Abstract
Signaling networks play crucial roles in the changes leading to malignancy. In melanoma, increased Wnt5A expression increases melanoma cell motility via activation of Protein Kinase C (PKC). PKC isoforms comprise a family of serine/threonine kinases that are involved in the transduction of signals for cell proliferation, differentiation and metastasis. The important role of PKC in processes leading to carcinogenesis and tumor cell invasion would render PKC a suitable target for cancer therapy, if not for its ubiquitous nature. Thus, targeting more tumor-specific pathways leading to PKC activation, such as the non-canonical Wnt pathway, may prove to be the key to targeting PKC in cancer. Here we summarize the current understanding of the Wnt/Calcium pathway and discuss methods of detecting activated/phosphorylated PKC as a result of Wnt signaling in malignant melanoma. We have shown that overexpression of Wnt5A results in the activation of PKC, while inhibition of Wnt5A via siRNA treatment results in its inactivation. In addition, the use of PKC activators and inhibitors has allowed us to study Wnt5A effects on downstream genes that may prove to be key targets for molecular therapy.
Keywords: melanoma, Wnt5A, Protein Kinase C (PKC)
1. Introduction
The Wnt/calcium pathway is one of the three major pathways by which Wnt proteins exert their intracellular signaling events (1). Dysregulation of Wnt signaling can cause developmental defects and is implicated in the genesis of several human cancers. In melanoma, cDNA microarray analysis identified Wnt5A as the gene that best discriminated highly aggressive melanomas from their less invasive counterparts (2). In a follow up study, we demonstrated that introducing Wnt5A into less aggressive melanomas resulted in an increase in their metastatic potential most likely via the activation of PKC and rises in intracellular calcium (3). The most recent study from our laboratory has combined the use of siRNA technology with microarray analysis, to identify the pathways and mechanisms by which Wnt5A might be mediating motility in melanoma cells. In this study, we used recombinant Wnt5A protein, as well as siRNA to validate our array observations, and furthermore more fully assessed the role of PKC in this process using activation and inhibition studies (4). In addition to our laboratory, many others have highlighted the importance of G-protein mediated signaling and the resultant activation of PKC and increases in intracellular calcium, in melanoma progression (5–7).
The PKC family consists of a number of serine-threonine kinases, which are divided into three major groups based on their activating factors (Table 1). PKC isoforms have been linked to carcinogenesis since PKC activators can act as tumor promoters and different PKC isoforms, especially PKC α and β, have been often linked to a malignant phenotype in melanoma (8, 9). We have confirmed this observation in cutaneous melanoma, and have shown that the PKC isoforms affected by Wnt5A are predominantly the conventional PKCs, PKC α, β and γ (3). In its non-phosphorylated state, PKC resides in the cytosol. Binding of a hormone or other effector molecule to the membrane receptor, results in the activation of phospholipase C (PLC) via a G-protein-dependent phenomenon. The activated PLC hydrolyzes phosphatidylinositol-4,5-bisphosphate (PIP2) to produce DAG and inositol-1,4,5- triphosphate (IP3) from plasma membrane phospholipids. DAG activates conventional PKC isoforms, while IP3 causes the releases of Ca2+ from intracellular stores, which in turn potentiates the activation of these PKC isoforms. The binding of Ca2+ causes PKC translocation to the plasma membrane, where it interacts with DAG and is transformed into a fully active enzyme, which is capable of phosphorylating specific substrates on serine/threonine residues, as reviewed in (8). Commercial antibodies that recognize the individual isoforms of PKC, both in phosphorylated and non-phosphorylated forms, are readily available. These antibodies give us the ability to determine activity of PKC via Western blot analysis. These phospho-antibodies are very important tools, because of some caveats that should be noted when working with antibodies against non-phosphorylated proteins, specifically, as we discuss here, antibodies against total PKC. These antibodies are marketed as antibodies to “total” protein, as they are often designed to peptides from the protein sequence. Thus, these antibodies should technically recognize both phosphorylated and non-phosphorylated proteins. However, our current data indicate that, in fact, these antibodies may recognize phosphorylation-sensitive epitopes, resulting in a decreased ability to recognize phosphorylated protein. For example, when treating Wnt5A low cells with recombinant Wnt5A, we see that as the phosphorylated form of a protein increases, the intensity of the band recognized by the antibody to the corresponding non-phosphorylated isoform decreases (4). The converse is true for knocking down Wnt5A in Wnt5A high cells- decreases in Wnt5A correspond to decreased in PO4-PKC, but to increases in PKC α,β and γ. This is only true for molecules that activate the existing pool of PKC, such as Wnt5A, and not for chemical agents such as PMA that appear to act via an increase in the protein expression as well as in its activation. This is also true for the chemical inhibitors we have tested, that decrease the overall expression of PKC. In order to determine if these antibodies were indeed phosphorylation sensitive, duplicate sets of samples were subjected to SDS-PAGE, then transferred to PVDF. According to the protocol by Maya et al (10), the membranes were cut in half, separating the duplicate blots, and one set of samples was blocked and probed as usual, but the other was first incubated in alkaline phosphatases for half- an-hour. Phosphatase treatment significantly increased the ability of antibodies against PKC α,β and γ to recognize these isoforms (4). These data, some of which are included as examples below, should provide a strong caution to researchers, as the use of the antibodies to the non-phosphorylated form of PKC could result in a misinterpretation of data, even when doing what are considered gold standard assays for PKC activation such as Western analysis of membrane extracts as compared to cytosolic extracts. In such studies for example, these antibodies could indicate a depletion in membrane-bound PKC, where there may in fact be an increase in membrane-bound phosphorylated PKC. Thus, antibodies to both the phosphorylated and non-phosphorylated isoforms should be used when assessing the effects of signaling molecules on PKC activation. Additionally, we suggest the use of other techniques such as substrate-based assays, or the use of commercially available GFP-tagged PKC isoforms followed by confocal microscopy to determine if activation or de-activation of a pathway of interest can affect the translocation of PKC to the membrane. The methods we use including transfection of GFP-PKC, confocal microscopy and Western analysis of PKC and PO4-PKC are provided in detail below.
