Abstract
Plant peroxidases are involved in numerous cellular processes in plant development and stress responses. Four plasma membrane-bound peroxidases have been identified and characterized in maize (Zea mays L.) roots. In the present study, maize seedlings were treated with different stresses and signal compounds, and a functional analysis of these membrane-bound class III peroxidases (pmPOX1, pmPOX2a, pmPOX2b, and pmPOX3) was carried out. Total guaiacol peroxidase activities from soluble and microsomal fractions of maize roots were compared and showed weak changes. By contrast, total plasma membrane and washed plasma membrane peroxidase activities, representing peripheral and integral membrane proteins, revealed strong changes after all of the stresses applied. A proteomic approach using 2D-PAGE analysis showed that pmPOX3 was the most abundant class III peroxidase at plasma membranes of control plants, followed by pmPOX2a >pmPOX2b >pmPOX1. The molecular mass (63 kDa) and the isoelectric point (9.5) of the pmPOX2a monomer were identified for the first time. The protein levels of all four enzymes changed in response to multiple stresses. While pmPOX2b was the only membrane peroxidase down-regulated by wounding, all four enzymes were differentially but strongly stimulated by methyl jasmonate, salicylic acid, and elicitors (Fusarium graminearum and Fusarium culmorum extracts, and chitosan) indicating their function in pathogen defence. Oxidative stress applied as H2O2 treatment up-regulated pmPOX2b >pmPOX2a, while pmPOX3 was down-regulated. Treatment with the phosphatase inhibitor chantharidin resulted in distinct responses.
Keywords: Class III peroxidases, methyl jasmonate, oxidative stress, plasma membrane-bound, salicylic acid, Zea mays L
Introduction
Class III peroxidases (EC 1.11.1.7; donor: H2O2 oxidoreductases; secretory pathway) belong to a large multigenic protein family. In maize, approximately 200 genes correspond with this protein class (Mika et al., 2008). Even more isoenzymes can be generated by post-transcriptional and post-translational modifications (Tognolli et al., 2002; Welinder et al., 2002), and more than 50% of them were estimated to show a stress-induced expression (Passardi et al., 2004). Artificial phenolic substrates like guaiacol can be used to detect all class III peroxidases present independent of their different in vivo substrate specificities. Probably due to this high number of isoforms, and due to the heterogeneous regulation of their expression, peroxidases are involved in numerous cellular processes during plant development and stress response (Hiraga et al., 2001; Kawano, 2003; Passardi et al., 2005; Cosi and Dunand, 2009). The majority of class III peroxidases are soluble apoplastic and cell wall-bound isoenzymes. In addition, four membrane-bound peroxidases were identified in plasma membrane (PM) preparations of maize (Zea mays L.) roots (Mika and Lüthje, 2003; Mika et al., 2008). The biochemical properties and enzyme activities of these peroxidases were characterized in detail (Mika and Lüthje, 2003). The full-length amino acid sequences of pmPOX1 (ZmPrx1), pmPOX2b (ZmPrx70), and pmPOX3 (ZmPrx66) were identified. A first in silico sequence analysis of the membrane-bound class III peroxidases, suggested a function of these peroxidases in oxidative stress at the apoplastic site of the plant PM (Mika et al., 2008).
Due to two possible catalytic cycles, peroxidative and hydroxylic, peroxidases can detoxify or generate reactive oxygen species like •OH and HOO•, polymerize cell wall compounds, and regulate H2O2 levels (Passardi et al., 2005). Large amounts of H2O2 are produced at the PM due to cellular processes, as a response to stress factors, or to external sources in plant–pathogen interactions (Schraudner et al., 1996; Bolwell et al., 2002; Schützendübel and Polle, 2002; Minibayeva et al., 2009). Oxidative stress causes lipid peroxidation and changes in membrane permeability (Cakmak et al., 1987; Qiu and Liang, 1995; Rawyler et al., 2002). Due to these observations it seems likely that PM-bound peroxidases could be substantially involved in the detoxification of the cell and/or membrane repair of the PM, and could probably play an important role to maintain the cell functions under different stress conditions. This hypothesis is further supported by solubilization properties of the tightly membrane-bound haem peroxidases (Mika and Lüthje, 2003) suggesting their localization in microdomains (so-called lipid rafts), and the identification of a PM-bound peroxidase in detergent-resistant membrane fractions of Medicago truncatula (Lefevbre et al., 2007). A localization in microdomains may allow PM-bound peroxidases to co-localize with ROS producing and detoxifying enzymes in the membrane. Thus PM-bound peroxidases could probably not only detoxify H2O2 directly at the site of origin to ensure the optimal protection of the membrane, but could also protect specific functional regions of the PM (Lüthje, 2008). However, despite the knowledge about the exact location of a peroxidase, it is often difficult to reveal the function(s) of each single enzyme due to (i) the large number of similar isoenzymes, (ii) their broad substrate specificity, (iii) multiple possible functions, and (iv) the ability of other isoforms to replace the role of a missing peroxidase in knock-out experiments (Hiraga et al., 2001; Mika et al., 2004).
When plants are attacked by pathogens, they defend themselves with an arsenal of defence mechanisms, that includes de novo protein synthesis which is regulated through a complex and interconnected network of signalling pathways. These pathways mainly involve the two signalling molecules methyl jasmonate and salicylic acid (Koornneef and Pieterse, 2008; Yuan and Lin, 2008; Almagro et al., 2009). Methyl jasmonate-mediated signalling pathways are implicated in the regulation of antiherbivore defences, wounding, and the induction of systemic acquired resistance (Vlot et al., 2008), while the salicylic acid pathway is associated with defence responses against pathogens. However, depending on the pathogen species there are many exceptions and network interactions between both pathways (Smith et al., 2009). In addition, H2O2 has been demonstrated to be involved in pathogen defence and signalling under oxidative stress (Desikan et al., 2004; Mika et al., 2004; Bienert et al., 2006; Stone and Yang, 2006). The gene expression of several soluble class III peroxidases is induced by these factors (Hiraga et al., 2001). Microbe or elicitor-induced signal transduction pathways lead to (i) the reinforcement of cell walls and lignification, (ii) the production of antimicrobial metabolites (phytoalexins), and (iii) the production of active oxygen species and active nitrogen species (Almagro et al., 2009). Elicitors are pathogen-derived messengers that induce plant responses against herbivores or pathogens (Paré et al., 2005). Among the proteins induced during host plant defence, soluble class III plant peroxidases are well known to participate directly or indirectly in a broad range of physiological processes, such as lignin and suberin formation, cross-linking of cell wall components, and synthesis of phytoalexins, or to participate in the metabolism of active oxygen species, switching on the hypersensitive response, a form of programmed host cell death at the infection site associated with limited pathogen development (Almagro et al., 2009). Phosphorylation and dephosphorylation events take place during cell signalling in oxidative stress (Felix et al., 1994) and are involved in protein biosynthesis and degradation processes (Stulemeijer and Joosten, 2008). A partial gene analysis of pmPOX2b revealed several putative cis-regulatory elements that suggested a regulation of its gene by wounding, methyl jasmonate, salicylic acid, and elicitors (Mika et al., 2008). In addition, the 5′-untranslated region of pmPOX2b contained seven successive elements with sequence similarity to the consensus sequence of antioxidant-responsive elements (ARE), strongly suggesting a regulation of the gene by oxidative stress (Haslekås et al., 2003).
The aim of the present study was the investigation of the regulation of class III peroxidases under oxidative stress with a special focus on PM-bound isoenzymes. Since gene expression does not always reflect protein abundance at the plant PM, i.e. the average correlation observed in eukaryotes is about 0.5 (Greenbaum et al., 2003), a functional analysis was carried out by biochemical and proteomic approaches. Total guaiacol peroxidase activity was measured in soluble and microsomal fractions of maize roots after wounding or treatment with different signalling compounds (H2O2, methyl jasmonate, salicylic acid, and elicitors), and compared with changes in PM-bound peroxidase activities. The changes of the protein levels of four plasma membrane-bound class III peroxidases were monitored in response to these multiple stresses.
