Abstract
The presence of a defect in mature articular cartilage can lead to degenerative changes of the joint. This is in part caused by the inability of cartilage to regenerate tissue that is capable of spanning a fissure or crack. In this study, we hypothesized that introduction of a biodegradable cell-seeded nanofibrous hydrogel, Puramatrixtm, into a cartilage gap would facilitate the generation of a mechanically stable interface. The effects of chondrocyte incorporation within the hydrogel and supplementation with transforming growth factor-ß3 (TGF-β3), a known regulator of cell growth and differentiation, on cartilage integration were examined mechanically and histologically as a function of cell density and incubation time. When supplemented with TGF-ß3, the cell-seeded hydrogel exhibited abundant matrix generation within the hydrogel and a corresponding increase in maximum push-out stress as compared to all other groups. Furthermore, initial cell seeding density affected interfacial strength in a time-dependent manner. This study suggests that a cell-seeded TGF-ß3 supplemented hydrogel can encourage integration between two opposing pieces of articular cartilage.
Keywords: nanofibrous hydrogel, chondrocytes, cartilage repair, TGF-β3, tissue engineering
1. INTRODUCTION
Mature articular cartilage does not possess an intrinsic ability to heal. Therefore, the presence of cartilage defects can lead to long-term degenerative changes of the joint [Mankin et al., 1982; Hunter 1995]. Attempts at treating cartilage defects early in the course of the problem using techniques such as microfracture, lavage and debridement have thus far led to variable, often unsatisfactory clinical outcomes [Moseley et al., 2002; Williams et al., 2007]. Mosaicplasty, the transplantation of autogenous osteochondral plugs from non-weight bearing areas has had reasonable short-term success, but faces many unresolved technical challenges, not least of which is the lack of integration between the graft and the host tissue [Bartz et al., 2001; Jurvelin et al., 2000]. Contributing factors include chondrocyte death adjacent to the cut surface [Zhang et al., 2005] and little propensity for chondrocytes to migrate out of their native matrix [Bos et al., 2005].
Techniques such as enzymatic digestion to free cells from their dense matrix and growth factor stimulation to encourage cellular migration and proliferation have been explored in an effort to encourage improved integration [van de Breevaart et al 2004; Bos et al., 2002]. Digestive enzymes have also been used in conjunction with biological glues [Jurgensen et al., 1997] in an attempt to increase chondrocyte adhesion to the digested matrix [Lee et al., 2000]. Finding the ideal adhesive, however, has been non-trivial, as researchers have to balance the requirements of a strong and durable interface, without detrimentally affecting the health of adjacent cartilage [Hunter and Levenston 2004; Peretti et al., 2006; Englert et al., 2007]. In 2002, Kisiday et al., developed a method to encapsulate chondrocytes within a novel self-assembling peptide hydrogel and found that the scaffold could support a stable chondrocyte phenotype and facilitate the time dependent synthesis and accumulation of extra cellular matrix components. However, the ability of such a scaffold to result in an integrative matrix that can span a cartilage fissure, or gap, and reestablish a stable interface is unclear.
In this study, we hypothesized that introduction of a biodegradable cell-seeded nanofibrous filler material into a cartilage-cartilage interface would facilitate the generation of a mechanically stable interface. A commercially available hydrogel, (Puramatrix™, 3DM Inc, MA) [Zhang et al., 1993; Kisiday et al., 2002] was injected into an in vitro cartilage gap model. The effects of cell incorporation within the hydrogel and supplementation with transforming growth factor- ß3 (TGF-ß3) on cartilage integration were examined mechanically and histologically as a function of cell density and incubation time.
