Abstract
Objective
We investigated whether Kupffer cell phagocytosis is differentially regulated following hypoxia (by breathing hypoxic gas) and trauma-hemorrhage. We hypothesized that the differences might result from a differential activation of hypoxia-inducible factor (HIF)-1α and phosphoinositide 3-kinase (PI3K)/Akt pathway under those conditions.
Background
HIF-1α is a biologic O2 sensor enabling adaptation to hypoxia. Studies have shown that under hypoxic conditions, HIF-1α enhances macrophage phagocytosis. Trauma-hemorrhage also produces a hypoxic insult with HIF-1α activation; however, macrophage phagocytosis is suppressed under those conditions. Thus, signaling molecules other than HIF-1α should be taken into consideration in the regulation of macrophage phagocytosis following cellular hypoxia or trauma-hemorrhage.
Methods
Male C3H/HeN mice were subjected to sham operation, trauma-hemorrhage (laparotomy, 90 minutes hemorrhagic shock, MAP 35 ± 5 mm Hg followed by resuscitation) or hypoxia (5% O2 for 120 minutes). The trauma-hemorrhage and hypoxia groups received Wortmannin (PI3K inhibitor), YC-1 (HIF-1α inhibitor) or vehicle at the time of maximum bleedout in the trauma-hemorrhage group or at a PaO2 of 30 mm Hg during hypoxic air inhalation. Mice were killed 2 hours later and samples/cells collected.
Results
While the systemic and Kupffer cell hypoxic states were similar in the trauma-hemorrhage and hypoxia groups, phagocytic capacity was suppressed following trauma-hemorrhage but enhanced in the hypoxia group. Kupffer cells from both groups showed increased HIF-1α activation, which was prevented by Wortmannin or YC-1 treatment. The increase in Kupffer cell phagocytosis following hypoxemia was also prevented by Wortmannin or YC-1 treatment. Akt activation was suppressed in the trauma-hemorrhage group, but enhanced in the hypoxia group. Wortmannin and YC-1 treatment prevented the increase in Akt activation.
Conclusions
These findings indicate that the suppression of Kupffer cell phagocytosis following trauma-hemorrhage is independent of cellular hypoxia and activation of HIF-1α, but it is possibly related to suppression of the Akt activation.
Phagocytosis is the process by which microbial pathogens are engulfed by macrophages and represents the first line of defense against bacterial infection.1,2 As the sites of infection are usually located inside injured tissue with poor perfusion, those effector cells of the innate immune system must be able to maintain phagocytic function under hypoxic conditions. Exposure of cells to hypoxia leads to an increase in the expression of hypoxia-inducible factor-1α (HIF-1α),3 which binds to HIF-1β and induces transcription of various hypoxia related elements that enables cellular adaptation to hypoxia.4 Evidence suggests that hypoxia enhances macrophage phagocytosis in an HIF-1α-dependent manner.5,6 Furthermore, such HIF-1α-dependent enhancement of macrophage phagocytosis had not only been tested in cultured cells, but also in vivo by testing cells isolated from animals which had inhaled hypoxic air.5 In such model, cellular hypoxia was produced by systemic hypoxemia, which was the result of inhaling hypoxic air (henceforth referred to as hypoxia model).
In contrast, trauma-hemorrhage/resuscitation is characterized by decreased hemoglobin and decreased tissue perfusion which often results in insufficient oxygen delivery to the tissue and produces cellular hypoxia, and as a result causes severe immunosuppression.7–12 The phagocytic capacity of phagocytes, such as Kupffer cells, is impaired following trauma-hemorrhage.13 However, our previous studies in heart and liver also showed that HIF-1α was up-regulated, but not down-regulated, following trauma-hemorrhage.14,15 Thus, it can be postulated that the HIF-1α signal transduction pathways are differentially regulated under different conditions of cellular hypoxia (ie, induced by hypoxic gas mixture or trauma-hemorrhage), as phagocytic capacities are improved or impaired, respectively, under those conditions.
Phosphoinositide 3-kinase (PI3K) and its downstream signaling molecule, Akt, have been shown to play an important role in regulating phagocytosis.16 PI3K/Akt is involved in the up-regulation of HIF-1α during cellular hypoxia17 and inhibition of PI3K/Akt blocks the phagocytic capacity of macrophages.18 It can therefore be postulated that the impact of hypoxia on phagocytosis may be via PI3K/Akt activation and HIF-1α induction.
MATERIALS AND METHODS
Mouse Models of Trauma-Hemorrhage and Hypoxia
In this study, we used 2 different models to produce Kupffer cell hypoxia. These 2 models are routinely performed in our laboratory.19 –21 The severity of Kupffer cell hypoxia produced by these 2 models was evaluated by pimonidazole staining to ensure they were comparable (explained in the following section).
Male C3H/HeN mice, 8-weeks old and weighing 22–25 g (Charles River Laboratories, Wilmington, MA) were fasted overnight before the experiment but were allowed water ad libitum. All experiments were performed in adherence with National Institutes of Health (NIH; Bethesda, MD) “Guidelines for the Care and Use of Laboratory Animals” and approved by the Institutional Animal Care and Use Committee of the University of Alabama at Birmingham (Birmingham, AL).
For the trauma-hemorrhage model, animals were anesthetized with isoflurane (Minrad, Bethlehem, PA) and restrained in a supine position. A 2-cm midline laparotomy was performed, which was then closed in 2 layers with sutures (Ethilon 6/0, Ethicon, Somerville, NJ). Both femoral arteries and a femoral vein were then cannulated with polyethylene-10 tubing (Becton Dickinson, Sparks, MD). Blood pressure was measured via one of the arterial catheters, using a blood pressure analyzer (Micro-Med, Louisville, KY). Upon awakening, the mice were bled rapidly through the other arterial catheter to a mean arterial blood pressure (MAP) of 35 ± 5 mm Hg within 10 minutes. This MAP was maintained by slowly withdrawing more blood to reach a blood loss of 60% of the total blood volume while still maintaining MAP at 35 ± 5 mm Hg (maximum bleedout). The entire hypotension interval was 90 minutes. At the end of that interval, the animals were resuscitated via the venous line with 4 times the shed blood volume, using Ringer lactate. After ligating the blood vessels, catheters were removed; the incisions were flushed with lidocaine and closed with sutures. Sham-operated animals underwent laparotomy and the same groin dissection, which included the cannulation and ligation of the femoral arteries and vein, but neither hemorrhage nor resuscitation was carried out.
