Abstract
The recent identification of activating mutations in NOTCH1 in the majority of T-cell acute lymphoblastic leukemias (T-ALL) has brought major interest towards targeting the NOTCH signaling pathway in this disease. Small molecule γ-secretase inhibitors (GSIs) which block a critical proteolytic step required for NOTCH1 activation can effectively block the activity of NOTCH1 mutant alleles. However, the clinical development of GSIs has been hampered by their low cytotoxicity against human T-ALL and the development of significant gastrointestinal toxicity derived from inhibition of NOTCH signaling in the gut. Improved understanding of the oncogenic mechanisms of NOTCH1 and the effects of NOTCH inhibition in leukemic cells and the intestinal epithelium are required for the design of effective anti-NOTCH1 therapies in T-ALL.
Keywords: T-ALL, NOTCH1, targeted therapy, γ-secretase inhibitors, mutation, MYC, PTEN
Introduction
T-cell acute lymphoblastic leukemia (T-ALL) is a highly aggressive hematologic malignancy characterized by infiltration of the bone marrow with immature lymphoblasts expressing a T-cell immunephenotype. T-ALL represents 10% to 15% of pediatric and 25% of adult acute lymphoblastic leukemia cases.[1–3] Patients with T-ALL commonly present large tumor burdens at diagnosis with high numbers of circulating leukemic cells in peripheral blood, mediastinal masses and frequent infiltration of the central nervous system at the time of diagnosis.
In the early days of combination chemotherapy, patients with T-ALL were recognized as a clinically distinct group associated with a particularly high risk of relapse. Thus, when early chemotherapy protocols started to make significant progress in improving overall survival in B-precursor ALL, the prognosis of T-ALL patients remained invariably poor, with cure rates under 10%.[4] Although the specific biological and molecular mechanisms that account for the aggressiveness and poor therapy response in T-ALL remain to be elucidated, the introduction of intensified chemotherapy for patients with this disease has led to a remarkable improvement in outcome over the last decades. Thus, 5-year relapse-free survival rates in T-ALL are now over 75% in children and adolescents[5–9] and over 50% in adults.[10],[11–16] Still, the prognosis for those patients who present with primary resistant disease or relapse after the induction of complete remission remains particularly poor, underscoring the need to develop more effective antileukemic drugs.[17–21] This imperative is further supported by studies showing that gains in leukemia-free survival with intensified chemotherapy have been achieved at the cost of significant increases in the incidence of life-threatening and incapacitating toxicities.[22] In this context, the identification of activating mutations in NOTCH1 in over 50% of T-ALL cases has brought much interest in the developing of molecularly tailored anti-NOTCH1 therapies for the treatment of T-ALL.[23]
NOTCH1
The NOTCH1 receptor is a ligand activated transcription factor that directly transduces extracellular signals at the cell membrane to changes in gene expression in the nucleus. In essence, the NOTCH1 signaling pathway has three components: (i) the delta-like and jagged family of ligands (Delta-like 1, 3 and 4; and Jagged 1 and 2), which are transmembrane proteins expressed in the surface of signaling cells; (ii) the NOTCH1 receptor and (iii) the CSL (CBF1, suppressor of hairless, lag1) DNA binding protein (Figure 1).
FIGURE 1. THE NOTCH1 SIGNALING PATHWAY.
The NOTCH1 receptor is activated upon interaction with Delta-like and Jagged ligands expressed the surface of a neighbor cell. This ligand-receptor interaction results in a conformational change in the LNR repeats of NOTCH1 and the dissociation of the two subunits of the HD domain, which is followed by cleavage of the receptor, first by an ADAM metalloprotease (S2 cleavage) and subsequently by the γ-secretase complex (S3 cleavage). This latter proteolytic processing releases the intracellular domains of NOTCH1 (ICN1) from the membrane. ICN1 interacts with DNA via the CSL DNA binding protein and recruits the MAML1 coactivator to activate the expression of NOTCH1 target genes.
The mature NOTCH1 protein is expressed at the cell surface as a heterodimeric protein consisting of a N-terminal and a C-terminal NOTCH fragments.[24, 25] These two subunits are generated from a precursor NOTCH1 polypeptide that is postranslationally cleaved by a furin protease during its transport to the membrane through the trans-Golgi network.[24, 25] The extracellular portion of NOTCH1 includes multiple epithelial growth factor (EGF) repeats responsible for ligand-receptor interaction, and a negative regulatory region consisting of three (Lin 12-Notch repeats (LNR) and a heterodimerization (HD) domain (Figure 1). This HD domain has an N-terminal component in the extracellular subunit of the receptor and a C-terminal component located in the extracellular stub of the transmembrane and intracellular subunit of NOTCH1. The LNR-HD complex holds together the two subunits of the receptor and keeps NOTCH1 in a resting state.[26] Thus, in the absence of ligand, the LNR repeats fold over the HD domain to form a molecular lock that stabilizes the interaction of the two HD subunits together.[26] Upon interaction with its ligands, NOTCH1 undergoes a conformational change in the LNR repeats, which destabilizes the interaction between the two subunits of the HD domain. As result, the C-terminal HD subunit is exposed as substrate for ADAM10, a metalloprotease expressed at the cell surface, which cleaves the extracellular portion of the receptor in the so called S2 site located 12–13 amino acids external to the plasma membrane (Figure 1).