2. METHODS
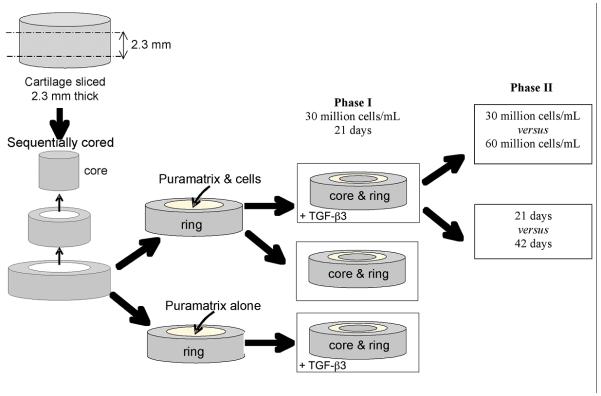
The study design consisted of two phases. The first phase explored the effects of cell incorporation and TGF-ß3 supplementation on cartilage integration across a Puramatrix filled cartilage gap by analyzing the following three groups; TGF-ß3 and cells, cells only, and TGF-ß3 only. The second phase was designed to explore the effect of varying cell seeding density (30 versus 60 million cells/mL) and incubation time (21 versus. 42 days) on interface strength in the TGF-ß3 and cells group. The study design is outlined in Figure 1.
Figure 1.
Schematic of study design. An osteochondral core of harvested, articular cartilage is sliced to a thickness of 2.3 mm; and sequentially cored. The outer ring is filled with the hydrogel (either with or without cells). When the inner core is replaced, three groups result: TGF-ß3 and cells; cells only; and TGF-ß3 only.
Cores of articular cartilage (8 mm diameter) were harvested from the femoral condyles and patella grooves of immature bovine knees (age of animals 3-6 months), Fresh Farm’s Beef Inc., Rutland, VT. The superficial and deep zones were removed using a scalpel and samples were sliced to a thickness of 2.3 mm using a custom designed slicing apparatus.
Chondrocytes enzymatically isolated from the knees of immature bovines [Huang et al., 2008] were suspended in 10% sucrose solution at twice their final concentration, and mixed at a ratio of 1:1 with Puramatrix™ (BD Biosciences, Franklin Lakes, NJ) to form a cell/gel solution of 0.5% Puramatrix™ containing either 30 million cells/ml or 60 million cells/ml.
Each piece of cartilage tissue was spot-dried and sequentially cored with 3.5 mm and 4.0 mm diameter biopsy punches to construct an inner core/outer ring with a peripheral gap of 0.25 mm. The inner core was removed, the outer ring was filled with Puramatrix either with or without cells and the corresponding inner core was replaced. Excess gel was removed and the Puramatrix™ was induced to self-assemble by exposure to 3 washes of DMEM for 5 minutes each.
The assembled construct was placed into a 12 well plate which was filled with 3 ml of chondrogenic medium (CM) consisting of DMEM supplemented with 0.1 μM dexamethasone, 50 μg/mL ascorbate, 40 μg/mL L-proline, 100 μg/mL sodium pyruvate, 1X ITS+ premix, 1X PSF supplemented with 1% antiobiotic/antimycotic, either with or without 10 ng/mL TGF-ß3 (R&D Systems, Minneapolis, MN) supplementation [Huang et al., 2008]. Culture medium was changed twice weekly.
After 21 days of culture, two samples from each group in phase I of the study (see Fig, 1) were sequentially dehydrated and embedded in paraffin, sectioned into 8 μm thick slices, stained with Alcian Blue and Eosin, and microscopically examined.
After 21 or 42 days of incubation, 5 samples from each group were subjected to a push out test using an Enduratec ELF3200 testing machine (Enduratec Systems, Minnetonka, MN). The thickness of each sample was measured using a micrometer. Each sample was loaded at a rate of 0.2 mm/s until complete push out of the inner core occurred. Load and displacement were recorded at a frequency of 20 Hz throughout testing. Stress was computed by normalizing the maximum load by the surface area of the interface between the gel and the outer ring. The maximum stress for each push-out test was computed.
Inter-group differences were explored using Kruskal-Wallis One Way Analysis of Variance on Ranks followed by pairwise multiple comparisons using either Dunn’s method where p<0.05 was considered significant, or Tukey’s test (α=0.05). All statistical analysis was performed using Sigma Stat v.3 (Systat Software, Inc., San Jose, CA).
