Abstract
Sex is increasingly recognized as a major factor in the outcome of patients who have trauma and sepsis. Moreover, sex steroids influence chemokine/adhesion molecule expression and neutrophil accumulation. Heat shock proteins, heat shock factor 1, and peroxisome proliferator–activated receptor γ coactivator 1 are regulated by the estrogen receptors and consequently contribute to organ protection after trauma-hemorrhage. Additionally, sex steroids regulate inflammatory cytokines, leading to increased morbidity and mortality. This article deals with trauma-hemorrhage and examines the following: 1) the evidence for sex differences; 2) the mechanisms by which sex hormones affect organ protection; 3) the tissue-specific effect of sex hormone receptors; and 4) the effect of genomic and nongenomic (i.e. membrane-initiated steroid signaling) pathways of sex hormones after trauma. The available information indicates that sex steroids modulate cardiovascular responses after trauma. Thus, alteration or modulation of the prevailing hormone milieu at the time of injury seems to be a novel therapeutic option for improving outcome after injury.
Keywords: Sex, shock, estrogen receptors, androgen receptors
INTRODUCTION
Severe hemorrhage, which often occurs with trauma, is known to produce many life-threatening sequelae. Numerous advances in the past have improved short-term survival of patients in intensive care units; however, despite these advances, sepsis and multiple organ failure still remain as the leading causes of morbidity and mortality in severely injured patients who survive the initial trauma (1–24). It is well known that sex is increasingly recognized to be a major factor in the outcome of patients after stress conditions such as trauma and sepsis (1, 2, 4–6, 25–32). Findings from both clinical studies and experimental models suggest that women are more tolerant to major injuries than men (1, 2, 4–6, 25–28). Several lines of evidence indicate that men and women respond differently to trauma. In this regard, findings from clinical studies suggest that premenopausal women have a lower incidence of infection, pneumonia, sepsis, and multiple organ failure than men after trauma (33, 34). In our retrospective analysis of blunt trauma patients (26, 35), we found that male patients had a significantly higher risk of death compared with premenopausal female patients. Furthermore, sex differences have been noted in organ function, and the potential reasons for the differences have been the subject of extensive research (5, 6, 36). This article provides a brief overview of potential use of novel therapeutic agents after trauma and a discussion of the possible mechanism of the salutary effects of sex hormones.
SEX DIMORPHISM IN TRAUMA/SEPSIS
Clinical studies
Clinical studies have shown that women have a lower incidence of pneumonia, sepsis, and multiple organ failure than men after trauma (37–39). In a study by Frink et al. (34) involving polytraumatized patients, women showed lower IL-6 and IL-8 levels, which were associated with less multiple organ dysfunction syndrome and sepsis. In another study, Wichmann et al. (40) showed that women developed sepsis less frequently than men during the clinical course in surgical intensive care units, although once sepsis developed, the mortality rate was the same for men and women. Consistent with these findings, Offner et al. (38) reported that male sex is associated with an increased risk of major infection after trauma. Some studies reported a benefit only in women older than 50 years (35), whereas others showed a benefit only in women younger than 50 years (37, 41). On the other hand, although men have been reported to have a higher incidence of pneumonia, which is described as the most common infection after severe trauma (39), an established diagnosis of pneumonia was associated with higher mortality in women (42). In another study, female sex predicted increased mortality in critically ill surgical patients with documented infection and after certain elective or emergency surgical procedures (43). Some investigators suggest that there is no difference between the sexes in mortality after trauma (42, 44), whereas other studies demonstrated a higher mortality rate in women older than 80 years compared with age-matched men (43). A recent study by Deitch et al. (45) involving more than 4,000 patients showed that hormonally active women tolerate shock-trauma better than men. Although most studies demonstrated an advantage for women, a definitive answer regarding the role of sex in the outcome after trauma and sepsis remains controversial. The reasons for the differences in results are several and include lack of knowledge of the hormonal status of the patient at the time of injury.
Croce et al. (46) found no sex differential for penetrating or blunt trauma patients. In addition, they separated blunt trauma patients into those 40 years or younger or older than 50 years and found no sex-related mortality difference. After a similar analysis, the data from George et al. (26) showed a statistically significant sex difference in the 50 years or younger age group, that is, male patients had a 2.5 times higher risk of death than women after trauma.
Another reason for the different results may be that the hormonal status of the victims was not accounted for at the time of injury, and thus, women in different phases of the menstrual cycle (i.e. with different circulating 17β-estradiol [E2] levels) were included. This may explain why similar findings are not observed in different studies. More precise patient grouping by cycle status would allow a clearer understanding of the hormone-related relationship to outcome. In addition, future clinical trials should include data with respect to oral contraception, hormone replacement therapy, or surgical history. Such factors also affect the hormone pattern in an individual and for similar reasons as cycle status; this information may provide a clearer understanding of a hormone pattern that is beneficial in a particular circumstance of trauma. Thus, more clinical studies that include hormone measurement at the time of injury are needed to understand the role of sex and sex steroids in posttrauma pathogenesis.
Animal studies
In contrast to findings in humans, animal studies have consistently found advantages for women under stressful conditions. Ovariectomized women have worse cardiac functional recovery after trauma-hemorrhage (T-H), which was markedly improved by the administration of E2 (47). Furthermore, women in the proestrus state (with highest E2 levels during the estrus cycle) showed normal cardiac and pulmonary function after T-H and resuscitation (48, 49). In contrast, women in diestrus state (the lowest E2 levels during the estrus cycle) had depressed cardiac and hepatic function similar to men after T-H (50). The beneficial effects of E2 were also evident in men that were treated with E2 as a therapeutic modality (5, 6, 51). Moreover, E2 maintained cardiovascular and liver functions under stress conditions (5, 52). Studies have also shown that administration of progesterone after T-H in sex steroid–deficient women improved cardiovascular functions (53). Because castration (51) or androgen receptor antagonism (25, 54) improved/restored organ functions after T-H, a pivotal role for the male sex hormone androgen has been suggested in producing the depression in organ functions after T-H (6).
The animal model of trauma and hemorrhage provides a basis for experimental investigation of the immune and physiologic responses to a controlled insult. However, the model may more closely resemble a clinical situation of penetrating, rather than blunt trauma, because there is a lack of diffuse tissue injury. Accordingly, it must be pointed out that the model yields only soft tissue injury without direct organ injury. Although exceedingly useful for the study of a soft tissue injury response (without internal organ damage) and potential therapeutic interventions, the animal model used may not adequately represent the clinical picture of either blunt or complex penetrating trauma. Similarly, differences in trauma patients must be considered: blunt- and penetrating-injured patients may vary with respect to comorbidities, injury patterns, and treatment schemes. Therefore, a clear definition of the patient population to which laboratory findings can be applied is necessary to bring the laboratory findings into the clinical arena in both a safe and efficient manner.
During menopause, luteinizing hormone and follicle-stimulating hormone levels increase to those well greater than premenopausal levels; estradiol and estrone levels decrease; and, to a lesser extent, as a function of age, androstenedione and testosterone levels decline, characterizing adrenopause (55). The murine model of T-H does not provide insight into the effects of the hormonal fluctuations associated with human menopause because female mice cycle their life span and do not experience menopause. In addition, human postmenopausal prolactin pulsatile frequencies and levels resemble those of men, rather than those of premenopausal women (56). Therefore, the beneficial effects of prolactin described by Zellweger et al. (57) may contribute to the mortality differences observed in blunt trauma patients, and the presumed E2 effect may be a marker for premenopausal prolactin levels and pulsatile frequencies. Unfortunately, a clinical determination of the importance of prolactin would be complicated by the pulsatile frequency and diurnal variations, highlighting the importance of laboratory investigations.
It should be noted that similarities and differences exist between the clinical and laboratory data, suggesting that the patient hormonal milieu may affect outcome. Additional research is needed to clarify the patient subpopulations for which the sex differential is most evident; however, prospective studies in which cycle status, medical history, and surgical history are recorded will provide the basis for potential intervention should the hormonal status impart a clinically apparent difference in outcome. Alternatively, it would seem that it may be easier to administer E2 to patients in the emergency department and determine if the incidence of organ dysfunction, complications, and length of hospital stay decrease when E2 is used as an adjunct to treatment.
SEX STEROID HORMONES/RECEPTORS AND ORGAN FUNCTION
The functions of vital organs such as heart, liver, lung, and intestine are compromised after T-H in men but not in proestrus women. Studies have shown that cardiac function, as determined by cardiac output, stroke volume, contractility, and total peripheral resistance, was markedly depressed after T-H in men and women in estrus, metestrus, and diestrus phases, and in ovariectomized women, despite fluid resuscitation. However, these parameters were maintained in proestrus women after T-H (4, 58). Furthermore, administration of E2 in men and ovariectomized women protected cardiac function after T-H (4). Similarly, T-H produces lung, liver, and intestinal tissue edema within a few hours after injury, and, like cardiac function, tissue edema was not observed in proestrus women or in men treated with E2 after T-H (59, 60). Additional findings indicate that an increase in lung myeloperoxidase (MPO) activity, neutrophil chemokines (e.g. cytokine-induced neutrophil chemoattractant [CINC] 1, CINC-3], and intercellular adhesion molecule 1 (ICAM-1) expression was observed in the diestrus, estrus, and ovariectomized animals (48). The maintenance of lung inflammatory markers after T-H in proestrus females was associated with the highest levels of E2, whereas all other stages of the estrus cycle had significantly lower plasma E2 levels (4, 48, 58). Although E2 levels were relatively higher in females in estrus and metestrus cycles compared with the levels seen in ovariectomized females, the finding of increased lung injury markers in those animals suggests that E2 levels in the estrus and metestrus cycles were not sufficient to attenuate lung injury after T-H. Thus, female hormones are, at least in part, responsible for improving/maintaining organ functions after T-H. 17β-Estradiol seems to be a causative factor in the maintenance of organ function both in males and females after T-H, and, thus, its administration in males and ovariectomized or postmenopausal females after T-H should be helpful in preventing organ dysfunction under such conditions. Because only a single dose of E2 is recommended for the treatment of T-H, one would not expect any ill/side effects of this treatment. In this regard, prolonged hormone replacement therapy has been suggested to cause breast cancer and endometrial cancer and increases the potential of coagulation (61). Such adverse effects would not be expected from administration of a single dose of E2. Although clinical studies dealing with E2 in trauma patients have not been conducted so far, we hope to perform such studies in the near future.
Sex hormone–mediated effects are primarily mediated by their receptors. This concept was supported by studies that showed that coadministration of E2 with ICI 182,780, a selective estrogen receptor (ER) antagonist, abolished the salutary effects of E2 on cardiac function after T-H (47). Moreover, progesterone-mediated cardioprotection was associated with increased progesterone receptor activity in the left ventricle and increased circulating blood volume after T-H (53). Administration of flutamide, an androgen receptor antagonist, restored cardiac and hepatic functions and decreased intestinal neutrophil infiltration after T-H (5, 25, 62). Flutamide administration after T-H also increased cardiac E2 levels and ER expression through up-regulation of aromatase activity, which converts testosterone to E2 (25). It has also been reported that E2 causes rapid stimulation of endothelial NO production in an ER-dependent manner in human endothelial cells (63) and fetal pulmonary artery endothelium (64).