[27, 28] After S2 cleavage, NOTCH1 is further processed by the γ-secretase complex, a transmembrane enzyme, which catalyzes a final endomembrane proteolytic cleavage (S3), required to release the intracellular domains of the receptor (ICN1) from the membrane (Figure 1)[29–34] Upon γ-secretase cleavage ICN1 rapidly translocates to the nucleus to form a complex with the CSLDNA binding protein via its RAM and ankyrin repeat domains (Figure 1). In the absence of NOTCH activation CSL serves as docking site for transcriptional corepresors including SMRT, CIR, SHARP, SIN3A, SAP30 and histone deacetylases HDAC1 and 2, which keep NOTCH1-CSL target genes inactive.[35–37] Binding of ICN1 to CSL displaces these corepressors and recruits the MAML1 coactivator to the complex to induce the transcriptional activation of NOTCH1-CSL target genes such as HES1 (Figure 1). Importantly, transcriptional activation is coupled with termination of NOTCH1 signaling via proteasomal degradation of ICN1. Thus, MAML1 recruiting of the RNA polymerase II holoenzyme results in cyclin-dependent kinase 8 phosphorylation of the PEST domain of ICN1,[38] which is subsequently recognized and targeted for proteasomal degradation by the FBXW7-SCF ubiquitin ligase complex.[35, 38, 39]
NOTCH1 signaling plays a crucial role in the specification of hematopoietic progenitors to the T-cell lineage.[40–43] Thus, immunodeficient mice transplanted with precursor cells expressing a constitutively active form of NOTCH1 develop into T-cells and fail to generate B-cells.[42] Conversely, inactivation of Notch1 in bone marrow or immature lymphoid precursors blocks the development of T-cells and favors their differentiation towards the B-cell lineage.[43–47] In addition, NOTCH1 signaling is essential for proliferation and survival of immature thymocytes[48] and regulates lineage decisions between αβ vs. γδ T-cell lineages.[49]
Role of NOTCH1 in T-ALL
The first evidence linking aberrant NOTCH1 signaling to the pathogenesis of T-ALL came from the analysis of the t(7;9)(q34;q34.3) translocation, a recurrent chromosomal abnormality present in 1% of human T-ALL cases.[50] Characterization of the chromosomal breakpoints associated with this rearrangement showed that this translocation truncates the NOTCH1 gene and misplaces it to the vicinity of the TCRB locus.[50, 51] As result, t(7;9)(q34;q34.3) bearing cells express very high levels of the a truncated and constitutively active form of NOTCH1.[50] The oncogenic activity of aberrant NOTCH1 signaling in T-cell transformation was further demonstrated when mice transplanted with hematopoietic progenitors cells transduced with viruses driving the expression of ICN1 developed T-ALL.[52–54] Similarly, transgenic mice expressing ICN1 in hematopoietic progenitor cells or in immature thymocytes developed T-cell tumors[52, 55] and NOTCH1 activation was found to be a common event in retrovirally induced T-cell neoplasias in mice.[56]
Although the molecular mechanisms by which aberrant NOTCH1 signaling contributes to T-cell transformation are not fully understood yet, analysis of proviral integration sites in mouse T-cell tumors generated by insertional mutagenesis has shown that oncogenic Notch1 can cooperate with other transcription factor oncogenes such as c-MYC,[56] E2A-PBX[57] and Ikaros.[58] Moreover, transduction of bone marrow progenitors deficient in pre-TCR signaling (rag2−/− or slp76−/−) with constitutively active forms of NOTCH1 fails to induce leukemia, demonstrating that aberrant NOTCH1 signaling requires the cooperation of additional signal transduction pathways to exert its leukemogenic potential.[54, 59]
Mutations in the NOTCH1 gene
Despite the accumulating evidence of the strong oncogenic role of NOTCH1 activation in T-cells, the role of aberrant NOTCH1 signaling in human T-ALL seemed to be limited to the very rare T-ALL cases harboring the t(7;9) translocation. This account changed drastically with the identification of activating mutations in the NOTCH1 gene in over 50% of human T-ALL samples (Table 1 and Figures 2 and 3).[23] Notably, NOTCH1 mutations can be found across multiple molecular groups of T-ALL defined by the expression of transcription factor oncogenes such as TLX1, TLX2, TAL1, LYL1, MLL-ENL, HOXA9 and CAML-AF10,[23, 60, 61] which sites aberrant activation of NOTCH1 at the center of T-ALL oncogenesis.
Table 1.
Oncogenic events leading to aberrant NOTCH1 signaling in T-ALL
| Mutation | Mechanism of action | Prevalence in T-ALL |
|---|---|---|
| t(7;9)(q34;q34) t(9;14)(q34.3;q11.2) |
NOTCH1 truncation and translocation to the TCR locus. Overexpression and ligand independent activation | 1% |
| NOTCH1 HD class I | Ligand hypersensitivity or independent activation | 40–45% |
| NOTCH1 HD class II | Ligand hypersensitivity or independent activation | 1% |
| NOTCH1 JME | Ligand hypersensitivity or independent activation | 3% |
| NOTCH1 ΔPEST | Increased activated NOTCH1 protein stability | 10–20% |
| FBXW7 mutations | Increased NOTCH1, c-MYC, Cyclin E, JUN and mTOR protein stability | 15% |
FIGURE 2. STRUCTURE AND MECHANISM OF ACTION OF ONCOGENIC FORMS OF NOTCH1.
NOTCH1 mutations located in the HD domain of the receptor increase ICN1 levels by inducing ligand independent NOTCH1 signaling. Truncations of the PEST domain of NOTCH1 (ΔPEST mutations) result in accumulation of ICN1 in the nucleus by impairing the degradation of activated NOTCH1.
