Abstract
Most, if not all, cell types and tissues display several aspects of polarization. In addition to the ubiquitous epithelial cell polarity along the apical-basolateral axis, many epithelial tissues and organs are also polarized within the plane of the epithelium. This is generally referred to as planar cell polarity (PCP; or historically, tissue polarity). Genetic screens in Drosophila pioneered the discovery of core PCP factors, and subsequent work in vertebrates has established that the respective pathways are evolutionarily conserved. PCP is not restricted only to epithelial tissues but is also found in mesenchymal cells, where it can regulate cell migration and cell intercalation. Moreover, particularly in vertebrates, the conserved core PCP signaling factors have recently been found to be associated with the orientation or formation of cilia. This review discusses new developments in the molecular understanding of PCP establishment in Drosophila and vertebrates; these developments are integrated with new evidence that links PCP signaling to human disease.
Keywords: cell polarity, drosophila, organ patterning, frizzled-pcp signaling, ciliopathies
HISTORY OF PLANAR CELL POLARITY STUDIES
The coordination of cellular polarization is an important feature of development and critical for organ function. Epithelial apical-basolateral polarity enables organs and tissues to perform vectorial functions, including transport of fluid or directed secretion of specialized components. In addition, most epithelial tissues require a second axis of polarity, commonly referred to as planar cell polarity (PCP), which is within the plane of an epithelium. This type of polarity is, however, not restricted to epithelial tissues, but is also found in mesenchymal cell types throughout animal development.
Historically, the study of PCP originated from work in arthropods (e.g., 45, 75; then referred to as tissue polarity), and elegant genetic analyses in Drosophila, where most adult cuticular structures show PCP-type polarization, put the problem firmly on the map some 25 years ago (45, 144). PCP studies in Drosophila have been most prominently studied in the fly wing, eye, abdomen, and notum (for reviews on Drosophila PCP, see References 1, 73, 74, 91; see also examples in Figure 1). The initial studies were soon followed by systematic genetic screens, molecular cloning, and functional analyses of Drosophila PCP factors (Table 1) (reviewed in References 1, 73, 128).
Figure 1.
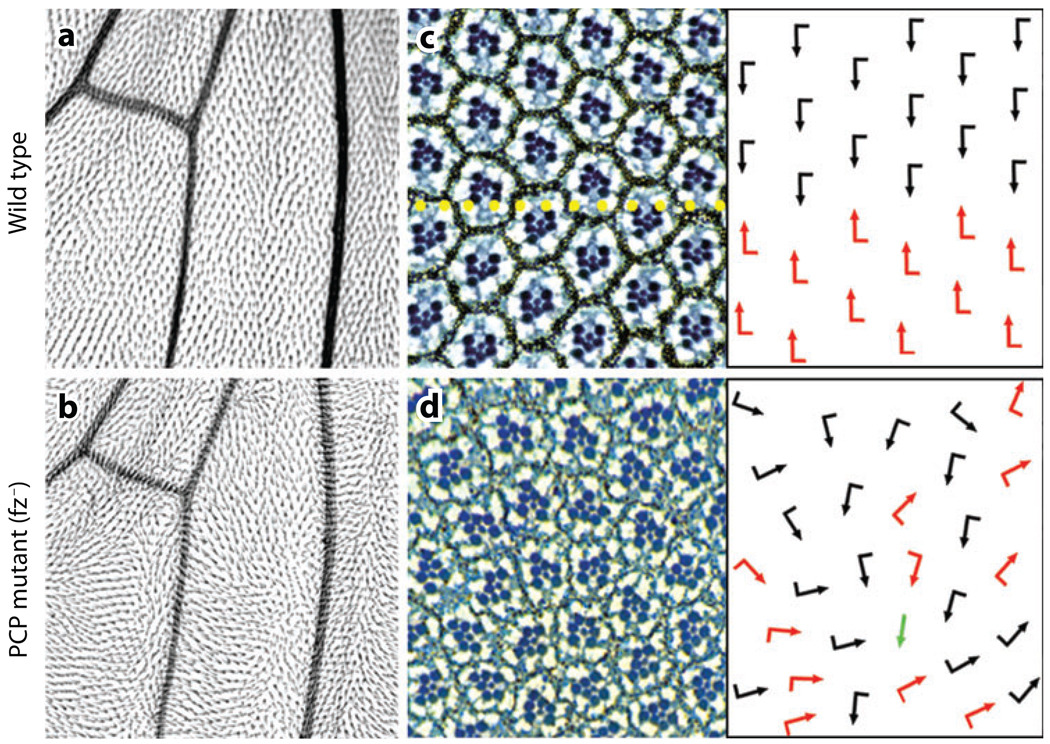
Typical examples of PCP features in Drosophila. PCP effects in the wing (a,b) and eye (c,d). Wild type is shown in panels a and c (note the regular arrangements in both tissues), and the mutant appearance (fz−) of same tissues is shown in b and d. (c and d) The right panels show the orientation of ommatidia schematically by arrows: black and red arrows represent the dorsal and ventral orientations (not mirror image symmetry in wild type/c) and loss of it in the mutant scenario. Occasional symmetrical ommatida (green arrow) are also found in the mutants.
Table 1.
PCP factors in Drosophila and vertebrates
| Drosophila gene | Tissues affected in Drosophila |
Molecular features | Vertebrate homologues involved in PCP (see also Table 2) |
Selected associated PCP-specific references |
|---|---|---|---|---|
| Core Fz/PCP components | ||||
| frizzled (fz) | All adult tissues | Seven-pass transmembrane receptor; binds Wnt ligands; binds Dsh; recruits Dsh and Dgo to membrane |
Fz3 (mouse) | (32, 47, 144, 151, 153, 168) |
| Fz6 (mouse) | ||||
| Fz7 (Xenopus) | ||||
| dishevelled (dsh) | All adult tissues | Cytoplasmic protein containing DIX, PDZ, DEP domains; recruited to membrane by Fz; binds Fz, Pk, Stbm, and Dgo; undergoes extensive phosphorylation |
Dvll, 2, and 3 (mammals) | (5, 13, 126, 133, 148) |
| XDsh (Xenopus) | ||||
|
prickle (pk) (a.k.a. prickle-spiny legs) |
All adult tissues | Cytoplasmic protein with 3 LIM domains and PET domain; recruited to membrane by Stbm; physically interacts with Dsh, Stbm, and Dgo; competes with Dgo for Dsh binding |
pk1 and 2 (mouse, zebrafish) |
(15, 46, 61, 135, 140, 143) |
|
strabismus (stbm)/ Van Gogh (Vang) |
All adult tissues | Novel 4-pass transmembrane protein; binds Pk, Dsh, and Dgo; recruits Pk to membrane |
trilobite (tri; zebrafish) | (20, 60, 62, 95, 136, 155) |
|
Vangl1 (mouse; function unknown) | ||||
| Vangl2 (looptail; mouse) | ||||
|
flamingo (fmi)/ starry night (stan) |
All adult tissues | Cadherin with seven-pass transmembrane receptor features; capable of homophillic cell adhesion |
Celsr1 (mouse) | (27, 30, 141) |
| diego (dgo) | Most adult tissues, notum only in GOF |
Cytoplasmic ankyrin repeat protein; recruited to membrane by Fz; binds Dsh, Stbm, and Pk; competes with Pk for Dsh binding |
Diversin (ankyrin repeat domain 6; ankrd6) |
(29, 40, 61, 93, 117, 123) |
| Inversin (invs) | ||||
| Fz/PCP signaling regulators | ||||
|
Casein Kinase 1ε (CK1ε; discs overgrown/dco) |
Eye and wing* | S/T protein kinase; acts positively on Fz/PCP signaling; regulates Dsh localization |
CK1 epsilon | (24, 72, 131) |
| widerborst (wdb) | Wing, not eye | Regulatory subunit of the PP2A phosphatase |
N.D. | (51) |
|
G protein o α47A (G-oα47A; brokenheart, bkh) |
Wing* | The α subunit of the Go protein; has asymmetric localization in the wing, which requires Fz. Also shown to regulate Fz localization in the wing |
Galpha proteins | (3, 64, 79, 149) |
| Fat/Dachsous PCP group factors | ||||
| fat (ft) | All adult tissues | Divergent member of the Cadherin superfamily; heterophillic interaction with Ds; cytoplasmic domain binds Atrophin |
mFat4 | (16, 74, 82, 86, 87, 116, 122, 160) |
| dachsous (ds) | All adult tissues | Divergent member of the Cadherin superfamily; heterophillic interaction with Fat |
N.D. | (16, 74, 82, 86, 87, 122, 160) |
| four-jointed (fj) | All adult tissues | Type-2 transmembrane protein; possibly functions in Golgi to modify Ds |
N.D. | (122, 130, 166, 167) |
| atrophin (atro) | Eye and wing | Transcriptional corepressor; binds to cytoplasmic domain of Fat |
N.D. | (38) |
| PCP effectors | ||||
| Fz/PCP signaling effectors | ||||
| dDAAM | ? | Formin-homology (FH) domain protein that binds both Dsh and RhoA. The Drosophila homolog is with other FH proteins |
XDaam1 | (49, 88) |
| PCP defects only observed in GOF | ||||
| dRac1 | Eye and wing* | Small GTPase of Rho subfamily, acts downstream of Dsh |
Xrac1 (Xenopus) | (35, 39, 48, 96) |
| dRhoA | Eye and wing* | Rho family of small GTPases, acts downstream of Dsh |
RhoA | (39, 49, 96, 129, 134) |
| misshapen (msn) | Eye, wing and notum* | STE20-like S/T protein kinase acting downstream of Dsh |
(103) | |
| Rho Kinase (drok) | Eye and wing* | RhoA effector protein; S/T kinase | rho kinase 2 (rok; zebrafish) | (83, 154) |
| Tissue-specific PCP effectors (in Drosophila) | ||||
| inturned (in) | Wing | A cytoplasmic protein with a putative PDZ domain that requires Fz/PCP for localization to the proximal side of wing cells where Stbm and Pk are also localized |
inturned (Xint; Xenopus) | (105, 107, 164) |
| fuzzy (fy) | Wing | A putative four-pass transmembrane protein that genetically interacts with inturned |
fuzzy (Xfy; Xenopus) | (22, 105) |
| fritz (frtz) | Wing | Coiled coil WD40 Protein | N.D. | (23) |
|
multiple wing hair (mwh) |
Wing | Molecular data is unavailable, but genetically mwh is downstream of fz and may work in parallel to fy |
N.D. | (2, 25, 156) |
| nemo (nmo) | Eye | S/T protein kinase (MAPK superfamily; Nlk subfamily) |
Nlk members | (19, 137) |
| Vertebrate-specific PCP factors | ||||
| Member of Wnt family of secreted glycoproteins |
Wnt5/pipetail (ppt; zebrafish) |
(52, 68) | ||
| Member of Wnt family of secreted glycoproteins |
Wnt11/silberblick (slb; zebrafish) |
(52) | ||
| Member of Wnt family of secreted glycoproteins |
Wnt7a (mouse) | (28) | ||
| Glypican family member, required for Wnt signaling |
Glypican 4/6/knypek (kny; zebrafish) |
(138) | ||
| Receptor Tyrosine kinase family member |
PTK7 (protein tyrosine kinase 7; mouse) |
(81) | ||
| Xpkt7 (Xenopus) | ||||
| S/T protein kinase of PKC superfamily |
PKCδ (protein kinase Cδ Xenopus) |
(69) | ||
Other tissues were not tested.
N.D.: not determined.
More recently, many vertebrate tissues and developmental processes have been shown to display typical PCP features (for reviews on PCP features, see References 65, 142, 145, 152). First, analyses in Xenopus (133, 148) and zebrafish (62, 84) of the process of convergent extension (CE) during gastrulation and neurulation indicated that orthologues of Drosophila PCP factors are key players in this context. For example, several CE mutants identified in forward genetic screens in zebrafish turned out to be orthologues of Drosophila PCP genes (reviewed in Reference 97). Thus most of the core PCP genes are now known to be important for both epithelial polarization and CE during gastrulation, supporting the commonality of PCP establishment not only across species but also between different polarized cell types and organs. In addition, in mammals, “hairy” organs such as the inner ear and skin/epidermis are beautiful examples of epithelia with PCP features (e.g., 47, 95, 151). In particular, the PCP hair pattern defects in the mouse are strikingly reminiscent of the actin–hair pattern defects in Drosophila wings, and PCP abnormalities in the inner ear are very similar to those in the fly retina (Figure 2) (e.g., 152). Taken together, the study of PCP generation has come full circle—from studying hair patterns on flies, to the studies of eye development, convergent extension, and cochlear development, and now back to studying hair patterns, only this time in mammals.
Figure 2.
