Abstract
The slow-wilting soybean [Glycine max (L.) Merr.] genotype, PI 416937, exhibits a limiting leaf hydraulic conductance for transpiration rate (TR) under high vapour pressure deficit (VPD). This genotype has a constant TR at VPD greater than 2 kPa, which may be responsible for its drought tolerance as a result of soil water conservation. However, the exact source of the hydraulic limitation between symplastic and apoplastic water flow in the leaf under high VPD conditions are not known for PI 416937. A comparison was made in the TR response to aquaporin (AQP) inhibitors between PI 416937 and N01-11136, a commercial genotype that has a linear TR response to VPD in the 1–3.5 kPa range. Three AQP inhibitors were tested: cycloheximide (CHX, a de novo synthesis inhibitor), HgCl2, and AgNO3. Dose–response curves for the decrease in TR following exposure to each inhibitor were developed. Decreases in TR of N01-11136 following treatment with inhibitors were up to 60% for CHX, 82% for HgCl2, and 42% for AgNO3. These results indicate that the symplastic pathway terminating in the guard cells of these soybean leaves may be at least as important as the apoplastic pathway for water flow in the leaf under high VPD. While the decrease in TR for PI 416937 was similar to that of N01-11136 following exposure to CHX and HgCl2, TR of PI 416937 was insensitive to AgNO3 exposure. These results indicate the possibility of a lack of a Ag-sensitive leaf AQP population in the slow-wilting line, PI 416937, and the presence of such a population in the commercial line, N01-11136.
Keywords: Aquaporin, hydraulic flow, soybean, transpiration
Introduction
Leaves are the major site of plant water loss via transpiration. To protect plants from excessive water loss, natural selection has resulted in limitations in liquid water flow within leaves as part of the whole-plant hydraulic system (Sack et al., 2004). Across species, the leaf hydraulic limitation is thought to represent about 30% of the whole-plant resistance to water flow, and, in some cases, can be as high as 80–98% (Sack et al., 2003; Sack and Holbrook, 2006). However, many aspects of the leaf hydraulic system and its connection with plant transpiration rate (TR) remain poorly understood and are currently a matter of extensive research (Sack et al., 2003, 2004; Cochard et al., 2004, 2007; Tyree et al., 2005; Hachez et al., 2008; Ye et al., 2008). Hydraulic resistances to water flow inside the leaf have been linked to environmental variables such as temperature and light (Sack et al., 2004; Cochard et al., 2007). Recently, Levin et al. (2007) showed that leaf hydraulic conductance of intact plants of Arabidopsis thaliana was higher under high relative humidity (77%) when compared to those measured under low relative humidity (17%), but this response was not isolated from the possibility of hydraulic or chemical signals from the roots.
The soybean genotype PI 416937 expresses a slow-wilting phenotype under water-deficit conditions in the field (Sloane et al., 1990), which may be a result of restricted water use resulting in soil water conservation. This slow-wilting genotype was reported by Fletcher et al. (2007) as having no further increase in TR once a VPD threshold of about 2 kPa was exceeded. In addition, phenotyping of commercial and recombinant inbred line populations that had PI 416937 in their pedigree resulted in a large genetic variability in TR response to VPD (Sadok and Sinclair, 2009a, b). Such variability indicated a complex inheritance for the trait and it was concluded that there may be more than one mechanism controlling the TR limitation trait associated with VPD.
The results of Sinclair et al. (2008) indicated that the source of the maximum TR response in PI 416937 was associated with a limited hydraulic conductance for water flow from the leaf xylem into the guard cells, which was not observed in two other genotypes studied. One possibility to explain these observations is a lower symplastic conductance (i.e. possibly aquaporin [AQP]-mediated water transport) in the leaf hydraulic pathway of PI 416937 as compared to the other genotypes. Although it is still unclear whether water moves principally apoplastically or symplastically in the leaf (Sack and Holbrook, 2006; Heinen et al., 2009), increasing evidence indicates that the symplastic, AQP-mediated path leading into the guard cells is important, on the basis of biophysical (Sack et al., 2004; Ye et al., 2008) and chemical (e.g. AQP gating by inhibitors; Nardini et al., 2005; Cochard et al., 2007) experiments.
In this study, based on a chemical approach, it is investigated whether symplastic differences in water flow from the xylem leading into the guard cells of two soybean genotypes was associated with differences in TR response to high VPD conditions. The slow-wilting genotype (PI 416937) was compared with genotype (N01-11136) with a linear increase in TR over the entire VPD range from 1–3.5 kPa. The effect on TR in response to AQP inhibitors under high VPD was measured on de-rooted plants. The approach using de-rooted plants differs from previous investigations using leaf protoplasts (Morillon and Chrispeels, 2001; Volkov et al., 2007), leaf tissues/discs (Terashima and Ono, 2002), or single leaves (Cochard et al., 2007). This use of whole, de-rooted plants may be important because leaf AQP activity may differ in planta from conditions prevailing in protoplasts, or vary for leaves depending on the location of the sampled tissue (Volkov et al., 2007; Hachez et al., 2008). In addition, the removal of roots nullifies the possible confounding influence of root characteristics, which may occur in whole-plant studies (Voicu and Zwiazek, 2004; Levin et al., 2007).
Dose–response curves were constructed for the TR response to exposure to four concentrations of three protein inhibitors that possibly have differing influences on AQP families and regulation processes. This approach contrasts with a common approach based just on the use of mercurials that are known to have a large array of toxic side-effects in addition to being ineffective against certain AQPs. Recent reviews highlighted the need for using an array of AQP inhibitors besides mercurials (Kaldenhoff et al., 2008; Maurel et al., 2008).