Table 1. PKC Isoforms.
All forms are monomeric. The βI and βII differ in their C-terminal amino acid residues, and the forms are the result of alternative splicing of the 3’exon.
| Isoform | Type | Calcium Dependence |
Phorbol stimulation |
Amino acids |
Predicted MW (kDa) |
|---|---|---|---|---|---|
| alpha (α) | Conventional | Yes | Yes | 672 | 76.8 |
| beta I (βI) | Conventional | Yes | Yes | 673 | 76.9 |
| beta II (βII) | Conventional | Yes | Yes | 671 | 76.8 |
| gamma (γ) | Conventional | Yes | Yes | 697 | 78.4 |
| delta (δ) | Novel | No | Yes | 673 | 77.5 (r) |
| epsilon (ε) | Novel | No | Yes | 737 (r) | 83.5 (r) |
| eta (η) | Novel | No | Yes | 680 | 77.6 |
| mu (μ) | Novel | No | Yes | 912 (m) | 115 (m) |
| theta (θ) | Novel | No | Yes | 706 | 82 (r) |
| zeta (ζ) | Atypical | No | No | 592 (r) | 67.7 (r) |
| lamda (λ) | Atypical | No | No | 586 | 67.2 |
2. Materials
2. 1. Cell Culture and Lysis Reagents
2.1.1
RPMI (UACC 903, UACC 647, M93-047, 1273 and 1205LU melanoma cell lines), EMEM (C-32 cells) or McCoy’s (G361 cells) medium (all from Invitrogen, Carlsbad, CA) supplemented with 10% fetal bovine serum (FBS, Invitrogen, Carlsbad, CA), 100 units/ml Penicillin and streptomycin and 4mM L-glutamine. C-32 cells only were additionally supplemented with 1% non-essential amino acids (Invitrogen, Carlsbad, CA). All cell lines were cultured at 37°C in 5% CO2/95% air, and the medium was replaced every two to three days.
2.1.2
Triple E Solution (Invitrogen, Carlsbad, CA) containing 0.25% ethylenediamine tetraacetic acid (EDTA) (1mM).
2.1.3
Phorbol 12-myristate 13-acetate (PMA, Sigma Aldrich, St. Louis, MO) is dissolved at 200uM in dimethyl sulfoxide (DMSO) and stored in single use aliquots at −20°C.
2.1.4
Gö 6983 and GF109293X (Calbiochem, San Diego, CA) are dissolved at 1mM in DMSO and stored in single use aliquots at −20°C.
2.1.5
Recombinant Wnt3A and Wnt5A (R&D Biosystems, Minneapolis, MN) are reconstituted in sterile PBS containing 0.1% BSA to a stock concentration of 10mg/mL and stored in single use aliquots at −20C.
2.1.6
RIPA buffer for cell lysis: 20mM Tris-HCl pH 7.5, 150mM NaCl EDTA1% Triton X-100, 1 tablet protease inhibitor cocktail (Complete, Roche, Indianapolis, IN) per 50ml total buffer solution; 1mM Na3VO4,1ml Phosphatase inhibitor cocktail 1 (Sigma Aldrich, St. Louis, MO) per 100ml total buffer. This buffer is stored in single use aliquots at −80°C (See Note 1)
2.1.7
BCA protein assay kit (Pierce, Rockford, IL) for quantitating protein lysates.
2.1.8
Spectrophotometer set to 570nM.
2.2. Transfection Reagents
2.2.1
PDest-Wnt5A was designed using the Gateway cloning system (The Harvard Gene Therapy Initiative), and is available from the authors upon request.
2.2.2
Wnt5A siRNA was designed using Invitrogen’s online design tools, which designs 21nt siRNA according to the Tushcl rules of siRNA design. SiRNAs are ordered from Qiagen, and are ordered in both rhodamine tagged (3’-UTR) and untagged versions (See Note 2). The sequence that works most efficiently as demonstrated in Dissanayake et al (REF) is: AAGACCTGGTCTACATCGACC.
2 . 2 . 3
GFP-PKCβ II was obtained from Invitrogen (Carlsbad, CA)
2.2.4
Lipofectamine Plus system, from Invitrogen (Carlsbad, CA).
2.3. Reagents for Confocal Microscopy
2.3.1
Glass slide chambers, single well.
2.3.2
Coverslips
2.3.3
Prolong Gold (Invitrogen, Carlsbad, CA)
2.4. SDS-Polyacrylamide Gel Electrophoresis (SDS-PAGE)
2.4.1
4–20% Tris-glycine pre-cast Novex mini gels (Invitrogen, Carlsbad, CA) Store at 4°C.
2.4.2
Running buffer (10X) Tris/Glycine buffer (Bio-Rad, Hercules, CA) Store at room temperature.
2.4.3
Prestained molecular weight markers: Kaleidoscope markers (Bio-Rad, Hercules, CA) Store at −20°C.
2.4.4
4x loading buffer :12% SDS (w/v), 40% glycerol (v/v), 0.2M Tris-HCl (v/v), 0.004% Bromo Phenol Blue (BPB) (w/v), 0.05% BME (v/v). Store at 4°C.