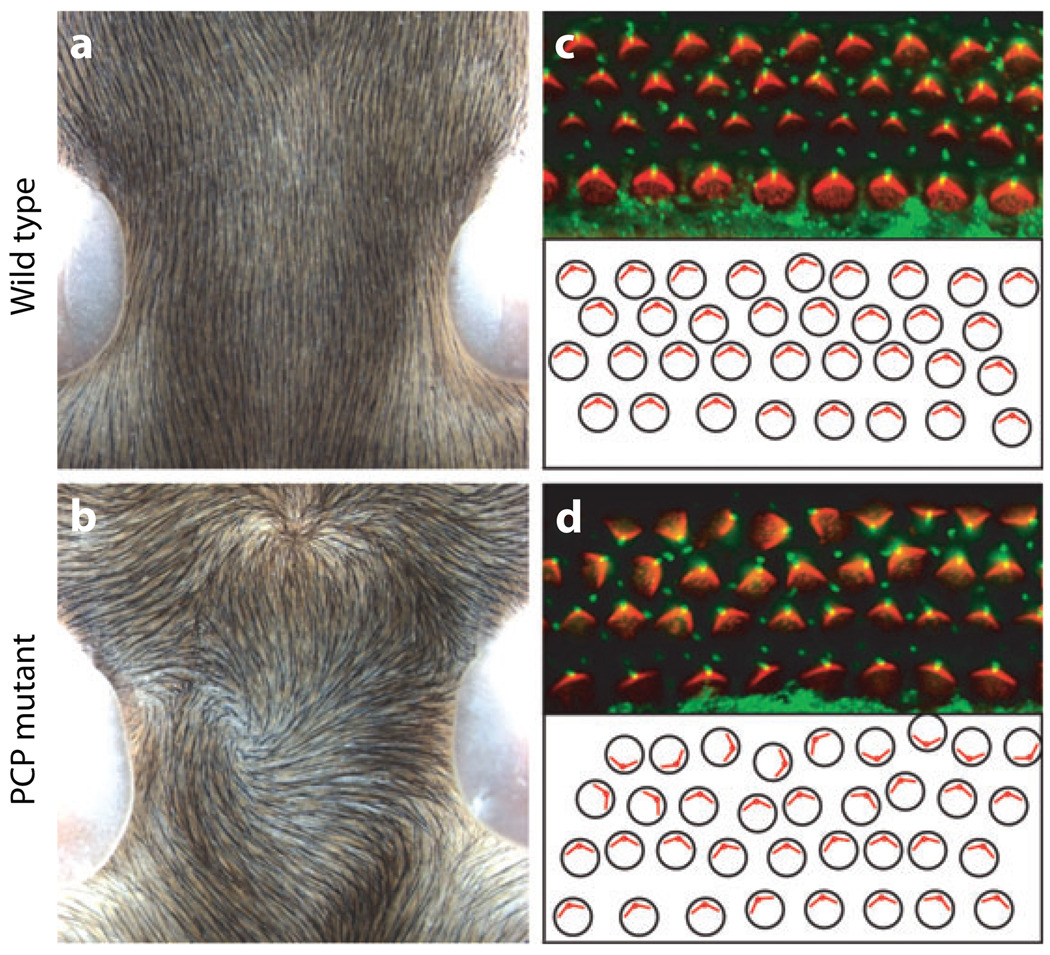
Examples of PCP features in mammals. PCP features of the mouse skin (a,b) and the inner ear (c,d). (a,b) Dorsal view of mouse neck and the orientation of the skin hair in wild-type mice (a) and fz3 mutant mice (b). Note random whorls, swirls, and waves in the mutant genotype as compared to the normal anterior-posterior orientation in a. In a and b anterior is up. (c,d) Orientation of sensory hair cells of the mammalian (mouse) chochlea (inner ear). Each cell contains polarized bundles of actin-based stereocilia (labeled red with phalloidin) and a tubulin-based kinocilium (labeled with antiacetylated tubulin in green). In PCP mutants these bundles still form but their orientation becomes randomized [d; Looptail/Vangl2 (stbm) mutant]. The lower panels in c and d show schematic representation of the cellular (actin bundle) orientation reflecting their randomized appearance in the mutant. The original pictures were kindly provided by Jeremy Nathans.
The evolutionary conservation and parallels of the Drosophila PCP factors during vertebrate development and homeostasis made the analysis of PCP generation an important and mainstream feature of developmental studies in many organisms and medical contexts. Unraveling the molecular and cellular mechanisms of the establishment of PCP is presently one of the frontiers in developmental genetics and cell biology. How individual cells that are hundreds of cell diameters apart acquire the same orientation within an organ (or the plane of an epithelial field) or how mesenchymal cells generate uniform polarization leading to ordered cellular migration and intercalation are fascinating biological and biomedical problems. In addition, recent discoveries suggest that the PCP factors play a critical role in many diseases, and these factors in particular have been linked to genetic syndromes associated with ciliary functions.
Despite significant progress in dissecting the molecular aspects of PCP establishment over the past 10 years, our mechanistic and cellular understanding of this process is still very rudimentary. Several good reviews have been published recently addressing specific PCP issues in Drosophila (e.g., 1, 76, 118, 165) and in vertebrates (e.g., 65, 142, 145, 152). We thus first summarize only the key features of PCP generation and then focus on recent findings of potential links of PCP factors to ciliary morphogenesis and function, and to human disease. We apologize for the omission of research areas and viewpoints that have not been included here.
CONSERVED FACTORS IN PLANAR CELL POLARITY SIGNALING
The current data indicate that there are two evolutionarily conserved sets of PCP factors that act together to coordinate PCP establishment: the Frizzled (Fz)/Flamingo (Fmi) core genes (Table 1; see below) and the Fat/Dachsous (Ds) PCP system (Table 1 and see below). The relationship between the two sets of factors is unresolved, and views and models of their interaction and integration differ (see below).
The Frizzled/Flamingo Core Group
The core Fz/PCP gene cassette is highly conserved across species and tissues and is now understood in more detail than that of the Fat/Ds system. The components of the Fz/Fmi system include Fz and Fmi (a.k.a. Starry night/Stan), Dishevelled (Dsh; Dvl in mammals), Prickle (Pk), Strabismus/Van Gogh (Stbm/Vang), and Diego (Dgo; Diversin and Inversin in vertebrates) (reviewed in References 1, 73, 128, 142, 152). In brief, historically Fz and Dsh have been the central PCP signaling molecules (potentially acting on the downstream effectors of, for example, the Rho subfamily of GTPases; see also Table 1), with the other core Fz-Fmi/PCP factors that regulate Fz-Dsh localization and/or signaling activity. For example, Stbm/Vang and Pk antagonize Fz-Dsh activity (60, 61, 136, 140, 155). Pk is recruited to the membrane by Stbm (60), binds Dsh, and antagonizes its Fz-mediated membrane recruitment (140). Stbm also binds Dsh and thus might affect its function directly (8, 104). In general, as deduced from the Drosophila data the Fz-Dsh and Stbm/Vang-Pk pairs are thought to antagonize each other, and their localization becomes resolved to mutually exclusive domains at opposite poles of each cell (Figure 3; see below). In addition, Diego promotes Fz-Dsh activity, colocalizes with Fz-Dsh, and binds Dsh directly (29, 40, 61). This interaction is thought to protect Dsh from the antagonistic effect of the Stbm-Pk complex (61).
Figure 3.
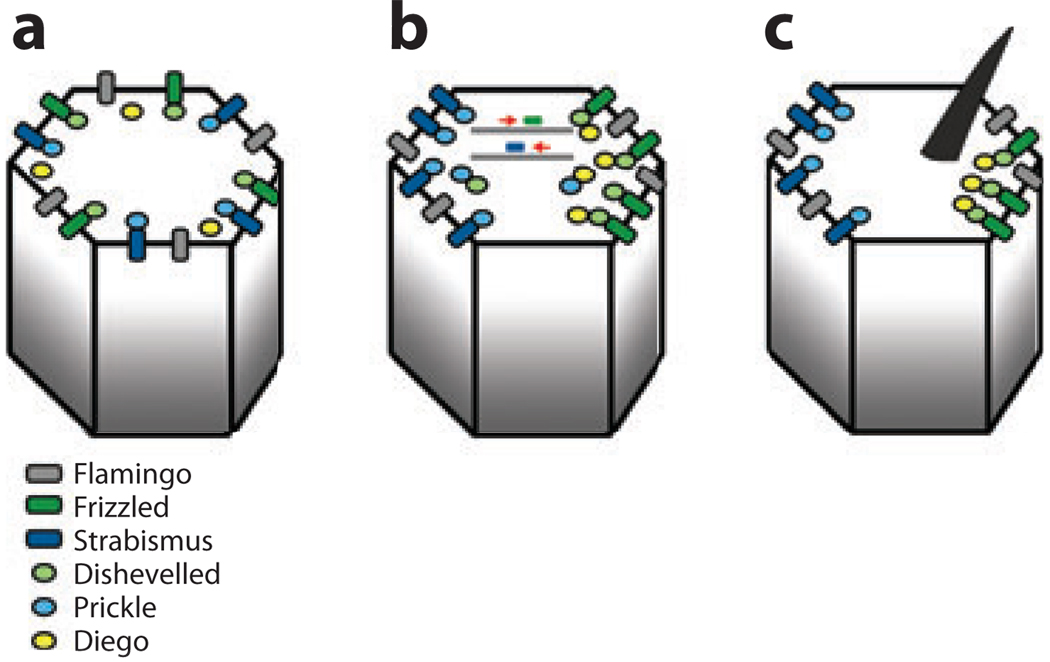
Schematic presentation of the generation of asymmetric core PCP protein localization in Drosophila wing cells. In pupal wing cells, the core PCP proteins of the Fz/Fmi cassette become asymmetrically localized to proximal and distal cell membranes. Proximal is left and distal is right in all panels. A single cell at different stages maturing from left to right is shown in the respective panels a–c. (a) Schematic of the localization of the core PCP proteins prior to any asymmetry detection at the onset of their interactions. (b) During polarization the Fz-Dsh-Dgo complexes become enriched at the distal end of each cell, whereas the Stbm-Pk complexes concentrate proximally. Fz has been seen to travel distally on microtubule-associated particles (121), and something similar might also happen for Stbm/Vang. (c) Final stage of polarization with all complexes resolved in either distal (Fz-associated protein or proximal cell ends. An actin-based hair is formed close to the distal vertex of each cell (where Fz-Dsh are localized). The Drosophila pupal wing is the only tissue in which the localization of all core PCP proteins has been analyzed; all but Dsh have also been analyzed in the Drosophila eye.
The role of the atypical cadherin Fmi is the least understood in this group (30, 120, 141). Fmi is thought to serve a homophilic adhesion function; it colocalizes with both the Fz-Dsh and the Stbm/Vang-Pk complexes, and is also genetically required in both complexes. The role of Fmi needs to be addressed in more detail. In particular, Fmi still has no known molecular PCP interaction partner.
It remains an open question how the localization and interactions will play out in the vertebrate Fz/PCP system. Although the asymmetric localization of some of the PCP factors has been documented in some vertebrate tissues (e.g., in the mouse inner ear or during zebrafish gastrulation and neurulation), a complete data set and thus an equivalent model to Drosophila do not yet exist. The current examples include the asymmetric localization of Vangl2, Dvl2, and mFz3 and mFz6 in the mouse inner ear (95, 150, 151), and the localization of Pk and Dsh during zebrafish convergent extension processes (20, 162; see below).
Genetic studies in Drosophila have identified additional genes that act within the Fz/PCP group as either downstream effectors or regulatory genes. The CKIε kinase has emerged as a potential regulator of Fz/PCP signaling in Drosophila (72, 131), and a related PCP function has been proposed in vertebrates (89). Genetic interactions indicate that CKIε acts positively on Fz-Dsh activity (72, 131). CKIε is apically enriched like Fz and Dsh and required for asymmetric Dsh localization, but it is itself not asymmetrically localized within the proximo-distal axis in wing cells (131). Surprisingly, its kinase activity is dispensable for Fz/PCP signaling (72).
An additional factor thought to participate in Fz-Dsh/PCP signaling is the heterotrimeric G protein subunit Gαo. Genetic evidence supports its role in both canonical Fz-Dsh/β-catenin signaling and Fz-Dsh/PCP signaling (64). Biochemical studies in heterologous cell culture systems also support a role of heterotrimeric G proteins in both Fz signaling pathways (3, 79, 149). However, the mechanistic role of the G proteins (and their associated regulators) remains unclear for either Fz-Dsh/PCP or canonical Fz-Dsh/β-catenin signaling. Importantly, the physical interaction of the candidate G protein(s) with Fz family receptors, a hallmark feature of seven pass-transmembrane G protein coupled receptors, has not been demonstrated. Moreover, in Drosophila, Gαo does not colocalize with the Fz-Dsh complex (or the Stbm-Pk complex) in wing cells, but it displays a diffuse proximally enriched localization (64). Thus, the role(s) of the G proteins in Fz-Dsh signaling remains to be resolved.
The Fat/Dachsous PCP Group
A second group of regulators has been shown in Drosophila to affect PCP establishment in all tissues analyzed. Its main components are the very large protocadherins Fat (Ft) and Dachsous (Ds) (82, 114, 160), as well as Four-jointed (Fj) (130, 166, 167). Fat and Ds can interact heterophilically across cell boundaries (86, 87). In addition to Fat, Ds, and Fj, the Atrophin (Atro; a.k.a. Grunge/Gug) protein has been linked to this group (37, 38). Although atro shows similar genetic behavior to Fat in the eye, how it is linked to Fat mechanistically is unclear. Atro has been shown to associate with the intracellular domain of Fat (38). However, it is a transcriptional corepressor in all other contexts and thus this might suggest that the Fat-Ds interaction could act via transcriptional regulation.
Fj is thought to modulate the activity of Ds and possibly its interaction with Fat (130). Fj appears to be acting in the Golgi and, based on phenotypic similarities and genetic studies, is proposed to modulate the extracellular domain of Ds and thus affect its “activity” (130). This interpretation is supported by the observation that, while fat expression is uniform, ds and fj are expressed in opposing gradients in the eye (160) and also in the wing, albeit to a lesser extent. Moreover, it is sufficient that either Ds or Fj are expressed in a graded manner (122).