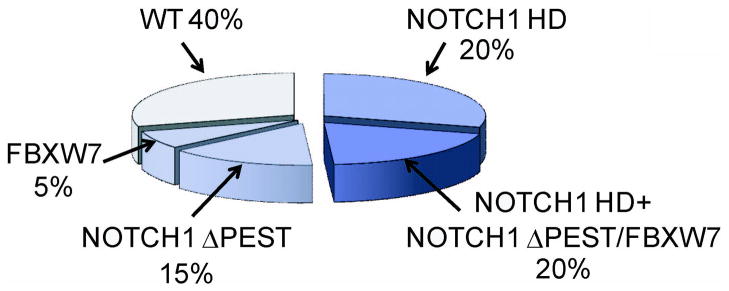
FIGURE 3. PREVALENCE OF ACTIVATING MUTATIONS IN NOTCH1 IN T-ALL.
HD and ΔPEST mutations account for the majority of activating mutations in NOTCH1 in T-ALL. An additional 1% of patients harbor translocations involving NOTCH1 and the TCR loci. NOTCH1 JME mutations related to HD mutant alleles are found in 3% of all T-ALLs. Finally about 15% of T-ALL cases harbor deletions or mutations in FBXW7, which are functionally related to NOTCH1 ΔPEST mutant alleles.
NOTCH1 mutations found in T-ALL are located at specific hotspots and affect critical negative regulatory elements built in the structure of the protein. The most frequent class of NOTCH1 mutations, present in 40–45% of human T-ALLs, are single amino acid substitutions or short in frame insertions or deletions in exons 26 and 27, which encode the N-terminal and C-terminal components of the heterodimerization domain, respectively (Figures 2 and 3).[23] Most of these so called HD mutations result in ligand independent activation or ligand hypersensitivity by disrupting the structure of the self-inactivating HD-LNR repeat complex.[62] A second class of HD mutations found in rare cases of T-ALL consist of in-frame insertions located in the 3′ region of exon 27, which expose the S2 processing site to ADAM cleavage by displacing it outside the protective HD-LNR complex.[23, 62] A related, but functionally distinct class of mutations present in 3% of T-ALLs, are located in exon 28 and consist of in frame insertions in the extracellular juxtamembrane portion of the receptor.[63] These so called JME, for JuxtaMembrane Expansion, mutations displace the HD-LNR domains away from the plasma membrane and result in increased S2 cleavage probably by inducing some conformational change in the HD-LNR complex.[63] An additional 10–20% of T-ALL cases harbor NOTCH1 mutations located in the 3′ end of exon 34 and consist of premature stop codons and frameshift insertions or deletions, which result in truncations of the PEST domain in the C-terminal region of the protein (Figures 2 and 3).[23] In contrast with mutations located in the extracellular portion of NOTCH1, all of which result in increased S2 and S3 cleavage, truncations of the intracellular PEST domain lead to aberrantly prolonged NOTCH1 signaling in the nucleus by impairing the proteasomal degradation of ICN1. A related mechanism leading to increased nuclear levels of activated NOTCH1 protein are deletions and mutations in FBXW7, which encodes the F-box protein responsible for targeting ICN1 to proteasomal degradation in the nucleus[64–66] (Figure 3). An important observation is that HD mutations frequently coexist in cis with truncations of the PEST domain or with FBXW7 mutations, which results in very high levels of ICN1 driven by the combined action of increased ligand independent S2 and S3 cleavage at the cell surface and impaired degradation of ICN1 in the nucleus (Figure 3) [23]. This finding suggests that there is continuous pressure during the process T-cell transformation to acquire higher levels of NOTCH signaling. Notably, the initiating role of NOTCH1 mutations in the leukemogenic process has been demonstrated by the identification of a NOTCH1 mutation in a T-ALL patient at both diagnosis and 7 years before the development of full blown leukemia [67]. However, NOTCH1 mutations may also play a role as secondary mutations involved in disease progression as indicated by the identification of NOTCH1 mutations in subclonal populations [68].
Targeting NOTCH1 Signaling in T-Cell Lymphoblasts
A fundamental mechanistic aspect of the oncogenic receptors encoded by NOTCH1 mutant alleles is that they are dependent on the S3, γ-secretase-mediated cleavage of the receptor for activation. Importantly, the presenilin γ-secretase complex has gained much attention as therapeutic target because of its role in the generation of pathogenic amyloid deposits in the brain of Alzheimer’s disease patients.[69] Consequently, small molecule γ-secretase inhibitors, or GSIs, originally developed for the treatment of Alzheimer’s disease could be exploited for the inhibition of oncogenic NOTCH1 signaling in T-ALL.[70]
Inhibition of NOTCH1 signaling with GSIs in T-ALL results in rapid clearance of ICN1 and transcriptional downregulation of NOTCH1 target genes.[23, 51, 71] These biochemical changes are coupled with decreased growth and proliferation characterized by G0/G1 cell cycle arrest and a decrease in cell size in some cell lines.[23, 51, 71–74] Following on these results, an open label, non-randomized, phase I clinical trial was initiated to test the activity of the MK-0752 GSI in relapsed T-ALL patients.[75] Unfortunately, the results of this study were less than encouraging, showing very limited antitumor activity and major gastrointestinal toxicity presumably resulting from inhibition of NOTCH signaling in the gut.[75]
The poor results of this clinical trial highlight several major difficulties in the clinical development of anti-NOTCH1 therapies for T-ALL. First, in contrast with mouse tumors in which inhibition of NOTCH1 signaling results in dramatic responses and high levels of apoptosis, GSIs seem to exert only a cytostatic effect against human T-ALLs.[23, 51] Moreover, primary resistance to NOTCH1 inhibition is highly prevalent in human T-ALL cell lines suggesting that this may also be a significant clinical problem in the treatment of primary T-ALL cases.[23, 73] Finally, the intestinal epithelium seems to be very sensitive to systemic inhibition of NOTCH signaling[75–79] and GSI treatment is associated with dose limiting gastrointestinal toxicity resulting from the accumulation of mucus secreting goblet cells in the gut.[75, 76] A problem that is probably accentuated by the fact that GSIs developed for the treatment of Alzheimer’s disease are formulated as oral drugs.