2.4.5
XCell SureLock Novex Mini-Cell (Invitrogen, Carlsbad, CA) for running mini-gels.
2.5. Western blotting for active PKC
2.5.1
Transfer buffer: 25x Novex Tris/Gly transfer buffer (Invitrogen, Carlsbad, CA), 20% (v/v) methanol. Store at room temperature, but cool to 4°C for use.
2.5.2
Filter paper sandwich 0.2 micron PVDF membranes (Invitrogen, Carlsbad, CA)
2.5.3
Tris-buffered saline (TBS) with Tween (TBS-T): Prepare from 10X TBS (Quality Biological Inc, Gaithersburg, MD), 1% Tween-20 (Sigma, St. Louis, MO).
2.5.4
Blocking buffer: 5% (w/v) nonfat dry milk (BioRad non-fat blocking solution) in TBS-T.
2.5.5
Primary antibody dilution buffer: 5% (w/v) nonfat dry milk in TBS-T.
2.5.6
Secondary antibody: anti-rabbit IgG conjugated to horseradish peroxidase (HRP) (Amersham Biosciences, Buckinghamshire, UK)
2.5.7
ECL-Plus reagent and high performance chemiluminescence film (Hyperfilm™ ECL (Amersham Biosciences, Buckinghamshire, UK).
2.5.8
Mini-PROTEAN 3 cell transfer system (Bio-Rad, Hercules, CA) for transfer of gel.
2.6. Antibodies
PO4-Pan-PKC (1:1000) and β-tubulin (1:1000) antibodies were obtained from Cell Signaling Technology Inc (Danvers, MA); Wnt5A-biotinylated antibody (1:100) was obtained from R&D Biosystems (Minneapolis, MN). Antibodies to PKC isoforms (non-phosphorylated) were obtained from BD Biosciences (San Jose, CA).
2.7. Stripping and reprobing blots
Stripping buffer: 50mM Tris-Chloride pH6.8, 2% SDS, 2% BME.
Wash buffer: Tris-buffered saline (TBS) with Tween (TBS-T).
Sealed plastic box.
Water bath with shaker at 55°C in fume hood.
3. Methods
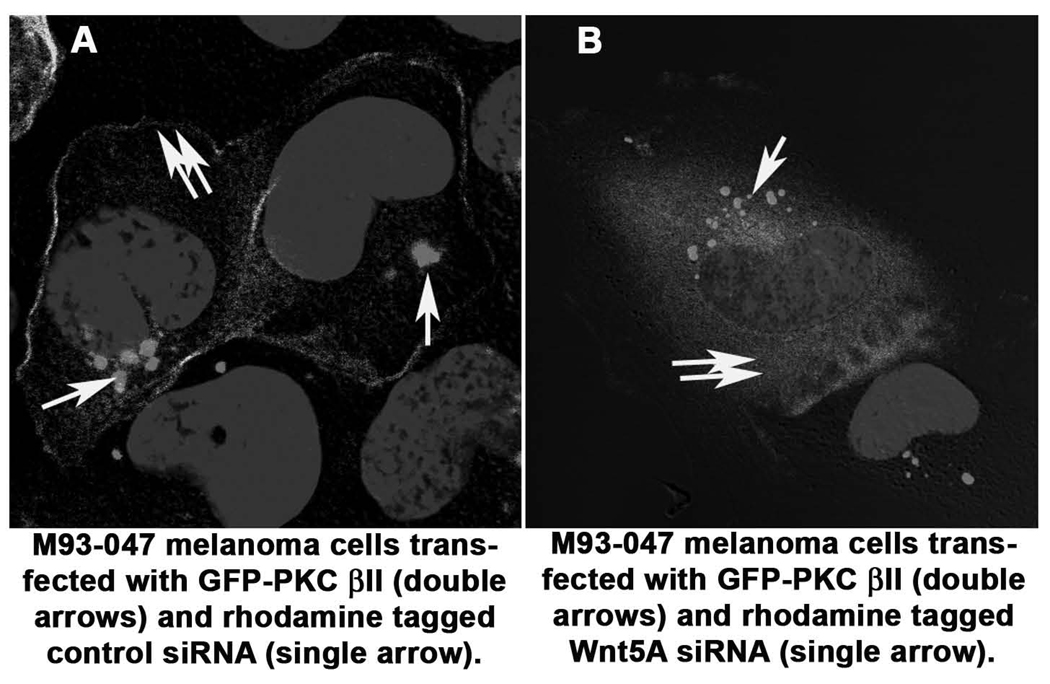
PKC is considered active when at the membrane. Because Wnt5A has been shown to increase the phosphorylation of PKC (3, 11), we recently used siRNA against Wnt5A to determine if inhibition of Wnt5A corresponded to an inhibition of PKC phosphorylation, and recombinant Wnt5A to determine if increasing Wnt5A could increase PO4-PKC levels (4). Subsequently, we investigated whether PKC translocated from its active site at the membrane of the cell, upon inhibition of Wnt5A, for further confirmation of PKC deactivation. To perform this experiment, we examined the conventional PKC, PKCβII. Cells were transiently transfected with a GFP-tagged PKCβII vector and a rhodamine-tagged control siRNA, and then examined the cells using confocal microscopy. Cells with endogenously high Wnt5A showed PKCβII expression predominantly at the membrane (Figure 1A, GFP-PKC double arrows, rhodamine tagged siRNA, single arrow). When co-transfected with rhodamine tagged A2-RNAi (Wnt5A-A2Rh) against Wnt5A GFP-PKCβII moved from the membrane into the cytoplasm (Figure 1B, GFP-PKC double arrows, rhodamine tagged siRNA, single arrow).