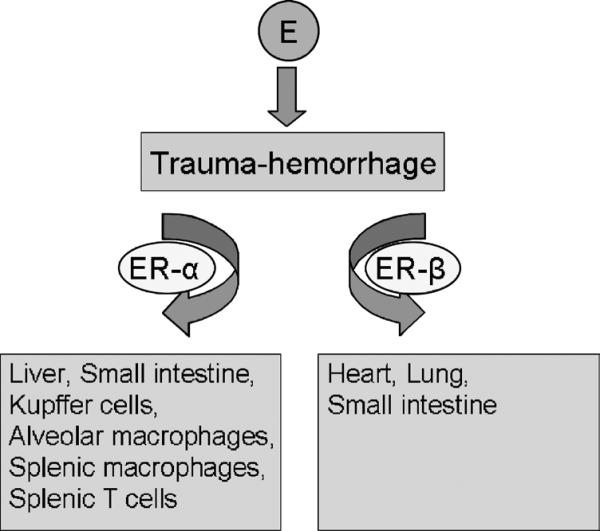
ER-α reduced inflammatory response in liver and small intestine, and ER-β attenuated inflammatory response in lung and small intestine
Action of E2 is mainly mediated by two ERs designated ER-α and ER-β (2, 27). Previous studies have shown tissue-specific expression of subtypes of ERs in different tissues (65). For example, the liver was found to be rich in ER-α, whereas the lung was found to be rich in ER-β. Alternatively, the intestine is rich in both ER-α and ER-β. Recent studies have shown the role of ERs in E2-mediated protection of various organ functions after T-H. These studies used ER-α– and ER-β–specific agonists propylpyrazole triol (PPT) and diarylpropionitrile (DPN). Propylpyrazole triol is a selective agonist for the ER-α subtype and is the most potent agonist for ER-α of a series of tetrasubstituted pyrazole analogs (66). Propylpyrazole triol binds to ER-α with high affinity, displaying 410-fold binding selectivity over ER-β (58). Diarylpropionitrile, on the other hand, acts as an agonist on both ER subtypes but has a 70-fold higher relative binding affinity and 170-fold higher relative estrogenic potency in transcription assays with ER-β than ER-α (59). The liver, small intestine, and lung are considered critical organs in the development of delayed organ dysfunction in patients who have traumatic injuries and severe blood loss (67). Multiple organ dysfunction or failure secondary to a systemic inflammatory response remains the major cause of mortality and morbidity after trauma (68). Neutrophils are the principal cells involved in host defense against acute bacterial and fungal infections (69), and, thus, these cells have a protective effect. However, under low flow conditions, the infiltration of these cells may cause tissue damage (70). Neutrophil movement and migration are mediated by multiple adhesion molecules on the neutrophils and endothelial cell surfaces and chemotactic factors. Among adhesion molecules, ICAM-1 is an important mediator in the firm adhesion of neutrophils to the vascular endothelium and is markedly up-regulated after T-H (71).
With regard to chemokines, rat CINC-1 and CINC-3 are members of the IL-8 family and are potent chemotactic factors for neutrophils. Chemotaxis of neutrophils is an important functional response to chemokines and is a key event in the recruitment of neutrophils in inflammation. Using CINC antibodies, studies have shown that CINC-1 and CINC-3 contribute significantly to the influx of neutrophils in rat inflammation models, including lung injury (72) and LPS-induced inflammation (73). Additional studies indicate that CINC-1 levels correlated with tissue MPO activity (an index of neutrophil infiltration) after T-H (48, 60). Studies have also indicated that E2-induced reduction of MPO activity, chemoattractants CINC-1 and CINC-3, and ICAM-1 after T-H are mediated via ER-α activation in the liver, via ER-β activation in the lung, and via both ER-α and ER-β in the small intestine (60). Those findings are consistent with ER mRNA expression in the liver, small intestine, and lung (i.e. ER-α mRNA expression is highest in the liver, and ER-β mRNA expression is greatest in the lung) (60). Thus, such differences in distribution of ER subtypes in various tissues contribute to the selective role of ER-α or ER-β in different tissues (65). Furthermore, administration of the ER-α agonist PPT attenuated hepatic injury and decreased the expression of the nuclear factor–κB (NF-κB), activating protein 1 (AP-1), and iNOS in the liver after T-H (74). The iNOS is significantly up-regulated in the liver after hemorrhagic shock and is thought to be one of the major contributors of hepatic injury after hemorrhagic shock or sepsis (75). Furthermore, a positive correlation between hepatic injury and increased iNOS expression has been shown (74). The production of proinflammatory mediators is regulated by NF-κB and AP-1 (76). A recent study has demonstrated cross talk between ER and NF-κB at several levels (77). Estrogen receptor also activates transcription at alternative sites such as AP-1 (78). Administration of DPN, on the other hand, attenuated T-H–mediated increase in protein concentration, lactate dehydrogenase activity, nitrate/nitrite, and IL-6 levels in bronchoalveolar fluid and iNOS expression, nitrate/nitrite, and IL-6 levels in the lung (36). In addition, it seems that the salutary effects of E2 on attenuation of iNOS expression and NO production in the lung are receptor dependent. Support for this suggestion comes from the study that showed that administration of E2 with ER antagonist ICI 182,780 abolished the salutary effects of E2 in the lung (79). Another study provided further evidence that after T-H, E2-induced attenuation of lung injury was mediated via ER-β activation (36).
iNOS has been previously shown to be overexpressed in rodent lung after hemorrhagic shock (80). Hierholzer et al. (81) have also reported that iNOS inhibition resulted in a marked reduction of lung injury produced by hemorrhagic shock. These findings support the view that an enhanced formation of NO from iNOS plays an important role in producing lung injury after hemorrhagic shock (81).
ER-β–Mediated cardiac protection by up-regulation of heat shock proteins
Studies have also shown that DPN improved cardiac function and increased heat shock proteins (HSPs) 32, 60, 70, and 90, and heat shock factor 1 (HSF-1) DNA binding activity in the heart after T-H (82). 17β-Estradiol has been reported to provide protection against vascular injury even in mice in which ER-α has been disrupted (83). Moreover, the expression of ER-β, but not of ER-α, is stimulated after vascular injury in male rats (84). Furthermore, studies using ER-α or ER-β knockout mice suggest that ER-β plays a role in cardioprotection after I/R (85). The HSPs are an important family of endogenous, protective proteins. In this regard, HSP70 is induced by brief ischemia, and overexpression of HSP70 protects cells and tissues against various forms of stress (86). Conversely, underexpression resulting from treatment with antisense oligonucleotides to HSP70 increases susceptibility to hypoxia and reoxygenation injury (87). Overexpression of other HSPs, including HSP32 and HSP60, is also reported to be protective against cardiac injury (88). Heat shock protein synthesis is controlled by a family of transcription factors, the HSFs. Four HSFs have been identified, but only HSF-1 has been shown to regulate the expression of HSPs in response to ischemia, hypoxia, heat, stretch, or injury (89). Heat and hypoxia activate HSF-1, which is present in the cytoplasm in an inactive, monomeric form. With stress, trimerization and phosphorylation occur, after which HSF-1 migrates to the nucleus. In the nucleus, HSF-1 binds to the heat-shock element, which is present in the promoter of the stress response gene, and then initiates HSP transcription and synthesis. Heat shock protein 90 is known to bind to intracellular steroid receptor, including the ERs (90). It has also been suggested that HSP90 complexes with HSF-1 in cardiomyocytes (90). Interactions involving HSP90 and ERs and the binding between HSP90 and HSFs thus represent an important element in the activation of HSF-1 by E2 (90). Several studies have examined the effects of E2 and sex on cardiac HSP expression (90, 91). Those studies have shown that female rat hearts have twice as much HSP70 as male hearts (91). Ovariectomy reduced the level of HSP70 in female hearts, and this could be prevented by E2 administration (91). Additional studies showed that 10 h of E2 treatment doubled the level of HSP70 in adult cardiomyocytes from male rats (90). Consistent with these findings, other studies have observed overexpression of heart HSP32 after T-H with E2 administration (88). Up-regulation of HSP synthesis is considered to be a powerful physiological, endogenous route for protecting crucial cellular homeostatic mechanisms against deleterious external factors. Physiological stresses ranging from myocardial ischemia to genetic mutations produce a disease state in which protein damage and misfolded protein structures are a common denominator (92). Multiple endogenous pathways are involved in restoring cellular homeostasis, but one well-characterized mechanism that involves protein folding is the heat shock family of stress proteins, that is, HSPs (75, 93). There are several potential mechanisms by which HSPs produce cardioprotective effects. Heat shock proteins are generally thought to be useful in correcting the folding of many proteins and restoring their functional structures (93). Moreover, HSPs target denatured proteins to the lysosome for degradation as molecular chaperones (93). These functions of HSPs as molecular chaperones play important roles in maintaining the normal cell functions and promoting cell survival. Heat shock proteins are also known to regulate the process of programmed cell death/apoptosis. One major pathway of apoptosis involves the release of cytochrome c from mitochondria. Cytochrome c, in turn, binds to a protein known as apoptosis protease activator protein 1 (Apaf-1) and triggers its oligomerization. This complex then attracts the inactive unprocessed proform of the proteolytic enzyme caspase 9, which is then cleaved to its active form, thereby initiating apoptosis. Heat shock proteins have been shown to inhibit this process at various points. In this regard, HSP90 has been shown to bind to Apaf-1 and prevent its binding to cytochrome c (94). Furthermore, HSP70 prevents oligomerized Apaf-1 from recruiting procaspase 9 (95). Studies have also suggested an antiapoptotic role of HSP60 (96, 97). Over-expression of HSP60 inhibits myocardial apoptosis in response to ischemic injury (96). Furthermore, a recent study has shown that reducing HSP60 expression with antisense oligonucleotides is associated with an increase in Bax and a reduction in Bcl-2, which induces apoptosis of cardiomyocytes (97). These findings raise the possibility that HSP60 may regulate apoptosis through modulation of the Bcl-2 family (97). In addition, HSP90 has been shown to bind to eNOS and stimulate its activity (98). Thus, the HSPs protect cells via multiple mechanisms that target key cellular components and regulatory processes. A previous study suggests that E2-mediated restoration of cardiac function after T-H is due in part to ER-dependent up-regulation of peroxisome proliferator–activated receptor (PPAR) γ coactivator 1 (PGC-1α) (2). The nuclear coactivator PGC-1α, known for its role in cellular metabolism, regulates a number of genes required for lipid metabolism and adenosine triphosphate (ATP) production by activating transcription factor PPAR-α and mitochondrial transcription factor A (Tfam), respectively (5). It is well known that lipids produce ATP through mitochondrial fatty acid β-oxidation. The PPAR-α regulates genes involved in lipid transport and mitochondrial fatty acid β-oxidation, including FAT/CD36, and medium-chain acyl-coenzyme A dehydrogenase (99). The Tfam transactivates mitochondrial DNA (mtDNA)–encoded gene cytochrome c oxidase subunit I that is required for mitochondrial ATP production (5). Studies have shown that DPN treatment also attenuated the decrease in cardiac mitochondrial ATP, abrogated the T-H–induced lipid accumulation, and normalized PGC-1α, Tfam, and cytochrome c oxidase subunit I after T-H (2). Likewise, it has been reported that PGC-1α expression can be induced by transcription factor CREB (cyclic adenosine monophosphate response element binding). Peroxisome proliferator–activated receptor γ coactivator 1 mRNA levels were reduced in the CREB knockout mice, and sequence analysis of the mouse PGC-1α promoter reveals a full consensus CREB binding site (100). Studies have also shown that E2 increased the enhancer activity of CREB binding and CREB protein levels (101). Moreover, the effects of E2 are through both ER-α and ER-β to increase CREB phosphorylation (102). Furthermore, ER antagonist ICI 182,780 was able to completely block the increase in CREB phosphorylation induced by E2 in hippocampal cell line (102). However, it remains to be determined whether ER-β–mediated PGC-1α up-regulation after T-H is through CREB phosphorylation. Furthermore, it has long been thought that sex hormone receptors, including ERs, are localized in the cytoplasm and nucleus of the cell. However, there is evidence indicating that ERs are also localized in mitochondria, and they enhance the level of mtDNA-encoded transcription directly (103). A recent study has shown that E2 enhances the mitochondrial level of ERs and increases the transcriptional levels of several mtDNA-encoded genes required for mitochondrial respiratory complex (MRC) proteins and MRC activity (103). These observations suggest that mtDNA-encoded MRC could be a direct target for E2 action in the mitochondrial ERs. Studies have also examined the role of mitochondria in E2-mediated cardioprotection after T-H (27). In those studies, rats received PPT, DPN, or E2 after T-H, and the effects of these treatments were examined on mitochondrial ER (mtER) α, mtER-β, mitochondrial estrogen response element-binding activity, and mtDNA-encoded genes for MRC-I and MRC-IV proteins (27). To determine the role of MRC-IV in DPN-mediated cardioprotection, a group of DPN-treated rats was cotreated with MRC-IV inhibitor sodium cyanide. The results showed that DPN or E2 treatment after T-H normalized cardiac mtER-β expression and increased mtER-β DNA binding activity. This was accompanied by an increase in MRC-IV gene expression and activity; MRC-I gene expression remained unchanged. Inhibition of MRC-IV in DPN-treated T-H rats by sodium cyanide abolished the DPN-mediated cardioprotection, ATP production, mitochondrial cytochrome c release, caspase 3 cleavage, and apoptosis (27). Thus, E2- and ER-β–mediated cardioprotection after T-H seems, at least in part, to be mediated via mtER-β–dependent MRC-IV activity and inhibition of mitochondrial apoptotic signaling pathways.
