Abstract
Mechanical ventilation at high tidal volume may cause pulmonary capillary leakage and acute lung inflammation culminating in ventilator-induced lung injury. Iloprost is a stable synthetic analogue of prostaglandin I2 used for treatment of pulmonary hypertension, which also showed endothelium-dependent anti-edemagenic effects in the models of lung injury. To test the hypothesis that iloprost may attenuate lung inflammation and lung endothelial barrier disruption caused by pathologic lung distension and coagulation system component thrombin, we used cell and animal two-hit models of ventilator-induced lung injury. Mice received triple injection of iloprost (2 μg/kg, intravenous instillation) at 0, 40 and 80 min after onset of high tidal volume (HTV) mechanical ventilation (30 ml/kg, 4 hrs) combined with administration of thrombin receptor activating peptide 6 (TRAP6, 3 × 10−7 mol/mouse, intratracheal instillation). After 4 hrs of ventilation, bronchoalveolar lavage (BAL), histological analysis, and measurements of Evans blue accumulation in the lung tissue lung were performed. Effects of iloprost on endothelial barrier dysfunction were further assessed in pulmonary endothelial cells (EC) exposed to thrombin and pathologic (18%) cyclic stretch. Combination of HTV and TRAP6 enhanced accumulation of neutrophils in BAL fluid and lung parenchyma, increased BAL protein content and endothelial permeability judged by Evans blue extravasation in the lung tissue. These effects were markedly attenuated by iloprost. Application of 18% cyclic stretch to pulmonary EC enhanced thrombin-induced EC paracellular gap formation and Rho-GTPase-mediated phosphorylation of regulatory myosin light chains and myosin phosphatase. Iloprost markedly inhibited Rho-kinase mediated site-specific phosphorylation of myosin phosphatase, and prevented cyclic stretch- and thrombin-induced endothelial monolayer disruption. This study characterizes for the first time the protective effects of iloprost in the in vitro and in vivo two-hit models of VILI and supports consideration of iloprost as a new therapeutic treatment of VILI.
Keywords: ventilator-induced lung injury, vascular permeability, cyclic stretch, prostacyclin, thrombin, Rho
INTRODUCTION
Although ventilator support is an indispensable treatment for critically ill patients, ventilator-induced lung injury (VILI) with the associated multi-organ dysfunction may lead to significant morbidity and mortality and thus remains one of the most important problems in the management of patients in the intensive care unit [1]. Pathologic lung over-distention is associated with mechanical ventilation at high tidal volumes and compromises the blood-gas barrier, increases lung permeability, and may culminate in VILI and pulmonary edema [2, 3]. Activation of coagulation system and elevation of pro-coagulant and endothelium activating mediator thrombin is also linked to the development of VILI [4, 5]. Clinical investigations and experimental models of ALI/ARDS, VILI, and pneumonia show beneficial effects of anticoagulant therapy [4–7]. Vascular leak observed in VILI patients is associated with increased levels of edemagenic and inflammatory mediators such as thrombin, histamine, TNF-alpha, IL-8, and IL-1 [2, 8–10]. However, the significance of the interactions between the edemagenic agents and pathological lung mechanical distension in progression of VILI-associated vascular leak and pulmonary edema has been only recently recognized. A model of pulmonary endothelial cells (EC) exposed to controlled levels of cyclic stretch (CS) and agonist stimulation is a unique tool that reproduces VILI conditions in vitro. Using this model, we have described differential effects of physiologic and pathologic CS magnitudes on the agonist-induced EC barrier disruption [11–13]. Previous reports by others and our data suggest that attenuation of Rho activity and stimulation of Rac-dependent mechanisms reduces lung vascular leak induced by pathologic cyclic stretch and inflammatory agents and promotes barrier recovery in the in vitro and in vivo models of ALI/VILI [11, 12, 14–16].
Prostacyclin PGI2, a product of cyclooxygenase, has been implicated in the regulation of vascular function, wound repair, inflammatory processes, and acute lung injury. Aerosolized prostacyclin shows marked protection against hyperoxic lung injury or lung damage caused by ischemia/reperfusion, and increased levels of prostacyclin stable metabolites have been associated with less severe respiratory distress [17, 18]. We have previously demonstrated potent barrier-protective effects of prostacyclin stable analog beraprost in human pulmonary EC [19]. Our and other studies have shown that barrier-protective effects of prostacyclin on pulmonary EC are mediated by cAMP-activated protein kinase (PKA), cAMP-activated guanine exchange factor Epac, that triggers its effector GTPase Rap1, and downstream signaling to Tiam1/Vav2 and Rac GTPase [19–21]. In turn, Rac activation leads to actin cytoskeletal remodeling and enhancement of adherens junctions [19].
In the current study, we examined effects of stable prostacyclin analog iloprost on EC barrier disruption induced by pathologic CS stimulation and thrombin in the in vitro model of VILI. Protective effects of iloprost were further evaluated in vivo, in a two-hit model of lung injury induced by protease activated receptor 1 (PAR1) receptor ligand TRAP6 and mechanical ventilation at high tidal volume.
MATERIALS AND METHODS
Reagents
Di-phospho-MLC and GEF-H1 antibodies were obtained from Cell Signaling (Beverly, MA); phospho-Thr850-MYPT antibodies were purchased from Upstate Biotechnology (Lake Placid, NY). TRAP6 was obtained from AnaSpec (San Jose, CA). All reagents for immunofluorescence staining were purchased from Molecular Probes (Eugene, OR). Unless specified, biochemical reagents were obtained from Sigma (St. Louis, MO).
Rats were randomized to concurrently receive an intravenous bolus of sterile PBS or OxPAPC (1.5 mg/kg) via the jugular vein at the initiation of mechanical ventilation.
Mechanical ventilation protocol
Adult male C57BL/6J mice, 8–10-week-old, with average weight 20–25 g (Jackson Laboratories, Bar Harbor, ME) were anesthetized with an intraperitoneal injection of ketamine (75 mg/kg) and acepromazine (1.5 mg/kg). Tracheotomy was performed and the trachea was cannulated with a 20-gauge one inch catheter (Penn-Century, Philadelphia, PA), which was tied into place to prevent air leak. The animals were placed on mechanical ventilator (Harvard Apparatus, Boston, MA) for 4 hr with high tidal volume ventilation (30 ml/kg, 75 breaths per minute and 0 PEEP, HTV), as we have previously described [14]. Control animals were anesthetized and allowed to breathe spontaneously. Mice were given a single dose of sterile saline solution or intratracheal (i/t) TRAP6 (3 × 10−7 mol/mouse) at the initiation of mechanical ventilation. Mice were randomized to concurrently receive sterile saline solution or iloprost (2 μg/kg, intravenous (i/v)) at three time points (0, 40, and 80 min) during mechanical ventilation. Intravenous injection into internal jugular vein suggests specific iloprost delivery to the lung vascular endothelium. Experimental animals yielded the following groups: control, iloprost, TRAP6, HTV, iloprost + HTV, TRAP6/HTV, and iloprost + TRAP6/HTV. After the experiment, animals were sacrificed by exsanguination under anesthesia. BAL was performed using 1 ml of sterile Hanks Balanced Saline Buffer. The BAL protein concentration was determined by a modified Lowry colorimetric assay using a Bio-Rad DC protein assay kit (Bio-Rad Laboratories, Hercules, CA). BAL inflammatory cell counting was performed using a standard hemacytometer technique [22, 23]. All experimental protocols involving the use of animals were approved by the University of Chicago Institutional Animal Care & Use Committee for the humane treatment of experimental animals. The animals were housed in pathogen-free conditions in the University of Chicago Animal Care Facilities where they were cared for in accordance with institutional and National Institutes of Health (NIH) guidelines.
