Abstract
This clinical trial compared two brief alcohol use interventions in prenatal clinics: Early Start (ES), a substance abuse screening and treatment program integrated with prenatal care focused on abstention (n=298), and Early Start Plus (ESP), adding a computerized drink-size assessment tool and intervention focused on drinking less (n=266). Controls were untreated alcohol users (n=344). Controls had higher adverse neonatal and maternal outcome rates. Findings favored ESP for preterm labor and ES for low birth weight. No differences between ES and ESP were statistically significant. ESP provides clinicians with an innovative assessment tool that creates open dialogue about drinking during pregnancy.
Keywords: computerized assessment, health services research, maternal outcomes, neonatal outcomes, prenatal alcohol abuse
Introduction
Alcohol use during pregnancy is a serious problem with adverse effects on mothers and their babies (Armstrong et al., 2003; Day et al., 1989; Jacobson & Jacobson, 1994; Sratton & Battaglia, 1996). Studies have shown that alcohol use is related to fetal alcohol spectrum disorders (Warren & Foudin, 2001), fetal alcohol syndrome (Jones & Smith, 1973), low birth weight (Little, 1977; Jaddoe et al., 2007; Armstrong et al, 2003), preterm delivery (Jaddoe et al., 2007), preterm labor (Jaddoe et al., 2007), intrauterine fetal demise (Burd, Roberts, Olson & Odendaal, 2007), and placental abruption (Tikkanen, Nuutila, Hiilesmaa, Paavonen, Ylikorkala, 2006). In the literature there is a range of definitions for high risk drinking among pregnant women. For example, the National Center on Birth Defects and Developmental Disabilities, Centers for Disease Control and Prevention & Department of Health and Human Services' definition (2004) is 7 or more drinks per week or 5 or more drinks on any one occasion. In Flynn, Marcus, Barry & Blow's (2003) study, higher risk drinking is defined less strictly as one drink or more per week and/or one or more drinking binge during pregnancy. Risk factors related to alcohol problems during pregnancy include single marital status, age between 21-25, unemployment and cigarette smoking (Leonardson & Loudenburg, 2003; Leonardson, Loudenburg, & Struck, 2007). Protective factors include being married and full-time housewife status (Leonardson & Loudenburg, 2003; Leonardson et al., 2007) and healthy maternal behaviors, such as regular exercise, prenatal vitamin use, prenatal care, and participation in prenatal classes (Broekhuizen, Utrie, & Van Mullem, 1992; Faden, Hanna, & Graubard, 1997).
Although about 95% of pregnant women surveyed in the general population are aware that they should abstain from alcohol (Kaskutas & Graves, 1994), recent published estimates report that 5% to 30% of pregnant women screened in prenatal clinics are current drinkers (Armstrong et al., 2001; Kaskutas, 2000). To reduce the incidence and severity of birth defects and other negative birth outcomes associated with alcohol consumption during pregnancy (Armstrong et al., 2003; Day et al., 1989; Jacobson & Jacobson, 1994; Sratton & Battaglia, 1996), brief interventions in prenatal clinics are a high priority (National Institute on Alcohol Abuse and Alcoholism [NIAAA], 2005). These brief interventions reinforce abstinence decisions (Chang, Wilkins-Haug, Berman & Goetz, 1999) and lead to less drinking among the heaviest drinkers or even abstinence (Chang et al., 2005; Floyd, O'Connor, Bertrand, & Sokol, 2006; O'Connor & Whaley, 2007). Following recommendations by the Surgeon General (United States Federal Drug Administration [FDA], 1981), the American Medical Association (1983), and the Institute of Medicine (1996), most interventions during pregnancy are abstention-based, although there is evidence that any reduction in drinking during pregnancy is beneficial (Rosett, Ouellette, Weiner, & Owens, 1978; Rosett, Weiner, & Edelin, 1983; Weiner & Larsson, 1987).
The purpose of the study reported here was to compare two brief interventions in prenatal care clinics, one that focuses on abstention (usual care group) and another that focuses on helping women drink less when they say that they cannot abstain (intervention group). The intervention under study here assesses women's drink sizes and provides an opportunity for an honest dialog which can help women realize how much alcohol they are really drinking. Counseling such women to cut down their drinking may offer an option that is beneficial (although probably less so than abstinence) to the health of the fetus, even if the advice is only followed intermittently throughout gestation. This is especially relevant given that it has been reported that about a third of the women who drank during pregnancy indicated having 3 or more drinks on the days they drank (Office of Applied Studies, 1997).
The intervention is an enhancement to an existing prenatal substance abuse screening and treatment program in Kaiser Permanente Northern California (KPNC) called Early Start (Armstrong et al., 2001). The unique feature of this program is that the substance abuse treatment provider, the Early Start Specialist, is located in the obstetric clinic where she conducts risk assessments, educational sessions, and brief interventions integrated with routine prenatal care visits.