ER-α prevented the increase in Kupffer cell cytokine production and mitogen-activated protein kinase activation
Mitogen-activated protein kinases (MAPKs) are signaling molecules that are important in immune response (104). There are three major MAPK-dependent pathways: p38, extracellular-regulated protein kinase 1/2, and c-Jun NH2-terminal kinase. All three MAPK families are activated by dual phosphorylation on both adjacent threonine and tyrosine residues. The MAPK families are involved in the expression of proinflammatory and anti-inflammatory cytokines, including IL-6, TNF-α, and IL-10, via inducing the activation of a number of transcription factors (1). Kupffer cells are the largest tissue macrophage population in the body. Previous studies have shown that Kupffer cells are the major source of IL-6, TNF-α, and IL-10, and contribute to the increased circulatory levels of these cytokines (105). The increased circulatory cytokines were associated with immune cell depression and an increased susceptibility to sepsis (6). Therefore, Kupffer cells seem to play a key role in producing the immunosuppression after T-H. Previous studies have demonstrated that proestrus female animals, with high circulatory levels of E2, do not show increased cytokine production by Kupffer cells, and they have normal immune functions compared with male mice after T-H (6). Studies have also shown that administration of a single dose of E2 after T-H improves macrophage and lymphocyte functions (106, 107). Blockade of ERs by administration of EM-800, however, abolished the salutary effects of E2 (108), suggesting that the salutary effects of E2 are mediated via ERs. Another study showed that cytokine production increased after T-H; however, PPT or E2 administration after T-H normalized Kupffer cell cytokine production (109). Although DPN attenuated increased production of these cytokines, Kupffer cell capacity to produce the cytokines remained significantly higher than sham. Propylpyrazole triol or E2 also prevented T-H–induced activation of MAPKs in Kupffer cells (109). However, DPN did not prevent MAPK activation. Because PPT administration after T-H was more effective in decreasing Kupffer cell cytokine production and MAPK activation than DPN, it seems that the salutary effects of E2 on Kupffer cell functions are mediated predominantly via ER-α and normalization of MAPK after T-H.
ER-α–Mediated macrophages and immune cell function
Other findings indicated that IL-6 and TNF-α production by splenic macrophages, alveolar macrophages, and peripheral blood mononuclear cells decreased after T-H (110, 111). This was accompanied by suppression of the phosphorylation of p38, c-Jun NH2-terminal kinase, and extracellular-regulated protein kinase 1/2. In contrast to cytokine production and MAPK activation, the DNA binding of NF-κB was increased after T-H. Administration of the ER-α agonist PPT or E2 after T-H restored the production of IL-6 and TNF-α by splenic macrophages to the levels observed in sham-operated animals. Moreover, PPT or E2 administration also prevented the suppression of MAPK activity and the activation of NF-κB in macrophages. Although administration of ER-β agonist DPN attenuated the decrease in macrophage cytokine production, the levels were not restored to those observed in sham-operated animals. In addition, DPN administration had no effect on the activation of MAPKs and NF-κB. These findings suggest that the effects of E2 on macrophages are mediated predominantly via ER-α. As for T cells, studies indicated that ER-α plays a predominant role in mediating the salutary effects of E2 on T cells after T-H, and that such effects are likely mediated via normalization of MAPK, NF-κB, and AP-1 signaling pathways (112). Thus, ER subtypes have tissue compartment–specific roles in mediating the protective effects of E2 on organ and immune cell functions after T-H (Fig. 1).
Fig. 1. Tissue-specific role of estrogen in various organs and immune cells after trauma-hemorrhage.
Protective effects of estrogen after trauma-hemorrhage are mediated via ER-α in the liver, Kupffer cells, alveolar macrophages, splenic macrophages, and splenic T cells, and via ER-β in the heart and lung. Tissue-specific effect of estrogen is not found in the small intestine.
GENOMIC AND NONGENOMIC EFFECTS
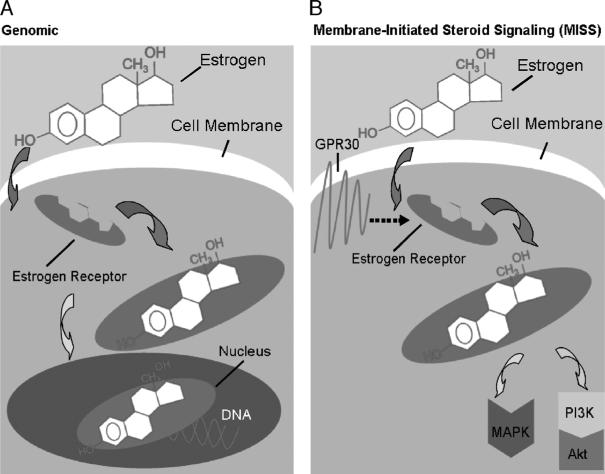
Sex steroid–mediated effects were suggested to be regulated by two different mechanisms, genomic and nongenomic (recently termed “membrane-initiated steroid signaling” [MISS]) (113, 114). Sex hormones such as E2 and androgen have traditionally been described as transcription factors, acting within the nucleus of target cells to regulate the transcription of E2- or androgen-sensitive genes (115). However, new information has been identified regarding signal transduction via ERs that does not involve transcriptional gene regulation (116–118). Furthermore, there is growing evidence that ERs can also mediate signaling cascades at the cell membrane and in the cytoplasm (119). Given that the rapidity of activation makes modulation of gene transcription less likely, and that the effects are not blocked by inhibitors of protein or RNA synthesis, these extranuclear mechanisms are commonly referred to as nonnuclear, nongenomic, or MISS effects of E2 (114, 119). As shown in Figure 2A, the mechanisms of the genomic pathway require an interaction between the nuclear ERs with HSF-1 and HSP90, which in turn regulate HSP70, HSP60, and HSP32 gene expressions (82). Moreover, ERs also interact with PGC-1α, which is a key regulator of ATP production and regulates a number of genes required for mitochondrial function (2, 5). Nuclear transcription of E2-regulated genes is also influenced by other factors that contribute to normalized cell functions after T-H and sepsis (5, 36, 52, 60, 120). Thus, it can be suggested that the underlying mechanism by which E2 regulates gene expressions is via a nuclear (genomic) action of ERs.
Fig. 2. A, Estrogen-mediated genomic action.
When an estrogen molecule binds to an estrogen receptor, the hormone-receptor complex then moves to the nucleus, where it binds to DNA and initiates transcription. B, Estrogen-mediated nongenomic action, membrane-initiated steroid signaling. In the case of the nongenomic, that is, MISS estrogen signaling pathway, estrogen receptors are located in the cell membrane. Their activity is linked to the PI3K/Akt or MAPK pathway, resulting in a rapid, nonnuclear effect, and is associated with the novel growth factor GPR30.
Nongenomic effects occur through classic ER-α and ER-β located at the plasma membrane (121) or through a recently described novel membrane–associated ER, G protein–coupled receptor 30 (GPR30) (122) (Fig. 2B). To exclude the influence of genomic events mediated by the nuclear ERs, investigators often use membrane-impermeable conjugates of E2 such as E2 conjugated to bovine serum albumin (BSA; E2-BSA) (117, 118, 123). Several lines of evidence indicate that E2-BSA acts only on membrane ERs. First, by using a fluorescent form, E2-BSA–fluorescein isothiocyanate (FITC; E2-BSA conjugated to FITC), E2-BSA has been shown with confocal (124) and regular microscopy (121) to bind only to the plasma membrane even after a prolonged (40 min) incubation that far exceeded the application time sufficient for achieving rapid E2 actions (125). Second, this membrane binding is due specifically to E2 because BSA-FITC devoid of E2 does not bind to the plasma membrane (124). Third, E2 binds to membrane ERs because 1) no binding was observed in cells devoid of ERs (121); and 2) the binding of E2-BSA-FITC to plasma membrane was blocked by ER antagonist ICI 182,780 (121). There is, however, evidence that E2-BSA does not bind to classical ERs (126). Finally, E2-BSA does not always act like E2 (127), indicating that direct E2/ER functions have been excluded.
