Abstract
T cell proliferation and survival are regulated by the cytokine receptor common γ chain (γc)3-associated cytokines IL-2, IL-7 and IL-15 while IL-4, another γc-associated cytokine, is thought to primarily affect T cell quality rather than quantity. In contrast, our experiments reveal that endogenously produced IL-4 is a direct, non-redundant and potent stimulator of CD8+ T cell proliferation in antigen- and pathogen-induced CD8+ T cell responses. These stimulatory effects of IL-4 are observed in both BALB/c and C57BL/6 mice and activate both naïve and memory/activated phenotype CD8+ T cells, although the former are stimulated less than the latter. IL-4 effects are IL-7- and IL-15-independent, but MHC class I-dependent stimulation appears to be required for the mitogenic effect of IL-4 on naïve phenotype CD8+ T cells. Thus, endogenously produced IL-4 is an important regulator of quantitative as well as qualitative aspects of T cell immunity.
Keywords: Rodent, T Cells, Cell Proliferation, Cytokine Receptors, Cytokines
Introduction
T cell proliferation and survival are regulated by cytokines and MHC and costimulatory molecules on antigen presenting cells (1, 2). IL-2, IL-7, and IL-15, are particularly important for T cell homeostasis. IL-7 is critical for T lymphopoiesis and promotes survival of naïve, activated, and memory T cells (3), while IL-15 selectively promotes proliferative renewal of activated/memory CD8+ T cells (4). The role of IL-2 is more complex. IL-2- and IL-2Rα-deficient mice develop T lymphoproliferation because IL-2 is required for survival of T regulatory cells that limit conventional T cell proliferation (5). However, increased levels of IL-2 can also stimulate conventional T cells (6). All of these cytokines bind to receptors that contain cytokine receptor common γ chain (γc) (7) and activate Stat5 transcription factors that are essential for their T cell stimulatory effects (8).
In contrast, although IL-4 binds to a constitutively expressed receptor on T cells that includes γc (9) and is known to promote T cell survival, it fails to directly stimulate T cell proliferation in vitro (10, 11) and relatively little is known about its role in T cell homeostasis and expansion in vivo. In vivo studies have shown that treatment of mice with IL-4, in the form of IL-4/anti-IL-4 mAb complexes, can strongly stimulate CD8+ T cell proliferation (12) and that IL-4 produced by NKT cells in response to the synthetic ligand, α-galactosylceramide, can act through a Sta6-dependent mechanisms to enhance proliferation by donor CD8+ T cells transferred into an irradiated host (13). In contrast, T cell proliferation has never been shown to be influenced by IL-4 produced in response to a T cell-dependent Ag or an infectious agent. In fact, some in vivo studies suggest that IL-4 suppresses CD8+ T cell function in models of virus and worm infection, tumor rejection, and trauma (14–17) although other studies suggest that IL-4 is important for CD8+ T cell-mediated tumor rejection (18), CD8+ T cell-mediated host protection against malaria parasites (19), and the generation of CD8+ memory T cells (20). These apparently discrepant results led us to investigate the in vivo effects of exogenous and endogenously produced IL-4 on CD8+ T cells. We find that IL-4 has two opposite effects: 1) a direct, activating effect that potently promotes proliferation and survival, which is described in this paper; and 2) an indirect effect that inhibits and kills activated T cells, which will be described in a future publication.
Materials and Methods
Mice
Female BALB/c, C57BL/6, C57BL/6 IL-15-deficient and C57BL/6 β2-microglobulin-deficient mice were purchased from Taconic Farms. BALB/c SCID mice were purchased from Jackson Laboratory. BALB/c IL-4-deficient (21) and IL-4Rα-deficient (22) mice were a gift of Nancy Noben-Trauth (George Washington University). BALB/c Thy1.1 mice were a gift of Richard Dutton (Trudeau Institute). P14 TCR transgenic mice (23), whose T cells express a TCR specific for amino acids 33–41 of the lymphocytic choriomeningitis virus (LCMV) glycoprotein (gp) peptide, were a gift of Michael Jordan (Cincinnati Children’s Hospital Medical Center). B6.SJL Ptprca mice were purchased from Taconic. IL-4Rα-deficient C57BL/6 mice were a gift of Frank Brombacher (University of Cape Town). These strains were bred at Cincinnati Children’s Hospital Medical Center. All mice were age-, sex- and strain-matched with controls in each experiment. All mouse studies were approved by IACUCs and the Cincinnati Veterans Affairs Medical Center and Cincinnati Children’s Hospital Medical Center.
Antibodies and immunological reagents
The following hybridomas and plasmacytoma were produced as ascites in Pristane-primed athymic nude or BALB/c mice, and Abs were purified from ascites by (NH4)2SO4 precipitation and DE-52 (Whatman) cation exchange column chromatography: BVD4-1D11.2 (rat IgG2b anti-IL-4), GK1.5 (rat IgG2b anti-CD4), RA3-6B2 (rat IgG2a anti-mouse CD45R/B220), 2.43 (rat IgG2b anti-CD8), MPC-11 (control mouse IgG2b), 2.4G2 (rat IgG2b anti-mouse FcγRII/III/IV), 2D1 (mouse IgG1 anti-hen egg lysozyme, used as a control), M25 (mouse IgG2b anti-IL-7). 4-3 (mouse IgG1 anti-IL-4Rα) was a gift of Dr. Joel Tocker (Amgen). GaMD was produced and purified as described (24). Goat anti-mouse IL-4Rα antibody (GaMIL-4Rα) was produced by immunizing a goat s.c. with recombinant IL-4Rα (Amgen) in CFA and boosting with the same Ag in IFA. Immune serum was affinity purified on a column of the same Ag bound to Sepharose. Some antibodies were labeled with Sulfo-NHS-LC-biotin (Pierce), or Alexa Fluor 647 (Molecular Probes). PerCP- or PE-anti-CD8, biotin-anti-Ly6C, biotin-anti-CD44, APC-anti-CD62L, FITC-anti-I-Ab, PE-anti-CD19, FITC-anti-CD4, PE-anti-Thy1.2, anti-TCR Vβ8.1/8.2, FITC-anti-CD45.2, streptavidin-PE and streptavidin-PerCP were purchased from BD Biosciences. gp33-41 peptide from LCMV gp (sequence KAVYNFATM) was a gift of Joel Collier (University of Chicago).
Cytokines
Recombinant mouse IL-4 was purchased from PeproTech.
IL-4C
A long-acting formulation of IL-4 (IL-4C) was prepared by mixing IL-4 and BVD4-1D11 at a 2:1 molar (1:6 weight) ratio, which was then diluted in 1% autologous mouse serum in saline to the appropriate concentration for injection of mice. IL-4C has an in vivo half-life of ~24 h (unlike IL-4, which has an in vivo half-life of a few minutes) and slowly dissociates to maintain high IL-4 levels for 3–5 days (25). IL-4C does not fix complement or bind avidly to low affinity IgG receptors, because it contains a single IgG molecule. Neither can it bind to IL-4Rs (BVD4-1D11 is a blocking mAb). Studies in many experimental systems demonstrate that IL-4C has no effects in IL-4Rα-deficient mice and that all effects of IL-4C can be replicated by frequent injections of larger amounts of IL-4 (26–29).
Cell Sorting
Single cell suspensions were negatively sorted for CD8+ cells with a Milteny Biotec autoMACS; then positively sorted for either CD8+ cells or for CD44lowLy6ClowCD8+ cells with a BD FACSVantage.
Carboxyfluorescein diacetate, succinimidyl ester (CFSE) labeling
Single cell suspensions of cells at 20 × 106/ml in PBS were mixed with an equal volume of 2.5 μM/ml CFSE in PBS (Molecular Probes). Cells were incubated for 5 minutes at room temperature in the dark. Labeling was stopped by addition of FBS and cells were washed twice with PBS.
In vivo 5-bromo-2′-deoxyuridine (BrdU) labeling
Mice were injected i.p. 24 and 16 hr prior to staining, unless stated otherwise, with 0.2 ml of a 3 mg/ml solution of BrdU (Sigma).
Cultures
CFSE-labeled spleen cells were cultured at 10 × 106 cells/ml with or without IL-4 (20 ng/ml). ToPro3 (Molecular Probes) was used to gate out dead cells.
Immunofluorescence staining
Cells were stained for 30 min on ice with 1 μg each of appropriately labeled Abs. All staining was performed in the presence of 1 μg of unlabeled anti-mouse FcγRII/III mAb (24G2). Samples were stained for BrdU incorporation using instructions provided by BD Pharmingen in staining kit 559619. All samples were analyzed on a FACSCalibur equipped with a red diode laser (Becton Dickinson). Data analysis was performed with CellQuest software (BD). Light scatter gates were set to exclude most nonlymphoid cells and cells that had died before fixation except in the cases where ToPro3 exclusion was used to gate out dead cells.
Parasite inoculation
Mice were inoculated with 500 infective N. brasiliensis larvae (30) or 60–70 S. mansoni cercariae (31).
Allergen inoculation
Anesthetized mice were inoculated intratracheally with 50 μl (125 μg) of house dust mite antigen (Greer Labs) on d 0, 6, 13 and 16. Mice were sacrificed on d 17 and BAL was performed. Single cell suspensions of lung cells were prepared by librase digestion, followed by filtration through a strainer and through nylon gause.
Statistics
A 1-tailed t test was used to test the hypothesis that IL-4 was increasing T cell proliferation or number. A 2-tailed t test was used when 2 groups were compared in the absence of a pre-existing hypothesis. p values >.05 are reported as “not significant” (NS).
