Abstract
Purpose
To investigate the possibility that Angiopoietin-like 7 (ANGPTL7) protein is involved in the pathogenesis of glaucoma.
Methods
Primary human trabecular meshwork (TM) cells and corneoscleral explants were stimulated with either dexamethasone (DEX) or transforming growth factor β (TGFβ), and ANGPTL7 protein secreted into culture medium was determined by Western blot analysis. The effect of stable overexpression of ANGPTL7 in transfected immortalized TM cell lines on collagen expression was investigated by immunocytochemistry. Localization of ANGPTL7 protein in human eyes was determined by immunohistochemistry. The concentration of ANGPTL7 protein in aqueous humor (AH) from patients with glaucoma and control patients was compared by Western blot analysis. The beagle model of primary open-angle glaucoma (POAG) was used to correlate ANGPTL7 protein levels in canine AH with disease progression.
Results
TGFβ and DEX stimulated secretion of ANGPTL7 protein by TM cells and corneoscleral explants. Overexpression of ANGPTL7 by immortalized TM cell lines increased expression of type I collagen. Expression of ANGPTL7 protein was located in the corneal stroma, near the limbus, and throughout the sclera, with lower expression in the TM. In the lamina cribrosa, ANGPTL7 expression was associated with the cribriform plates. The concentration of ANGPTL7 protein was elevated in AH from patients with glaucoma and increased as disease progressed in POAG beagle dogs.
Conclusions
Induction of ANGPTL7 secretion by glaucoma stimuli and increased concentration of ANGPTL7 in glaucomatous AH suggest that ANGPTL7 is overexpressed in glaucoma. Since overexpression of ANGPTL7 increases collagen expression, a potential disease mechanism, ANGPTL7 could have a pathogenic role in glaucoma, and may serve as a potential therapeutic target.
Glaucoma is a complex set of diseases that cause visual impairment and blindness due to death of retinal ganglion cells, with elevated intraocular pressure (IOP) as an important risk factor. There are probably multiple pathways leading to glaucoma involving interactions between genetic and environmental factors1,2 and changes in gene expression patterns. Many studies have used microarray technology to search for genes that may have altered expression in glaucoma and therefore may be pathogenic.3–11 A challenge now is to choose specific candidates from these lists for further evaluation.
We chose to investigate the possible involvement in glaucoma of angiopoietin-like 7 (ANGPTL7), also known as cornea-derived transcript 6 (CDT6), in part because it has been identified by microarray to be a highly induced mRNA in response to either dexamethasone (DEX) or transforming growth factor β (TGFβ) treatment of trabecular meshwork (TM) cells.8,9,11 ANGPTL7 was also identified as possibly being associated with primary open-angle glaucoma (POAG) in a proteomics study of TM tissue.12 The chromosomal location of the ANGPTL7 gene (1p36.22)13 is within the GLC3B locus (1p36.2-1p36.1) of primary congenital glaucoma,14 further supporting ANGPTL7 as a candidate glaucoma gene.
ANGPTL7 is a member of the angiopoietin-like (ANGPTL) family of proteins that have high sequence and structural homology to the angiopoietins, which are important regulators of angiogenesis.13 Currently, seven members of the ANGPTL family have been identified (ANGPTL1-7), all of which are secreted proteins that form multimers via amino-terminal coiled-coil domains and contain carboxyl-terminal fibrinogen-like domains. All ANGPTL proteins studied to date have been shown to be involved in blood vessel formation or neovascularization in several models,15–17 including corneal angiogenesis assays.17,18 In addition, ANGPTL proteins have been demonstrated to play an important role in lipid metabolism by inhibition of phospholipid lipase.19–22 The lipid and vascular functions are mediated by distinct protein domains, with the amino-terminal coiled-coil domain mediating lipid metabolism22–24 and the carboxyl-terminal fibrinogen-like domain mediating vascular effects.18 ANGPTL proteins appear to be bi-functional, with both functions demonstrated for most of the family members studied.
ANGPTL7 was first identified as highly expressed in a human corneal cDNA library,25 and the protein has been shown to be secreted as disulfide-linked multimers.26 Because of its corneal location, it was initially speculated that ANGPTL7 may have antiangiogenic properties. In a mouse tumor model, vascularization of tumors overexpressing ANGPTL7 was distorted, with deposits of collagen types I and V and proteoglycans near vascular endothelia.26 Melanoma cell lines transfected with ANGPTL7 also produced more collagen types I and V than did the vector-transfected control cells.26
The potential to alter collagen homeostasis makes ANGPTL7 an attractive candidate for involvement in glaucoma’s pathogenesis. To investigate this possibility, we extended previous microarray findings to the protein level. Consistent with previous studies, ANGPTL7 protein was increased in response to either DEX or TGFβ treatment of TM cells, and TM cell lines transfected with ANGPTL7 expressed more type I collagen. ANGPTL7 protein concentration was elevated in AH from patients with glaucoma and correlated with disease progression in the beagle model of POAG, supporting the possibility that ANGPTL7 is involved in the pathogenesis of glaucoma.
Methods
Tissue Samples
All human samples were obtained in accordance with provisions of the Declaration of Helsinki, under approval of Institutional Review Board of Vanderbilt University Medical Center. Canine AH was obtained in compliance with the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research and with protocols approved by the Institutional Animal Care and Use Committees of the University of Florida, the University of Pennsylvania, and Vanderbilt University.
Stimulation of Primary TM Cell Lines with DEX and TGFβ
The human primary TM cell lines TM86 and TM61, provided by W. Daniel Stamer, PhD, University of Arizona (Tucson, AZ), were cultured as described.27 Confluent layers of cells (passages 4 and 5) in wells of 48-well plates in 250 μL medium were used. For TGFβ experiments, the cells were rinsed several times with serum-free DMEM before addition of either vehicle control, TGFβ1 or TGFβ2 (both at 10 ng/mL) or no addition. A concentration of 10 ng/mL TGFβ2 has been shown to stimulate submaximal production of fibronectin and plasminogen activator inhibitor-1 in cultured TM cells.28 TGFβ1 and -β2 (R&D Systems, Minneapolis, MN) were solubilized in vehicle consisting of 4 mM HCl in endotoxin-tested water containing 1 mg/mL low endotoxin and fatty-acid-free albumin (Sigma-Aldrich, St. Louis, MO). After 48 hours, media were collected and centrifuged and supernatants stored at −20°C. The cells were harvested by trypsinization, pelleted, and re-suspended in 100 μL media and counted. For DEX experiments, DMEM/10% FBS was added, containing either 100 nM DEX solubilized in absolute ethanol (Sigma-Aldrich) or vehicle (ethanol, 1:1000), or no addition, with replenishment of media and additions every 2 days. After 10 days, the cell layers were rinsed several times with serum-free DMEM and then additions continued in serum-free DMEM. After 48 hours, the media were harvested as in the TGFβ experiments. The variation in number of cells per well was <20%. For Western blot analysis, 40 μL of cell supernatant was loaded for each sample.
Corneoscleral Explant Culture
Corneoscleral explants were cultured based on previous work.29 Corneal button rims left over from cornea transplants and free of ocular diseases were sliced sagittally into approximately equal-sized pieces (~8 mm along radial axis), which included scleral tissue posterior to the limbus, the TM, and ~3 mm cornea anterior to the limbus. The explants were placed in 48-well plates containing 0.5 mL DMEM with 876 mg/mL L-glutamine, including antibiotic/antimycotic mixture for the first 4 days and cultured in a humidified 37°C incubator with 5% CO2, with media changes every 2 days. After 6 to 8 days, the medium was harvested (day 0) and replaced with medium containing either 10 ng/mL TGFβ1 or 100 nM DEX, or the appropriate vehicle controls. The medium was harvested and replaced with fresh medium, with additions every 48 hours. Medium was centrifuged and supernatants stored at −20°C. The explants appeared viable as assessed by H&E staining of tissue fixed at the end of experiments. Equal volumes (40 μL) of supernatant were used for Western blot analysis.
Western Blot Detection of ANGPTL7
Samples were mixed with 6× Laemmli SDS sample buffer, with or without 14 mM β-mercaptoethanol (β-ME), and loaded into wells of 1-mm-thick, 10% or 12% precast polyacrylamide gels (Bio-Rad, Hercules, CA). After electrophoretic separation, the proteins were transferred to PVDF membrane. The resultant blots were blocked in PBS/1% casein for 1 hour, incubated overnight with 0.2 μg/mL goat anti-human ANGPTL7 antibody AF914 (R&D Systems) at 4°C, washed four times with PBS/0.1% Tween-20, and incubated with 0.4 μg/mL rabbit anti-goat IgG antibody conjugated to Alexa Fluor 680 (Invitrogen, Carlsbad, CA) in PBS, 1% casein, 0.1% Tween-20 and 0.02% SDS for 45 minutes, washed three times with PBS and 0.1% Tween-20, and two times with PBS, and dried. Blots were imaged and band intensities and molecular weights determined using an infrared imaging system (Odyssey; Li-Cor Biosciences, Lincoln, NE).