The functional relationship of the Fat/Ds group to the Fz/Fmi PCP core group remains an open question. It has been suggested that Fat/Ds acts upstream of Fz/PCP signaling (82, 160), largely based on data in the fly eye. For this system it has been suggested that Fz/Fmi-PCP signaling loses its bias between the R3 and R4 cells in the fat/ds mutant situation and a random decision is made (160), whereas in wild type, higher Fz/PCP signaling in the R3 precursor relative to R4 is key to properly specifying it as R3; PCP establishment in the fly eye is determined by the interactions and specific fates of the R3/R4 precursors (91). However, more recently, a very detailed and complete set of results presented by Casal and colleagues (16), from analysis of PCP establishment and the interactions between the Fat/Ds and Fz/Fmi groups in the abdomen, strongly suggest that the two signaling cassettes act in parallel and reinforce correct PCP establishment through their independent parallel inputs. This analysis took advantage of the nonautonomous properties of some of the core PCP factors [first described for fz (144)] and established that, for example, the nonautonomous redirection of wild-type cells, as caused by either fat or ds mutant clones, does not require the presence of Fz or Fmi. The simplest interpretation of these very extensive data is that the two signaling cassettes act independently of each other in parallel (16; see also 76). This conclusion is further supported by the observation that in certain tissues (for example, the larval denticle belts) neither pathway shows defects in a single loss-of-function scenario, but PCP defects become apparent when components of both cassettes are mutant (16). This observation suggests that sometimes the two cassettes act redundantly in parallel, again supporting a parallel input. With these new data in hand, it is possible to reinterpret the available data in the eye (160) along the same lines and conclude that Fz/Fmi-PCP signaling is “normal” in fat mutant tissue, but as the two pathways act independently, in a nonredundant manner in the eye, a randomized R3/R4 fate decision is the result for mutants of either. Despite an active but unresolved debate, the evidence now clearly favors the parallel, independent function of the Fat/Ds and Fz/Fmi cassettes as proposed by Lawrence et al. (16, 76).
TISSUE-SPECIFIC PCP SIGNALING EFFECTOR PROTEINS
Both signaling cassettes are required in many different tissues (from flies to mammals) and the associated cellular responses are very diverse, ranging from the regulation of cytoskeletal organization, cell adhesion and movement, nuclear signaling, and orientation of the mitotic spindle to probably more yet unknown cellular functions (reviewed in 118, 152). Thus, besides the core PCP factors described above, many tissue-specific PCP signaling effectors have been identified in Drosophila (see also Table 1). Three main tissue responses are known in Drosophila: (a) formation of actin hairs (trichomes) on a specific side of the cell as a result of cytoskeletal rearrangements in, for example, the wing or the abdomen; (b) cell fate specification as a result of nuclear signaling in the eye; and (c) orientation of the mitotic spindle in sensory organ precursor cells on the thorax and abdomen (for detailed reviews, see References 1, 73, 91, 132). Each of these tissues is thought to have some tissue-specific PCP effectors. For example, the genes fuzzy and inturned appear to be specific to the cytoskeletal rearrangements occurring in wing cells (and related cellular polarization in other cells of the adult cuticle). They have no obvious effect in the eye. Similarly, the kinase encoded by nemo appears to be an eye-specific effector for the ommatidial rotation process, and it has no obvious PCP phenotypes in the wing (90).
In contrast to these, there are also Fz/PCP effectors that function in all tissues analyzed. Most prominently, this group includes the Rho family GTPases (35, 39, 96, 129) and dROK, the Drosophila orthologue of mammalian Rho-associated kinase ROCK (154). DRok, for example, appears to affect a specific step of PCP establishment in the wing and the eye, e.g., the number wing hairs formed per cell in the wing and ommatidial rotation in the eye.
Although the Rho-family GTPases are also required in several contexts for vertebrate PCP establishment (48, 49), it is not yet established what the cellular effector pool will be in vertebrates. Note that in some vertebrate PCP contexts, some of these effectors from Drosophila have been implicated as well. For example, Fuzzy and Inturned appear critical for the formation of cilia (105), but here they act upstream of Dvl and Rho (105, 106). Moreover, in zebrafish CE Nlk (Nemolike kinase) mutants enhance the CE-specific Wnt11/silberblick–associated cell movement defects (137). More studies are needed to correlate the respective functions between the different organisms.
PAR PROTEINS AND PCP ESTABLISHMENT
Several recent studies suggest that there is also a link between the PAR proteins and PCP establishment. A recent study has identified physical interactions between an ArfGAP (XGAP) and several PAR proteins as essential in the PCP context of convergent extension (CE) in Xenopus (58). The XGAP and the PAR proteins (PAR-6, 14-3-3e/PAR-5, and aPKC) localize to mediolateral ends of cells during the CE process, where they are mutually dependent on each other’s localization, suggesting the existence of stable, polarized PAR complexes. These observations suggest an intriguing relationship between the PAR protein complexes and the core Fz/PCP factors. Although it requires the comparison of two different systems (Xenopus and zebrafish), it is intriguing to speculate that the PAR/aPKC complex and the Fz/PCP complexes display antagonistic interactions. For example in zebrafish, in cells undergoing CE, Pk and Dsh are asymmetrically localized within the anterior-posterior axis, with Pk localizing to the anterior end of cells (20) and Dsh localizing to the posterior of the same cells (162), whereas the XGAP-PAR-aPKC complex is localized to the mediolateral ends of cells during Xenopus CE. If these localizations are conserved across species, this would indeed suggest an antagonistic interaction between the complexes containing aPKC and core Fz/PCP factors. Support for this model comes from studies of PCP establishment in the Drosophila eye. Here, Fz/Fmi levels are selectively increased in the R3/R4 pair by antagonizing aPKC in the two critical cells for Fz/PCP signaling via PAR-3 (Bazooka). In other cells during eye patterning, where PCP signaling is low, aPKC phosphorylates Fz and thus reduces its levels by a yet unknown mechanism (33).
Taken together with the mutually inhibitory interactions between the Fz-Dsh and Stbm-Pk complexes (see above), antagonistic interactions among all three complexes (the aPKC complex and the two PCP complexes) lead in mesenchymal cells to an anterior Stbm-Pk complex, a posterior Fz-Dsh complex [as are both factually seen in zebrafish (20, 162)], and a mediolateral aPKC/PAR complex. This would provide cells undergoing CE with all polarity information needed for convergence to and intercalation at the midline. Consistent with this model, the PAR-1 kinase can phosphorylate and positively regulate Dsh during CE (102) and PAR-1 localization appears to be mutually exclusive with that of aPKC/PAR-3/PAR-6 in several cellular contexts. However, this model remains speculative, and further experimental studies are needed to dissect these interactions in more detail.
HOW IS PCP GLOBALLY COORDINATED OR THE CELLULAR ASYMMETRY GENERATED ACROSS TISSUES?
Although much information has been gained by studying PCP in Drosophila, one question has not been answered. How is the global polarization—the initial asymmetry—within whole tissues established? The Fz receptor-family are the receptors of the secreted growth factors of the Wnt family, and functions as such in canonical Wnt/β-catenin signaling (e.g., 80). Thus the Drosophila Wnts are good candidates to serve as a polarizing cue in PCP as well. However, to date no published experimental evidence supports this assumption, and it remains unknown how whole fields of cells are initially polarized in Drosophila. In vertebrates, the Wnt5a and Wnt11 family members have been clearly linked to PCP-type signaling; for example, the zebrafish mutants silberblick (Wnt11) and pipetail (Wnt5a) have typical convergent extension phenotypes (52, 77). The experimental evidence, however, suggests a permissive role rather than an instructive role for these vertebrate Wnts. Recent additions to canonical Wnt signaling in vertebrates suggest that a Fz ligand could also be unrelated to Wnts. For example, Norrin is a high-affinity ligand for mammalian Fz4 (shown in mouse and human), activating canonical signaling (159). It shares no apparent sequence similarity to Wnts, and no homologues of Norrin are present in the fly genome. This observation might suggest, however, that other secreted factors might regulate the activity of Fz in PCP. Nevertheless, despite serious efforts by the Drosophila PCP community to find a Fz ligand for PCP signaling, it appears that the Fz signaling activity in PCP is largely ligand independent or, in other words, constitutive. This conclusion, although mainly based on gain-of-function data (12, 17, 113, 127, 157, 158), taken together with the lack of any known ligand is rather compelling.
What then are the global polarizing signal(s) and mechanism(s)? As mentioned above, it has been proposed that the Fat/Ds system could serve as an upstream polarizing cue for the Fz/Fmi-PCP group. Members of the Fat/Ds group are expressed in gradients (e.g., Ds in the eye and the wing) and their graded expression is under the control of canonical Wg-signaling (86, 160). As attractive as this model first appears, there are obvious shortcomings. First, the gradients of the Fat/Ds group have opposite slopes in the eye and wing relative to the presumed polarization of the field, as deduced through the analysis of the Fz/Fmi core factors (86, 160), arguing against a general polarizing mechanism. Second, as discussed above, it appears likely that the Fat/Ds and Fz/Fmi “cassettes” function in parallel to each other (16, 76). As there are no known Fz ligands in PCP in Drosophila, maybe extracellular ligands should be sought for the other transmembrane core PCP factors in that group, Vang/Stbm and/or Fmi. A global polarization of these would suffice to create a polarized field and maybe this could provide clues for the global polarization cue. Or can Drosophila learn a trick from vertebrates here?
PCP IN VERTEBRATES
Although the fly is amenable to phenotypic analysis of PCP, studying PCP in vertebrates remains difficult. Most PCP genes have only one isoform in Drosophila, whereas in vertebrates there are often numerous isoforms (for example 3 Dvls and 10 Fzs in the mouse). Furthermore, some isoforms have nonoverlapping expression patterns, with the result that knocking out one PCP mouse gene will most likely not lead to full PCP defects, as is the case in Drosophila. Therefore, the study of PCP generation in vertebrates has been hampered by redundancy and requires a detailed analysis of as many tissues as possible. Double and triple knockouts are very often inevitable, making it a tedious business (152).
In the fly, the tissues that are typically scored for PCP defects are mostly external epithelia and therefore easily accessible. So far, only a few vertebrate or mammalian tissues have been shown to involve clear aspects of PCP. At present, the tissues that require PCP genes to organize their cells in the plane are cells that undergo convergent extension during gastrulation, neurulation, and possibly also cardiac outflow tract development (53). Inner ear sensory cells use PCP to organize their characteristic stereociliary bundles. In the skin, hair follicle orientation as well as eyelid closure requires PCP (152). Recent data also indicate that PCP signaling is involved in renal development and that defective PCP signaling leads to polycystic kidney disease (124).
The list of affected tissues in PCP mutants (Table 2) will likely grow. Most tissues, in particular epithelia, require a three-dimensional organization during development and also in adulthood to maintain their function. PCP controls organization in the planar dimension and should therefore be active at some point in most tissues. Likely candidates are, for example, tissues that perform a vectorial function in the plane of the epithelium, such as the respiratory epithelium that transports mucus aborally through coordinated ciliary beating, or the oviduct epithelium that transports the fertilized ovum to the uterus, as was already proposed 10 years ago (34).
Table 2.
Vertebrate PCP phenotypes
| Tissues affected (defects) | Genes | References |
|---|---|---|
| CNS (neural tube closure defect) | Fz3/6, Dvl1/2, Vangl1/2, Celsr1, Fy, Int BBS4/6, PTK7 |
(27, 50, 66, 67, 81, 95, 105, 111, 115, 150, 151) |
| Cochlea (stereocilia patterning) | Fz3/6, Dvl1/2 Vangl2, Wnt5a, BBS4/6, PTK7 | (81, 95, 115, 150, 151) |
| Skin (hair patterning) | Fz6, Inv/NPHP2 | (47, 123) |
| Cardiac (outflow tract abnormalities) | Dvl2, Vangl2 | (50, 111) |
| Renal (polycystic kidneys) | Fat4, BBS genes, NPHP genes | (55, 92, 116) |
| CNS (axon guidance) | Fz3, Celsr2/3, | (119, 153) |
| Anterior-posterior body plan (shortened body axis)/convergent extension |
Virtually all PCP genes analyzed in this context display this phenotypic feature |
See throughout the text and above in this Table |
Insight into mammalian PCP has mostly been obtained through analysis of the Looptail mouse, which harbors a truncating mutation of Vangl2 (or Strabismus). There are only two Vangl genes and they often appear to act nonredundantly, the reason why looptail mice display a relatively broad spectrum of PCP defects. In contrast, individual knockouts of single Dvl genes (there are three) show minimal defects. The two Vangl (strabismus) genes were also found to be redundant in some contexts, for example, in the control of neural tube formation, orientation of the stereociliary bundles in cochlea, and organization of major extracardiac vessels (139). Homozygous double mutant mice could not be recovered, which represents another caveat of mammalian PCP analysis compared to the fly, where many PCP mutations produce viable animals.
PCP AND CILIA
PCP in vertebrates seems to be a strikingly connected with one particular organelle: the cilium. Cilia are cellular appendages that have recently attracted close attention due to their crucial involvement in human disease (54, 85, 110). In fact, although the primary cilium has long been studied, it was the province of only a small but dedicated group of researchers until its importance became clear early this decade. A new disease class has even been established, termed ciliopathies (6, 54, 85). Ciliopathies, which are caused by defects in cilia formation and function, mainly consist of genetic syndromes such as the autosomal-dominant polycystic kidney disease (ADPKD), Bardet-Biedl syndrome (BBS), Meckel-Gruber syndrome (MKS), Oro-facio-digital syndrome (OFD), and nephronophthisis (NPHP). Except for ADPKD, these syndromes are rare. However, the spectrum of clinical signs and symptoms includes such common and important features as obesity, retinitis pigmentosa, neural tube defects, and polycystic kidney disease (6).