Method
Participants
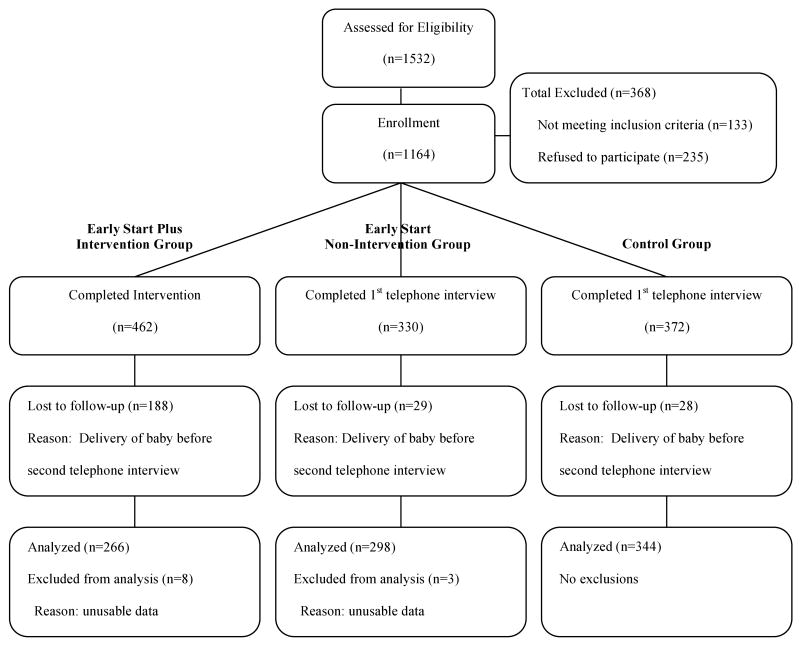
A total of 1,069 women were assessed positively for alcohol at the Early Start Plus (n=618) and Early Start (n=451) sites and were referred to the study; 1001 were found to be eligible (Early Start Plus, n=584; Early Start, n=417) and 79% in each group consented to participate in the study (Early Start Plus, n=462); Early Start, n=330). At the control sites, 463 women had questionnaires positive for alcohol use risk and were referred to the study; of these, 398 were found to be eligible, and almost all (94%, n=372) consented. See Figure 1, Recruitment and Retention Flow Chart, for details. Reasons for ineligibility included late to prenatal care, transferring prenatal care to a non-study site or out of KPNC, and pregnancy loss.
Figure 1.
Early Start Plus Recruitment Flow Chart
The Intervention
The Early Start Plus interventions were conducted by the Early Start Program Specialists, either licensed clinical social workers or other trained substance abuse treatment providers. The Specialists led the women through a computerized intervention that used drink size to create a discussion about drinking during pregnancy. The goal of the intervention was to help pregnant women recognize how much they actually drank, using calibrated glassware and beverage containers, along with computerized graphics designed to define true volume for specific alcoholic beverage types. The intervention promoted abstinence; however, if that was not an obtainable goal, women were taught ways to cut down as much as possible during the remaining months of their pregnancy. Specifically, the Specialist asked women to select their usual drinking vessel(s) and to indicate their usual pour or fill level for each alcoholic beverage they drank in the 12 months before becoming pregnant and in the past 30 days. A set of nine actual glass vessels was available, augmented by colored photographs of 90 vessels for use when none of the actual glasses matched a woman's usual beverage container. Each photograph included one of the original nine vessels so the woman could visualize the scale of the other pictured vessels. Each vessel that was not a bottle or can was labeled with a set of letters marking various heights to which the vessel might be filled. If the woman chose a glass as her usual vessel, she was asked how high she would usually fill it with the beverage not including ice, water or other mixers. She responded by giving the letter that most accurately designated her pour level (calibrated to indicate fluid ounces). If she instead chose a bottle or can, she was asked how many drinks she considered that to be. We defined standard drink sizes as 12 ounces for beer, 4 ounces for wine, 1 ounce for spirits, 8 ounces for malt liquor, 3 ounces for fortified wine, and 12 ounces for wine coolers. The intervention was conducted the first time at a median of 21 weeks gestation (Intraquartile range [IQR] 17-27) and took 20-25 minutes to complete on average. A follow-up intervention was conducted later in the pregnancy when possible but was not required for inclusion in this analysis. See Witbrodt et al. (2007a) for a more detailed description of the vessels drink size assessment and intervention. The women in all three study groups were interviewed twice by telephone regarding their alcohol use. The interview asked the women to quantify their consumption of each beverage type they consumed in the 30 days prior to the interview and in the 12 months before they became pregnant using standard drink sizes. The first interviews were conducted at a median of 16 weeks (IQR 13-21) in the Early Start Plus group, 17 weeks (IQR 12-23) in the Early Start group and 13 weeks (IQR 11-17) in the control group. All study participants had working telephones. The data collected at the interventions and interviews is not reported here.