Transfection of Immortalized TM Cell Lines
The stably transformed human TM cell lines NTM-5 and GTM-3, provided by Abbot F. Clark, PhD, Alcon Laboratories (Fort Worth, TX), and the immortalized human TM cell line iHTM,30 provided by Vincent Raymond, PhD, Laval University (Québec City, Canada) were cultured as described.31,32 Two different constructs were used: one with ANGPTL7 cDNA obtained through the Harvard Institute of Proteomics (Boston, MA) subcloned into the pLP-CMVneo vector (BD-Clontech, Mountain View, CA), the other with ANGPTL7 cDNA subcloned into the pCMV6-Neo vector (Origene Technologies, Rockville, MD). Transfection-quality vector DNA was prepared with an endotoxin-free kit (Maxi-prep; Qiagen, Valencia, CA) and inserts verified by DNA sequencing. Transfections were performed in serum-free medium (OptiMEM) with a transfection reagent (Lipofectamine 2000; Invitrogen). For transient transfections, cell supernatant was harvested 24 to 48 hours after transfection for anti-ANGPTL7 Western blot analysis. To generate stable lines, we cultured the cells in medium containing 1 mg/mL G418 for several weeks after transfection, resulting in four separate lines of ANGPTL7 and four of vector-transfected lines. Expression of ANGPTL7 was confirmed by Western blot of the cell supernatants.
Immunocytochemistry of Collagens
Cell lines stably transfected with ANGPTL7 or empty vector were plated in eight-well plastic chamber slides and allowed to reach confluence. The cells were then rinsed with ice-cold PBS; fixed in methanol for 5 minutes at −20°C; rinsed with PBS, blocked for 1 hour with PBS and 2% goat serum, incubated with 20 μg/mL of rabbit anti-collagen type I, rabbit anti-collagen type V antibodies (Rockland, Gilbertsville, PA), or purified rabbit IgG (Jackson ImmunoResearch, West Grove, PA) for 1 hour at 4°C; washed; and incubated 1 hour with 5 μg/mL goat anti-rabbit IgG conjugated to Alexa Fluor 594 (Invitrogen). The slides were imaged with a microscope (model AX70; Olympus, Center Valley, PA) equipped for fluorescence microscopy and a digital camera controlled with the accompanying software (Spot; Diagnostic Instruments, Sterling Heights, MI).
Immunohistochemistry
Human cadaveric eyes obtained through the National Disease Research Interchange (NDRI, Philadelphia, PA) and Michael P. Fautsch, PhD, Mayo Clinic College of Medicine (Rochester, MN), and normal canine eyes were fixed in 4% paraformaldehyde (PFA), embedded in paraffin, and cut into 5-μm-thick sections. Sections were placed on glass slides, deparaffinated, and rehydrated through graded alcohols. After treatment with 3% hydrogen peroxide and blocking with 5% normal rabbit serum (Jackson ImmunoResearch, West Grove, PA), sections were incubated overnight at 4°C with 0.5 μg/mL of either goat anti-human ANGPTL7 antibody AF914 (R&D Systems) or purified goat IgG (Jackson ImmunoResearch). Immunodetection was performed with biotinylated rabbit anti-goat secondary antibody and peroxidase-labeled streptavidin (Jackson ImmunoResearch), with a red chromogen (Nova Red; Vector Laboratories, Burlingame, CA). The slides were then counterstained in hematoxylin. Phase-contrast microscopy was preformed with the microscope (model AX70; Olympus), camera and software as described earlier.
Western Blot Analysis of TM and Cornea Tissue Extracts
TM and cornea tissue from the limbal region were microdissected from corneal button rims under a dissecting microscope. The protein was extracted by mincing tissue in extraction buffer (150 mM LiCl, 50 mM Tris/pH 7.5, 1 mM dithiothreitol, 1% lithium dodecyl sulfate, and protease inhibitors), triturated through a 26-gauge needle, followed by removal of insoluble debris by centrifugation. A fluorescence-based protein assay33 was used to detect total protein concentration in duplicate 5-μL samples (Nano-orange Protein Assay; Invitrogen), according to the manufacturer’s protocol. Fluorescence was read on a fluorescence microplate reader (Spectra Max M2; Molecular Devices, Sunnyvale, CA). An equal amount of protein per lane was loaded for each sample for anti-ANGPTL7 Western blot analysis, as just described. The blots were reprobed with a mouse anti-glyceraldehyde-3-phosphate dehydrogenase (GAPDH) antibody (MAB374; Chemicon International, Temecula, CA) used at 1:300 dilution, followed by detection with an infrared dye-conjugated goat anti-mouse IgG antibody (IRDye 800; Rockland) used at 1:10,000 and scanned with an infrared scanner (Odyssey; Li-Cor Biosciences), as described earlier.
Human and Canine AH
Human AH (50–150 μL) was obtained at the time of cataract or glaucoma procedures and stored at −80°C. All samples were obtained by a single surgeon (RK) with consistent technique, placed immediately in sealed sterile tubes and frozen. Canine AH was withdrawn from laboratory-quality mongrel dogs of various ages and from affected dogs in the POAG beagle colony at 6-month intervals. POAG disease status was evaluated by measurement of IOP, examination of the iridocorneal angle by gonioscopy, slit lamp examination of the anterior segment, and ophthalmoscopic examination of the optic nerve34,35 and scored as very mild, mild, mild-moderate, or moderate. IOP was measured with a handheld tonometer (Tonopen; Medtronic, Jacksonville, FL) with the dogs in the sitting position under topical anesthesia. Protein concentration was determined by protein assay (Nano-orange; Invitrogen), as described earlier. To compare concentration of ANGPTL7, we used equal volumes of AH (20 μL) of each sample for anti-ANGPTL7 Western blot analysis.
Results
Secretion of ANGPTL7 Protein by Cultured TM Cells and Corneoscleral Explants
To extend previous findings at the mRNA level,8,9 we stimulated primary human TM cells with DEX, TGFβ1, or TGFβ2, to determine whether ANGPTL7 protein is also increased by these stimuli. Since ANGPTL7 is secreted,26 we looked at protein released into the medium by Western blot analysis. Stimulation for 12 days with 100 nM DEX, or for 2 days with 10 ng/mL of either TGFβ1 or -β2 resulted in doublets of immunoreactive bands with mobilities of approximately 45 and 48 kDa (Figs. 1A, 1B). After cleavage of a predicted amino-terminal signal, ANGPTL7 would be a 320-amino-acid protein with the predicted molecular weight of 37 kDa. The higher than predicted apparent molecular weights of the bands in our study are consistent with a previous anti-ANGPTL7 Western blot analysis with a different antibody.26 The distinct doublets separated by ~3 kDa in our Western blot analysis could be glycosylated and nonglycosylated forms, since ANGPTL7 has three potential N-glycosylation sites, although other modifications are possible.
Figure 1.