For the hypoxia model, the animals were placed in a chamber (35 × 25 × 15 cm) with an inlet and an outlet, through which moist hypoxic gas (95% N2–5% O2) flowed at a rate of 5 L/min for 2 hours. The animals were killed at 2 hours after the end of resuscitation or hypoxic air inhalation. To exclude the possibility that the constant airflow in the chamber may affect oxygenation and hydration status of the animals, we compared the hemoglobin and arterial partial pressure of oxygen (PaO2) levels of sham animals placed inside the chamber (with compressed air flowing at a rate of 5 L/min) or in room air in a preliminary study (data not shown). The results showed that there were no differences under those conditions.
Animal Groups
Mice were randomly assigned to sham, trauma-hemorrhage, or hypoxia groups. Each group of animals received Wortmannin (PI3K inhibitor, Sigma, St. Louis, MO), YC-1 (HIF-1α inhibitor, A.G. Scientific, San Diego, CA), or vehicle at the time of maximum bleedout or 30 minutes after the induction of hypoxia. In a pilot study, it was determined that the PaO2 levels consistently dropped to 30 mm Hg at 30 minutes after the onset of hypoxia. In addition, the maximum bleedout time also was usually around 30 minutes. The experimental drugs were given as follows in a volume of 100 μL/mouse: Wortmannin 1 mg/Kg body weight (BW) dissolved in normal saline, YC-15 mg/Kg BW, dissolved in 1 part ethanol, 1 part Cremophor EL (Fluka, St. Louis, MO), and 4 parts normal saline. The solvent for YC-1 was also used as vehicle.
Measurement of PaO2 and Hemoglobin
In separate experiments, 100 μL of arterial blood was withdrawn into a heparinized syringe at the end of resuscitation or hypoxic air inhalation. The PaO2 and hemoglobin levels were determined immediately using an OSM hemoximeter (Radiometer, Copenhagen, Denmark).
Preparation of Kupffer Cells
Kupffer cells were isolated as previously described.22 In brief, the portal vein was catheterized with a 27-gauge needle, and the liver was perfused with 20 mL of Hanks balanced salt solution (HBSS; GIBCO, Grand Island, NY) at 37°C, which was immediately followed by perfusion with 15 mL of 0.05% collagenase IV (Worthington, Lakewood, NJ) in HBSS with 0.5 mM CaCl2 (Sigma) at 37°C. The liver was then removed and transferred to a Petri dish containing the above mentioned collagenase IV solution. The liver was minced, incubated for 15 minutes at 37°C, and passed through a sterile mesh stainless steel screen into a beaker containing 40 mL of cold HBSS with 10% FBS. The hepatocytes were removed by centrifugation at 50 g for 3 minutes. The residual cell suspension was washed twice by centrifugation at 800 g for 10 minutes at 4°C in HBSS. The cells were then resuspended in complete William’s E medium containing 10% FBS and antibiotics (50 U/mL penicillin, 50 μg/mL streptomycin, and 20 μg/mL gentamycin, all from GIBCO) and layered over 16% Histodenz (Sigma) in HBSS and centrifuged at 3000 g for 45 minutes at 4°C. After removing the nonparenchymal cells from the interface, the cells were washed twice by centrifugation (800 g, 10 minutes, 4°C) in complete William’s E medium. The cells were then resuspended in complete RPMI 1640 medium or plated in a 96-well plate at a cell density of 5 × 106 cells/mL.
Determination of Kupffer Cell Hypoxia
A Hypoxyprobe-1 kit (Natural Pharmacia International, Burlington, MA) which contains pimonidazole and FITC-conjugated antipimonidazole antibody was used for detection of Kupffer cell hypoxia in vivo. Pimonidazole is known to form a reduced adduct and bind to proteins if intracellular PO2 is below 10 mm Hg and has been used to characterize cellular or tissue hypoxia.23 In these studies, cellular hypoxia in vivo was detected by intravenous injection of pimonidazole before sham operation, trauma-hemorrhage, or hypoxia at a dosage of 60 mg/Kg. Upon harvesting, Kupffer cells were prepared as described above, washed and fixed in 70% ethanol. After washing in PBS twice, the cells were blocked with anti-CD16/32 antibody (eBioscience, San Diego, CA) for 15 minutes and stained with APC-conjugated anti-F4/80 antibody or its isotype control (eBioscience). The macrophages were then permeabilized with 0.1% Triton-X100 and stained for 2 hours with FITC-conjugated antipimonidazole antibody or isotype control. Analysis was carried out using a BD LSRII flow cytometer (BD Biosciences, Mountain View, CA) after cells were washed and resuspended in flow cytometry staining buffer (PBS containing 0.1% bovine serum albumin).
Measurement of Activated HIF-lα by ELISA
A commercially available ELISA kit was used to measure the levels of activated HIF-lα (R&D Systems, Minneapolis, MN). In brief, nuclear extracts of Kupffer cells were prepared by solubilizing cells in lysis buffer provided by the manufacturer and the protein concentrations of each sample were determined using the Bio-Rad Dc protein assay (Bio-Rad Laboratories, Hercules, CA). A biotinylated oligonucleotide containing a consensus HIF-1α binding site was then incubated with nuclear extracts for 30 minutes. Following incubation, the HIF-1α-oligonucleotide complex was added to the wells of a plate, which was coated with HIF-1α capture antibody. After incubation for 2 hours, the unbound materials were washed away. A standard streptavidin-HRP format was used for detection and the optical densities were read at 450 nm followed by 570 nm subtraction on a spectrophotometer (Bio-Tek Instruments, Winooski, VT).
Measurement of Phospho-Akt by ELISA
The relative amount of phospho-Akt (p-Akt) protein in Kupffer cells was determined using Duoset IC ELISA kit for p-Akt (R&D Systems). Briefly, lysis buffer was prepared according to the manufacturer’s instructions and a 96-well microplate was coated with p-Akt capture antibody and incubated overnight at room temperature before use. Isolated Kupffer cells were solubilized in lysis buffer at a concentration of 1 × 107 cells/mL and the cell lysates were added into wells at a volume of 100 μL/well and incubated for 2 hours. The p-Akt detection antibody was then added to each well after the washing procedure, followed by 2 hours incubation. The relative amount of p-Akt protein was then determined using a standard streptavidin-HRP format and the absorbances were read at 450 nm followed by 570 nm subtraction. The value of p-Akt was normalized to the protein content of each group before statistical analysis.