Results are presented from experiments using cycloheximide (CHX), which specifically targets the inhibition of the de novo synthesis process and two metallic ions, mercury (HgCl2) and silver (AgNO3). Cycloheximide is known to inhibit peptide initiation and extension (O'Brig et al., 1971) and has been previously used on plants for water transport studies (Moshelion et al., 2002; Voicu and Zwiazek, 2004; Cochard et al., 2007). For instance, on walnut leaves, a 100 μM CHX treatment reduced leaf hydraulic conductance by about 65% and this decrease was attributed to an inhibited AQP (Cochard et al., 2007). Both silver (Ag) and mercury (Hg) react with the sulphydryl group of a cysteine. However, the specific interaction between Ag+ and a histidine (in addition to cysteine) and the non-reversibility of the Ag+ effect by mercaptoethanol strongly indicate different inhibition modes between Ag and Hg (Niemietz and Tyerman, 2002). In contrast to CHX and Hg, there have only been a few studies on the effects of Ag on water transport in plants.
Materials and methods
Plant material
Soybean line PI 416937 (maturity group VI) is a plant introduction from Japan with unknown parentage (Pantalone et al., 1999; Carter et al., 2003). PI 416937 was identified as a slow-wilting genotype in the field (Sloane et al., 1990; Hudak and Patterson, 1995; King et al., 2009). The second line, N01-11136 (maturity group VII) is a new cultivar (developed by T Carter, ARS-USDA, Raleigh, NC) that has PI 416937 in its pedigree. These genotypes were selected based on differences in their TR response to VPD under well-watered greenhouse conditions. In the 0.8–3.2 kPa VPD range, TR of PI 416937 reaches a maximum value at a VPD of about 2 kPa, and maintains a constant TR as VPD is increased further (Fletcher et al., 2007; Sinclair et al., 2008). By contrast, the TR response to VPD of genotype N01-11136 showed a continuous linear increase in TR over the same VPD range (Sadok and Sinclair, 2009a).
Seeds were sown in pots filled with 1.5–3 kg of composted garden soil (Miracle-Gro lawn products, Inc., Marysville, OH) containing slow-release fertilizer (1.5 g N kg–1, 0.2 g P kg–1, 0.8 g K kg–1). Three to four seeds inoculated with Bradyrhyzobium japonicum (Nitragin, Inc., Brookfield, WI) were sown in each pot. The plants were grown in a greenhouse with the temperature regulated for a minimum temperature of 20 °C and maximum temperature of 33 °C. Pots were watered every 1–2 d. Seven to 15 d after sowing, each pot was thinned to one plant.
Plants were grown for approximately 4 weeks to vegetative stages ranging from V2 to V3 (2–3 unfolded trifoliolate leaves, respectively). At that time, pots were over-irrigated daily for 2–3 d. On the afternoon of the day prior to the experiment, two or three replicate plants per genotype (i.e. 4–6 plants) were gently removed from the soil and de-rooted. Although it was found that de-rooting the plants underwater was not necessary to avoid an impact on TR (data not shown), in nearly all cases de-rooting was done by cutting the base of the plant stem underwater. Immediately after cutting, the cut stems were placed in 125 ml beakers containing de-ionized water and placed in a dark room overnight (approximately 14 h) under a temperature maintained at 20.3 °C (±0.18 SE). The following morning, the plants were moved from the dark room and transferred to a new set of 125 ml beakers containing fresh de-ionized water. Laboratory film (Parafilm ‘M’®, Pechiney Plastic Packaging, Chicago, IL) was used to seal the stems in the beakers to avoid direct water evapouration. A small hole was made in the film to avoid negative pressure inside the sealed beaker due to water loss.
Experiments
The impact of each AQP inhibitor was measured simultaneously on 4–6 plants placed in a test chamber with a stable atmosphere of approximately 3.8 kPa. A stable VPD was achieved by continuously flowing about 40 l min−1 of air into the chamber. The air was dried by first pumping air through two PVC cylindrical cartridges (l=0.68 m, d=0.03 m) filled with a silica gel desiccant (SiO2, 10–18 Mesh, S161–500, Fisher Scientific, Fair Lawn, NJ). The air in the chamber was vigorously mixed with two 80 mm diameter fans (ASAF-B83, Cooler Master, Fremont, CA, USA) and three 40 mm diameter fans (EC4010M12C, Evercool Thermal Corp., LTD., San-Chung City, Taiwan, ROC). The top of the test chamber was covered with a 5 mm thick Plexiglas sheet above which water-cooled lamps provided a photosynthetic photon flux density at 1062 μmol m−2 s−1 (±4.8 SE) at plant level. Temperature and relative humidity were measured every 10 min inside the chamber by two pocket humidity/temperature pens (Extech Instruments, League City, TX). Temperature in the test chamber was maintained at 34–35 °C (temperature amplitude: up to 1.1 °C). For soybean, it has previously been shown that significant effects of temperature on photosynthetic rates or heat shock proteins accumulation occur above this range, typically in the 36–40 °C range (Key et al., 1985; Campbell et al., 1990; Hsieh et al., 1992; Vu et al., 1997).
Experiments were initiated by acclimatizing the plants for approximately 80 min in the test chamber until water loss rates were at steady-state. Following acclimation, beakers containing the plants were weighed every 20 min on a balance with a resolution of 0.01 g (Model XP-300, Denver Instrument, Denver, CO). After four consecutive weighings, the plants were quickly transferred and stems sealed into beakers containing a solution of an AQP inhibitor. The new beakers with the plants were immediately weighed and placed back into the test chamber. The whole process typically lasted from 120–180 s.