Materials and methods
Stress and effector treatments of maize seedlings
Maize seedlings (Zea mays L. cv. GBL, Saatenunion, Hannover, Germany) were cultivated in hydroponic cultures in the dark as described earlier (Lüthje et al., 1998). Effectors were added to the hydroponic culture medium of 5-d-old seedlings under green light, and cautiously but thoroughly mixed. Wounding experiments were performed as cutting of the roots in 2–5 cm long root segments. Cantharidin stock solutions were dissolved in acetone. The final concentration of the solvent during the treatment was ≤0.02%. Control experiments were always performed in parallel using the same procedures and conditions, while effector solutions were replaced by equal amounts of hydroponic medium or solvent. Each sample was analysed using 2–3 independent stress experiments, each experiment with approximately 900 maize seedlings, and 900 control plants that were handled in parallel.
Elicitor preparation
Crude preparations of elicitor from Fusarium graminearum and Fusarium culmorum were prepared by standard procedures (García-Muniz et al., 1998). Conidial suspensions (provided by Frank Maier, Biocenter Klein Flottbek, Hamburg, Germany) were cultivated on agar plates (Corn-meal agar, Difco) at 28 °C until mycelium and new conidia covered the surface of the plate. Fungal mycelium and conidia were collected after the addition of 10 ml sterile water and shaking of the plate (50 U min−1) for 2 h at room temperature. The covering solution containing the whole suspension was sonicated for 2 min in a Labsonic 1510 apparatus (B Braun, Melsunger AG, Germany). The protein concentration of the suspension was determined according to Bradford (1976), then autoclaved at 115 °C for 35 min and used as elicitor extract stock solution.
Chitosan was prepared as described elsewhere (Kauss et al., 1989).
Isolation of microsomes, PMs, and soluble proteins
A soluble protein fraction containing soluble proteins from all the cell compartments, microsomes, and PM was prepared from stressed 5-d-old maize roots by differential centrifugation, and aqueous two-phase partitioning as described earlier (Lüthje et al., 1998). Briefly, primary roots (80–130 g fresh weight) were harvested, and washed with cold hydroponic medium. The harvest time did not exceed 30 min. Roots were homogenized (three times for 15 s) in a Waring blender in 200 ml of cold HEPES-KOH buffer (250 mM sucrose, 50 mM HEPES, 1 mM EDTA, pH 7.5) supplemented with 1 mM dithiothreitol and 1% polyvinylpyrrolidone. Crude extracts were filtered through a nylon net (125 μm mesh; Hydrobios, Kiel, Germany), supplemented with 1 mM phenylmethylsulphonyl fluoride, and microsomal fractions were isolated by differential centrifugation (10 min at 10 000 g and 30 min at 50 000 g). Plasma membranes were purified from microsomal fractions by six steps of phase partitioning [36 g phase system consisting of 6.5% (w/w) dextran T500 (Pharmacia, Freiburg, Germany), 6.5% (w/w) polyethylene glycol 3350 (Sigma Aldrich, Taufkirchen, Germany), 250 mM sucrose, 5 mM KC1, 5 mM phosphate buffer, pH 7.8]. Isolated PM were washed in 25 mM sodium acetate-HCl, pH 4.0, 500 mM KCl, 1 mM EDTA, and 0.01% (w/v) Triton X-100 for 30 min at 4 °C under continuous stirring to remove peripheral and adsorbed soluble proteins (Mika and Lüthje, 2003). Washed plasma membranes were pelleted at 105 000 g for 45 min at 4 °C, and resuspended in acetate buffer (25 mM sodium acetate-HCl, pH 4.0, 1 mM EDTA). The final membrane pellets were stored at −80 °C until use.
The purity of the plasma membrane preparations was estimated by a plant cell compartment antibody marker set (Agrisera, Vännäs, Sweden; see Supplementary Fig. S1 at JXB online). Polyclonal antibodies against H+-ATPase (plasma membrane), V-ATPase (tonoplast), Sar1 (endoplasmic reticulum, ER), Sec21 (Golgi apparatus), and cytochrome c oxidase (COXII, inner mitochondrial membrane) were used for Western-blot analysis. According to the protocol of the distributor, 1 μg protein was loaded on the gel for each sample.
Solubilization of membrane proteins
Washed PM (50–200 μg protein) were solubilized with CHAPS at a detergent:protein ratio of 30:1 (w/v) in the presence of 0.5 mM aminocaproic acid in acetate buffer (25 mM sodium acetate-HCl, pH 4.0, and 1 mM EDTA). After incubation for 1 h at 4 °C, solubilised proteins were separated by centrifugation at 13 000 rpm for 1 h at 4 °C (Heraeus Biofuge Type fresco, Kendro, Osterode, Germany).
The PM-bound peroxidase pmPOX2a was further purified from solubilizates of control plants as described earlier (Mika et al., 2008). Briefly, CHAPS/ACA solubilizates were separated by cation exchange chromatography using an HPLC-System (AKTA, GE Healthcare, Freiburg, Germany), peak fractions were concentrated using Centricon YM-10 concentrators (Millipore, Bedford, MA), and purified by additional size exclusion chromatography.
2D-electrophoresis
Solubilized membrane proteins were separated in the first dimension by isoelectric focusing with mini gels (7.5% (w/v) acrylamide, 0.2% (w/v) N,N’-methylene bisacrylamide, 3 M urea, 2% (w/v) CHAPS, 0.1% (v/v) TEMED, 1% (w/v) ammonium persulphate) for 3 h at 4 °C with 10 mM phosphoric acid and 20 mM NaOH as anode and cathode buffer, respectively. For a better resolution, different pH gradients [pH 3–10, pH 8–11; 2% ampholyte (Amersham Pharmacia Biotech, Freiburg, Germany)] were used for different PM-bound peroxidases. pmPOX3 was visible in both gradients allowing standardization. 24 μl sample were mixed with 8 μl loading buffer (8% ampholyte, 8% (w/v) CHAPS, 40% glycerol). Isoelectric points were calculated in comparison with the pH of gel segments derived from control lanes. Gel lanes of the first dimension were equillibrated in 62.5 mM TRIS-HCl, pH 8.8, 10% glycerol, 0.8% SDS, 0.02% bromophenol blue for 30 min on a shaker (50 min−1) and subjected to gradient sodium dodecylsulphate-polyacrylamide gel electrophoresis (SDS-PAGE) with 4% sample gels and 4–18% gradient polyacrylamide slab gels under non-reducing conditions. Peroxidase protein spots were detected by staining with 1% (v/v) guaiacol and 0.03% (v/v) H2O2 in 250 mM sodium acetate–HCl, pH 5.0 for 30 min. As described earlier (Mika et al., 2008), under these conditions this detects the haem groups of class III peroxidases. These contain exactly one prosthetic group per monomer and subunit. Thus, the orange colour detected is directly proportional to the protein abundance of the pmPOX. Control and stressed samples were purified, separated, stained, and photographed in parallel and on the same image. Images were taken by a digital camera (Camedia C-4000 ZOOM, Olympus Optical Co. Ltd., Indonesia). For each gel, several pictures were subjected to image analysis (n=3), and protein spot intensities were evaluated by Fitswork (Version 3.93, http://freenet-homepage.de/JDierks/softw.htm), and ImageJ (Version 1.37; http://rsbweb.nih.gov /ij/). pmPOX were identified in comparison with identified isoelectric points and molecular masses of purified proteins from control plants separated under the same conditions.