Results
Endogenously produced IL-4 contributes to T cell expansion during Th2 responses
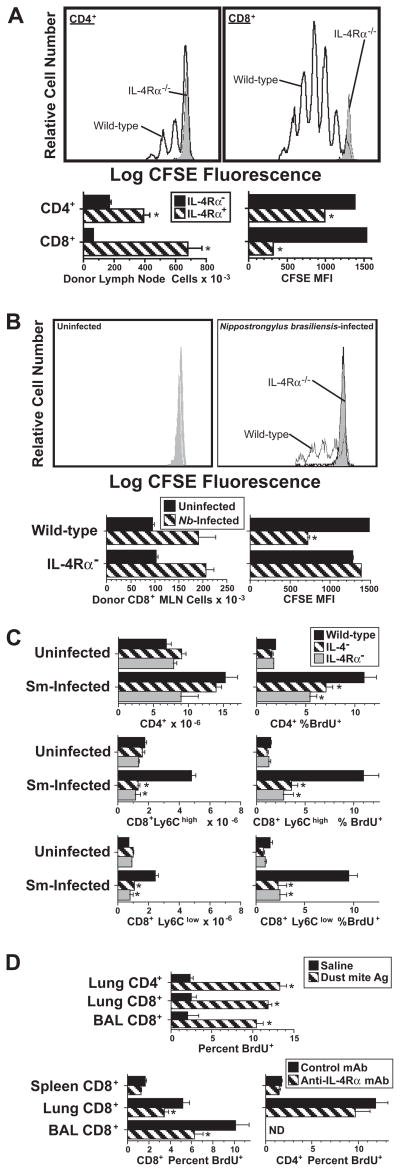
Recent studies demonstrate that injection of mice with IL-4, in the form of IL-4/anti-IL-4 mAb complexes, can stimulate CD8+ T cells in unimmunized mice to proliferate (12). To determine whether IL-4 generated during a Th2 immune response is sufficient to stimulate CD8+ T cell proliferation, we first investigated whether immunization with a T cell-dependent Ag, goat anti-mouse IgD antiserum (GaMD), or inoculation with the nematode parasite, Nippostrongylus brasiliensis, would have this effect. Mice were immunized with GaMD or inoculated with N. brasiliensis because these stimuli induce strong Th2 responses in IL-4Rα-deficient mice (32). Both GaMD immunization and N. brasiliensis inoculation induced dramatic proliferation by wild-type, but not IL-4Rα-deficient donor CD8+ T cells transferred into IL-4Rα-deficient recipients, with ~10-fold greater accumulation of IL-4Rα+ than IL-4Rα− donor CD8+ T cells in the GaMD system (Figures 1A and B). Much stronger splenic CD8+ T cell division (BrdU incorporation) and accumulation were also observed in S. mansoni-infected wild-type mice than in IL-4- or IL-4Rα-deficient mice; the ~3-fold increase in splenic CD8+ T cell number observed in infected wild-type mice was entirely IL-4 and IL-4Rα-dependent (Figure 1C). This was true both for CD8+ T cells that had expressed large amounts of a marker for memory/activated CD8+ cells, Ly6C (33, 34) and for CD8+ T cells that expressed only low amounts of this marker.
Figure 1. IL-4 produced during an immune response promotes CD8+ T cell proliferation.
A. BALB/c IL-4Rα-deficient mice were immunized with GaMD and injected i.v. 3 d later with 5 × 107 CFSE-labeled spleen cells from BALB/c wild-type or IL-4Rα-deficient mice. Axillary lymph node cells from recipients sacrificed 3 d later were stained for CD4 and CD8 and analyzed for numbers of CFSE+ CD4+ and CD8+ cells and CFSE fluorescence. CFSE staining histograms of cells from IL-4Rα-deficient mice are filled with grey; CFSE staining histograms of cells from wild-type mice are unfilled. N = 4. * signifies p <.05 compared to cells from IL-4Rα-deficient mice.
B. BALB/c IL-4Rα-deficient mice were left uninfected or were inoculated s.c. with 500 Nippostrongylus brasiliensis third-stage larvae on d 0 and injected i.v. on d 6 (when strong IL-4 secretion is first observed) with 5 × 107 CFSE-labeled spleen cells from BALB/c wild-type or IL-4Rα-deficient mice. Mesenteric lymph node cells from recipients sacrificed 3 d later were stained for CD8 and analyzed for numbers of CFSE+ CD8+ cells and their CFSE fluorescence. CFSE staining histograms of donor cells from IL-4Rα-deficient mice are filled with grey; CFSE staining histograms of donor cells from wild-type mice are unfilled. N = 3–4. * signifies p <.05 compared to cells from IL-4Rα-deficient mice.
C. Wild-type, IL-4-deficient, and IL-4Rα-deficient mice were inoculated with S. mansoni and injected twice with BrdU 7.5 wk later. Spleen cells obtained 1 d after that were stained for CD4, CD8, Ly6C and BrdU and analyzed for numbers of CD4+, CD8+Ly6Clow and CD8+Ly6Chigh cells and the percent of each cell type that had incorporated BrdU. N = 3–4; * signifies p <.05 compared to cells from wild-type mice.
D. Upper panel: BALB/c mice were inoculated i.t. on d 0, 6, 13, and 16 with saline or house dust mite Ag and injected twice with BrdU on d 16. BAL and lung lymphoid cells obtained 1 d later were stained for CD4, CD8 and BrdU and analyzed to determine percentages of CD4+ and CD8+ cells that had incorporated BrdU. N = 5; * signifies p <.05 compared to cells from saline-treated mice.
Lower panel: BALB/c mice were inoculated i.t. with saline or Ag as in the upper panel, injected with anti-IL-4Rα mAb or control mAb on d 15, and pulsed twice with BrdU on d 16. Spleen, BAL and lung cells obtained 1 d later were stained for CD4, CD8 and BrdU and analyzed for percentages of CD4+ and CD8+ T cells that had incorporated BrdU. N = 5; * signifies p <.05 compared to cells from control mAb-treated mice.
Because pulmonary CD8+ T cells contribute to airway hyperresponsiveness in a mouse model of asthma (35), we also determined whether pulmonary CD8+ T cell proliferation induced by inhalation of dust mite allergen is IL-4-dependent. Because Stat6, which is activated by IL-4R ligation (36–38), is important for cell homing to the lungs during a Th2 response (39), we used wild-type rather than IL-4Rα-deficient mice for this experiment. Intratracheal inoculation with dust mite allergen was used to induce a strong pulmonary Th2 response before treating mice with anti-IL-4Rα or control mAbs 2 d prior to terminating the experiment (Figure 1D, lower panels). Results demonstrated that: 1) dust mite allergen inoculation increased DNA synthesis (BrdU incorporation) by pulmonary T cells ~5-fold (Figure 1D, upper panel); and 2) anti-IL-4Rα mAb treatment significantly suppressed DNA synthesis (BrdU incorporation) by CD8+ T cells in the lungs and bronchi, but did not significantly affect DNA synthesis by lung CD4+ T cells or splenic CD4+ or CD8+ T cells (Figure 1D, lower panels). Thus, endogenously produced IL-4 can stimulate substantial CD8+ T cell proliferation and accumulation during a Th2 response.
IL-4 stimulates T cell proliferation in vivo but not in vitro
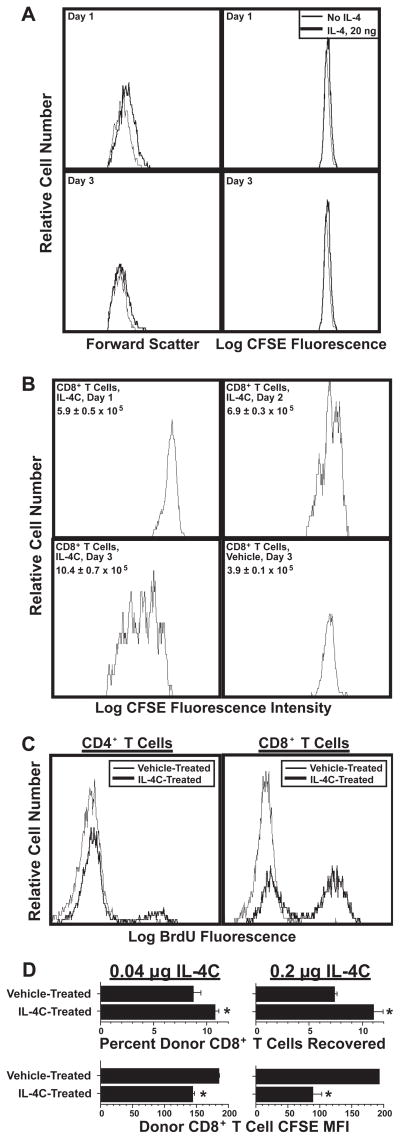
The above-described observations are consistent with previous reports of IL-4 stimulation or enhancement of CD8+ T cell proliferation in vivo (12, 13), but seemed to contradict in vitro studies that demonstrated that IL-4 enhances T cell survival (10, 11) and TCR crosslinking-dependent T cell proliferation (40), but fails to induce T cell proliferation in the absence of additional stimuli. Our own results confirm the negative in vitro finding: IL-4 stimulation initially induces a slight increase in T cell size (forward light scatter), but fails to induce cell division (Figure 2A). Because of this apparent discrepancy we repeated in vivo studies in which otherwise unstimulated mice are injected with recombinant IL-4 (12). Because IL-4 has a very short in vivo half-life, we treated mice with a long-acting formulation of recombinant IL-4 (IL-4/anti-IL-4 mAb complexes (IL-4C)) (25, 41). IL-4C stimulated large increases in T cell size and DNA synthesis (CFSE dilution) in vivo. IL-4-driven proliferation was evident 2 d after IL-4C injection and dramatic by d 3 (Figure 2B). IL-4C stimulated significant DNA synthesis (BrdU incorporation) by CD4+ T cells and considerably greater DNA synthesis by CD8+ T cells in 2–3 d (Figure 2C). Although the stimulatory effect of IL-4 was dose-related, even 40 ng of IL-4 (as IL-4C), an amount that increases B cell class II MHC expression less than endogenously produced IL-4 in worm-infected mice (25, 42), induced significant CD8+ T cell proliferation and accumulation (Figure 2D). Thus, physiological quantities of IL-4 induce CD8+ T cell proliferation and accumulation in vivo and IL-4-induced T cell proliferation must be co-stimulated by factors that are present only in vivo or blocked by inhibitors that are present only in vitro.