Kupffer Cell Phagocytosis Assay
Because lysosomes are acidified to kill ingested pathogens following phagocytosis, pHrodo E. coli bioparticle conjugates (Invitrogen Molecular Probes, Eugene, OR) which emit strong fluorescence in acidic surroundings were used to evaluate Kupffer cell phagocytic capacity. For the in vitro phagocytosis assay, the isolated Kupffer cells were plated in a 96-well plate at a density of 5 × 106 cells/mL and incubated overnight before 15 μg of the particles (dissolved in RPMI 1640 at a concentration of 1 mg/mL) were added to each well. After incubation for another 1 hour, the Kupffer cells were harvested and resuspended in flow cytometry staining buffer. The Kupffer cells were then incubated at 4°C with purified anti-CD16/CD32 Fc blocking antibody for 15 minutes. In the presence of the blocking antibody, the cells were then incubated on ice with APC-conjugated anti-F4/80 antibody for 45 minutes. After washing the cells twice in staining buffer, the cells were resuspended in 200 μL of staining buffer and analyzed using the LSRII flow cytometer. The cell populations gated as F4/80-positive were analyzed. Data analysis was carried out using the FACSDiva software (BD Biosciences).
Statistical Analysis
Statistical analysis was performed using Sigma-Stat computer software (SPSS, Chicago, IL). Statistical significance was assumed if probability values of less than 0.05 were obtained. Comparisons between groups were performed using one-way ANOVA followed by Tukey test. Results are expressed as mean ± SEM of 6 animals in each group.
RESULTS
Hemoglobin and PaO2 Levels Following Trauma-Hemorrhage and Hypoxia
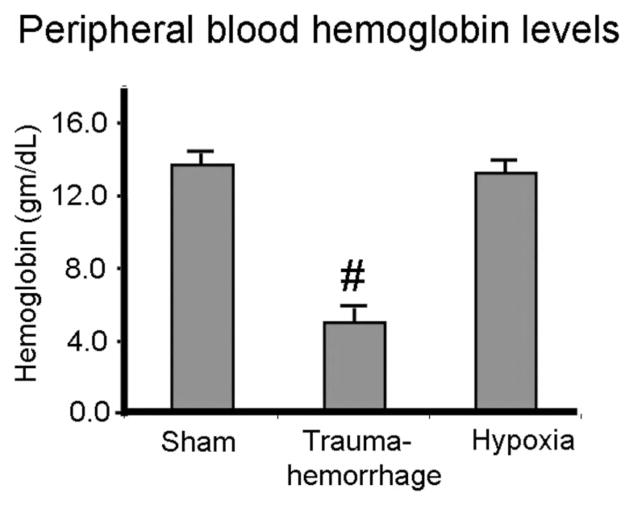
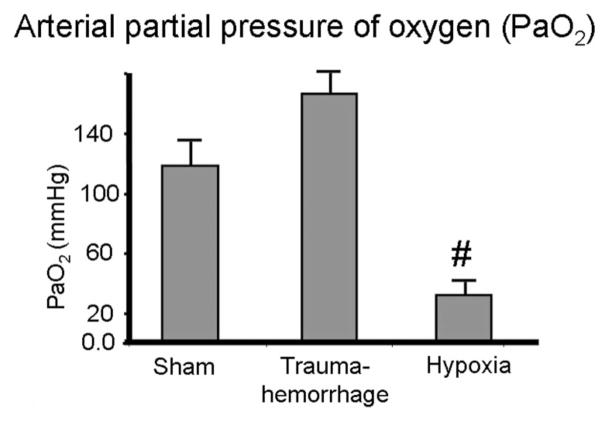
As shown in Figure 1, mice in the sham and hypoxia groups had a normal hemoglobin level of 14 g/dL. Mice in the trauma-hemorrhage group, after losing 60% of their circulating blood volume and resuscitation with crystalloid solution, had a hemoglobin level of 5.8 g/dL. In contrast, the PaO2 levels of sham and trauma-hemorrhage mice were similar and significantly higher than those of the hypoxia group (Fig. 2).
FIGURE 1.
Hemoglobin levels of peripheral blood following trauma-hemorrhage or hypoxia. Male C3H/HeN mice were subjected to sham treatment, trauma-hemorrhage or hypoxia as described in Materials and Methods. At the end of resuscitation or hypoxia, arterial blood hemoglobin levels were determined. Data are mean ± SE; n = 6 animals/ group. #P < 0.05 compared with sham and hypoxia group.
FIGURE 2.
Arterial partial pressure of oxygen (PaO2) following trauma-hemorrhage or hypoxia. Male C3H/HeN mice were subjected to sham treatment, trauma-hemorrhage or hypoxia as described in Materials and Methods. At the end of resuscitation or hypoxia, arterial blood PaO2 levels were determined. Data are mean ± SE; n = 6 animals/group. #P < 0.05 compared with sham and trauma-hemorrhage group.
Hypoxic Status of Kupffer Cells Following Trauma-Hemorrhage and Hypoxia
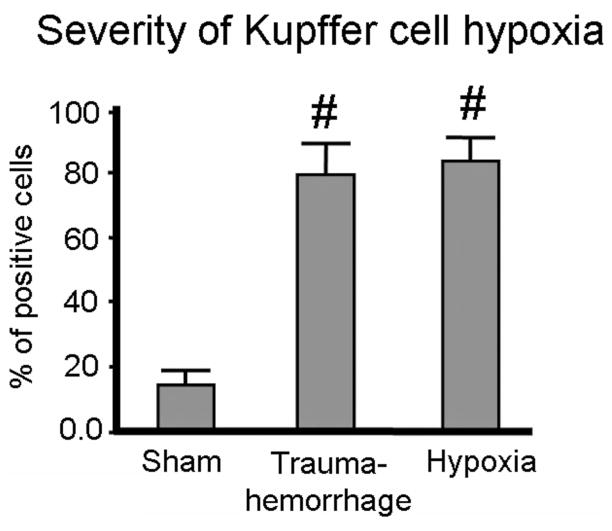
The pimonidazole was injected before trauma-hemorrhage and hypoxia procedure. As shown in Figure 3, approximately 80% of Kupffer cells from trauma-hemorrhage and hypoxemic hypoxia groups were positive for pimonidazole staining, indicating profound hypoxia in those cells. In contrast, less than 15% of Kupffer cells from sham group were hypoxic (Fig. 3).
FIGURE 3.
Severity of hypoxia of Kupffer cells following trauma-hemorrhage or hypoxia. Pimonidazole was injected intravenously before trauma-hemorrhage or hypoxic air inhalation at a dosage of 60 mg/Kg. Upon harvesting, Kupffer cells were prepared as described in Materials and Methods and stained with APC-conjugated anti-F4/80 antibodies and FITC-conjugated antipimonidazole antibodies. Data are mean ± SE; n = 6 animals/group. #P < 0.05 compared with sham.