Figure 1. Confocal Microscopy for PKC Activation.
Using a GFP-tagged PKCβII expression vector, cells were treated with either control siRNA or siRNA against Wnt5A. In cells co-transfected with a control siRNA (single arrow), PKC (double arrows) remains at its active site in the membrane (A). When these cells are cotransfected with siRNA against Wnt5A (single arrow), PKC (double arrows) moves into the cytoplasm (B).
3.1. Assessment of PKC translocation using GFP-tagged PKC and Confocal Microscopy
3.1.1
Passage all cells when approaching confluence with Trypsin/EDTA (TripleE) to provide new maintenance cultures in T-75 tissue culture dishes and experimental cultures on 1 well glass slide chambers (see Note 4). One slide chamber is required for each experimental data point. Typically we seed between 0.3 -7.5×105 cells per slide chamber for experimental cultures that would require 60%–70% confluency after 16 hours. This should be calculated for each cell line depending on its doubling time. Rinse the cultures twice with cell culture media (without serum) and incubate for a further 1–3 hours in serum free media before transfection.
3.1.2
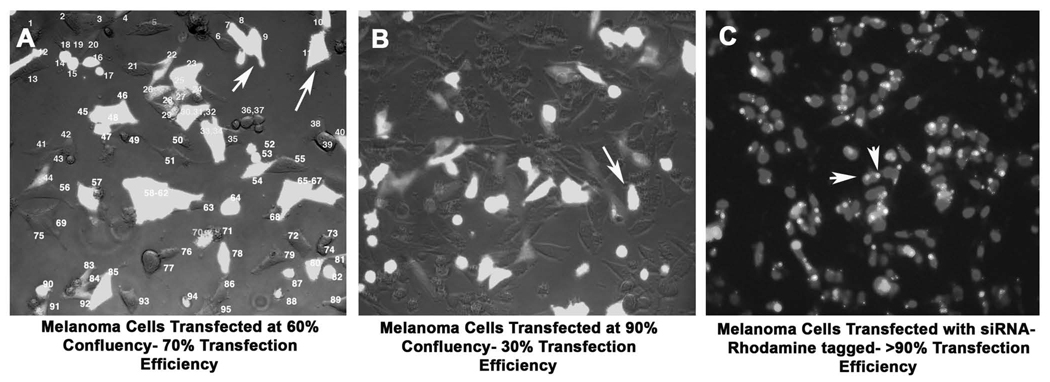
Transfect Wnt5A siRNA or plasmid DNA into cells (60–70% confluency) using Lipofectamine Plus (Invitrogen, Carlsbad, CA). Allow the Plus reagent to incubate with DNA/ siRNA for 15 minutes, and then incubate this complex with Lipofectamine for a further 15 minutes, prior to adding to the serum-starved cells (See Note 5). After five to six hours of transfection, replace the medium with fresh serum-containing medium. Transfection efficiencies are usually upwards of 90% for siRNA oligos as gauged by transfection with rhodamine tagged siRNAs, and siRNAs are routinely transfected at a concentration of 150nM. For the PCDNA-Wnt5A vector and GFP-tagged PKCβII vector, transfect approximately 1 µg of DNA/ 35mm dish to achieve transfection efficiencies around 75%. Interestingly, transfection efficiency is highly dependent on melanoma cell confluency, and cell densities higher than 80% result in inefficient transfection (Figure 2).
Figure 2. Transfection Efficiency Based on Cell Confluency.
When melanoma cells are transfected at efficiencies of 50–60% (A), the efficiency of transfection of a GFP-plasmid (arrows) is around 70–75%. Cells transfected at higher confluencies (B), lose efficiency of transfection dramatically, dropping down to about 25–30%. SiRNA transfection efficiency, as gauged by the rhodamine fluorescence (arrows) in this image (C) is consistently high, around 90–95%.
3.1.3
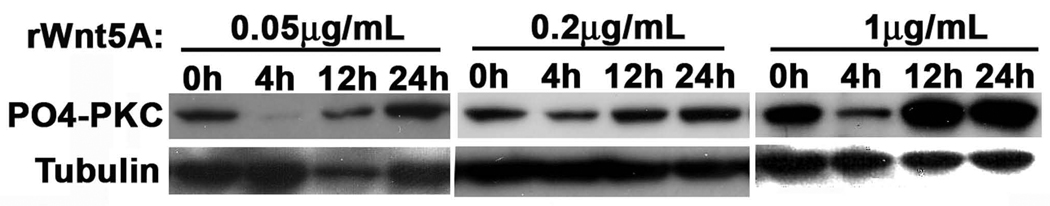
To test effects of Wnt5A on GFP-tagged PKC, either cotransfect Wnt5A-A2 siRNA (Wnt5A high cells), or add recombinant Wnt5A to transfected cells (Wnt5A low cells). For recombinant Wnt5A treatments, after testing a range of concentrations, and time points (Figure 3), a concentration of 0.2µg/ml for 16 hours in serum free medium was decided upon as ideal for our melanoma cells (See Note 6). Researchers attempting this in different cells should assess the ideal times and concentrations for their cells. Additionally, assess whether 24 hours post transfection or 48 hours post transfection gives the best results in each individual case. We find (for our cells) that when co-transfecting GFP-tagged PKC and Wnt5A siRNA, 48 hours is an effective time point. For Wnt5A treatments, we prefer to treat cells 24 hours post-transfection for maximal results. PKC inhibitors and activators are also added at 24 hours post-transfection (see Notes 7 and 8).