Conclusions
Better understanding of the oncogenic mechanisms controlled by NOTCH1 and of the genes and pathways mediating the effects of GSIs in the leukemic clone and the intestinal epithelium are clearly needed to overcome the difficulties of targeting NOTCH1 signaling in T-cell lymphoblasts. Gene expression profiling studies have shown that the genetic program controlled by NOTCH1 in T-ALL is dominated by genes involved in cellular metabolism including nucleotide, protein and ribosome biosynthesis.[71, 72] Moreover, identification of NOTCH1 direct target genes via ChIP-on-chip analysis showed that oncogenic NOTCH1 controls this cell growth and metabolism program via direct regulation of multiple anabolic genes and pathways, but also indirectly via upregulation of the cMYC oncogene.[71, 72, 80] Additional data has linked the oncogenic activity of NOTCH1 with the NFκB signaling pathway in T-ALL.[81] Thus, NOTCH signaling may turn on or enhance NFKB activity by increasing NFKB expression,[65] enhancing NFκB nuclear retention,[82] and via activation of IκB kinase.[83] Similarly, NOTCH1 seems to enhance the activity of the PI3K-AKT-mTOR signaling pathway at least in part via HES1-mediated transcriptional downregulation of the PTEN tumor suppressor gene.[73, 74, 84] Overall these studies highlight the central role of NOTCH1 as a central regulator of cell growth and metabolism in T-ALL and identify potential therapeutic strategies to enhance the activity of GSIs via inhibition of NFKB and the PI3K-AKT-mTOR pathways.[65, 73, 74] Finally, analysis of GSI sensitive and resistant T-ALL cell lines has shown that mutational loss of PTEN and consequent constitutive activation of the PI3K-AKT-mTOR pathway can induce GSI resistance by bypassing the requirement for NOTCH1 signaling for continuous growth and metabolism in the leukemic clone.[73] Notably, PTEN mutations, which result in complete loss of PTEN protein expression, have been found in 10–15% of T-ALL cases at diagnosis suggesting that analysis of PTEN expression by flow cytometry may identify patients with poor response to GSI treatment.[73]
Future Directions
Improved strategies for the clinical application of GSIs and NOTCH inhibition therapies will probably include: (i) the use of combination therapies that will increase the antileukemic effects of these drugs and improve their therapeutic window; (ii) the use of biomarkers to predict the response of T-ALL tumor cells to GSI treatment; (iii) new parenteral drug formulations aiming to avoid the toxic effects of inhibiting NOTCH signaling in the gut; and (iv) new classes of NOTCH1 inhibitory molecules such as anti-NOTCH1 inhibitory antibodies, which could be engineered to specifically block the NOTCH1 receptor in the leukemic clone.
Acknowledgments
Funding: This work was supported by the National Institutes of Health (R01CA120196 and R01CA129382 to A.F.); the WOLF Foundation (A.F), the Swim Across America Foundation, the Leukemia and Lymphoma Society (grants 1287-08 and 6237-08 to A.F.), the Charlotte Geyer Foundation (A.F.). Adolfo Ferrando is a Leukemia & Lymphoma Society Scholar. Teresa Palomero is a recipient of a Young Investigator Award from the Alex’s Lemonade Stand Foundation.
Footnotes
Conflict of Interest Page
Research funding from Merck (A.F) for preclinical testing of gamma-secretase inhibitors in T-ALL. No other conflicts of interest. ”
Contributor Information
Teresa Palomero, Assistant Professor of clinical Pathology, Department of Pathology, Institute for Cancer Genetics, Columbia University, New York, 10032
Adolfo Ferrando, Assistant Professor, Department of Pediatrics, Department of Pathology, Institute for Cancer Genetics, Columbia University, New York, 10032
References
- 1.Ferrando AA, Look AT. Clinical implications of recurring chromosomal and associated molecular abnormalities in acute lymphoblastic leukemia. Semin Hematol. 2000;37:381–395. doi: 10.1016/s0037-1963(00)90018-0. [DOI] [PubMed] [Google Scholar]
- 2.Pui CH, Relling MV, Downing JR. Acute lymphoblastic leukemia. N Engl J Med. 2004;350:1535–1548. doi: 10.1056/NEJMra023001. [DOI] [PubMed] [Google Scholar]
- 3.Pui C. Childhood leukemiased. Cambridge: Cambridge University Press; 1999. [Google Scholar]
- 4.Silverman LB, Sallan SE. Acute lymphoblastic leukemia. In: Nathan DG, Ginsburg D, Look AT, editors. Hematology of infancy and childhood. Philadelphia: W.B. Saunders; 2000. pp. 575–592. [Google Scholar]
- 5.Chessells JM, Bailey C, Richards SM. Intensification of treatment and survival in all children with lymphoblastic leukaemia: results of UK Medical Research Council trial UKALL X. Medical Research Council Working Party on Childhood Leukaemia. Lancet. 1995;345:143–148. doi: 10.1016/s0140-6736(95)90164-7. [DOI] [PubMed] [Google Scholar]