Figure 2. IL-4 induces T cell proliferation in vivo but not in vitro.
A. CFSE-labeled BALB/c spleen cells were cultured with or without IL-4. Cells were stained for CD8 after 1 or 3 d of culture and analyzed for CFSE fluorescence and forward light scatter. Similar results were obtained when cultures were supplemented daily with IL-4 (not shown).
B. 5.7 × 107 CFSE-labeled BALB/c spleen cells were transferred into recipient BALB/c mice, which were then injected i.p. every other day with vehicle or IL-4C (5 μg IL-4/30 μg anti-mouse IL-4 mAb) in vehicle. Spleen cells from recipient mice sacrificed 1, 2 or 3 d after cell transfer were analyzed for number of CD8+ CFSE+ cells and CD8+ T cell CFSE fluorescence.
C. BALB/c mice were injected i.p. on d 0 and 2 with vehicle or IL-4C as in “B” and twice with BrdU on d 2. Spleen cells obtained on d 3 were stained for CD4, CD8, and BrdU and analyzed for BrdU staining on CD4+ and CD8+ cells.
D. BALB/c IL-4Rα-deficient mice were injected i.v. with 5.3 × 106 (left panels) or 5.0 × 106 (right panels) CFSE-labeled wild-type BALB/c spleen cells. Recipients were also injected i.p. every other day with vehicle or IL-4C (0.04/0.24 μg (left panels) or 0.2/1.2 μg (right panels)). Spleen cells from recipient mice sacrificed 5 d after cell transfer were stained for CD8 and IL-4Rα and analyzed by flow cytometry for numbers of CD8+IL-4Rα+ cells and CFSE fluorescence. N = 4–5; * signifies p <.05 compared to cells from vehicle-treated mice.
IL-4 directly stimulates CD8+ T cells to proliferate
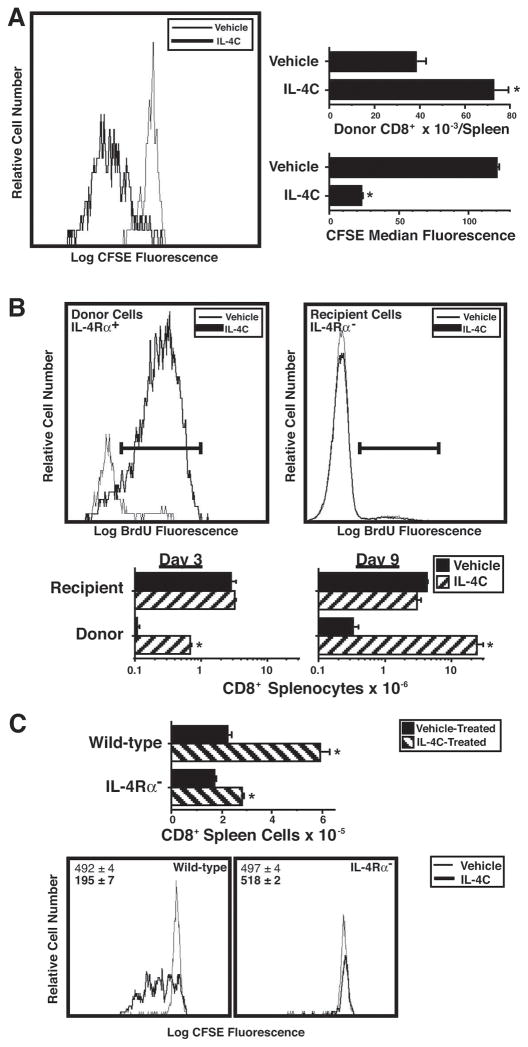
This in vitro/in vivo difference suggested that IL-4 might stimulate in vivo T cell proliferation indirectly. Both CFSE dilution and BrdU incorporation techniques were used to evaluate this possibility. CFSE-labeled, purified CD8+ T cells proliferated in response to IL-4C stimulation when transferred into IL-4Rα-deficient mice and nearly doubled in number over 3 d (Figure 3A). Even more dramatic results were observed when unlabeled, purified IL-4Rα+CD8+ T cells were transferred into IL-4Rα-deficient mice that were then stimulated with IL-4C for 3 or 9 d (Figure 3B). Greater than 80% of the transferred CD8+ T cells were synthesizing DNA by d 2–3 and recovery of transferred cells was ~6-fold greater in IL-4C-treated mice than in vehicle-treated mice at d 3. Donor cell number increased another 30–40-fold during the next 6 d of IL-4C treatment. In contrast, IL-4C treatment had no effect on the IL-4Rα-deficient host CD8+ T cells (Figure 3B) and stimulated proliferation by wild-type, but not IL-4Rα-deficient CFSE-labeled CD8+ T cells when both cell types were transferred into wild-type mice (Figure 3C). Thus, IL-4 directly stimulates CD8+ T cells to proliferate and has no indirect mitogenic effect on IL-4-unresponsive T cells.
Figure 3. IL-4 acts directly on CD8+ T cells to induce proliferation and accumulation.
A. BALB/c IL-4Rα-deficient mice were injected i.v. with 1.6 × 106 CFSE-labeled purified wild-type BALB/c splenic CD8+ cells, then injected i.p. every other day with vehicle or IL-4C (10 μg IL-4/60 μg anti-IL-4). Spleen cells obtained from recipient mice 3 d after cell transfer were stained for CD8, CD4 and IL-4Rα and analyzed for number and CFSE fluorescence of CD8+IL-4Rα+ cells. N = 4; * signifies p <.05 compared to cells from vehicle-treated mice.
B. BALB/c IL-4Rα-deficient mice were injected i.v. with 4 × 106 purified wild-type BALB/c splenic CD8+ cells on d 0, i.v. with anti-CD4 mAb on d 0 and 6; i.p. every other day with vehicle or IL-4C (10/60); and twice i.p. 1 d prior to sacrifice with BrdU. Spleen cells from recipient mice sacrificed 3 or 9 d after cell transfer were stained for IL-4Rα, CD8, and BrdU and analyzed for number and BrdU staining of CD8+IL-4Rα+ (donor) and CD8+IL-4Rα− (host) cells. Histograms are of cells from mice sacrificed 3 d after cell transfer. N = 3–4; * signifies p <.05 compared to cells from vehicle-treated mice.
C. Wild-type BALB/c mice were injected i.v. on d 0 with 4 × 107 CFSE-labeled wild-type or IL-4Rα-deficient splenic cells and injected i.p. every other day with vehicle or IL-4C (5/30). Spleen cells from mice sacrificed 3 d after cell transfer were stained for CD8 and IL-4Rα and analyzed for CFSE staining and number of donor CD8+IL-4Rα+ and IL-4Rα− cells. N = 4–5; * signifies p <.05 compared to cells from vehicle-treated mice.
Endogenously produced IL-4 can promote proliferation by bystander CD8+ cells and accumulation of antigen-activated CD8+ T cells
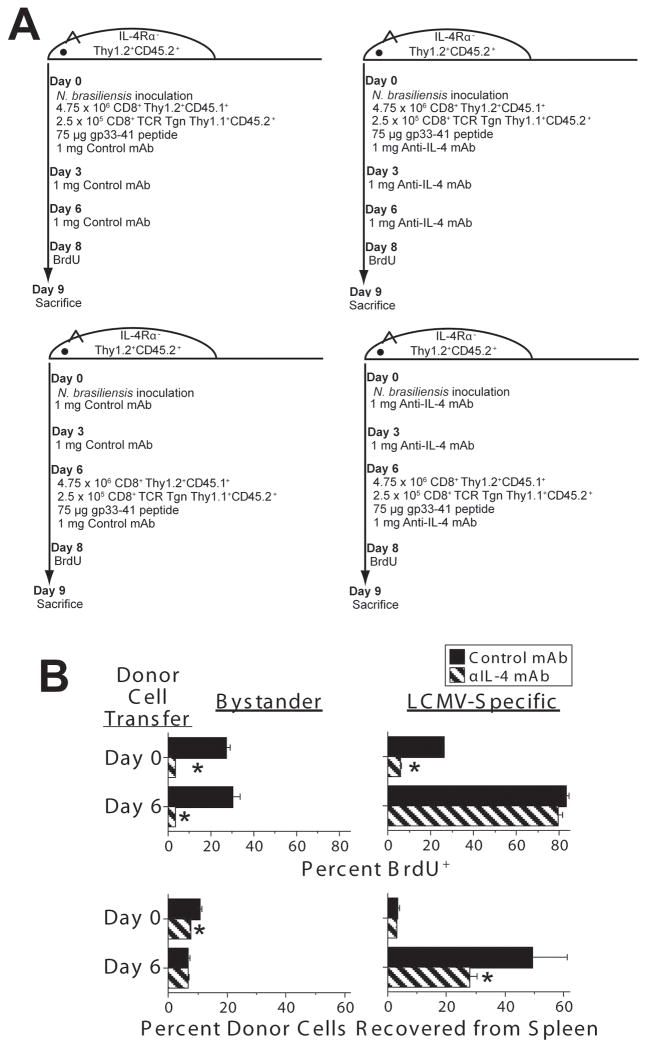
The very large percentage of CD8+ T cells that can be induced to incorporate BrdU by high concentrations of IL-4 suggested that this cytokine induces division by CD8+ T cells that are not being simultaneously stimulated by antigen (i.e., bystander cells). Relative effects of IL-4 on antigen-stimulated and bystander CD8+ T cells could theoretically be evaluated by transferring a mixture of antigen-specific TCR transgenic CD8+ T cells and conventional CD8+ T cells into recipient mice, immunizing these mice with an antigen that induces a Th2 response and is recognized by the transgenic TCR and comparing the responses of the TCR transgenic and conventional T cells. This approach was impractical, however, because of the non-availability of TCR transgenic CD8+ T cells that bind a peptide derived from an antigen that induces a Th2 response. Consequently, we required a more complex experimental protocol (Figure 4A) to compare the effects of endogenously produced IL-4 on antigen-activated vs. bystander CD8+ T cells.