Enzyme assays
Peroxidase activities of soluble proteins and membrane fractions were measured as oxidation of guaiacol (8.26 mM, ε=26.6 mm−1 cm−1) in the presence of 8.8 mM H2O2 within 1 min in 25 mM sodium acetate-HCl (pH 5.0). The assay (1 ml) contained 10 μl soluble proteins, 25 μl of 1:10 diluted microsomes, 25 μl PM or 25 μl washed PM vesicles, respectively. The reaction was started by the addition of guaiacol and followed spectrophotometrically (DU 7500, Beckmann, Munich, Germany) as the increase of absorbance at 470 nm. All rates were corrected by chemical control experiments, i.e. auto-oxidation rates of guaiacol under these assay conditions in the absence of active samples. Protein concentrations were determined according to Bradford (1976) in the presence of 0.01% Triton X-100. Data presented were calculated with Microcal Origin (version 5.0, Additive GmbH, Friedrichsdorf/TS, Germany), and significant differences were analysed using one-way ANOVA. When significant differences in data groups were found, single t tests were performed between each control and stress treatment data set.
Results
5-d-old maize seedlings were exposed to wound stress, or treated with different signal compounds to investigate different forms of oxidative stress. The effects of a broad range of compounds (H2O2, methyl jasmonate, salicylic acid, maize pathogen extracts, chitosan, and cantharidin) that are involved in multiple plant signalling cascades were investigated. For each factor, the total protein yield and total class III peroxidase activity was determined for the soluble protein fraction, the microsomal membrane protein fraction, and the non-washed and washed plasma membrane vesicle fraction, respectively.
Total soluble and membrane-bound class III peroxidase activities
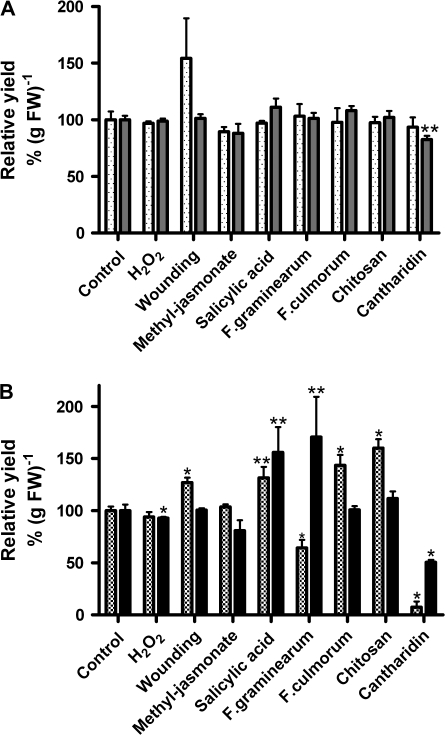
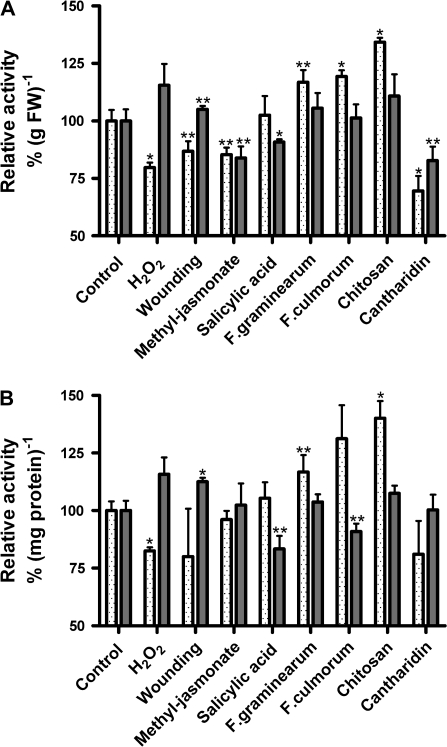
Table 1 reveals an overview of total protein yields and total guaiacol peroxidase activities of the four different protein fractions investigated in control plants. Figures 1A and 2 show the comparison of microsomal and soluble fractions after different stress treatments. Only cantharidin decreased the yield of soluble proteins, whereas the yield of membrane proteins did not change significantly (Fig. 1A). Guaiacol as artificial phenolic substrate detects mainly class III peroxidases. Relative guaiacol activity is shown on the basis of gram fresh weight of maize roots and on the basis of milligrams of protein. After treatment with H2O2, total guaiacol peroxidase activity of the membrane protein fraction was reduced by 20%. In contrast, the total activity of the soluble enzymes was not significantly changed (Fig. 2A, B). Wounding and treatment with methyl-jasmonate slightly reduced the guaiacol rates of the membrane fraction, while the total rate of the soluble proteins was less affected. Treatment with salicylic acid did not exhibit any effect on the total peroxidase activity of membrane proteins (Fig. 2A, B). The soluble guaiacol activity showed a weak, but significant reduction after treatment with salicylic acid.
Table 1.
Total soluble, microsomal, PM-bound, and wPM-bound protein yield and class III peroxidase activity of control plants
| Yield (μg protein g−1 FW) | Guaiacol peroxidase activity |
||
| (nmol min−1 g−1 FW) | (μmol min−1 mg−1 protein) | ||
| Soluble enzymes | 1817±63 | 13372±674 | 7.58±0.33 |
| Membrane-bound enzymes (microsomes) | 242±18 | 517±25 | 2.24±0.09 |
| PM-bound enzymes | 43.1±1.7 | 43.9±1.4 | 0.89±0.02 |
| wPM-bound enzymes | 4.8±0.3 | 15.4±0.4 | 3.40±0.10 |
Shown are means ±SE (n=30). The soluble, membrane-bound (microsomal), plasma membrane-bound, and washed plasma membrane-bound protein fractions of maize seedlings grown in hydroponic cultures were analysed. Plasma membranes were isolated representing peripheral and integral PM-bound proteins. By contrast, thoroughly washed PM vesicles contain mainly integral PM-bound proteins, and, thus, represent the total activity of the four pmPOX of the plant PM (for further details see Figs 1, 2, and 3). FW, fresh weight.
Fig. 1.
Effects of stress treatments on total soluble, microsomal, PM-bound, and wPM-bound protein yields from 5-d-old maize roots. Maize seedlings were exposed to different stressors as indicated. Each stress treatment was analysed using 2–3 independent experiments, i.e. each experiment with approximately 900 plant seedlings. Total protein yields were determined in triplicate in each experiment. Relative yields were calculated in comparison with direct controls that were handled in parallel. Shown are means ±SE (n=6–9; controls n=30). Values that were significantly different from the controls in t test analysis were marked at P < 0.001 (*) and P < 0.05 (**). H2O2 (2 mM); wounding (cutting); methyl-jasmonate (50 μM); salicylic acid (0.5 mM); F. graminearum extract (250 ng protein ml−1); F. culmorum extract (125 ng protein ml−1); chitosan (20 μg ml−1); cantharidin (20 μM); FW, fresh weight. For treatment times see Table 2. Soluble enzymes (grey bars), membrane-bound (microsomes) (dotted bars); PM-bound, checked bars; wPM-bound, solid bars.
Fig. 2.
Effects of stress treatments on total microsomal and soluble guaiacol peroxidase activities from 5-d-old maize roots. Maize seedlings were exposed to different stressors as indicated. Each treatment was analysed using 2–3 independent experiments. Total class III peroxidase activities were determined in triplicate in each experiment. Relative peroxidase activities were calculated in comparison with direct controls that were handled in parallel. Shown are means ±SE (n=6–9; controls n=30). Class III peroxidase activities were measured in the presence of 8.26 mM guaiacol and 8.8 mM H2O2. Values that were significantly different from the controls in t test analysis were marked at P < 0.001 (*) and P < 0.05 (**). H2O2 (2 mM); wounding (cutting); methyl-jasmonate (50 μM); salicylic acid (0.5 mM); F. graminearum extract (250 ng protein ml−1); F. culmorum extract (125 ng protein ml−1); chitosan (20 μg ml−1); cantharidin (20 μM); FW, fresh weight. For treatment times see Table 2. (Soluble enzymes, grey bars; membrane-bound enzymes (microsomes), dotted bars.