- 6.Pui CH, Evans WE. Acute lymphoblastic leukemia. N Engl J Med. 1998;339:605–615. doi: 10.1056/NEJM199808273390907. [DOI] [PubMed] [Google Scholar]
- 7.Rivera GK, Raimondi SC, Hancock ML, et al. Improved outcome in childhood acute lymphoblastic leukaemia with reinforced early treatment and rotational combination chemotherapy. Lancet. 1991;337:61–66. doi: 10.1016/0140-6736(91)90733-6. [DOI] [PubMed] [Google Scholar]
- 8.Schrappe M, Reiter A, Ludwig WD, et al. Improved outcome in childhood acute lymphoblastic leukemia despite reduced use of anthracyclines and cranial radiotherapy: results of trial ALL-BFM 90. German-Austrian-Swiss ALL-BFM Study Group. Blood. 2000;95:3310–3322. [PubMed] [Google Scholar]
- 9.Silverman LB, Gelber RD, Dalton VK, et al. Improved outcome for children with acute lymphoblastic leukemia: results of Dana-Farber Consortium Protocol 91–01. Blood. 2001;97:1211–1218. doi: 10.1182/blood.v97.5.1211. [DOI] [PubMed] [Google Scholar]
- 10.Thomas X, Boiron JM, Huguet F, et al. Outcome of treatment in adults with acute lymphoblastic leukemia: analysis of the LALA-94 trial. J Clin Oncol. 2004;22:4075–4086. doi: 10.1200/JCO.2004.10.050. [DOI] [PubMed] [Google Scholar]
- 11.Annino L, Vegna ML, Camera A, et al. Treatment of adult acute lymphoblastic leukemia (ALL): long-term follow-up of the GIMEMA ALL 0288 randomized study. Blood. 2002;99:863–871. doi: 10.1182/blood.v99.3.863. [DOI] [PubMed] [Google Scholar]
- 12.Linker C, Damon L, Ries C, Navarro W. Intensified and shortened cyclical chemotherapy for adult acute lymphoblastic leukemia. J Clin Oncol. 2002;20:2464–2471. doi: 10.1200/JCO.2002.07.116. [DOI] [PubMed] [Google Scholar]
- 13.Hunault M, Harousseau JL, Delain M, et al. Better outcome of adult acute lymphoblastic leukemia after early genoidentical allogeneic bone marrow transplantation (BMT) than after late high-dose therapy and autologous BMT: a GOELAMS trial. Blood. 2004;104:3028–3037. doi: 10.1182/blood-2003-10-3560. [DOI] [PubMed] [Google Scholar]
- 14.Czuczman MS, Dodge RK, Stewart CC, et al. Value of immunophenotype in intensively treated adult acute lymphoblastic leukemia: cancer and leukemia Group B study 8364. Blood. 1999;93:3931–3939. [PubMed] [Google Scholar]
- 15.Goldstone AH, Richards SM, Lazarus HM, et al. In adults with standard-risk acute lymphoblastic leukemia, the greatest benefit is achieved from a matched sibling allogeneic transplantation in first complete remission, and an autologous transplantation is less effective than conventional consolidation/maintenance chemotherapy in all patients: final results of the International ALL Trial (MRC UKALL XII/ECOG E2993) Blood. 2008;111:1827–1833. doi: 10.1182/blood-2007-10-116582. [DOI] [PubMed] [Google Scholar]
- 16.Fielding AK, Richards SM, Chopra R, et al. Outcome of 609 adults after relapse of acute lymphoblastic leukemia (ALL); an MRC UKALL12/ECOG 2993 study. Blood. 2007;109:944–950. doi: 10.1182/blood-2006-05-018192. [DOI] [PubMed] [Google Scholar]
- 17.Barrett AJ, Horowitz MM, Pollock BH, et al. Bone marrow transplants from HLA-identical siblings as compared with chemotherapy for children with acute lymphoblastic leukemia in a second remission. N Engl J Med. 1994;331:1253–1258. doi: 10.1056/NEJM199411103311902. [DOI] [PubMed] [Google Scholar]
- 18.Biggs JC, Horowitz MM, Gale RP, et al. Bone marrow transplants may cure patients with acute leukemia never achieving remission with chemotherapy. Blood. 1992;80:1090–1093. [PubMed] [Google Scholar]
- 19.Dopfer R, Henze G, Bender-Gotze C, et al. Allogeneic bone marrow transplantation for childhood acute lymphoblastic leukemia in second remission after intensive primary and relapse therapy according to the BFM- and CoALL-protocols: results of the German Cooperative Study. Blood. 1991;78:2780–2784. [PubMed] [Google Scholar]
- 20.Forman SJ, Schmidt GM, Nademanee AP, et al. Allogeneic bone marrow transplantation as therapy for primary induction failure for patients with acute leukemia. J Clin Oncol. 1991;9:1570–1574. doi: 10.1200/JCO.1991.9.9.1570. [DOI] [PubMed] [Google Scholar]
- 21.Schroeder H, Gustafsson G, Saarinen-Pihkala UM, et al. Allogeneic bone marrow transplantation in second remission of childhood acute lymphoblastic leukemia: a population-based case control study from the Nordic countries. Bone Marrow Transplant. 1999;23:555–560. doi: 10.1038/sj.bmt.1701617. [DOI] [PubMed] [Google Scholar]
- 22.Ochs J, Mulhern R. Long-term sequelae of therapy for childhood acute lymphoblastic leukaemia. Baillieres Clin Haematol. 1994;7:365–376. doi: 10.1016/s0950-3536(05)80208-2. [DOI] [PubMed] [Google Scholar]
- 23.Weng AP, Ferrando AA, Lee W, et al. Activating mutations of NOTCH1 in human T cell acute lymphoblastic leukemia. Science. 2004;306:269–271. doi: 10.1126/science.1102160. [DOI] [PubMed] [Google Scholar]