3. RESULTS
All cell seeded hydrogels exhibited cell viability and matrix generation after 21 days of incubation. The cell seeded hydrogels that had been supplemented with TGF-ß3 manifested robust matrix generation within the interface, and the hydrogel remained in intimate contact with cartilage on either side of the gap (Fig. 2a). Interface matrix generation within the group that was not supplemented with TGF-ß3 was not as robust (Fig. 2b). Although cells were found within the initially acellular group, little matrix was produced by these cells (Fig. 2c).
Figure 2.

Histological analysis of interface. Alcian blue-esosin stained sections of the interface between the inner core and outer ring articular cartilage components at culture day 21. (A) Puramtrix hydrogel containing both TGF-ß3 and chondrocytes; (B) hydrogel containing chondrocytes only; and (C) hydrogel containing TGF-ß3 only. Robust cartilage formation in intimate contact with the native cartilage components was seen only in the cell-seeded hydrogel in the presence of TGF-ß3. Bar = 100 μm (A & B), or 50 μm (C).
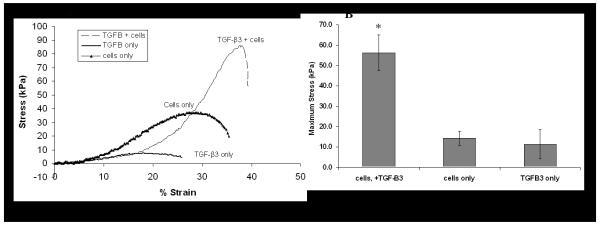
Mechanical testing of samples revealed a well-defined peak in load that corresponded to push-out (Fig 3a). After 21 days of incubation, the maximum stress before push-out for the cell seeded, TGF-ß3 supplemented group was significantly higher than both other groups (p<0.05); whereas there was no significant difference between the acellular and the non-TGF-ß3 treated cell seeded groups (>0.05) (Fig 3b).
Figure 3.
Push-out mechanical testing of interface strength. (A) The stress-strain curve obtained at day 21 showed highest level of integration in the TGF-ß3 and cells group. (B) Mean maximal stress values (n = 5) were seen in the TGF-ß3 plus cells group, while the cells only and TGF-ß3 only groups showed similar values (*, p<0.05, compared to TGF-ß3 only or cells only group).
Seeding with 60 million cells/mL vs. 30 million cells/mL did not result in a statistically significant increase in interface strength at 21 days; however after 42 days of culture a statistically significant increase in strength was found in the sample seeded with 60 million cells/mL. There was a statistically significant time-dependent increase in maximum stress at push-out in the TGF-ß3 supplemented, cell seeded group regardless of initial cell seeding density (30 versus 60 million cells/ml) (Fig. 4).
Figure 4.
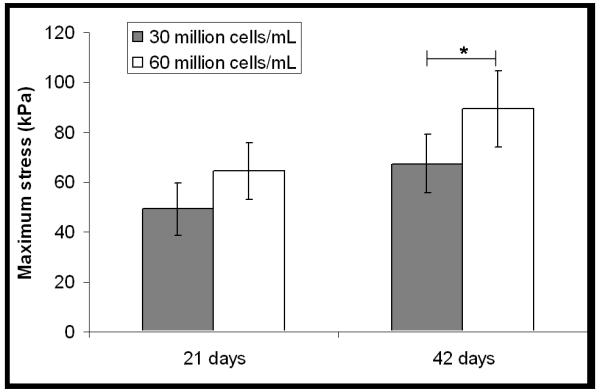
Effect of cell seeding density on mechanical strength of interface. A statistically significant, cell-dependent difference (*, p<0.05; n = 5) was seen only later in culture (42 days)
4. DISCUSSION
Integration across a cartilage fissure or gap has been an elusive outcome based on current approaches. This study has demonstrated the potential application of a degradable nano-fibrous cell seeded hydrogel to foster integration across a cartilage gap. When supplemented with TGF-ß3, a known regulator of cell growth and differentiation, the cell-seeded hydrogel exhibited abundant matrix generation within the hydrogel and a corresponding increase in maximum push-out stress as compared to all other groups; in addition, initial cell seeding density affected interfacial strength in a time-dependent manner. Lack of TGF-ß3 supplementation detrimentally affected the histological appearance and mechanical strength of the interface. Thus, in order to replicate and optimize integration in vivo, initiating or increasing TGF-ß3 production, perhaps via prior gene transduction (via plasmid or viral mechanisms), or other methods for controlled growth factor release in the local environment, might be required.