Phagocytic Capacity of Kupffer Cells Following Trauma-Hemorrhage and Hypoxia
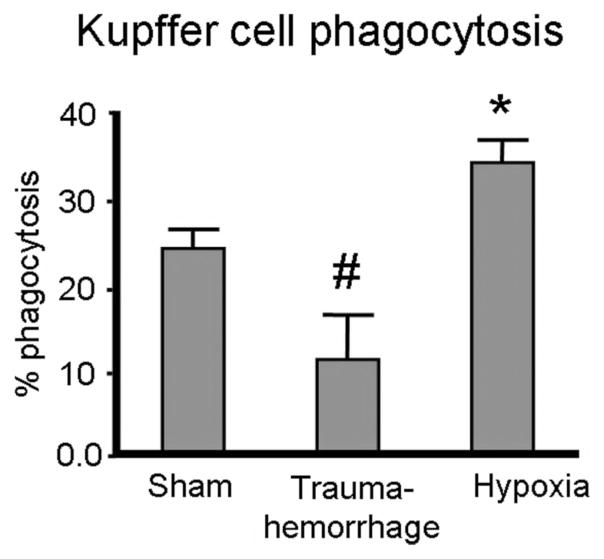
Although both of these models produced similar Kupffer cell hypoxia, trauma-hemorrhage and hypoxia differentially affected Kupffer cell in vitro phagocytic capacity (Fig. 4). Compared with the sham group, trauma-hemorrhage suppressed Kupffer cell phagocytic capacity by 50%. In contrast, cellular hypoxia due to hypoxemia enhanced Kupffer cell phagocytic capacity by almost 40% compared with sham cells (Fig. 4).
FIGURE 4.
Phagocytic capacities of isolated Kupffer cells following trauma-hemorrhage or hypoxia. Kupffer cells were isolated as described in Materials and Methods. Following overnight incubation, the cells were cultured with bioparticles for 1 hour and phagocytosis determined as described in Materials and Methods. Data are mean ± SE; n = 6 animals/group. #P < 0.05 compared with sham and hypoxia; *P < 0.05 compared with sham and trauma-hemorrhage.
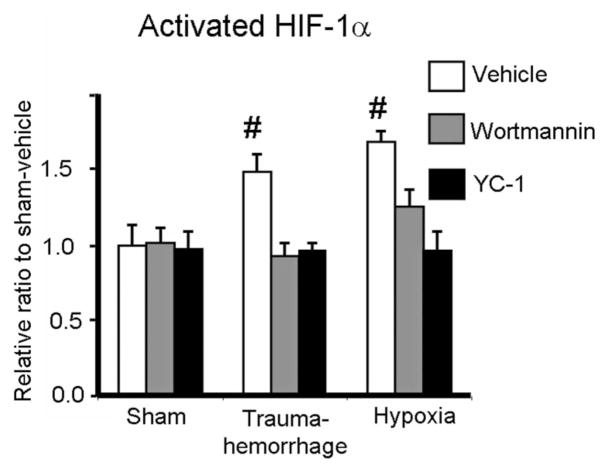
Activated HIF-1α Levels of Kupffer Cells Following Trauma-Hemorrhage and Hypoxia
Since trauma-hemorrhage-induced cellular hypoxia and breathing hypoxic gas-induced cellular hypoxia differentially affected Kupffer cell phagocytic capacity, even though the severity of hypoxia was similar between these 2 groups, we then examined whether there was any difference in the regulation of HIF-1α between the groups. Compared with shams, the expression of activated HIF-1α was similarly increased following trauma-hemorrhage or hypoxia. Administration of Wortmannin or YC-1 during trauma-hemorrhage or hypoxia normalized activated HIF-1α expression to sham levels (Fig. 5).
FIGURE 5.
Expression of activated HIF-1α in Kupffer cells following trauma-hemorrhage or hypoxia. Male C3H/HeN mice were subjected to sham treatment, trauma-hemorrhage or hypoxia as described in Materials and Methods. Each group of animals was given Wortmannin (1 mg/Kg body weight, BW), YC-1 (5 mg/Kg BW) or vehicle at maximum bleedout time or 30 minutes after induction of hypoxia. Upon harvesting, Kupffer cells were isolated, nuclear proteins were extracted, and HIF-1α levels were determined as described in Materials and Methods. Data are mean ± SE; n = 6 animals/group. #P < 0.05 compared with all the other groups.
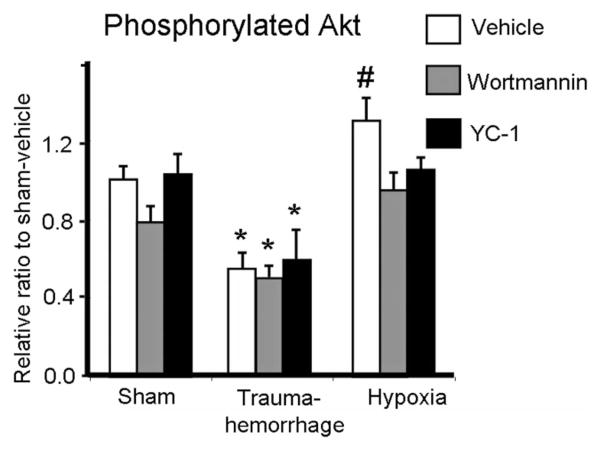
Effect of Trauma-Hemorrhage and Hypoxia on Kupffer Cell Akt Phosphorylation
As shown in Figure 6, trauma-hemorrhage-induced hypoxia and breathing hypoxic gas differentially affected Kupffer cell Akt phosphorylation. Following trauma-hemorrhage, the p-Akt levels were significantly decreased compared with shams. In contrast, hypoxia induced a significant increase in p-Akt levels compared with shams. The effects of hypoxia on p-Akt levels were abolished by Wortmannin or YC-1 administration. Furthermore, administration of Wortmannin or YC-1 in sham or trauma-hemorrhage mice did not affect Akt phosphorylation compared with vehicle.
FIGURE 6.
Expression of phosphorylated Akt in Kupffer cells following trauma-hemorrhage or hypoxia. Male C3H/HeN mice were subjected to sham treatment, trauma-hemorrhage or hypoxia as described in Materials and Methods. Each group of animals was given Wortmannin (1 mg/kg body weight, BW), YC-1 (5 mg/kg BW) or vehicle at maximum bleedout time or 30 minutes after induction of hypoxia. Kupffer cell phospho-Akt was determined as described in Materials and Methods. The value of p-Akt was normalized to the protein content. Data are mean ± SE; n= 6 animals/group. *P < 0.05 compared with sham and hypoxia groups; #P < 0.05 compared with all the other groups.