By contrast, the three elicitors tested, i.e. chitosan, and extracts of Fusarium graminearum and F. culmorum, slightly increased the total peroxidase activities of the membrane fraction, while the total amount of soluble peroxidases did not reveal a significant change (Fig. 2A, B). Treatment with the phosphatase inhibitor cantharidin reduced both membrane-bound and soluble activities, on the basis of gram fresh weight, whereas the specific activity (μmol min−1 mg−1 protein) of soluble guaiacol peroxidases appeared not to be affected due to the change in the total protein yield.
Total plasma membrane and washed plasma membrane peroxidase activities
In order to achieve a more detailed picture of stress and signal compound impact on the membranes and peroxidases of maize roots, plasma membranes (PM) and thoroughly washed plasma membrane (wPM) vesicles were investigated (Figs 1B, 3).
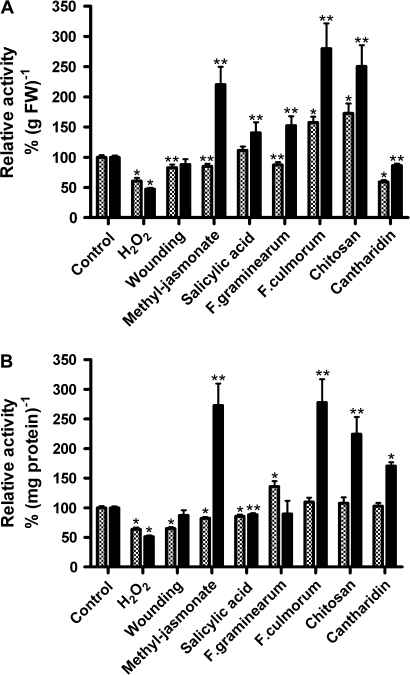
Fig. 3.
Effects of stress treatments on total PM-bound and wPM-bound guaiacol peroxidase activities from 5-d-old maize roots. Maize seedlings were exposed to different stressors as indicated. Each treatment was analysed using 2–3 independent repetitions. Plasma membranes were isolated representing peripheral and integral PM-bound proteins. By contrast, thoroughly washed PM vesicles contain mainly integral PM-bound proteins, and thus represent the total activity of the four pmPOX of the plant PM. Relative peroxidase activities were determined in triplicate for each independent experiment. Shown are means ±SE (n=6–9; controls n=30; further details see Fig. 2). Class III peroxidase activities were measured in presence of the peroxidase substrates guaiacol and H2O2. Values that were significantly different from the controls in t test analysis were marked at levels of P < 0.001 (*) and P < 0.05 (**). PM-bound, checked bars; wPM-bound enzymes, solid bars.
The measurements with PM vesicles represent the total activities of integral and peripheral membrane-bound peroxidases including some activities of soluble peroxidases that bind to the vesicles during the purification procedure (Bérczi and Asard, 2003). The latter are not removable without the removal of non-covalent bound peripheral membrane proteins as well. The activities of wPM representing only the integral membrane protein fraction and, thus, the total activities of the four pmPOX, in comparison with the peroxidase activities of the PM and the soluble fraction, at least show the possible tendencies in the regulation of peripheral membrane bound enzymes. After wounding and treatment with salicylic acid, F. culmorum, and chitosan the PM protein yields were significantly increased, while decreased values were found in F. graminearum and cantharidin-treated samples (Fig. 1B). The wPM protein yield was enhanced by salicylic acid and F. graminearum, and was slightly decreased by H2O2. By contrast, cantharidin reduced the yield to 50% (Fig. 1B).
As shown in Fig. 3, the specific guaiacol peroxidase activity of PM vesicles derived from seedlings after wounding or treatment with H2O2, methyl-jasmonate or salicylic acid, respectively, was reduced by 20–40% (Fig. 3A, B). The effects on the wPM fractions were similar to the PM fractions with the exception of methyl jasmonate, which revealed a strongly induced guaiacol activity. A stimulation of total guaiacol peroxidase activity was also found for wPM with all the elicitors tested (Fig. 3B).
2D-PAGE analysis of the four single pmPOX
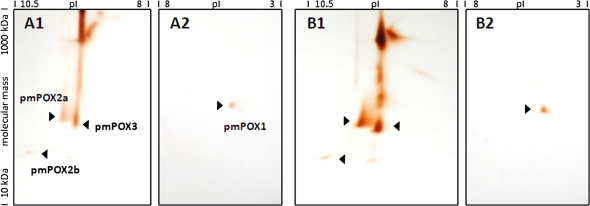
In addition to the guaiacol peroxidase activities, the stress-dependent protein abundance of the four PM-bound peroxidases (pmPOX1, pmPOX2a, pmPOX2b, and pmPOX3) present at the plant wPM was analysed in detail by 2D electrophoresis. For each treatment 6–8 two-dimensional PAGE gels (n=18–24 gel pictures) of two to three independent experiments and the same amount of control gels were analysed. A comparison between exemplary gels representing samples treated with salicylic acid and its direct controls is shown in Fig. 4. The up-regulation of all four pmPOX by salicylic acid is clearly visible.
Fig. 4.
2D-PAGE analysis of pmPOX after treatment with salicylic acid. Shown are exemplary gels for a control (A) and salicylic acid treatment (B). Solubilizates of washed PM were separated by isoelectric focusing in the first dimension with two different overlapping ampholyte gradients (A1, B1, pH range 10–8; A2, B2, pH range 8–3) to allow optimal separation of pmPOX, followed by a modified non-reducing gradient SDS-PAGE in the second dimension. Thus, oligomers and haem proteins remain intact. Protein spots were stained with guaiacol and H2O2. As described earlier (Mika et al., 2008), under these conditions this detects the haem groups of class III peroxidases, i.e. visualizing the protein abundance of the pmPOX (for details see the Materials and methods). pmPOX1, pmPOX2a, pmPOX2b, and pmPOX3 are visible. All four pmPOX are up-regulated in salicylic acid-treated plants.
The PM-bound peroxidases were identified by staining with the peroxidase substrates guaiacol and H2O2 in comparison with known pI and MW values for pmPOX1, pmPOX2b, and pmPOX3. By contrast, the isoelectric point (9.5) and molecular mass (63 kDa) of pmPOX2a were revealed for the first time, based on a 2D-PAGE analysis of the purified protein after cation exchange chromatography and size exclusion of control wPM solubilizates (data not shown). Thus, the corresponding spot for the monomeric pmPOX2a was easily identified in the 2D-PAGE analysis of stressed wPM solubilizates (Fig. 4). The non-reducing SDS-PAGE used in the second dimension allows the in-gel detection of haem groups of class III peroxidases (b-type cytochromes) by peroxidase substrates as described earlier (Thomas et al., 1976; Mika et al., 2008), and the quantification of the spot intensity allows an estimation of the regulation of the different isoenzymes on the protein level due to their constant amount of prosthetic groups.