Figure 4. Endogenously produced IL-4 enhances bystander CD8+ T cell proliferation and antigen-specific CD8+ T cell accumulation.
C57BL/6 IL-4Rα-deficient mice were inoculated with N. brasiliensis infective larvae on d 0 and injected i.v. with spleen cells that included 4.75 × 106 B6.SJL Ptprca CD8+ and 2.5 × 105 P14 TCR transgenic CD8+ T cells on d 0 or d 6. All donor cells were IL-4Rα-sufficient. All mice were injected i.v. with 75 μg of peptide GP33-41 2 hours after cell transfer. Mice were also injected i.p. on d 0, 3, and 6 with 1 mg of BVD4-1D11 anti-IL-4 mAb or J1.2 isotype control mAb. Mice were injected twice i.p. with BrdU on d 8 and sacrificed on d 9. Cells were stained for Thy1.1 and TCR Vβ8.1/8.2 to identify donor bystander and antigen-specific CD8+ T cells and for BrdU to identify cells that had synthesized DNA on the day prior to sacrifice. N = 4; * signifies p<0.05. The experimental protocol is diagramed in A, with results shown in B.
To do this, C57BL/6 IL-4Rα-deficient mice were inoculated with N. brasiliensis, which stimulates a relatively normal Th2 response in these mice. At the time of worm inoculation or 6 d later, when IL-4 production becomes considerable, these mice were inoculated with spleen cells that included 5 × 106 CD8+ T cells, 95% of which were from B6.SJL Ptprca mice (C57BL/6 background, CD45.1+ Thy1.2+) and 5% of which were from P14 mice (C57BL/6 background, Vβ8+ TCR transgene specific for LCMV gp33-41, CD45.2+ Thy1.1+). Both donor mouse strains were IL-4Rα-sufficient. To determine the effects of IL-4 on the antigen-specific and bystander donor CD8+ T cells, mice were injected i.p. with 1 mg of a blocking anti-IL-4 mAb (BVD4-1D11) or an isotype-matched control mAb (J1.2) on the day of worm inoculation and 3 and 6 d later. All mice were inoculated with 75 μg of gp33-41 peptide 2 h after the transfer of donor cells, injected twice i.p. with BrdU 8 d after worm inoculation and sacrificed 1 d later. Flow cytometry was used to differentiate donor gp33-41-specific T cells, which expressed Thy1.1, CD45.2, and Vβ8, from non-Ag-specific (bystander) donor cells (Thy 1.2+, CD45.1+) and host cells (Thy1.2+ CD45.2+), and to determine the percentages of antigen-specific and bystander cells that had divided (incorporated BrdU) during the 24 hr prior to sacrifice.
Results of this experiment (Figure 4B) demonstrate that donor bystander CD8+ T cells were induced to divide by endogenously produced IL-4 regardless of whether they were infused 9 or 3 d prior to sacrifice, but increased in number (percent recovered from the spleen) only if infused 9 d prior to sacrifice. Even then, the increase in number was quite small. gp33-41-specific donor CD8+ T cells were affected similarly to bystander CD8+ T cells when infused 9 d prior to sacrifice, but proliferated to a much greater extent, with or without IL-4, when infused 3 d prior to sacrifice. IL-4 affected the gp33-41-sepcific cells infused 3 d prior to sacrifice, however, by substantially increasing the percent that was recovered from spleen.
The difference in donor antigen-specific CD8+ cell recovery and BrdU incorporation when these cells were administered 0 vs. 6 d after N. brasiliensis may reflect the short half-life of the gp33-41 peptide, so that gp33-41-specific CD8+ cells no longer were responding to this antigen (and had mostly been eliminated) if inoculated, along with the peptide 9 d prior to sacrifice. We also suspect that an IL-4 contribution to antigen-specific T cell BrdU incorporation would have been observed had mice been administered a considerably lower dose of GP33-41 peptide. However, at a minimum, these observations demonstrate that IL-4 can induce bystander CD8+ T cell division and, under some circumstances, contribute to the accumulation of antigen-specific CD8+ T cells.
IL-4 is a more potent mitogen for memory than naïve CD8+ cells
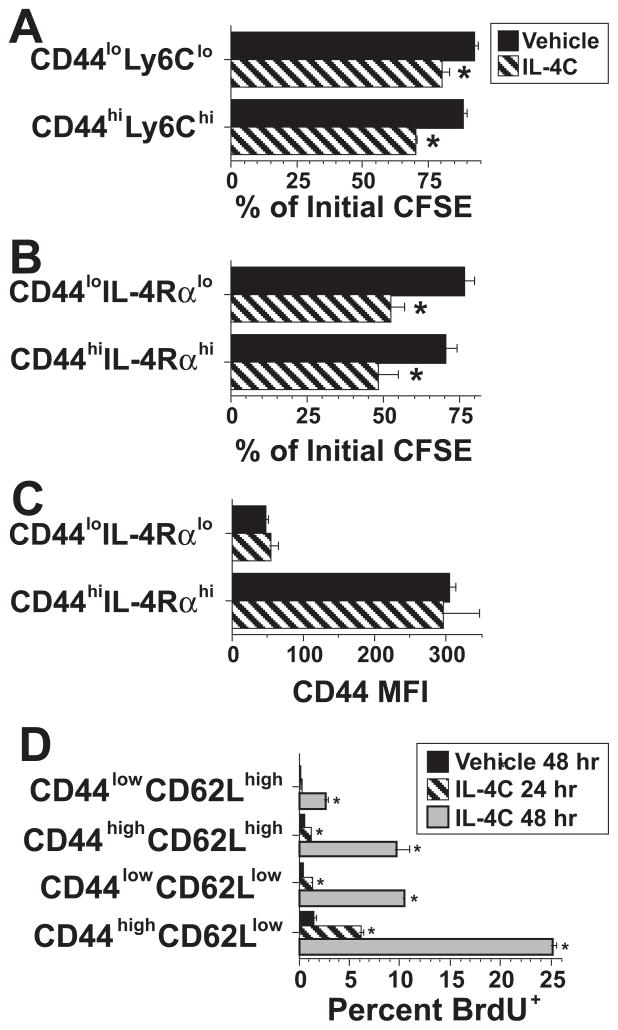
Because IL-4R is constitutively expressed on T cells (43), naïve T cells might be able to proliferate in response to IL-4. To test this, T cells, in two separate experiments, were sorted into CD44lowLy6Clow (naïve) and CD44highLy6Chigh (effector/memory) populations (Figure 5A), or CD44lowIL-4Rαlow (naïve) and CD44highIL-4Rαhigh (effector/memory) populations (Figure 5B), labeled with CFSE, and transferred into wild-type hosts that were then stimulated for 3 d with vehicle or IL-4C. Donor cell recovery in host spleens was insufficient in both experiments to clearly determine the average number of times donor cells had divided, even when over 2 million spleen cells were analyzed from each mouse. In addition, inability to detect cells that had divided > 2 times may have underestimated CFSE dilution in IL-4C-treated mice. However, enough donor cells were recovered to demonstrate greater CFSE dilution of both naïve and memory phenotype donor cells when recipient mice where treated with IL-4C than when they received saline (Figure 5A and B). Furthermore, evidence that CD44high and CD44low cells retained this phenotype after transfer and stimulation with IL-4C for 3 days (Figure 5C) demonstrated that this marker could be used to track cell division by IL-4C stimulated naïve and memory phenotype cells without having to perform cell transfer experiments with sorted cells.
Figure 5. In vivo IL-4 activation of naïve and activated/memory CD8+ T cells.
A. CD44lowLy6Clow (naïve) and CD44highLy6Chigh (effector/memory) wild-type BALB/c CD8+ spleen cells were purified by sequential magnetic and electronic cell sorting and CFSE-labeled. Wild-type BALB/c mice were injected i.v. with 7 × 105 of these cells and injected i.p. every other day with vehicle or IL-4C (5/30). Spleen cells from recipient mice sacrificed 3 d after cell transfer were stained for CD8 and analyzed for CFSE on CD8+CFSE+ cells. Mean CFSE fluorescence was determined for the entire population of CFSE+ CD8+ cells and for CFSE+ CD8+ cells that had the highest CFSE fluorescence. Percent CFSE dilution was determined by dividing the first number by the second. N = 4; * signifies p <.05 compared to cells from vehicle-treated mice.
B. BALB/c wild-type CD8+ T cells were sorted into CD44lowIL-4Rαlow (naïve) and CD44highIL-4Rαhigh (effector/memory) populations, labeled with CFSE and transferred into BALB/c IL-4Rα-deficient mice, which were then injected every other day with vehicle or IL-4C (5/30). Spleen cells from recipient mice sacrificed 3 d after cell transfer were stained for CD8 and analyzed for CFSE and CD44 on CD8+CFSE+ cells. CFSE dilution was determined as in “A.” N = 4; * signifies p <.05 compared to cells from vehicle-treated mice.
C. CD44 median fluorescence intensity was determined for CFSE+ CD8+ spleen cells from mice in the experiment described in “B” that had been infused with purified CD44lowIL-4Rαlow or CD44highIL-4Rαhigh CD8+ CFSE-labeled spleen cells and treated with vehicle or IL-4C.