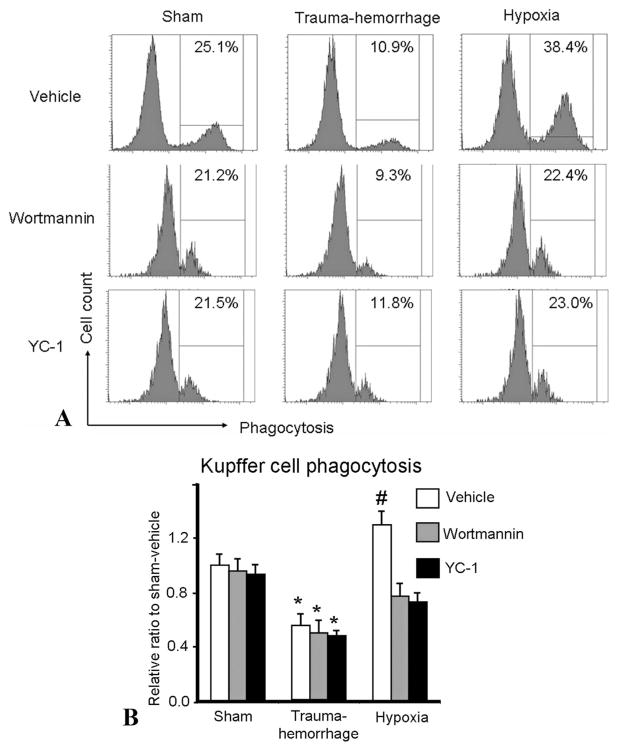
The Effect of P13K/Akt and HIF-1α Inhibition on Kupffer Cell Phagocytosis Following Trauma-Hemorrhage and Hypoxia
Following trauma-hemorrhage, Kupffer cell phagocytosis was significantly impaired compared with the sham group (Fig. 7). In contrast, hypoxia significantly enhanced Kupffer cell phagocytosis compared with shams. Similar to Akt phosphorylation, administration of Wortmannin or YC-1 reduced phagocytosis in the hypoxia group and the levels were similar to shams. Wortmannin or YC-1 did not alter Kupffer cell phagocytosis in the sham or trauma-hemorrhage groups.
FIGURE 7.
Kupffer cell phagocytic capacities following trauma-hemorrhage or hypoxia. Male C3H/HeN mice were subjected to sham treatment, trauma-hemorrhage or hypoxia as described in Materials and Methods. Each group of animals was given Wortmannin (1 mg/Kg body weight [BW]), YC-1 (5 mg/Kg BW) or vehicle at maximum bleedout time or 30 minutes after induction of hypoxia. Kupffer cells were isolated and cultured overnight. Following overnight incubation, the cells were cultured with bioparticles for 1 hour and phagocytosis was determined as described in Materials and Methods. A, The marked numbers in the gated region of representative histograms indicate the percentage of Kupffer cells that had ingested bioparticles. B, Data are shown as mean ± SE; n = 6 animals/ group. *P < 0.05 compared with sham and hypoxia groups; #P < 0.05 compared with all the other groups.
DISCUSSION
The eradication of invading microorganisms depends initially on innate immune response. Phagocytes, such as macrophages, play a key role in innate immunity and are part of the first line of defense as they recognize, ingest, and kill pathogens. However, invasion of pathogens often occurs at the site of tissue injury where blood and O2 supply are compromised. Therefore, successful control of infection requires phagocytes to function effectively even under a hypoxic environment. HIF-1α is a well-known transcription factor that is up-regulated following hypoxia and is recognized to play an essential role in myeloid cell inflammatory response.24 Peyssonnaux et al compared bacterial killing capacities of wild type, HIF-1α-null or vHL (von Hippel-Lindau tumor suppressor protein)-null macrophages and demonstrated that HIF-1α expression regulates the bactericidal capacity of phagocytes.6 Furthermore, functional inactivation of HIF-1α greatly inhibited cell motility, invasiveness, and adhesion of macrophages.24 –26 Thus, up-regulation of HIF-1α plays an important role in bacterial killing and in inflammatory responses.
Although trauma-hemorrhagic shock creates systemic and cellular hypoxic conditions, our results did not demonstrate an increased phagocytic capacity of Kupffer cells under those conditions. On the contrary, phagocytic capacity was suppressed after trauma-hemorrhage. This raises the question about the role of HIF-1α in the regulation of Kupffer cell phagocytosis following trauma-hemorrhage. Accordingly, we compared the similarity of cellular hypoxia between trauma-hemorrhage and the hypoxic animal model used in this study. Although adequate oxygenation of the tissue requires both normal oxygen tension and sufficient hemoglobin,27,28 either normal systemic PaO2 but low hemoglobin level in trauma-hemorrhage model or low PaO2 but normal hemoglobin level in hypoxemic model could result in tissue/cellular hypoxia. Using pimonidazole as a marker of hypoxia, our results showed that a similar percentage (80%) of the Kupffer cells from trauma-hemorrhage and hypoxia groups were positive for pimonidazole staining. Thus, a comparable hypoxic status of Kupffer cells was induced in the trauma-hemorrhage and hypoxia models.
Because hypoxia stabilizes HIF-1α,29 it was not surprising that the levels of activated HIF-1α in Kupffer cells were similarly increased following trauma-hemorrhage and hypoxia. Several studies have shown that increased HIF-1α levels contribute to the enhanced bactericidal capacity of myeloid cells following hypoxia.5,6 In the current study, we further demonstrated that hypoxia up-regulated Kupffer cell HIF-1α expression and enhanced phagocytic capacity. Although myeloid cell activities are regulated by HIF-1α,30,31 this does not explain our findings that Kupffer cells had a decreased phagocytic capacity following trauma-hemorrhage, even though HIF-1α levels were elevated. Thus, a pathway independent of HIF-1α must also be important in regulating Kupffer cell phagocytosis under those conditions.
The PI3K/Akt pathway is a key signal transduction pathway involved in the process of phagocytosis.18,32,33 Studies have shown that Akt activation was increased during phagocytosis.34,35 Furthermore, overexpression of Akt in macrophages enhanced phagocytic capacity.36 Previous studies from our laboratory have also shown that Akt activation in various tissues and cell types was decreased following trauma-hemorrhage.37– 40 Our present results reveal that although PI3K/Akt activation was inhibited following trauma-hemorrhage, it was increased after breathing hypoxic gas, thus indicating that PI3K/Akt activation is differentially regulated following those conditions. This hypoxia-induced activation of PI3K/Akt signaling has also been noted in several other cell types.41– 43 Consistent with those studies showing that the increased HIF-1α expression following hypoxia was regulated by PI3K/Akt signaling,1,44,45 we found that accumulation of HIF-1α following hypoxia was diminished if Wortmannin was administered during hypoxia. Furthermore, Wortmannin administration during hypoxia suppressed Kupffer cell phagocytosis. Therefore, it can be suggested that there is a crucial link between PI3K/Akt and HIF-1α activation in the enhancement of Kupffer cell phagocytosis. This notion is further supported by the observation that P13K/Akt down-regulation following trauma-hemorrhage was associated with suppressed Kupffer cells phagocytic capacity, even though HIF-1α expression was increased under these conditions.