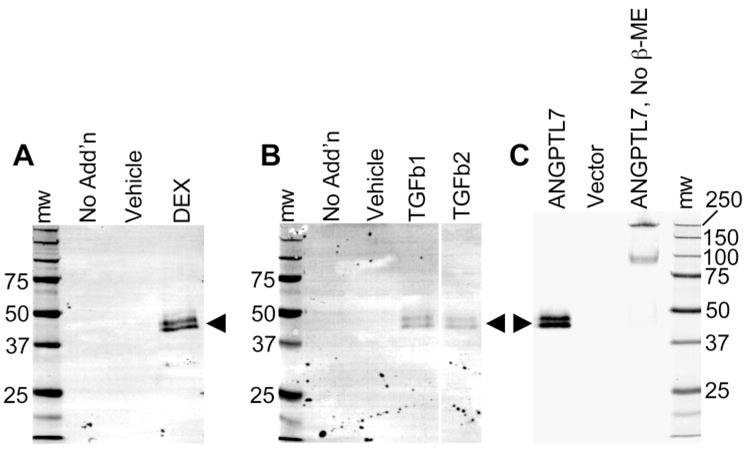
ANGPTL7 protein was secreted by primary human TM cells stimulated by DEX, TGFβ1, or TGFβ2. A goat antibody against human ANGPTL7 was used for Western blot analysis of medium collected from primary human TM cells in culture (A, B) treated for 12 days with no addition, vehicle only, or 100 nM DEX (A) or for 2 days either no addition, vehicle only, or 10 ng/mL TGFβ1 or TGFβ2 (B), as labeled for each lane. To confirm antibody specificity, Western blot was performed on culture medium from immortalized TM cell lines (NTM-5 shown) transiently transfected with either vector containing cDNA encoding ANGPTL7 (ANGPTL7) or empty vector (Vector) (C). (A–C, arrowheads): doublet bands of approximately 45 and 48 kDa, corresponding to ANGPTL7 protein. Under nonreducing conditions, higher molecular weight bands of 95 and 250 kDa were seen (C; ANGPTL7, No β-ME), corresponding to nonreduced multimers of ANGPTL7. The volume of cell culture medium loaded in each lane was 40 μL for TGFβ and DEX stimulations (A, B) and 2.5 μL for transient transfections (C). The DEX and TGFβ stimulation experiments (A, B) were repeated three to five times with two different primary TM cell lines, and TGFβ stimulation repeated twice with an immortalized TM cell line (GTM-3); transient transfections (C) were repeated twice each with three different immortalized cell lines, all with the same results as shown.
To test specificity of the antibody used in our experiments, we transiently transfected immortalized TM cell lines with expression vectors containing ANGPTL7 cDNA or empty vector, and performed anti-ANGPTL7 Western blot analysis on culture medium conditioned by the cells. No immunoreactive bands were detected in medium from vector-only transfected cells, whereas doublet bands appeared in medium from ANGPTL7-transfected cells (Fig. 1C, ANGPTL7), identical with that observed in response to DEX or TGFβ stimulation of primary TM cell lines (Figs. 1A, 1B), confirming specificity of the antibody for ANGPTL7. For further biochemical confirmation, we ran Western blot analysis after SDS-PAGE under nonreducing conditions, because previous work showed that ANGPTL7 forms multimers dependent on disulfide bonds.26 Similarly, we found immunoreactive bands at approximately 95 and 250 kDa in the absence of reducing agent (Fig. 1C, ANGPTL7, No β-ME), which probably correspond to nonreduced multimers of ANGPTL7. We conclude that DEX, TGFβ1, and TGFβ2 induce secretion of ANGPTL7 protein by primary TM cells in culture.
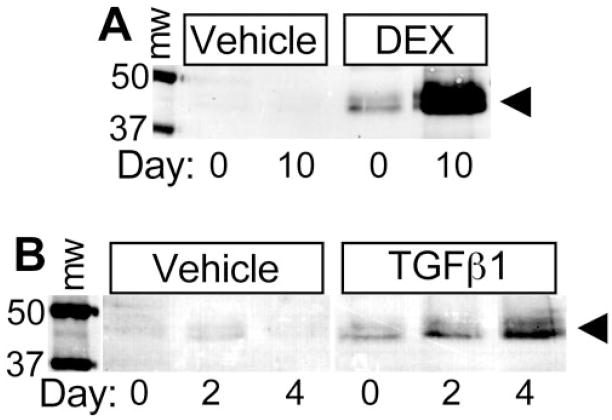
To see whether these findings would apply to intact tissue, we stimulated corneoscleral explants with DEX or TGFβ1 and measured ANGPTL7 released into the medium by Western blot analysis. ANGPTL7 protein was increased in medium from explants stimulated with DEX or TGFβ1, relative to those treated with the corresponding vehicle control (Fig. 2). These results suggest that, as with TM cell monolayers, TGFβ or DEX increases ANGPTL7 protein expression in intact corneoscleral explants.
Figure 2.
Secretion of ANGPTL7 protein by human corneoscleral explants is enhanced by DEX and TGFβ1. Corneoscleral explants were cultured in serum-free DMEM, with culture medium changed every 2 days. On day 0, the media were collected and replaced with media containing either vehicle control or 100 nM DEX for 10 days (A) or 10 ng/mL TGFβ1 for 2 to 4 days (B). ANGPTL7 protein in the medium from days 0 and 10 for DEX or vehicle (A) or days 0, 2, and 4 for TGFβ1 or vehicle (B) was determined by Western blot (40 μL medium in each lane). Molecular weight markers are shown in kilodaltons. Arrowhead: doublet bands of ANGPTL7 are indicated. The results are representative of three independent experiments with tissue from three different donors.
Effect of ANGPTL7 Overexpression on Type I Collagen Expression by Immortalized TM Cell Lines
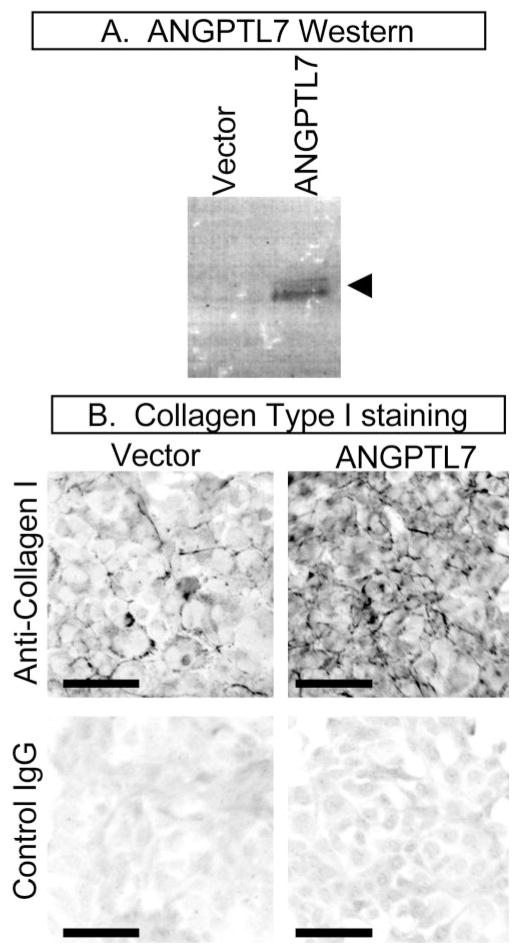
It has been shown that melanoma cells transfected with ANGPTL7 express more collagens type I and V.26 To determine whether this result extends to TM cells, we stably transfected immortalized human TM cell lines with expression vectors containing either cDNA for ANGPTL7, or no additional insert (empty vector). As detected by Western blot analysis of cell culture medium, ANGPTL7 protein expression was greater for ANGPTL7-transfected cells, compared with cells transfected with empty vector (Fig. 3A).
Figure 3.
Stable transfection of immortalized TM cell lines caused increased type I collagen expression. The immortalized cell line iHTM was transfected with either empty expression vector (Vector) or expression vector containing cDNA encoding ANGPTL7 (ANGPTL7). After antibiotic selection, overexpression of ANGPTL7 protein was confirmed by Western blot analysis of 40 μL of cell culture medium (A), and immunocytochemistry was preformed on confluent monolayers using an antibody against collagen type I (Anti-collagen I) or negative control IgG (Control IgG) (B). (A, arrowhead): ANGPTL7-reactive doublet bands of 45 and 48 kDa. Staining for type I collagen was greatest for ANGPTL7-transfected cells (B, top right) compared with vector-transfected cells (B, top left). Data are representative of six experiments with GTM-3 and iHTM cells. Scale bars, 50 μm.
To investigate whether overexpression of ANGPTL7 protein affects collagen expression, immunocytochemistry was performed on the stably transfected cell lines using either an anti-collagen type I antibody or negative control IgG (Fig. 3B). Immunoreactivity for collagen type I was stronger for ANGPTL7-transfected cell lines (Fig. 3B, top right) compared with cell lines transfected with empty vector (Fig. 3B, top left). Staining for collagen type V showed no obvious difference between the cell lines (data not shown). These experiments suggest that a modest increase in expression of ANGPTL7 results in increased expression of type I collagen by immortalized TM cells. Overall, these results support the previous findings that increased expression of ANGPTL7 could lead to increased expression of extracellular matrix, which is a plausible disease mechanism for glaucoma.
ANGPTL7 Protein Expression in Human Eyes
ANGPTL7 was originally identified as cornea-specific,25 but its mRNA is also expressed by TM cells in culture8,9,11,36,37 and in human TM tissue.36 A search of the current NEIbank cDNA expression libraries (http://neibank.nei.nih.gov/index.shtml/National Eye Institute, Bethesda, MD) revealed significant expression of ANGPTL7 mRNA in the whole eye, cornea, and TM libraries, but almost no detectable mRNA expression of any other ANGPTL family members.