The total spot intensities as an indicator of the protein levels of the four class III peroxidases were 18.4 for pmPOX1, 84.9 for pmPOX2a, 22.2 for pmPOX2b, and 100.0 for pmPOX3 in control plants (n=21 gels/63 gel pictures), when they were normalized to the predominant enzyme. The intensity of the four pmPOX spots is higher in 2D gels using wPM in comparison to PM, since the amount of the pmPOX referred to protein concentration is higher, i.e. the specific activity increased. As shown in Table 2, the four membrane-bound class III peroxidases were differentially regulated by the treatments tested. H2O2 had a different effect on the protein level of each single pmPOX: pmPOX1 was not affected, while an increase in spot intensity was observed for pmPOX2b >pmPOX2a, whereas a strong decrease was observed for pmPOX3. Wounding caused a significant decrease in intensity for pmPOX2b. The methyl jasmonate-treatment increased the intensity of all four pmPOX: pmPOX3 >pmPOX2b >pmPOX2a >pmPOX1. Salicylic acid showed a strong up-regulation for pmPOX2b, whereas pmPOX3 >pmPOX1 >pmPOX2a were less affected.
Table 2.
Effects of different stress factors and signal compounds on the protein level of all four pmPOX from maize seedlings
| Treatment | Treatment times (h) | Relative protein spot intensity (%) |
|||
| pmPOX1 | pmPOX2a | pmPOX2b | pmPOX3 | ||
| Control | 100.0±17.1 (21/63) | 100.0±15.7 (24/72) | 100.0±18.0 (21/63) | 100.0±15.3 (24/72) | |
| H2O2 | 1 | 111.0±44.9 (5/15) | 158.9±24.4 (6/18)** | 244.8±60.8 (4/12)** | 47.7±4.3 (6/18)* |
| Wounding | 1 | 100.3±5.1 (6/18) | 87.4±11.2 (8/24) | 52.7±4.1 (6/18)* | 79.5±9.3 (8/24) |
| Methyl jasmonate | 1 | 147.9±14.6 (6/18)** | 251.8±25.3 (6/18)* | 267.3±21.2 (4/12)* | 628.0±166.2 (6/18)* |
| Salicylic acid | 1 | 347.0±81.1 (4/12)* | 188.8±39.7 (6/18)** | 877.4±306.5 (4/12)** | 422.3±87.4 (6/18)* |
| F. graminearum extract | 4 | 113.5±18.2 (4/12) | 65.3±6.9(6/18)* | 233.0±42.5 (4/12)** | 113.8±23.0 (6/18) |
| F. culmorum extract | 4 | 183.0±40.9 (4/12)** | 182.9±23.1 (6/18)* | 165.3±23.8 (4/12)* | 241.0±14.9 (6/18)* |
| Chitosan | 4 | 136.7±23.2 (4/12)** | 169.7±20.0 (6/18)* | 256.7±71.5 (4/12)** | 981.7±99.0 (6/18)* |
| Cantharidin | 1 | 72.3±12.3 (4/12)** | 59.4±5.9 (6/18)* | 192.4±25.1 (4/12)** | 98.6±15.3 (6/18) |
5-d-old maize seedlings were exposed to different stressors as indicated. Each treatment was analysed using 2–3 independent experiments, i.e. each experiment with approximately 900 plant seedlings. Treated and control plants and samples were handled in parallel throughout all subsequent steps. For each experiment solubilizates from isolated wPM were repeatedly separated by 2D PAGE analysis (isoelectric focusing and non-reducing modified gradient SDS-PAGE, Fig. 4). As described earlier, thus, oligomers and haem proteins remain intact (Mika and Lüthe, 2003). PM-bound peroxidases were identified by staining with guaiacol and H2O2. Relative spot intensities representing protein abundance were analysed and calculated in comparison with direct controls. Isoelectric focusing was performed in two different overlapping ampholyte gradients to provide optimal separation of the pmPOX. Shown are means ±SE (n=analysed 2D gels/gel pictures). SE of controls for single independent treatments were ≤4%. Values that were significantly different from controls in t test analysis were marked at P < 0.001 (*) and P < 0.05 (**). The proteomic analysis revealed strong changes in protein biosynthesis of all four pmPOX proteins after different treatments.
Elicitors, in general, induced the peroxidases, with the exception of the F. graminearum extract that decreased the intensity of pmPOX2a significantly and was without an effect on pmPOX1 and pmPOX3. Cantharidin decreased the intensity of pmPOX2a >pmPOX1, and increased the intensity of pmPOX2b. Again intensity of pmPOX3 was not significantly affected.
Overall the strongest decrease in spot intensity occurred for pmPOX2b by wounding. A maximal up-regulation of 980% was revealed for pmPOX3 after treatment with chitosan. None of the PAGE gels revealed new spots after any of the treatments.
Discussion
After treatment with the different effectors total activities of soluble and microsomal fractions were compared. However, only slight changes were observed for the total activities of microsomal fractions and soluble peroxidases with the majority of stresses (Figs 1A, 2). Exceptions were H2O2, chitosan, and cantharidin with effects of ≥20–30% on the peroxidase activity of microsomal fractions. This observation may be explained by the large number of isoenzymes in both fractions that might be differentially regulated. Some of the isoenzymes may be up-regulated whereas others are down-regulated, the overall reaction may cause either no effect or weak changes.
In contrast to microsomal fractions, PM preparations showed multiple strong changes in peroxidase activity by the effectors tested (Fig. 3). The presence of soluble peroxidases, which may be attached to the PM by electrostatic interaction, appears to falsify the results in unwashed PM fractions. The results for PM vesicles in comparison with the total peroxidase activities of the soluble enzymes and wPM vesicles, indicated the presence of possible peripheric membrane-bound peroxidases only in plants treated with cantharidin, while all other apparent contradictory results between PM and wPM fractions (i.e. methyl jasmonate) are easily explained by the attachment of soluble proteins to the PM. Bolwell et al. (2002) showed that soluble apoplastic peroxidases can bind to plasma membranes of French bean (Phaseolus vulgaris L.) in the presence of certain stresses.
In control plants, the purified plasma membrane-bound peroxidases showed guaiacol activities of 61.9±24.1 for pmPOX1, 26.5±2.4 for pmPOX2a, 6.9±1.1 for pmPOX2b, and 54.7±1.8 μmol min−1 mg−1 protein for pmPOX3 after cation exchange chromatography and size exclusion (Mika et al., 2008). Thus, pmPOX1 contributes to the total activity in non-stressed plants with 41.3%, followed by pmPOX3 with 36.5%, pmPOX2a with 17.6%, and pmPOX2b with 4.6%. The expression levels of the four haem proteins in response to stresses and signal compounds were investigated using a proteomic approach with isoelectric focusing and a non-reducing, modified SDS-PAGE as 2D analysis had several advantages. (i) As already stated above, the non-reducing state of the SDS-PAGE allows the in-gel detection of the haem groups of class III peroxidases by peroxidase substrates (Thomas et al., 1976; Mika et al., 2008), and the quantification of the spot intensity allows an estimation of the regulation of the different isoenzymes on the protein level due to their constant amount of prosthetic groups. (ii) In contrast, an activity gel staining of native 2D-PAGE gels with guaiacol and hydrogen peroxide would detect their prosthetic groups and enzyme activity, and create a mixture of protein abundance and peroxidase activity data. (iii) In addition, the natural substrates of the four pmPOX might differ. Despite a detailed analysis of the substrate specificity of the pmPOX (Mika and Lüthje, 2003), the in vivo substrates of these enzymes at the outside of the plant PM remain unclear. Thus, the enzyme activites of the pmPOX that are already constitutively present at the membrane could be additionally regulated, depending on the levels of their natural substrates and regulating factors to provide even faster adaptations to stress responses.