Figure 3. Concentration and time course determination for rWnt5A treatment.
Cells were assayed for their ability to increase PO4-PKC in response to rWnt5A treatment, at differing concentrations, and over differing times. 16–24 hours was ultimately determined to be the ideal time-course for Wnt5A treatment. Note that at 4 hours Wnt5A effects are consistently dampened, perhaps due to receptor recycling.
3.1.4
After treatment with agents of interest, fix the cells using either 95% methanol, or 4% formaldehyde. We prefer to use 95% methanol, as this effectively permeabilizes membranes, while fixing proteins that are in the membrane to the membrane, without allowing leaching into the cytoplasm. Rinse chambers once with PBS, and use ice-cold methanol, and fix the cells for 20 minutes at room temperature. Break off the chambers and store the slides in PBS, pH 7.4 at 4°C until ready to stain.
3.1.5
Use Prolong Gold, with or without DAPI (we prefer with DAPI for easier visualization). Drop 100 µL onto the slide, then coverslip. Allow the slides to cure in the dark at room temperature for at least 24 hours (See Note 9). Our data indicate that Wnt5A high cells will have GFP-PKC at the membranes, with some cytoplasmic expression, while Wnt5A low cells will have predominantly cytoplasmic PKCβII. Treatment with siRNA against Wnt5A reduces membrane-associated PKC in Wnt5A high cells (Figure 1), where treatment with recombinant Wnt5A increases membrane associated PKC, as does treatment with phorbol ester. Image cells using confocal microscopy to accurately determine membrane association. Expect that about 20% of your cells will not be membrane or cytoplasmic as expected, i.e., in Wnt5A high cells, you will still have about 20% of cells that have some significant cytoplasmic staining, and in Wnt5A low cells, you will come across a proportion that have some membrane staining.
3.2. Assessment of PKC activation by Western Blotting
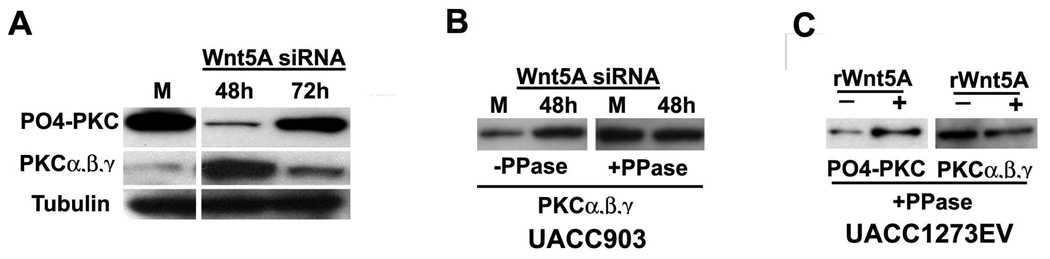
The activation of PKC is under the control of three distinct phosphorylation events, specifically Threonine 500 at the activation loop, the Threonine 641 autophosphorylation site and the Serine 660 hydrophobic site at the carboxy terminus of PKCβII (12). The pan PO4-PKC antibody used in our studies detects endogenous levels of PKC α, β and γ isoforms only when phosphorylated at a carboxy-terminal residue homologous to serine 660 at PKCβII. The integrity of the total protein loaded per sample was assessed by re-probing blots for β-tubulin, a conventional housekeeping gene widely used in many protein expression analysis studies. Using the PO4-PKC antibody as well as antibody to non-phosphorylated PKC we see that Wnt5A appears to be activating only the existing pool of PKC, as inhibiting Wnt5A decreases PO4-PKC, but increases non-phosphorylated PKC (Figure 4a), and increasing Wnt5A produces the reverse effect. PKC antibodies are phosphorylation sensitive, and this is determined by probing Western blots with antibodies against PKC isoforms before and after phosphatase treatment (Figure 4b, c). Perhaps due to the technique used (phosphatasing of PVDF, where phosphatase treatment may be incomplete), phosphatase treatment does not result in complete ablation of the ability of the phospho-antibody to recognize PO4-PKC (Figure 4c).
Figure 4. Wnt5A affects the existing pool of PKC.
A. Wnt5A inhibition results in a decrease in PO4-PKC, that is maximal at 24–48 hours. 72 hours post-transfection, Wnt5A siRNA effects begin to decrease, and PO4-PKC levels start to increase again. Conversely, non-phosphorylated PKC levels increase with Wnt5A knockdown, as PO4-PKC decreases, and when PO4-PKC starts to increase again, non-phosporylated PKC levels decrease. B. This is attributable to the fact that the PKC antibodies recognize phosphorylation sensitive epitopes. This is shown by probing two identical membranes with antibodies against PKC, and subjecting one to phosphatase treatment (right panel), but not the other (left panel). C. Treating cells with rWnt5A increases PO4-PKC (shown, left panel) but decreases non-phosphorylated PKC (shown in Dissanayake et al). Phosphatasing the membrane results in an increase of the ability of the non-phospho antibody to recognize PKC (right panel).
3.2.1
Prepare cells as described above, with the exception that they should be grown in 35mm dishes or 6-well plates. For our cells to achieve 60–70% confluency the following day, required seeding densities in these dishes are 0.75 to 1 × 106. Researchers should determine this for each cell line. Transfect or treat cells as described in Section 3.1.1–2.