Following the standard Early Start protocol, women were identified as at risk for prenatal substance abuse during pregnancy based on their responses to a screening questionnaire using a standard protocol, including history of alcohol and drug use, tolerance, and alcohol and drug use since pregnancy and in the 12 months before pregnancy (Armstrong et al., 2001). At the Early Start Plus and Early Start sites, women identified as at risk were referred to the Early Start Specialist at their facility for an in person in-depth assessment. If a woman was determined to be at risk specifically for alcohol use during pregnancy based on the assessment, she was eligible to be recruited for the study. At the control sites, Early Start Screening questionnaires were classified in the same manner, but no assessments or interventions were conducted. Women whose questionnaires were positive for risk of alcohol use during pregnancy were eligible for recruitment. Women were recruited into the study between May 1, 2000 and June 30, 2004; the last follow-up interview was completed on February 28, 2005.
Design and Procedure
In this cluster randomized clinical trial, 15 obstetric clinics in KPNC offering standard prenatal care services augmented by Early Start were randomized to one of two study groups: the enhanced intervention group called Early Start Plus (receiving Early Start counseling plus a drink size assessment and intervention aimed at reducing risk) (Witbrodt et al., 2007a) or the usual care group (receiving Early Start counseling but no drink size assessment or intervention). Randomization was stratified by clinic size to ensure equal numbers of women in each arm. Neither Specialists nor participants were blinded to the intervention. A third study group, which included women from two large and 3 satellite clinics that offered standard prenatal care but no Early Start services, served as a control group at risk for alcohol use during pregnancy but who received no Early Start counseling.
Outcomes
Maternal and infant outcomes were obtained from KPNC electronic databases. Five infant outcomes were studied: assisted ventilation, low birth weight (<2500g), preterm delivery (<35 weeks gestational age), Neonatal Intensive Care Unit (NICU) admission, and rehospitalization within 2 weeks of discharge from birth hospitalization. Five maternal outcomes were considered: preterm labor, preeclampsia, placenta previa, placental abruption, and rehospitalization within 2 weeks of discharge from delivery hospitalization.
Analysis
Since sites, not subjects, were randomized in this trial, we first studied potential differences among women seen in Early Start Plus clinics, Early Start clinics, and the control clinics where no form of Early Start was implemented. These analyses focused on demographics and smoking status, using Fisher exact tests adjusted for multiple comparisons (MULTEST procedure in SAS) to compare the three study groups in pairs and were used to inform our multivariate analyses. We also compared the urine toxicology results between women at Early Start Plus and Early Start clinics (urine toxicology tests were not done in the control clinics) using a Chi-square test. Secondly, we compared infant and maternal outcome rates among the three study groups (Early Start Plus, Early Start, and controls) in pairs, again using Fisher exact tests adjusted for multiple comparisons. The third set of analyses compared maternal and infant outcomes for study participants in all three groups adjusted for potential confounders (maternal age, ethnicity, marital status, and maternal education). Here we used non-linear mixed models analysis to account for the randomization by site (NLMIXED procedure in SAS).
The KPNC Institutional Review Board approved this study on July 20, 1999.
Results
Table 1 provides comparisons of demographic characteristics and risk factors of the three study groups. The Early Start Plus group was significantly older and more likely to be black than the Early Start group. The Early Start Plus group and the Early Start group were more likely to be black, unmarried, less educated, lower income, and smokers before pregnancy than the control group. The Early Start group was also significantly more likely to be younger and smoke during pregnancy than the control group.
Table 1. Demographic Characteristics by Study Group.
| Study Group (%) | P-values* | ||||||
|---|---|---|---|---|---|---|---|
| Characteristic | ESP (n=266) |
ES (n=298) |
C (n=344) |
Comparison Category | ESP vs ES | ESP vs C | ES vs C |
| Maternal age | |||||||
| 15-21 years | 29% | 42% | 24% | age >21 | 0.004 | 0.41 | 0.0001 |
| 22-25 years | 25 | 18 | 24 | ||||
| 26-30 years | 18 | 20 | 27 | ||||
| 31-44 years | 29 | 20 | 24 | ||||
| Maternal race | |||||||
| White | 29 | 45 | 65 | not white | 0.0001 | 0.0001 | 0.0001 |
| Black | 16 | 9 | 4 | not black | 0.03 | 0.0001 | 0.03 |
| Hispanic | 32 | 24 | 11 | ||||
| Asian | 9 | 4 | 3 | ||||
| Other | 14 | 18 | 17 | ||||
| Married/partner | 59 | 60 | 75 | Not married | 0.92 | 0.0001 | 0.0001 |
| Education (≤ high school) |
47 | 45 | 31 | > H.S. | 0.89 | 0.0001 | 0.002 |
| Annual income (< $25,000) |
36 | 44 | 25 | ≥ $25,000 | 0.24 | 0.01 | 0.0001 |
| Smoked prepregnancy | 56 | 64 | 45 | No smoking | 0.17 | 0.008 | 0.0001 |
| Smoked since pregnancy | 29 | 34 | 23 | No smoking | 0.37 | 0.26 | 0.003 |
| Positive urine screen† | 18 | 18 | None | All negative | 0.99 | --- | --- |
ESP, Early Start Plus; ES, Early Start; C, Control
Based on pair-wise comparisons using Fisher exact test, as computed by the MULTEST procedure in SAS
For one or more of the following: Alcohol, barbiturates, benzodiazepines, cocaine, methamphetamines, amphetamines, opiates, PCP, THC.