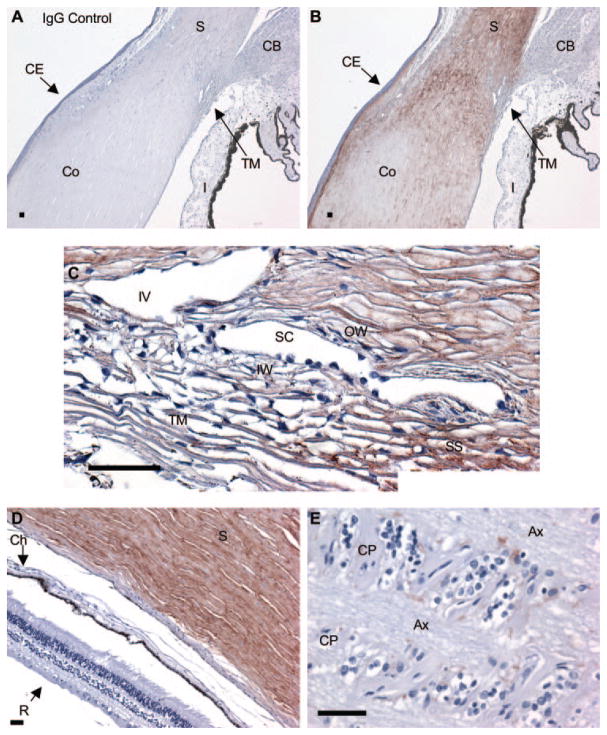
To determine the distribution of ANGPTL7 protein, we performed anti-ANGPTL7 immunohistochemistry with hematoxylin counterstaining on sagittal sections of normal cadaveric human eyes (Fig. 4). In all experiments, staining with ANGPTL7 antibody (Figs. 4B–E) was compared to staining with goat IgG as negative control, which resulted only in blue staining from the hematoxylin counterstain, with no positive immunoreactivity (Fig. 4A).
Figure 4.
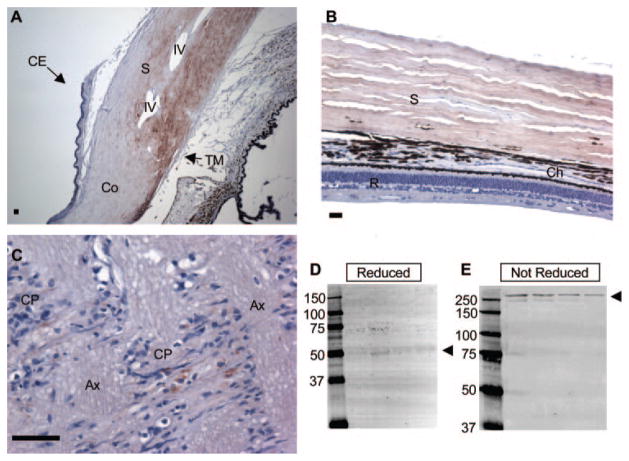
ANGPTL7 protein expression in human eyes. Immunohistochemistry was performed on sections of paraformaldehyde-fixed human cadaveric eyes using negative control IgG (A) or anti-ANGPTL7 antibody (B–E) and detection with red substrate followed by hematoxylin counterstain. Brown: positive immunoreactivity; blue: hematoxylin counterstain. Staining with negative control IgG resulted in no detectable immunoreactivity (A). Low-power view of the angle region showing ANGPTL7 expression in the sclera (S) and cornea (Co), with strongest staining in the limbus, decreasing toward the cornea (B). The Iris (I), ciliary body (CB), and corneal epithelium (CE) were negative for ANGPTL7 (B). Higher-power view of TM and Schlemm’s canal (SC) region, including scleral spur (SS) and intrascleral vein (IV), showing positive reactivity for ANGPTL7 in the inner wall (IW) and outer wall (OW) of Schlemm’s canal (C). Positive ANGPTL7 immunoreactivity was found in the sclera (S), but the retina (R) and choroid (Ch) were negative (D). Positive ANGPTL7 staining was found in the cribriform plates (CP) of the lamina cribrosa (E). Ax, axon bundles. Scale bar, 50 μm.
ANGPTL7 immunoreactivity (brown staining) was apparent in the corneal stroma near the limbus, but decreased in intensity toward the central cornea (Fig. 4B). In the anterior cornea, a band of strong staining was found in the region of Bowman’s layer. ANGPTL7 immunoreactivity appeared in the TM, but at lower intensity than in the adjacent scleral spur (Fig. 4C). Endothelial cells lining Schlemm’s canal stained positive for ANGPTL7 (Fig. 4C). Strong staining was detected throughout the entire sclera (Figs. 4B, 4D). In the optic nerve, ANGPTL7 immunoreactivity was localized to the cribriform plates (Fig. 4E). ANGPTL7 expression was not detected in the ciliary body, iris, corneal epithelium (Fig. 4B), retina, or choroid (Fig. 4D).
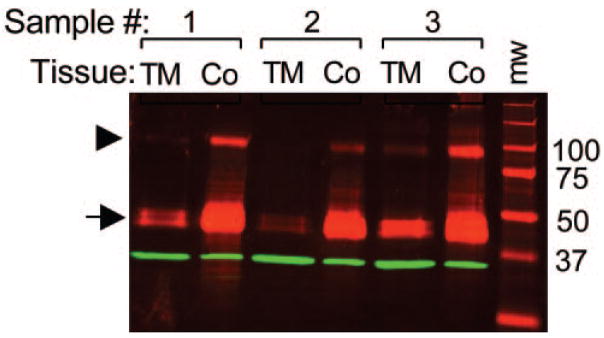
As a validation of the immunohistochemistry results, we used Western blot analysis to test the finding that ANGPTL7 expression is higher in the cornea than in the TM. Protein extracts were made from TM and corneal tissue near the limbus dissected from corneal button rims for anti-ANGPTL7 Western blot analysis, with equal amounts of protein loaded in each lane. Consistent with the immunohistochemistry, ANGPTL7 doublet bands were more intense for corneal protein extracts than for extracts of TM tissue (Fig. 5).
Figure 5.
ANGPTL7 protein expression was higher in the cornea compared with the TM, consistent with immunohistochemistry results. Anti-ANGPTL7 Western blot analysis was performed on 3 μg of protein extracted from TM or cornea tissue (lanes labeled TM or Co, respectively) microdissected from three different samples of cadaveric human tissue (labeled samples 1–3). Rightmost lane: molecular weight in kilodaltons. ANGPTL7 immunoreactivity and the molecular weight standard appear as red fluorescence. Arrow: ANGPTL7 doublet; arrowhead: nonreduced dimmers. As a control for equal protein loading, the blot was reprobed with anti-GAPDH antibody. Anti-GAPDH immunoreactivity appears as green fluorescence at the expected molecular weight of 36 kDa and indicates approximately equal protein loading.
Elevation of ANGPTL7 Protein Concentration in Human Glaucomatous AH
Since we found that TGFβ2 treatment of TM cells caused increased secretion of ANGPTL7 into the culture medium, and because previous work showed that ANGPTL7 mRNA is upregulated in the TM from perfused anterior segments exposed to elevated pressure (Comes N, et al. IOVS 2006;47:ARVO E-Abstract 1853), we predicted that ANGPTL7 expression would be higher in glaucoma patients which tend to have elevated IOP and TGFβ2. Since ANGPTL7 is a secreted protein, we predicted further that the concentration of ANGPTL7 would be higher in AH of patients with glaucoma compared with control specimens.
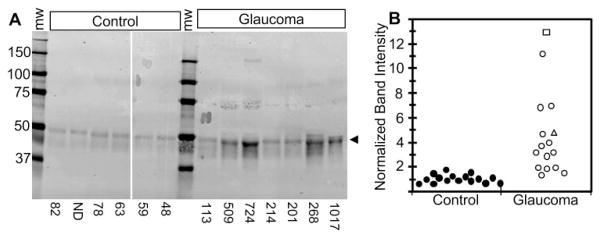
To compare the concentration of ANGPTL7 protein in AH, Western blot analyses were performed on equal volumes (20 μL) of AH from at least five control patients and five patients with glaucoma in each gel. ANGPTL7 was detectable in all control and glaucoma samples as diffuse bands of ~48 kDa (Fig. 6A). In all gels, bands from control samples were faint, whereas bands from most glaucoma samples were darker. In a few glaucoma samples with dark bands, doublets could be seen. Band intensities from several gels were normalized and combined (Fig. 6B). The median band intensity was ~3-fold higher for AH from patients with POAG compared with control samples.