The phosphatidylinositol 3-kinase/Akt pathway mediated the nongenomic effects of estrogen in the heart and small intestine
Nongenomic activation of the phosphatidylinositol 3-kinase (PI3K)/Akt signaling cascade by E2 has been observed in multiple cell types (128, 129). Furthermore, studies have shown that activation of the PI3K pathway protects organs or cells against I/R injury and hypoxia through suppression of the cell death machinery (130). One of the downstream targets of the PI3K pathway is the serine/threonine [kinase] Akt (129, 131, 132). Previous studies have suggested that an increase in Akt activity improved left-ventricle contractile recovery after transient ischemia (133). Recent studies using E2-BSA have suggested that E2-mediated cardioprotection and attenuation of intestinal inflammation are mediated via a nongenomic pathway after T-H (117, 118). Moreover, the biologic effects of E2-BSA are receptor-dependent because administration of a selective ER antagonist ICI 182,780 along with E2-BSA abolished the E2-BSA–induced cardioprotection and attenuation of intestinal injury after T-H. The findings further indicated that the PI3K/Akt pathway plays a major role in mediating the nongenomic effects of E2. Phosphatidylinositol 3-kinase/Akt has been reported to play an important role in the cell survival pathway (134). Studies have also reported that PI3K/Akt signal has an antiapoptotic activity through different mechanisms, for example, by phosphorylation of Akt, it induces Bcl-2-associated death promoter (BAD) phosphorylation and thus inhibits its translocation into the mitochondria and binding to Bcl-2 (134). Another study has shown that E2-BSA induced Akt phosphorylation and prevented DNA fragmentation in cardiomyocytes (117). These findings were further confirmed by using PI3K inhibitor Wortmannin, which inhibited the E2-BSA–mediated protective effects on cardiomyocyte apoptosis. Phosphatidylinositol 3-kinases constitute a family of evolutionarily conserved lipid kinases that regulate a vast array of fundamental cellular responses, including proliferation, protection from apoptosis, superoxide production, cell migration, and adhesion (135, 136). These responses result from the activation of membrane-trafficking protein such as the serine/threonine [kinase] Akt. Another study indicated that at 2 h after T-H, intestinal MPO activity, IL-6, ICAM-1, CINC-1, and CINC-3 levels were markedly increased in male rats (118). Administration of a single dose of E2-BSA during resuscitation attenuated the increase in those inflammatory markers. Administration of E2-BSA also prevented the T-H–induced decrease in intestinal PI3K expression and Akt activity. Administration of ER antagonist ICI 182,780 or the PI3K inhibitor Wortmannin along with E2-BSA after T-H prevented the E2-BSA–induced effects previously cited. These studies collectively suggest that the salutary effects of E2-BSA seem to be mediated via ERs and up-regulation of the PI3K/Akt pathway. Understanding the exact mechanism by which E2-BSA induces Akt activation is essential for understanding the nongenomic protective effects of E2-BSA after T-H. It is quite possible that there are two mechanisms for the activation of Akt by E2-BSA. First, studies have demonstrated that activation of growth factors plays an important role in the PI3K pathway, which in turn activates Akt. It is therefore possible that E2-BSA treatment could activate certain growth factors (126). Second, the E2 receptor has been shown to activate PI3K activity by association with the p85α regulatory subunit of PI3K in a ligand-dependent manner (137). Despite this information, additional studies are needed to clarify the exact mechanism by which E2-BSA produces its effects on the PI3K/Akt pathway. However, it should be noted that compared with E2 treatment, the improvement/restoration of cardiac function and attenuation of intestinal injury after E2-BSA treatment were not complete. This finding indicates that surface and nuclear ERs are required for the full action of E2 and, therefore, for the complete restoration of cardiac function after T-H. Thus, although the genomic and nongenomic actions of E2 can be segregated, it should be noted that both actions are interdependent, and that genomic and nongenomic pathways work synergistically in the regulation of organ functions.
MAPK mediated nongenomic effects of estrogen on macrophages and Kupffer cells
Studies have also shown that E2 and E2-BSA induced the activation of MAPK signaling in neuron and endothelial cells, suggesting that E2 regulates activation of MAPKs through nongenomic pathway (138, 139). Because activation of MAPKs is implicated in the regulation of inflammatory responses after trauma and sepsis, it is likely that MAPKs play an important role in E2-mediated immunoprotection after T-H (1, 109, 140). Previous studies have shown that estrogen-mediated activation of the nongenomic pathway improves macrophage cytokine production after T-H (141). Additional findings further indicated that the MAPK pathway plays a major role in attenuating Kupffer cells and splenic macrophage alterations of IL-6, TNF-α, and IL-10 production, and transcription factor (NF-κB and AP-1) activation after T-H (141). In addition, recent findings have shown that E2 uses GPR30 in activating the PKA pathway in isolated hepatocytes and attenuating hepatic injury after T-H (123). However, more studies are needed to fully understand the role of GPR30 in E2-mediated effects on organ function after T-H.
CONCLUSIONS
There is increasing evidence that sex hormones maintain organ function after trauma or sepsis. Studies have examined the role of E2 in posttrauma pathogenesis. The findings indicate that T-H impairs the function of many organs. Administration of E2 after T-H as an adjunct to fluid resuscitation normalizes organ function. The findings also indicate that there are two major receptors, ER-α and ER-β, which mediate E2 actions. Studies have shown that tissue-specific expression of subtypes of ER mediate the protective effects of E2 on organ function after T-H. Estrogen receptors are widely located in the cytoplasm and on the nuclear membrane, but there is evidence that ERs are also found on the plasma membrane. In addition, G-protein, particularly GPR30, is implicated in mediating E2 action. Thus, alteration or modulation of the prevailing hormonal milieu at the time of injury seems to be a novel therapeutic option for improving cardiovascular function under those conditions. However, this complex network needs additional elucidation in future experimental studies and clinical trials (142–147).
ACKNOWLEDGMENT
The authors also thank Bobbi Smith for help and assistance in preparing this article.
This work was supported by the National Institutes of Health (grant nos. R37 GM39519 and R01 GM37127).
REFERENCES
- 1.Kher A, Wang M, Tsai BM, Pitcher JM, Greenbaum ES, Nagy RD, Patel KM, Wairiuko GM, Markel TA, Meldrum DR. Sex differences in the myocardial inflammatory response to acute injury. Shock. 2005;23:1–10. doi: 10.1097/01.shk.0000148055.12387.15. [DOI] [PubMed] [Google Scholar]
- 2.Hsieh YC, Choudhry MA, Yu HP, Shimizu T, Yang S, Suzuki T, Chen J, Bland KI, Chaudry IH. Inhibition of cardiac PGC-1alpha expression abolishes ERbeta agonist–mediated cardioprotection following trauma-hemorrhage. FASEB J. 2006;20:1109–1117. doi: 10.1096/fj.05-5549com. [DOI] [PubMed] [Google Scholar]
- 3.Finnerty CC, Herndon DN, Chinkes DL, Jeschke MG. Serum cytokine differences in severely burned children with and without sepsis. Shock. 2007;27:4–9. doi: 10.1097/01.shk.0000235138.20775.36. [DOI] [PubMed] [Google Scholar]
- 4.Choudhry MA, Schwacha MG, Hubbard WJ, Kerby JD, Rue LW, Bland KI, Chaudry IH. Gender differences in acute response to trauma-hemorrhage. Shock. 2005;24:101–106. doi: 10.1097/01.shk.0000191341.31530.5e. [DOI] [PubMed] [Google Scholar]
- 5.Hsieh YC, Yang S, Choudhry MA, Yu HP, Rue LW, III, Bland KI, Chaudry IH. PGC-1 upregulation via estrogen receptors: a common mechanism of salutary effects of estrogen and flutamide on heart function after trauma-hemorrhage. Am J Physiol Heart Circ Physiol. 2005;289:H2665–H2672. doi: 10.1152/ajpheart.00682.2005. [DOI] [PubMed] [Google Scholar]
- 6.Angele MK, Schwacha MG, Ayala A, Chaudry IH. Effect of gender and sex hormones on immune responses following shock. Shock. 2000;14:81–90. doi: 10.1097/00024382-200014020-00001. [DOI] [PubMed] [Google Scholar]
- 7.Baue AE. A debate on the subject “Are SIRS and MODS important entities in the clinical evaluation of patients?” The con position. Shock. 2000;14:590–593. doi: 10.1097/00024382-200014060-00003. [DOI] [PubMed] [Google Scholar]
- 8.Bruhn A, Verdant C, Vercruysse V, Su F, Vray B, Vincent JL. Effects of dexamethasone on macrophage migration inhibitory factor production in sepsis. Shock. 2006;26:169–173. doi: 10.1097/01.shk.0000225416.27742.cb. [DOI] [PubMed] [Google Scholar]
- 9.Rossaint R, Cerny V, Coats TJ, Duranteau J, Fernandez-Mondejar E, Gordini G, Stahel PF, Hunt BJ, Neugebauer E, Spahn DR. Key issues in advanced bleeding care in trauma. Shock. 2006;26:322–331. doi: 10.1097/01.shk.0000225403.15722.e9. [DOI] [PubMed] [Google Scholar]