D. BALB/c mice were treated with vehicle or IL-4C (5/30) for 24 or 48 hr and pulsed with BrdU for 4 hr prior to sacrifice. Spleen cells were prepared, counted and stained for CD8, CD44 and CD62L. BrdU incorporation was determined for CD8+ T cells from each of the 4 populations shown. N = 4; * signifies p <.05 compared to the same cell population from vehicle-treated mice.
Based on this observation, we evaluated the effect of in vivo IL-4C stimulation on BrdU incorporation by splenic CD44lowCD62Lhigh (naïve) and CD44highCD62Llow (effector memory) CD8+ T cells after 24 or 48 hr of in vivo stimulation with IL-4C. CD62L was used as a second marker to help differentiate splenic naïve vs. memory CD8+ T cells because it, like CD44, is not affected by IL-4 over a 3 day period, while in vivo IL-4C treatment upregulates IL-4Rα expression and downregulates Ly6C expression (data not shown). IL-4 induced greater and more rapid proliferation by the effector memory phenotype cells than the naïve phenotype cells, but some naïve phenotype cells were proliferating by 48 hrs (Figure 5D). Thus, IL-4 can stimulate DNA synthesis by naïve CD8+ T cells, but is a more potent mitogen for memory CD8+ cells.
T cell development in the absence of IL-4 does not prevent IL-4 responsiveness
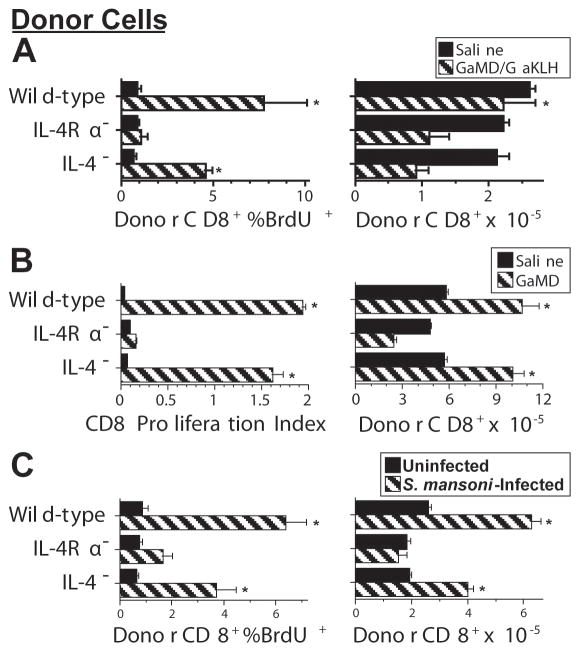
The greater proliferation of IL-4Rα-sufficient than IL-4Rα-deficient CD8+ T cells during Th2 responses might result from abnormal T cell development in the absence of IL-4, from the failure of IL-4Rα-deficient T cells to respond to IL-4 produced during the Th2 response, or from both effects. To evaluate these possibilities, we compared proliferative responses by equal numbers of donor CD8+ T cells from BALB/c background wild-type, IL-4-deficient, and IL-4Rα-deficient mice when transferred into GaMD-immunized IL-4Rα-deficient or wild-type mice or S. mansoni-infected wild-type mice (Figure 6, panels A, B and C, respectively). Results of these experiments demonstrate that CD8+ T cells from IL-4-deficient mice resemble CD8+ T cells from wild-type more closely than IL-4Rα-deficient mice in their proliferative responses. However, decreased accumulation of donor CD8+ T cells from IL-4-deficient mice when transferred into GaMD-immunized wild-type mice (Figure 6A) and a trend towards less proliferation of CD8+ T cells from IL-4-deficient than wild-type mice is all 3 experiments suggest that developmental effects of IL-4 on CD8+ T cells may also increase their responsiveness to IL-4 during a Th2 response.
Figure 6.
CD8+ T cells from IL-4-deficient mice proliferate in response to endogenously produced IL-4.A. BALB/c congenic mice that express Thy 1.1 (4/gp) were immunized i.p. with 0.2 ml of saline or GaMD on d 0, boosted i.p. with 0.2 ml of saline or GaKLH on d 3, injected with spleen cells from wild-type, IL-4Rα-deficient, or IL-4-deficient BALB/c donors that contained 4.5 × 106 CD8+ T cells on d 4, injected twice with BrdU on d 6, and sacrificed on d 7. Number and BrdU incorporation by donor (Thy1.2) splenic CD8+ T cells were determined by Coulter counting and flow cytometry. An asterisk indicated a significant response to GaMD/GaKLH compared to CD8+ T cells from GaMD/GaKLH-treated IL-4Rα-deficient mice in this experiment. Asterisks in the experiments shown in panels B and C similar indicate significant responses to GaMD or S. mansoni infection, respectively, by T cells from wild-type or IL-4-deficient mice as compared to T cells from IL-4Rα-deficient mice.
B. BALB/c IL-4Rα-deficient mice were immunized s.c. with GaMD on d 0 and injected with equal numbers of CFSE-labeled spleen cells from BALB/c wild-type, IL-4Ra-deficient, or IL-4-deficient mice on d 3 and sacrificed on d6. Numbers and proliferation indices (average number of cell divisions) of donor CD8+ T cells were determined by Coulter counting and flow cytometry.
C. BALB/c congenic mice that express Thy 1.1 were left untreated (n = 6) or inoculated with 60–70 S. mansoni cercaria on d 0 (n = 8) and injected with spleen cells from wild-type, IL-4Rα-deficient, or IL-4-deficient BALB/c donors that contained 3.5 × 106 CD8+ T cells on d 39, injected twice with BrdU on d 52, and sacrificed on d 53. Number and BrdU incorporation by donor (Thy1.2) splenic CD8+ T cells were determined by Coulter counting and flow cytometry.
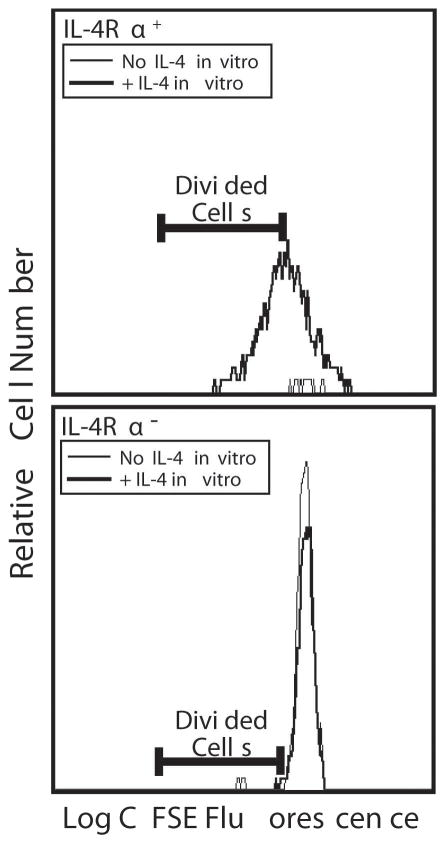
IL-4-activated CD8+ T cells become IL-4-dependent
T cells activated in vivo by IL-4 might stay activated, die, or revert to a resting state once exposure to IL-4 terminates. To distinguish among these possibilities, cells from wild-type mice were transferred into IL-4Rα-deficient mice, which were then stimulated with IL-4C for 5 d. Spleen cells from these IL-4C-stimulated mice were labeled with CFSE and cultured with or without IL-4. IL-4Rα+ (donor) CD8+ T cells from these mice continued to proliferate ex vivo in the presence of IL-4, albeit less rapidly than in vivo, but died in the absence of IL-4 (Figure 7, upper panel). In contrast, IL-4Rα− (host) CD8+ T cells survived to some extent ex vivo and were not affected by IL-4 (Figure 7, lower panel). Thus, IL-4-activated CD8+ T cells die without continuing stimulation. Consistent results were observed in vivo when ongoing IL-4-induced T cell proliferation and accumulation was blocked by anti-IL-4Rα mAb (not shown).
Figure 7. IL-4-activated CD8+ T cells become IL-4-dependent.
BALB/c IL-4Rα-deficient mice were injected with 75 × 106 spleen cells from wild-type BALB/c mice on d 0 and injected with IL-4C that contained 5 μg of IL-4 on d 0, 2 and 4. Mice were sacrificed on d 5 and their spleen cells were labeled with CFSE and cultured at 107 cells/ml with or without 20 ng/ml of IL-4. Cells were harvested 3 d later, stained for CD8 and IL-4Rα and with To-Pro 3 (to identify dead cells). Stained cells were analyzed by flow cytometry for fluorescence intensity of CFSE on living IL-4Rα+ (donor) and IL-4Rα− (host) cells. Results for IL-4Rα+ cells are shown in the upper panel; for IL-4Rα− cells in the lower panel.