Figure 6.
ANGPTL7 protein concentration was elevated in AH from POAG patients. Anti-ANGPTL7 Western blot was performed on equal volumes of AH (20 μL) from patients without glaucoma undergoing cataract procedures (Control, n = 16) or from patients with glaucoma undergoing glaucoma or cataract procedures (Glaucoma, n = 16). Three separate gels were run, each containing samples from five to eight control subjects and five to eight patients with glaucoma. A representative gel is shown (A). Within each gel, band intensities were normalized to the average of the control band intensities, allowing for comparison between all control (filled symbols) and glaucomatous (open symbols) AH (B). The difference between normalized band intensities for control (1.0 ± 0.33, mean ± SD) and POAG AH (3.8 ± 2.76) was significant (P < 0.001, two-tailed student’s t-test). The ages of the patients ranged from 48 to 84 years (69.4 ± 15.6, mean ± SD) for control and 40 to 93 years (71.4 ± 15.6) for glaucoma. (○) patients with POAG; (□) a patient with uveitic glaucoma treated with topical steroids; (△) patient with pigmentary glaucoma. The total protein concentration (micrograms per milliliter) is listed at the bottom of each lane. ND, not determined.
The total protein concentration in AH was found to be higher for POAG eyes, ranging from 92 to 1126 μg/mL (median = 224 μg/mL), compared with control eyes, which ranged from 48 to 84 μg/mL (median = 70 μg/mL). These protein concentrations are similar to those reported previously,38 validating the method of AH collection and confirming the generalized increase in total protein in glaucomatous AH. No significant correlation was detected between normalized band intensity and total protein concentration of the glaucoma patient samples (r = 0.35, P > 0.05). From these data, we conclude that the concentration of ANGPTL7 protein is elevated in AH from patients with glaucoma, consistent with elevated expression of this secreted protein.
Increase in ANGPTL7 Protein Concentration with Disease Progression in the Beagle POAG Model
The familial glaucoma colony of beagles was established from a single founder and first reported in 1972.39 Glaucoma in the beagle model is inherited as an autosomal recessive trait35 characterized by an open iridocorneal angle40 and bilateral increases in IOP beginning at 9 to 18 months of age,34 due to decreased outflow facility of the AH.41 Optic nerve cupping,34 loss of large-diameter optic nerve axons,42 and vision loss occur as disease progresses. In advanced stages of the disease, narrowing and closing of the iridocorneal angle occurs, due to lens subluxation.39 This well-established beagle model of human POAG offers a significant advantage because, unlike humans, the dogs are not receiving antiglaucoma medications at the time of the experiment. In addition, it is possible to sample AH from the POAG beagles at various times during the course of the disease.
Since canine ANGPTL7 is 97% identical with the human protein, we performed immunohistochemistry on canine tissue and Western blot analysis of canine AH, using the anti-human ANGPTL7 antibody. The ANGPTL7 staining pattern for canine eyes (Figs. 7A–C) was very similar to that in human eyes (Fig 4). As with human tissue, ANGPTL7 staining (brown) was strong in the limbal area of the cornea, with gradual reduction in staining intensity toward the central cornea (Fig. 7A). Also similar to humans, ANGPTL7 staining was found throughout the sclera, but not in the corneal epithelium (Fig. 7A), retina, or choroid (Fig. 7B). Within the canine optic nerve, ANGPTL7 immunoreactivity was found within the cribriform plates, similar to the staining pattern in the human optic nerve (Fig. 4E). No immunoreactivity was observed with negative control IgG substituted for the anti-ANGPTL7 antibody (data not shown). In Western blot analysis of canine AH, the antibody against human ANGPTL7 recognized canine ANGPTL7 under reducing conditions as immunoreactive bands of ~50 kDa, similar to human AH (Fig. 7D, cf. Fig. 6A) and corresponding to monomers of canine ANGPTL7. However, the bands were faint and diffuse under reducing conditions and difficult to detect. Under nonreducing conditions, prominent well-defined bands of ~250 kDa and fainter bands of ~210 kDa were detected (Fig. 7E), indicative of multimers of ANGPTL7. This is expected, since ANGPTL7 and other ANGPTL family members form disulfide-linked multimers which, under nonreducing conditions, display several high molecular weight bands corresponding to various degrees of oligomerization.17,24,26 The shift in molecular weight is similar to human ANGPTL7 immunoreactivity in ANGPTL7-transfected TM cells, which showed 48-kDa bands in the presence of reducing reagent and a ~250-kDa band without it (Fig. 1C).
Figure 7.
ANGPTL7 protein was expressed in canine eyes and AH. Anti-ANGPTL7 immunohistochemistry was performed on sections from normal dog eyes (A–C). Brown: ANGPTL7 immunoreactivity; blue: hematoxylin counterstain. Low-power view of the angle shows expression of ANGPTL7 in the limbal area of the cornea, decreasing toward the central cornea (A). ANGPTL7 is expressed in the sclera (S), but the retina (R) and choroid (Ch) were negative (B). In the lamina cribrosa, the cribriform plates (CP) stained positive for ANGPTL7 (C). ANGPTL7 Western blot was performed on AH from control dogs without eye disease, after separation by SDS-PAGE under reducing (D) or nonreducing (E) conditions. Four separate samples (20 μL/lane) are shown for each condition, with molecular weight markers in the leftmost lane indicated in kDa. Arrowhead: ANGPTL7 immunoreactive bands. CE, corneal epithelia; IV, intrascleral veins; Ax, axon bundles. Scale bar, 50 μm.
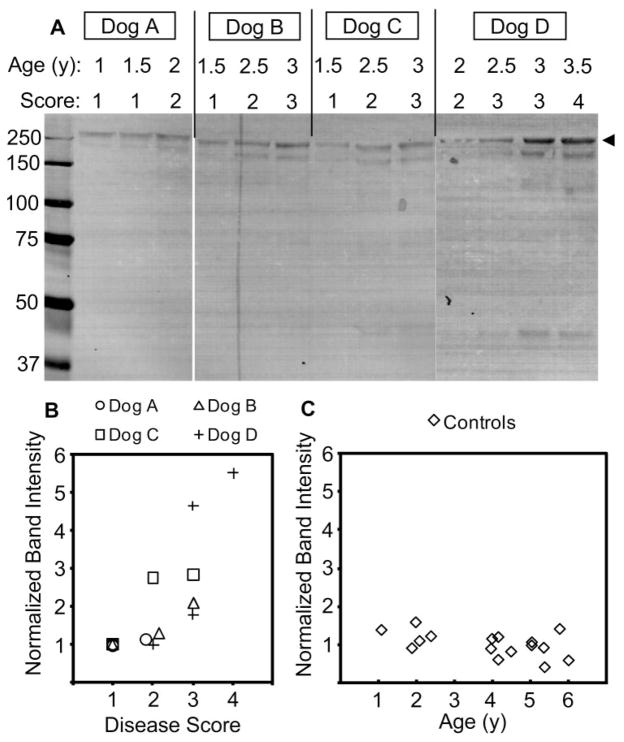
To determine the relationship between the concentration of ANGPTL7 protein and disease progression in the POAG beagles, we performed Western blot analysis with equal volumes (20 μL) of AH from POAG beagles (Fig. 8A). AH samples were obtained from individual dogs at 6-month intervals as disease progressed from very mild to moderate glaucoma, as assessed by clinical examination. To ensure reliable detection of ANGPTL7 with the limited volume of AH available at each time point, we separated the samples by SDS-PAGE under nonreducing conditions, which resulted in stronger band immunoreactivity compared with reducing conditions (Figs. 7D, 7E). In each of the four POAG beagles examined, the intensity of the ANGPTL7 bands increased as disease progressed. For comparison, the intensities of the upper 250-kDa band were quantified and normalized to the intensity of the earliest AH sample collected from each dog (Fig. 8B).
Figure 8.
Increased ANGPTL7 protein concentration in canine AH correlated with disease progression in the beagle POAG model. Equal volumes (20 μL) of AH humor sampled from POAG beagle dogs at 6-month intervals was separated by nonreducing SDS-PAGE, followed by anti-ANGPTL7 Western blot analysis (A). At each AH sampling, the dogs were examined and scored for severity of glaucoma (1, very mild; 2, mild; 3, mild-moderate; and 4, moderate). Leftmost lane: molecular weight in kilodaltons. The band intensities were quantified and normalized to the band intensity of the earliest sample for each dog (B). The top band (A, arrowhead) was chosen for quantification. The age-dependent increase in ANGPTL7 was determined by anti-ANGPTL7 Western blot analysis of equal volumes (20 μL) of AH obtained from healthy laboratory-quality mongrel dogs aged 1 to 6 years and band intensities normalized to the average band intensity for the gel (C).