- 10.Moore EE, Moore FA, Harken AH, Johnson JL, Ciesla D, Banerjee A. The two-event construct of postinjury multiple organ failure. Shock. 2005;24(Suppl 1):71–74. doi: 10.1097/01.shk.0000191336.01036.fe. [DOI] [PubMed] [Google Scholar]
- 11.Holcomb JB, Jenkins D, Rhee P, Johannigman J, Mahoney P, Mehta S, Cox ED, Gehrke MJ, Beilman GJ, Schreiber M, et al. Damage control resuscitation: directly addressing the early coagulopathy of trauma. J Trauma. 2007;62:307–310. doi: 10.1097/TA.0b013e3180324124. [DOI] [PubMed] [Google Scholar]
- 12.Kobbe P, Vodovotz Y, Kaczorowski D, Mollen KP, Billiar TR, Pape HC. Patterns of cytokine release and evolution of remote organ dysfunction after bilateral femur fracture. Shock. 2008;30:43–47. doi: 10.1097/SHK.0b013e31815d190b. [DOI] [PubMed] [Google Scholar]
- 13.Tsujimoto H, Ono S, Efron PA, Scumpia PO, Moldawer LL, Mochizuki H. Role of Toll-like receptors in the development of sepsis. Shock. 2008;29:315–321. doi: 10.1097/SHK.0b013e318157ee55. [DOI] [PubMed] [Google Scholar]
- 14.Cotton BA, Guy JS, Morris JA, Jr, Abumrad NN. The cellular, metabolic, and systemic consequences of aggressive fluid resuscitation strategies. Shock. 2006;26:115–121. doi: 10.1097/01.shk.0000209564.84822.f2. [DOI] [PubMed] [Google Scholar]
- 15.Kamoun WS, Shin MC, Keller S, Karaa A, Huynh T, Clemens MG. Induction of biphasic changes in perfusion heterogeneity of rat liver after sequential stress in vivo. Shock. 2005;24:324–331. doi: 10.1097/01.shk.0000180618.98692.ee. [DOI] [PubMed] [Google Scholar]
- 16.Gauglitz GG, Song J, Herndon DN, Finnerty CC, Boehning DF, Barral JM, Jeschke MG. Characterization of the inflammatory response during acute and postacute phases after severe burn. Shock. 2008;30:503–507. doi: 10.1097/SHK.0b013e31816e3373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Murakami K, Enkhbaatar P, Yu YM, Traber LD, Cox RA, Hawkins HK, Tompkins RG, Herndon D, Traber DL. L-Arginine attenuates acute lung injury after smoke inhalation and burn injury in sheep. Shock. 2007;28:477–483. doi: 10.1097/shk.0b013e31804a59bd. [DOI] [PubMed] [Google Scholar]
- 18.Acosta JA, Hoyt DB, Schmid-Schonbein GW, Hugli TE, Anjaria DJ, Frankel DA, Coimbra R. Intraluminal pancreatic serine protease activity, mucosal permeability, and shock: a review. Shock. 2006;26:3–9. doi: 10.1097/01.shk.0000209557.31457.ae. [DOI] [PubMed] [Google Scholar]
- 19.Yu M, Wang H, Ding A, Golenbock DT, Latz E, Czura CJ, Fenton MJ, Tracey KJ, Yang H. HMGB1 signals through Toll-like receptor (TLR) 4 and TLR2. Shock. 2006;26:174–179. doi: 10.1097/01.shk.0000225404.51320.82. [DOI] [PubMed] [Google Scholar]
- 20.Wheeler DS, Lahni PM, Denenberg AG, Poynter SE, Wong HR, Cook JA, Zingarelli B. Induction of endotoxin tolerance enhances bacterial clearance and survival in murine polymicrobial sepsis. Shock. 2008;30:267–273. doi: 10.1097/shk.0b013e318162c190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Purcell EM, Dolan SM, Kriynovich S, Mannick JA, Lederer JA. Burn injury induces an early activation response by lymph node CD4+ T cells. Shock. 2006;25:135–140. doi: 10.1097/01.shk.0000190824.51653.32. [DOI] [PubMed] [Google Scholar]
- 22.Kawasaki T, Choudhry MA, Schwacha MG, Fujimi S, Lederer JA, Bland KI, Chaudry IH. Trauma-hemorrhage inhibits splenic dendritic cell proinflammatory cytokine production via a mitogen-activated protein kinase process. Am J Physiol Cell Physiol. 2008;294:C754–C764. doi: 10.1152/ajpcell.00494.2007. [DOI] [PubMed] [Google Scholar]
- 23.MacConmara MP, Maung AA, Fujimi S, McKenna AM, Delisle A, Lapchak PH, Rogers S, Lederer JA, Mannick JA. Increased CD4+ CD25+ T regulatory cell activity in trauma patients depresses protective TH1 immunity. Ann Surg. 2006;244:514–523. doi: 10.1097/01.sla.0000239031.06906.1f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Remick DG, Ward PA. Evaluation of endotoxin models for the study of sepsis. Shock. 2005;24(Suppl 1):7–11. doi: 10.1097/01.shk.0000191384.34066.85. [DOI] [PubMed] [Google Scholar]
- 25.Hsieh YC, Yang S, Choudhry MA, Yu HP, Bland KI, Schwacha MG, Chaudry IH. Flutamide restores cardiac function after trauma-hemorrhage via an estrogen-dependent pathway through upregulation of PGC-1. Am J Physiol Heart Circ Physiol. 2006;290:H416–H423. doi: 10.1152/ajpheart.00865.2005. [DOI] [PubMed] [Google Scholar]
- 26.George RL, McGwin G, Jr, Windham ST, Melton SM, Metzger J, Chaudry IH, Rue LW., III Age-related gender differential in outcome after blunt or penetrating trauma. Shock. 2003;19:28–32. doi: 10.1097/00024382-200301000-00006. [DOI] [PubMed] [Google Scholar]
- 27.Hsieh YC, Yu HP, Suzuki T, Choudhry MA, Schwacha MG, Bland KI, Chaudry IH. Upregulation of mitochondrial respiratory complex IV by estrogen receptor–beta is critical for inhibiting mitochondrial apoptotic signaling and restoring cardiac functions following trauma-hemorrhage. J Mol Cell Cardiol. 2006;41:511–521. doi: 10.1016/j.yjmcc.2006.06.001. [DOI] [PubMed] [Google Scholar]
- 28.Meldrum DR. Estrogen increases protective proteins following trauma and hemorrhage. Am J Physiol Regul Integr Comp Physiol. 2006;290:R809–R811. doi: 10.1152/ajpregu.00802.2005. [DOI] [PubMed] [Google Scholar]
- 29.Deitch EA, Feketeova E, Lu Q, Zaets S, Berezina TL, Machiedo GW, Hauser CJ, Livingston DH, Xu DZ. Resistance of the female, as opposed to the male, intestine to I/R-mediated injury is associated with increased resistance to gut–induced distant organ injury. Shock. 2008;29:78–83. doi: 10.1097/shk.0b013e318063e98a. [DOI] [PubMed] [Google Scholar]
- 30.Sperry JL, Friese RS, Frankel HL, West MA, Cuschieri J, Moore EE, Harbrecht BG, Peitzman AB, Billiar TR, Maier RV, et al. Male gender is associated with excessive IL-6 expression following severe injury. J Trauma. 2008;64:572–578. doi: 10.1097/TA.0b013e3181650fdf. [DOI] [PubMed] [Google Scholar]
- 31.Sharma R, Markel TA, Wang Y, Crisostomo PR, Wang M, Sando IC, Weil BR, Meldrum DR. Proestrus female rats are more resistant to right ventricular pressure overload. Shock. 2008;30:318–323. doi: 10.1097/SHK.0b013e318164e981. [DOI] [PubMed] [Google Scholar]
- 32.Deitch EA, Senthil M, Brown M, Caputo F, Watkins A, Anjaria D, Badami C, Pisarenko V, Doucet D, Lu Q, et al. Trauma-shock–induced gut injury and the production of biologically active intestinal lymph is abrogated by castration in a large animal porcine model. Shock. 2008;30:135–141. doi: 10.1097/shk.0b013e318161724f. [DOI] [PubMed] [Google Scholar]
- 33.Schroder J, Kahlke V, Book M, Stuber F. Gender differences in sepsis: genetically determined? Shock. 2000;14:307–310. [PubMed] [Google Scholar]
- 34.Frink M, Pape HC, van GM, Krettek C, Chaudry IH, Hildebrand F. Influence of sex and age on MODS and cytokines after multiple injuries. Shock. 2007;27:151–156. doi: 10.1097/01.shk.0000239767.64786.de. [DOI] [PubMed] [Google Scholar]
- 35.George RL, McGwin G, Jr, Metzger J, Chaudry IH, Rue LW., III The association between gender and mortality among trauma patients as modified by age. J Trauma. 2003;54:464–471. doi: 10.1097/01.TA.0000051939.95039.E6. [DOI] [PubMed] [Google Scholar]
- 36.Yu HP, Hsieh YC, Suzuki T, Shimizu T, Choudhry MA, Schwacha MG, Chaudry IH. Salutary effects of estrogen receptor–beta agonist on lung injury after trauma-hemorrhage. Am J Physiol Lung Cell Mol Physiol. 2006;290:L1004–L1009. doi: 10.1152/ajplung.00504.2005. [DOI] [PubMed] [Google Scholar]
- 37.Mostafa G, Huynh T, Sing RF, Miles WS, Norton HJ, Thomason MH. Gender-related outcomes in trauma. J Trauma. 2002;53:430–434. doi: 10.1097/00005373-200209000-00006. [DOI] [PubMed] [Google Scholar]
- 38.Offner PJ, Moore EE, Biffl WL. Male gender is a risk factor for major infections after surgery. Arch Surg. 1999;134:935–938. doi: 10.1001/archsurg.134.9.935. [DOI] [PubMed] [Google Scholar]
- 39.Gannon CJ, Pasquale M, Tracy JK, McCarter RJ, Napolitano LM. Male gender is associated with increased risk for postinjury pneumonia. Shock. 2004;21:410–414. doi: 10.1097/00024382-200405000-00003. [DOI] [PubMed] [Google Scholar]
- 40.Wichmann MW, Inthorn D, Andress HJ, Schildberg FW. Incidence and mortality of severe sepsis in surgical intensive care patients: the influence of patient gender on disease process and outcome. Intensive Care Med. 2000;26:167–172. doi: 10.1007/s001340050041. [DOI] [PubMed] [Google Scholar]
- 41.Wohltmann CD, Franklin GA, Boaz PW, Luchette FA, Kearney PA, Richardson JD, Spain DA. A multicenter evaluation of whether gender dimorphism affects survival after trauma. Am J Surg. 2001;181:297–300. doi: 10.1016/s0002-9610(01)00582-7. [DOI] [PubMed] [Google Scholar]
- 42.Napolitano LM, Greco ME, Rodriguez A, Kufera JA, West RS, Scalea TM. Gender differences in adverse outcomes after blunt trauma. J Trauma. 2001;50:274–280. doi: 10.1097/00005373-200102000-00013. [DOI] [PubMed] [Google Scholar]
- 43.Eachempati SR, Hydo L, Barie PS. Gender-based differences in outcome in patients with sepsis. Arch Surg. 1999;134:1342–1347. doi: 10.1001/archsurg.134.12.1342. [DOI] [PubMed] [Google Scholar]