Three inhibitors were applied: cycloheximide (CHX), mercury chloride (HgCl2) and silver nitrate (AgNO3). Four concentrations were tested for each inhibitor (10, 50, 100, and 200 μM for CHX and 10, 100, 200, and 500 μM for HgCl2 and AgNO3). In most cases, each concentration for each inhibitor was tested simultaneously on three plants of each genotype. The solutions were prepared 1–5 d before the experiments and stored in darkness at 4 °C. CHX solutions were obtained by dissolving in a 0.05% (v/v) aqueous dimethyl sulphoxide solution, according to the protocol of Cochard et al. (2007). Tests showed that dimethyl sulphoxide did not affect the TR of either genotype (data not shown). Silver nitrate solutions were placed in opaque, dark brown 30 ml glass bottles (Fisher Scientific, Suwanee, GA) to prevent silver precipitation. Since it has previously been shown that NO3− ions can reduce TR in de-rooted plants (Wilkinson et al., 2007), the effects of KNO3 solutions were tested at 10, 100, 200, and 500 μM on 3–6 replicate plants on genotype N01-11136 which exhibited TR sensitivity to AgNO3. There was no sensitivity to KNO3 except for a TR decrease (29%) as a result of the 500 μM KNO3 treatment (data not shown).
Simultaneously with the 20 min-step weighings, conditions in the test chamber were recorded. Weighings were stopped when the water loss rates reached a plateau in water loss rate for 4–6 consecutive measurements. Overall, the AQP inhibitor treatments lasted approximately 180 min, 270 min, and 390 min for CHX, AgNO3, and HgCl2, respectively. The leaf area of each plant was measured using an area meter (Model LI-3100, Li-Cor, Lincoln, NE) and TR of each plant was expressed per unit leaf area.
Data analysis
To facilitate comparison between genotypes, a normalized transpiration rate (NTR) following the inhibitor treatment was calculated for each plant as the ratio between the TR value and the average of the four TR values before inhibitor treatment (TR0).
For each inhibitor and concentration, a Boltzmann sigmoid equation (Equation 1) was fitted to the averaged TR to describe the decrease in TR following inhibitor treatment;
| (1) |
where TRP is the value of the eventual plateau in TR following the inhibitor treatment, V50 is the time at which the TR has decreased halfway between TR0 and TRP, and C is a coefficient which represents the steepness of the curve.
The fraction of the total transpiration rate associated with exposure to AQP inhibitors was calculated for each individual from the drop in TR (DTR, %):
| (2) |
The value of DTR assumed for TRP in PI 416937 when it was insensitive to an inhibitor was calculated based on the measurements during the same 80–120 min period when a plateau in the response for N01-11136 plants was observed. For each genotype, DTR and NTR values were the average of 3–6 individuals. All statistical analysis and fittings were carried out on GraphPad Prism (GraphPad Software Inc., San Diego, CA, 1996).
Results
In total, 15 experiments were performed to document for each genotype the TR response to four concentrations of three different aquaporin (AQP) inhibitors under high VPD conditions (Table 1). In all experiments except one, three replicate plants per genotype (i.e. six plants) were studied simultaneously. In the remaining case (E11), two replicate plants per genotype (i.e. four plants) were studied. As a check for the consistency of the responses, some experiments have been replicated (E1, E3, and E11). Overall, for each inhibitor, the number of replicate plants varied from three to six for both genotypes (Table 1).
Table 1.
Summary of the experiments including sowing and measurement dates, inhibitor, and vapour pressure deficit (VPD) and temperature during measurement
| Experiment | Replicate/genotypea | Sowing date | Measurement date | Inhibitor (μM) | VPD (kPa)b | Temperature (°C)b |
| E1 | 3 | 19/9/2008 | 7/10/2008 | CHX (100) | 3.5±0.03 | 34.3±0.07 |
| E2 | 3 | 19/9/2008 | 8/10/2008 | CHX (200) | 3.4±0.02 | 34.4±0.06 |
| E3 | 3 | 28/9/2008 | 15/10/2008 | CHX (50) | 3.6±0.02 | 34.6±0.05 |
| E4 | 3 | 28/9/2008 | 16/10/2008 | CHX (10) | 3.6±0.02 | 34.7±0.05 |
| E5 | 3 | 5/10/2008 | 3/11/2008 | CHX (50) | 3.6±0.02 | 34.6±0.06 |
| E6 | 3 | 5/10/2008 | 4/11/2008 | CHX (100) | 3.6±0.02 | 34.4±0.06 |
| E7 | 3 | 23/10/2008 | 17/11/2008 | HgCl2 (200) | 4.2±0.02 | 34.3±0.05 |
| E8 | 3 | 23/10/2008 | 18/11/2008 | HgCl2 (100) | 4.3±0.03 | 34.3±0.06 |
| E9 | 3 | 10/12/2008 | 7/1/2009 | HgCl2 (10) | 3.6±0.03 | 34.8±0.06 |
| E10 | 3 | 4/1/2009 | 3/2/2009 | HgCl2 (500) | 4.3±0.04 | 34.5±0.08 |
| E11 | 2 | 4/1/2009 | 4/2/2009 | AgNO3 (100) | 4.5±0.02 | 34.3±0.11 |
| E12 | 3 | 4/1/2009 | 6/2/2009 | AgNO3 (100) | 4.6±0.04 | 34.0±0.13 |
| E13 | 3 | 12/1/2009 | 20/2/2009 | AgNO3 (500) | 3.9±0.06 | 33.9±0.14 |
| E14 | 3 | 12/1/2009 | 23/2/2009 | AgNO3 (200) | 3.8±0.05 | 34.1±0.12 |
| E15 | 3 | 26/3/2009 | 21/4/2009 | AgNO3 (10) | 3.5±0.02 | 34.5±0.05 |
Number of replicate plants per genotype.
Average and standard errors of the values measured during the experiments.
Experimental conditions
A first condition for analysing the data across 15 experiments was that the chamber environment was similar across experiments. Overall, the average temperature of all experiments was 34.4 °C (±0.06 SE). There was less than 1 °C difference between the lowest and highest values (33.9 °C for E13 to 34.8 °C for E9; Table 1). VPD values were slightly more variable, averaging 3.8 kPa (±0.09 SE) across experiments with 1.15 kPa difference between the lowest and highest average values (3.4 kPa for E2 to 4.6 kPa for E12; Table 1). Photosynthetic photon flux density values were the most stable, averaging 1062 μmol m−2 s−1 (±4.8 SE) at canopy level.