- 24.Rand MD, Grimm LM, Artavanis-Tsakonas S, et al. Calcium depletion dissociates and activates heterodimeric notch receptors. Mol Cell Biol. 2000;20:1825–1835. doi: 10.1128/mcb.20.5.1825-1835.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sanchez-Irizarry C, Carpenter AC, Weng AP, Pear WS, Aster JC, Blacklow SC. Notch subunit heterodimerization and prevention of ligand-independent proteolytic activation depend, respectively, on a novel domain and the LNR repeats. Mol Cell Biol. 2004;24:9265–9273. doi: 10.1128/MCB.24.21.9265-9273.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Aster JC, Pear WS, Blacklow SC. Notch Signaling in Leukemia. Annu Rev Pathol. 2008;3:587–613. doi: 10.1146/annurev.pathmechdis.3.121806.154300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mumm JS, Schroeter EH, Saxena MT, et al. A ligand-induced extracellular cleavage regulates gamma-secretase-like proteolytic activation of Notch1. Mol Cell. 2000;5:197–206. doi: 10.1016/s1097-2765(00)80416-5. [DOI] [PubMed] [Google Scholar]
- 28.Brou C, Logeat F, Gupta N, et al. A novel proteolytic cleavage involved in Notch signaling: the role of the disintegrin-metalloprotease TACE. Mol Cell. 2000;5:207–216. doi: 10.1016/s1097-2765(00)80417-7. [DOI] [PubMed] [Google Scholar]
- 29.Yu G, Nishimura M, Arawaka S, et al. Nicastrin modulates presenilin-mediated notch/glp-1 signal transduction and betaAPP processing. Nature. 2000;407:48–54. doi: 10.1038/35024009. [DOI] [PubMed] [Google Scholar]
- 30.Chen F, Yu G, Arawaka S, et al. Nicastrin binds to membrane-tethered Notch. Nat Cell Biol. 2001;3:751–754. doi: 10.1038/35087069. [DOI] [PubMed] [Google Scholar]
- 31.Shah S, Lee SF, Tabuchi K, et al. Nicastrin functions as a gamma-secretase-substrate receptor. Cell. 2005;122:435–447. doi: 10.1016/j.cell.2005.05.022. [DOI] [PubMed] [Google Scholar]
- 32.Schroeter EH, Kisslinger JA, Kopan R. Notch-1 signalling requires ligand-induced proteolytic release of intracellular domain. Nature. 1998;393:382–386. doi: 10.1038/30756. [DOI] [PubMed] [Google Scholar]
- 33.De Strooper B, Annaert W, Cupers P, et al. A presenilin-1-dependent gamma-secretase-like protease mediates release of Notch intracellular domain. Nature. 1999;398:518–522. doi: 10.1038/19083. [DOI] [PubMed] [Google Scholar]
- 34.Struhl G, Greenwald I. Presenilin is required for activity and nuclear access of Notch in Drosophila. Nature. 1999;398:522–525. doi: 10.1038/19091. [DOI] [PubMed] [Google Scholar]
- 35.Lai EC. Protein degradation: four E3s for the notch pathway. Curr Biol. 2002;12:R74–78. doi: 10.1016/s0960-9822(01)00679-0. [DOI] [PubMed] [Google Scholar]
- 36.Stancheva I, Collins AL, Van den Veyver IB, Zoghbi H, Meehan RR. A mutant form of MeCP2 protein associated with human Rett syndrome cannot be displaced from methylated DNA by notch in Xenopus embryos. Mol Cell. 2003;12:425–435. doi: 10.1016/s1097-2765(03)00276-4. [DOI] [PubMed] [Google Scholar]
- 37.Mumm JS, Kopan R. Notch signaling: from the outside in. Dev Biol. 2000;228:151–165. doi: 10.1006/dbio.2000.9960. [DOI] [PubMed] [Google Scholar]
- 38.Fryer CJ, White JB, Jones KA. Mastermind recruits CycC:CDK8 to phosphorylate the Notch ICD and coordinate activation with turnover. Mol Cell. 2004;16:509–520. doi: 10.1016/j.molcel.2004.10.014. [DOI] [PubMed] [Google Scholar]
- 39.Weinmaster G, Kintner C. Modulation of notch signaling during somitogenesis. Annu Rev Cell Dev Biol. 2003;19:367–395. doi: 10.1146/annurev.cellbio.19.111301.115434. [DOI] [PubMed] [Google Scholar]
- 40.Jaleco AC, Neves H, Hooijberg E, et al. Differential effects of Notch ligands Delta-1 and Jagged-1 in human lymphoid differentiation. J Exp Med. 2001;194:991–1002. doi: 10.1084/jem.194.7.991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pear WS, Radtke F. Notch signaling in lymphopoiesis. Semin Immunol. 2003;15:69–79. doi: 10.1016/s1044-5323(03)00003-4. [DOI] [PubMed] [Google Scholar]
- 42.Pui JC, Allman D, Xu L, et al. Notch1 expression in early lymphopoiesis influences B versus T lineage determination. Immunity. 1999;11:299–308. doi: 10.1016/s1074-7613(00)80105-3. [DOI] [PubMed] [Google Scholar]
- 43.Radtke F, Wilson A, Stark G, et al. Deficient T cell fate specification in mice with an induced inactivation of Notch1. Immunity. 1999;10:547–558. doi: 10.1016/s1074-7613(00)80054-0. [DOI] [PubMed] [Google Scholar]
- 44.Wilson A, MacDonald HR, Radtke F. Notch 1-deficient common lymphoid precursors adopt a B cell fate in the thymus. J Exp Med. 2001;194:1003–1012. doi: 10.1084/jem.194.7.1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Izon DJ, Punt JA, Pear WS. Deciphering the role of Notch signaling in lymphopoiesis. Curr Opin Immunol. 2002;14:192–199. doi: 10.1016/s0952-7915(02)00321-7. [DOI] [PubMed] [Google Scholar]