The experimental isoelectric point and relative molecular mass were determined for the pmPOX2a monomer for the first time, while the values for pmPOX1, pmPOX2a, and pmPOX3 were known by earlier 1-dimensional isoelectric focusing, SDS-PAGE analysis, and size exclusion chromatography (Mika et al., 2008). After purification by cation exchange chromatography and size exclusion, pmPOX2a eluted in fractions with average molecular masses of 155 kDa (Mika and Lüthje, 2003). However, the present combined 2D-PAGE analysis of both wPM and the purified protein resulted in the detection of the enzyme by guaiacol and hydrogen peroxide. The difference between the native molecular mass and the value derived from 2D gels is often found, and can be explained by post-translational modifications, the formation of protein aggregates during the purification procedure, or the participation of pmPOX2a in a protein complex within the plant PM (Mika and Lüthje, 2003). The 63 kDa molecular mass of the pmPOX2a protein spot is typical for monomeric glycosylated class III peroxidases, as already described for pmPOX2b, and pmPOX3 and other soluble plant peroxidases (Mika et al., 2008). Isoelectric points of class III peroxidases show the complete range of the spectrum, while molecular masses are often within a range of 28–60 kDa (Hiraga et al., 2001). The 2D-PAGE analysis revealed additional high molecular mass spots (Fig. 4) that either comprise residual amounts of pmPOX3 due to aggregate formation during the purification procedure, or might be haem proteins. It was shown for in-gel staining with other peroxidase substrates, i.e. tetramethylbenzidine and hydrogen peroxide, that, in addition to class III peroxidases, haem and copper-containing proteins were non-specifically stained (Miller and Nicholas, 1984). The native molecular mass of pmPOX3 (40 kDa) in size exclusion chromatography fractions did not suggest the existence of a pmPOX3-containing complex.
Oxidative stress
H2O2 plays a crucial role in plant development and cell signalling (Desikan et al., 2004). Total activity of wPM decreased after H2O2 treatment in order to induce oxidative stress directly (Fig. 3). The increase in intensity of pmPOX2a and pmPOX2b in comparison to the controls (Table 2) suggests an up-regulation of these isoenzymes by H2O2. The discrepancy between total activity and protein up-regulation of pmPOX2a and pmPOX2b can be explained by the simultaneous down-regulation of pmPOX3 (Table 2), which is usually the dominant peroxidase at the protein level and reveals a high activity level. In contrast to pmPOX2a and pmPOX2b, the down-regulation of pmPOX3 suggests that this enzyme might not be involved in membrane protection. Due to these results and the indications that pmPOX could be part of lipid rafts (Lüthje, 2008) it can be hypothesized that the overall peroxidase activity at the PM might be reduced to allow H2O2 signalling, while certain microdomains of the membrane and, thus, specific functions might be protected. A thylakoid-bound peroxidase in chloroplasts contains an additional hydrophobic domain at the C-terminus that is missing in soluble cytosolic and stroma ascorbate peroxidases, and it seems to enable the protein to bind next to photosystem I to the thylakoid membrane (Asada et al., 1996). This microcompartmentation of the membrane-bound peroxidase is an elegant solution to protect the thylakoid membrane against destruction and to detoxify H2O2 directly at the site of origin.
Mechanical stress
Wounding, applied as cutting, caused a strong decrease in the guaiacol activity of unwashed PM preparations, whereas no effect was observed with washed PM (Fig. 3). The stronger effect of wounding on the activity of unwashed PM may be explained partially by the contribution of soluble proteins attached to the PM during preparation (Bérczi and Asard, 2003). The decrease in total guaiacol activity of washed PM suggested that PM-bound peroxidases may not be involved in the wound response. This hypothesis was further supported by the decrease in abundance of pmPOX2b (Table 2). The decreases of the protein levels of pmPOX2a and pmPOX3 were not significant and pmPOX1 was not affected. In contrast to PM-bound peroxidases from maize roots, extracellular soluble peroxidases isolated from wheat roots were demonstrated to be involved in the wound response (Minibayeva et al., 2003, 2009).
The total guaiacol activity of wPM revealed a strong increase after treatment with the plant hormone methyl jasmonate (Fig. 3). Data shown in Table 2 demonstrated the up-regulation of all PM isoenzymes by methyl jasmonate, and pmPOX2a showed its highest response to this signal compound. This observation suggests a function of PM-bound peroxidases in systemic acquired resistance and/or herbivore stress. The low responses of the pmPOX after cutting do not exclude reactions of the pmPOX to herbivores. The strong up-regulation of all pmPOX at the protein level in the presence of methyl jasmonate could indicate their involvement in herbivore stress as well. Hiraga et al. (2000) showed that different types of wounding triggered the expression of different class III peroxidases from rice (Oryza sativa L.), thus, representing an even more complicated network of responses. Soluble peroxidases are well known as pathogen-related proteins involved in systemic acquired resistance and plant defence reactions (Almagro et al., 2009).
Both methyl jasmonate and salicylic acid (0.1–0.5 mM) increase plant tolerance against oxidative stress (Yuan and Lin, 2008). As shown in Fig. 3, treatment with salicylic acid caused weak effects on total activities of PM preparations. In contrast to this observation, the spot intensity of all four PM-bound peroxidases increased and suggested an up-regulation of the enzymes. However, data observed for PM preparations appear contradictory to the increases in intensity. As shown in Fig. 3, the effects on total activity depend on the basis used for calculation. The specific activity in μmol min−1 mg−1 protein decreased, whereas total activity in nmol min−1 g−1 FW increased. It may be speculated, that besides peroxidases, several other proteins were up- or down-regulated by the treatment. A significant up-regulation of other proteins changes the specific activity calculated for the peroxidases.
Pathogen defence
Up-regulation of PM-bound peroxidases by methyl jasmonate and salicylic acid suggests not only a function in mechanical stress but also in pathogen response. The classical answer of animal and plant cells to pathogen attack is an oxidative burst, a sudden production of active oxygen species appearing briefly and/or hours after certain stresses (Kawano, 2003; Mika et al., 2004; Torres and Dangl, 2005; Sumimoto et al., 2008). The production of reactive oxygen species can be observed after the application of fungal elicitors as well (Paré et al., 2005). In the present study, chitosan and extracts of F. graminearum and F. culmorum were used for the induction of an oxidative burst. Both fungi are major pathogens of cereals. As shown in Fig. 3, treatment with all three elicitors increased guaiacol peroxidase activity of wPM fractions. This observation was confirmed by the increase in protein abundance for PM-bound peroxidases (Table 2). However, the F. graminearum extract caused a decrease in intensity of pmPOX2a, whereas the other two elicitors increased the intensity. This suggested a differential regulation of pmPOX2a in the responses of maize plants against the different microbes. Recent data suggest a fine-tuning of the pathogen defence and the corresponding signal cascades to single species and their properties (Koornneef and Pieterse, 2008). Pathogen defence responses include cell wall strengthening by lignin and suberin formation, cross-linking of cell wall components, biosynthesis of defence molecules, i.e. phytoalexins, and the metabolism of active oxygen species (Mika et al., 2004; Almagro et al., 2009). Peroxidases can be involved in all of these processes (Almagro et al., 2009). The high affinity of several pmPOX for hydroxycinnamyl alcohols (Mika and Lüthje, 2003) that are used by apoplastic peroxidases for lignin biosynthesis (Hiraga et al., 2001) in combination with their very strong increases in abundance after contact of the plants to pathogen elicitors suggest a function in cell wall strengthening besides membrane protection.
The contribution of protein phosphorylation and dephosphorylation in signal transduction during plant–pathogen interaction has been demonstrated frequently (Stulemeijer and Joosten, 2008). Treatment of plants with the phosphatase inhibitor cantharidin results in a decrease of total guaiacol peroxidase activity in PM preparations (Fig. 3). The effect on washed PM, however, indicated a weak decrease in peroxidase activity. As shown in Table 2, the protein level of pmPOX2b was shown to be up-regulated, whereas pmPOX2a and pmPOX1 were significantly down-regulated after treatment with cantharidin. These data suggest a differential regulation of PM-bound peroxidases. Elicitor treatment of cell cultures induced a rapid change in the phosphorylation status of extracellular peroxidases (Chevasa et al., 2005). Sequence analysis of the pmPOX revealed 15–21 putative phosphorylation sites in each of the proteins (NetPhos2.0 prediction tool; Blom et al., 1999). Thus, results observed after treatment with cantharidin could be based on direct regulation by phosphorylation/dephosphorylation of the proteins or indirect regulation due to effects on de novo biosynthesis or the degradation of peroxidases.
