;94:870–6. doi: 10.1172/JCI117408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Hung SH, Lin YT, Chu CY, Lee CC, Liang TC, Yang YH, et al. Staphylococcus colonization in atopic dermatitis treated with fluticasone or tacrolimus with or without antibiotics. Ann Allergy Asthma Immunol. 2007;98:51–6. doi: 10.1016/S1081-1206(10)60859-9. [DOI] [PubMed] [Google Scholar]
- 84.Berth-Jones J, Damstra RJ, Golsch S, Livden JK, Van Hooteghem O, Allegra F, et al. Twice weekly fluticasone propionate added to emollient maintenance treatment to reduce risk of relapse in atopic dermatitis: randomised, double blind, parallel group study. BMJ. 2003;326:1367. doi: 10.1136/bmj.326.7403.1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Peserico A, Stadtler G, Sebastian M, Fernandez RS, Vick K, Bieber T. Reduction of relapses of atopic dermatitis with methylprednisolone aceponate cream twice weekly in addition to maintenance treatment with emollient: a multicentre, randomized, double-blind, controlled study. Br J Dermatol. 2008;158:801–7. doi: 10.1111/j.1365-2133.2008.08436.x. [DOI] [PubMed] [Google Scholar]
- 86.Wollenberg A, Reitamo S, Girolomoni G, Lahfa M, Ruzicka T, Healy E, et al. Proactive treatment of atopic dermatitis in adults with 0.1% tacrolimus ointment. Allergy. 2008;63:742–50. [PubMed] [Google Scholar]
- 87.Breneman D, Fleischer AB, Jr., Abramovits W, Zeichner J, Gold MH, Kirsner RS, et al. Intermittent therapy for flare prevention and long-term disease control in stabilized atopic dermatitis: a randomized comparison of 3-times-weekly applications of tacrolimus ointment versus vehicle. J Am Acad Dermatol. 2008;58:990–9. doi: 10.1016/j.jaad.2008.02.008. [DOI] [PubMed] [Google Scholar]
- 88.Paller AS, Eichenfield LF, Kirsner RS, Shull T, Jaracz E, Simpson EL, et al. Three times weekly tacrolimus ointment reduces relapse in stabilized atopic dermatitis: a new paradigm for use. Pediatrics. 2008;122:e1210–8. doi: 10.1542/peds.2008-1343. [DOI] [PubMed] [Google Scholar]
- 89.Wollenberg A, Bieber T. Proactive therapy of atopic dermatitis--an emerging concept. Allergy. 2009;64:276–8. doi: 10.1111/j.1398-9995.2008.01803.x. [DOI] [PubMed] [Google Scholar]
- 90.Gauger A, Mempel M, Schekatz A, Schäfer T, Ring J, Abeck D. Silver-coated textiles reduce Staphylococcus aureus colonization in patients with atopic eczema. Dermatology. 2003;207:15–21. doi: 10.1159/000070935. [DOI] [PubMed] [Google Scholar]
- 91.Gauger A, Fischer S, Mempel M, Schaefer T, Foelster-Holst R, Abeck D, et al. Efficacy and functionality of silver-coated textiles in patients with atopic eczema. J Eur Acad Dermatol Venereol. 2006;20:534–41. doi: 10.1111/j.1468-3083.2006.01526.x. [DOI] [PubMed] [Google Scholar]
- 92.Takei S, Arora YK, Walker SM. Intravenous immunoglobulin contains specific antibodies inhibitory to activation of T cells by staphylococcal toxin superantigens. J Clin Invest. 1993;91:602–7. doi: 10.1172/JCI116240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Jolles S. A review of high-dose intravenous immunoglobulin treatment for atopic dermatitis. Clin Exp Dermatol. 2002;27:3–7. doi: 10.1046/j.0307-6938.2001.00955.x. [DOI] [PubMed] [Google Scholar]