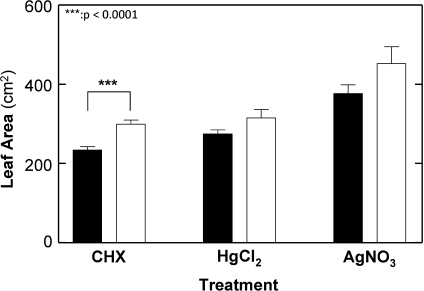
A second condition to allow comparison among experiments was a similarity in leaf areas among genotypes since the AQP inhibitors effect may depend on plant size. In only two CHX experiments were leaf areas significantly different between PI 416937 and N01-11136 (E3 and E5 at P <0.05 and P <0.01, respectively) out of a total of 15 experiments. Except for the CHX treatment, pooling leaf areas across all experiments for a given inhibitor treatment did not result in significant differences in leaf areas between genotypes (Fig. 1).
Fig. 1.
Leaf area differences between genotypes N01-11136 (closed bars) and PI 416937 (open bars) during the three AQP inhibitors treatments. Data are the mean of the 18, 12 and 14 observations for CHX, Hg, and Ag treatments, respectively. Error bars are the standard error of the mean. ***P <0.0001.
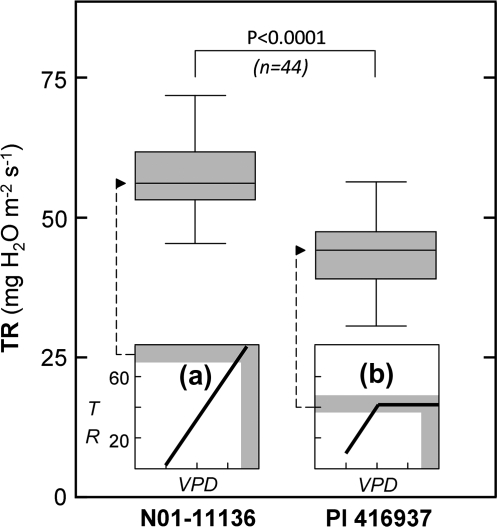
A third condition for these experiments was insensitivity of TR in N01-11136 and PI 416937 to de-rooting. This is necessary to avoid drastic changes in (i) their TR response to high evaporative demand or (ii) the differences between genotypes in TR response to high VPD. The average TR0 value of de-rooted PI 416937 was 43.5 (±0.92 SE) mg H2O m−2 s−1, matching the maximum TR value previously reported on intact plants of this genotype by Fletcher et al. (2007) of 42 mg H2O m−2 s−1 (Fig. 2, inset b). TR of de-rooted N01-11136 (average of 58 mg H2O m−2 s−1 ±0.99 SE) was significantly higher (P <0.0001) than that of de-rooted PI 416937 (Fig. 2). The higher TR for N01-11136 is consistent with the expected differences in TR values at high VPD of intact plants between genotypes. Overall, the fulfilment of this condition indicates little or no involvement of the root system in the leaf TR response under high evapourative demand for both genotypes at least for the duration of the experiments.
Fig. 2.
The differences in transpiration rates (TR) of the two studied de-rooted genotypes observed before the treatments match those previously observed on intact whole plants under high VPD conditions [insets (a) and (b)]. The box and whiskers represent TR values that were measured for 80 min, before the AQP inhibitors treatments, under high VPD conditions (3.8 kPa±0.4 SD). Each value (n) represents the average of the four consecutive TR measurements of a single individual. Insets (a) and (b): TR versus VPD regressions previously established for the studied genotypes by Sadok and Sinclair (2009a) and Fletcher et al. (2007), respectively (see Materials and methods for details). Horizontal grey areas in insets (a) and (b) represent the TR values that can be inferred from the corresponding formalisms for high VPD values in the 3.5–4 kPa range (vertical grey areas).
TR response to the AQP inhibitors
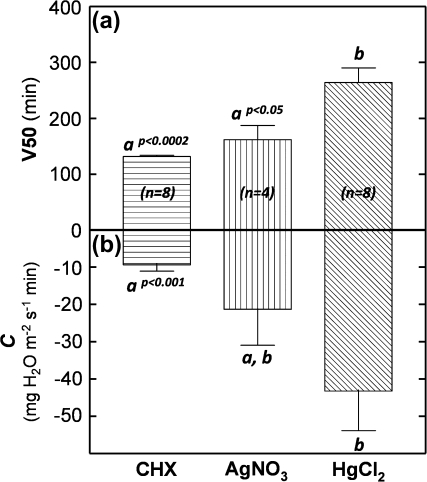
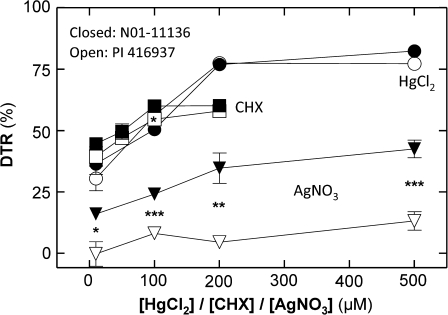
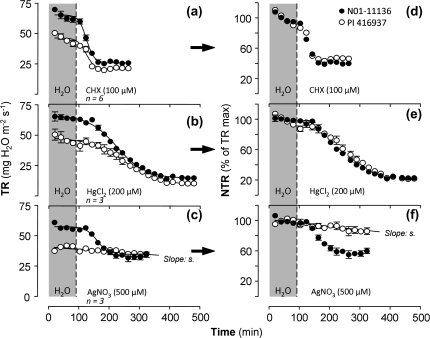
The analysis of the TR response to CHX treatments, regardless of concentration and genotype, revealed that, after about 40 min of exposure to the inhibitor, TR began to decrease. Transpiration rate decreased (V50=132 min ±2.6 SE; Fig. 3a) in a dose-dependent way (Fig. 4) and eventually established a plateau TR, as exemplified in Fig. 5a for a CHX concentration of 100 μM. The resulting DTR values were non-significantly different between PI 416937 and N01-11136 with the marginal exception of the 100 μM treatment, for which the difference was significant at P <0.05 (Figs 4, 5d). Overall, across a 10–200 μM CHX concentration range, DTR values were similar between genotypes, ranging from 39.4% ±3.8 SE to 57.9% ±1.2 SE for PI 416937 and 44.6% ±0.6 SE to 60.1% ±2.1 SE for N01-11136 (Fig. 4). For both genotypes, DTR did not significantly change when concentrations were increased from 100 μM to 200 μM CHX.