Histological assessment of lung injury
Left lungs were intratracheally instilled with 10% formaldehyde from 20 cm height, immersed in 10% formaldehyde for at least 24 hours and then embedded in paraffin. After deparaffinization and dehydration, the lungs were cut into 4-μm sections, and stained with hematoxylin and eosin. Alveolar fluid accumulation and neutrophil infiltration as indices of lung leak and inflammation were evaluated by bright field microscopy of lung tissue sections at × 40 magnification.
Assessment of pulmonary vascular leakage by Evans blue
This analysis was done as we have described previously [23]. Briefly, Evans blue dye (EBD, 30 ml/kg, i/v) was injected into the external jugular vein 2 hrs before termination of experiment to assess vascular leak. In brief, at the end of ventilation, thoracotomy was performed, and the lungs were perfused free of blood with PBS containing 5 mM EDTA. Both left lung and right lung were excised and imaged by Kodak digital camera. After imaging lungs were blotted dry, weighed, homogenized in PBS (1 ml/100 μg tissue) and used for quantitative analysis of EB tissue accumulation as described elsewhere [24]. Homogenized tissue was incubated with 2 volume formamide (18 h, 60°C), centrifuged at 12,000 g for 20 min. Optical density of the supernatant was determined by spectrophotometry at 620 nm and 740 nm. The concentration of extravasated Evans blue dye (μg of Evans blue dye per g lung) in lung homogenates was calculated against a standard curve.
Cell culture under cyclic stretch
Human pulmonary artery endothelial cells (HPAEC) and cell culture basal medium (EBM-2) with growth supplements were obtained from Lonza (Allendale, NJ), cultured according to the manufacturer’s protocol, and used at passages 5–9. All CS experiments were performed using FX-4000T Flexcell Tension Plus system (Flexcell International, McKeesport, PA) equipped with 25 mm BioFlex Loading station, as previously described [13, 25]. In brief, after 72 hr of culture on collagen I-coated Flexcell plates, cells were exposed to high magnitude cyclic stretch (18% linear elongation, sinusoidal wave, 25 cycles/min) to recapitulate the mechanical stresses experienced by the alveolar endothelium at high tidal volume mechanical ventilation [25–27]. At two hours, plates were treated vehicle or iloprost (200 ng/ml) for 15 min followed by treatment with thrombin (0.1 U/ml) for another 15 min with continuous exposure to cyclic stretch. Control BioFlex plates with static EC culture were placed in the same cell culture incubator and processed similarly to CS-preconditioned cells. At the end of experiment, cell lysates were collected for western blot analysis, or CS-exposed endothelial monolayers were fixed with 3.7% formaldehyde and used for immunofluorescence staining as previously described [11, 28, 29].
Immunoblotting
After stimulation cells were lysed, and protein extracts were separated by SDS-PAGE, transferred to nitrocellulose membrane, and probed with specific antibodies as previously described [28]. Levels of phosphorylated or activated proteins were normalized to the total protein content.
Statistical analysis
Results are expressed as means ± SD of three to ten independent experiments. Stimulated samples were compared to the controls using the unpaired Student’s t-test. For multiple-group comparisons, a one-way variance analysis (ANOVA) and post hoc multiple comparisons tests were used. P<0.05 was considered statistically significant.
RESULTS
Effects of iloprost on VILI-induced lung inflammation and barrier dysfunction
The following experiments attempted to reproduce the two-hit model of lung injury induced by high tidal volume (HTV) mechanical ventilation and TRAP6, the thrombin-derived non-thrombogenic peptide that serves as a PAR1 receptor ligand. During optimization of experimental conditions, TRAP6 was tested in vivo at two concentrations (6×10−7 mol/mouse and 3×10−7 mol/mouse, i/t, 4 hr). Higher TRAP6 dose increased protein content of bronchoalveolar lavage fluid (BAL) in spontaneously ventilated mice (0.36±0.06 mg/ml vs. 0.14±0.04 mg/ml in control, p<0.05), which was attenuated by iloprost (0.19±0.07 mg/ml, p<0.05), but increased lethality in mice exposed to HTV. Lower doses of TRAP6 (3×10−7 mol/mouse) used in the following experiments with HTV did not affect BAL cell count and protein concentration (1.35 ± 0.53 × 105 cells/ml vs. 1.44 ± 0.41 × 105 cells/ml in control, and 0.16±0.05 mg/ml vs. 0.17±0.03 mg/ml in control, respectively).
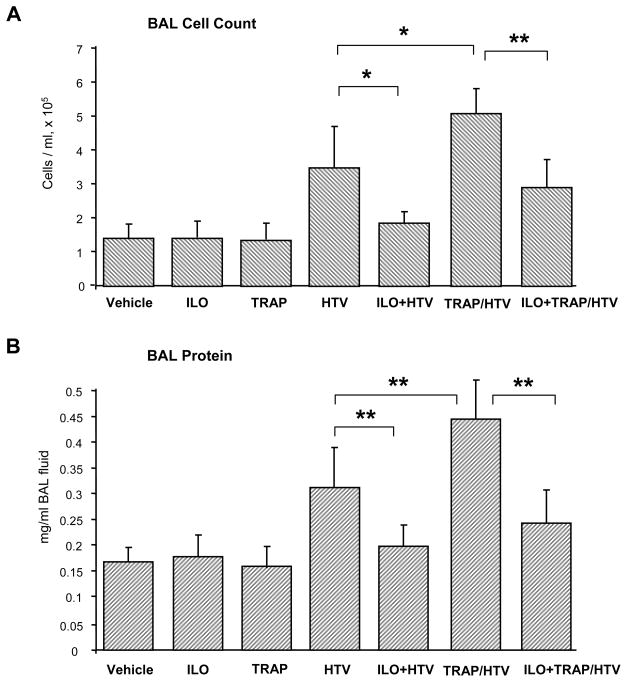
Mice were treated with TRAP6 at the onset of HTV mechanical ventilation (30 ml/kg, 4 hr). Instillation of iloprost or sterile saline was performed at three time points (0, 40, and 80 min) during mechanical ventilation. Control experiments were conducted using animals breathing spontaneously without TRAP6 instillation. Bronchoalveolar lavage (BAL) fluid was collected after 4 hr of HTV, and lung injury was evaluated by measurements of cell counts and protein concentration in BAL fluid. TRAP6 administration further promoted HTV-induced lung injury detected by increased BAL cell counts and protein concentration (Figure 1). HTV alone induced more than 2-fold increase in BAL cell count, as compared to controls (3.49 ± 1.18 × 105 cells/ml vs. 1.44 ± 0.41 × 105 cells/ml, p<0.03). Combination of TRAP6 and HTV induced nearly 1.5-fold increase in BAL cell count in comparison to HTV alone (5.09 ± 0.75 × 105 cells/ml vs. 3.49 ± 1.18 × 105 cells/ml, p<0.03). Importantly, iloprost instillation dramatically decreased cell counts in both, HTV and TRAP6/HTV models (HTV alone: 1.89 ± 0.31 × 105 cells/ml vs. 3.49 ± 1.18 × 105 cells/ml, p<0.03; TRAP6/HTV: 2.95 ± 0.83 × 105 cells/ml vs. 5.09 ± 0.75 × 105 cells/ml, p<0.005) (Figure 1A). HTV induced nearly 2-fold increase in the total protein concentration in BAL fluid (0.32 ± 0.08 mg/ml vs. 0.17 ± 0.03 mg/ml in controls, p<0.005) reflecting increased lung vascular permeability. TRAP6 injection further stimulated HTV-induced elevation of BAL protein content (0.44 ± 0.08 mg/ml vs. 0.32 ± 0.08 mg/ml, p<0.03 for HTV alone). Again, iloprost markedly decreased protein accumulation in BAL from both, HTV- and TRAP6/HTV-treated animals (0.18 ± 0.04 mg/ml vs. 0.32 ± 0.08 mg/ml for HTV, p<0.005; and 0.25 ± 0.07 mg/ml vs. 0.44 ± 0.08 mg/ml for TRAP6/HTV, p<0.005) (Figure 1B). BAL cell count and protein content were similar in iloprost-treated and untreated animals in the absence of mechanical ventilation.