- 46.Maillard I, Weng AP, Carpenter AC, et al. Mastermind critically regulates Notch-mediated lymphoid cell fate decisions. Blood. 2004;104:1696–1702. doi: 10.1182/blood-2004-02-0514. [DOI] [PubMed] [Google Scholar]
- 47.Radtke F, Ferrero I, Wilson A, Lees R, Aguet M, MacDonald HR. Notch1 deficiency dissociates the intrathymic development of dendritic cells and T cells. J Exp Med. 2000;191:1085–1094. doi: 10.1084/jem.191.7.1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Schmitt TM, Ciofani M, Petrie HT, Zuniga-Pflucker JC. Maintenance of T cell specification and differentiation requires recurrent notch receptor-ligand interactions. J Exp Med. 2004;200:469–479. doi: 10.1084/jem.20040394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Washburn T, Schweighoffer E, Gridley T, et al. Notch activity influences the alphabeta versus gammadelta T cell lineage decision. Cell. 1997;88:833–843. doi: 10.1016/s0092-8674(00)81929-7. [DOI] [PubMed] [Google Scholar]
- 50.Ellisen LW, Bird J, West DC, et al. TAN-1, the human homolog of the Drosophila notch gene, is broken by chromosomal translocations in T lymphoblastic neoplasms. Cell. 1991;66:649–661. doi: 10.1016/0092-8674(91)90111-b. [DOI] [PubMed] [Google Scholar]
- 51.Palomero T, Barnes KC, Real PJ, et al. CUTLL1, a novel human T-cell lymphoma cell line with t(7;9) rearrangement, aberrant NOTCH1 activation and high sensitivity to gamma-secretase inhibitors. Leukemia. 2006;20:1279–1287. doi: 10.1038/sj.leu.2404258. [DOI] [PubMed] [Google Scholar]
- 52.Pear WS, Aster JC, Scott ML, et al. Exclusive development of T cell neoplasms in mice transplanted with bone marrow expressing activated Notch alleles. J Exp Med. 1996;183:2283–2291. doi: 10.1084/jem.183.5.2283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Aster J, Pear W, Hasserjian R, et al. Functional analysis of the TAN-1 gene, a human homolog of Drosophila notch. Cold Spring Harb Symp Quant Biol. 1994;59:125–136. doi: 10.1101/sqb.1994.059.01.016. [DOI] [PubMed] [Google Scholar]
- 54.Allman D, Karnell FG, Punt JA, et al. Separation of Notch1 promoted lineage commitment and expansion/transformation in developing T cells. J Exp Med. 2001;194:99–106. doi: 10.1084/jem.194.1.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Deftos ML, Bevan MJ. Notch signaling in T cell development. Curr Opin Immunol. 2000;12:166–172. doi: 10.1016/s0952-7915(99)00067-9. [DOI] [PubMed] [Google Scholar]
- 56.Girard L, Hanna Z, Beaulieu N, et al. Frequent provirus insertional mutagenesis of Notch1 in thymomas of MMTVD/myc transgenic mice suggests a collaboration of c-myc and Notch1 for oncogenesis. Genes Dev. 1996;10:1930–1944. doi: 10.1101/gad.10.15.1930. [DOI] [PubMed] [Google Scholar]
- 57.Feldman BJ, Hampton T, Cleary ML. A carboxy-terminal deletion mutant of Notch1 accelerates lymphoid oncogenesis in E2A-PBX1 transgenic mice. Blood. 2000;96:1906–1913. [PubMed] [Google Scholar]
- 58.Beverly LJ, Capobianco AJ. Perturbation of Ikaros isoform selection by MLV integration is a cooperative event in Notch(IC)-induced T cell leukemogenesis. Cancer Cell. 2003;3:551–564. doi: 10.1016/s1535-6108(03)00137-5. [DOI] [PubMed] [Google Scholar]
- 59.Felli MP, Vacca A, Calce A, et al. PKC theta mediates pre-TCR signaling and contributes to Notch3-induced T-cell leukemia. Oncogene. 2005;24:992–1000. doi: 10.1038/sj.onc.1208302. [DOI] [PubMed] [Google Scholar]
- 60.Ferrando AA, Neuberg DS, Staunton J, et al. Gene expression signatures define novel oncogenic pathways in T cell acute lymphoblastic leukemia. Cancer Cell. 2002;1:75–87. doi: 10.1016/s1535-6108(02)00018-1. [DOI] [PubMed] [Google Scholar]
- 61.Soulier J, Clappier E, Cayuela JM, et al. HOXA genes are included in genetic and biologic networks defining human acute T-cell leukemia (T-ALL) Blood. 2005;106:274–286. doi: 10.1182/blood-2004-10-3900. [DOI] [PubMed] [Google Scholar]
- 62.Malecki MJ, Sanchez-Irizarry C, Mitchell JL, et al. Leukemia-associated mutations within the NOTCH1 heterodimerization domain fall into at least two distinct mechanistic classes. Mol Cell Biol. 2006;26:4642–4651. doi: 10.1128/MCB.01655-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sulis ML, Williams O, Palomero T, et al. NOTCH1 extracellular juxtamembrane expansion mutations in T-ALL. Blood. 2008;112:733–740. doi: 10.1182/blood-2007-12-130096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.O’Neil J, Grim J, Strack P, et al. FBW7 mutations in leukemic cells mediate NOTCH pathway activation and resistance to gamma-secretase inhibitors. J Exp Med. 2007;204:1813–1824. doi: 10.1084/jem.20070876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Thompson BJ, Buonamici S, Sulis ML, et al. The SCFFBW7 ubiquitin ligase complex as a tumor suppressor in T cell leukemia. J Exp Med. 2007;204:1825–1835. doi: 10.1084/jem.20070872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Malyukova A, Dohda T, von der Lehr N, et al. The tumor suppressor gene hCDC4 is frequently mutated in human T-cell acute lymphoblastic leukemia with functional consequences for Notch signaling. Cancer Res. 2007;67:5611–5616. doi: 10.1158/0008-5472.CAN-06-4381. [DOI] [PubMed] [Google Scholar]