It could be argued that, based on a physiologic point of view, it is relatively difficult to compare trauma-hemorrhage with pure hypoxia with an attendant decrease in oxygen tension. While both models similarly induced cellular hypoxia, the consequences produced during trauma-hemorrhage were probably an important element that made those cellular responses different from pure hypoxia.46 In future studies, a modified trauma-hemorrhage model that eliminates the effect of low perfusion (such as by bleeding the animals from one femoral artery while replacing volume with crystalloid through the femoral vein) might be necessary to clarify the role of hypoxia on the regulation of P13K/Akt activation. Furthermore, considering the therapeutic potential of Akt activation to maintain innate immunity following trauma-hemorrhage, administration of an Akt activator/agonist might well be an option.
The elevated HIF-1α levels following trauma-hemorrhage may result from either stabilization of the protein from degradation by hypoxia or increased HIF-1α production through activation of various pathways.47 Other than PI3K/Akt signaling, the mitogen-activated protein kinase (MAPK) reaction cascades such as p38-MAPK are another major pathway involved in HIF-1α activation.47– 49 In this regard, Kupffer cell p38-MAPK was activated following trauma-hemorrhage and that resulted in increased inflammatory cytokine production.50 HIF-1α has been shown to mediate the increased inflammatory response in cardiomyocytes51 and Kupffer cells (unpublished data) under such conditions. Taken together with those reports demonstrating the critical role of HIF-1α on regulating inflammatory cell function,31 it can be concluded that the increased HIF-1α expression following trauma-hemorrhage plays a role in the inflammatory response of Kupffer cells via activation of the p38-MAPK pathway.
It could also be argued that the present study used only one time point, ie, 2 hours after trauma-hemorrhage or hypoxia, and thus it remains unknown whether similar differences in Kupffer cell phagocytosis between trauma-hemorrhage and hypoxia exist for longer intervals of time. Although other time intervals have not been examined in this study, our previous studies have shown that if a depression or enhancement of immune cellular function was evident at 2 hours, it was also evident at 24 and 48 hours after an insult.19,52–54 Based upon those studies,19,52–54 it would appear that the differences in Kupffer cell phagocytosis following trauma-hemorrhage and hypoxia would be expected to exist for longer periods of time after those insults.
In summary, this current study demonstrated that cellular hypoxia and up-regulation of HIF-1α was not the exclusive factor that controlled phagocytosis. The results of cellular responses to decreased oxygen tension could be differentially regulated by different factors that induce cellular hypoxia.
Acknowledgments
Supported by NIH grant RO1 GM37127.
The authors thank Ms. Bobbi Smith for her skill and assistance in preparing this manuscript and for her editorial assistance.
References
- 1.Underhill DM, Ozinsky A. Phagocytosis of microbes: complexity in action. Annu Rev Immunol. 2002;20:825–852. doi: 10.1146/annurev.immunol.20.103001.114744. [DOI] [PubMed] [Google Scholar]
- 2.Jutras I, Desjardins M. Phagocytosis: at the crossroads of innate and adaptive immunity. Annu Rev Cell Dev Biol. 2005;21:511–527. doi: 10.1146/annurev.cellbio.20.010403.102755. [DOI] [PubMed] [Google Scholar]
- 3.Nogami Y, Kinoshita M, Takase B, et al. Liposome-encapsulated hemoglobin transfusion rescues rats undergoing progressive hemodilution from lethal organ hypoxia without scavenging nitric oxide. Ann Surg. 2008;248:310–319. doi: 10.1097/SLA.0b013e3181820c80. [DOI] [PubMed] [Google Scholar]
- 4.Brahimi-Horn MC, Pouyssegur J. Oxygen, a source of life and stress. FEBS Lett. 2007;581:3582–3591. doi: 10.1016/j.febslet.2007.06.018. [DOI] [PubMed] [Google Scholar]
- 5.Anand RJ, Gribar SC, Li J, et al. Hypoxia causes an increase in phagocytosis by macrophages in a HIF-1alpha-dependent manner. J Leukoc Biol. 2007;82:1257–1265. doi: 10.1189/jlb.0307195. [DOI] [PubMed] [Google Scholar]
- 6.Peyssonnaux C, Datta V, Cramer T, et al. HIF-1alpha expression regulates the bactericidal capacity of phagocytes. J Clin Invest. 2005;115:1806–1815. doi: 10.1172/JCI23865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kobbe P, Kaczorowski DJ, Vodovotz Y, et al. Local exposure of bone components to injured soft tissue induces toll-like-receptor-4 dependent systemic inflammation with acute lung injury. Shock. 2008;30:686–691. doi: 10.1097/SHK.0b013e31816f257e. [DOI] [PubMed] [Google Scholar]
- 8.Meng X, Ao L, Song Y, et al. Signaling for myocardial depression in hemorrhagic shock: roles of Toll-like receptor 4 and p55 TNF-alpha receptor. Am J Physiol Regul Integr Comp Physiol. 2005;288:R600–R606. doi: 10.1152/ajpregu.00182.2004. [DOI] [PubMed] [Google Scholar]
- 9.Frankel DA, Acosta JA, Anjaria DJ, et al. Physiologic response to hemorrhagic shock depends on rate and means of hemorrhage. J Surg Res. 2007;143:276–280. doi: 10.1016/j.jss.2007.01.031. [DOI] [PubMed] [Google Scholar]
- 10.Noel G, Guo X, Wang Q, et al. Postburn monocytes are the major producers of TNF-alpha in the heterogeneous splenic macrophage population. Shock. 2007;27:312–319. doi: 10.1097/01.shk.0000239753.75088.5e. [DOI] [PubMed] [Google Scholar]