The in vitro assessment of the ability of enzymatic digestion to foster integration has been largely limited to models in which two pieces of articular cartilage are placed in direct apposition to each other [Tam et al., 2007; van de Breevaart Bravenboer et al., 2004; Moretti et al., 2005]. In vivo, a gap or fissure across which the chondrocytes must migrate and lay down matrix in order to facilitate integration is likely to exist. The ability of freed and migrating cells to cross a gap is uncertain and has received considerably less scientific attention. Accordingly, the model chosen in this study was designed such that a gap of 2.5 mm existed between the inner core and outer ring.
Given the novelty of our gap model, it is difficult to compare directly the magnitude of the maximum stress measured during push-out to that measured in other studies. For example, Moretti et al. (2004) measured maximum push out strengths of 71-161 kPa in disk-ring models, values that are within the range of that measured in our study for the TGF-ß3 and cell supplemented group. However, our values are an order of magnitude below that reported by van de Breevaart Bravenboer et al., 2004, who measured interface strengths of 1.32 ± 0.15 MPa in disk-annulus cartilage models, both sides of which had been enzymatically digested and placed in direct contact. Had a gap been present, it is likely that the interface strength would have been reduced.
The potential of adhesives or biological glues to create an initial interfacial bond between adjacent pieces of cartilage tissue is appealing [Wilson et al., 2005]. However, balancing the need for a strong and lasting bond without detrimentally affecting the health of adjacent tissue has made finding the ideal adhesive difficult [Hunter and Levenston 2004; Peretti et al., 2006; Englert et al., 2007]. In our study, PuraMatrix was used in lieu of an adhesive for these reasons. It is a peptide based hydrogel with water content of greater than 99%. Injected as a gel, it can self-assemble into three dimensional nano-fbrous structure when exposed to a salt-solution [Zhang et al., 1993] (in our case, self-assembly was induced by exposure to DMEM). The final gel exhibits fiber diameters of about 10 nm and pore size ranges from 50-200 μm [Wang et al., 2007]. Although not immediately adhesive, the peptide sequence of the gel has been previously shown to promote the attachment and migration of a variety of cells, ranging from hepatocytes [Wang et al., 2007], neural cells [Semino et al., 2004; Holmes et al., 2000] and more recently chondrocytes [Kisiday et al., 2002]. Prior to this study, the ability of PuraMatrix to enhance integration across a cartilage gap had not been explored.
Chondrocyte death in articular cartilage wound edges [Zhang et al., 2005] and the subsequent lack of matrix producing cells in the interface area are considered to be major causes of impaired integrative articular cartilage repair [Bos et al., 2005]. Accordingly, efforts have been made to allow chondrocytes to migrate through their dense matrix towards an interface by combining local enzymatic digestion of the matrix with growth factor stimulation. For example, decreasing proteoglycan content close to the defect edge via exposure to hyaluronidase or collagenase has shown promising histological results [Bos et al., 2004; Quinn et al., 2002]. Cell death appears to be localized to approximately 400 μm within the affected edge, and is temporal in nature. Growth factor supplementation, such as with TGF-ß3, has been shown to enhance proteoglycan content in the wound edges of articular cartilage (Bos et al., 2001). For this reason, TGF-ß3 was used as a supplement in this study and was found to significantly increase the maximum stress prior to push-out. Although the mechanism by which TGF-ß3 supplementation increased the interface strength was not further explored, our findings suggest that the ability to continuously deliver growth factor to cells would be required to maximize integration in vivo.
The mechanism by which integration occurred in this model is unclear. It has been suggested by Sah and colleagues that chondrocytes at the edge of a wound can secrete and deposit matrix molecules which form part of a collagen network to span the fissure [DiMicco and Sah 2001; DiMicco et al., 2002; McGowan and Sah, 2005]. It is possible that the secreted matrix from both sides of the interface in our model (from the gel and the native tissue) is contributing to the adhesion. Furthermore, the histological presence of chondrocytes within the acellular group suggests that chondrocytes can actually migrate into this material, which could further assist integration.