Figure 1. Effects of iloprost on BAL cell count and protein content in the two-hit VILI model.
Mice were subjected to mechanical ventilation at high tidal volume (HTV, 30 ml/kg, 4 hr) with or without TRAP6 injection (3×10−7 mol/mouse i/t), or left spontaneously ventilated. Animals were treated with iloprost (2 μg/kg, i/v) or sterile saline at three time points (0, 40, and 80 min) during mechanical ventilation. Cell count (A) and measurements of protein concentration (B) were performed in BAL fluid taken from control and experimental animals; n=6–10 per condition; *p<0.03, **p<0.005.
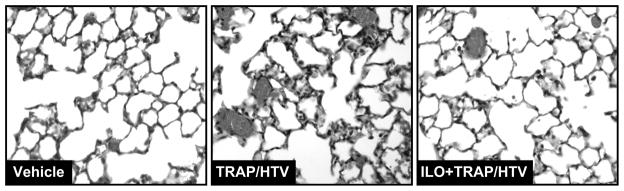
Histological analysis of lung paraffin-embedded tissue sections stained with hematoxylin and eosin revealed neutrophil accumulation in the lung parenchyma, alveolar accumulation of protein enriched fluid, and areas of alveolar hemorrhage indicative of vascular disruption caused by TRAP6/HTV. These effects were diminished in iloprost-treated mice (Figure 2). Treatment with iloprost alone caused no histological changes in the mice lungs, as compared with control animals (data not shown).
Figure 2. Histological assessment of iloprost effect in the VILI model.
Histological assessment of lung injury was performed in lung tissue samples from control and TRAP6/HTV treated mice with or without iloprost instillation. Whole lungs were fixed in formaldehyde, embedded in paraffin, and used for histological evaluation by hematoxylin and eosin staining; n=4–6 per condition; magnification × 40.
Effects of iloprost against TRAP6/HTV-induced vascular leak
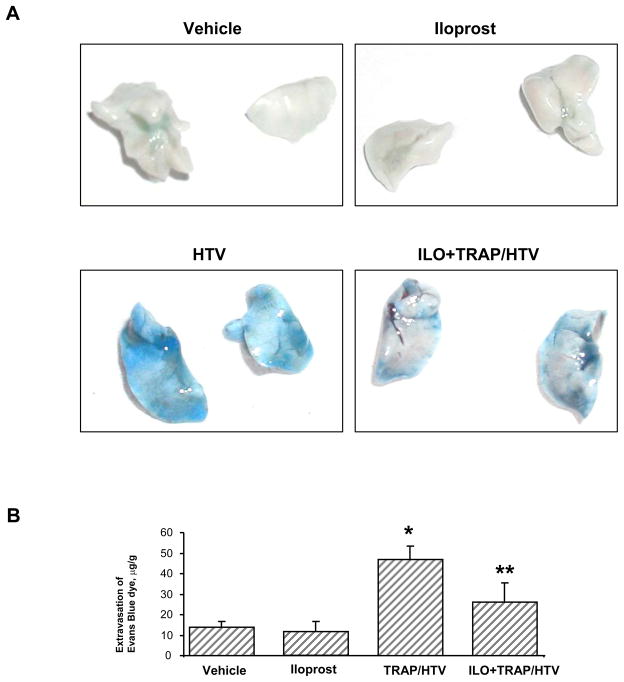
Protective effects of iloprost against lung vascular leak caused by high tidal volume mechanical ventilation in the presence of TRAP6 were assessed by measurements of Evans blue extravasation into the lung tissue. Evans blue dye was intravenously injected 2 hr before termination of ventilation. TRAP6/HTV induced prominent Evans blue leakage from the vascular space into the lung parenchyma, which was markedly suppressed by iloprost administration (Figure 3A). These results were confirmed by quantitative analysis of Evans blue-labeled albumin extravasation in the lung samples (Figure 3B). Evans blue dye accumulation was significantly elevated in the lungs from animals subjected to mechanical ventilation in the presence of TRAP6 (46.99 ± 6.47 μg/g wet weight lung vs. 13.87 ± 2.89 μg/g wet weight lung in non-treated controls, p<0.01). Iloprost instillation markedly reduced TRAP6/HTV-induced tissue accumulation of Evans blue (26.05 ± 9.57 μg/g wet weight lung vs. 46.99 ± 6.47 μg/g wet weight lung, p<0.05). There was no significant difference in the Evans blue dye accumulation in iloprost-treated animals and controls in the absence of HTV (12.12 ± 4.72 μg/g wet weight lung vs. 13.87 ± 2.89 μg/g wet weight lung). Taken together, our results show potent protective effect of iloprost in the in vitro and in vivo two-hit models of VILI and vascular leak.
Figure 3. Effects of iloprost on lung vascular leak in the VILI model.
Effects of iloprost (2 μg/kg, i/v) on the TRAP6/HTV-induced vascular leak were evaluated visually by Evans blue accumulation in the lung tissue samples (A). In addition, quantitative analysis of Evans blue labeled albumin extravasation was performed by spectrophotometric analysis of Evans blue extracted from the lung tissue samples (B); n=4–8 per condition; *p<0.01 for TRAP6/HTV vs. non-treated controls; **p<0.05 for iloprost + TRAP6/HTV vs. TRAP6/HTV.
Effects of iloprost on cytoskeletal remodeling induced by combination of thrombin and pathologic CS
Using pulmonary EC preconditioned at pathologically relevant CS levels (18% CS), we evaluated protective effects of iloprost on EC barrier dysfunction induced by 18% CS and thrombin. Effects of pathologic CS were judged by enhanced paracellular gap formation and activation of actomyosin contraction indicated by increased myosin light chain (MLC) phosphorylation [11].