Unadjusted rates of the neonatal and maternal outcomes studied are presented in Table 2. Although none of the differences are statistically significant at the 0.05 level (adjusted for multiple comparisons), in many cases, the Early Start Plus group had the lowest rates of adverse health outcomes and the control group had the highest rates, with the Early Start group having intermediate rates. For example, the rates for low birth weight were 3.2% in the Early Start Plus group, 3.7% in the Early Start group, and 6.6% in the control group. Differences of a larger magnitude were found for preterm delivery < 35 weeks, where less than half of one percent of the Early Start Plus group had preterm deliveries, compared to 1.1% in Early Start and 2.2% in the control group. A similar pattern was seen for infant rehospitalization and preterm labor. However, for two outcomes, assisted ventilation and preeclampsia, results slightly favored Early Start above Early Start Plus and for NICU admission the rates were virtually identical. The unadjusted rates for placenta previa, placental abruption and maternal rehospitalization were negligible and were not included in Table 2. Rates for all baby and maternal outcomes combined were also examined but no comparisons were significant.
Table 2. Unadjusted Rates of Neonatal and Maternal Outcomes by Study Group.
| Unadjusted rates (%)* | |||
|---|---|---|---|
| Outcomes | ESP | ES | C |
| Neonatal Outcomes | |||
| Assisted ventilation | 2.0 | 0.7 | 2.2 |
| Low birth weight (<2500gm) |
3.2 | 3.7 | 6.6 |
| Preterm delivery (<35 wks gestational age) |
0.4 | 1.1 | 2.2 |
| NICU admission | 10.8 | 10.2 | 10.4 |
| Rehospitalization† | 1.6 | 2.9 | 4.1 |
| Maternal Outcomes | |||
| Preterm labor | 6.4 | 7.3 | 9.1 |
| Preeclampsia | 5.2 | 4.8 | 4.1 |
ESP, Early Start Plus; ES, Early Start; C, Control; NICU, Neonatal Intensive Care Unit
All p-values > 0.05 based on pair-wise comparisons using Fisher exact test, as computed by the MULTEST procedure in SAS
Within 2 weeks of discharge from birth/delivery hospitalization
In multivariate analyses, odds ratios for each infant and maternal outcome, comparing all three groups pair-wise, are reported in Table 3, adjusted for maternal age, ethnicity, marital status and maternal education. Only two significant differences in odds emerged: between Early Start and controls for low birth weight and between Early Start Plus and controls for preterm labor. No differences in odds ratios were found between Early Start Plus and Early Start. The odds of having a low birth weight infant were lower among women seen in the Early Start clinics compared to the control sites (OR=0.28, p=.02). The odds of maternal preterm labor were lower for women seen in the Early Start Plus clinics compared to the control sites (OR=0.44, p=.04).
Table 3. Adjusted Odds Ratios* for Selected Infant and Maternal Outcomes by Study Group.
| Odds Ratios (95% CI, p-value) | |||
|---|---|---|---|
| Outcome | ESP vs C | ES vs C | ESP vs ES |
| Neonatal outcomes | |||
| Assisted ventilation | 0.80 (0.19-3.35; 0.75) | 0.05 (0.00-2.34; 0.12) | 5.58 (0.49-64.72; 0.16) |
| Low birth weight (<2500gm) |
0.49 (0.20-1.25; 0.12) | 0.28 (0.10-0.80; 0.02) | 0.91 (0.29-2.83; 0.86) |
| Preterm delivery (<35 wks) |
0.08 (0.00-1.99; 0.11) | 0.30 (0.05-1.82; 0.17) | 0.37 (0.03-5.25; 0.43) |
| NICU admission | 0.78 (0.20-2.97; 0.70) | 0.65 (0.16-2.64; 0.52) | 1.24 (0.38-4.01; 0.70) |
| Rehospitalization† | 0.27 (0.04-1.77; 0.16) | 0.62 (0.17-2.29; 0.44) | 1.95 (0.43-8.94; 0.36) |
| Maternal outcomes | |||
| Preterm labor | 0.44 (0.20-0.97; 0.04) | 0.55 (0.27-1.14; 0.10) | 0.91 (0.36-2.31; 0.84) |
| Preeclampsia | 0.70 (0.27-1.79; 0.42) | 0.54 (0.21-1.40; 0.19) | 1.47 (0.58-3.74; 0.39) |
ESP, Early Start Plus; ES, Early Start; C, Control; NICU, Neonatal Intensive Care Unit
Estimated from Non-linear mixed models (SAS procedure NLMIXED) regressions coefficients, controlled for maternal age, ethnicity, marital status, and maternal education
Within 2 weeks of birth/delivery discharge
Discussion
Prior to the Vessels Intervention that was introduced in the Early Start Plus group, there was no standardized way of assessing for actual quantity and frequency patterns of alcohol use by Early Start patients. In addition, if a patient continued to drink alcohol in pregnancy and did not want to quit but could agree to cut down her drinking, Early Start Specialists had no tools available to assist them with conversations aimed not at abstinence but at harm reduction. As Early Start is an abstinence based program, it was awkward for the Specialists to enter into harm-reduction conversations without feeling like they were somehow “giving permission” to the women to drink. The Vessels Intervention provided a comfortable tool for successfully navigating these difficult conversations. This is probably the most significant contribution of the intervention.