3.2.2
Label microfuge tubes for each sample and have a 22G 1 1/2 “ needle attached to a 1CC syringe, cell lysis buffer (RIPA), an ice bucket, and a centrifuge set at 4°C. For adherent cells, rinse flasks once with PBS, then add RIPA directly onto the dish. We use 150µLof RIPA per well of a 6 well plate. Incubate the plate on ice for 5 minutes. Using a cell scraper, scrape the cells, and place them in a microfuge tube on ice. Shear the cells using a syringe, by pushing them through the syringe 7 times. Incubate on ice for 30 minutes and centrifuge at 15,000 X g for 10 minutes. Quantitate using the Pierce BCA protein quantitation assay (Pierce, CA). (See Note 10).
3.2.3
Mix 50 µg of each protein lysate with 4X loading dye, heat denature at 95°C for 10 minutes in a heat block and run out on 4–20% Tris-glycine pre-cast Novex mini gel at 100 volts for approximately 2 hrs.
3.2.4
Transfer as follows- these directions assume the use of a Bio-Rad Mini-PROTEAN 3 cell transfer system. Prepare a tray of transfer buffer that is large enough to lay out a transfer cassette with 1 piece of foam and 3mm thick filter paper submerged on one side. Cut the PVDF membrane in one corner to allow orientation to be tracked, and immerse in methanol followed by two washes in distilled water. Submerge the wet membrane in the transfer buffer on top of the filter paper.
3.2.5
Disconnect the gel unit from the power supply and dissemble. Remove and discard stacking gel, and lay the separating gel on top of the PVDF membrane. Wet another sheet of filter paper in the transfer buffer and carefully lay it on top of the gel, ensuring that no bubbles are trapped in the resulting sandwich, which can be done by firmly rolling a 15ml centrifuge tube, or pipette across the sandwich. Lay the second wet foam sheet on top and close the transfer cassette.
3.2.6
Place the cassette into the transfer tank such that the PVDF membrane is between the gel and the anode. It is critical that this orientation is maintained or the proteins will be lost from the gel into the buffer rather than transferred to the PVDF membrane.
3.2.7
Place the lid on the tank and activate the power supply. Transfers are done at 4 degrees preferably with a magnetic stir-bar in the tank activated to maintain a temperature between 10–15 degrees in the tank. Transfers can be accomplished at either 30 mA overnight or 100 mA for 2 hours, but the overnight transfer is preferable.
3.2.8
Once the transfer system is complete, take the cassette out of the tank and carefully dissemble, removing the top sponge and sheet of filter paper. Discard the gel and immerse the PVDF membrane containing the transferred protein in TBS-T. The colored molecular weight markers should be clearly visible on the membrane.
3.2.9
Incubate the PVDF membrane in 10ml blocking buffer (5% milk, w/v in TBS-T) for 1h at room temperature on a rocking platform (see Note 11).
3.2.10
Discard the blocking buffer and add a primary antibody in 5% milk made up in TBST (See Note 12). The membrane is incubated overnight at 4°C on a rocking platform. Alternately the membrane can be incubated with antibody in a 50ml tube on a rotating wheel at 4°C.
3.2.11
Remove the primary antibody after the overnight incubation step and wash the membrane three times for 5 min each with 10ml of TBS-T.
3.2.12
Freshly prepare the secondary antibody for each experiment at a 1:2000 dilution in blocking buffer and add this to the membrane for 1 hour at room temperature with gentle agitation on a rocking platform. Alternately, as in the case of the primary antibody, the membrane can be incubated with secondary antibody in a 50 ml tube on a rotating wheel at 4°C.
3.2.13
Discard the secondary antibody and wash the membrane three times for 5 minutes each with TBS-T.
3.2.14
During the final wash, mix 1 ml of solution A of ECL plus reagent with 25µLof solution B and apply immediately to the membrane removed from the final wash. Rotate the ECL plus reagents by hand for 1 min to ensure even coverage.
3.2.15
Remove the membrane from the ECL reagents, blot with Kim-Wipes, and then place between the leaves of polythene sheet protector, and place in an X-ray film cassette.
3.2.16
Expose the membrane is to film for a suitable exposure time, typically 1–2 minutes.
3.3. Stripping and reprobing blots
3.3.1
The PKC and β-tubulin antibodies work on stripped and blocked membranes. The Wnt5A antibody works only on fresh blots.
3.3.2
For stripping, warm stripping buffer (10ml per membrane – see note 13) to 55°C and add to the membrane. Incubate the membrane for 20 min with gentle agitation in a water bath in the fume hood. Once the blot is stripped and washed extensively (3x with 10 ml TBS-T, each wash for 10 minutes), it can be re-probed with desired primary antibody immediately. Further blocking with milk for 1 hour is not necessary. Incubation with primary antibody is followed by secondary antibody, and ECL-plus detection as above.
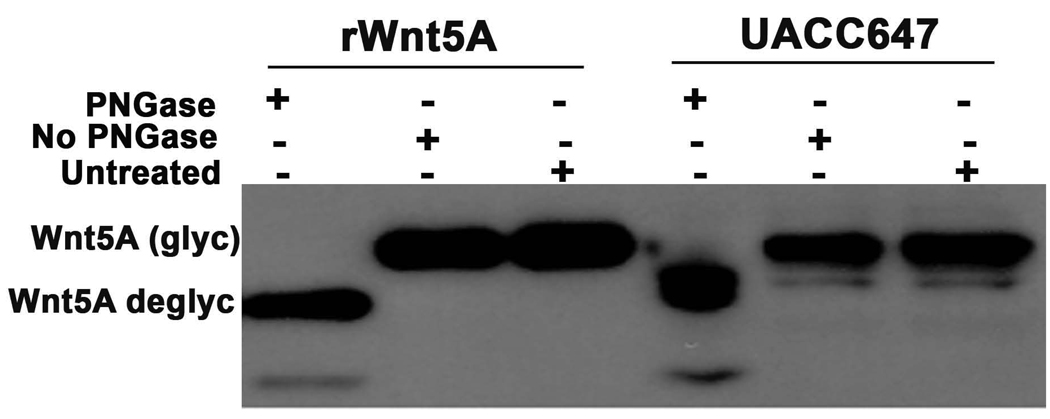
Figure 5. Wnt5A is glycosylated in melanoma cells.