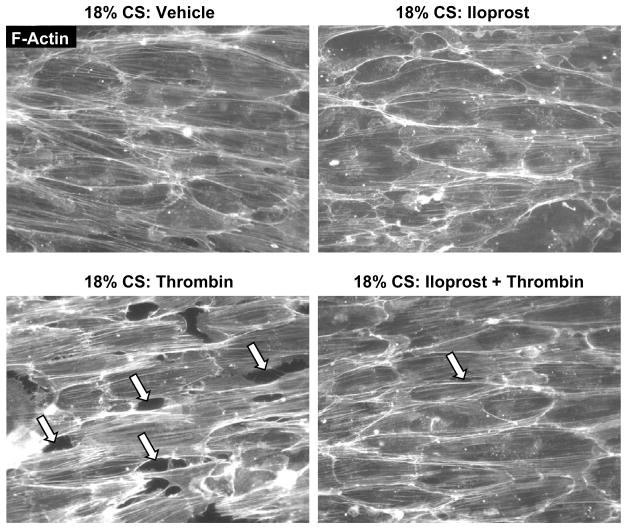
Pulmonary EC preconditioned at 18% CS (2.5 hr) were pretreated with iloprost or vehicle prior to thrombin challenge with continuous CS exposure. In agreement with our previous studies [11, 30], CS preconditioning at 18% elongation during 2.5 hr caused cytoskeletal reorientation without affecting EC monolayer integrity, while thrombin challenge caused rapid monolayer disruption and increased central stress fiber formation. Pretreatment with iloprost abolished paracellular gap formation and markedly reduced stress fiber formation leading to preservation of monolayer integrity in CS- and thrombin-stimulated EC (Figure 4). Collectively, these results strongly suggest the mechanism of the iloprost-induced reduction in actin stress fibers and paracellular gap formation in EC exposed to 18% CS and thrombin via iloprost-induced attenuation of Rho signaling.
Figure 4. Effects of iloprost on EC monolayer integrity in the in vitro model of VILI.
EC monolayers grown on Flexcell plates were preconditioned at pathologic (18%) levels of CS for 2.5 hr and stimulated with thrombin (0.1 U/ml, 15 min) with or without iloprost (200 ng/ml, 15 min) pretreatment. F-actin was visualized by immunofluorescence staining with Texas-Red phalloidin. Paracellular gaps are marked by arrows. Results are representative of four independent experiments.
Effects of iloprost on Rho pathway activation induced by thrombin and pathologic CS
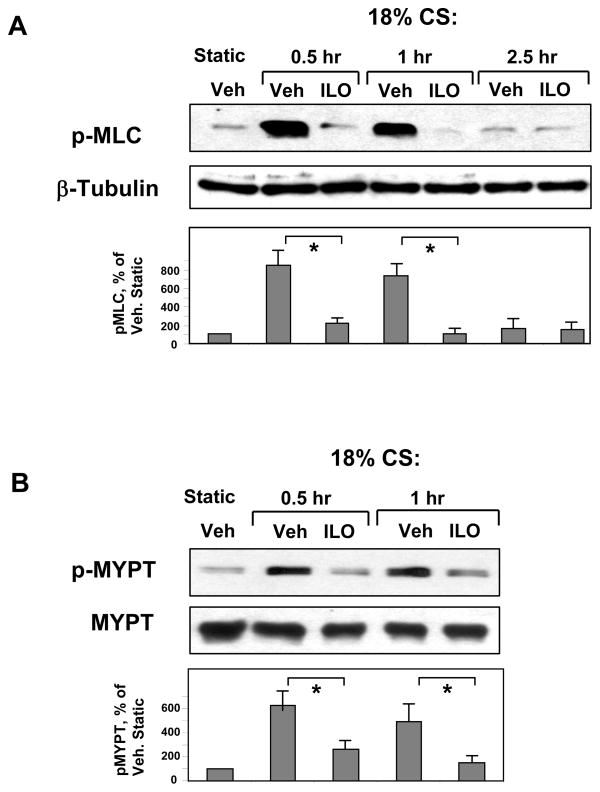
Protective effects of iloprost on EC monolayer integrity in thrombin- and CS-challenged cells were next linked with state of MLC and MYPT phosphorylation. Human pulmonary EC monolayers were treated with iloprost or vehicle prior to CS exposure (18% CS; 0.5 – 2.5 hr). Activation of Rho cascade was monitored by phosphorylation of Rho downstream targets: myosin-binding subunit of myosin-associated phosphatase type 1 (MYPT) at Rho-kinase-specific phosphorylation site (Thr850), and MLC. In contrast to static conditions, acute 18% CS induced phosphorylation of both, MLC (Figure 5A) and MYPT (Figure 5B). EC pretreatment with iloprost prior to CS exposure abolished CS-induced increases in MLC and MYPT phosphorylation.
Figure 5. Effects of iloprost on MLC and MYPT phosphorylation in the in vitro model of VILI.
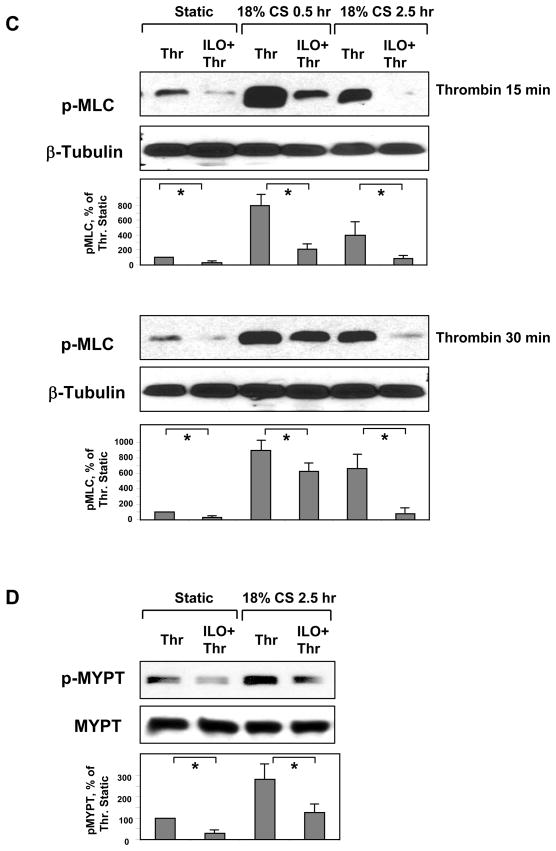
Pulmonary EC grown to confluence on Flexcell plates were pre-treated with iloprost (200 ng/ml, 15 min) and exposed to 18% CS for indicated time periods. Phosphorylation of MLC (A) and MYPT (B) was detected by western blot with phospho-specific antibodies. HPAEC were further stimulated with thrombin (0.1 U/ml, 15 or 30 min) with or without iloprost (200 ng/ml, 15 min) pretreatment. Phosphorylated MLC (C) and MYPT (D) were detected by immunoblotting with specific antibodies. Results are representative of three to six independent experiments.