Fig. 3.
Differences in the TR decrease induced by the three AQP inhibitors (averaged across all concentrations) as expressed by V50 (a) and the C (b) parameters of the Boltzmann fits. Bars with horizontal, vertical, and inclined lines refer to CHX (both genotypes), AgNO3 (N01-11136 only), and HgCl2 (both genotypes) treatments. Standard errors are indicated for each bar (n ranging from 4–8). Bars with different letters are significantly different at the indicated P-values.
Fig. 4.
Dose–response curves of the drop in transpiration (DTR) induced by different AQP inhibitors for soybean genotypes N01-11136 and PI 416937. Closed/open symbols refer to genotypes N01-11136 and PI 416937, respectively. Circles, squares, and inverted triangles correspond to HgCl2, CHX, and AgNO3 treatments, respectively. Data are the mean of 3–6 observations (see Table 1 for details) and error bars represent standard error of the mean. Error bars for some points are invisible when they are smaller than the size of the symbols (*, **, and ***: P <0.05, P <0.02, and P <0.005, respectively).
Fig. 5.
Examples of time-courses of transpiration rate (TR) response to the three different AQP inhibitors: 100 μM CHX (a, d), 200 μM HgCl2 (b, e), and 500 μM AgNO3 (c, f) under constant, high VPD conditions for two de-rooted soybean genotypes (N01-11136: closed circles and PI 416937: open circles). Grey areas highlight the 80 min sequence where maximum TR values (TR0) were measured under de-ionized water treatment. (a–c) Time-courses of absolute TR. (d, f) Time-courses of the corresponding normalized TR values (NTR, expressed in % of the average of TR maximum values, see Materials and methods for details). The number of replicate plants (n=3–6 ±SE) are indicated. Error bars for some points are invisible when they are smaller than the size of the symbols. Curves on (a), (b) and (c) are Bolzmann sigmoidal fittings.
Similar to the CHX response, Hg treatments did not result in significant differences in DTR between PI 416937 and N01-11136 for any of the four concentrations in the 10–500 μM HgCl2 range (Fig. 4). However, as exemplified by TR in Fig. 5b and e and compiled in the dose–response curve in Fig. 4, Hg treatments resulted in different effects on TR when compared to CHX, on the basis of three observations. First, DTR values induced by Hg treatments ranged to higher and lower values than those caused by CHX. Second, the maximum DTR values were reached at a concentration of 200 μM HgCl2, twice the CHX concentration for which the maximum DTR was observed (Fig. 4). Third, the time from TR0 to TRP was much longer for Hg (V50=264 min ±26.2 SE; Fig. 3a) with a C value almost four times less than for the TR kinetics observed under CHX treatments (Fig. 3b).
In contrast to the above inhibitors, Ag induced a striking difference in DTR between PI 416937 and N01-11136 for all four tested concentrations in the 10–500 μM range (Fig. 4). For the lower AgNO3 concentrations, a TR of PI 416937 was insensitive to silver (non-significant slopes). At the highest concentration of 500 μM AgNO3, TR of PI 416967 exhibited a small but significant linear decrease (slope at 0.018±0.004 SE, Fig. 5c). By contrast, the TR of N01-11136 exhibited a sharp decrease across all Ag concentrations (V50=162 min ±25.4 SE, Fig. 3a) that started only 40 min after the treatment and stabilized at 120 min with a TRP of 42.5% ±3.6 SE (Fig. 4). Compared with the effects of CHX and Hg, Ag treatments induced markedly lower DTR values for N01-11136 which ranged from 15.9% ±2.5 SE to 42.5% ±3.6 SE. These values were lower than those of the other metallic ion, Hg, indicating an inhibition by Hg roughly twice as strong as that resulting from the Ag treatments. The decrease time from TR0 to TRP of Ag treatments for N01-11136 was significantly shorter (P <0.05) than that observed for Hg treatments, indicating a faster mode of action for silver.
Discussion
This study showed that de-rooted plants under high VPD conditions could be used to study the TR response to AQP inhibitors. Side-by-side comparisons of de-rooted plants of two soybean genotypes with different sensitivities to VPD showed similar responses when treated with two AQP inhibitors (CHX and Hg). However, a major difference between genotypes was observed in their response to a third inhibitor, Ag. The TR of a slow-wilting line (PI 416937) was virtually insensitive to AgNO3 exposure of concentrations from 10–500 μM.
Cycloheximide inhibition
Possible side-effects resulting from long exposure to CHX may be marginal in our study for two main reasons. First, root exposure to 1 mM CHX for 265 min in aspen roots (Voicu and Zwiazek, 2004) did not result in changes in O2 uptake indicating that the effect of CHX was not the result of metabolic disruption. In this study with soybean leaves, the response to CHX treatment stabilized after 80 min, a duration that is comparable to that of the study by Cochard et al. (2007) to examine AQPs in walnut leaves (≈60 min). Second, the swiftness of the effect of CHX on TR observed in all experiments, reflected by (i) the early start of the decrease in TR (40 min after the inhibitor is added) or (ii) the steep slope and low V50 values (Fig. 3a, b) is consistent with the possibility of an inhibition of protein synthesis directly involved in the water flow within the leaf.