To see whether the increase of ANGPTL7 protein was an effect of aging, independent of glaucoma disease status, we performed anti-ANGPTL7 Western blot analysis on AH collected from healthy laboratory-quality mongrel dogs of various ages. ANGPTL7 band intensities for these samples were normalized to the average band intensity within a single gel containing 16 samples from dogs aged 1 to 6 years. No obvious trend toward increased ANGPTL7 concentration with age was seen (Fig. 8C). These results suggest that increased ANGPTL7 protein concentration correlates with POAG progression in the beagle model of POAG.
Correlation of Increased Expression of ANGPTL7 with Disease in a Patient with Neovascular Glaucoma
The higher concentration of ANGPTL7 in glaucomatous AH suggests increased expression of ANGPTL7, which could induce collagen deposition. Although the source of the increased ANGPTL7 in the AH is not known, localized production by the TM is possible, since we showed that TGFβ2 induces ANGPTL7 protein secretion by TM cells in vitro and elevated pressure upregulates ANGPTL7 mRNA in the TM from perfused anterior segments (Comes N, et al. IOVS 2006;47:ARVO E-Abstract 1853). To further investigate the possibility that increased expression of ANGPTL7 occurs in glaucoma, we performed anti-ANGPTL7 immunohistochemistry on eyes of patients with glaucoma and control subjects without glaucoma.
Immunohistochemical comparisons between individual patients were complicated by individual variability of expression, significant differences in tissue procurement and fixation, and the rarity of obtaining cadaveric eyes with glaucoma with a well-documented ocular history. Comparisons of ANGPTL7 immunoreactivity among three patients with glaucoma and three control samples (six eyes from each group) revealed one patient with glaucoma with clearly more intense immunostaining for ANGPTL7 in the TM, compared with control subjects (data not shown).
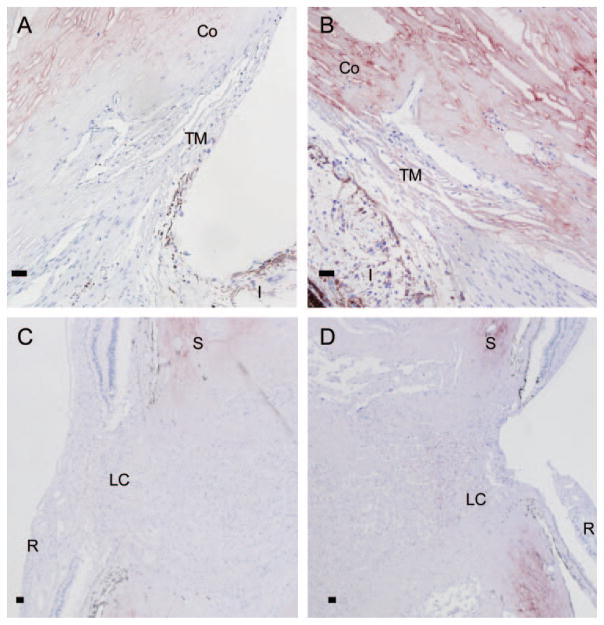
We also obtained a pair of eyes from a patient who had severely elevated IOP secondary to neovascular glaucoma in one eye, whereas the fellow eye remained unaffected. Closure of the angle was apparent, as well as characteristic glaucomatous optic nerve cupping in the affected eye (Figs. 9B, 9D). IOP was normal in the unaffected eye, which retained an open angle and normal optic nerve appearance (Figs. 9A, 9C). Anti-ANGPTL7 immunoreactivity was greater for the affected eye in both the TM (Fig. 9B) and lamina cribrosa (Fig. 9D), compared with the unaffected eye (Fig. 9A, 9C), suggesting that ANGPTL7 expression may increase with disease progression, as found in the beagle POAG model (Fig. 8). These results also suggest that the increased ANGPTL7 concentration found in the AH of patients with glaucoma (Fig. 6) may result from localized increases in ANGPTL7 expression, which could increase collagen deposition.
Figure 9.
Elevated expression of ANGPTL7 protein in human TM and lamina cribrosa of an eye with high IOP secondary to neovascular glaucoma. Anti-ANGPTL7 immunohistochemistry was performed on sections of paraformaldehyde-fixed eyes from a patient with history of highly elevated IOP secondary to neovascular glaucoma in one eye (B, D), whereas the fellow eye was unaffected (A, C). The unaffected eye had a normal open-angle appearance (A) whereas angle closure was apparent in the affected eye (B). The appearance of the optic nerve was normal in the unaffected eye (C), whereas the affected eye had obvious optic nerve cupping, characteristic of glaucomatous optic nerve damage (D). ANGPTL7 immunoreactivity (brown) was higher in the TM region (B) and in the lamina cribrosa (D) of the glaucomatous eye, compared with the unaffected eye (A, C). Blue: hematoxylin counterstain. Co, cornea; S, sclera; I, iris; LC, lamina cribrosa; R, retina. Scale bar, 50 μm.
Discussion
The purpose of this study was to investigate the possibility that ANGPTL7 is involved in glaucoma pathogenesis, as suggested by the fact that it has been identified in microarray studies as a highly induced mRNA in cultured TM cells stimulated with TGFβ or DEX.8,9,11 Our findings that ANGPTL7 protein secretion increases in response to either TGFβ or DEX treatment of TM cells and corneoscleral explants extend the previous microarray findings to the protein level and place them in a more physiological context with the intact explant tissue. Steroids can induce IOP elevation, and therefore DEX treatment of TM cells may induce changes analogous to those that occur in susceptible patients. Similarly, TGFβ2 can increase IOP28,43 and has an elevated concentration in glaucomatous AH44–48 and therefore may contribute to glaucoma. Our findings suggest that elevated TGFβ concentration or steroid treatment stimulates production of ANGPTL7 protein, which would then be secreted into the AH.
Consistent with increased expression and secretion, we found that ANGPTL7 protein concentration is elevated in AH of patients with glaucoma and increases with disease progression in the beagle model of POAG. The source of the ANGPTL7 is not known, but our histology results suggest localized production by the sclera, cornea, and TM as likely sources, since ANGPTL7 protein was expressed at significant levels by these tissues. Increased ANGPTL7 immunoreactivity in the affected eye with high IOP compared with the fellow unaffected eye of a patient with neovascular glaucoma supports increased localized expression of ANGPTL7 in glaucoma. The cause of increased ANGPTL7 protein is not known, but transcriptional regulation by TGFβ2 is a likely possibility, since TGFβ2 is elevated in the AH of patients with glaucoma44–48 and has been shown in microarray studies to increase ANGPTL7 mRNA.8,9,11
A complicating factor in studying samples of AH from patients with glaucoma is that with few exceptions, patients are receiving antiglaucoma medications, usually of more than one class and with various durations and levels of compliance. Dogs from the spontaneous POAG beagle model were not on active medication at the time of the study, and therefore the potential for confounding pharmacologic effects was greatly diminished. Another advantage of the canine model is that repeated sampling of AH at regular intervals is possible, which allowed us to follow changes occurring with documented disease progression for individual dogs. Our results from dogs of the POAG beagle colony are consistent with results from POAG patients, suggesting that the increase of ANGPTL7 protein concentration in the AH is not an artifact of anti-glaucoma medications.
ANGPTL7 has been shown to induce distorted vascular morphology and to increase the expression of proteoglycans and collagens types I and V.26 Changes in collagen occur in glaucomatous TM and may restrict the outflow of AH, causing ocular hypertension.49 Also, changes in collagen deposition in the lamina cribrosa may cause impairment of axonal transport in the optic nerve head, possibly leading to glaucomatous optic nerve damage.50 In support of this, it has been shown that transgenic mice overexpressing type I collagen develop ocular hypertension and optic nerve damage, similar to human POAG.51,52 Elevated ANGPTL7 protein concentration in glaucomatous AH, as found in this study, suggests increased expression of ANGPTL7, which could induce changes in the extracellular matrix and thereby contribute to glaucoma.