UACC647 melanoma cells have high levels of Wnt5A and are highly metastatic. To determine if the Wnt5A in these cells was glycosylated, protein lysates were subjected to treatment with PNGaseF, a de-glycosylase. Post- treatment with this enzyme, Wnt5A migrated at around 38–40kDa as compared to its migration at 42–44kDa pre-treatment. Recombinant Wnt5A was also run as control, and demonstrated the same de-glycosylation.
Footnotes
The phosphorylation and activation of PKC occurs on its threonine and serine residues, and PKC in turn is a serine/threonine kinase. Therefore it is important that the RIPA buffer used in the extraction of protein contains inhibitors of serine/threonine protein phosphatase such as PP1 and PP2A (Phosphatase Inhibitor Cocktail 1, Sigma) and Sodium Orthovanadate (Na3VO4), which protect these PKC phosphorylation sites. Protease and phosphatase inhibitors are quite unstable in aqueous solution and are usually degraded with repeated thaw-freeze cycles. Therefore, RIPA buffer should not be subjected to such repeated thaw freeze cycles and should be stored in single use aliquots.
We have tried other siRNAs (such as Dharmacon smartpool siRNA) against Wnt5A, and found them ineffective, as determined by Western analysis. Also, when using rhodamine-tagged siRNA, fluorescence can be overwhelming when using confocal microscopy, so we dilute our tagged with non-tagged siRNAs 1:3.
When performing Western Analysis of Wnt5A in melanoma, researchers should note that there are often two bands present, one of which in some lines in non-specific, and in some lines represents the glycosylated or unglycosylated version of Wnt5A. To ascertain which band is Wnt5A it is advisable to perform a glycosylase assay. To do this, use PNGaseF. Set up the following three conditions- in the example shown here we also subjected the recombinant protein to the assay as a positive control- Untreated protein, protein lysate subjected to PNGaseF treatment, and protein lysate subjected to identical treatment, with no enzyme. We use PNGaseF from New England Biosciences, which allows for the use of protein lysates extracted in RIPA. The enzyme comes with denaturing buffer at 10X, NP-40, and a 10X G7 reaction buffer. Incubate up 50–100µg of protein (RIPA lysates are fine, but NP-40 MUST be added to counteract inhibitory effects of SDS) with 1X Denaturing Buffer at 100°C for 10 minutes. After the addition of NP-40 and G7 Buffer, add 2µL of PNGase F/ 10µL of reaction volume, and incubate the reaction for 1 hour at 37°C. Visualize the results by Western analysis, probing for Wnt5A as described in the methods. A sample gel is shown in Figure 5.
Many researchers like to use multi-well chamber slides, and treat different well on the same slide. We appreciate that this should be the ideal experimental situation, however it has been our experience that when treating with reagents that are in solution in the medium, the evaporation, and condensation that occurs on the lids of these chambers can sometimes cause a cross reaction, affecting untreated cells.
It is vital to test the effects of the plus reagent on each cell line that will be used. It can be toxic to some. This applies to other agents also. For example, we find that Lipofectamine 2000 is highly toxic to many of our cells, and Lipofectamine alone results in an inefficient transfection when using DNA vectors. SiRNA however can be transfected very efficiently using only Lipofectamine with no additional reagents.
Some notes on the use of recombinant Wnt5A: Early time points of Wnt5A treatment such as 4 hours should be avoided due to the consistent deactivation of PKC across a wide range of concentrations of Wnt5A, that may be indicative of receptor internalization, prior to recycling. As with Wnt5A siRNA treatments it should be noted that increases in PO4-PKC upon treatment of melanoma cells with recombinant Wnt5A, result in a decrease in the non-phosphorylated forms of PKC. Finally, R&D Systems estimates that Wnt5A activity, as gauged by its ability to inhibit Wnt3A, is 5–25 times lower than that of Wnt3A. Thus, when using assays where Wnt3A is used as a control, cells should be treated at a concentration 0.04µg/mL for the same time points.
To study the role of PKC phosphorylation in the Wnt/Ca2+ pathway in melanoma, we also employed PKC specific activators and inhibitors to determine if we could mimic Wnt5A effects via PKC activation, and also if Wnt5A could still mediate its effects in the presence of PKC inhibitors. Our recent data implicating Wnt5A in the epithelial to mesenchymal transition in melanoma cells indicates that for many of the Wnt5A mediated effects that we see, Wnt5A requires PKC. To assess PKC activation effects, melanoma cell lines low in Wnt5A (UACC1273EV and C-32) were incubated with 200nM PMA over a 24 hour time period. Increases in PKC could be detected as early as 30 minutes peaking at 4–12 hours in both cell lines, but with sustained effects to 24 hours. Many studies have suggested that prolonged PMA activation can result in PKC depletion, but this is not the case in our cells (4, 13).