The DTR of both genotypes caused by the four concentrations of CHX indicates an inhibiting effect on a protein-mediated, symplastic/transcellular water pathway in the leaves that would require de novo synthesis. The involvement of such a process in the protein-mediated water pathway on whole-plant hydraulics has been established by Voicu and Zwiazek (2004), and its specific involvement in the leaf-based water flow was demonstrated by Cochard et al. (2007), who established that such involvement was dependent on the PPFD. Under high-light conditions (≈1400 μmol m−2 s−1), Cochard et al. (2007) found that 100 μM CHX reduced leaf hydraulic conductance by approximately 65%. The present study with soybean extends the range of environmental variables responsible for such responses to high VPD conditions and indicates that symplastic water pathway terminating in the guard cells may account for up to ≈60% (at 100 μM CHX) of the overall hydraulic pathway in the leaf (Fig. 4). The response to CHX was not significantly different between PI 416937 and N01-11136 indicating that the CHX-sensitive pathway may not be linked to their differences in TR responses to high VPD (Fig. 2).
Inhibition from CHX is reported to be a consequence of its negative effects on peptide initiation and extension (O'Brig et al., 1971). Therefore, toxic doses of CHX or high exposure times to this inhibitor may result in a metabolic disruption (Zhang et al., 1995). In our study, three CHX treatments were at concentrations equal to or lower than 100 μM, a concentration that was used by Cochard et al. (2007) as a physiological concentration on single leaves of walnut to study the involvement of AQPs in modulating water flow inside the leaf in response to light. These CHX concentrations were also much lower than those used in the study of Voicu and Zwiazek (2004) on aspen seedlings (1 mM) and that of Moshelion et al. (2002) carried out on protoplasts (2 mM). The speed of the response to the CHX treatment is consistent with the fact that transcriptional regulation of AQP genes can operate in less than 60 min (Kawasaki et al., 2002). This indicates that CHX could act by decreasing turnover of AQPs and/or of different elements essential to AQP activity, at the transcript and/or protein levels as suggested by the complex regulation of AQP (reviewed by Javot and Maurel, 2002).
Mercury inhibition
Mercurials are the most commonly used AQP inhibitors in the literature. They act through covalent modification of cysteine residues within the water pore and in other regions of the protein causing either block or conformational changes leading to inhibition of water transport (Niemietz and Tyerman, 2002). However, Hg has been reported to have a large array of side-effects depending on the dose and the duration of the treatments (Tyerman et al., 1999; Javot and Maurel, 2002; Niemietz and Tyerman, 2002; Kaldenhoff et al., 2008; Maurel et al., 2008). In our study, all HgCl2 concentrations were equal or less than 500 μM, a concentration at which inhibition on sap flux of tomato roots were reversed partially by mercaptoethanol (Maggio and Joly, 1995). At 200 μM HgCl2, Nardini et al. (2005) showed that the effects of mercury on the hydraulic conductance of sunflower leaves were completely reversed by 30 mM mercaptoethanol. Further, Levin et al. (2007) found that concentrations below 50 μM HgCl2 were ineffective on leaf hydraulic conductance and suggested the use of higher concentrations. North et al. (2004) showed the decrease in conductance of Agave deserti roots caused by 25 μM HgCl2 and it was reversed by 20 mM mercaptoethanol. Consequently, previous studies indicate the use of HgCl2 at concentrations below 200 μM would involve minimal side-effect damages, especially the 10 μM HgCl2 treatment in our study with soybean.
Overall, DTR values as a result of Hg treatment indicated that a symplastic/transcellular pathway involving proteins sensitive to Hg account for 36% (10 μM) to 82% (500 μM) of the leaf TR under high VPD. Interestingly, these values are consistent with previous studies reporting mercurials reducing hydraulic conductivities in plants. For instance, hydraulic conductivities of roots from different plant species were reduced by mercurials by values ranging from 32–90% (reviewed in Javot and Maurel, 2002). Further, a non-significant difference in DTR values between PI 416937 and N01-11136 indicated that putative Hg-sensitive AQP populations are not responsible for the difference in TR response to VPD observed between the two genotypes.
Silver inhibition
Silver inhibits AQPs by a different mechanism than that of Hg, which may result from the difference in the sizes between Hg2+ and Ag+ ions, the specific interaction between Ag and a histidine (in addition to cysteine), and the structure of the AQP pores (Niemietz and Tyerman, 2002). Exposure of de-rooted soybean plants to AgNO3 resulted in a striking difference in the DTR response of genotypes PI 416937 and N01-11136. In contrast to N01-11136, TR of PI 416937 was virtually insensitive to AgNO3 when exposed to 10–500 μM AgNO3. This study offers the first demonstration of an intraspecies difference in response to Ag at least in the 10–200 μM range.
Since Sinclair et al. (2008) have shown that the constant TR of PI 416937 when subjected to VPD greater than 2 kPa resulted from a limited hydraulic conductance within the leaf, the results of this current study indicate that this TR limitation may be the result of a lack a specific silver-sensitive AQP in the leaves of PI 416937. Extending this conclusion to intact plants is supported by the fact that the TR of untreated, de-rooted plants under high VPD of both genotypes closely matched those previously reported on intact plants.
The results of N01-11136 revealed a dose-dependent silver inhibition of leaf AQPs of this genotype, which is a type of inhibition infrequently reported in the literature. The only direct reference of plant tissue water permeability being affected by silver treatment was in the study of Niemietz and Tyerman (2002) carried out with soybean peribacteroid membrane and sugar beet plasma membrane vesicles. Based on the corresponding DTR values (Fig. 4, black inverted triangles), an inhibition caused solely by Ag+ may account for up to 35% (200 μM AgNO3) of the hydraulic pathway. In this case it is highly unlikely that the silver concentrations tested may have had significant side-effects, given that, in the same concentration range, TR of PI 416937 was not affected. Further, TR response to Ag in N01-11136 as described by the V50 parameter was as swift as that of the CHX treatment (non-significant difference; Fig. 3a), reinforcing the idea that silver probably inhibited proteins directly involved in leaf water flow under high VPD.