- 44.Gannon CJ, Napolitano LM, Pasquale M, Tracy JK, McCarter RJ. A statewide population-based study of gender differences in trauma: validation of a prior single-institution study. J Am Coll Surg. 2002;195:11–18. doi: 10.1016/s1072-7515(02)01187-0. [DOI] [PubMed] [Google Scholar]
- 45.Deitch EA, Livingston DH, Lavery RF, Monaghan SF, Bongu A, Machiedo GW. Hormonally active women tolerate shock-trauma better than do men: a prospective study of over 4000 trauma patients. Ann Surg. 2007;246:447–453. doi: 10.1097/SLA.0b013e318148566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Croce MA, Fabian TC, Malhotra AK, Bee TK, Miller PR. Does gender difference influence outcome? J Trauma. 2002;53:889–894. doi: 10.1097/00005373-200211000-00013. [DOI] [PubMed] [Google Scholar]
- 47.Jarrar D, Wang P, Knoferl MW, Kuebler JF, Cioffi WG, Bland KI, Chaudry IH. Insight into the mechanism by which estradiol improves organ functions after trauma-hemorrhage. Surgery. 2000;128:246–252. doi: 10.1067/msy.2000.107376. [DOI] [PubMed] [Google Scholar]
- 48.Yu HP, Yang S, Hsieh YC, Choudhry MA, Bland KI, Chaudry IH. Maintenance of lung myeloperoxidase activity in proestrus females after trauma-hemorrhage: upregulation of heme oxygenase–1. Am J Physiol Lung Cell Mol Physiol. 2006;291:L400–L406. doi: 10.1152/ajplung.00537.2005. [DOI] [PubMed] [Google Scholar]
- 49.Schneider CP, Nickel EA, Samy TS, Schwacha MG, Cioffi WG, Bland KI, Chaudry IH. The aromatase inhibitor, 4-hydroxyandrostenedione, restores immune responses following trauma-hemorrhage in males and decreases mortality from subsequent sepsis. Shock. 2000;14:347–353. doi: 10.1097/00024382-200014030-00019. [DOI] [PubMed] [Google Scholar]
- 50.Jarrar D, Wang P, Cioffi WG, Bland KI, Chaudry IH. The female reproductive cycle is an important variable in the response to trauma-hemorrhage. Am J Physiol Heart Circ Physiol. 2000;279:H1015–H1021. doi: 10.1152/ajpheart.2000.279.3.H1015. [DOI] [PubMed] [Google Scholar]
- 51.Angele MK, Knoferl MW, Schwacha MG, Ayala A, Cioffi WG, Bland KI, Chaudry IH. Sex steroids regulate pro- and anti-inflammatory cytokine release by macrophages after trauma-hemorrhage. Am J Physiol. 1999;277:C35–C42. doi: 10.1152/ajpcell.1999.277.1.C35. [DOI] [PubMed] [Google Scholar]
- 52.Szalay L, Shimizu T, Suzuki T, Yu HP, Choudhry MA, Schwacha MG, Rue LW, III, Bland KI, Chaudry IH. Estradiol improves cardiac and hepatic function after trauma-hemorrhage: role of enhanced heat shock protein expression. Am J Physiol Regul Integr Comp Physiol. 2006;290:R812–R818. doi: 10.1152/ajpregu.00658.2005. [DOI] [PubMed] [Google Scholar]
- 53.Kuebler JF, Jarrar D, Bland KI, Rue L, III, Wang P, Chaudry IH. Progesterone administration after trauma and hemorrhagic shock improves cardiovascular responses. Crit Care Med. 2003;31:1786–1793. doi: 10.1097/01.CCM.0000063441.41446.23. [DOI] [PubMed] [Google Scholar]
- 54.Wichmann MW, Angele MK, Ayala A, Cioffi WG, Chaudry IH. Flutamide: a novel agent for restoring the depressed cell-mediated immunity following soft-tissue trauma and hemorrhagic shock. Shock. 1997;8:242–248. [PubMed] [Google Scholar]
- 55.Lobo R. Menopause. In: Goldman L, Bennett J, editors. Cecil Textbook of Medicine. W.B. Sanders Company; Philadelphia, PA: 2000. pp. 1360–1366. [Google Scholar]
- 56.Katznelson L, Riskind PN, Saxe VC, Klibanski A. Prolactin pulsatile characteristics in postmenopausal women. J Clin Endocrinol Metab. 1998;83:761–764. doi: 10.1210/jcem.83.3.4675. [DOI] [PubMed] [Google Scholar]
- 57.Zellweger R, Wichmann MW, Ayala A, DeMaso CM, Chaudry IH. Prolactin: a novel and safe immunomodulating hormone for the treatment of immuno-depression following severe hemorrhage. J Surg Res. 1996;63:53–58. doi: 10.1006/jsre.1996.0222. [DOI] [PubMed] [Google Scholar]
- 58.Yang S, Choudhry MA, Hsieh YC, Hu S, Rue LW, III, Bland KI, Chaudry IH. Estrus cycle: influence on cardiac function following trauma-hemorrhage. Am J Physiol Heart Circ Physiol. 2006;291:H2807–H2815. doi: 10.1152/ajpheart.00195.2006. [DOI] [PubMed] [Google Scholar]
- 59.Frink M, Hsieh YC, Hsieh CH, Pape HC, Choudhry MA, Schwacha MG, Chaudry IH. Keratinocyte-derived chemokine plays a critical role in the induction of systemic inflammation and tissue damage after trauma-hemorrhage. Shock. 2007;28:576–581. doi: 10.1097/shk.0b013e31814b8e0d. [DOI] [PubMed] [Google Scholar]
- 60.Yu HP, Shimizu T, Hsieh YC, Suzuki T, Choudhry MA, Schwacha MG, Chaudry IH. Tissue-specific expression of estrogen receptors and their role in the regulation of neutrophil infiltration in various organs following trauma-hemorrhage. J Leukoc Biol. 2006;79:963–970. doi: 10.1189/jlb.1005596. [DOI] [PubMed] [Google Scholar]
- 61.Gruber CJ, Tschugguel W, Schneeberger C, Huber JC. Production and actions of estrogens. N Engl J Med. 2002;346:340–352. doi: 10.1056/NEJMra000471. [DOI] [PubMed] [Google Scholar]
- 62.Yu HP, Choudhry MA, Shimizu T, Hsieh YC, Schwacha MG, Yang S, Chaudry IH. Mechanism of the salutary effects of flutamide on intestinal myeloperoxidase activity following trauma-hemorrhage: up-regulation of estrogen receptor-{beta}–dependent HO-1. J Leukoc Biol. 2006;79:277–284. doi: 10.1189/jlb.0705363. [DOI] [PubMed] [Google Scholar]
- 63.Caulin-Glaser T, Garcia-Cardena G, Sarrel P, Sessa WC, Bender JR. 17beta-Estradiol regulation of human endothelial cell basal nitric oxide release, independent of cytosolic Ca2+ mobilization. Circ Res. 1997;81:885–892. doi: 10.1161/01.res.81.5.885. [DOI] [PubMed] [Google Scholar]
- 64.Lantin-Hermoso RL, Rosenfeld CR, Yuhanna IS, German Z, Chen Z, Shaul PW. Estrogen acutely stimulates nitric oxide synthase activity in fetal pulmonary artery endothelium. Am J Physiol. 1997;273:L119–L126. doi: 10.1152/ajplung.1997.273.1.L119. [DOI] [PubMed] [Google Scholar]
- 65.Kuiper GG, Carlsson B, Grandien K, Enmark E, Haggblad J, Nilsson S, Gustafsson JA. Comparison of the ligand binding specificity and transcript tissue distribution of estrogen receptors alpha and beta. Endocrinology. 1997;138:863–870. doi: 10.1210/endo.138.3.4979. [DOI] [PubMed] [Google Scholar]
- 66.Yang S, Choudhry MA, Hsieh YC, Hu S, Rue LW, III, Bland KI, Chaudry IH. Estrus cycle: influence on cardiac function following trauma–hemorrhage. Am J Physiol Heart Circ Physiol. 2006;291:H2807–H2815. doi: 10.1152/ajpheart.00195.2006. [DOI] [PubMed] [Google Scholar]
- 67.Adams CA, Jr, Hauser CJ, Adams JM, Fekete Z, Xu DZ, Sambol JT, Deitch EA. Trauma-hemorrhage–induced neutrophil priming is prevented by mesenteric lymph duct ligation. Shock. 2002;18:513–517. doi: 10.1097/00024382-200212000-00005. [DOI] [PubMed] [Google Scholar]
- 68.Wu LL, Tang C, Liu MS. Altered phosphorylation and calcium sensitivity of cardiac myofibrillar proteins during sepsis. Am J Physiol Regul Integr Comp Physiol. 2001;281:R408–R416. doi: 10.1152/ajpregu.2001.281.2.R408. [DOI] [PubMed] [Google Scholar]
- 69.Malech HL, Gallin JI. Current concepts: immunology. Neutrophils in human diseases. N Engl J Med. 1987;317:687–694. doi: 10.1056/NEJM198709103171107. [DOI] [PubMed] [Google Scholar]
- 70.Angle N, Hoyt DB, Coimbra R, Liu F, Herdon-Remelius C, Loomis W, Junger WG. Hypertonic saline resuscitation diminishes lung injury by suppressing neutrophil activation after hemorrhagic shock. Shock. 1998;9:164–170. doi: 10.1097/00024382-199803000-00002. [DOI] [PubMed] [Google Scholar]
- 71.Dayal SD, Hasko G, Lu Q, Xu DZ, Caruso JM, Sambol JT, Deitch EA. Trauma/hemorrhagic shock mesenteric lymph upregulates adhesion molecule expression and IL-6 production in human umbilical vein endothelial cells. Shock. 2002;17:491–495. doi: 10.1097/00024382-200206000-00009. [DOI] [PubMed] [Google Scholar]
- 72.Shanley TP, Schmal H, Warner RL, Schmid E, Friedl HP, Ward PA. Requirement for C-X-C chemokines (macrophage inflammatory protein–2 and cytokine-induced neutrophil chemoattractant) in IgG immune complex-induced lung injury. J Immunol. 1997;158:3439–3448. [PubMed] [Google Scholar]
- 73.Iida M, Watanabe K, Tsurufuji M, Takaishi K, Iizuka Y, Tsurufuji S. Level of neutrophil chemotactic factor CINC/gro, a member of the interleukin-8 family, associated with lipopolysaccharide-induced inflammation in rats. Infect Immun. 1992;60:1268–1272. doi: 10.1128/iai.60.4.1268-1272.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Shimizu T, Yu HP, Suzuki T, Szalay L, Hsieh YC, Choudhry MA, Bland KI, Chaudry IH. The role of estrogen receptor subtypes in ameliorating hepatic injury following trauma-hemorrhage. J Hepatol. 2007;46:1047–1054. doi: 10.1016/j.jhep.2007.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Menezes JM, Hierholzer C, Watkins SC, Billiar TR, Peitzman AB, Harbrecht BG. The modulation of hepatic injury and heat shock expression by inhibition of inducible nitric oxide synthase after hemorrhagic shock. Shock. 2002;17:13–18. doi: 10.1097/00024382-200201000-00003. [DOI] [PubMed] [Google Scholar]
- 76.Meldrum DR, Shenkar R, Sheridan BC, Cain BS, Abraham E, Harken AH. Hemorrhage activates myocardial NFkappaB and increases TNF-alpha in the heart. J Mol Cell Cardiol. 1997;29:2849–2854. doi: 10.1006/jmcc.1997.0506. [DOI] [PubMed] [Google Scholar]
- 77.Kalaitzidis D, Gilmore TD. Transcription factor cross-talk: the estrogen receptor and NF-kappaB. Trends Endocrinol Metab. 2005;16:46–52. doi: 10.1016/j.tem.2005.01.004. [DOI] [PubMed] [Google Scholar]