- 11.Meldrum DR. G-protein-coupled receptor 30 mediates estrogen’s non-genomic effects after hemorrhagic shock and trauma. Am J Pathol. 2007;170:1148–1151. doi: 10.2353/ajpath.2007.070025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shelley O, Murphy T, Paterson H, et al. Interaction between the innate and adaptive immune systems is required to survive sepsis and control inflammation after injury. Shock. 2003;20:123–129. doi: 10.1097/01.shk.0000079426.52617.00. [DOI] [PubMed] [Google Scholar]
- 13.Rana MW, Ayala A, Dean RE, et al. Decreased Fc receptor expression on macrophages following simple hemorrhage as observed by scanning immunoelectron microscopy. J Leukoc Biol. 1990;48:512–518. doi: 10.1002/jlb.48.6.512. [DOI] [PubMed] [Google Scholar]
- 14.Nickel EA, Hsieh CH, Chen JG, et al. Estrogen suppresses cardiac IL-6 after trauma-hemorrhage via a hypoxia-inducible factor-1α-mediated pathway. Shock. 2009;31:354–358. doi: 10.1097/SHK.0b013e3181862fdd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kan WH, Hsu JT, Schwacha MG, et al. Selective inhibition of iNOS attenuates trauma-hemorrhage/resuscitation-induced hepatic injury. J Appl Physiol. 2008;105:1076–1082. doi: 10.1152/japplphysiol.90495.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Williams DL, Ozment-Skelton T, Li C. Modulation of the phosphoinositide 3-kinase signaling pathway alters host response to sepsis, inflammation, and ischemia/reperfusion injury. Shock. 2006;25:432–439. doi: 10.1097/01.shk.0000209542.76305.55. [DOI] [PubMed] [Google Scholar]
- 17.Zhong H, Chiles K, Feldser D, et al. Modulation of hypoxia-inducible factor 1alpha expression by the epidermal growth factor/phosphatidylinositol 3-kinase/PTEN/AKT/FRAP pathway in human prostate cancer cells: implications for tumor angiogenesis and therapeutics. Cancer Res. 2000;60:1541–1545. [PubMed] [Google Scholar]
- 18.Araki N, Johnson MT, Swanson JA. A role for phosphoinositide 3-kinase in the completion of macropinocytosis and phagocytosis by macrophages. J Cell Biol. 1996;135:1249–1260. doi: 10.1083/jcb.135.5.1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hsieh CH, Frink M, Hsieh YC, et al. The role of MIP-1α in the development of systemic inflammatory response and organ injury following trauma hemorrhage. J Immunol. 2008;181:2806–2812. doi: 10.4049/jimmunol.181.4.2806. [DOI] [PubMed] [Google Scholar]
- 20.Zheng R, Pan G, Thobe BM, et al. MyD88 and Src are differentially regulated in Kupffer cells of males and proestrus females following hypoxia. Mol Med. 2006;12:65–73. doi: 10.2119/2006-00030.Zheng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Thobe BM, Frink M, Choudhry MA, et al. Src family kinases regulate p38 MAPK-mediated IL-6 production in Kupffer cells following hypoxia. Am J Physiol Cell Physiol. 2006;291:C476–C482. doi: 10.1152/ajpcell.00076.2006. [DOI] [PubMed] [Google Scholar]
- 22.Frink M, Hsieh YC, Thobe BM, et al. TLR4 regulates Kupffer cell chemokine production, systemic inflammation and lung neutrophil infiltration following trauma-hemorrhage. Mol Immunol. 2007;44:2625–2630. doi: 10.1016/j.molimm.2006.12.009. [DOI] [PubMed] [Google Scholar]
- 23.Hofer SO, Mitchell GM, Penington AJ, et al. The use of pimonidazole to characterise hypoxia in the internal environment of an in vivo tissue engineering chamber. Br J Plast Surg. 2005;58:1104–1114. doi: 10.1016/j.bjps.2005.04.033. [DOI] [PubMed] [Google Scholar]
- 24.Walmsley SR, Cadwallader KA, Chilvers ER. The role of HIF-1alpha in myeloid cell inflammation. Trends Immunol. 2005;26:434–439. doi: 10.1016/j.it.2005.06.007. [DOI] [PubMed] [Google Scholar]
- 25.Walmsley SR, Sheares KK, Sobolewski A, et al. New insights into oxygen sensing at a cellular level. Thorax. 2004;59:90–92. doi: 10.1136/thorax.2003.017020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Walmsley SR, Print C, Farahi N, et al. Hypoxia-induced neutrophil survival is mediated by HIF-1alpha-dependent NF-kappaB activity. J Exp Med. 2005;201:105–115. doi: 10.1084/jem.20040624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Moore FA, Nelson T, McKinley BA, et al. Massive transfusion in trauma patients: tissue hemoglobin oxygen saturation predicts poor outcome. J Trauma. 2008;64:1010–1023. doi: 10.1097/TA.0b013e31816a2417. [DOI] [PubMed] [Google Scholar]
- 28.Rosenthal G, Morabito D, Cohen M, et al. Use of hemoglobin-based oxygen-carrying solution-201 to improve resuscitation parameters and prevent secondary brain injury in a swine model of traumatic brain injury and hemorrhage: laboratory investigation. J Neurosurg. 2008;108:575–587. doi: 10.3171/JNS/2008/108/3/0575. [DOI] [PubMed] [Google Scholar]
- 29.Zarember KA, Malech HL. HIF-1alpha: a master regulator of innate host defenses? J Clin Invest. 2005;115:1702–1704. doi: 10.1172/JCI25740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cramer T, Johnson RS. A novel role for the hypoxia inducible transcription factor HIF-1alpha: critical regulation of inflammatory cell function. Cell Cycle. 2003;2:192–193. [PubMed] [Google Scholar]
- 31.Cramer T, Yamanishi Y, Clausen BE, et al. HIF-1alpha is essential for myeloid cell-mediated inflammation. Cell. 2003;112:645–657. doi: 10.1016/s0092-8674(03)00154-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cox D, Tseng CC, Bjekic G, et al. A requirement for phosphatidylinositol 3-kinase in pseudopod extension. J Biol Chem. 1999;274:1240–1247. doi: 10.1074/jbc.274.3.1240. [DOI] [PubMed] [Google Scholar]
- 33.Qian Y, Corum L, Meng Q, et al. PI3K induced actin filament remodeling through Akt and p70S6K1: implication of essential role in cell migration. Am J Physiol Cell Physiol. 2004;286:C153–C163. doi: 10.1152/ajpcell.00142.2003. [DOI] [PubMed] [Google Scholar]