Conclusions
The present study demonstrates the involvement of membrane-bound class III peroxidases from maize seedlings in stress responses for the first time. The regulation of pmPOX2b confirmed the predictions made due to the occurrence of cis-regulatory elements found by gene analysis (Mika et al., 2008). The total peroxidase activity of the maize root wPM and the protein abundance of all four pmPOX were changed after five or more treatment types, indicating a tight control and adjustment of the enzymes to multiple stress functions. As postulated before, the tremendous amount of peroxidase isoenzymes and their tight, differential, and overlapping regulation seem to have evolved to deal with every possible stress situation (Passardi et al., 2005). Using the strongest effects on the pmPOX as guidelines, the results of the present study indicate a regulation of all four pmPOX by methyl-jasmonate and salicylic acid signalling. The data at hand showed that all four membrane-bound peroxidases are involved in pathogen defence with different responses to each elicitor, and probably different roles. pmPOX2b showed the broadest response of all four enzymes, reacted to every stress and effector used, and revealed the highest up-regulation by H2O2 treatment. Thus, the present study suggests a broad function of PM-bound peroxidases in oxidative stress. In the future, more detailed analysis approaches might shed light on the complex functional network of plasma membrane-bound, apoplastic, and cell wall peroxidases.
Supplementary data
Supplementary data can be found at JXB online.
Supplementary Fig. S1. Marker analysis of plasma membrane-enriched fractions.
Supplementary Material
Acknowledgments
The authors wish to thank Aslihan Ayar, Janis Hantke, and Natalie Cyranck, for excellent technical assistance. This work was supported by the Deutsche Forschungsgemeinschaft (DFG Lu-668/4-2) and by the University of Hamburg (PhD student's grant no. HmbNFG to DH).
References
- Almagro L, Gómez Ros LV, Belchi-Navarro S, Bru R, Ros Barceló A, Pedreño MA. Class III peroxidases in plant defence reactions. Journal of Experimental Botany. 2009;60:377–390. doi: 10.1093/jxb/ern277. [DOI] [PubMed] [Google Scholar]
- Asada K, Miyake C, Ogawa K, Hossain MA. Microcompartmentation of ascorbate peroxidase and regeneration of ascorbate from ascorbate radical: its dual role in chloroplasts. In: Obinger C, Burner U, Ebermann R, Penel C, Greppin H, editors. Proceedings of the IV international symposium on plant peroxidases: biochemistry and physiolology. Switzerland: University of Vienna, Austria and University of Geneva; 1996. pp. 163–167. [Google Scholar]
- Bérczi A, Asard H. Soluble proteins, an often overlooked contaminant in plasma membrane preparations. Trends in Plant Science. 2003;8:250–251. doi: 10.1016/S1360-1385(03)00100-6. [DOI] [PubMed] [Google Scholar]
- Bienert GP, Schjoerring JK, Jahn TP. Membrane transport of hydrogen peroxide. Biochimica et Biophysica Acta. 2006;1758:994–1003. doi: 10.1016/j.bbamem.2006.02.015. [DOI] [PubMed] [Google Scholar]
- Blom N, Gammeltoft S, Brunak S. Sequence- and structure-based prediction of eukaryotic protein phosphorylation sites. Journal of Molecular Biology. 1999;294:1351–1362. doi: 10.1006/jmbi.1999.3310. [DOI] [PubMed] [Google Scholar]
- Bolwell GP, Bindschedler LV, Blee KA, Butt VS, Davies DR, Gardner SL, Gerrish C, Minibayeva F. The apoplastic oxidative burst in response to biotic stress in plants: a three-component system. Journal of Experimental Botany. 2002;53:1367–1376. [PubMed] [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein–dye binding. Analytical Biochemistry. 1976;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Cakmak I, van de Wetering DAM, Marschner H, Bienfait HF. Involvement of superoxide radical in extracellular ferric reduction by iron-deficient bean roots. Plant Physiology. 1987;85:310–314. doi: 10.1104/pp.85.1.310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chevasa S, Simon WJ, Yu YL, Yalpani N, Slabas AR. Pathogen elicitor-induced changes in the maize extracellular matrix proteome. Proteomics. 2005;5:4894–4904. doi: 10.1002/pmic.200500047. [DOI] [PubMed] [Google Scholar]
- Cosio C, Dunand C. Specific functions of individual class III peroxidase genes. Journal of Experimental Botany. 2009;60:391–408. doi: 10.1093/jxb/ern318. [DOI] [PubMed] [Google Scholar]
- Desikan R, Cheung MK, Bright J, Henson D, Hancock JT, Neill SJ. ABA, hydrogen peroxide and nitric oxide signalling in stomatal guard cells. Journal of Experimental Botany. 2004;55:205–212. doi: 10.1093/jxb/erh033. [DOI] [PubMed] [Google Scholar]
- Dietz KJ, Tavakoli N, Kluge C, Mimura T, Sharma SS, Harris GC, Chardonnens AN, Golldack D. Significance of the V-type ATPase for the adaptation to stressful growth conditions and its regulation on the molecular and biochemical level. Journal of Experimental Botany. 2001;52:1969–1980. doi: 10.1093/jexbot/52.363.1969. [DOI] [PubMed] [Google Scholar]
- Felix G, Regenass M, Spanu P, Boller T. The protein phosphatase inhibitor calyculin A mimics elicitor action in plant cells and induces rapid hyperphosphorylation of specific proteins as revealed by pulse labelling with [32P] phosphate. Proceedings of the National Academy of Sciences, USA. 1994;91:952–956. doi: 10.1073/pnas.91.3.952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- García-Muniz N, Martínez-Izquierdo JA, Puigdomènech P. Induction of mRNA accumulation corresponding to a gene encoding a cell wall hydroxyproline-rich glycoprotein by fungal elicitors. Plant Molecular Biology. 1998;38:623–632. doi: 10.1023/a:1006056000957. [DOI] [PubMed] [Google Scholar]
- Greenbaum D, Colangelo C, Williams K, Gerstein M. Comparing protein abundance and mRNA expression levels on a genomic scale. Genome Biology. 2003;4:117. doi: 10.1186/gb-2003-4-9-117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haslekås C, Grini PE, Nordgard SH, Thorstensen T, Viken MK, Nygaard V. ABI3 mediates expression of the peroxiredoxin antioxidant AtPER1 gene and induction by oxidative stress. Plant Molecular Biology. 2003;53:313–326. doi: 10.1023/b:plan.0000006937.21343.2a. [DOI] [PubMed] [Google Scholar]
- Hiraga S, Sasaki K, Ito H, Ohashi Y, Matsui H. A large family of class III plant peroxidases. Plant and Cell Physiology. 2001;42:462–468. doi: 10.1093/pcp/pce061. [DOI] [PubMed] [Google Scholar]
- Hiraga S, Yamamoto K, Ito H, Sasaki K, Matsui H, Honma M, Nagamura Y, Sasaki T, Ohashi Y. Diverse expression profile of 21 rice peroxidase genes. FEBS Letters. 2000;471:245–250. doi: 10.1016/s0014-5793(00)01409-5. [DOI] [PubMed] [Google Scholar]
- Kauss H, Jeblick W, Domard A. The degrees of polymerization and N-accetylation of chitosan determine its ability to elicit callose formation in suspension cells and protoplasts of Catharanthus roseus. Planta. 1989;178:385–392. doi: 10.1007/BF00391866. [DOI] [PubMed] [Google Scholar]