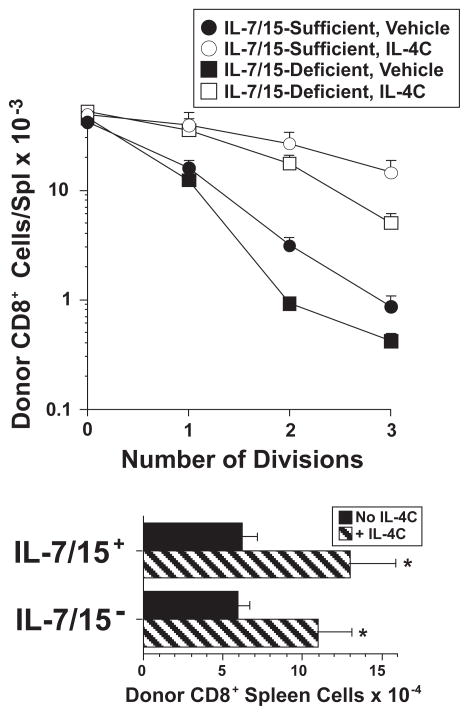
IL-4-induced CD8+ T cell proliferation is IL-7- and IL-15-independent
IL-4C might independently activate CD8+ T cells to proliferate in vivo or simply enhance the mitogenicity of endogenous IL-7 or IL-15. To differentiate between these possibilities, we compared the ability of IL-4C to stimulate proliferation by purified, CFSE-labeled CD8+ wild-type T cells that had been transferred into anti-IL-7 mAb-treated IL-15-deficient mice or control mAb-treated wild-type mice. Both wild-type and IL-15-deficient mice were on a C57BL/6 background. IL-4C induced donor CD8+ T cells to divide in both conditions, although somewhat greater cell division was seen in the presence of IL-7 and IL-15 (Figure 8). Thus, IL-4: 1) stimulates proliferation by C57BL/6, as well as BALB/c CD8+ T cells; and 2) stimulates IL-7/IL-15-independent cell division, which can be enhanced by basal levels of IL-7 and/or IL-15.
Figure 8. IL-4-induced CD8+ T cell proliferation is IL-7- and IL-15-independent.
C57BL/6 CD8+ spleen cells were purified and labeled with CFSE. 1.75 × 106 of these cells were transferred into either C57BL/6 wild-type mice that had been injected 24 hr earlier with 3 mg of control mAb or C57BL/6 IL-15-deficient mice that had been injected 24 hr earlier with 3 mg of anti-IL-7 mAb. Recipient mice were injected on the day of cell transfer and 2 d later with vehicle or IL-4C (5/30) and also received a second dose of control mAb or anti-IL-7 mAb 2 d after cell transfer. Spleen cells from recipients sacrificed 3 d after cell transfer were stained for CD4 and CD8 and analyzed to determine number of CD8+CFSE+ cells and CFSE fluorescence intensity, which was then used to determine number of cell divisions. N = 3–4; * indicated p <.05 compared to CD8+ T cells from the same mouse strain that received the same treatment by received no IL-4C.
MHC class I is required for IL-4 to stimulate naïve CD8+ T cells to proliferate
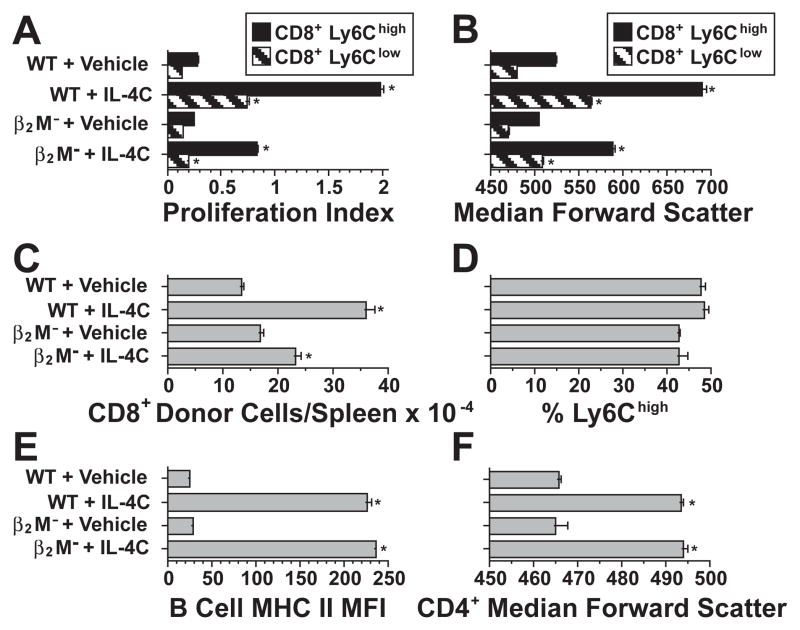
To determine if IL-4 induction of CD8+ T cell proliferation requires an interaction with MHC class I, purified wild-type CD8+ T cells were CFSE-labeled and transferred into C57BL/6 background wild-type or β2-microglobulin-deficient mice, which lack MHC class I, and treated with IL-4C or vehicle for 3 d. Because IL-4C has a shorter half-life in β2-microglobulin-deficient mice than in wild-type mice (44), the former hosts were injected with IL-4C twice a day, while wild-type hosts were injected every other day. IL-4C induced considerably greater proliferation (Figure 9A), increase in cell size (9B) and recovery (9C) of donor CD8+ T cells in wild-type than in β2-microglobulin-deficient hosts. Donor CD8+ T cells that expressed large amounts of Ly6C proliferated and increased in size more than Ly6Clow CD8+ T cells (Figures 9A and 9B). Ly6Clow cells proliferated well in response to IL-4C in wild-type hosts but barely proliferated in β2-microglobulin-deficient hosts (Figure 9A). Differences in the responses of Ly6C high and low cells reflected differences in the biological behavior of activated/memory vs. naïve cells, rather than an IL-4-induced increase in Ly6C expression; in fact, IL-4 treatment decreases Ly6C expression in vivo (not shown). Consistent with this, the percentage of Ly6Chigh CD8+ T cells was not changed by IL-4C treatment in wild-type or β2-microglobulin-deficient hosts (Figure 9D), even though DNA synthesis was greater for the Ly6Chigh cells. It is also unlikely that differences in CD8+ T cell responses in wild-type vs. β2-microglobulin-deficient hosts reflect differences in IL-4C concentration, because host B cell class II MHC expression and CD4+ T cell size increased similarly in response to IL-4C in both strains (Figures 9E and 9F). Thus, MHC class I stimulation enhances IL-4-induced activation of both naïve and memory/activated CD8+ T cells and is required for IL-4 to induce naive cells to proliferate.
Figure 9. MHC class I stimulation contributes to the CD8+ T cell response to IL-4.
2.4 × 106 CFSE-labeled purified CD8+ T cells were transferred into C57BL/6 wild-type mice, which were then injected i.p. every other day with vehicle or IL-4C (5/30), or into C57BL/6 β2-microglobulin-deficient mice, which were then injected twice daily with vehicle or IL-4C (5/30). Mice were sacrificed 3 d after cell transfer and their cells were stained for CD4, CD8, Ly6C, B220, CD19 and/or I-Ab and analyzed for percent of CFSE+ cells that were CD8+Ly6Clow or CD8+Ly6Chigh. N = 4.
A. Proliferation index (average number of cell divisions) of donor CD8+ cells recovered from recipient spleens. * indicates p < .05 as compared to the same cell population from vehicle-treated mice in all panels in this figure. B. Median forward light scatter of donor CD8+ T cells. C. Number of donor (CFSE+) CD8+ cells recovered from host spleens. D. Percent of donor CFSE+CD8+ cells that were Ly6Chigh. Differences between vehicle- and IL-4C-treated mice were insignificant. E. Host B cell median MHC class II expression. Differences between wild-type and β2-microglobulin-deficient mice were insignificant in this panel and panel F. F. Host CD4+ T cell median forward light scatter.
Discussion
Our observations demonstrate that IL-4 has substantial nonredundant mitogenic effects on CD8+ T cells in vivo. IL-4 has been shown previously to promote CD8+ T cell growth and differentiation (45); IL-4 produced by invariant NKT cells in response to a synthetic ligand has been shown to enhance CD8+ T cell proliferation during homeostatic expansion (13) and exogenous IL-4 has been shown to induce CD8+ T cell proliferation in otherwise unstimulated mice (12). However, it has not previously been shown that endogenously produced IL-4 influences CD8+ T cell growth during an immune response to a T dependent Ag or infectious agent. We demonstrate that IL-4 produced during the course of a Th2 response is sufficient to stimulate CD8+ T cell proliferation. This was particularly clear when CFSE-labeled cells were transferred into GaMD-stimulated wild-type mice 3 d after immunization. Donor IL-4Rα-sufficient CD4+ and CD8+ T cells proliferated vigorously and increased >2-fold and >10-fold, respectively, as compared to IL-4Rα-deficient donor cells, during the subsequent 3 d (the period of strongest IL-4 production). Because GaMD is not a typical Ag, we also studied the effects of IL-4 produced during N. brasiliensis infection on transferred T cells, the effects of IL-4 produced during S. mansoni infection on splenic T cells; and the effects of IL-4 produced in response to dust mite allergen on pulmonary T cells. We again saw IL-4Rα- and IL-4-dependent stimulation of CD8+ T cell proliferation. Although differences in responses made by wild-type vs. IL-4Rα-deficient CD8+ T cells in these systems might partially result from possible effects of IL-4 on CD8+ T cell development, this appears to be less important than the ability to respond to IL-4; CD8+ T cells from IL-4-deficient mice proliferate to nearly the same extent as CD8+ T cells from wild-type mice when transferred into GaMD-immunized or S. mansoni-inoculated wild-type mice.
Our evidence of IL-4 stimulatory effects on CD8+ T cells in mouse models of allergic airway disease is particularly intriguing. Recent studies by Gelfand and colleagues in a mouse model of asthma demonstrate that airway hyperresponsiveness and allergic inflammation in this model depend on IL-13 production by pulmonary CD8+ T cells (35). It seems likely that IL-4 drives the CD8+ T cell IL-13 response because repeated airway inoculation of mice with strong allergens, such as dust mite fecal pellets or Ascaris pseudocoelomic fluid, induces a strong pulmonary IL-4 response (46) that is required for induction of both pulmonary IL-13 production (F. Finkelman, unpublished data) and pulmonary CD8+ T cell proliferation and IL-4 promotes the differentiation of CD8+ T cells into effector cells that secrete Th2 cytokines (47).