Our study has limitations; only one group (TGF-ß3 supplemented cell seeded) was incubated for the longer time-point of 42 days. It is possible that cells which were not supplemented by TGF-ß3 may eventually secrete increased matrix comparable to their supplemented counterparts by this later time-point. Furthermore, the interfacial properties required to ensure a long-term mechanically stable interface in vivo is unclear. As second limitation of the study is that chondrocytes were the only cell type used in the model. Recent work has suggested that adult mesenchymal stem cells have enhanced growth potential when seeded within Puramatrix, suggesting that chondrocytes may not be the only cell source suitable for this application [Mauck et al., 2008; Kisiday et al., 2008].
In conclusion, we have demonstrated the feasibility of using a nano-fibrous, growth factor supplemented filler material towards integrating across gaps in articular cartilage. When seeded with cells and supplemented with TGF-ß3, the filler material appeared to act as a conduit for chondrocyte movement whilst also supporting cell proliferation and matrix generation. The approach herein proposed could be adapted to integrate osteochondral grafts with surrounding cartilage or to encourage mechanically viable integration across fissures, in a clinical setting.
Acknowledgments
The authors would like to thank Hospital for Special Surgery for funding this study, and the support of the Intramural Research Program of the NIAMS, NIH (ZO1 AR41131).
REFERENCES
- 1.Bartz RL, Kamaric E, Noble PC, Lintner D, Bocell J. Topographic matching of selected donor and recipient sites for osteochondral autografting of the articular surface of the femoral condyles. American Journal of Sports Medicine. 2001;29:207–12. doi: 10.1177/03635465010290021501. [DOI] [PubMed] [Google Scholar]
- 2.Bos PK, DeGroot J, Budde M, Verhaar JA, van Osch GJ. Specific enzymatic treatment of bovine and human articular cartilage: implications for integrative cartilage repair. Arthritis and Rheumatism. 2002;46:976–85. doi: 10.1002/art.10208. [DOI] [PubMed] [Google Scholar]
- 3.Bos PK, van Osch GJ, Frenz DA, Verhaar JA, Verwoerd-Verhoef HL. Growth factor expression in cartilage wound healing: temporal and spatial immunolocalization in a rabbit auricular cartilage wound model. Osteoarthritis Cartilage. 2001;9:382–9. doi: 10.1053/joca.2000.0399. [DOI] [PubMed] [Google Scholar]
- 4.Bos PK, van Osch GJ, Frenz DA, Verhaar JA, Verwoerd-Verhoef HL. Growth factor expression in cartilage wound healing: temporal and spatial immunolocalization in a rabbit auricular cartilage wound model. Osteoarthritis Cartilage. 2001;9:382–9. doi: 10.1053/joca.2000.0399. [DOI] [PubMed] [Google Scholar]
- 5.Bush PG, Wokosin DL, Hall AC. Two-versus one photon excitation laser scanning microscopy: critical importance of excitation wavelength. Frontiers in Bioscience. 2007;1:2646–57. doi: 10.2741/2261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chang C, Lauffenburger DA, Morales TI. Motile chondrocytes from newborn calf: migration properties and synthesis of collagen II. Osteoarthritis Cartilage. 2003;11:603–12. doi: 10.1016/s1063-4584(03)00087-6. [DOI] [PubMed] [Google Scholar]
- 7.DiMicco MA, Sah RL. Integrative cartilage repair: adhesive strength is correlated with collagen deposition. Journal of Orthopaedic Research. 2001;19:1105–12. doi: 10.1016/S0736-0266(01)00037-7. [DOI] [PubMed] [Google Scholar]