The essential structure of cilia consists of nine peripheral microtubule doublets, the axoneme, which emerges from the basal body (i.e., the mother centriole in a centrosome). The axoneme is surrounded by a membrane lipid bilayer that is continuous with the plasma membrane. The assembly and maintenance of the cilium depends critically on the existence and function of a dedicated transport system, called intraflagellar transport (IFT), that transports cargo in and out of the cilium (85). Based on whether the axoneme includes an additional central pair of microtubules, cilia are classified as 9+2 or 9+0 cilia. 9+2 cilia tend to be motile, whereas 9+0 cilia tend to be immotile, sensory cilia (85). Also, in contrast to motile cilia, which are always numerous, only one non-motile cilium usually occurs per cell. Almost all cell types in the human body are ciliated, which also explains why so many tissues are affected in pleiotropic ciliopathies such as the Bardet-Biedl syndrome.
Motile cilia often function in fluid or mucus transport, whereas sensory cilia are mechanosensors and chemosensors. Recently, a number of signaling pathways have been shown to depend on ciliary function such as Hedgehog, PDGF, and Wnt signaling pathways. In particular, for the mammalian Hedgehog pathway the relationship between cilia, both transmembrane proteins in the Hedgehog pathway—Patched and Smoothened—and the processing of Gli transcription factors has been addressed in detail (reviewed in Reference 57). Impaired hedgehog signaling caused by ciliary defects also contributes to several clinical features included in the ciliopathies (e.g., poly-dactyly and rostral neural tube defects) (146).
The first hint that defective PCP signaling could be involved in ciliopathies came from studies on the nephronophthisis type II gene inversin. Inversin is a ciliary protein that shares sequence similarity and domain architecture with the PCP core component Diego. Inversin was found to directly interact with Dvl and also to regulate Dvl stability (123). Dsh/Dvl is shared by both Wnt signaling pathways, and both pathways are thought to require distinct subcellular pools of Dvl (158). Within the PCP pathway, Dsh/Dvl has to be tightly associated with the plasma membrane, whereas in canonical Wnt signaling, Dsh/Dvl shuttles between different subcellular compartments, including nucleus, cytoplasm, and plasma membrane (59, 147). Inversin downregulates cytoplasmic but not membrane-bound Dvl pools. Both proteins also translocate together to the membrane during epithelial cell differentiation. Inversin thus appears to negatively regulate the canonical Wnt signaling pathway while promoting PCP signaling (123). This becomes manifest in impaired CE movements during gastrulation and cystic kidneys in Xenopus and zebrafish morphants, respectively. Inv mutant mice suffer from polycystic kidney disease, situs inversus, and hepatobiliary and cardiac abnormalities. They also display abnormal fur patterning reminiscent of Fz6 −/− mice (47, 123).
Recently it was found that other proteins that belong to the ciliary and centrosomal machinery such as the Bardet-Biedl (BBS) proteins, the nephronophthisis protein NPHP3, the ciliary kinesin KIF3A and the oro-facial-digital syndrome protein OFD1, are also able to function as molecular switches in Wnt signaling pathways (11, 26, 42). For example, the disruption of basal bodies by knockdown of BBS proteins compromises proteasomal degradation of Dvl and β-catenin and, therefore, the intracellular Wnt response. The depletion of BBS proteins also impairs CE movements in zebrafish (42, 115). These defects can be partially rescued by coinjection of mRNA for a membrane-bound version of Dvl, including a N-terminal myristoylation and palmitoylation signal. This suggests that the subcellular distribution and regulation of Dvl degradation might be influenced directly by the cilium and the basal body (42).
PCP AND NEURAL TUBE DEFECTS
BBS mouse models also interact genetically with the looptail mouse with respect to neural tube closure and stereociliary bundle orientation in the cochlea (115). These phenotypes are typical mammalian PCP defects, and should therefore be discussed in more detail.
Neural tube closure is the result of neurulation, a process in which the neural plate bends up and eventually fuses to form the hollow tube that will become the brain and the spinal cord. The driving force of neural tube closure is provided and maintained by cells undergoing CE movements. Neural tube closure normally initiates at three sites along the rostral–caudal axis but occurs in the brain in a manner very different from that in the spinal cord. Human neural tube defects (NTDs) are therefore classified based on their location in the rostral-caudal axis. Forebrain and midbrain closure defects are termed anencephaly or encephalocele, whereas hindbrain and spinal cord closure defects are referred to as craniorachischisis (146, 161). Partial closure of the spinal cord, or spina bifida, represents the mildest and also most common human NTD. Combined with the other NTDs, it occurs in 1 to 2 infants per 1000 births, which makes it the second most common human birth defect. Population- and family-based studies indicate a complex multigenic cause of NTDs.
This notion was challenged recently by Gros and colleagues, who found mutations in the PCP core component Vangl1 in patients with familial and sporadic NTDs (66). The respective patients mainly exhibited defects in the caudal neural tube, including craniorachischisis. Moreover, it was shown that the Vangl mutations disrupted the physical interaction with Dvl. These results, for the first time, place core PCP genes within the realm of human disease and suggest that screening for mutations in Vangl1 and in other PCP components should be considered in affected families.
In light of the looptail mouse phenotype, these findings were, however, not so surprising. Like the patients, these mice suffer from more caudal defects such as craniorachischisis (67). Although Dvl2−/− mice also display some rostral defects, it is believed that the PCP pathway is responsible for caudal NTDs (50). In contrast, the Hedgehog pathway accounts for most of the rostral defects (146). The distinction is not entirely consistent as, for example, in Patched1 null mice both rostral and caudal defects are seen (44). It seems that both pathways act at different stages during neurulation. Whereas the Hedgehog pathway regulates neural plate bending and specification of ventral neural cell fates, the PCP pathway drives neural tube closure (31).
Since Hedgehog signaling critically depends on intact ciliary stucture and function, there is a strong correlation of the neural tube phenotypes in mouse mutants with abnormal cilia, such as IFT mutant mice, with the Hedgehog signaling phenotypes (36). In the case of PCP signaling, the correlation is less clear, as null or partial loss-of-function IFT mutants typically do not display PCP-like NTDs. The role for cilia in PCP-dependent neural tube closure therefore remains obscure.
In Xenopus embryos, depletion of the PCP effector proteins Inturned and Fuzzy (see Table 1) leads to open neural tubes and defective cilia formation (105). Both proteins were found to be required for the organization of a subapical actin cytoskeletal network essential to anchor basal bodies to the apical membrane—a prerequisite for ciliogenesis. The conclusion from this observation would be that PCP signaling functions upstream of cilia signaling. This is, however, again in contrast with the observed phenotypes in mouse models. Mouse mutants in the core PCP factors have so far not been shown to display defects in ciliogenesis. However, as described above, the analysis might be impaired by redundancy between related PCP genes. Therefore, a more systematic approach is needed.
Recent studies have also shown that the subapical cytoskeleton is important for apical constriction. Apical constriction initiates cell shape changes that underlie neural tube bending and closure. Dominant-negative Dsh, for example, disrupts apical constriction by preventing apical Rho accumulation (70). As both ciliogenesis and apical constriction depend on an intact subapical cytoskeleton, it would be interesting to see whether disrupted PCP signaling affects both processes equally or whether one defect is the result of the other. A very interesting question is also whether cilia are required in cells undergoing CE movements and, if so, whether they perform motile or sensory functions.
PCP AND THE COCHLEA
The vertebrate system in which the relationship between cilia and PCP signaling is probably best understood is the mammalian inner ear, in particular the organ of Corti (94). In the organ of Corti the sensory hair cells form rows of cells with V-shaped organized stereociliary bundles on the apical surface (see Figure 3). The stereociliary bundles consist of a large single kinocilium (a true sensory cilium) located on the abneural side of the cell and an actin-based stereociliary bundle organized in a staircase pattern, which points in the abneural direction. Hair bundle orientation is important for inner ear function as it allows the hair cells to sense the direction of mechanical stimulation.
The morphological polarization of sensory hair cells is under the control of the PCP signaling pathway. The first evidence for this came again from the looptail and the circletail mice (which affects the Scribble gene) (95). In both mouse mutants the precise arrangement of stereociliary bundles is disrupted. The picture resembles the situation in the Drosophila compound eye where the ommatidia are randomly organized in PCP mutants. Hair bundle orientation defects in the organ of Corti have since been described in Ptk7 and Celsr1/Flamingo knockout and Dvl1/Dvl2 and Fz3/Fz6 double knockout mice (27, 81, 150, 151) (see also Table 2). The organ of Corti can also be explanted and cultured in vitro for several days. Using this system, it was shown that the application of Wnt ligands can influence the orientation of stereociliary bundles, suggesting Wnts as a permissive factor in the orientation process (28). However, this role has not been addressed in a loss-of-function experiment.
The inner ear has also emerged as a good vertebrate system to study asymmetric protein localization of PCP core factors. The respective studies have already produced some surprises: In contrast to the situation in Drosophila where Stbm and Fz are on opposite poles, Vangl2 and the Fz receptors mFz3 and mFz6 localize to the same side of the inner ear sensory cells (151). Fz3 and Fz6 displayed remarkable redundancy in this process where at the protein level one Fz can compensate for the loss of the other (151). As Dvl2 is found on the opposite side of Fz, the membrane protein that would anchor Dvl2 at the membrane is not yet known. Other Fz receptors may play a role in this context and/or another Dvl member may be involved. The PCP signaling pathway operating in the mammalian inner ear thus seems to have similarities to, but also clear differences from, the Drosophila PCP pathway.
A recent study by Chen and colleagues examined the relationship between PCP and IFT mutants in the context of sensory hair polarization (63). Using conditional knockout mice for IFT88/Polaris and Kif3a, the authors asked whether kinocilia are required for normal development of the mouse inner ear sensory epithelium. Loss of the kinocilium due to IFT88 and Kif3a inactivation caused a misorientation of stereociliary bundles similar to that in the core PCP mutants. However, in some cells the bundles appeared to be mislocalized to a more central location on the apical surface. This finding also correlated with a circular and symmetric arrangement of the bundles. When examining PCP protein localization in the IFT88 mutant cochlea, the PCP factors Fz3 and Vangl2 were found to retain their normal, asymmetric localization patterns in the mutant hair cells. These data have two important implications: first, the cilium is required for the actin-based stereociliary bundle positioning, and second, PCP protein localization does not appear to be downstream of ciliary function. One conclusion could also be that stereociliary bundles fail to respond to the positioning cues provided by PCP signaling when the cilium is missing (4).
Similar precise cilia and basal body positioning can be found in the mouse node, an embryonic tissue that regulates left-right body asymmetry (56). Here, the node cells place almost all of their basal bodies near the posterior end of their apical dome-like surface. The motile cilia formed by these basal bodies are tilted posteriorly in an angle that allows them to perform their characteristic rotation movements (101). The importance for this precise positioning is reflected in the phenotype of inv mutant mice. In the nodes of these mice basal bodies are mispositioned, leading to mistilting and uncoordinated beating of the cilia. As a consequence, the flow generated by these cilia is slower and has a meandering streamline (101).
It will be interesting to investigate core PCP mouse mutants with regard to basal body positioning and ciliary beating in the node and also other tissues that require coordinated ciliary beating. This analysis should include close examination of the subapical actin cytoskeleton and its role in the anchoring of basal bodies. In zebrafish, Fz2 was found to be involved in left-right asymmetry regulation (100). Depletion of Fz2 by using morpholino olignucleotides resulted in decreased cilia length and number. In addition, Fz2 morphants phenocopied the morphants for duboraya, a novel ciliogenesis factor (100). Duboraya function appeared to be dependent on Fz2-mediated noncanonical Wnt signaling and also required for an intact subapical actin cytoskeleton in cells lining the Kupffer’s vesicle and the pronephric duct (100).
In summary, the PCP pathway seems to have a role in the organization of a subapical actin cytoskeleton, which in turn is a prerequisite for proper basal body and cilia positioning. Precise positioning could be required for coordinated beating of motile cilia populations and for the execution of selective sensory cilia function.
PCP AND POLYCYSTIC KIDNEY DISEASE
The question whether the PCP factors and PCP signaling act upstream or downstream of cilia function might be different and possibly more complicated in the kidney. Here, defective PCP signaling has been brought into connection with polycystic kidney disease (PKD) etiology (124, 125).
PKD is one of the most common monogenic diseases. ADPKD occurs in 1 to 1000 individuals and accounts for up to 10% of all cases of end-stage renal disease. Due to lack of treatment options, affected individuals will inevitably undergo renal transplantation or require dialysis. The ADPKD symptom is caused by mutations in Polycystin-1 and -2 and is the main PKD type. Other PKD forms include autosomal-recessive PKD, nephronophthisis, BBS or MKS, in which PKD is part of a syndromic complex and typically affects infants or children (55).
The decisive pathogenetic events for all PKD forms are thought to occur during renal development (112). There is also strong evidence that defects in ciliary function cause PKD. All PKD gene products, for example, localize to the primary cilium and/or basal body, and for some specific ciliary functions have been identified (98, 99). Moreover, the kidney-specific ablation of cilia leads to renal cysts (78). Cilia can be found on the apical surface of tubular epithelial cells where they are supposed to function as mechanosensors of fluid flow occurring in the tubular lumen. The mechanical stimulus is transduced by the calcium channel Polycystin-2 (probably with the help of Polycystin-1), which leads to an increase in cytosolic calcium (99). The signaling events triggered by this increase in calcium are poorly understood.