Our observation that IL-4 promotes CD8+ T cell proliferation during helminth infections raises the possibility that T cell effects of this cytokine contribute to host protection against non-helminthic pathogens. Indeed, development of CD8+ effector memory cells is decreased in IL-4- and IL-4Rα-deficient mice that have been inoculated with the malarial protozoan Plasmodium berghei, and IL-4- and IL-4Rα-deficient mice that have recovered from a primary P. berghei infection show increased susceptibility to a second infection with this parasite (19, 48). These defects in IL-4- and IL-4Rα-deficient mice have been traced to a direct stimulatory effect of IL-4 on CD8+ T cells in normal, P. berghei-infected mice during the first few days after parasite inoculation. This early effect of IL-4 on CD8+ T cell-dependent immunity is consistent with observations that erythrocyte parasitemia is more persistent in IL-4-deficient mice and Stat6-deficient mice during a primary infection with the related parasite P. chabaudi (49).
Having demonstrated that endogenously produced IL-4 has biologically important stimulatory effects on CD8+ T cell responses to immune stimulation, we injected mice with IL-4C to define characteristics and mechanisms of IL-4 stimulation of T cell proliferation and accumulation. These studies reveal that the mitogenic effect of IL-4 on T cells is rapid and direct: it acts in < 24 hr, stimulates proliferation by purified IL-4Rα+CD8+ T cells that had been transferred into IL-4Rα-deficent hosts, and fails to stimulate proliferation by IL-4Rα− CD8+ T cells in wild-type hosts. This is consistent with previous observations that T cells constitutively express the type 1 IL-4R (43) and that other γc-related cytokines have prominent mitogenic effects on T cells, with a greater effect on CD8+ than CD4+ cells (50, 51). The mitogenic effect of IL-4 is enhanced by IL-7 and/or IL-15, the γc-related cytokines most associated with T cell proliferation and survival (1, 4), but is still considerable in the simultaneous absence of both of these cytokines. The mitogenic effect of IL-4 is greater on memory/activated CD8+ T than on naïve, resting CD8+ T cells, but it is dramatic even for the latter population in both BALB/c and C57BL/6 background mice (Figures 5 and 9, respectively). An MHC class I-dependent effect, presumably submitogenic CD8/TCR stimulation, contributes to IL-4 induction of proliferation by both naïve and memory/effector cells and is particularly important for the former population (Figure 9). Indeed, the importance of MHC class I for mitogenic effects of IL-4 may be underestimated by our results, inasmuch as some MHC class I-dependent signaling may have been induced in donor CD8+ T cells by MHC class I present on those cells. MHC class I potentiation of in vivo IL-4 mitogenicity may account for the failure of IL-4 to stimulate T cell proliferation in vitro, where the intercellular contacts that may be required for MHC class I-CD8/TCR interactions are likely to be less pronounced than they are in vivo.
Taken together, our observations alter appreciation of T cell homeostasis and expansion by demonstrating that IL-4, like IL-15, has an important, non-redundant role that particularly promotes CD8+ T cell activation and memory. The IL-4 effect is important in infectious disease and allergy and is mediated primarily by separate signaling pathways that promote DNA synthesis and survival. A subsequent paper will present evidence that these rapid T cell stimulatory effects of IL-4 are balanced by slower induction of a Stat6-dependent regulatory pathway in which non-T cells suppress T cell proliferation and activated T cell survival.
Acknowledgments
We appreciate advice from Christopher Karp, William Paul and Mark Boothby, the gift of mAb 4-3 from Amgen Corporation, LCMV gp33-41 from Joel Collier and the gift of mice from Richard Dutton, Michael Grusby, Frank Brombacher, Michael Jordan and Nancy Noben-Trauth.
Footnotes
Disclosures: The authors have no conflicts of interest to disclose.
References
- 1.Bradley LM, Haynes L, Swain SL. IL-7: maintaining T-cell memory and achieving homeostasis. Trends Immunol. 2005;26:172–176. doi: 10.1016/j.it.2005.01.004. [DOI] [PubMed] [Google Scholar]
- 2.Boyman O, Cho JH, Tan JT, Surh CD, Sprent J. A major histocompatibility complex class I-dependent subset of memory phenotype CD8+ cells. J Exp Med. 2006;203:1817–1825. doi: 10.1084/jem.20052495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lee SK, Surh CD. Role of interleukin-7 in bone and T-cell homeostasis. Immunol Rev. 2005;208:169–180. doi: 10.1111/j.0105-2896.2005.00339.x. [DOI] [PubMed] [Google Scholar]
- 4.Surh CD, Sprent J. Regulation of mature T cell homeostasis. Semin Immunol. 2005;17:183–191. doi: 10.1016/j.smim.2005.02.007. [DOI] [PubMed] [Google Scholar]
- 5.Malek TR. The main function of IL-2 is to promote the development of T regulatory cells. J Leukoc Biol. 2003;74:961–965. doi: 10.1189/jlb.0603272. [DOI] [PubMed] [Google Scholar]
- 6.Boyman O, Kovar M, Rubinstein MP, Surh CD, Sprent J. Selective stimulation of T cell subsets with antibody-cytokine immune complexes. Science. 2006;311:1924–1927. doi: 10.1126/science.1122927. [DOI] [PubMed] [Google Scholar]
- 7.Nakajima H, Shores EW, Noguchi M, Leonard WJ. The common cytokine receptor γ chain plays an essential role in regulating lymphoid homeostasis. J Exp Med. 1997;185:189–195. doi: 10.1084/jem.185.2.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kelly J, Spolski R, Imada K, Bollenbacher J, Lee S, Leonard WJ. A role for Stat5 in CD8+ T cell homeostasis. J Immunol. 2003;170:210–217. doi: 10.4049/jimmunol.170.1.210. [DOI] [PubMed] [Google Scholar]
- 9.Nelms K, Keegan AD, Zamorano J, Ryan JJ, Paul WE. The IL-4 receptor: signaling mechanisms and biologic functions. Annu Rev Immunol. 1999;17:701–738. doi: 10.1146/annurev.immunol.17.1.701. [DOI] [PubMed] [Google Scholar]
- 10.Vella A, Teague TK, Ihle J, Kappler J, Marrack P. Interleukin 4 (IL-4) or IL-7 prevents the death of resting T cells: stat6 is probably not required for the effect of IL-4. J Exp Med. 1997;186:325–330. doi: 10.1084/jem.186.2.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hu-Li J, Shevach EM, Mizuguchi J, Ohara J, Mosmann T, Paul WE. B cell stimulatory factor 1 (interleukin 4) is a potent costimulant for normal resting T lymphocytes. J Exp Med. 1987;165:157–172. doi: 10.1084/jem.165.1.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Boyman O, Kovar M, Rubinstein MP, Surh CD, Sprent J. Selective stimulation of T cell subsets with antibody-cytokine immune complexes. Science. 2006;311:1924–1927. doi: 10.1126/science.1122927. [DOI] [PubMed] [Google Scholar]
- 13.Ueda N, Kuki H, Kamimura D, Sawa S, Seino K, Tashiro T, Fushuku K, Taniguchi M, Hirano T, Murakami M. CD1d-restricted NKT cell activation enhanced homeostatic proliferation of CD8+ T cells in a manner dependent on IL-4. Int Immunol. 2006;18:1397–1404. doi: 10.1093/intimm/dxl073. [DOI] [PubMed] [Google Scholar]
- 14.Terabe M, Matsui S, Noben-Trauth N, Chen H, Watson C, Donaldson DD, Carbone DP, Paul WE, Berzofsky JA. NKT cell-mediated repression of tumor immunosurveillance by IL-13 and the IL-4R-STAT6 pathway. Nat Immunol. 2000;1:515–520. doi: 10.1038/82771. [DOI] [PubMed] [Google Scholar]
- 15.Marshall MA, Jankovic D, Maher VE, Sher A, Berzofsky JA. Mice infected with Schistosoma mansoni develop a novel non-T-lymphocyte suppressor population which inhibits virus-specific CTL induction via a soluble factor. Microbes Infect. 2001;3:1051–1061. doi: 10.1016/s1286-4579(01)01499-x. [DOI] [PubMed] [Google Scholar]
- 16.Bronte V, Serafini P, De Santo C, Marigo I, Tosello V, Mazzoni A, Segal DM, Staib C, Lowel M, Sutter G, Colombo MP, Zanovello P. IL-4-induced arginase 1 suppresses alloreactive T cells in tumor-bearing mice. J Immunol. 2003;170:270–278. doi: 10.4049/jimmunol.170.1.270. [DOI] [PubMed] [Google Scholar]
- 17.Makarenkova VP, Bansal V, Matta BM, Perez LA, Ochoa JB. CD11b+/Gr-1+ myeloid suppressor cells cause T cell dysfunction after traumatic stress. J Immunol. 2006;176:2085–2094. doi: 10.4049/jimmunol.176.4.2085. [DOI] [PubMed] [Google Scholar]