The results of this study indicated that, without the influence of the root system, soybean leaves under high VPD conditions may have active Hg/Ag-sensitive AQPs that require de novo synthesis, and may account for up to 82% of the hydraulic pathway terminating in the guard cells. Although previous studies indicated the importance of the symplastic pathway in the leaf hydraulics for different species under different developmental and environmental conditions (Sack et al., 2004; Nardini et al., 2005; Tyree et al., 2005; Cochard et al., 2007; Ye et al., 2008), this study offers direct evidence that the symplastic pathway could be involved in the response of TR to high VPD. Given the fact that PI 416937 has restricted hydraulic flow at high VPD (Sinclair et al., 2008) and displays slow-wilting capability in the field, the current results now indicate that this may be the result of a lack of Ag-sensitive symplastic pathway in PI 416937.
Acknowledgments
We are thankful to Mr Andrew Schreffler for building the test chamber used in this study. We also thank the reviewers of the manuscript for their recommendations. We gratefully acknowledge the generous financial support of the United Soybean Board and a grant from the National Research Initiative, US Department of Agriculture.
References
- Campbell WJ, Allen LH, Jr, Bowes G. Response of soybean canopy photosynthesis to CO2 concentration, light, and temperature. Journal of Experimental Botany. 1990;41:427–433. [Google Scholar]
- Carter TE, Jr, Burton JW, Bowman DT, Cui Z, Zhou X, Villagarcia MR, Fountain MO, Niewoehner AS. Registration of ‘N7001’ soybean. Crop Science. 2003;43:1126–1127. [Google Scholar]
- Cochard H, Nardini A, Coll L. Hydraulic architecture of leaf blades: where is the main resistance? Plant, Cell and Environment. 2004;27:1257–1267. [Google Scholar]
- Cochard H, Venisse J-S, Barigah TS, Brunel N, Herbette S, Guilliot A, Tyree MT, Sakr S. Putative role of aquaporins in variable hydraulic conductance of leaves in response to light. Plant Physiology. 2007;143:122–133. doi: 10.1104/pp.106.090092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fletcher AL, Sinclair TR, Allen LH., Jr Transpiration responses to vapour pressure deficit in well watered ‘slow-wilting’ and commercial soybean. Environmental and Experimental Botany. 2007;61:145–151. [Google Scholar]
- Hachez C, Heinen RB, Draye X, Chaumont F. The expression pattern of plasma membrane aquaporins in maize leaf highlights their role in hydraulic regulation. Plant Molecular Biology. 2008;68:337–353. doi: 10.1007/s11103-008-9373-x. [DOI] [PubMed] [Google Scholar]
- Heinen RB, Ye Q, Chaumont F. Role of aquaporins in leaf physiology. Journal of Experimental Botany. 2009;60:2971–2985. doi: 10.1093/jxb/erp171. [DOI] [PubMed] [Google Scholar]
- Hsieh M-H, Chen J-T, Jinn T-L, Chen Y-M, Lin C-Y. A class of soybean low molecular weight heat shock proteins: immunological study and quantitation. Plant Physiology. 1992;99:1279–1284. doi: 10.1104/pp.99.4.1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudak CM, Patterson RP. Vegetative growth analysis of a drought-resistant soybean plant introduction. Crop Science. 1995;35:464–471. [Google Scholar]
- Javot H, Maurel C. The role of aquaporins in root water uptake. Annals of Botany. 2002;90:301–313. doi: 10.1093/aob/mcf199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaldenhoff R, Ribas-Carbo M, Flexas Sans J, Lovisolo C, Heckwolf M, Uehlein N. Aquaporins and plant water balance. Plant, Cell and Environment. 2008;31:658–666. doi: 10.1111/j.1365-3040.2008.01792.x. [DOI] [PubMed] [Google Scholar]
- Kawasaki S, Borchert C, Deyholos M, Wang H, Brazille S, Kawai K, Galbraith D, Bohnert HJ. Gene espression profiles during the initial phase of salt stress in rice. The Plant Cell. 2001;13:889–905. doi: 10.1105/tpc.13.4.889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Key JL, Kimpel JA, Lin CY, Nagao RT, Vierling E, Czarnecka E, Gurley WB, Roberts JK, Mansfield MA, Edelman L. The heat shock response in soybean. In: Key JL, Kosuge T, editors. Cellular and molecular biology of plant stress. New York: Alan R. Liss, Inc.; 1985. pp. 161–179. [Google Scholar]
- King CA, Purcell LC, Brye KR. Differential wilting among soybean genotypes in response to water deficit. Crop Science. 2009;49:290–298. [Google Scholar]
- Levin M, Lemcoff JH, Cohen S, Kapulnik Y. Low air humidity increases leaf-specific hydraulic conductance of Arabidopsis thaliana (L.) Heynh (Brassicaceae) Journal of Experimental Botany. 2007;58:3711–3718. doi: 10.1093/jxb/erm220. [DOI] [PubMed] [Google Scholar]
- Maggio A, Joly RJ. Effects of mercuric chloride on the hydraulic conductivity of tomato root systems (evidence for a channel-mediated water pathway) Plant Physiology. 1995;109:331–335. doi: 10.1104/pp.109.1.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maurel C, Verdoucq L, Luu D-T, Santoni V. Plant aquaporins: membrane channels with multiple integrated functions. Annual Review of Plant Biology. 2008;59:595–624. doi: 10.1146/annurev.arplant.59.032607.092734. [DOI] [PubMed] [Google Scholar]