Although the intervention did not result in significantly lower rates of neonatal and maternal outcomes when comparing the two types of interventions, in several cases the analyses suggested that the women in the Early Start Plus group had more favorable outcomes than the women in the Early Start group. For example, the Early Start Plus group had a lower unadjusted rate of preterm labor than the Early Start group and the controls, and significantly lower odds of preterm labor than the controls. This finding is highly relevant. About 9% of pregnant women in KPNC are hospitalized for preterm labor. Given that there are about 30,000 deliveries per year in this region, any reduction in this rate would have a positive effect on the pregnant population and have the additional benefit of lower costs. A related outcome is preterm delivery, where we found in the unadjusted analyses that women in Early Start Plus had about one-third the rate of preterm delivery of those in Early Start (0.4 vs. 1.1 respectively). Preterm babies have increased morbidity and mortality as well as costs of care, so any reduction in this rate is beneficial. Taken together, these results suggest that Early Start Plus appears to be helpful in reducing the incidence of preterm events. Future research with the Vessels Intervention will attempt to understand the mechanism(s) through which this might be occurring.
Another important finding was the significantly lower odds of low birth weight infants in the Early Start group compared to the controls. While this does not inform our trial comparing Early Start Plus to Early Start, this result highlights the inherent value of high-intensity interventions placed in prenatal clinics which are targeted towards high risk women. Low birth weight infants are usually also preterm and have increased morbidity, mortality and costs of care (Little 1977).
The nearly identical high NICU admission rates for the 3 study groups were not surprising. Many NICU admissions are for highly discretionary reasons, such as “rule out sepsis”, and babies of women who are identified as substance users during pregnancy are often admitted for observation, even if there is documentation that the mother is no longer using.
There are a number of important limitations to our study. The first concerns the comparability of the study groups on demographics and risk factors. In general, the control group had a better demographic profile in terms of characteristics that put women at risk (such as low income, higher age) and traditional risk factors (such as smoking before or during pregnancy). Nevertheless, the control group had generally worse baby and maternal outcomes than the two Early Start groups, which emerged as significant for two outcomes (low birth weight and preterm labor) in the multivariate analyses. This pattern of results highlights the value of both the Early Start and the Early Start Plus interventions, since women treated in those groups would otherwise have been thought to have had worse, not better, outcomes than those in the control group.
A second limitation stems from the smaller than anticipated sample size, which was due to difficulties with recruitment in this multi-site trial. We based our original power estimations on the comparison of rates of a combined maternal and neonatal outcome measure. This variable had a positive value if the mother-infant pair had any of the neonatal and maternal outcomes considered and was negative if they had none. We estimated that the Early Start Plus rate would be 12%, Early Start would be 14-16% and the controls would be 18%. To achieve 80% power with a 2-sided test and alpha = 0.05, we needed 555 women in each group to detect the difference between a control group rate of 18% and an Early Start Plus rate of 12%. We noted in our proposal that we would not have sufficient power to detect differences between Early Start Plus and Early Start but expected the rate for Early Start Plus to be lower than the rate for Early Start and that any reduction in the rate would have a public health impact. The results of the study were: Early Start Plus rate = 14.3% (n=251), Early Start rate = 14.9% (n=275), and control rate = 19.5% (n=318). The final sample sizes were about half of what was anticipated, but the rates in the ESP and control groups were higher than predicted. Given the sample size we achieved, we had 30% power to detect significance of the difference we found at the 0.05 level. Thus our power was limited, compromising our ability to detect significant results.