Cyst formation tends to occur during the proliferative phases in renal development (41, 112). The cells still remain differentiated since even in cysts the epithelial barrier function is maintained. This factor distinguishes PKD from cancerous diseases. Presumably, a defect in the temporal control of differentiation or in the orientation of tubule growth occurs during renal development. Normal tubule growth is most likely driven by convergent extension movements and oriented cell division—two processes that are both controlled by the PCP pathway (7, 9, 43, 65) (see Figure 4). Although convergent extension movements have not yet been described for the developing kidney, oriented cell divisions were recently observed by Fischer and colleagues. They found that during postnatal life there is massive cell proliferation in the maturing nephron. Because of a strict alignment of the mitotic spindle with the tubule axis, this growth phase does not lead to an increase in tubule diameter. In contrast, cystic kidneys display a randomization of the mitotic angle of the dividing tubular epithelial cells that could account for cyst formation (41). Another group also demonstrated that in mouse models lacking cilia, spindle orientation defects can be seen during development and also during repair processes following kidney injury (108). Taken together, these results suggest that the PCP pathway might regulate the planar growth of developing and injured tubules in the kidney. Aberrant PCP signaling could therefore cause uncoordinated growth, which might lead to an increase of tubular diameter and, thus, cyst formation. This hypothesis was strengthened recently by the finding that mice mutant for Fat4, an orthologue of Drosophila Fat, exhibit polycystic kidney disease in addition to PCP phenotypes in the cochlea and the neural tube. mFat4 mutant kidneys also showed genetic interaction with Vangl2 and Fjx, the orthologue of Drosophila fj. The mitotic angle was slightly randomized in dividing tubular epithelial cells of Fat4−/− kidneys. In addition, Fat4 localized to the primary cilia in interphase Madin-Darby canine kidney cells (116).
Figure 4.
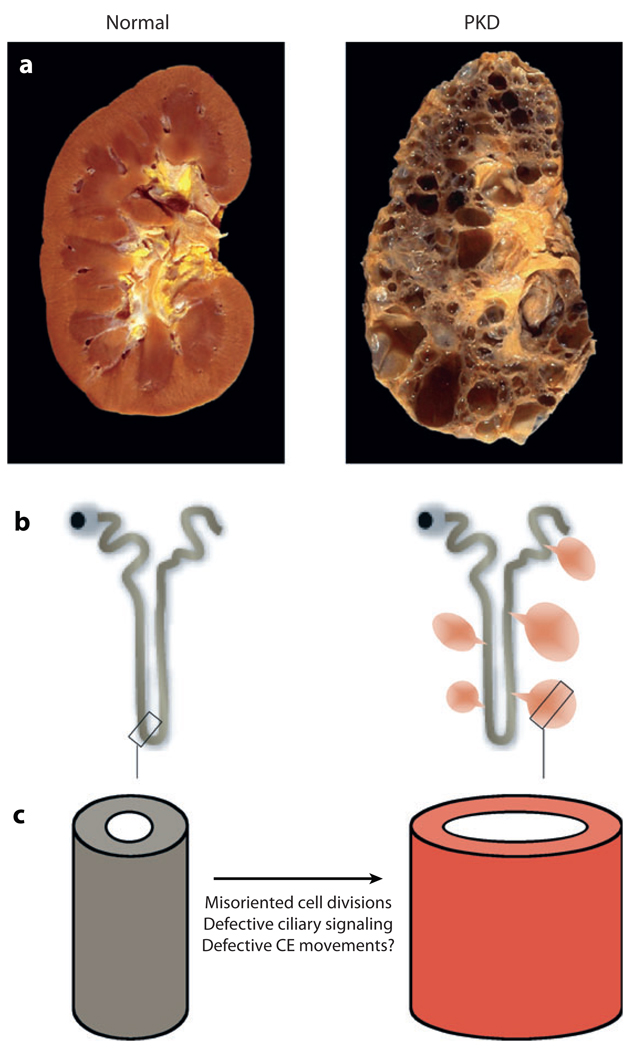
PCP and polycystic kidney disease (a) Photographs of a healthy (left) and a polycystic kidney (right). (b) Schematic diagram of the nephron, the functional unit of the kidney. In ADPKD, cysts arise from all segments of the nephron (right panel). (c) Cyst formation is thought to be caused, in part, by defective PCP signaling. The PCP pathway regulates the cell division plane of epithelial cells in the elongating tube during renal development. More speculatively, elongation of the growing tube could also be driven by convergent extension-like movements. The cilium might provide directional cues for the regulation of these two processes.
How could cilia be involved in setting up the division plane for tubular epithelial cells? Cilia may well sense and transmit the directional cues for spindle orientation before onset of mitosis. During mitosis, cilia are not present, as the centrosomes are required for the spindle apparatus by forming the spindle poles. Another possibility is therefore that the centrosomes but not the cilia are directly involved in spindle orientation. Centrosomes could also be involved in polarization events that occur right after mitosis. Ciruna et al. (20) found that in neuroepithelial cells in zebrafish the PCP pathway is necessary to reestablish cell polarity that is transiently lost during mitotic cell division and to reintegrate daughter cells into the tissue. Strikingly, neural tube defects in the Vangl2/trilobite mutants can be rescued by blocking cell division (20). Since the inhibition of the cell cycle is an effective treatment in murine models of PKD, this effect may be due, at least in part, to the PCP pathway performing similar functions in the mouse nephron as in the zebrafish neural tube (14).
A COMMON RIGHT DIRECTION FOR PCP IN DIFFERENT SPECIES
The diversity of tissues in mammals poses a challenge in the search for a unifying theme of the PCP pathway across species and organs. Each tissue has likely found its own variation on a theme in utilizing the PCP core pathway to develop and maintain its three-dimensional architecture. Cilia can be envisioned as global navigation devices providing each cell in a tissue with positional information (10). This characterization is especially true for sensory cilia, which could indeed be helping or even instructing the PCP pathway in its tissue-shaping task. Multiciliated cells, on the other hand, could be instructed by the PCP pathway to perform their vectorial functions across the plane of the epithelium. Park and colleagues recently demonstrated that Dsh functions in a ciliogenetic pathway together with Inturned and Rho to anchor basal bodies at the apical plasma membrane (106). Dsh was found to localize asymmetrically near the base of the cilia regulating the sort of planar polarization that underlies directional beating of cilia. These authors also reported that Dsh functions to link basal bodies with membraneous vesicles that then fuse with the plasma membrane and thus appear to govern the final step of basal body docking to the plasma membrane. The vesicles contain members of the Exocyst complex, which are involved in polarized membrane trafficking. The Exocyst complex has previously been shown to regulate the trafficking of PCP core factors such as Flamingo in the Drosophila wing (21). In the same system, the dsh1 mutation compromises the polarized distribution of Flamingo (141). Apart from the Exocyst complex, Dsh also seems to associate with other components of the vesicular trafficking machinery, suggesting that one mechanism for Dsh function might lie in the regulation of membrane trafficking (18, 71, 163).
Although several caveats still exist, these examples nicely demonstrate how work carried out in different systems—mammalian and Drosophila—can lead to a unifying hypothesis about the function of PCP core factors. It is hoped that we will see more of these examples, leading in the future to a more complete understanding of the molecular mechanisms underlying the roles of PCP signaling pathway(s).
PCP is a molecular pathway with big potential. Every tissue or organ can make use of it in one way or the other for coupling cell-cell communication with tissue development and function. The PCP field has already worked its way into a number of tissues and diseases of different species. Nevertheless, much work is needed to find a common right direction.
ACKNOWLEDGMENTS
We thank Carlo Iomini, William Gault, and Jennifer Zallen for careful reading of and comments on the manuscript. We are grateful to John Wallingford and Helen McNeill for sharing data prior to publication, and to Jeremy Nathans and Jennifer Zallen for pictures and drawings. We thank all members of the Mlodzik lab for continuous discussions and support. Work in the Mlodzik lab is supported by grants from the NIH/NIGMS and the NIH/NEI. M.S. is supported by a postdoctoral fellowship from the Deutsche Forschungsgemeinschaft.
Footnotes
DISCLOSURE STATEMENT
The authors are not aware of any biases that might be perceived as affecting the objectivity of this review.
LITERATURE CITED
- 1.Adler PN. Planar signaling and morphogenesis in Drosophila. Dev. Cell. 2002;2:525–535. doi: 10.1016/s1534-5807(02)00176-4. [DOI] [PubMed] [Google Scholar]
- 2.Adler PN, Liu J, Charlton J. Cell size and the morphogenesis of wing hairs in Drosophila. Genesis. 2000;28:82–91. doi: 10.1002/1526-968x(200010)28:2<82::aid-gene60>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 3.Ahumada A, Slusarski DC, Liu X, Moon RT, Malbon CC, Wang HY. Signaling of rat Frizzled-2 through phosphodiesterase and cyclic GMP. Science. 2002;298:2006–2010. doi: 10.1126/science.1073776. [DOI] [PubMed] [Google Scholar]
- 4.Axelrod JD. Basal bodies, kinocilia and planar cell polarity. Nat. Genet. 2008;40:10–11. doi: 10.1038/ng0108-10. [DOI] [PubMed] [Google Scholar]
- 5.Axelrod JD, Miller JR, Shulman JM, Moon RT, Perrimon N. Differential requirement of Dishevelled provides signaling specificity in the Wingless and planar cell polarity signaling pathways. Genes Dev. 1998;12:2610–2622. doi: 10.1101/gad.12.16.2610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Badano JL, Mitsuma N, Beales PL, Katsanis N. The ciliopathies: an emerging class of human genetic disorders. Annu. Rev. Genomics Hum Genet. 2006;7:125–148. doi: 10.1146/annurev.genom.7.080505.115610. [DOI] [PubMed] [Google Scholar]
- 7.Baena-Lopez LA, Baonza A, Garcia-Bellido A. The orientation of cell divisions determines the shape of Drosophila organs. Curr. Biol. 2005;15:1640–1644. doi: 10.1016/j.cub.2005.07.062. [DOI] [PubMed] [Google Scholar]
- 8.Bastock R, Strutt H, Strutt D. Strabismus is asymmetrically localised and binds to Prickle and Dishevelled during Drosophila planar polarity patterning. Development. 2003;130:3007–3014. doi: 10.1242/dev.00526. [DOI] [PubMed] [Google Scholar]
- 9.Bellaiche Y, Gho M, Kaltschmidt JA, Brand AH, Schweisguth F. Frizzled regulates localization of cell-fate determinants and mitotic spindle rotation during asymmetric cell division. Nat. Cell Biol. 2001;3:50–57. doi: 10.1038/35050558. [DOI] [PubMed] [Google Scholar]
- 10.Benzing T, Walz G. Cilium-generated signaling: a cellular GPS? Curr. Opin. Nephrol. Hypertens. 2006;15:245–249. doi: 10.1097/01.mnh.0000222690.53970.ca. [DOI] [PubMed] [Google Scholar]
- 11.Bergmann C, Fliegauf M, Bruchle NO, Frank V, Olbrich H, et al. Loss of nephrocystin-3 function can cause embryonic lethality, Meckel-Gruber-like syndrome, situs inversus, and renal-hepatic-pancreatic dysplasia. Am. J. Hum Genet. 2008;82:959–970. doi: 10.1016/j.ajhg.2008.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Boutros M, Mihaly J, Bouwmeester T, Mlodzik M. Signaling specificity by Frizzled receptors in Drosophila. Science. 2000;288:1825–1828. doi: 10.1126/science.288.5472.1825. [DOI] [PubMed] [Google Scholar]
- 13.Boutros M, Mlodzik M. Dishevelled:at the crossroads of divergent intracellular signaling pathways. Mech. Dev. 1999;83:27–37. doi: 10.1016/s0925-4773(99)00046-5. [DOI] [PubMed] [Google Scholar]
- 14.Bukanov NO, Smith LA, Klinger KW, Ledbetter SR, Ibraghimov-Beskrovnaya O. Long-lasting arrest of murine polycystic kidney disease with CDK inhibitor roscovitine. Nature. 2006;444:949–952. doi: 10.1038/nature05348. [DOI] [PubMed] [Google Scholar]
- 15.Carreira-Barbosa F, Concha ML, Takeuchi M, Ueno N, Wilson SW, Tada M. Prickle 1 regulates cell movements during gastrulation and neuronal migration in zebrafish. Development. 2003;130:4037–4046. doi: 10.1242/dev.00567. [DOI] [PubMed] [Google Scholar]
- 16.Casal J, Lawrence PA, Struhl G. Two separate molecular systems, Dachsous/Fat and Starry night/Frizzled, act independently to confer planar cell polarity. Development. 2006;133:4561–4572. doi: 10.1242/dev.02641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen CM, Strapps W, Tomlinson A, Struhl G. Evidence that the cysteine-rich domain of Drosophila Frizzled family receptors is dispensable for transducing Wingless. Proc. Natl. Acad. Sci USA. 2004;101:15961–15966. doi: 10.1073/pnas.0407103101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen W, ten Berge D, Brown J, Ahn S, Hu LA, et al. Dishevelled 2 recruits beta-arrestin 2 to mediate Wnt5A-stimulated endocytosis of Frizzled 4. Science. 2003;301:1391–1394. doi: 10.1126/science.1082808. [DOI] [PubMed] [Google Scholar]
- 19.Choi K-W, Benzer S. Rotation of photoreceptor clusters in the developing Drosophila eye requires the nemo gene. Cell. 1994;78:125–136. doi: 10.1016/0092-8674(94)90579-7. [DOI] [PubMed] [Google Scholar]