- 8.DiMicco MA, Sah RL. Integrative cartilage repair: adhesive strength is correlated with collagen deposition. Journal of Orthopaedic Research. 2001;19:1105–12. doi: 10.1016/S0736-0266(01)00037-7. [DOI] [PubMed] [Google Scholar]
- 9.Englert C, Blunk T, Müller R, von Glasser SS, Baumer J, Fierlbeck J, Heid IM, Nerlich M, Hammer J. Bonding of articular cartilage using a combination of biochemical degradation and surface cross-linking. Arthritis Research and Therapy. 2007;9:R47. doi: 10.1186/ar2202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Erickson IE, Huang AH, Chung C, Li RT, Burdick JA, Mauck RL. Differential Maturation and Structure-Function Relationships in MSC and Chondrocyte Seeded Hydrogels. Tissue Engineering. 2009 doi: 10.1089/ten.tea.2008.0099. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Holmes TC, de Lacalle S, Su X, Liu G, Rich A, Zhang S. Extensive neurite outgrowth and active synapse formation on self-assembling peptide scaffolds. Proceedings of the National Academy of Sciences U S A. 2000;97:6728–33. doi: 10.1073/pnas.97.12.6728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Huang AH, Yeger-McKeever M, Stein A, Mauck RL. Tensile properties of engineered cartilage formed from chondrocyte- and MSC-laden hydrogels. Osteoarthritis and Cartilage. 2008;16:1074–82. doi: 10.1016/j.joca.2008.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hunter CJ, Levenston ME. Maturation and integration of tissue-engineered cartilages within an in vitro defect repair model. Tissue Engineering. 2004;10:736–46. doi: 10.1089/1076327041348310. [DOI] [PubMed] [Google Scholar]
- 14.Hunter W. Of the structure and disease of articulating cartilages. Clinical Orthopaedics and Related Research. 1995;317:3–6. [PubMed] [Google Scholar]
- 15.Jurgensen K, Aeschlimann D, Cavin V, Genge M, Hunziker EB. A new biological glue for cartilage-cartilage interfaces: tissue transglutaminase. Journal of Bone and Joint Surgery (American) 1997;79:185–93. doi: 10.2106/00004623-199702000-00004. [DOI] [PubMed] [Google Scholar]
- 16.Jurvelin JS, Arokoski JP, Hunziker EB, Helminen HJ. Topographical variation of the elastic properties of articular cartilage in the canine knee. Journal of Biomechanics. 2000;33:669–75. doi: 10.1016/s0021-9290(00)00007-5. [DOI] [PubMed] [Google Scholar]
- 17.Kaplonyi G, Zimmerman I, Frenyo AD, Farkas T, Nemes G. The use of fibrin adhesive in the repair of chondral and osteochondral injuries. Injury. 1998;19:267–72. doi: 10.1016/0020-1383(88)90043-5. [DOI] [PubMed] [Google Scholar]
- 18.Kisiday J, Jin M, Kurz B, Hung H, Semino C, Zhang S, Grodzinsky AJ. Self-assembling peptide hydrogel fosters chondrocyte extracellular matrix production and cell division: implications for cartilage tissue repair. Proceedings of the National Academy of Sciences. 2002;99:9996–10001. doi: 10.1073/pnas.142309999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kisiday JD, Kopesky PW, Evans CH, Grodzinsky AJ, McIlwraith CW, Frisbie DD. Evaluation of adult equine bone marrow- and adipose-derived progenitor cell chondrogenesis in hydrogel cultures. Journal of Orthopaedic Research. 2008;26:322–31. doi: 10.1002/jor.20508. [DOI] [PubMed] [Google Scholar]
- 20.Lee MC, Sung KL, Kurtis MS, Akeson WH, Sah RL. Adhesive force of chondrocytes to cartilage. Effects of chondroitinase ABC. Clinical Orthopaedics and Related Research. 2000;370:286–94. doi: 10.1097/00003086-200001000-00029. [DOI] [PubMed] [Google Scholar]