Next experiments tested effects of iloprost on thrombin-induced MLC and MYPT phosphorylation in EC exposed to 30 min (acute CS) or 2.5 hr of 18% CS. Cells were treated with iloprost or vehicle prior to CS exposure followed by thrombin stimulation after 30 min or 2.5 hr of CS. MLC phosphorylation in response to thrombin (15 or 30 min) was markedly exacerbated at 30 min and 2.5 hr of CS preconditioning, as compared to static thrombin-stimulated controls. Iloprost significantly suppressed thrombin-induced MLC phosphorylation in static and CS-preconditioned EC (Figure 5C). Similar to effects on MLC phosphorylation, 2.5-hr CS preconditioning increased thrombin-induced phosphorylation of MYPT at Rho-kinase specific site, which was dramatically attenuated by iloprost (Figure 5D).
DISCUSSION
Acute lung injury and ARDS are severe conditions associated with excessive fluid accumulation in the lung. These events are due to impaired alveolar fluid clearance by alveolar epithelium and lung vascular leak caused by increased endothelial permeability [4, 31–33]. Increased coagulation and thrombin activation is an important pathogenic mechanism of ALI and ARDS [34]. Thrombin triggers mechanisms of lung vascular injury and increased vascular permeability secondary to intravascular coagulation [35]. Studies using pulmonary endothelial cell cultures described the mechanism of thrombin-induced endothelial permeability which involves cytoskeletal remodeling, assembly of F-actin stress fibers, and their association with activated myosin leading to actomyosin-driven cell contraction, disruption of cell junctions and formation of paracellular gaps – the hallmarks of endothelial barrier compromise [36]. In turn, MLC phosphorylation in endothelial cells is controlled by a balance between Ca2+/calmodulin regulated myosin light chain kinase and Rho kinase-regulated myosin light chain phosphatase [37–39].
Clinical observations show that vascular leak in VILI patients is associated with increased levels of edemagenic and inflammatory mediators [2, 8–10], and combination of pathologic mechanical stress with inflammatory mediators may synergize leading to development of ARDS. Such two-hit model is currently a focus of translational and clinical studies exploring the mechanisms of lung injury and associated barrier dysfunction [31, 40]. This study tested the two-hit animal model of acute lung injury induced by high tidal volume mechanical ventilation and thrombin-derived signaling peptide TRAP6 lacking pro-thrombotic activity. TRAP6 at high doses increased lung permeability in spontaneously ventilated mice, but also increased lethality. Of note, lower doses of TRAP6 did not affect BAL cell and protein content, but further increased parameters of lung vascular permeability induced by high tidal volume mechanical ventilation. These results show synergistic effects of high tidal volume mechanical ventilation and TRAP6 on development of lung vascular leak in murine model.
Our data indicate that Rho pathway is essential, but not a sole mechanism activated by thrombin/TRAP6 and mechanical ventilation. Proposed mechanisms and molecular pathways governing mechanochemical signaling during development of VILI are numerous. Mechanical strain induces activation of non-receptor tyrosine kinases including p60Src, focal adhesion kinase, integrin-mediated signaling, and MAP kinase cascades [25, 41, 42]. Mechanical ventilation also activates small GTPase Rho [43, 44]. The role of MLC kinase in mechanical ventilation-induced lung dysfunction is less studied, however its pharmacological inhibition augments capillary fluid leak after experimental ventilation-induced injury [45]. On the other hand, thrombin also activates multiple signaling pathways in the lung. In pulmonary endothelium thrombin triggers various signaling cascades including activation of small GTPase Rho, Ca2+/calmodulin-dependent protein kinase, MLC kinase, protein kinase C, phospholipase C, and inhibition of adenylate cyclase, which could promote lung injury and vascular leak [36, 46].
Our recent report showed that Rho signaling contributes to the development of ventilator-induced vascular leak and lung injury. Inhibition of Rho-associated kinase by injection of pharmacological inhibitor Y-27632 decreased BAL protein content in the mice exposed to high tidal volume mechanical ventilation [14]. Thus, activation of Rho caused by mechanical stress and increased levels of circulating thrombin may be a critical mechanism leading to development of VILI. We have previously developed an in vitro two-hit model of pulmonary EC barrier dysfunction in cultured endothelial cells exposed to high magnitude cyclic stretch (18% linear elongation, 25 cycles/min) and ALI-associated mediators. In these models, 18% CS markedly enhanced EC barrier disruption induced by thrombin and VEGF, and synergistic effects of agonists and mechanical stress were mediated by Rho GTPase [11, 12]. Of note, our previous report showed that TRAP6 also increased permeability in the pulmonary EC cultures in a Rho-dependent manner [47].
Experimental and clinical evidence suggest that cAMP stimulation may accelerate the resolution of pulmonary edema in the presence of acute lung injury [33]. Clinically relevant concentrations of beta2-adrenergic agonists stimulate airspace fluid clearance in normal lungs and reduce pulmonary edema in acid aspiration-induced lung injury by further activation of alveolar fluid clearance and inhibition of endothelial permeability in a cAMP-dependent manner [48]. In the other report, infusion of prostacyclin (PGI2) and its stable analogue 6-alpha-carba-PGI2 reduced pulmonary transvascular fluid and protein fluxes after intravascular coagulation induced by thrombin [49]. Although beneficial role of prostaglandins and their stable analogs has been shown in ALI caused by repeated lung lavage [50] and ischemia-reperfusion [51], iloprost was however, not effective in the models of oleic acid induced lung injury [52], while Iloprost effects on the ventilator induced lung injury and vascular leak remain to be characterized.
The results of this study show that lung injury and increased vascular leakiness caused by HTV and TRAP6 may be partially reversed by iloprost. These results are further supported by in vitro data, which show that iloprost dramatically attenuated disruption of EC monolayers exposed to 18% CS and thrombin. These protective effects were further linked to attenuation of Rho signaling manifested by inhibition of Rho-kinase specific MYPT phosphorylation and reduction of phospho-MLC levels. Elevated intracellular cAMP concentrations induced by prostacyclin and its stable analogs activate PKA signaling and recently described PKA-independent Epac/Rap1 signaling cascade [53–56]. PKA reduces endothelial myosin light chain kinase activity, which may decrease pool of phosphorylated MLC, and cause relaxation of actomyosin complex, stabilization of F-actin filaments and strengthening of cell-matrix adhesions [55, 57, 58]. PKA also affects Rho signaling. One potential mechanism is PKA-mediated phosphorylation of Rho-GDP dissociation inhibitor, a negative regulator of Rho, leading to Rho inactivation [57] and abrogation of thrombin-induced EC hyper-permeability. On the other hand, cAMP-sensitive guanine exchange factor Epac and its effector GTPase Rap1 may activate Rac and Rac-dependent mechanisms of barrier enhancement via activation of Rac-specific GEFs Tiam1 and Vav2 [19]. Activated Rac may also negatively regulate Rho via several potential mechanisms of Rac/Rho crosstalk [59, 60]. These potential mechanisms of lung protection by prostacyclin analogs await further investigation.