We had hypothesized better maternal and infant outcomes for Early Start Plus compared to Early Start, but found no differences in the multivariate analyses. However, several other recent, well-designed alcohol trials have similarly failed to detect differences between study groups. For example, Project MATCH found similar drinking outcomes for motivation enhancement therapy, cognitive behavioral therapy, and 12-step facilitation (Project MATCH Research Group, 1996); trials comparing medical and social model treatment did not find different abstinence rates for patients treated in day hospital or community residential treatment (Witbrodt et al., 2007b), nor for those randomized to clinical vs. social model day treatment (Kaskutas, Witbrodt, & French, 2004). Also see McKay, Alterman, McLellan, Snider, & O'Brien (1995) for similar results with male-only samples.
We believe this trend across studies reflects the very high quality of treatment delivery, regardless of modality, as well as common therapeutic ingredients that are present in seemingly diverse treatment approaches (NIAAA, 2006). Certainly that is the case here. Early Start is an intensive abstinence-based, patient-focused prenatal program in which highly skilled therapists provide compassionate support throughout pregnancy. In a recent study (Goler, Armstrong, Taillac & Osejo, 2008), substance abusing women treated by the Early Start Program were found to have significantly better neonatal and maternal outcomes than untreated substance abusing women. Perhaps it is not surprising that the Early Start Plus Group did not do significantly better than the Early Start Group; it was being compared to an already very high standard of care.
A key contribution of Early Start Plus is that its computerized assessment of drink sizes provided a guided framework in which the same high caliber of therapists (the Early Start Specialists) could discuss drinking during pregnancy without feeling they were giving women permission to drink. We had hoped to be able to compare drinking outcomes between the Early Start Plus and the Early Start groups, to measure the effectiveness of Early Start Plus in reducing heavy drinking and to determine whether it also resulted in less abstention than Early Start (which was abstention-focused and did not encourage discussions about how to drink less). Toward that end, telephone interviews near the end of pregnancy were planned to provide the trial with drinking measures that would have allowed us to study changes that occurred in drinking following the Early Start Plus and the Early Start interventions. However, only about half the women in the Early Start Plus group completed a follow-up interview (compared to over 90% in the Early Start and the control groups), thus leaving a very small sample size for studying drinking among women who received Early Start Plus. Another limitation with the follow-up interviews among women in Early Start Plus arises from their heightened awareness of standard drink sizes; the follow-up interviews asked women about their drinking in terms of standard drinks, thus introducing still another bias to the drinking data of women in the Early Start Plus group.
One final point about Early Start Plus is how well received it was by the Early Start Specialists and clients alike. The Specialists felt that the intervention was a useful tool in their assessments of drinking during pregnancy, and that it was indispensable for women who were resistant to abstinence. Perhaps the most valuable use of the computerized program and actual vessels models is as an assessment tool for substance abuse treatment providers, including licensed clinical social workers, which genuinely facilitates an in-depth discussion with pregnant women about their drinking. We hope to study this in subsequent research, and to increase the reliability and validity of drinking assessments in prenatal clinics using the computerized tool and the models of drinking vessels. The computerized intervention is available from the authors upon request.
Acknowledgments
This project was supported by a grant (RO1 AA12486) from the National Institute on Alcohol Abuse and Alcoholism. The authors thank Rachael Korcha, MA for invaluable help in data base development and computer programming.
Contributor Information
Mary Anne Armstrong, Kaiser Permanente Medical Care Program, Division of Research, Oakland, California.
Lee Ann Kaskutas, Alcohol Research Group, Emeryville, California, School of Public Health, University of California, Berkeley, California.
Jane Witbrodt, Alcohol Research Group, Emeryville, California.
Cosette J. Taillac, Kaiser Foundation Health Plan, Patient Care Services, Oakland, California.
Yun-Yi Hung, Kaiser Permanente Medical Care Program, Division of Research, Oakland, California.
Veronica M. Osejo, Kaiser Foundation Health Plan, Patient Care Services, Oakland, California.
Gabriel J. Escobar, Kaiser Permanente Medical Care Program, Division of Research, Oakland, California, Department of Pediatrics, Kaiser Permanente Medical Center, Walnut Creek, California.