- Morillon R, Chrispeels MJ. The role of ABA and the transpiration stream in the regulation of the osmotic water permeability of leaf cells. Proceedings of the National Academy of Sciences, USA. 2001;98:14138–14143. doi: 10.1073/pnas.231471998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moshelion M, Becker D, Biela A, Uehlein N, Hedrich R, Otto B, Levi H, Moran N, Kaldenhoff R. Plasma membrane aquaporins in the motor cells of Samanea saman: diurnal and circadian regulation. The Plant Cell. 2002;14:727–739. doi: 10.1105/tpc.010351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nardini A, Salleo S, Andri S. Circadian regulation of leaf hydraulic conductance in sunflower (Helianthus annuus L. cv. Margot) Plant, Cell and Environment. 2005;28:750–759. [Google Scholar]
- Niemietz CM, Tyerman SD. New potent inhibitors of aquaporins: silver and gold compounds inhibit aquaporins of plant and human origin. FEBS Letters. 2002;531:443–447. doi: 10.1016/s0014-5793(02)03581-0. [DOI] [PubMed] [Google Scholar]
- North GB, Martre P, Nobel PS. Aquaporins account for variations in ydraulic conductance for metabolically active root regions of Agave deserti in wet, dry, and rewetted soil. Plant, Cell and Environment. 2004;27:219–228. [Google Scholar]
- O'Brig TG, Culp WJ, McKeenan WL, Boyd H. The mechanism by which cycloheximide and related glutarimide antibiotics inhibit peptide synthesis in reticulocyte ribosomes. Journal of Biological Chemistry. 1971;246:174–181. [PubMed] [Google Scholar]
- Pantalone VR, Rebetzke GJ, Burton JW, Carter TE, Jr., Israel DW. Soybean PI 416937 root system contributes to biomass accumulation in reciprocal grafts. Agronomy Journal. 1999;91:840–844. [Google Scholar]
- Sack L, Cowan PD, Jaikumar N, Holbrook NM. The ‘hydrology’ of leaves: co-ordination of structure and function in temperate woody species. Plant, Cell and Environment. 2003;26:1343–1356. [Google Scholar]
- Sack L, Holbrook NM. Leaf hydraulics. Annual Review of Plant Biology. 2006;57:361–381. doi: 10.1146/annurev.arplant.56.032604.144141. [DOI] [PubMed] [Google Scholar]
- Sack L, Streeter CM, Holbrook NM. Hydraulic analysis of water flow through leaves of sugar maple and red oak. Plant Physiology. 2004;134:1824–1833. doi: 10.1104/pp.103.031203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadok W, Sinclair TR. Genetic variability of transpiration response to vapour pressure deficit among soybean (Glycine max [L.] Merr.) cultivars. Crop Science. 2009a;49:955–960. [Google Scholar]
- Sadok W, Sinclair TR. Genetic variability of transpiration response to vapour pressure deficit among soybean (Glycine max [L.] Merr.) genotypes selected from a recombinant inbred line population. Field Crops Research. 2009b;113:156–160. [Google Scholar]
- Sinclair TR, Zwieniecki MA, Holbrook NM. Low leaf hydraulic conductance associated with drought tolerance in soybean. Physiologia Plantarum. 2008;132:446–451. doi: 10.1111/j.1399-3054.2007.01028.x. [DOI] [PubMed] [Google Scholar]
- Sinclair TR, Hammer GL, van Oosterom EJ. Potential yield and water-use efficiency benefits in sorghum from limited maximum transpiration rate. Functional Plant Biology. 2005;32:945–952. doi: 10.1071/FP05047. [DOI] [PubMed] [Google Scholar]
- Sloane RJ, Patterson RP, Carter TE. Field drought tolerance of a soybean plant introduction. Crop Science. 1990;30:118–123. [Google Scholar]
- Terashima I, Ono K. Effects of HgCl2 on CO2 dependence of leaf photosynthesis: evidence indicating involvement of aquaporins in CO2 diffusion across the plasma membrane. Plant and Cell Physiology. 2002;43:70–78. doi: 10.1093/pcp/pcf001. [DOI] [PubMed] [Google Scholar]
- Tyerman SD, Bohnert HJ, Maurel C, Steudle E, Smith JA. Plant aquaporins: their molecular biology, biophysics and significance for plant water relations. Journal of Experimental Botany. 1999;50:1055–1071. [Google Scholar]
- Tyree MT, Nardini A, Salleo S, Sack L, El Omari B. The dependence of leaf hydraulic conductance on irradiance during HPFM measurements: any role for stomatal response? Journal of Experimental Botany. 2005;56:737–744. doi: 10.1093/jxb/eri045. [DOI] [PubMed] [Google Scholar]
- Voicu MC, Zwiazek JJ. Cycloheximide inhibits root water flow and stomatal conductance in aspen (Populus tremuloides) seedlings. Plant, Cell and Environment. 2004;27:199–208. [Google Scholar]
- Volkov V, Hachez C, Moshelion M, Draye X, Chaumont F, Fricke W. Water permeability differs between growing and non-growing barley leaf tissues. Journal of Experimental Botany. 2007;58:377–390. doi: 10.1093/jxb/erl203. [DOI] [PubMed] [Google Scholar]
- Vu JCV, Allen LH, Jr, Boote KJ, Bowes G. Effects of elevated CO2 and temperature on photosynthesis and Rubisco in rice and soybean. Plant, Cell and Environment. 1997;20:68–76. [Google Scholar]
- Wilkinson S, Bacon MA, Davies WJ. Nitrate signalling to stomata and growing leaves: interactions with soil drying, ABA and xylem sap pH in maize. Journal of Experimental Botany. 2007;58:1705–1716. doi: 10.1093/jxb/erm021. [DOI] [PubMed] [Google Scholar]
- Ye Q, Holbrook NM, Zwieniecki MA. Cell-to-cell pathway dominates xylem–epidermis hydraulic connection in Tradescantia fluminensis (Vell. Conc.) leaves. Planta. 2008;227:1311–1317. doi: 10.1007/s00425-008-0703-7. [DOI] [PubMed] [Google Scholar]
- Zhang G, Archambault DJ, Slaski JJ, Taylor GJ. Effects of protein synthesis inhibitor on uptake of aluminium in aluminium-resistant and aluminium-sensitive cultivars of wheat. Journal of Plant Physiology. 1995;147:457–462. [Google Scholar]