- 20.Ciruna B, Jenny A, Lee D, Mlodzik M, Schier AF. Planar cell polarity signalling couples cell division and morphogenesis during neurulation. Nature. 2006;439:220–224. doi: 10.1038/nature04375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Classen AK, Anderson KI, Marois E, Eaton S. Hexagonal packing of Drosophila wing epithelial cells by the planar cell polarity pathway. Dev. Cell. 2005;9:805–817. doi: 10.1016/j.devcel.2005.10.016. [DOI] [PubMed] [Google Scholar]
- 22.Collier S, Gubb D. Drosophila tissue polarity requires the cell-autonomous activity of the fuzzy gene, which encodes a novel transmembrane protein. Development. 1997;124:4029–4037. doi: 10.1242/dev.124.20.4029. [DOI] [PubMed] [Google Scholar]
- 23.Collier S, Lee H, Burgess R, Adler P. The WD40 repeat protein fritz links cytoskeletal planar polarity to frizzled subcellular localization in the Drosophila epidermis. Genetics. 2005;169:2035–2045. doi: 10.1534/genetics.104.033381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cong F, Schweizer L, Varmus H. Casein kinase Iepsilon modulates the signaling specificities of dishevelled. Mol. Cell Biol. 2004;24:2000–2011. doi: 10.1128/MCB.24.5.2000-2011.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cong J, Geng W, He B, Liu J, Charlton J, Adler PN. The furry gene of Drosophila is important for maintaining the integrity of cellular extensions during morphogenesis. Development. 2001;128:2793–2802. doi: 10.1242/dev.128.14.2793. [DOI] [PubMed] [Google Scholar]
- 26.Corbit KC, Shyer AE, Dowdle WE, Gaulden J, Singla V, et al. Kif3a constrains beta-catenin-dependent Wnt signalling through dual ciliary and nonciliary mechanisms. Nat. Cell Biol. 2008;10:70–76. doi: 10.1038/ncb1670. [DOI] [PubMed] [Google Scholar]
- 27.Curtin JA, Quint E, Tsipouri V, Arkell RM, Cattanach B, et al. Mutation of Celsr1 disrupts planar polarity of inner ear hair cells and causes severe neural tube defects in the mouse. Curr. Biol. 2003;13:1129–1133. doi: 10.1016/s0960-9822(03)00374-9. [DOI] [PubMed] [Google Scholar]
- 28.Dabdoub A, Donohue MJ, Brennan A, Wolf V, Montcouquiol M, et al. Wnt signaling mediates reorientation of outer hair cell stereociliary bundles in the mammalian cochlea. Development. 2003;130:2375–2384. doi: 10.1242/dev.00448. [DOI] [PubMed] [Google Scholar]
- 29.Das G, Jenny A, Klein TJ, Eaton S, Mlodzik M. Diego interacts with Prickle and Strabismus/Van Gogh to localize planar cell polarity complexes. Development. 2004;131:4467–4476. doi: 10.1242/dev.01317. [DOI] [PubMed] [Google Scholar]
- 30.Das G, Reynolds-Kenneally J, Mlodzik M. The atypical cadherin flamingo links Frizzled and Notch signaling in planar polarity establishment in the Drosophila eye. Dev. Cell. 2002;2:656–666. doi: 10.1016/s1534-5807(02)00147-8. [DOI] [PubMed] [Google Scholar]
- 31.De Marco P, Merello E, Mascelli S, Capra V. Current perspectives on the genetic causes of neural tube defects. Neurogenetics. 2006;7:201–221. doi: 10.1007/s10048-006-0052-2. [DOI] [PubMed] [Google Scholar]
- 32.Djiane A, Riou J, Umbhauer M, Boucaut J, Shi D. Role of frizzled 7 in the regulation of convergent extension movements during gastrulation in Xenopus laevis. Development. 2000;127:3091–3100. doi: 10.1242/dev.127.14.3091. [DOI] [PubMed] [Google Scholar]
- 33.Djiane A, Yogev S, Mlodzik M. The apical determinants aPKC and dPatj regulate Frizzled-dependent planar cell polarity in the Drosophila eye. Cell. 2005;121:621–631. doi: 10.1016/j.cell.2005.03.014. [DOI] [PubMed] [Google Scholar]
- 34.Eaton S. Planar polarity in Drosophila and vertebrate epithelia. Curr. Opin Cell Biol. 1997;9:860–866. doi: 10.1016/s0955-0674(97)80089-0. [DOI] [PubMed] [Google Scholar]
- 35.Eaton S, Auvinen P, Luo L, Jan YN, Simons K. CDC42 and Rac1 control different actin-dependent processes in the Drosophila wing disc epithelium. J. Cell Biol. 1995;131:151–164. doi: 10.1083/jcb.131.1.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Eggenschwiler JT, Anderson KV. Cilia and developmental signaling. Annu. Rev. Cell Dev. Biol. 2007;23:345–373. doi: 10.1146/annurev.cellbio.23.090506.123249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Erkner A, Roure A, Charroux B, Delaage M, Holway N, et al. Grunge, related to human Atrophin-like proteins, has multiple functions in Drosophila development. Development. 2002;129:1119–1129. doi: 10.1242/dev.129.5.1119. [DOI] [PubMed] [Google Scholar]
- 38.Fanto M, Clayton L, Meredith J, Hardiman K, Charroux B, et al. The tumor-suppressor and cell adhesion molecule Fat controls planar polarity via physical interactions with Atrophin, a transcriptional corepressor. Development. 2003;130:763–774. doi: 10.1242/dev.00304. [DOI] [PubMed] [Google Scholar]
- 39.Fanto M, Weber U, Strutt DI, Mlodzik M. Nuclear signaling by Rac and Rho GTPases is required in the establishment of epithelial planar polarity in the Drosophila eye. Curr. Biol. 2000;10:979–988. doi: 10.1016/s0960-9822(00)00645-x. [DOI] [PubMed] [Google Scholar]
- 40.Feiguin F, Hannus M, Mlodzik M, Eaton S. The Ankyrin repeat protein Diego mediates Frizzled-dependent planar polarization. Dev. Cell. 2001;1:93–101. doi: 10.1016/s1534-5807(01)00010-7. [DOI] [PubMed] [Google Scholar]
- 41.Fischer E, Legue E, Doyen A, Nato F, Nicolas JF, et al. Defective planar cell polarity in polycystic kidney disease. Nat. Genet. 2006;38:21–23. doi: 10.1038/ng1701. [DOI] [PubMed] [Google Scholar]
- 42.Gerdes JM, Liu Y, Zaghloul NA, Leitch CC, Lawson SS, et al. Disruption of the basal body compromises proteasomal function and perturbs intracellular Wnt response. Nat. Genet. 2007;39:1350–1360. doi: 10.1038/ng.2007.12. [DOI] [PubMed] [Google Scholar]
- 43.Gong Y, Mo C, Fraser SE. Planar cell polarity signalling controls cell division orientation during zebrafish gastrulation. Nature. 2004;430:689–693. doi: 10.1038/nature02796. [DOI] [PubMed] [Google Scholar]
- 44.Goodrich LV, Milenkovic L, Higgins KM, Scott MP. Altered neural cell fates and medulloblastoma in mouse patched mutants. Science. 1997;277:1109–1113. doi: 10.1126/science.277.5329.1109. [DOI] [PubMed] [Google Scholar]
- 45.Gubb D, García-Bellido A. A genetic analysis of the determination of cuticular polarity during development in Drosophila melanogaster. J. Embryol. Exp. Morphol. 1982;68:37–57. [PubMed] [Google Scholar]
- 46.Gubb D, Green C, Huen D, Coulson D, Johnson G, et al. The balance between isoforms of the prickle LIM domain protein is critical for planar polarity in Drosophila imaginal discs. Genes Dev. 1999;13:2315–2327. doi: 10.1101/gad.13.17.2315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Guo N, Hawkins C, Nathans J. Frizzled6 controls hair patterning in mice. Proc. Natl. Acad. Sci USA. 2004;101:9277–9281. doi: 10.1073/pnas.0402802101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Habas R, Dawid IB, He X. Coactivation of Rac and Rho by Wnt/Frizzled signaling is required for vertebrate gastrulation. Genes Dev. 2003;17:295–309. doi: 10.1101/gad.1022203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Habas R, Kato Y, He X. Wnt/Frizzled activation of rho regulates vertebrate gastrulation and requires a novel formin homology protein Daam1. Cell. 2001;107:843–854. doi: 10.1016/s0092-8674(01)00614-6. [DOI] [PubMed] [Google Scholar]
- 50.Hamblet NS, Lijam N, Ruiz-Lozano P, Wang J, Yang Y, et al. Dishevelled 2 is essential for cardiac outflow tract development, somite segmentation and neural tube closure. Development. 2002;129:5827–5838. doi: 10.1242/dev.00164. [DOI] [PubMed] [Google Scholar]
- 51.Hannus M, Feiguin F, Heisenberg CP, Eaton S. Planar cell polarization requires Widerborst, a B’ regulatory subunit of protein phosphatase 2A. Development. 2002;129:3493–3503. doi: 10.1242/dev.129.14.3493. [DOI] [PubMed] [Google Scholar]
- 52.Heisenberg CP, Tada M, Rauch GJ, Saude L, Concha ML, et al. Silberblick/Wnt11 mediates convergent extension movements during zebrafish gastrulation. Nature. 2000;405:476–481. doi: 10.1038/35011068. [DOI] [PubMed] [Google Scholar]
- 53.Henderson DJ, Phillips HM, Chaudhry B. Vang-like 2 and noncanonical Wnt signaling in outflow tract development. Trends Cardiovasc Med. 2006;16:38–45. doi: 10.1016/j.tcm.2005.11.005. [DOI] [PubMed] [Google Scholar]
- 54.Hildebrandt F, Otto E. Cilia and centrosomes: a unifying pathogenic concept for cystic kidney disease? Nat. Rev. Genet. 2005;6:928–940. doi: 10.1038/nrg1727. [DOI] [PubMed] [Google Scholar]
- 55.Hildebrandt F, Zhou W. Nephronophthisis-associated ciliopathies. J. Am. Soc. Nephrol. 2007;18:1855–1871. doi: 10.1681/ASN.2006121344. [DOI] [PubMed] [Google Scholar]
- 56.Hirokawa N, Tanaka Y, Okada Y, Takeda S. Nodal flow and the generation of left-right asymmetry. Cell. 2006;125:33–45. doi: 10.1016/j.cell.2006.03.002. [DOI] [PubMed] [Google Scholar]
- 57.Huangfu D, Anderson KV. Signaling from Smo to Ci/Gli: conservation and divergence of Hedgehog pathways from Drosophila to vertebrates. Development. 2006;133:3–14. doi: 10.1242/dev.02169. [DOI] [PubMed] [Google Scholar]
- 58.Hyodo-Miura J, Yamamoto TS, Hyodo AC, Iemura S, Kusakabe M, et al. XGAP, an ArfGAP, is required for polarized localization of PAR proteins and cell polarity in Xenopus gastrulation. Dev. Cell. 2006;11:69–79. doi: 10.1016/j.devcel.2006.04.019. [DOI] [PubMed] [Google Scholar]
- 59.Itoh K, Brott BK, Bae GU, Ratcliffe MJ, Sokol SY. Nuclear localization is required for Dishevelled function in Wnt/beta-catenin signaling. J. Biol. 2005;4:3. doi: 10.1186/jbiol20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Jenny A, Darken RS, Wilson PA, Mlodzik M. Prickle and Strabismus form a functional complex to generate a correct axis during planar cell polarity signaling. EMBO J. 2003;22:4409–4420. doi: 10.1093/emboj/cdg424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Jenny A, Reynolds-Kenneally J, Das G, Burnett M, Mlodzik M. Diego and Prickle regulate Frizzled planar cell polarity signalling by competing for Dishevelled binding. Nat. Cell Biol. 2005;7:691–697. doi: 10.1038/ncb1271. [DOI] [PubMed] [Google Scholar]
- 62.Jessen JR, Topczewski J, Bingham S, Sepich DS, Marlow F, et al. Zebrafish trilobite identifies new roles for Strabismus in gastrulation and neuronal movements. Nat. Cell Biol. 2002;4:610–615. doi: 10.1038/ncb828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Jones C, Roper VC, Foucher I, Qian D, Banizs B, et al. Ciliary proteins link basal body polarization to planar cell polarity regulation. Nat. Genet. 2008;40:69–77. doi: 10.1038/ng.2007.54. [DOI] [PubMed] [Google Scholar]
- 64.Katanaev VL, Ponzielli R, Semeriva M, Tomlinson A. Trimeric G protein-dependent frizzled signaling in Drosophila. Cell. 2005;120:111–122. doi: 10.1016/j.cell.2004.11.014. [DOI] [PubMed] [Google Scholar]
- 65.Keller R. Shaping the vertebrate body plan by polarized embryonic cell movements. Science. 2002;298:1950–1954. doi: 10.1126/science.1079478. [DOI] [PubMed] [Google Scholar]
- 66.Kibar Z, Torban E, McDearmid JR, Reynolds A, Berghout J, et al. Mutations in VANGL1 associated with neural-tube defects. N. Engl. J. Med. 2007;356 doi: 10.1056/NEJMoa060651. 1432-27. [DOI] [PubMed] [Google Scholar]
- 67.Kibar Z, Vogan KJ, Groulx N, Justice MJ, Underhill DA, Gros P. Ltap, a mammalian homolog of Drosophila Strabismus/Van Gogh, is altered in the mouse neural tube mutant Loop-tail. Nat. Genet. 2001;28:251–255. doi: 10.1038/90081. [DOI] [PubMed] [Google Scholar]
- 68.Kilian B, Mansukoski H, Barbosa FC, Ulrich F, Tada M, Heisenberg CP. The role of Ppt/Wnt5 in regulating cell shape and movement during zebrafish gastrulation. Mech Dev. 2003;120:467–476. doi: 10.1016/s0925-4773(03)00004-2. [DOI] [PubMed] [Google Scholar]