Our finding that ANGPTL7-overexpression increases immunostaining for type I collagen in TM cell lines is similar to previous findings in transfected melanoma cells.26 One implication is that responsiveness to ANGPTL7 may not be restricted to specialized cell types, but could be a more general capability. In addition to inhibiting conventional outflow, increases in collagen content of the sclera could restrict AH outflow through the uveal–scleral pathway, which could result in elevated IOP. Increased ANGPTL7 could affect collagen in the posterior segment, which could affect the biomechanical properties of the lamina cribrosa and perhaps cause increased susceptibility to mechanical damage to optic nerve axons or retinal vasculature due to elevated IOP-induced stress. In this context, it is interesting to note that ANGPTL7 mRNA was identified as highly expressed in the retina of the DBA/2J mouse glaucoma model.5
In conclusion, our findings that expression of ANGPTL7 protein was induced by either DEX or TGFβ and that the concentration of ANGPTL7 was elevated in glaucomatous AH support the hypothesis that ANGPTL7 could be involved in glaucoma pathogenesis. Our data suggest an overall hypothesis that elevated TGFβ2 levels in glaucomatous AH could cause increased ANGPTL7 expression, which in turn could induce collagen changes, or through other mechanisms, contribute to the pathogenesis of glaucoma. Further examination of the mechanism and source of increased ANGPTL7 expression and of how ANGPTL7 may contribute to glaucoma through extracellular matrix homeostasis or other mechanisms should contribute to the understanding and treatment of glaucoma.
Acknowledgments
Supported by grants from Fight for Sight and Research to Prevent Blindness, a Challenge Grant to Vanderbilt Eye Institute; National Eye Institute Grant K12-EY15398; and the Foundation Fighting Blindness.
The authors thank Jennifer Harvey for performing immunohistochemistry; Ed MacKay for help with canine samples, David Calkins for support with microscopy; our colleagues at Vanderbilt Eye Institute, Jeffrey Kammer and Laura Wayman, for kindly providing AH samples; and Uyen Tran for generously supplying cornea explant tissue.
Footnotes
Disclosure: J. Kuchtey, None; M.E. Källberg, None; K.N. Gelatt, None; T. Rinkoski, None; A.M. Komàromy, None; R.W. Kuchtey, None
References
- 1.Libby RT, Gould DB, Anderson MG, John SW. Complex genetics of glaucoma susceptibility. Annu Rev Genomics Hum Genet. 2005;6:15–44. doi: 10.1146/annurev.genom.6.080604.162209. [DOI] [PubMed] [Google Scholar]
- 2.Wiggs JL. Genetic etiologies of glaucoma. Arch Ophthalmol. 2007;125:30–37. doi: 10.1001/archopht.125.1.30. [DOI] [PubMed] [Google Scholar]
- 3.Miyahara T, Kikuchi T, Akimoto M, et al. Gene microarray analysis of experimental glaucomatous retina from cynomolgus monkey. Invest Ophthalmol Vis Sci. 2003;44:4347–4356. doi: 10.1167/iovs.02-1032. [DOI] [PubMed] [Google Scholar]
- 4.Ahmed F, Brown KM, Stephan DA, et al. Microarray analysis of changes in mRNA levels in the rat retina after experimental elevation of intraocular pressure. Invest Ophthalmol Vis Sci. 2004;45:1247–1258. doi: 10.1167/iovs.03-1123. [DOI] [PubMed] [Google Scholar]
- 5.Steele MR, Inman DM, Calkins DJ, Horner PJ, Vetter ML. Microarray analysis of retinal gene expression in the DBA/2J model of glaucoma. Invest Ophthalmol Vis Sci. 2006;47:977–985. doi: 10.1167/iovs.05-0865. [DOI] [PubMed] [Google Scholar]
- 6.Vittitow J, Borras T. Genes expressed in the human trabecular meshwork during pressure-induced homeostatic response. J Cell Physiol. 2004;201:126–137. doi: 10.1002/jcp.20030. [DOI] [PubMed] [Google Scholar]
- 7.Vittal V, Rose A, Gregory KE, Kelley MJ, Acott TS. Changes in gene expression by trabecular meshwork cells in response to mechanical stretching. Invest Ophthalmol Vis Sci. 2005;46:2857–2868. doi: 10.1167/iovs.05-0075. [DOI] [PubMed] [Google Scholar]
- 8.Zhao X, Ramsey KE, Stephan DA, Russell P. Gene and protein expression changes in human trabecular meshwork cells treated with transforming growth factor-beta. Invest Ophthalmol Vis Sci. 2004;45:4023–4034. doi: 10.1167/iovs.04-0535. [DOI] [PubMed] [Google Scholar]
- 9.Lo WR, Rowlette LL, Caballero M, et al. Tissue differential microarray analysis of dexamethasone induction reveals potential mechanisms of steroid glaucoma. Invest Ophthalmol Vis Sci. 2003;44:473–485. doi: 10.1167/iovs.02-0444. [DOI] [PubMed] [Google Scholar]
- 10.Ishibashi T, Takagi Y, Mori K, et al. cDNA microarray analysis of gene expression changes induced by dexamethasone in cultured human trabecular meshwork cells. Invest Ophthalmol Vis Sci. 2002;43:3691–3697. [PubMed] [Google Scholar]
- 11.Rozsa FW, Reed DM, Scott KM, et al. Gene expression profile of human trabecular meshwork cells in response to long-term dexamethasone exposure. Mol Vis. 2006;12:125–141. [PubMed] [Google Scholar]
- 12.Bhattacharya SK, Rockwood EJ, Smith SD, et al. Proteomics reveal Cochlin deposits associated with glaucomatous trabecular meshwork. J Biol Chem. 2005;280:6080–6084. doi: 10.1074/jbc.M411233200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Katoh Y, Katoh M. Comparative integromics on angiopoietin family members. Int J Mol Med. 2006;17:1145–1149. [PubMed] [Google Scholar]
- 14.Akarsu AN, Turacli ME, Aktan SG, et al. A second locus (GLC3B) for primary congenital glaucoma (Buphthalmos) maps to the 1p36 region. Hum Mol Genet. 1996;5:1199–1203. doi: 10.1093/hmg/5.8.1199. [DOI] [PubMed] [Google Scholar]
- 15.Kubota Y, Oike Y, Satoh S, et al. Cooperative interaction of angiopoietin-like proteins 1 and 2 in zebrafish vascular development. Proc Natl Acad Sci USA. 2005;102:13502–13507. doi: 10.1073/pnas.0501902102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Galaup A, Cazes A, Le Jan S, et al. Angiopoietin-like 4 prevents metastasis through inhibition of vascular permeability and tumor cell motility and invasiveness. Proc Natl Acad Sci USA. 2006;103:18721–18726. doi: 10.1073/pnas.0609025103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Oike Y, Ito Y, Maekawa H, et al. Angiopoietin-related growth factor (AGF) promotes angiogenesis. Blood. 2004;103:3760–3765. doi: 10.1182/blood-2003-04-1272. [DOI] [PubMed] [Google Scholar]
- 18.Camenisch G, Pisabarro MT, Sherman D, et al. ANGPTL3 stimulates endothelial cell adhesion and migration via integrin alpha vbeta 3 and induces blood vessel formation in vivo. J Biol Chem. 2002;277:17281–17290. doi: 10.1074/jbc.M109768200. [DOI] [PubMed] [Google Scholar]
- 19.Koishi R, Ando Y, Ono M, et al. Angptl3 regulates lipid metabolism in mice. Nat Genet. 2002;30:151–157. doi: 10.1038/ng814. [DOI] [PubMed] [Google Scholar]
- 20.Koster A, Chao YB, Mosior M, et al. Transgenic angiopoietin-like (angptl)4 overexpression and targeted disruption of angptl4 and angptl3: regulation of triglyceride metabolism. Endocrinology. 2005;146:4943–4950. doi: 10.1210/en.2005-0476. [DOI] [PubMed] [Google Scholar]
- 21.Oike Y, Akao M, Yasunaga K, et al. Angiopoietin-related growth factor antagonizes obesity and insulin resistance. Nat Med. 2005;11:400–408. doi: 10.1038/nm1214. [DOI] [PubMed] [Google Scholar]