- 21.Mankin HJ. The response of articular cartilage to mechanical injury. Journal of Bone and Joint Surgery (American) 1982;64:460–6. [PubMed] [Google Scholar]
- 22.McGowan KB, Sah RL. Treatment of cartilage with beta-aminopropionitrile accelerates subsequent collagen maturation and modulates integrative repair. Journal of Orthopaedic Research. 2005;23:594–601. doi: 10.1016/j.orthres.2004.02.015. [DOI] [PubMed] [Google Scholar]
- 23.Moretti M, Wendt D, Schaefer D, Jakob M, Hunziker EB, Heberer M, Martin I. Structural characterization and reliable biomechanical assessment of integrative cartilage repair. Journal of Bim,echanics. 2005;38(9):1846–54. doi: 10.1016/j.jbiomech.2004.08.021. [DOI] [PubMed] [Google Scholar]
- 24.Moseley JB, O’Malley K, Petersen NJ, Menke TJ, Brody BA, Kuykendall DH, Hollingsworth JC, Ashton CM, Wray NP. A controlled trial of arthroscopic surgery for osteoarthritis of the knee. New England Journal of Medicine. 2002;347:81–8. doi: 10.1056/NEJMoa013259. [DOI] [PubMed] [Google Scholar]
- 25.Peretti GM, Xu JW, Bonassar LJ, Kirchhoff CH, Yaremchuk MJ, Randolph MA. Review of injectable cartilage engineering using fibrin gel in mice and swine models. Tissue Engineering. 2006;12:1151–68. doi: 10.1089/ten.2006.12.1151. [DOI] [PubMed] [Google Scholar]
- 26.Quinn TM, Hunziker EB. Controlled enzymatic matrix degradation for integrative cartilage repair: effects on viable cell density and proteoglycan deposition. Tissue Engineering. 2002;8:799–806. doi: 10.1089/10763270260424150. [DOI] [PubMed] [Google Scholar]
- 27.Zhang S, Holmes T, Lockshin C, Rich A. Spontaneous Assembly of a Self-Complementary Oligopeptide to Form a Stable Macroscopic Membrane. Proceedings of the National Academy of Sciences. 1993;90:3334–3338. doi: 10.1073/pnas.90.8.3334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Semino CE, Kasahara J, Hayashi Y, Zhang S. Entrapment of migrating hippocampal neural cells in three-dimensional peptide nanofiber scaffold. Tissue Engineering. 2004;10:643–55. doi: 10.1089/107632704323061997. [DOI] [PubMed] [Google Scholar]
- 29.Tam HK, Srivastava A, Colwell CW, Jr, D’Lima DD. In vitro model of full-thickness cartilage defect healing. Journal of Orthopaedic Research. 2007;25:1136–44. doi: 10.1002/jor.20428. [DOI] [PubMed] [Google Scholar]
- 30.van de Breevaart Bravenboer J, In der Maur CD, Bos PK, van Rensen IH, Weinans H, Feenstra L, Verhaar JA, van O GJ. Increased Interfacial Strength Of Transplanted Cartilage In Vivo Following Enzymatic Treatment Of Wound Edges. Trans. Orthopaedic Research Society. 2003;28:0188. [Google Scholar]
- 31.Wang S, Nagrath D, Chen PC, Berthiaume F, Yarmush ML. Three-dimensional primary hepatocyte culture in synthetic self-assembling peptide hydrogel. Tissue Engineering Part A. 2008;14:227–36. doi: 10.1089/tea.2007.0143. [DOI] [PubMed] [Google Scholar]
- 32.Williams RJ, 3rd, Harnly HW. Microfracture: indications, technique, and results. Instructional Course Lectures. 2007;56:419–28. [PubMed] [Google Scholar]
- 33.Wilson DJ, Chenery DH, Bowring HK, Wilson K, Turner R, Maughan J, West PJ, Ansell CW. Physical and biological properties of a novel siloxane adhesive for soft tissue applications. Journal of Biomaterials and Science, Polymers Edition. 2005;16:449–72. doi: 10.1163/1568562053700200. [DOI] [PubMed] [Google Scholar]
- 34.Zhang Z, McCaffery JM, Spencer RG, Francomano CA. Growth and integration of neocartilage with native cartilage in vitro. Journal of Orthopaedic Research. 2005;23:433–9. doi: 10.1016/j.orthres.2004.08.028. [DOI] [PubMed] [Google Scholar]