- Kawano T. Roles of the reactive oxygen species-generating peroxidase reaction in plant defense and growth induction. Plant Cell Reports. 2003;21:829–837. doi: 10.1007/s00299-003-0591-z. [DOI] [PubMed] [Google Scholar]
- Koornnef A, Pieterse CM. Cross talk in defence signalling. Plant Physiology. 2008;146:839–844. doi: 10.1104/pp.107.112029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefevbre B, Furt F, Hartmann M-A, et al. Characterization of lipid rafts from Medicago truncatula root plasma membranes: a proteomic study reveals the presence of a raft associated redox system. Plant Physiology. 2007;144:402–418. doi: 10.1104/pp.106.094102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipka V, Panstruga R. Dynamic cellular responses in plant microbe interactions. Current Opinion in Plant Biology. 2005;8:625–631. doi: 10.1016/j.pbi.2005.09.006. [DOI] [PubMed] [Google Scholar]
- Lüthje S. Plasma membrane redox systems: lipid rafts and protein assemblies. In: Lüttge U, Beyschlag W, Murata J, editors. Progress in botany. Vol. 69. Heidelberg\: Springer; 2008. pp. 169–200. [Google Scholar]
- Lüthje S, Van Gestelen P, Córdoba-Pedregosa MC, Gonzáles-Reyes JA, Asard H, Villalba JM, Böttger M. Quinones in plant plasma membranes: a missing link? Protoplasma. 1998;205:43–51. [Google Scholar]
- Mika A, Buck F, Lüthje S. Membrane-bound class III peroxidases: identification, biochemical properties and sequence analysis of isoenzymes purified from maize (Zea mays L.) roots. Journal of Proteomics. 2008;71:412–424. doi: 10.1016/j.jprot.2008.06.006. [DOI] [PubMed] [Google Scholar]
- Mika A, Minibayeva F, Beckett R, Lüthje S. Possible functions of extracellular peroxidases in stress-induced generation and detoxification of active oxygen species. Phytochemistry Reviews. 2004;3:173–193. [Google Scholar]
- Mika A, Lüthje S. Properties of guaiacol peroxidase activities isolated from corn root plasma membranes. Plant Physiology. 2003;132:1489–1498. doi: 10.1104/pp.103.020396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller DJ, Nicholas DJD. 3,3′,5,5′-Tetramethylbenzidine/H2O2 staining is not specific for heme proteins separated by gel electrophoresis. Analytical Biochemistry. 1984;140:577–580. doi: 10.1016/0003-2697(84)90209-4. [DOI] [PubMed] [Google Scholar]
- Minibayeva F, Lüthje S, Kolesnikov O, Chasov A, Beckett RP, Vylegzhanina N, Buck F, Böttger M. Wound-induced apoplastic peroxidase activities: their roles in the production and detoxification of reactive oxygen species. Plant, Cell Environment. 2009;32:497–508. doi: 10.1111/j.1365-3040.2009.01944.x. [DOI] [PubMed] [Google Scholar]
- Minibayeva F, Mika A, Lüthje S. Salicylic acid changed the properties of extracellular peroxidase activity secreted from wounded wheat (Triticum aestivum L.) roots. Protoplasma. 2003;221:67–72. doi: 10.1007/s00709-002-0071-2. [DOI] [PubMed] [Google Scholar]
- Paré PW, Farag MA, Krishnamachari V, Zhang H, Ryu CM, Kloepper JW. Elicitors and priming agents initiate plant defense response. Photosynthesis Research. 2005;85:149–159. doi: 10.1007/s11120-005-1001-x. [DOI] [PubMed] [Google Scholar]
- Passardi F, Cosio C, Penel C, Dunand C. Peroxidases have more functions than a Swiss army knife. Plant Cell Reports. 2005;24:255–265. doi: 10.1007/s00299-005-0972-6. [DOI] [PubMed] [Google Scholar]
- Passardi F, Longet D, Penel C, Dunand C. The class III peroxidase multigenic family in rice and its evolution in land plants. Phytochemistry. 2004;65:1879–1893. doi: 10.1016/j.phytochem.2004.06.023. [DOI] [PubMed] [Google Scholar]
- Qiu QS, Liang HG. Lipid peroxidation caused by the redox system of plasma membranes from wheat roots. Journal of Plant Physiology. 1995;145:261–265. [Google Scholar]
- Rawyler A, Arpagaus S, Braendle R. Impact of oxygen stress and energy availability on membrane stability of plant cells. Annals of Botany (London) 2002;90:499–507. doi: 10.1093/aob/mcf126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schraudner M, Langebartels C, Sandermann H., Jr Plant defence systems and ozone. Biochemical Society Transactions. 1996;24:456–461. doi: 10.1042/bst0240456. [DOI] [PubMed] [Google Scholar]
- Schützendübel A, Polle A. Plant responses to abiotic stresses: heavy metal-induced oxidative stress and protection by mycorrhization. Journal of Experimental Botany. 2002;53:1351–1365. [PubMed] [Google Scholar]
- Smith JL, De Moraes CM, Mescher MC. Jasmonate- and salicylate-mediated plant defense responses to insect herbivores, pathogens and parasitic plants. Pest Management Science. 2009 doi: 10.1002/ps.1714. 10.1002/ps.1714 DOI. [DOI] [PubMed] [Google Scholar]
- Stone JR, Yang S. Hydrogen peroxide: a signaling messenger. Antioxidants and Redox Signalling. 2006;8:243–270. doi: 10.1089/ars.2006.8.243. [DOI] [PubMed] [Google Scholar]
- Stulemeijer IJ, Joosten MH. Post-translational modification of host proteins in pathogen-triggered defence signalling in plants. Molecular Plant Pathology. 2008;9:545–560. doi: 10.1111/j.1364-3703.2008.00468.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sumimoto H. Structure, regulation and evolution of NOX-family NADPH oxidases that produce reactive oxygen species. FEBS Journal. 2008;275:3249–3277. doi: 10.1111/j.1742-4658.2008.06488.x. [DOI] [PubMed] [Google Scholar]
- Thomas PE, Ryan D, Levin W. An improved staining procedure for the detection of the peroxidase activity of cytochrome P-450 on sodium dodecyl sulphate polyacrylamide gels. Analytical Biochemistry. 1976;75:168–176. doi: 10.1016/0003-2697(76)90067-1. [DOI] [PubMed] [Google Scholar]
- Tognolli M, Penel C, Greppin H, Simon P. Analysis and expression of the large class III peroxidase gene family in Arabidopsis thaliana. Gene. 2002;288:129–138. doi: 10.1016/s0378-1119(02)00465-1. [DOI] [PubMed] [Google Scholar]
- Torres MA, Dangl JL. Functions of the respiratory burst oxidase in biotic interactions, abiotic stress and development. Current Opinion in Plant Biology. 2005;8:397–403. doi: 10.1016/j.pbi.2005.05.014. [DOI] [PubMed] [Google Scholar]
- Vlot AC, Klessig DF, Park SW. Systemic acquired resistance: the elusive signal(s) Current Opinion in Plant Biology. 2008;11:436–442. doi: 10.1016/j.pbi.2008.05.003. [DOI] [PubMed] [Google Scholar]
- Welinder KG, Justesen AF, Kjaersgard IV, Jensen RB, Rasmussen SK, Jespersen HM, Duroux L. Structural diversity and transcription of class III peroxidases from Arabidopsis thaliana. European Journal of Biochemistry. 2002;269:6063–6081. doi: 10.1046/j.1432-1033.2002.03311.x. [DOI] [PubMed] [Google Scholar]
- Yuan S, Lin HH. Role of salicylic acid in plant abiotic stress. Zeitschrift fur Naturforschung c. 2008;63:313–320. doi: 10.1515/znc-2008-5-601. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.