- 34.Indik ZK, Park JG, Hunter S, et al. The molecular dissection of Fc gamma receptor mediated phagocytosis. Blood. 1995;86:4389–4399. [PubMed] [Google Scholar]
- 35.Tilton B, Andjelkovic M, Didichenko SA, et al. G-Protein-coupled receptors and Fcgamma-receptors mediate activation of Akt/protein kinase B in human phagocytes. J Biol Chem. 1997;272:28096–28101. doi: 10.1074/jbc.272.44.28096. [DOI] [PubMed] [Google Scholar]
- 36.Ganesan LP, Wei G, Pengal RA, et al. The serine/threonine kinase Akt Promotes Fc gamma receptor-mediated phagocytosis in murine macrophages through the activation of p70S6 kinase. J Biol Chem. 2004;279:54416–54425. doi: 10.1074/jbc.M408188200. [DOI] [PubMed] [Google Scholar]
- 37.Yu HP, Hsieh YC, Suzuki T, et al. The PI3K/Akt pathway mediates the nongenomic cardioprotective effects of estrogen following trauma-hemorrhage. Ann Surg. 2007;245:971–977. doi: 10.1097/01.sla.0000254417.15591.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yu HP, Hsieh YC, Suzuki T, et al. Mechanism of the nongenomic effects of estrogen on intestinal myeloperoxidase activity following trauma-hemorrhage: up-regulation of the PI-3K/Akt pathway. J Leukoc Biol. 2007;82:774–780. doi: 10.1189/jlb.0307182. [DOI] [PubMed] [Google Scholar]
- 39.Hsu JT, Kan WH, Hsieh CH, et al. Mechanism of estrogen-mediated attenuation of hepatic injury following trauma-hemorrhage: Akt-dependent HO-1 up-regulation. J Leukoc Biol. 2007;82:1019–1026. doi: 10.1189/jlb.0607355. [DOI] [PubMed] [Google Scholar]
- 40.Hsu JT, Kan WH, Hsieh YC, et al. Mechanism of estrogen-mediated improvement in cardiac function following trauma-hemorrhage: p38-dependent normalization of cardiac Akt phosphorylation and glycogen levels. Shock. 30:372–378. doi: 10.1097/SHK.0b013e318164f25c. [DOI] [PubMed] [Google Scholar]
- 41.Sung SM, Jung DS, Kwon CH, et al. Hypoxia/reoxygenation stimulates proliferation through PKC-dependent activation of ERK and Akt in mouse neural progenitor cells. Neurochem Res. 2007;32:1932–1939. doi: 10.1007/s11064-007-9390-1. [DOI] [PubMed] [Google Scholar]
- 42.Kwon DS, Kwon CH, Kim JH, et al. Signal transduction of MEK/ERK and PI3K/Akt activation by hypoxia/reoxygenation in renal epithelial cells. Eur J Cell Biol. 2006;85:1189–1199. doi: 10.1016/j.ejcb.2006.06.001. [DOI] [PubMed] [Google Scholar]
- 43.Alvarez-Tejado M, Naranjo-Suarez S, Jimenez C, et al. Hypoxia induces the activation of the phosphatidylinositol 3-kinase/Akt cell survival pathway in PC12 cells: protective role in apoptosis. J Biol Chem. 2001;276:22368–22374. doi: 10.1074/jbc.M011688200. [DOI] [PubMed] [Google Scholar]
- 44.Belaiba RS, Bonello S, Zahringer C, et al. Hypoxia Up-Regulates Hypoxia-Inducible Factor-1α Transcription by Involving Phosphatidylinositol 3-Kinase and Nuclear Factor κB in Pulmonary Artery Smooth Muscle Cells. Mol Biol Cell. 2007;18:4691–4697. doi: 10.1091/mbc.E07-04-0391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sun HL, Liu YN, Huang YT, et al. YC-1 inhibits HIF-1 expression in prostate cancer cells: contribution of Akt/NF-kappaB signaling to HIF-1alpha accumulation during hypoxia. Oncogene. 2007;26:3941–3951. doi: 10.1038/sj.onc.1210169. [DOI] [PubMed] [Google Scholar]
- 46.Gutierrez G, Reines HD, Wulf-Gutierrez ME. Clinical review: hemorrhagic shock. Crit Care. 2004;8:373–381. doi: 10.1186/cc2851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hellwig-Burgel T, Stiehl DP, Wagner AE, et al. Review: hypoxia-inducible factor-1 (HIF-1): a novel transcription factor in immune reactions. J Interferon Cytokine Res. 2005;25:297–310. doi: 10.1089/jir.2005.25.297. [DOI] [PubMed] [Google Scholar]
- 48.Sang N, Stiehl DP, Bohensky J, et al. MAPK signaling up-regulates the activity of hypoxia-inducible factors by its effects on p300. J Biol Chem. 2003;278:14013–14019. doi: 10.1074/jbc.M209702200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gusterson R, Brar B, Faulkes D, et al. The transcriptional co-activators CBP and p300 are activated via phenylephrine through the p42/p44 MAPK cascade. J Biol Chem. 2002;277:2517–2524. doi: 10.1074/jbc.M104626200. [DOI] [PubMed] [Google Scholar]
- 50.Hsieh YC, Frink M, Thobe BM, et al. 17Beta-estradiol downregulates Kupffer cell TLR4-dependent p38 MAPK pathway and normalizes inflammatory cytokine production following trauma-hemorrhage. Mol Immunol. 2007;44:2165–2172. doi: 10.1016/j.molimm.2006.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hsieh YC, Choudhry MA, Yu HP, et al. Inhibition of cardiac PGC-1alpha expression abolishes ERbeta agonist-mediated cardioprotection following trauma-hemorrhage. FASEB J. 2006;20:1109–1117. doi: 10.1096/fj.05-5549com. [DOI] [PubMed] [Google Scholar]
- 52.Yu HP, Shimizu T, Hsieh YC, et al. Tissue-specific expression of estrogen receptors and their role in the regulation of neutrophil infiltration in various organs following trauma-hemorrhage. J Leukoc Biol. 2006;79:963–970. doi: 10.1189/jlb.1005596. [DOI] [PubMed] [Google Scholar]
- 53.Wang M, Markel T, Crisostomo P, et al. Deficiency of TNFR1 protects myocardium through SOCS3 and IL-6 but not p38 MAPK or IL-1beta. Am J Physiol Heart Circ Physiol. 2007;292:H1694–H1699. doi: 10.1152/ajpheart.01063.2006. [DOI] [PubMed] [Google Scholar]
- 54.Terrell AM, Crisostomo PR, Wairiuko GM, et al. Jak/STAT/SOCS signaling circuits and associated cytokine-mediated inflammation and hypertrophy in the heart. Shock. 2006;26:226–234. doi: 10.1097/01.shk.0000226341.32786.b9. [DOI] [PubMed] [Google Scholar]