- 94.Grice EA, Kong HH, Conlan S, Deming CB, Davis J, Young AC, et al. Topographical and temporal diversity of the human skin microbiome. Science. 2009;324:1190–2. doi: 10.1126/science.1171700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Elston DM. Community-acquired methicillin-resistant Staphylococcus aureus. J Am Acad Dermatol. 2007;56:1–16. doi: 10.1016/j.jaad.2006.04.018. [DOI] [PubMed] [Google Scholar]
- 96.Lee MC, Rios AM, Aten MF, Mejias A, Cavuoti D, McCracken GH, Jr, et al. Management and outcome of children with skin and soft tissue abscesses caused by community-acquired methicillin-resistant Staphylococcus aureus. Pediatr Infect Dis J. 2004;23:123–7. doi: 10.1097/01.inf.0000109288.06912.21. [DOI] [PubMed] [Google Scholar]
- 97.Gupta J, Grube E, Ericksen MB, Stevenson MD, Lucky AW, Sheth AP, et al. Intrinsically defective skin barrier function in children with atopic dermatitis correlates with disease severity. J Allergy Clin Immunol. 2008;121:725–30. doi: 10.1016/j.jaci.2007.12.1161. [DOI] [PubMed] [Google Scholar]
- 98.Jensen JM, Pfeiffer S, Witt M, Bräutigam M, Neumann C, Weichenthal M, et al. Different effects of pimecrolimus and betamethasone on the skin barrier in patients with atopic dermatitis. J Allergy Clin Immunol. 2009;123:1124–33. doi: 10.1016/j.jaci.2009.03.032. [DOI] [PubMed] [Google Scholar]
- 99.Akdis CA, Akdis M, Bieber T, Bindslev-Jensen C, Boguniewicz M, Eigenmann P, et al. Diagnosis and treatment of atopic dermatitis in children and adults: European Academy of Allergology and Clinical Immunology/American Academy of Allergy, Asthma and Immunology/PRACTALL Consensus Report. J Allergy Clin Immunol. 2006;118:152–69. doi: 10.1016/j.jaci.2006.03.045. [DOI] [PubMed] [Google Scholar]
- 100.Bork K, Brauninger W. Increasing incidence of eczema herpeticum: analysis of seventy-five cases. J Am Acad Dermatol. 1988;19:1024–9. doi: 10.1016/s0190-9622(88)70267-4. [DOI] [PubMed] [Google Scholar]
- 101.Oren E, Banerji A, Camargo CA., Jr. Vitamin D and atopic disorders in an obese population screened for vitamin D deficiency. J Allergy Clin Immunol. 2008;121:533–4. doi: 10.1016/j.jaci.2007.11.005. [DOI] [PubMed] [Google Scholar]
- 102.Schauber J, Gallo RL. Antimicrobial peptides and the skin immune defense system. J Allergy Clin Immunol. 2008;122:261–6. doi: 10.1016/j.jaci.2008.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Hata TR, Kotol P, Jackson M, Nguyen M, Paik A, Udall D, et al. Administration of oral vitamin D induces cathelicidin production in atopic individuals. J Allergy Clin Immunol. 2008;122:829–31. doi: 10.1016/j.jaci.2008.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Sidbury R, Sullivan AF, Thadhani RI. Randomized controlled trial of vitamin D supplementation for winter-related atopic dermatitis in Boston: a pilot study. Br J Dermatol. 2008;159:245–7. doi: 10.1111/j.1365-2133.2008.08601.x. [DOI] [PubMed] [Google Scholar]
- 105.Otto M. Targeted immunotherapy for staphylococcal infections: focus on anti-MSCRAMM antibodies. BioDrugs. 2008;22:27–36. doi: 10.2165/00063030-200822010-00003. [DOI] [PubMed] [Google Scholar]
- 106.Wang R, Braughton KR, Kretschmer D, Bach TH, Queck SY, Li M, et al. Identification of novel cytolytic peptides as key virulence determinants for community-associated MRSA. Nat Med. 2007;13:1510–4. doi: 10.1038/nm1656. [DOI] [PubMed] [Google Scholar]