Bolus-type intravenous iloprost administration used in this study has certain limitations. First, it does not allow the maintenance of constant circulating iloprost levels. Together with limited half-life of iloprost, this protocol may underestimate the potency of iloprost therapy in VILI model. Other approaches such as utilization of osmotic pump or inhalation protocols may be more efficient protective strategies in this model. Another aspect of iloprost treatment is its hypotensive effect, which may cause undesirable side effects and, on the other hand mimic “decreased vascular permeability” by reducing hydrostatic capillary pressure and transcapillary filtration rates. In the additional studies (unpublished data), mice were exposed to mechanical ventilation with our without iloprost treatment followed by lung isolation and Kf,c measurements in the isolated perfused lungs. The results showed significant decrease in Kf,c in the isolated lungs from mice preconditioned with iloprost during ventilation protocol. These results show direct endothelial barrier-protective effect of iloprost in the VILI model.
Thus, to the best of our knowledge, this is the first report that addresses effects of iloprost on parameters of lung vascular leak and inflammation (BAL protein content and cell count, increased Evans Blue extravasation and neutrophil accumulation in lung tissue) induced by combined pathologic mechanical ventilation and treatment with thrombin peptide, which were linked with Rho- and MLC-dependent permeability changes in lung vascular endothelium. Inhaled iloprost improves gas exchange due to a decrease of pulmonary shunt acutely and as a long-term effect, possibly as a result of a reduction of lung edema formation [50]. Thus, increased number of blood-filled microvessels in the lungs of mice subjected to HTV and TRAP6 may indicate increased shunting and regional microvascular occlusion in this VILI model, which seems reduced in iloprost-treated mice.
In summary, this study shows potent protective effects of iloprost in the in vitro and murine VILI models and suggests beneficial effects of iloprost in the two-hit model of acute lung injury. These results suggest utilization of stable prostacyclin analogs as promising strategy for therapeutic treatment of VILI and ARDS.
Acknowledgments
Sources of support: National Heart, Lung, and Blood Institutes grants HL87823, HL76259, and HL58064 for KGB; HL89257 and the American Lung Association Biomedical Research Grant for AAB
List of abbreviations
- ALI
acute lung injury
- ARDS
acute respiratory distress syndrome
- BAL
bronchoalveolar lavage
- EC
endothelial cells
- HPAEC
Human pulmonary artery endothelial cells
- HTV
high tidal volume mechanical ventilation
- i/v
intravenous instillation
- i/t
intratracheal instillation
- MLC
myosin light chain
- MYPT
myosin-associated phosphatase type 1
- CS
cyclic stretch
- TRAP6
thrombin receptor activating peptide 6
- PAR1
protease activated receptor 1
- PKA
protein kinase A
- VILI
ventilator-induced lung injury
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Slutsky AS, Tremblay LN. Multiple system organ failure. Is mechanical ventilation a contributing factor? Am J Respir Crit Care Med. 1998;157(6 Pt 1):1721–5. doi: 10.1164/ajrccm.157.6.9709092. [DOI] [PubMed] [Google Scholar]
- 2.Dos Santos CC, Slutsky AS. Invited review: mechanisms of ventilator-induced lung injury: a perspective. J Appl Physiol. 2000;89(4):1645–55. doi: 10.1152/jappl.2000.89.4.1645. [DOI] [PubMed] [Google Scholar]
- 3.Uhlig S. Ventilation-induced lung injury and mechanotransduction: stretching it too far? Am J Physiol Lung Cell Mol Physiol. 2002;282(5):L892–6. doi: 10.1152/ajplung.00124.2001. [DOI] [PubMed] [Google Scholar]
- 4.Oeckler RA, Hubmayr RD. Ventilator-associated lung injury: a search for better therapeutic targets. Eur Respir J. 2007;30(6):1216–26. doi: 10.1183/09031936.00104907. [DOI] [PubMed] [Google Scholar]
- 5.Schultz MJ, Haitsma JJ, Zhang H, Slutsky AS. Pulmonary coagulopathy as a new target in therapeutic studies of acute lung injury or pneumonia--a review. Crit Care Med. 2006;34(3):871–7. [PubMed] [Google Scholar]
- 6.Russell JA. Management of sepsis. N Engl J Med. 2006;355(16):1699–713. doi: 10.1056/NEJMra043632. [DOI] [PubMed] [Google Scholar]
- 7.Ware LB, Camerer E, Welty-Wolf K, Schultz MJ, Matthay MA. Bench to bedside: targeting coagulation and fibrinolysis in acute lung injury. Am J Physiol Lung Cell Mol Physiol. 2006;291(3):L307–11. doi: 10.1152/ajplung.00157.2006. [DOI] [PubMed] [Google Scholar]
- 8.Narimanbekov IO, Rozycki HJ. Effect of IL-1 blockade on inflammatory manifestations of acute ventilator-induced lung injury in a rabbit model. Exp Lung Res. 1995;21(2):239–54. doi: 10.3109/01902149509068830. [DOI] [PubMed] [Google Scholar]
- 9.Pugin J, Dunn I, Jolliet P, Tassaux D, Magnenat JL, Nicod LP, et al. Activation of human macrophages by mechanical ventilation in vitro. Am J Physiol. 1998;275(6 Pt 1):L1040–50. doi: 10.1152/ajplung.1998.275.6.L1040. [DOI] [PubMed] [Google Scholar]
- 10.Vlahakis NE, Schroeder MA, Limper AH, Hubmayr RD. Stretch induces cytokine release by alveolar epithelial cells in vitro. Am J Physiol. 1999;277(1 Pt 1):L167–73. doi: 10.1152/ajplung.1999.277.1.L167. [DOI] [PubMed] [Google Scholar]
- 11.Birukova AA, Chatchavalvanich S, Rios A, Kawkitinarong K, Garcia JG, Birukov KG. Differential regulation of pulmonary endothelial monolayer integrity by varying degrees of cyclic stretch. Am J Pathol. 2006;168(5):1749–61. doi: 10.2353/ajpath.2006.050431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Birukova AA, Moldobaeva N, Xing J, Birukov KG. Magnitude-dependent effects of cyclic stretch on HGF- and VEGF-induced pulmonary endothelial remodeling and barrier regulation. Am J Physiol Lung Cell Mol Physiol. 2008 doi: 10.1152/ajplung.90236.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shikata Y, Rios A, Kawkitinarong K, DePaola N, Garcia JG, Birukov KG. Differential effects of shear stress and cyclic stretch on focal adhesion remodeling, site-specific FAK phosphorylation, and small GTPases in human lung endothelial cells. Exp Cell Res. 2005;304(1):40–9. doi: 10.1016/j.yexcr.2004.11.001. [DOI] [PubMed] [Google Scholar]