- 22.Sukonina V, Lookene A, Olivecrona T, Olivecrona G. Angiopoietin-like protein 4 converts lipoprotein lipase to inactive monomers and modulates lipase activity in adipose tissue. Proc Natl Acad Sci USA. 2006;103:17450–17455. doi: 10.1073/pnas.0604026103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ono M, Shimizugawa T, Shimamura M, et al. Protein region important for regulation of lipid metabolism in angiopoietin-like 3 (ANGPTL3): ANGPTL3 is cleaved and activated in vivo. J Biol Chem. 2003;278:41804–41809. doi: 10.1074/jbc.M302861200. [DOI] [PubMed] [Google Scholar]
- 24.Ge H, Yang G, Huang L, et al. Oligomerization and regulated proteolytic processing of angiopoietin-like protein 4. J Biol Chem. 2004;279:2038–2045. doi: 10.1074/jbc.M307583200. [DOI] [PubMed] [Google Scholar]
- 25.Peek R, van Gelderen BE, Bruinenberg M, Kijlstra A. Molecular cloning of a new angiopoietinlike factor from the human cornea. Invest Ophthalmol Vis Sci. 1998;39:1782–1788. [PubMed] [Google Scholar]
- 26.Peek R, Kammerer RA, Frank S, Otte-Holler I, Westphal JR. The angiopoietin-like factor cornea-derived transcript 6 is a putative morphogen for human cornea. J Biol Chem. 2002;277:686–693. doi: 10.1074/jbc.M105746200. [DOI] [PubMed] [Google Scholar]
- 27.Stamer WD, Seftor RE, Williams SK, Samaha HA, Snyder RW. Isolation and culture of human trabecular meshwork cells by extracellular matrix digestion. Curr Eye Res. 1995;14:611–617. doi: 10.3109/02713689508998409. [DOI] [PubMed] [Google Scholar]
- 28.Fleenor DL, Shepard AR, Hellberg PE, et al. TGFbeta2-induced changes in human trabecular meshwork: implications for intraocular pressure. Invest Ophthalmol Vis Sci. 2006;47:226–234. doi: 10.1167/iovs.05-1060. [DOI] [PubMed] [Google Scholar]
- 29.Acott TS, Kingsley PD, Samples JR, Van Buskirk EM. Human trabecular meshwork organ culture: morphology and glycosamino-glycan synthesis. Invest Ophthalmol Vis Sci. 1988;29:90–100. [PubMed] [Google Scholar]
- 30.Polansky JR, Weinreb RN, Baxter JD, Alvarado J. Human trabecular cells. I. Establishment in tissue culture and growth characteristics. Invest Ophthalmol Vis Sci. 1979;18:1043–1049. [PubMed] [Google Scholar]
- 31.Zhang X, Clark AF, Yorio T. Regulation of glucocorticoid responsiveness in glaucomatous trabecular meshwork cells by glucocorticoid receptor-beta. Invest Ophthalmol Vis Sci. 2005;46:4607–4616. doi: 10.1167/iovs.05-0571. [DOI] [PubMed] [Google Scholar]
- 32.Gobeil S, Rodrigue MA, Moisan S, et al. Intracellular sequestration of hetero-oligomers formed by wild-type and glaucoma-causing myocilin mutants. Invest Ophthalmol Vis Sci. 2004;45:3560–3567. doi: 10.1167/iovs.04-0300. [DOI] [PubMed] [Google Scholar]
- 33.Jones LJ, Haugland RP, Singer VL. Development and characterization of the NanoOrange protein quantitation assay: a fluorescence-based assay of proteins in solution. BioTechniques. 2003;34:850–854. 856, 858. doi: 10.2144/03344pt03. [DOI] [PubMed] [Google Scholar]
- 34.Gelatt KN, Peiffer RL, Jr, Gwin RM, Gum GG, Williams LW. Clinical manifestations of inherited glaucoma in the beagle. Invest Ophthalmol Vis Sci. 1977;16:1135–1142. [PubMed] [Google Scholar]
- 35.Gelatt KN, Gum GG. Inheritance of primary glaucoma in the beagle. Am J Vet Res. 1981;42:1691–1693. [PubMed] [Google Scholar]
- 36.Liton PB, Luna C, Challa P, Epstein DL, Gonzalez P. Genome-wide expression profile of human trabecular meshwork cultured cells, nonglaucomatous and primary open angle glaucoma tissue. Mol Vis. 2006;12:774–790. [PMC free article] [PubMed] [Google Scholar]
- 37.Tomarev SI, Wistow G, Raymond V, Dubois S, Malyukova I. Gene expression profile of the human trabecular meshwork: NEIBank sequence tag analysis. Invest Ophthalmol Vis Sci. 2003;44:2588–2596. doi: 10.1167/iovs.02-1099. [DOI] [PubMed] [Google Scholar]
- 38.Tripathi RC, Borisuth NS, Tripathi BJ, Gotsis SS. Quantitative and qualitative analyses of transferrin in aqueous humor from patients with primary and secondary glaucomas. Invest Ophthalmol Vis Sci. 1992;33:2866–2873. [PubMed] [Google Scholar]
- 39.Gelatt KN. Familial glaucoma in the beagle dog. J Am Anim Hosp Assoc. 1972;8:23. [Google Scholar]
- 40.Samuelson DA, Gum GG, Gelatt KN. Ultrastructural changes in the aqueous outflow apparatus of beagles with inherited glaucoma. Invest Ophthalmol Vis Sci. 1989;30:550–561. [PubMed] [Google Scholar]
- 41.Peiffer RL, Jr, Gum GG, Grimson RC, Gelatt KN. Aqueous humor outflow in beagles with inherited glaucoma: constant pressure perfusion. Am J Vet Res. 1980;41:1808–1813. [PubMed] [Google Scholar]
- 42.Brooks DE, Strubbe DT, Kubilis PS, et al. Histomorphometry of the optic nerves of normal dogs and dogs with hereditary glaucoma. Exp Eye Res. 1995;60:71–89. doi: 10.1016/s0014-4835(05)80085-5. [DOI] [PubMed] [Google Scholar]
- 43.Gottanka J, Chan D, Eichhorn M, Lütjen-Drecoll E, Ethier CR. Effects of TGF-beta2 in perfused human eyes. Invest Ophthalmol Vis Sci. 2004;45:153–158. doi: 10.1167/iovs.03-0796. [DOI] [PubMed] [Google Scholar]
- 44.Tripathi RC, Li J, Chan WF, Tripathi BJ. Aqueous humor in glaucomatous eyes contains an increased level of TGF-beta 2. Exp Eye Res. 1994;59:723–727. doi: 10.1006/exer.1994.1158. [DOI] [PubMed] [Google Scholar]
- 45.Picht G, Welge-Luessen U, Grehn F, Lütjen-Drecoll E. Transforming growth factor beta 2 levels in the aqueous humor in different types of glaucoma and the relation to filtering bleb development. Graefes Arch Clin Exp Ophthalmol. 2001;239:199–207. doi: 10.1007/s004170000252. [DOI] [PubMed] [Google Scholar]
- 46.Inatani M, Tanihara H, Katsuta H, et al. Transforming growth factor-beta 2 levels in aqueous humor of glaucomatous eyes. Graefes Arch Clin Exp Ophthalmol. 2001;239:109–113. doi: 10.1007/s004170000241. [DOI] [PubMed] [Google Scholar]
- 47.Ochiai Y, Ochiai H. Higher concentration of transforming growth factor-beta in aqueous humor of glaucomatous eyes and diabetic eyes. Jpn J Ophthalmol. 2002;46:249–253. doi: 10.1016/s0021-5155(01)00523-8. [DOI] [PubMed] [Google Scholar]
- 48.Ozcan AA, Ozdemir N, Canataroglu A. The aqueous levels of TGF-beta2 in patients with glaucoma. Int Ophthalmol. 2004;25:19–22. doi: 10.1023/b:inte.0000018524.48581.79. [DOI] [PubMed] [Google Scholar]
- 49.Gabelt BT, Kaufman PL. Changes in aqueous humor dynamics with age and glaucoma. Prog Retin Eye Res. 2005;24:612–637. doi: 10.1016/j.preteyeres.2004.10.003. [DOI] [PubMed] [Google Scholar]
- 50.Guo L, Moss SE, Alexander RA, et al. Retinal ganglion cell apoptosis in glaucoma is related to intraocular pressure and IOP-induced effects on extracellular matrix. Invest Ophthalmol Vis Sci. 2005;46:175–182. doi: 10.1167/iovs.04-0832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mabuchi F, Lindsey JD, Aihara M, Mackey MR, Weinreb RN. Optic nerve damage in mice with a targeted type I collagen mutation. Invest Ophthalmol Vis Sci. 2004;45:1841–1845. doi: 10.1167/iovs.03-1008. [DOI] [PubMed] [Google Scholar]
- 52.Aihara M, Lindsey JD, Weinreb RN. Ocular hypertension in mice with a targeted type I collagen mutation. Invest Ophthalmol Vis Sci. 2003;44:1581–1585. doi: 10.1167/iovs.02-0759. [DOI] [PubMed] [Google Scholar]