- 107.Ji G, Beavis R, Novick RP. Bacterial interference caused by autoinducing peptide variants. Science. 1997;276:2027–30. doi: 10.1126/science.276.5321.2027. [DOI] [PubMed] [Google Scholar]
- 108.Park J, Jagasia R, Kaufmann GF, Mathison JC, Ruiz DI, Moss JA, et al. Infection control by antibody disruption of bacterial quorum sensing signaling. Chem Biol. 2007;14:1119–27. doi: 10.1016/j.chembiol.2007.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Novick RP. Autoinduction and signal transduction in the regulation of staphylococcal virulence. Mol Microbiol. 2003;48:1429–49. doi: 10.1046/j.1365-2958.2003.03526.x. [DOI] [PubMed] [Google Scholar]
- 110.Stranger-Jones YK, Bae T, Schneewind O. Vaccine assembly from surface proteins of Staphylococcus aureus. Proc Natl Acad Sci U S A. 2006;103:16942–7. doi: 10.1073/pnas.0606863103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Clarke SR, Brummell KJ, Horsburgh MJ, McDowell PW, Mohamad SA, Stapleton MR, et al. Identification of in vivo-expressed antigens of Staphylococcus aureus and their use in vaccinations for protection against nasal carriage. J Infect Dis. 2006;193:1098–108. doi: 10.1086/501471. [DOI] [PubMed] [Google Scholar]
- 112.Weidenmaier C, Kokai-Kun JF, Kristian SA, Chanturiya T, Kalbacher H, Gross M, et al. Role of teichoic acids in Staphylococcus aureus nasal colonization, a major risk factor in nosocomial infections. Nat Med. 2004;10:243–5. doi: 10.1038/nm991. [DOI] [PubMed] [Google Scholar]
- 113.Buonpane RA, Churchill HRO, Moza B, Sundberg EJ, Peterson ML, Schlievert PM, et al. Neutralization of staphylococcal enterotoxin B by soluble, high-affinity receptor antagonists. Nature Med. 2007;13:725–9. doi: 10.1038/nm1584. [DOI] [PubMed] [Google Scholar]
- 114.Yang X, Buonpane RA, Moza B, Nur-ur Rahman AKM, Wang N, Schlievert PM, et al. Neutralization of multiple staphylococcal superantigens by a single-chain protein consisting of affinity-matured, variable domain repeats. J Invest Dermatol. 2008;198:344–8. doi: 10.1086/589776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Howell MD, Streib JE, Leung DY. Antiviral activity of human beta-defensin 3 against vaccinia virus. J Allergy Clin Immunol. 2007;119:1022–5. doi: 10.1016/j.jaci.2007.01.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Howell MD, Jones JF, Kisich KO, Streib JE, Gallo RL, Leung DY. Selective killing of vaccinia virus by LL-37: implications for eczema vaccinatum. J Immunol. 2004;172:1763–7. doi: 10.4049/jimmunol.172.3.1763. [DOI] [PubMed] [Google Scholar]
- 117.Savage PB, Li C, Taotafa U, Ding B, Guan Q. Antibacterial properties of cationic steroid antibiotics. FEMS Microbiol Lett. 2002;217:1–7. doi: 10.1111/j.1574-6968.2002.tb11448.x. [DOI] [PubMed] [Google Scholar]
- 118.Ding B, Taotofa U, Orsak T, Chadwell M, Savage PB. Synthesis and characterization of peptide-cationic steroid antibiotic conjugates. Org Lett. 2004;6:3433–6. doi: 10.1021/ol048845t. [DOI] [PubMed] [Google Scholar]
- 119.Howell MD, Streib JE, Kim BE, Lesley LJ, Dunlap AP, Geng D, et al. Ceragenins: A class of antiviral compounds to treat orthopox infections. J Invest Dermatol. 2009 June 11; doi: 10.1038/jid.2009.120. ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]