- 14.Nonas S, Birukova AA, Fu P, Xing J, Chatchavalvanich S, Bochkov VN, et al. Oxidized phospholipids reduce ventilator-induced vascular leak and inflammation in vivo. Crit Care. 2008;12(1):R27. doi: 10.1186/cc6805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nonas S, Miller I, Kawkitinarong K, Chatchavalvanich S, Gorshkova I, Bochkov VN, et al. Oxidized phospholipids reduce vascular leak and inflammation in rat model of acute lung injury. Am J Respir Crit Care Med. 2006;173(10):1130–8. doi: 10.1164/rccm.200511-1737OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tasaka S, Koh H, Yamada W, Shimizu M, Ogawa Y, Hasegawa N, et al. Attenuation of endotoxin-induced acute lung injury by the Rho-associated kinase inhibitor, Y-27632. Am J Respir Cell Mol Biol. 2005;32(6):504–10. doi: 10.1165/rcmb.2004-0009OC. [DOI] [PubMed] [Google Scholar]
- 17.Howard LS, Morrell NW. New therapeutic agents for pulmonary vascular disease. Paediatr Respir Rev. 2005;6(4):285–91. doi: 10.1016/j.prrv.2005.09.006. [DOI] [PubMed] [Google Scholar]
- 18.Ueno Y, Koike H, Annoh S, Nishio S. Anti-inflammatory effects of beraprost sodium, a stable analogue of PGI2, and its mechanisms. Prostaglandins. 1997;53(4):279–89. doi: 10.1016/s0090-6980(97)89601-3. [DOI] [PubMed] [Google Scholar]
- 19.Birukova AA, Zagranichnaya T, Alekseeva E, Fu P, Chen W, Jacobson JR, et al. Prostaglandins PGE2 and PGI2 promote endothelial barrier enhancement via PKA- and Epac1/Rap1-dependent Rac activation. Exp Cell Res. 2007;313(11):2504–20. doi: 10.1016/j.yexcr.2007.03.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fukuhara S, Sakurai A, Sano H, Yamagishi A, Somekawa S, Takakura N, et al. Cyclic AMP potentiates vascular endothelial cadherin-mediated cell-cell contact to enhance endothelial barrier function through an Epac-Rap1 signaling pathway. Mol Cell Biol. 2005;25(1):136–46. doi: 10.1128/MCB.25.1.136-146.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kooistra MR, Corada M, Dejana E, Bos JL. Epac1 regulates integrity of endothelial cell junctions through VE-cadherin. FEBS Lett. 2005;579(22):4966–72. doi: 10.1016/j.febslet.2005.07.080. [DOI] [PubMed] [Google Scholar]
- 22.Birukova AA, Fu P, Chatchavalvanich S, Burdette D, Oskolkova O, Bochkov VN, et al. Polar head groups are important for barrier protective effects of oxidized phospholipids on pulmonary endothelium. Am J Physiol Lung Cell Mol Physiol. 2007;292(4):L924–35. doi: 10.1152/ajplung.00395.2006. [DOI] [PubMed] [Google Scholar]
- 23.Fu P, Birukova AA, Xing J, Sammani S, Murley JS, Garcia JG, et al. Amifostine reduces lung vascular permeability via suppression of inflammatory signalling. Eur Respir J. 2009;33(3):612–24. doi: 10.1183/09031936.00014808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Moitra J, Sammani S, Garcia JG. Re-evaluation of Evans Blue dye as a marker of albumin clearance in murine models of acute lung injury. Transl Res. 2007;150(4):253–65. doi: 10.1016/j.trsl.2007.03.013. [DOI] [PubMed] [Google Scholar]
- 25.Birukov KG, Jacobson JR, Flores AA, Ye SQ, Birukova AA, Verin AD, et al. Magnitude-dependent regulation of pulmonary endothelial cell barrier function by cyclic stretch. Am J Physiol Lung Cell Mol Physiol. 2003;285(4):L785–97. doi: 10.1152/ajplung.00336.2002. [DOI] [PubMed] [Google Scholar]
- 26.Tschumperlin DJ, Oswari J, Margulies AS. Deformation-induced injury of alveolar epithelial cells. Effect of frequency, duration, and amplitude. Am J Respir Crit Care Med. 2000;162(2 Pt 1):357–62. doi: 10.1164/ajrccm.162.2.9807003. [DOI] [PubMed] [Google Scholar]
- 27.Tschumperlin DJ, Margulies SS. Alveolar epithelial surface area-volume relationship in isolated rat lungs. J Appl Physiol. 1999;86(6):2026–33. doi: 10.1152/jappl.1999.86.6.2026. [DOI] [PubMed] [Google Scholar]
- 28.Birukov KG, Bochkov VN, Birukova AA, Kawkitinarong K, Rios A, Leitner A, et al. Epoxycyclopentenone-containing oxidized phospholipids restore endothelial barrier function via Cdc42 and Rac. Circ Res. 2004;95(9):892–901. doi: 10.1161/01.RES.0000147310.18962.06. [DOI] [PubMed] [Google Scholar]
- 29.Birukova AA, Cokic I, Moldobaeva N, Birukov KG. Paxillin is Involved in the Differential Regulation of Endothelial Barrier by HGF and VEGF. Am J Respir Cell Mol Biol. 2008 doi: 10.1165/rcmb.2008-0099OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Birukova AA, Rios A, Birukov KG. Long-term cyclic stretch controls pulmonary endothelial permeability at translational and post-translational levels. Exp Cell Res. 2008;314(19):3466–77. doi: 10.1016/j.yexcr.2008.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. The Acute Respiratory Distress Syndrome Network. N Engl J Med. 2000;342(18):1301–8. doi: 10.1056/NEJM200005043421801. [DOI] [PubMed] [Google Scholar]
- 32.Matthay MA, Bhattacharya S, Gaver D, Ware LB, Lim LH, Syrkina O, et al. Ventilator-induced lung injury: in vivo and in vitro mechanisms. Am J Physiol Lung Cell Mol Physiol. 2002;283(4):L678–82. doi: 10.1152/ajplung.00154.2002. [DOI] [PubMed] [Google Scholar]
- 33.Matthay MA, Robriquet L, Fang X. Alveolar epithelium: role in lung fluid balance and acute lung injury. Proc Am Thorac Soc. 2005;2(3):206–13. doi: 10.1513/pats.200501-009AC. [DOI] [PubMed] [Google Scholar]
- 34.Nieuwenhuizen L, de Groot PG, Grutters JC, Biesma DH. A review of Pulmonary Coagulopathy in Acute Lung Injury, Acute Respiratory Distress Syndrome and Pneumonia. Eur J Haematol. 2009 doi: 10.1111/j.1600-0609.2009.01238.x. [DOI] [PubMed] [Google Scholar]
- 35.Malik AB, Horgan MJ. Mechanisms of thrombin-induced lung vascular injury and edema. Am Rev Respir Dis. 1987;136(2):467–70. doi: 10.1164/ajrccm/136.2.467. [DOI] [PubMed] [Google Scholar]
- 36.Mehta D, Malik AB. Signaling mechanisms regulating endothelial permeability. Physiol Rev. 2006;86(1):279–367. doi: 10.1152/physrev.00012.2005. [DOI] [PubMed] [Google Scholar]