References
- American Medical Association and Council on Scientific Affairs. Fetal effects of maternal alcohol use. Journal of the American Medical Association. 1983;249(18):2517–2521. doi: 10.1001/jama.249.18.2517. [DOI] [Google Scholar]
- Armstrong MA, Osejo V, Lieberman L, Carpenter DM, Pantoja PM, Escobar GJ. Perinatal substance abuse intervention in obstetric clinics decreases adverse neonatal outcomes. Journal of Perinatology. 2003;23(1):3–9. doi: 10.1038/sj.jp.7210847. [DOI] [PubMed] [Google Scholar]
- Armstrong MA, Lieberman L, Carpenter DM, Gonzales VM, Usatin MS, Newman L, et al. Early Start: an obstetric clinic-based, perinatal substance abuse intervention program. Quality Management in Health Care. 2001;9(2):6–15. doi: 10.1097/00019514-200109020-00004. Retrieved June 30, 2008, from http://web.ebscohost.com/ehost/pdf?vid=5&hid=9&sid=69434ff6-a6c8-45d2-bce1-2e3da5cb5fb3%40sessionmgr7. [DOI] [PubMed]
- Broekhuizen FF, Utrie J, Van Mullem C. Drug use or inadequate prenatal care? Adverse pregnancy outcome in an urban setting. American Journal of Obstetetrics and Gynecology. 1992;166(6):1747–1754. doi: 10.1016/0002-9378(92)91565-r. [DOI] [PubMed] [Google Scholar]
- Burd L, Roberts D, Olson M, Odendaal H. Ethanol and the placenta: A review. Journal of Maternal-Fetal and Neonatal Medicine. 2007;20(5):361–375. doi: 10.1080/14767050701298365. [DOI] [PubMed] [Google Scholar]
- Chang G, McNamara TK, Orav EJ, Koby D, Lavigne A, Ludman B, et al. Brief intervention for prenatal alcohol use: a randomized trial. Obstetrics & Gynecology. 2005;105(5 Pt 1):991–998. doi: 10.1097/01.AOG.0000157109.05453.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang G, Wilkins-Haug L, Berman S, Goetz MA. Brief intervention for alcohol use in pregnancy: a randomized trial. Addiction. 1999;94(10):1499–1508. doi: 10.1046/j.1360-0443.1999.941014996.x. [DOI] [PubMed] [Google Scholar]
- Day NL, Jasperse D, Richardson G, Robles N, Sambamoorthi U, Taylor P, et al. Prenatal exposure to alcohol: effect on infant growth and morphologic characteristics. Pediatrics. 1989;84(3):536–541. [PubMed] [Google Scholar]
- Faden VB, Hanna E, Graubard BI. The effect of positive and negative health behavior during gestation on pregnancy outcome. Journal of Substance Abuse. 1997;9:63–76. doi: 10.1016/s0899-3289(97)90006-7. [DOI] [PubMed] [Google Scholar]
- Floyd RL, O'Connor MJ, Bertrand J, Sokol R. Reducing Adverse Outcomes from Prenatal Alcohol Exposure: A Clinical Plan of Action. Alcoholism: Clinical and Experimental Research. 2006;30(8):1271–1275. doi: 10.1111/j.1530-0277.2006.00175.x. [DOI] [PubMed] [Google Scholar]
- Flynn HA, Marcus SM, Barry KL, Blow FC. Rates and Correlates of Alcohol Use Among Pregnant Women in Obstetrics Clinics. Alcoholism: Clinical and Experimental Research. 2003;27(1):81–87. doi: 10.1111/j.1530-0277.2003.tb02725.x. [DOI] [PubMed] [Google Scholar]
- Goler N, Armstrong MA, Taillac C, Osejo VM. Substance abuse treatment linked with prenatal visits reduces negative maternal and neonatal outcomes: Setting a new standard. Journal of Perinatology. 2008 doi: 10.1038/jp.2008.70. Advance online publication. Retrieved June 26, 2008. [DOI] [PubMed] [Google Scholar]
- Stratton K, Howe C, Battaglia F, editors. Institute of Medicine. Fetal alcohol syndrome: diagnosis, epidemiology, prevention and treatment. Washington D.C.: National Academy Press; 1996. p. 213. [Google Scholar]
- Jacobson JL, Jacobson SW. Prenatal exposure and neurobehavioral development: where is the threshold? Alcohol Health Research World. 1994;18(1):30–36. [PMC free article] [PubMed] [Google Scholar]
- Jaddoe VW, Bakker R, Hofman A, Mackenbach JP, Moll HA, Steegers EA, et al. Moderate alcohol consumption during pregnancy and the risk of low birth weight and preterm birth. The generation R study. Annals of Epidemiology. 2007;17(10):834–840. doi: 10.1016/j.annepidem.2007.04.001. [DOI] [PubMed] [Google Scholar]
- Jones KL, Smith DW. Pattern of malformation in offspring of chronic Alcoholic mothers. Lancet. 1973;1:1267–1271. doi: 10.1016/s0140-6736(73)91291-9. [DOI] [PubMed] [Google Scholar]
- Kaskutas LA, Graves K. Relationship between cumulative exposure to health messages and awareness and behavior-related drinking during pregnancy. American Journal of Health Promotion. 1994;9(2):115–124. doi: 10.4278/0890-1171-9.2.115. [DOI] [PubMed] [Google Scholar]