For PKC inhibition studies, a range of times, concentrations and inhibitors were tested, and ultimately, Gö 6983 (a specific inhibitor of PKC α, β, γ, δ, and ζ, but not μ) and GF 109203X (inhibitor of PKC α, β, δ, and ε) were used in an attempt to use two different inhibitors of the conventional PKC pathway, at concentration of 1µM each. Cells were pre-treated with the inhibitor over a 24 hr time course and assayed by western blot for phospho PKC. A decrease in phospho-PKC can be observed as early as 30 minutes after addition of inhibitor, with maximum inhibition occurring at 12–24 hrs. For studies in which the effect of PKC inhibition on rWnt5A treatment was examined, cells were pretreated with inhibitor for 12 hours, then media was changed, and fresh inhibitor and rWnt5A were added for 16 hours more.
Placement at 4°C “uncures” the slides, making the coverslips slide around, so always leave them out for at least one overnight (manufacturer recommends 80 hours) prior to examining them.
If cells are frozen at −80°C in RIPA and need to be homogenized at a later date do the following: Quick thaw the cell lysates in RIPA in a 37°C water bath, and homogenize them with a 22G 1 1/2 “ needle immediately. Let sit on ice for 30 minutes and spin samples down at 12,000 x g for 10 min at 4°C. Transfer supernatant to a new tube and measure concentration using Pierce BCA protein Assay kit.
The source of milk is very important. We prefer Biorad non-fat blocking solution, and switching to another company caused a loss of signal on our westerns.
The PKC antibodies from BD Biosciences are provided as a sampler kit of all the different isoforms. For total PKC analysis, mix the antibodies as recommended by the supplier. Be aware that not all the PKC isoforms are used at the same concentrations, but the volume provided is the same, so they are used up at different rates.
Western stripping buffer can be reused until the effect of BME is gone (this is evident when the solution no longer smells potent) if stored at 4°C.
References
- 1.Kuhl M, Sheldahl LC, Park M, Miller JR, Moon RT. The Wnt/Ca2+ pathway:a new vertebrate Wnt signaling pathway takes shape. Trends Genet. 2000;16:279–283. doi: 10.1016/s0168-9525(00)02028-x. [DOI] [PubMed] [Google Scholar]
- 2.Bittner M, Meltzer P, Chen Y, Jiang Y, Seftor E, Hendrix M, et al. Molecular classification of cutaneous malignant melanoma by gene expression profiling. Nature. 2000;406:536–540. doi: 10.1038/35020115. [DOI] [PubMed] [Google Scholar]
- 3.Weeraratna AT, Jiang Y, Hostetter G, Rosenblatt K, Duray P, Bittner M, et al. Wnt5a signaling directly affects cell motility and invasion of metastatic melanoma. Cancer Cell. 2002;1:279–288. doi: 10.1016/s1535-6108(02)00045-4. [DOI] [PubMed] [Google Scholar]
- 4.Dissanayake SK, Wade MS, Johnson CE, O'Connell MP, Leotlela PD, AD F, et al. The Wnt5a/ Pkc Pathway Mediates Motility In Melanoma Cells Via The Inhibition Of Metastasis Suppressors, And Initiation Of An Epithelial To Mesenchymal Transition. J Biol Chem. 2007 doi: 10.1074/jbc.M700075200. epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Parker C, Sherbet GV. Modulators of intracellular Ca2+ and the calmodulin inhibitor W-7 alter the expression of metastasis-associated genes MTS1 and NM23 in metastatic variants of the B16 murine melanoma. Melanoma Res. 1992;2:337–343. doi: 10.1097/00008390-199212000-00007. [DOI] [PubMed] [Google Scholar]
- 6.Li S, Huang S, Peng SB. Overexpression of G protein-coupled receptors in cancer cells: involvement in tumor progression. Int J Oncol. 2005;27:1329–1339. [PubMed] [Google Scholar]
- 7.Fink-Puches R, Helige C, Kerl H, Smolle J, Tritthart HA. Inhibition of melanoma cell directional migration in vitro via different cellular targets. Exp Dermatol. 1993;2:17–24. doi: 10.1111/j.1600-0625.1993.tb00194.x. [DOI] [PubMed] [Google Scholar]
- 8.Oka M, Kikkawa U. Protein kinase C in melanoma. Cancer Metastasis Rev. 2005;24:287–300. doi: 10.1007/s10555-005-1578-8. [DOI] [PubMed] [Google Scholar]
- 9.Lahn MM, Sundell KL. The role of protein kinase C-alpha (PKC-alpha) in melanoma. Melanoma Res. 2004;14:85–89. doi: 10.1097/00008390-200404000-00002. [DOI] [PubMed] [Google Scholar]
- 10.Maya R, Oren M. Unmasking of phosphorylation-sensitive epitopes on p53 and Mdm2 by a simple Western-phosphatase procedure. Oncogene. 2000;19:3213–3215. doi: 10.1038/sj.onc.1203658. [DOI] [PubMed] [Google Scholar]
- 11.Sheldahl LC, Park M, Malbon CC, Moon RT. Protein kinase C is differentially stimulated by Wnt and Frizzled homologs in a G-protein-dependent manner. Curr Biol. 1999;9:695–698. doi: 10.1016/s0960-9822(99)80310-8. [DOI] [PubMed] [Google Scholar]
- 12.Keranen LM, Dutil EM, Newton AC. Protein kinase C is regulated in vivo by three functionally distinct phosphorylations. Curr Biol. 1995;5:1394–1403. doi: 10.1016/s0960-9822(95)00277-6. [DOI] [PubMed] [Google Scholar]
- 13.Leotlela PD, Wade MS, Duray PH, Rhode MJ, Brown HF, Rosenthal DT, et al. Claudin-1 overexpression in melanoma is regulated by PKC and contributes to melanoma cell motility. Oncogene. 2006 doi: 10.1038/sj.onc.1210155. epub ahead of print. [DOI] [PubMed] [Google Scholar]