- 69.Kinoshita N, Iioka H, Miyakoshi A, Ueno N. PKC delta is essential for Dishevelled function in a noncanonical Wnt pathway that regulates Xenopus convergent extension movements. Genes Dev. 2003;17:1663–1676. doi: 10.1101/gad.1101303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kinoshita N, Sasai N, Misaki K, Yonemura S. Apical accumulation of Rho in the neural plate is important for neural plate cell shape change and neural tube formation. Mol. Biol. Cell. 2008 doi: 10.1091/mbc.E07-12-1286. In press? [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kishida S, Hamao K, Inoue M, Hasegawa M, Matsuura Y, et al. Dvl regulates endo- and exocytotic processes through binding to synaptotagmin. Genes Cells. 2007;12:49–61. doi: 10.1111/j.1365-2443.2006.01030.x. [DOI] [PubMed] [Google Scholar]
- 72.Klein TJ, Jenny A, Djiane A, Mlodzik M. CKIepsilon/discs overgrown promotes both Wnt-Fz/beta-catenin and Fz/PCP signaling in Drosophila. Curr. Biol. 2006;16:1337–1343. doi: 10.1016/j.cub.2006.06.030. [DOI] [PubMed] [Google Scholar]
- 73.Klein TJ, Mlodzik M. Planar cell polarization: an emerging model points in the right direction. Annu. Rev. Cell Dev. Biol. 2005;21:155–176. doi: 10.1146/annurev.cellbio.21.012704.132806. [DOI] [PubMed] [Google Scholar]
- 74.Lawrence PA, Casal J, Struhl G. Cell interactions and planar polarity in the abdominal epidermis of Drosophila. Development. 2004;131 doi: 10.1242/dev.01351. 4651-64. [DOI] [PubMed] [Google Scholar]
- 75.Lawrence PA, Shelton PMJ. The determination of polarity in the developing insect retina. J. Embryol. Exp. Morph. 1975;33:471–486. [PubMed] [Google Scholar]
- 76.Lawrence PA, Struhl G, Casal J. Planar cell polarity: one or two pathways? Nat. Rev. Genet. 2007;8:555–563. doi: 10.1038/nrg2125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Lele Z, Bakkers J, Hammerschmidt M. Morpholino phenocopies of the swirl, snailhouse, somitabun, minifin, silberblick, and pipetail mutations. Genesis. 2001;30:190–194. doi: 10.1002/gene.1063. [DOI] [PubMed] [Google Scholar]
- 78.Lin F, Hiesberger T, Cordes K, Sinclair AM, Goldstein LS, et al. Kidney-specific inactivation of the KIF3A subunit of kinesin-II inhibits renal ciliogenesis and produces polycystic kidney disease. Proc. Natl. Acad. Sci USA. 2003;100:5286–5291. doi: 10.1073/pnas.0836980100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Liu T, DeCostanzo AJ, Liu X, Wang H, Hallagan S, et al. G protein signaling from activated rat frizzled-1 to the beta-catenin-Lef-Tcf pathway. Science. 2001;292:1718–1722. doi: 10.1126/science.1060100. [DOI] [PubMed] [Google Scholar]
- 80.Logan CY, Nusse R. The Wnt signaling pathway in development and disease. Annu. Rev. Cell Dev. Biol. 2004;20:781–810. doi: 10.1146/annurev.cellbio.20.010403.113126. [DOI] [PubMed] [Google Scholar]
- 81.Lu X, Borchers AG, Jolicoeur C, Rayburn H, Baker JC, Tessier-Lavigne M. PTK7/CCK-4 is a novel regulator of planar cell polarity in vertebrates. Nature. 2004;430:93–98. doi: 10.1038/nature02677. [DOI] [PubMed] [Google Scholar]
- 82.Ma D, Yang CH, McNeill H, Simon MA, Axelrod JD. Fidelity in planar cell polarity signalling. Nature. 2003;421:543–547. doi: 10.1038/nature01366. [DOI] [PubMed] [Google Scholar]
- 83.Marlow F, Topczewski J, Sepich D, Solnica-Krezel L. Zebrafish Rho kinase 2 acts downstream of Wnt11 to mediate cell polarity and effective convergence and extension movements. Curr. Biol. 2002;12:876–884. doi: 10.1016/s0960-9822(02)00864-3. [DOI] [PubMed] [Google Scholar]
- 84.Marlow F, Zwartkruis F, Malicki J, Neuhauss SCF, Abbas L, et al. Functional interactions of genes mediating convergent extension, knypek and trilobite, during the partitioning of the eye primordium in zebrafish. Dev. Biol. 1998;203:382–393. doi: 10.1006/dbio.1998.9032. [DOI] [PubMed] [Google Scholar]
- 85.Marshall WF. The cell biological basis of ciliary disease. J. Cell Biol. 2008;180:17–21. doi: 10.1083/jcb.200710085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Matakatsu H, Blair SS. Interactions between Fat and Dachsous and the regulation of planar cell polarity in the Drosophila wing. Development. 2004;131:3785–3794. doi: 10.1242/dev.01254. [DOI] [PubMed] [Google Scholar]
- 87.Matakatsu H, Blair SS. Separating the adhesive and signaling functions of the Fat and Dachsous protocadherins. Development. 2006;133:2315–2324. doi: 10.1242/dev.02401. [DOI] [PubMed] [Google Scholar]
- 88.Matusek T, Djiane A, Jankovics F, Brunner D, Mlodzik M, Mihaly J. The Drosophila formin DAAM regulates the tracheal cuticle pattern through organizing the actin cytoskeleton. Development. 2006;133:957–966. doi: 10.1242/dev.02266. [DOI] [PubMed] [Google Scholar]
- 89.McKay RM, Peters JM, Graff JM. The casein kinase I family: roles in morphogenesis. Dev. Biol. 2001;235:378–387. doi: 10.1006/dbio.2001.0307. [DOI] [PubMed] [Google Scholar]
- 90.Mirkovic I, Charish K, Gorski SM, McKnight K, Verheyen EM. Drosophila nemo is an essential gene involved in the regulation of programmed cell death. Mech. Dev. 2002;119:9–20. doi: 10.1016/s0925-4773(02)00289-7. [DOI] [PubMed] [Google Scholar]
- 91.Mlodzik M. Planar polarity in the Drosophila eye: a multifaceted view of signaling specificity and cross-talk. EMBO J. 1999;18:6873–6879. doi: 10.1093/emboj/18.24.6873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Mochizuki T, Saijoh Y, Tsuchiya K, Shirayoshi Y, Takai S, et al. Cloning of inv, a gene that controls left/right asymmetry and kidney development. Nature. 1998;395:177–181. doi: 10.1038/26006. [DOI] [PubMed] [Google Scholar]
- 93.Moeller H, Jenny A, Schaeffer HJ, Schwarz-Romond T, Mlodzik M, et al. Diversin regulates heart formation and gastrulation movements in development. Proc. Natl. Acad. Sci. USA. 2006;103:15900–15905. doi: 10.1073/pnas.0603808103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Montcouquiol M, Crenshaw EB, 3rd, Kelley MW. Noncanonical Wnt signaling and neural polarity. Annu. Rev. Neurosci. 2006;29:363–386. doi: 10.1146/annurev.neuro.29.051605.112933. [DOI] [PubMed] [Google Scholar]
- 95.Montcouquiol M, Rachel RA, Lanford PJ, Copeland NG, Jenkins NA, Kelley MW. Identification of Vangl2 and Scrb1 as planar polarity genes in mammals. Nature. 2003;423:173–177. doi: 10.1038/nature01618. [DOI] [PubMed] [Google Scholar]
- 96.Munoz-Descalzo S, Gomez-Cabrero A, Mlodzik M, Paricio N. Analysis of the role of the Rac/Cdc42 GTPases during planar cell polarity generation in Drosophila. Int. J. Dev. Biol. 2007;51:379–387. doi: 10.1387/ijdb.062250sm. [DOI] [PubMed] [Google Scholar]
- 97.Myers DC, Sepich DS, Solnica-Krezel L. Convergence and extension in vertebrate gastrulae: cell movements according to or in search of identity? Trends Genet. 2002;18:447–455. doi: 10.1016/s0168-9525(02)02725-7. [DOI] [PubMed] [Google Scholar]
- 98.Nachury MV, Loktev AV, Zhang Q, Westlake CJ, Peranen J, et al. A core complex of BBS proteins cooperates with the GTPase Rab8 to promote ciliary membrane biogenesis. Cell. 2007;129:1201–1213. doi: 10.1016/j.cell.2007.03.053. [DOI] [PubMed] [Google Scholar]
- 99.Nauli SM, Alenghat FJ, Luo Y, Williams E, Vassilev P, et al. Polycystins 1 and 2 mediate mechanosensation in the primary cilium of kidney cells. Nat. Genet. 2003;33:129–137. doi: 10.1038/ng1076. [DOI] [PubMed] [Google Scholar]
- 100.Oishi I, Kawakami Y, Raya A, Callol-Massot C, Izpisua Belmonte JC. Regulation of primary cilia formation and left-right patterning in zebrafish by a noncanonical Wnt signaling mediator, duboraya. Nat. Genet. 2006;38:1316–1322. doi: 10.1038/ng1892. [DOI] [PubMed] [Google Scholar]
- 101.Okada Y, Takeda S, Tanaka Y, Belmonte JC, Hirokawa N. Mechanism of nodal flow: a conserved symmetry breaking event in left-right axis determination. Cell. 2005;121:633–644. doi: 10.1016/j.cell.2005.04.008. [DOI] [PubMed] [Google Scholar]
- 102.Ossipova O, Dhawan S, Sokol S, Green JB. Distinct PAR-1 proteins function in different branches of Wnt signaling during vertebrate development. Dev. Cell. 2005;8:829–841. doi: 10.1016/j.devcel.2005.04.011. [DOI] [PubMed] [Google Scholar]
- 103.Paricio N, Feiguin F, Boutros M, Eaton S, Mlodzik M. The Drosophila STE20-like kinase Misshapen is required downstream of the Frizzled receptor in planar polarity signaling. EMBO J. 1999;18:4669–4678. doi: 10.1093/emboj/18.17.4669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Park M, Moon RT. The planar cell polarity gene stbm regulates cell behaviour and cell fate in vertebrate embryos. Nat. Cell Biol. 2002;4:20–25. doi: 10.1038/ncb716. [DOI] [PubMed] [Google Scholar]
- 105.Park TJ, Haigo SL, Wallingford JB. Ciliogenesis defects in embryos lacking inturned or fuzzy function are associated with failure of planar cell polarity and Hedgehog signaling. Nat. Genet. 2006;38:303–311. doi: 10.1038/ng1753. [DOI] [PubMed] [Google Scholar]
- 106.Park TJ, Mitchell BJ, Abitua PB, Kintner C, Wallingford JB. Dishevelled controls apical docking and planar polarization of basal bodies in ciliated epithelial cells. Nat. Genet. 2008;40:871–879. doi: 10.1038/ng.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Park WJ, Liu J, Sharp EJ, Adler PN. The Drosophila tissue polarity gene inturned acts cell autonomously and encodes a novel protein. Development. 1996;122:961–969. doi: 10.1242/dev.122.3.961. [DOI] [PubMed] [Google Scholar]
- 108.Patel V, Li L, Cobo-Stark P, Shao X, Somlo S, et al. Acute kidney injury and aberrant planar cell polarity induce cyst formation in mice lacking renal cilia. Hum. Mol. Genet. 2008;17:1578–1590. doi: 10.1093/hmg/ddn045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Deleted in proof
- 110.Pazour GJ, Rosenbaum JL. Intraflagellar transport and cilia-dependent diseases. Trends Cell Biol. 2002;12:551–555. doi: 10.1016/s0962-8924(02)02410-8. [DOI] [PubMed] [Google Scholar]
- 111.Phillips HM, Rhee HJ, Murdoch JN, Hildreth V, Peat JD, et al. Disruption of planar cell polarity signaling results in congenital heart defects and cardiomyopathy attributable to early cardiomyocyte disorganization. Circ. Res. 2007;101:137–145. doi: 10.1161/CIRCRESAHA.106.142406. [DOI] [PubMed] [Google Scholar]
- 112.Piontek K, Menezes LF, Garcia-Gonzalez MA, Huso DL, Germino GG. A critical developmental switch defines the kinetics of kidney cyst formation after loss of Pkd1. Nat. Med. 2007;13:1490–1495. doi: 10.1038/nm1675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Povelones M, Howes R, Fish M, Nusse R. Genetic evidence that Drosophila frizzled controls planar cell polarity and Armadillo signaling by a common mechanism. Genetics. 2005;171:1643–1654. doi: 10.1534/genetics.105.045245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Rawls AS, Guinto JB, Wolff T. The cadherins fat and dachsous regulate dorsal/ventral signaling in the Drosophila eye. Curr. Biol. 2002;12:1021–1026. doi: 10.1016/s0960-9822(02)00893-x. [DOI] [PubMed] [Google Scholar]
- 115.Ross AJ, May-Simera H, Eichers ER, Kai M, Hill J, et al. Disruption of Bardet-Biedl syndrome ciliary proteins perturbs planar cell polarity in vertebrates. Nat. Genet. 2005;37:1135–1140. doi: 10.1038/ng1644. [DOI] [PubMed] [Google Scholar]
- 116.Saburi S, Hester I, Eremina V, Fischer E, Pontoglio M, et al. Loss of Fat4 disrupts PCP signalling and oriented cell division, leading to cystic kidney disease. Nat. Genet. 2008;40:1010–1015. doi: 10.1038/ng.179. [DOI] [PubMed] [Google Scholar]
- 117.Schwarz-Romond T, Asbrand C, Bakkers J, Kuhl M, Schaeffer HJ, et al. The ankyrin repeat protein Diversin recruits Casein kinase Iepsilon to the beta-catenin degradation complex and acts in both canonical Wnt and Wnt/JNK signaling. Genes Dev. 2002;16:2073–2084. doi: 10.1101/gad.230402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Seifert JR, Mlodzik M. Frizzled/PCP signalling: a conserved mechanism regulating cell polarity and directed motility. Nat. Rev. Genet. 2007;8:126–138. doi: 10.1038/nrg2042. [DOI] [PubMed] [Google Scholar]