- Kaskutas LA. Understanding drinking during pregnancy among urban American Indians and African Americans: health messages, risk beliefs, and how we measure consumption. Alcoholism Clinical & Experimental Research. 2000;24(8):1241–1250. doi: 10.1111/j.1530-0277.2000.tb02090.x. [DOI] [PubMed] [Google Scholar]
- Kaskutas LA, Witbrodt J, French MT. Outcomes and costs of day hospital treatment and nonmedical day treatment for chemical dependency. Journal of Studies on Alcohol. 2004;65(3):371–382. doi: 10.15288/jsa.2004.65.371. [DOI] [PubMed] [Google Scholar]
- Leonardson GR, Loudenburg R. Risk factors for alcohol use during pregnancy in a multistate area. Neurotoxicology and Teratology. 2003;25:651–8. doi: 10.1016/j.ntt.2003.07.002. [DOI] [PubMed] [Google Scholar]
- Leonardson GR, Loudenburg R, Struck J. Factors predictive of alcohol use during pregnancy in three rural states. Behavioral and Brain Functions. 2007;3(8) doi: 10.1186/1744-9081-3-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Little RE. Moderate alcohol use during pregnancy and decreased infant birth weight. American Journal of Public Health. 1977;67(12):1154–1156. doi: 10.2105/ajph.67.12.1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKay JR, Alterman AI, McLellan AT, Snider EC, O'Brien CP. The effect of random versus nonrandom assignment in a comparison of inpatient and day hospital rehabilitation for male alcoholics. Journal of Consulting and Clinical Psychology. 1995;653(1):70–78. doi: 10.1037/0022-006X.63.1.70. [DOI] [PubMed] [Google Scholar]
- National Center on Birth Defects and Developmental Disabilities, Centers for Disease Control and Prevention, Department of Health and Human Services. Fetal Alcohol Syndrome: Guidelines for Referral and Diagnosis. Washington D.C.: Department of Health and Human Services; 2004. [Google Scholar]
- National Institute on Alcohol Abuse and Alcoholism. Mechanisms of behavior change in the treatment of alcohol use disorders (R21) 2006. FRA-AA-07-005. [Google Scholar]
- National Institute on Alcohol Abuse and Alcoholism. Alcohol Alert. 2005. Brief Interventions; pp. 1–8. [Google Scholar]
- O'Connor MJ, Whaley SE. Brief Intervention for Alcohol Use by Pregnant Women. American Journal of Public Health. 2007;97(2):252–8. doi: 10.2105/AJPH.2005.077222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Office of Applied Studies. Substance use among women in the United States. Washington: Department of Health and Human Services, Substance Abuse and Mental Health Services Administration; 1997. [Google Scholar]
- Project MATCH Research Group. Project MATCH: Treatment main effects and matching results. Alcoholism Clinical & Experimental Research. 1996;20(8):196A–197A. doi: 10.1111/j.1530-0277.1996.tb01775.x. [DOI] [PubMed] [Google Scholar]
- Rosett HL, Ouellette EM, Weiner L, Owens E. Therapy of heavy drinking during pregnancy. Obstetrics & Gynecology. 1978;51(1):41–46. [PubMed] [Google Scholar]
- Rosett HL, Weiner L, Edelin KC. Treatment experience with pregnant problem drinkers. Journal of the American Medical Association. 1983;249(15):2029–2033. doi: 10.1001/jama.249.15.2029. [DOI] [PubMed] [Google Scholar]
- Sratton K, Battaglia CH, editors. Fetal alcohol syndrome: diagnosis, epidemiology, prevention, and treatment. Washington D.C.: National Academy Press; 1996. [Google Scholar]
- Tikkanen M, Nuutila M, Hiilesmaa V, Paavonen J, Ylikorkala O. Clinical presentation and risk factors of placental abruption. Acta Obstetricia et Gynecologica Scandinavica. 2006;85(6):700–705. doi: 10.1080/00016340500449915. [DOI] [PubMed] [Google Scholar]
- United States Food and Drug Administration. FDA drug bulletin. Washington, D.C.: 1981. [Google Scholar]
- Warren KR, Foudin LL. Alcohol-related birth defects – The past, present, and future. Alcohol Research and Health. 2001;25:153–158. [PMC free article] [PubMed] [Google Scholar]
- Weiner L, Larsson G. Clinical prevention of fetal alcohol effects: a reality. Alcohol Health Research World. 1987 Summer;:60–63. 92–93. [Google Scholar]
- Witbrodt J, Armstrong MA, Diehl S, Kaskutas LA, Taillac C, Osejo VM, et al. Using drink size to talk about drinking during pregnancy: Early Start Plus. Journal of Addictions Nursing. 2007a;18(4):199–206. doi: 10.1080/10884600701699420. [DOI] [Google Scholar]
- Witbrodt J, Bond J, Kaskutas LA, Weisner C, Jaeger G, Pating D, et al. Day Hospital and Residential Addiction Treatment: Randomized and Nonrandomized Managed Care Clients. Journal of Consulting and Clinical Psychology. 2007b;75(6):947–959. doi: 10.1037/0022-006X.75.6.947. [DOI] [PubMed] [Google Scholar]