- 67.Eguchi-Ishimae M, Eguchi M, Kempski H, Greaves M. NOTCH1 mutation can be an early, prenatal genetic event in T-ALL. Blood. 2008;111:376–378. doi: 10.1182/blood-2007-02-074690. [DOI] [PubMed] [Google Scholar]
- 68.Mansour MR, Duke V, Foroni L, et al. Notch-1 mutations are secondary events in some patients with T-cell acute lymphoblastic leukemia. Clin Cancer Res. 2007;13:6964–6969. doi: 10.1158/1078-0432.CCR-07-1474. [DOI] [PubMed] [Google Scholar]
- 69.Kimberly WT, Esler WP, Ye W, et al. Notch and the amyloid precursor protein are cleaved by similar gamma-secretase(s) Biochemistry. 2003;42:137–144. doi: 10.1021/bi026888g. [DOI] [PubMed] [Google Scholar]
- 70.Pollack SJ, Lewis H. Secretase inhibitors for Alzheimer’s disease: challenges of a promiscuous protease. Curr Opin Investig Drugs. 2005;6:35–47. [PubMed] [Google Scholar]
- 71.Palomero T, Lim WK, Odom DT, et al. NOTCH1 directly regulates c-MYC and activates a feed-forward-loop transcriptional network promoting leukemic cell growth. Proc Natl Acad Sci U S A. 2006;103:18261–18266. doi: 10.1073/pnas.0606108103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Weng AP, Millholland JM, Yashiro-Ohtani Y, et al. c-Myc is an important direct target of Notch1 in T-cell acute lymphoblastic leukemia/lymphoma. Genes Dev. 2006;20:2096–2109. doi: 10.1101/gad.1450406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Palomero T, Sulis ML, Cortina M, et al. Mutational loss of PTEN induces resistance to NOTCH1 inhibition in T-cell leukemia. Nat Med. 2007;13:1203–1210. doi: 10.1038/nm1636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Chan SM, Weng AP, Tibshirani R, Aster JC, Utz PJ. Notch signals positively regulate activity of the mTOR pathway in T-cell acute lymphoblastic leukemia. Blood. 2007;110:278–286. doi: 10.1182/blood-2006-08-039883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Deangelo D, Stone R, Silverman L, et al. A phase I clinical trial of the notch inhibitor MK-0752 in patients with T-cell acute lymphoblastic leukemia/lymphoma (T-ALL) and other leukemias. Journal of Clinical Oncology, 2006 ASCO Annual Meeting Proceedings Part I. 2006;24:6585. [Google Scholar]
- 76.Milano J, McKay J, Dagenais C, et al. Modulation of notch processing by gamma-secretase inhibitors causes intestinal goblet cell metaplasia and induction of genes known to specify gut secretory lineage differentiation. Toxicol Sci. 2004;82:341–358. doi: 10.1093/toxsci/kfh254. [DOI] [PubMed] [Google Scholar]
- 77.van Es JH, van Gijn ME, Riccio O, et al. Notch/gamma-secretase inhibition turns proliferative cells in intestinal crypts and adenomas into goblet cells. Nature. 2005;435:959–963. doi: 10.1038/nature03659. [DOI] [PubMed] [Google Scholar]
- 78.Wong GT, Manfra D, Poulet FM, et al. Chronic treatment with the gamma-secretase inhibitor LY-411,575 inhibits beta-amyloid peptide production and alters lymphopoiesis and intestinal cell differentiation. J Biol Chem. 2004;279:12876–12882. doi: 10.1074/jbc.M311652200. [DOI] [PubMed] [Google Scholar]
- 79.Searfoss GH, Jordan WH, Calligaro DO, et al. Adipsin, a biomarker of gastrointestinal toxicity mediated by a functional gamma-secretase inhibitor. J Biol Chem. 2003;278:46107–46116. doi: 10.1074/jbc.M307757200. [DOI] [PubMed] [Google Scholar]
- 80.Sharma VM, Calvo JA, Draheim KM, et al. Notch1 contributes to mouse T-cell leukemia by directly inducing the expression of c-myc. Mol Cell Biol. 2006;26:8022–8031. doi: 10.1128/MCB.01091-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Osipo C, Golde TE, Osborne BA, Miele LA. Off the beaten pathway: the complex cross talk between Notch and NF-kappaB. Lab Invest. 2008;88:11–17. doi: 10.1038/labinvest.3700700. [DOI] [PubMed] [Google Scholar]
- 82.Shin HM, Minter LM, Cho OH, et al. Notch1 augments NF-kappaB activity by facilitating its nuclear retention. EMBO J. 2006;25:129–138. doi: 10.1038/sj.emboj.7600902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Song LL, Peng Y, Yun J, et al. Notch-1 associates with IKKalpha and regulates IKK activity in cervical cancer cells. Oncogene. 2008;27:5833–5844. doi: 10.1038/onc.2008.190. [DOI] [PubMed] [Google Scholar]
- 84.Mungamuri SK, Yang X, Thor AD, Somasundaram K. Survival signaling by Notch1: mammalian target of rapamycin (mTOR)-dependent inhibition of p53. Cancer Res. 2006;66:4715–4724. doi: 10.1158/0008-5472.CAN-05-3830. [DOI] [PubMed] [Google Scholar]