- 119.Shima Y, Kengaku M, Hirano T, Takeichi M, Uemura T. Regulation of dendritic maintenance and growth by a mammalian 7-pass transmembrane cadherin. Dev. Cell. 2004;7:205–216. doi: 10.1016/j.devcel.2004.07.007. [DOI] [PubMed] [Google Scholar]
- 120.Shimada Y, Usui T, Yanagawa S, Takeichi M, Uemura T. Asymmetric colocalization of Flamingo, a seven-pass transmembrane cadherin, and Dishevelled in planar cell polarization. Curr. Biol. 2001;11:859–863. doi: 10.1016/s0960-9822(01)00233-0. [DOI] [PubMed] [Google Scholar]
- 121.Shimada Y, Yonemura S, Ohkura H, Strutt D, Uemura T. Polarized transport of Frizzled along the planar microtubule arrays in Drosophila wing epithelium. Dev. Cell. 2006;10:209–222. doi: 10.1016/j.devcel.2005.11.016. [DOI] [PubMed] [Google Scholar]
- 122.Simon MA. Planar cell polarity in the Drosophila eye is directed by graded Four-jointed and Dachsous expression. Development. 2004;131:6175–6184. doi: 10.1242/dev.01550. [DOI] [PubMed] [Google Scholar]
- 123.Simons M, Gloy J, Ganner A, Bullerkotte A, Bashkurov M, et al. Inversin, the gene product mutated in nephronophthisis type II, functions as a molecular switch between Wnt signaling pathways. Nat. Genet. 2005;37:537–543. doi: 10.1038/ng1552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Simons M, Walz G. Polycystic kidney disease: cell division without a c(l)ue? Kidney Int. 2006;70:854–864. doi: 10.1038/sj.ki.5001534. [DOI] [PubMed] [Google Scholar]
- 125.Singla V, Reiter JF. The primary cilium as the cell’s antenna: signaling at a sensory organelle. Science. 2006;313:629–633. doi: 10.1126/science.1124534. [DOI] [PubMed] [Google Scholar]
- 126.Sokol SY. Analysis of Dishevelled signalling pathways during Xenopus development. Curr. Biol. 1996;6:1456–1467. doi: 10.1016/s0960-9822(96)00750-6. [DOI] [PubMed] [Google Scholar]
- 127.Strapps WR, Tomlinson A. Transducing properties of Drosophila Frizzled proteins. Development. 2001;128:4829–4835. doi: 10.1242/dev.128.23.4829. [DOI] [PubMed] [Google Scholar]
- 128.Strutt D. Frizzled signalling and cell polarisation in Drosophila and vertebrates. Development. 2003;130:4501–4513. doi: 10.1242/dev.00695. [DOI] [PubMed] [Google Scholar]
- 129.Strutt DI, Weber U, Mlodzik M. The role of RhoA in tissue polarity and Frizzled signalling. Nature. 1997;387:292–295. doi: 10.1038/387292a0. [DOI] [PubMed] [Google Scholar]
- 130.Strutt H, Mundy J, Hofstra K, Strutt D. Cleavage and secretion is not required for Four-jointed function in Drosophila patterning. Development. 2004;131:881–890. doi: 10.1242/dev.00996. [DOI] [PubMed] [Google Scholar]
- 131.Strutt H, Price MA, Strutt D. Planar polarity is positively regulated by casein kinase Iepsilon in Drosophila. Curr. Biol. 2006;16:1329–1336. doi: 10.1016/j.cub.2006.04.041. [DOI] [PubMed] [Google Scholar]
- 132.Strutt H, Strutt D. Polarity determination in the Drosophila eye. Curr. Opin. Genet. Dev. 1999;9:442–446. doi: 10.1016/S0959-437X(99)80067-7. [DOI] [PubMed] [Google Scholar]
- 133.Tada M, Smith JC. Xwnt11 is a target of Xenopus Brachyury: regulation of gastrulation movements via Dishevelled, but not through the canonical Wnt pathway. Development. 2000;127:2227–2238. doi: 10.1242/dev.127.10.2227. [DOI] [PubMed] [Google Scholar]
- 134.Tahinci E, Symes K. Distinct functions of Rho and Rac are required for convergent extension during Xenopus gastrulation. Dev. Biol. 2003;259:318–335. doi: 10.1016/s0012-1606(03)00206-9. [DOI] [PubMed] [Google Scholar]
- 135.Takeuchi M, Nakabayashi J, Sakaguchi T, Yamamoto TS, Takahashi H, et al. The prickle-related gene in vertebrates is essential for gastrulation cell movements. Curr. Biol. 2003;13:674–679. doi: 10.1016/s0960-9822(03)00245-8. [DOI] [PubMed] [Google Scholar]
- 136.Taylor J, Abramova N, Charlton J, Adler PN. Van Gogh: a new Drosophila tissue polarity gene. Genetics. 1998;150:199–210. doi: 10.1093/genetics/150.1.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Thorpe CJ, Moon RT. nemo-like kinase is an essential coactivator of Wnt signaling during early zebrafish development. Development. 2004;131:2899–2909. doi: 10.1242/dev.01171. [DOI] [PubMed] [Google Scholar]
- 138.Topczewski J, Sepich DS, Myers DC, Walker C, Amores A, et al. The zebrafish glypican knypek controls cell polarity during gastrulation movements of convergent extension. Dev. Cell. 2001;1:251–264. doi: 10.1016/s1534-5807(01)00005-3. [DOI] [PubMed] [Google Scholar]
- 139.Torban E, Patenaude AM, Leclerc S, Rakowiecki S, Gauthier S, et al. Genetic interaction between members of the Vangl family causes neural tube defects in mice. Proc. Natl. Acad. Sci. USA. 2008;105:3449–3454. doi: 10.1073/pnas.0712126105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Tree DR, Shulman JM, Rousset R, Scott MP, Gubb D, Axelrod JD. Prickle mediates feedback amplification to generate asymmetric planar cell polarity signaling. Cell. 2002;109:371–381. doi: 10.1016/s0092-8674(02)00715-8. [DOI] [PubMed] [Google Scholar]
- 141.Usui T, Shima Y, Shimada Y, Hirano S, Burgess RW, et al. Flamingo, a seven-pass transmembrane cadherin, regulates planar cell polarity under the control of Frizzled. Cell. 1999;98:585–595. doi: 10.1016/s0092-8674(00)80046-x. [DOI] [PubMed] [Google Scholar]
- 142.Veeman MT, Axelrod JD, Moon RT. A second canon. Functions and mechanisms of beta-catenin-independent Wnt signaling. Dev. Cell. 2003;5:367–377. doi: 10.1016/s1534-5807(03)00266-1. [DOI] [PubMed] [Google Scholar]
- 143.Veeman MT, Slusarski DC, Kaykas A, Louie SH, Moon RT. Zebrafish prickle, a modulator of noncanonical Wnt/Fz signaling, regulates gastrulation movements. Curr. Biol. 2003;13:680–685. doi: 10.1016/s0960-9822(03)00240-9. [DOI] [PubMed] [Google Scholar]
- 144.Vinson CR, Adler PN. Directional noncell autonomy and the transmission of polarity information by the frizzled gene of Drosophila. Nature. 1987;329:549–551. doi: 10.1038/329549a0. [DOI] [PubMed] [Google Scholar]
- 145.Wallingford JB. Closing in on vertebrate planar polarity. Nat. Cell Biol. 2004;6:687–689. doi: 10.1038/ncb0804-687. [DOI] [PubMed] [Google Scholar]
- 146.Wallingford JB. Planar cell polarity, ciliogenesis and neural tube defects. Hum. Mol. Genet. 2006;15(Spec No 2):R227–R234. doi: 10.1093/hmg/ddl216. [DOI] [PubMed] [Google Scholar]
- 147.Wallingford JB, Habas R. The developmental biology of Dishevelled: an enigmatic protein governing cell fate and cell polarity. Development. 2005;132:4421–4436. doi: 10.1242/dev.02068. [DOI] [PubMed] [Google Scholar]
- 148.Wallingford JB, Rowning BA, Vogeli KM, Rothbacher U, Fraser SE, Harland RM. Dishevelled controls cell polarity during Xenopus gastrulation. Nature. 2000;405:81–85. doi: 10.1038/35011077. [DOI] [PubMed] [Google Scholar]
- 149.Wang HY, Malbon CC. Wnt signaling, Ca2+, and cyclic GMP: visualizing Frizzled functions. Science. 2003;300:1529–1530. doi: 10.1126/science.1085259. [DOI] [PubMed] [Google Scholar]
- 150.Wang J, Hamblet NS, Mark S, Dickinson ME, Brinkman BC, et al. Dishevelled genes mediate a conserved mammalian PCP pathway to regulate convergent extension during neurulation. Development. 2006;133:1767–1778. doi: 10.1242/dev.02347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Wang Y, Guo N, Nathans J. The role of Frizzled3 and Frizzled6 in neural tube closure and in the planar polarity of inner-ear sensory hair cells. J. Neurosci. 2006;26:2147–2156. doi: 10.1523/JNEUROSCI.4698-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Wang Y, Nathans J. Tissue/planar cell polarity in vertebrates: new insights and new questions. Development. 2007;134:647–658. doi: 10.1242/dev.02772. [DOI] [PubMed] [Google Scholar]
- 153.Wang Y, Zhang J, Mori S, Nathans J. Axonal growth and guidance defects in Frizzled3 knockout mice: a comparison of diffusion tensor magnetic resonance imaging, neurofilament staining, and genetically directed cell labeling. J. Neurosci. 2006;26:355–364. doi: 10.1523/JNEUROSCI.3221-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Winter CG, Wang B, Ballew A, Royou A, Karess R, et al. Drosophila Rho-associated kinase (Drok) links Frizzled-mediated planar cell polarity signaling to the actin cytoskeleton. Cell. 2001;105:81–91. doi: 10.1016/s0092-8674(01)00298-7. [DOI] [PubMed] [Google Scholar]
- 155.Wolff T, Rubin GM. Strabismus, a novel gene that regulates tissue polarity and cell fate decisions in Drosophila. Development. 1998;125:1149–1159. doi: 10.1242/dev.125.6.1149. [DOI] [PubMed] [Google Scholar]
- 156.Wong LL, Adler PN. Tissue polarity genes of Drosophila regulate the subcellular location for prehair initiation in pupal wing cells. J. Cell Biol. 1993;123:209–221. doi: 10.1083/jcb.123.1.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Wu J, Jenny A, Mirkovic I, Mlodzik M. Frizzled-Dishevelled signaling specificity outcome can be modulated by Diego in Drosophila. Mech. Dev. 2008;125:30–42. doi: 10.1016/j.mod.2007.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Wu J, Klein TJ, Mlodzik M. Subcellular localization of frizzled receptors, mediated by their cytoplasmic tails, regulates signaling pathway specificity. PLoS Biol. 2004;2:1004–1014. doi: 10.1371/journal.pbio.0020158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Xu Q, Wang Y, Dabdoub A, Smallwood PM, Williams J, et al. Vascular development in the retina and inner ear: control by Norrin and Frizzled-4, a high-affinity ligand-receptor pair. Cell. 2004;116:883–895. doi: 10.1016/s0092-8674(04)00216-8. [DOI] [PubMed] [Google Scholar]
- 160.Yang C, Axelrod JD, Simon MA. Regulation of Frizzled by Fat-like Cadherins during planar polarity signaling in the Drosophila compound eye. Cell. 2002;108:675–688. doi: 10.1016/s0092-8674(02)00658-x. [DOI] [PubMed] [Google Scholar]
- 161.Ybot-Gonzalez P, Savery D, Gerrelli D, Signore M, Mitchell CE, et al. Convergent extension, planar-cell-polarity signalling and initiation of mouse neural tube closure. Development. 2007;134:789–799. doi: 10.1242/dev.000380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Yin C, Kiskowski M, Pouille PA, Farge E, Solnica-Krezel L. Cooperation of polarized cell intercalations drives convergence and extension of presomitic mesoderm during zebrafish gastrulation. J. Cell Biol. 2008;180:221–232. doi: 10.1083/jcb.200704150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Yu X, Prekeris R, Gould GW. Role of endosomal Rab GTPases in cytokinesis. Eur. J. Cell Biol. 2007;86:25–35. doi: 10.1016/j.ejcb.2006.10.002. [DOI] [PubMed] [Google Scholar]
- 164.Yun UJ, Kim SY, Liu J, Adler PN, Bae E, et al. The inturned protein of Drosophila melanogaster is a cytoplasmic protein located at the cell periphery in wing cells. Dev. Genet. 1999;25:297–305. doi: 10.1002/(SICI)1520-6408(1999)25:4<297::AID-DVG3>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 165.Zallen JA. Planar polarity and tissue morphogenesis. Cell. 2007;129:1051–1063. doi: 10.1016/j.cell.2007.05.050. [DOI] [PubMed] [Google Scholar]
- 166.Zeidler MP, Perrimon N, Strutt DI. The four-jointed gene is required in the Drosophila eye for ommatidial polarity specification. Curr. Biol. 1999;9:1363–1372. doi: 10.1016/s0960-9822(00)80081-0. [DOI] [PubMed] [Google Scholar]
- 167.Zeidler MP, Perrimon N, Strutt DI. Multiple roles for four-jointed in planar polarity and limb patterning. Dev. Biol. 2000;228:181–196. doi: 10.1006/dbio.2000.9940. [DOI] [PubMed] [Google Scholar]
- 168.Zheng L, Zhang J, Carthew RW. Frizzled regulates mirror-symmetric pattern formation in the Drosophila eye. Development. 1995;121:3045–3055. doi: 10.1242/dev.121.9.3045. [DOI] [PubMed] [Google Scholar]