- 78.Kushner PJ, Agard DA, Greene GL, Scanlan TS, Shiau AK, Uht RM, Webb P. Estrogen receptor pathways to AP-1. J Steroid Biochem Mol Biol. 2000;74:311–317. doi: 10.1016/s0960-0760(00)00108-4. [DOI] [PubMed] [Google Scholar]
- 79.Cuzzocrea S, Santagati S, Sautebin L, Mazzon E, Calabro G, Serraino I, Caputi AP, Maggi A. 17beta-Estradiol antiinflammatory activity in carrageenan-induced pleurisy. Endocrinology. 2000;141:1455–1463. doi: 10.1210/endo.141.4.7404. [DOI] [PubMed] [Google Scholar]
- 80.Kiang JG, Lu X, Tabaku LS, Bentley TB, Atkins JL, Tsokos GC. Resuscitation with lactated Ringer solution limits the expression of molecular events associated with lung injury after hemorrhage. J Appl Physiol. 2005;98:550–556. doi: 10.1152/japplphysiol.00858.2004. [DOI] [PubMed] [Google Scholar]
- 81.Hierholzer C, Harbrecht B, Menezes JM, Kane J, MacMicking J, Nathan CF, Peitzman AB, Billiar TR, Tweardy DJ. Essential role of induced nitric oxide in the initiation of the inflammatory response after hemorrhagic shock. J Exp Med. 1998;187:917–928. doi: 10.1084/jem.187.6.917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Yu HP, Shimizu T, Choudhry MA, Hsieh YC, Suzuki T, Bland KI, Chaudry IH. Mechanism of cardioprotection following trauma-hemorrhagic shock by a selective estrogen receptor–beta agonist: up-regulation of cardiac heat shock factor–1 and heat shock proteins. J Mol Cell Cardiol. 2006;40:185–194. doi: 10.1016/j.yjmcc.2005.10.001. [DOI] [PubMed] [Google Scholar]
- 83.Iafrati MD, Karas RH, Aronovitz M, Kim S, Sullivan TR, Jr, Lubahn DB, O'Donnell TF, Jr, Korach KS, Mendelsohn ME. Estrogen inhibits the vascular injury response in estrogen receptor alpha–deficient mice. Nat Med. 1997;3:545–548. doi: 10.1038/nm0597-545. [DOI] [PubMed] [Google Scholar]
- 84.Lindner V, Kim SK, Karas RH, Kuiper GG, Gustafsson JA, Mendelsohn ME. Increased expression of estrogen receptor–beta mRNA in male blood vessels after vascular injury. Circ Res. 1998;83:224–229. doi: 10.1161/01.res.83.2.224. [DOI] [PubMed] [Google Scholar]
- 85.Gabel SA, Walker VR, London RE, Steenbergen C, Korach KS, Murphy E. Estrogen receptor beta mediates gender differences in ischemia/reperfusion injury. J Mol Cell Cardiol. 2005;38:289–297. doi: 10.1016/j.yjmcc.2004.11.013. [DOI] [PubMed] [Google Scholar]
- 86.Wang JL, Ke DS, Lin MT. Heat shock pretreatment may protect against heatstroke-induced circulatory shock and cerebral ischemia by reducing oxidative stress and energy depletion. Shock. 2005;23:161–167. doi: 10.1097/01.shk.0000150779.47107.d5. [DOI] [PubMed] [Google Scholar]
- 87.Nakano M, Mann DL, Knowlton AA. Blocking the endogenous increase in HSP 72 increases susceptibility to hypoxia and reoxygenation in isolated adult feline cardiocytes. Circulation. 1997;95:1523–1531. doi: 10.1161/01.cir.95.6.1523. [DOI] [PubMed] [Google Scholar]
- 88.Szalay L, Shimizu T, Schwacha MG, Choudhry MA, Rue LW, III, Bland KI, Chaudry IH. Mechanism of salutary effects of estradiol on organ function after trauma-hemorrhage: upregulation of heme oxygenase. Am J Physiol Heart Circ Physiol. 2005;289:H92–H98. doi: 10.1152/ajpheart.01247.2004. [DOI] [PubMed] [Google Scholar]
- 89.Nishizawa J, Nakai A, Komeda M, Ban T, Nagata K. Increased preload directly induces the activation of heat shock transcription factor 1 in the left ventricular overloaded heart. Cardiovasc Res. 2002;55:341–348. doi: 10.1016/s0008-6363(02)00404-2. [DOI] [PubMed] [Google Scholar]
- 90.Knowlton AA, Sun L. Heat-shock factor–1, steroid hormones, and regulation of heat-shock protein expression in the heart. Am J Physiol Heart Circ Physiol. 2001;280:H455–H464. doi: 10.1152/ajpheart.2001.280.1.H455. [DOI] [PubMed] [Google Scholar]
- 91.Voss MR, Stallone JN, Li M, Cornelussen RN, Knuefermann P, Knowlton AA. Gender differences in the expression of heat shock proteins: the effect of estrogen. Am J Physiol Heart Circ Physiol. 2003;285:H687–H692. doi: 10.1152/ajpheart.01000.2002. [DOI] [PubMed] [Google Scholar]
- 92.Kumarapeli AR, Wang X. Genetic modification of the heart: chaperones and the cytoskeleton. J Mol Cell Cardiol. 2004;37:1097–1109. doi: 10.1016/j.yjmcc.2004.07.004. [DOI] [PubMed] [Google Scholar]
- 93.Benjamin IJ, McMillan DR. Stress (heat shock) proteins: molecular chaperones in cardiovascular biology and disease. Circ Res. 1998;83:117–132. doi: 10.1161/01.res.83.2.117. [DOI] [PubMed] [Google Scholar]
- 94.Pandey P, Saleh A, Nakazawa A, Kumar S, Srinivasula SM, Kumar V, Weichselbaum R, Nalin C, Alnemri ES, Kufe D, et al. Negative regulation of cytochrome c–mediated oligomerization of Apaf-1 and activation of procaspase-9 by heat shock protein 90. EMBO J. 2000;19:4310–4322. doi: 10.1093/emboj/19.16.4310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Beere HM, Wolf BB, Cain K, Mosser DD, Mahboubi A, Kuwana T, Tailor P, Morimoto RI, Cohen GM, Green DR. Heat-shock protein 70 inhibits apoptosis by preventing recruitment of procaspase-9 to the Apaf-1 apoptosome. Nat Cell Biol. 2000;2:469–475. doi: 10.1038/35019501. [DOI] [PubMed] [Google Scholar]
- 96.Lin KM, Lin B, Lian IY, Mestril R, Scheffler IE, Dillmann WH. Combined and individual mitochondrial HSP60 and HSP10 expression in cardiac myocytes protects mitochondrial function and prevents apoptotic cell deaths induced by simulated ischemia-reoxygenation. Circulation. 2001;103:1787–1792. doi: 10.1161/01.cir.103.13.1787. [DOI] [PubMed] [Google Scholar]
- 97.Kirchhoff SR, Gupta S, Knowlton AA. Cytosolic heat shock protein 60, apoptosis, and myocardial injury. Circulation. 2002;105:2899–2904. doi: 10.1161/01.cir.0000019403.35847.23. [DOI] [PubMed] [Google Scholar]
- 98.Shi Y, Hutchins W, Ogawa H, Chang CC, Pritchard KA, Jr, Zhang C, Khampang P, Lazar J, Jacob HJ, Rafiee P, et al. Increased resistance to myocardial ischemia in the Brown Norway vs. Dahl S rat: role of nitric oxide synthase and Hsp90. J Mol Cell Cardiol. 2005;38:625–635. doi: 10.1016/j.yjmcc.2005.02.005. [DOI] [PubMed] [Google Scholar]
- 99.Erol E, Kumar LS, Cline GW, Shulman GI, Kelly DP, Binas B. Liver fatty acid binding protein is required for high rates of hepatic fatty acid oxidation but not for the action of PPARalpha in fasting mice. FASEB J. 2004;18:347–349. doi: 10.1096/fj.03-0330fje. [DOI] [PubMed] [Google Scholar]
- 100.Herzig S, Long F, Jhala US, Hedrick S, Quinn R, Bauer A, Rudolph D, Schutz G, Yoon C, Puigserver P, et al. CREB regulates hepatic gluconeo-genesis through the coactivator PGC-1. Nature. 2001;413:179–183. doi: 10.1038/35093131. [DOI] [PubMed] [Google Scholar]
- 101.Kanda N, Watanabe S. 17beta-Estradiol stimulates the growth of human keratinocytes by inducing cyclin D2 expression. J Invest Dermatol. 2004;123:319–328. doi: 10.1111/j.0022-202X.2004.12645.x. [DOI] [PubMed] [Google Scholar]
- 102.Wade CB, Dorsa DM. Estrogen activation of cyclic adenosine 5’-monophosphate response element–mediated transcription requires the extracellularly regulated kinase/mitogen-activated protein kinase pathway. Endocrinology. 2003;144:832–838. doi: 10.1210/en.2002-220899. [DOI] [PubMed] [Google Scholar]
- 103.Chen JQ, Eshete M, Alworth WL, Yager JD. Binding of MCF-7 cell mitochondrial proteins and recombinant human estrogen receptors alpha and beta to human mitochondrial DNA estrogen response elements. J Cell Biochem. 2004;93:358–373. doi: 10.1002/jcb.20178. [DOI] [PubMed] [Google Scholar]
- 104.Imahara SD, Jelacic S, Junker CE, O'Keefe GE. The influence of gender on human innate immunity. Surgery. 2005;138:275–282. doi: 10.1016/j.surg.2005.03.020. [DOI] [PubMed] [Google Scholar]
- 105.Schneider CP, Schwacha MG, Chaudry IH. The role of interleukin-10 in the regulation of the systemic inflammatory response following trauma-hemorrhage. Biochim Biophys Acta. 2004;1689:22–32. doi: 10.1016/j.bbadis.2004.01.003. [DOI] [PubMed] [Google Scholar]
- 106.Diodato MD, Knoferl MW, Schwacha MG, Bland KI, Chaudry IH. Gender differences in the inflammatory response and survival following haemorrhage and subsequent sepsis. Cytokine. 2001;14:162–169. doi: 10.1006/cyto.2001.0861. [DOI] [PubMed] [Google Scholar]
- 107.Raju R, Bland KI, Chaudry IH. Estrogen: a novel therapeutic adjunct for the treatment of trauma-hemorrhage–induced immunological alterations. Mol Med. 2008;14:213–221. doi: 10.2119/2008-00001.Raju. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Knoferl MW, Angele MK, Schwacha MG, Anantha Samy TS, Bland KI, Chaudry IH. Immunoprotection in proestrus females following trauma-hemorrhage: the pivotal role of estrogen receptors. Cell Immunol. 2003;222:27–34. doi: 10.1016/s0008-8749(03)00081-9. [DOI] [PubMed] [Google Scholar]
- 109.Suzuki T, Shimizu T, Yu HP, Hsieh YC, Choudhry MA, Bland KI, Chaudry IH. 17 beta–Estradiol administration following trauma-hemorrhage prevents the increase in Kupffer cell cytokine production and MAPK activation predominately via estrogen receptor–alpha. Surgery. 2006;140:141–148. doi: 10.1016/j.surg.2006.02.014. [DOI] [PubMed] [Google Scholar]