- 37.Garcia JG, Verin AD, Schaphorst KL. Regulation of thrombin-mediated endothelial cell contraction and permeability. Semin Thromb Hemost. 1996;22(4):309–315. doi: 10.1055/s-2007-999025. [DOI] [PubMed] [Google Scholar]
- 38.Verin AD, Cooke C, Herenyiova M, Patterson CE, Garcia JG. Role of Ca2+/calmodulin-dependent phosphatase 2B in thrombin-induced endothelial cell contractile responses. Am J Physiol. 1998;275(4 Pt 1):L788–99. doi: 10.1152/ajplung.1998.275.4.L788. [DOI] [PubMed] [Google Scholar]
- 39.Essler M, Amano M, Kruse HJ, Kaibuchi K, Weber PC, Aepfelbacher M. Thrombin inactivates myosin light chain phosphatase via Rho and its target Rho kinase in human endothelial cells. J Biol Chem. 1998;273(34):21867–74. doi: 10.1074/jbc.273.34.21867. [DOI] [PubMed] [Google Scholar]
- 40.Matute-Bello G, Frevert CW, Martin TR. Animal models of acute lung injury. Am J Physiol Lung Cell Mol Physiol. 2008;295(3):L379–99. doi: 10.1152/ajplung.00010.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Katsumi A, Orr AW, Tzima E, Schwartz MA. Integrins in mechanotransduction. J Biol Chem. 2004;279(13):12001–4. doi: 10.1074/jbc.R300038200. [DOI] [PubMed] [Google Scholar]
- 42.Geiger B, Bershadsky A. Assembly and mechanosensory function of focal contacts. Curr Opin Cell Biol. 2001;13(5):584–92. doi: 10.1016/s0955-0674(00)00255-6. [DOI] [PubMed] [Google Scholar]
- 43.Finigan JH, Boueiz A, Wilkinson E, Damico R, Skirball J, Pae HH, et al. Activated protein C protects against ventilator-induced pulmonary capillary leak. Am J Physiol Lung Cell Mol Physiol. 2009;296(6):L1002–11. doi: 10.1152/ajplung.90555.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Birukov KG. Small GTPases in mechanosensitive regulation of endothelial barrier. Microvasc Res. 2009;77(1):46–52. doi: 10.1016/j.mvr.2008.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Parker JC. Inhibitors of myosin light chain kinase and phosphodiesterase reduce ventilator-induced lung injury. J Appl Physiol. 2000;89(6):2241–8. doi: 10.1152/jappl.2000.89.6.2241. [DOI] [PubMed] [Google Scholar]
- 46.Dudek SM, Garcia JG. Cytoskeletal regulation of pulmonary vascular permeability. J Appl Physiol. 2001;91(4):1487–500. doi: 10.1152/jappl.2001.91.4.1487. [DOI] [PubMed] [Google Scholar]
- 47.Kawkitinarong K, Linz-McGillem L, Birukov KG, Garcia JG. Differential regulation of human lung epithelial and endothelial barrier function by thrombin. Am J Respir Cell Mol Biol. 2004;31(5):517–27. doi: 10.1165/rcmb.2003-0432OC. [DOI] [PubMed] [Google Scholar]
- 48.McAuley DF, Frank JA, Fang X, Matthay MA. Clinically relevant concentrations of beta2-adrenergic agonists stimulate maximal cyclic adenosine monophosphate-dependent airspace fluid clearance and decrease pulmonary edema in experimental acid-induced lung injury. Crit Care Med. 2004;32(7):1470–6. doi: 10.1097/01.ccm.0000129489.34416.0e. [DOI] [PubMed] [Google Scholar]
- 49.Perlman MB, Lo SK, Malik AB. Effect of prostacyclin on pulmonary vascular response to thrombin in awake sheep. J Appl Physiol. 1986;60(2):546–53. doi: 10.1152/jappl.1986.60.2.546. [DOI] [PubMed] [Google Scholar]
- 50.Dembinski R, Brackhahn W, Henzler D, Rott A, Bensberg R, Kuhlen R, et al. Cardiopulmonary effects of iloprost in experimental acute lung injury. Eur Respir J. 2005;25(1):81–7. doi: 10.1183/09031936.04.10085504. [DOI] [PubMed] [Google Scholar]
- 51.Wittwer T, Franke UF, Ochs M, Sandhaus T, Schuette A, Richter S, et al. Inhalative pre-treatment of donor lungs using the aerosolized prostacyclin analog iloprost ameliorates reperfusion injury. J Heart Lung Transplant. 2005;24(10):1673–9. doi: 10.1016/j.healun.2004.11.014. [DOI] [PubMed] [Google Scholar]
- 52.Hucklenbruch C, Hinder F, Berger C, Ertmer C, Lange M, Westphal M, et al. Effects of inhaled aerosolized iloprost and inhaled NO on pulmonary circulation and edema formation in ovine lung injury. Shock. 2008;30(1):75–80. doi: 10.1097/SHK.0b013e31815dd1ad. [DOI] [PubMed] [Google Scholar]
- 53.Mei FC, Qiao J, Tsygankova OM, Meinkoth JL, Quilliam LA, Cheng X. Differential signaling of cyclic AMP: opposing effects of exchange protein directly activated by cyclic AMP and cAMP-dependent protein kinase on protein kinase B activation. J Biol Chem. 2002;277(13):11497–504. doi: 10.1074/jbc.M110856200. [DOI] [PubMed] [Google Scholar]
- 54.Cullere X, Shaw SK, Andersson L, Hirahashi J, Luscinskas FW, Mayadas TN. Regulation of vascular endothelial barrier function by Epac, a cAMP-activated exchange factor for Rap GTPase. Blood. 2005;105(5):1950–5. doi: 10.1182/blood-2004-05-1987. [DOI] [PubMed] [Google Scholar]
- 55.Birukova AA, Zagranichnaya T, Alekseeva E, Bokoch GM, Birukov KG. Epac/Rap and PKA are novel mechanisms of ANP-induced Rac-mediated pulmonary endothelial barrier protection. J Cell Physiol. 2008;215(3):715–24. doi: 10.1002/jcp.21354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bos JL. Epac proteins: multi-purpose cAMP targets. Trends Biochem Sci. 2006 doi: 10.1016/j.tibs.2006.10.002. [DOI] [PubMed] [Google Scholar]
- 57.Qiao J, Huang F, Lum H. PKA inhibits RhoA activation: a protection mechanism against endothelial barrier dysfunction. Am J Physiol Lung Cell Mol Physiol. 2003;284(6):L972–80. doi: 10.1152/ajplung.00429.2002. [DOI] [PubMed] [Google Scholar]
- 58.Lampugnani MG, Giorgi M, Gaboli M, Dejana E, Marchisio PC. Endothelial cell motility, integrin receptor clustering, and microfilament organization are inhibited by agents that increase intracellular cAMP. Lab Invest. 1990;63(4):521–31. [PubMed] [Google Scholar]
- 59.Wong KW, Mohammadi S, Isberg RR. Disruption of RhoGDI and RhoA regulation by a Rac1 specificity switch mutant. J Biol Chem. 2006;281(52):40379–88. doi: 10.1074/jbc.M605387200. [DOI] [PubMed] [Google Scholar]
- 60.Herbrand U, Ahmadian MR. p190-RhoGAP as an integral component of the Tiam1/Rac1-induced downregulation of Rho. Biol Chem. 2006;387(3):311–7. doi: 10.1515/BC.2006.041. [DOI] [PubMed] [Google Scholar]