- 110.Suzuki T, Shimizu T, Yu HP, Hsieh YC, Choudhry MA, Bland KI, Chaudry IH. Estrogen receptor–alpha predominantly mediates the salutary effects of 17beta-estradiol on splenic macrophages following trauma-hemorrhage. Am J Physiol Cell Physiol. 2007;293:C978–C984. doi: 10.1152/ajpcell.00092.2007. [DOI] [PubMed] [Google Scholar]
- 111.Suzuki T, Shimizu T, Yu HP, Hsieh YC, Choudhry MA, Schwacha MG, Chaudry IH. Tissue compartment–specific role of estrogen receptor subtypes in immune cell cytokine production following trauma-hemorrhage. J Appl Physiol. 2007;102:163–168. doi: 10.1152/japplphysiol.00964.2006. [DOI] [PubMed] [Google Scholar]
- 112.Suzuki T, Shimizu T, Yu HP, Hsieh YC, Choudhry MA, Chaudry IH. Salutary effects of 17beta-estradiol on T-cell signaling and cytokine production after trauma-hemorrhage are mediated primarily via estrogen receptor–alpha. Am J Physiol Cell Physiol. 2007;292:C2103–C2111. doi: 10.1152/ajpcell.00488.2006. [DOI] [PubMed] [Google Scholar]
- 113.Song RX. Membrane-initiated steroid signaling action of estrogen and breast cancer. Semin Reprod Med. 2007;25:187–197. doi: 10.1055/s-2007-973431. [DOI] [PubMed] [Google Scholar]
- 114.Govind AP, Thampan RV. Membrane associated estrogen receptors and related proteins: localization at the plasma membrane and the endoplasmic reticulum. Mol Cell Biochem. 2003;253:233–240. doi: 10.1023/a:1026068017309. [DOI] [PubMed] [Google Scholar]
- 115.Kuiper GG, Enmark E, Pelto-Huikko M, Nilsson S, Gustafsson JA. Cloning of a novel receptor expressed in rat prostate and ovary. Proc Natl Acad Sci USA. 1996;93:5925–5930. doi: 10.1073/pnas.93.12.5925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Hsieh YC, Frink M, Hsieh CH, Choudhry MA, Schwacha MG, Bland KI, Chaudry IH. Downregulation of migration inhibitory factor is critical for estrogen-mediated attenuation of lung tissue damage following trauma-hemorrhage. Am J Physiol Lung Cell Mol Physiol. 2007;292:L1227–L1232. doi: 10.1152/ajplung.00479.2006. [DOI] [PubMed] [Google Scholar]
- 117.Yu HP, Hsieh YC, Suzuki T, Choudhry MA, Schwacha MG, Bland KI, Chaudry IH. The PI3K/Akt pathway mediates the nongenomic cardioprotective effects of estrogen following trauma-hemorrhage. Ann Surg. 2007;245:971–977. doi: 10.1097/01.sla.0000254417.15591.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Yu HP, Hsieh YC, Suzuki T, Choudhry MA, Schwacha MG, Bland KI, Chaudry IH. Mechanism of the nongenomic effects of estrogen on intestinal myeloperoxidase activity following trauma-hemorrhage: up-regulation of the PI-3K/Akt pathway. J Leukoc Biol. 2007;82:774–780. doi: 10.1189/jlb.0307182. [DOI] [PubMed] [Google Scholar]
- 119.Ho KJ, Liao JK. Nonnuclear actions of estrogen. Arterioscler Thromb Vasc Biol. 2002;22:1952–1961. doi: 10.1161/01.atv.0000041200.85946.4a. [DOI] [PubMed] [Google Scholar]
- 120.Yu HP, Shimizu T, Choudhry MA, Hsieh YC, Suzuki T, Bland KI, Chaudry IH. Mechanism of cardioprotection following trauma–hemorrhagic shock by a selective estrogen receptor–beta agonist: up-regulation of cardiac heat shock factor–1 and heat shock proteins. J Mol Cell Cardiol. 2006;40:185–194. doi: 10.1016/j.yjmcc.2005.10.001. [DOI] [PubMed] [Google Scholar]
- 121.Razandi M, Pedram A, Greene GL, Levin ER. Cell membrane and nuclear estrogen receptors (ERs) originate from a single transcript: studies of ERalpha and ERbeta expressed in Chinese hamster ovary cells. Mol Endocrinol. 1999;13:307–319. doi: 10.1210/mend.13.2.0239. [DOI] [PubMed] [Google Scholar]
- 122.Revankar CM, Cimino DF, Sklar LA, Arterburn JB, Prossnitz ER. A transmembrane intracellular estrogen receptor mediates rapid cell signaling. Science. 2005;307:1625–1630. doi: 10.1126/science.1106943. [DOI] [PubMed] [Google Scholar]
- 123.Hsieh YC, Yu HP, Frink M, Suzuki T, Choudhry MA, Schwacha MG, Chaudry IH. G protein–coupled receptor 30–dependent protein kinase A pathway is critical in nongenomic effects of estrogen in attenuating liver injury after trauma-hemorrhage. Am J Pathol. 2007;170:1210–1218. doi: 10.2353/ajpath.2007.060883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Guo Z, Krucken J, Benten WP, Wunderlich F. Estradiol-induced nongenomic calcium signaling regulates genotropic signaling in macrophages. J Biol Chem. 2002;277:7044–7050. doi: 10.1074/jbc.M109808200. [DOI] [PubMed] [Google Scholar]
- 125.Kow LM, Pfaff DW. The membrane actions of estrogens can potentiate their lordosis behavior–facilitating genomic actions. Proc Natl Acad Sci U S A. 2004;101:12354–12357. doi: 10.1073/pnas.0404889101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Stevis PE, Deecher DC, Suhandolnik L, Mallis LM, Frail DE. Differential effects of estradiol and estradiol-BSA conjugates. Endocrinology. 1999;140:5455–5458. doi: 10.1210/endo.140.11.7247. [DOI] [PubMed] [Google Scholar]
- 127.Abraham IM, Han SK, Todman MG, Korach KS, Herbison AE. Estrogen receptor beta mediates rapid estrogen actions on gonadotropin-releasing hormone neurons in vivo. J Neurosci. 2003;23:5771–5777. doi: 10.1523/JNEUROSCI.23-13-05771.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Honda K, Sawada H, Kihara T, Urushitani M, Nakamizo T, Akaike A, Shimohama S. Phosphatidylinositol 3-kinase mediates neuroprotection by estrogen in cultured cortical neurons. J Neurosci Res. 2000;60:321–327. doi: 10.1002/(SICI)1097-4547(20000501)60:3<321::AID-JNR6>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 129.Hisamoto K, Ohmichi M, Kurachi H, Hayakawa J, Kanda Y, Nishio Y, Adachi K, Tasaka K, Miyoshi E, Fujiwara N, et al. Estrogen induces the Akt-dependent activation of endothelial nitric-oxide synthase in vascular endothelial cells. J Biol Chem. 2001;276:3459–3467. doi: 10.1074/jbc.M005036200. [DOI] [PubMed] [Google Scholar]
- 130.Cai Z, Semenza GL. Phosphatidylinositol-3-kinase signaling is required for erythropoietin-mediated acute protection against myocardial ischemia/reperfusion injury. Circulation. 2004;109:2050–2053. doi: 10.1161/01.CIR.0000127954.98131.23. [DOI] [PubMed] [Google Scholar]
- 131.Fujio Y, Nguyen T, Wencker D, Kitsis RN, Walsh K. Akt promotes survival of cardiomyocytes in vitro and protects against ischemia-reperfusion injury in mouse heart. Circulation. 2000;101:660–667. doi: 10.1161/01.cir.101.6.660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Haynes MP, Sinha D, Russell KS, Collinge M, Fulton D, Morales–Ruiz M, Sessa WC, Bender JR. Membrane estrogen receptor engagement activates endothelial nitric oxide synthase via the PI3-kinase–Akt pathway in human endothelial cells. Circ Res. 2000;87:677–682. doi: 10.1161/01.res.87.8.677. [DOI] [PubMed] [Google Scholar]
- 133.Matsui T, Tao J, del Monte F, Lee KH, Li L, Picard M, Force TL, Franke TF, Hajjar RJ, Rosenzweig A. Akt activation preserves cardiac function and prevents injury after transient cardiac ischemia in vivo. Circulation. 2001;104:330–335. doi: 10.1161/01.cir.104.3.330. [DOI] [PubMed] [Google Scholar]
- 134.Rajesh KG, Suzuki R, Maeda H, Yamamoto M, Yutong X, Sasaguri S. Hydrophilic bile salt ursodeoxycholic acid protects myocardium against reperfusion injury in a PI3K/Akt dependent pathway. J Mol Cell Cardiol. 2005;39:766–776. doi: 10.1016/j.yjmcc.2005.07.014. [DOI] [PubMed] [Google Scholar]
- 135.Puri KD, Doggett TA, Douangpanya J, Hou Y, Tino WT, Wilson T, Graf T, Clayton E, Turner M, Hayflick JS, et al. Mechanisms and implications of phosphoinositide 3–kinase delta in promoting neutrophil trafficking into inflamed tissue. Blood. 2004;103:3448–3456. doi: 10.1182/blood-2003-05-1667. [DOI] [PubMed] [Google Scholar]
- 136.Cadwallader KA, Condliffe AM, McGregor A, Walker TR, White JF, Stephens LR, Chilvers ER. Regulation of phosphatidylinositol 3-kinase activity and phosphatidylinositol 3,4,5-trisphosphate accumulation by neutrophil priming agents. J Immunol. 2002;169:3336–3344. doi: 10.4049/jimmunol.169.6.3336. [DOI] [PubMed] [Google Scholar]
- 137.Simoncini T, Hafezi–Moghadam A, Brazil DP, Ley K, Chin WW, Liao JK. Interaction of oestrogen receptor with the regulatory subunit of phosphatidylinositol-3-OH kinase. Nature. 2000;407:538–541. doi: 10.1038/35035131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Chen DB, Bird IM, Zheng J, Magness RR. Membrane estrogen receptor–dependent extracellular signal–regulated kinase pathway mediates acute activation of endothelial nitric oxide synthase by estrogen in uterine artery endothelial cells. Endocrinology. 2004;145:113–125. doi: 10.1210/en.2003-0547. [DOI] [PubMed] [Google Scholar]
- 139.Carrer HF, Cambiasso MJ, Gorosito S. Effects of estrogen on neuronal growth and differentiation. J Steroid Biochem Mol Biol. 2005;93:319–323. doi: 10.1016/j.jsbmb.2004.12.007. [DOI] [PubMed] [Google Scholar]
- 140.Angele MK, Nitsch S, Knoferl MW, Ayala A, Angele P, Schildberg FW, Jauch KW, Chaudry IH. Sex-specific p38 MAP kinase activation following trauma-hemorrhage: involvement of testosterone and estradiol. Am J Physiol Endocrinol Metab. 2003;285:E189–E196. doi: 10.1152/ajpendo.00035.2003. [DOI] [PubMed] [Google Scholar]
- 141.Suzuki T, Yu HP, Hsieh YC, Choudhry MA, Bland KI, Chaudry IH. Estrogen-mediated activation of non-genomic pathway improves macrophages cytokine production following trauma-hemorrhage. J Cell Physiol. 2008;214:662–672. doi: 10.1002/jcp.21255. [DOI] [PubMed] [Google Scholar]