- 18.Schuler T, Kammertoens T, Preiss S, Debs P, Noben-Trauth N, Blankenstein T. Generation of tumor-associated cytotoxic T lymphocytes requires interleukin 4 from CD8+ T cells. J Exp Med. 2001;194:1767–1775. doi: 10.1084/jem.194.12.1767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Carvalho LH, Sano G, Hafalla JC, Morrot A, Curotto de Lafaille MA, Zavala F. IL-4-secreting CD4+ T cells are crucial to the development of CD8+ T-cell responses against malaria liver stages. Nat Med. 2002;8:166–170. doi: 10.1038/nm0202-166. [DOI] [PubMed] [Google Scholar]
- 20.Huang LR, Chen FL, Chen YT, Lin YM, Kung JT. Potent induction of long-term CD8+ T cell memory by short-term IL-4 exposure during T cell receptor stimulation. Proc Natl Acad Sci U S A. 2000;97:3406–3411. doi: 10.1073/pnas.060026497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Noben-Trauth N, Kohler G, Burki K, Ledermann B. Efficient targeting of the IL-4 gene in a BALB/c embryonic stem cell line. Transgenic Res. 1996;5:487–491. doi: 10.1007/BF01980214. [DOI] [PubMed] [Google Scholar]
- 22.Urban JF, Jr, Noben-Trauth N, Donaldson DD, Madden KB, Morris SC, Collins M, Finkelman FD. IL-13, IL-4Rα, and Stat6 are required for the expulsion of the gastrointestinal nematode parasite. Nippostrongylus brasiliensis Immunity. 1998;8:255–264. doi: 10.1016/s1074-7613(00)80477-x. [DOI] [PubMed] [Google Scholar]
- 23.Pircher HP, Burki K, Lang R, Hengartner H, Zinkernagel R. Tolerance induction in double specific T-cell receptor transgenic mice varies with antigen. Nature. 1989;342:559–561. doi: 10.1038/342559a0. [DOI] [PubMed] [Google Scholar]
- 24.Finkelman FD, Kessler SW, Mushinski JF, Potter M. IgD-secreting murine plasmacytomas: identification and partial characterization of two IgD myeloma proteins. J Immunol. 1981;126:680–687. [PubMed] [Google Scholar]
- 25.Finkelman FD, Madden KB, Morris SC, Holmes JM, Boiani N, Katona IM, Maliszewski CR. Anti-cytokine antibodies as carrier proteins. Prolongation of in vivo effects of exogenous cytokines by injection of cytokine-anti-cytokine antibody complexes. J Immunol. 1993;151:1235–1244. [PubMed] [Google Scholar]
- 26.Finkelman FD, Yang M, Perkins C, Schleifer K, Sproles A, Santeliz J, Bernstein JA, Rothenberg ME, Morris SC, Wills-Karp M. Suppressive effect of IL-4 on IL-13-induced genes in mouse lung. J Immunol. 2005;174:4630–4638. doi: 10.4049/jimmunol.174.8.4630. [DOI] [PubMed] [Google Scholar]
- 27.Perkins C, Wills-Karp M, Finkelman FD. IL-4 induces IL-13-independent allergic airway inflammation. J Allergy Clin Immunol. 2006;118:410–419. doi: 10.1016/j.jaci.2006.06.004. [DOI] [PubMed] [Google Scholar]
- 28.Strait RT, Morris SC, Smiley K, Urban JF, Jr, Finkelman FD. IL-4 exacerbates anaphylaxis. J Immunol. 2003;170:3835–3842. doi: 10.4049/jimmunol.170.7.3835. [DOI] [PubMed] [Google Scholar]
- 29.Morris SC, Dragula NL, Finkelman FD. IL-4 promotes Stat6-dependent survival of autoreactive B cells in vivo without inducing autoantibody production. J Immunol. 2002;169:1696–1704. doi: 10.4049/jimmunol.169.4.1696. [DOI] [PubMed] [Google Scholar]
- 30.Katona IM, Urban JF, Jr, Scher I, Kanellopoulos-Langevin C, Finkelman FD. Induction of an IgE response in mice by Nippostrongylus brasiliensis: characterization of lymphoid cells with intracytoplasmic or surface IgE. J Immunol. 1983;130:350–356. [PubMed] [Google Scholar]
- 31.Herbert DR, Holscher C, Mohrs M, Arendse B, Schwegmann A, Radwanska M, Leeto M, Kirsch R, Hall P, Mossmann H, Claussen B, Forster I, Brombacher F. Alternative macrophage activation is essential for survival during schistosomiasis and downmodulates T helper 1 responses and immunopathology. Immunity. 2004;20:623–635. doi: 10.1016/s1074-7613(04)00107-4. [DOI] [PubMed] [Google Scholar]
- 32.Finkelman FD, Morris SC, Orekhova T, Mori M, Donaldson D, Reiner SL, Reilly NL, Schopf L, Urban JF., Jr Stat6 regulation of in vivo IL-4 responses. J Immunol. 2000;164:2303–2310. doi: 10.4049/jimmunol.164.5.2303. [DOI] [PubMed] [Google Scholar]
- 33.Cerwenka A, Carter LL, Reome JB, Swain SL, Dutton RW. In vivo persistence of CD8 polarized T cell subsets producing type 1 or type 2 cytokines. J Immunol. 1998;161:97–105. [PubMed] [Google Scholar]
- 34.Pihlgren M, Dubois PM, Tomkowiak M, Sjogren T, Marvel J. Resting memory CD8+ T cells are hyperreactive to antigenic challenge in vitro. J Exp Med. 1996;184:2141–2151. doi: 10.1084/jem.184.6.2141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Miyahara N, Swanson BJ, Takeda K, Taube C, Miyahara S, Kodama T, Dakhama A, Ott VL, Gelfand E. Effector CD8+ T cells mediate inflammation and airway hyper-responsiveness. Nat Med. 2004;10:865–869. doi: 10.1038/nm1081. [DOI] [PubMed] [Google Scholar]
- 36.Kaplan MH, Schindler U, Smiley ST, Grusby MJ. Stat6 is required for mediating responses to IL-4 and for development of Th2 cells. Immunity. 1996;4:313–319. doi: 10.1016/s1074-7613(00)80439-2. [DOI] [PubMed] [Google Scholar]
- 37.Shimoda K, van Deursen J, Sangster MY, Sarawar SR, Carson RT, Tripp RA, Chu C, Quelle FW, Nosaka T, Vignali DA, Doherty PC, Grosveld G, Paul WE, Ihle JN. Lack of IL-4-induced Th2 response and IgE class switching in mice with disrupted Stat6 gene. Nature. 1996;380:630–633. doi: 10.1038/380630a0. [DOI] [PubMed] [Google Scholar]
- 38.Takeda K, Tanaka T, Shi W, Matsumoto M, Minami M, Kashiwamura S, Nakanishi K, Yoshida N, Kishimoto T, Akira S. Essential role of Stat6 in IL-4 signalling. Nature. 1996;380:627–630. doi: 10.1038/380627a0. [DOI] [PubMed] [Google Scholar]
- 39.Mohrs M, Shinkai K, Mohrs K, Locksley RM. Analysis of type 2 immunity in vivo with a bicistronic IL-4 reporter. Immunity. 2001;15:303–311. doi: 10.1016/s1074-7613(01)00186-8. [DOI] [PubMed] [Google Scholar]
- 40.Trenn G, Takayama H, Hu-Li J, Paul WE, Sitkovsky MV. B cell stimulatory factor 1 (IL-4) enhances the development of cytotoxic T cells from Lyt-2+ resting murine T lymphocytes. J Immunol. 1988;140:1101–1106. [PubMed] [Google Scholar]
- 41.Phelan JD, Orekov T, Finkelman FD. Mechanisms of enhancement of in vivo cytokine effects by anti-cytokine mAbs. J Immunol. 2008 doi: 10.4049/jimmunol.180.1.44. In press. [DOI] [PubMed] [Google Scholar]
- 42.Conrad DH, Ben-Sasson SZ, Le Gros G, Finkelman FD, Paul WE. Infection with Nippostrongylus brasiliensis or injection of anti-IgD antibodies markedly enhances Fc-receptor-mediated interleukin 4 production by non-B, non-T cells. J Exp Med. 1990;171:1497–1508. doi: 10.1084/jem.171.5.1497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Armitage RJ, Beckmann MP, Idzerda RL, Alpert A, Fanslow WC. Regulation of interleukin 4 receptors on human T cells. Int Immunol. 1990;2:1039–1045. doi: 10.1093/intimm/2.11.1039. [DOI] [PubMed] [Google Scholar]
- 44.Junghans RP, Anderson CL. The protection receptor for IgG catabolism is the β2-microglobulin-containing neonatal intestinal transport receptor. Proc Natl Acad Sci U S A. 1996;93:5512–5516. doi: 10.1073/pnas.93.11.5512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Croft M, Carter L, Swain SL, Dutton RW. Generation of polarized antigen-specific CD8 effector populations: reciprocal action of interleukin (IL)-4 and IL-12 in promoting type 2 versus type 1 cytokine profiles. J Exp Med. 1994;180:1715–1728. doi: 10.1084/jem.180.5.1715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Johnson JR, Wiley RE, Fattouh R, Swirski FK, Gajewska BU, Coyle AJ, Gutierrez-Ramos JC, Ellis R, Inman MD, Jordana M. Continuous exposure to house dust mite elicits chronic airway inflammation and structural remodeling. Am J Respir Crit Care Med. 2004;169:378–385. doi: 10.1164/rccm.200308-1094OC. [DOI] [PubMed] [Google Scholar]
- 47.Seder RA, Boulay JL, Finkelman F, Barbier S, Ben-Sasson SZ, Le Gros G, Paul WE. CD8+ T cells can be primed in vitro to produce IL-4. J Immunol. 1992;148:1652–1656. [PubMed] [Google Scholar]
- 48.Morrot A, Hafalla JC, Cockburn IA, Carvalho LH, Zavala F. IL-4 receptor expression on CD8+ T cells is required for the development of protective memory responses against liver stages of malaria parasites. J Exp Med. 2005;202:551–560. doi: 10.1084/jem.20042463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.von der Weid T, Kopf M, Kohler G, Langhorne J. The immune response to Plasmodium chabaudi malaria in interleukin-4-deficient mice. Eur J Immunol. 1994;24:2285–2293. doi: 10.1002/eji.1830241004. [DOI] [PubMed] [Google Scholar]
- 50.Geiselhart LA, Humphries CA, Gregorio TA, Mou S, Subleski J, Komschlies KL. IL-7 administration alters the CD4:CD8 ratio, increases T cell numbers, and increases T cell function in the absence of activation. J Immunol. 2001;166:3019–3027. doi: 10.4049/jimmunol.166.5.3019. [DOI] [PubMed] [Google Scholar]
- 51.Yajima T, Nishimura H, Ishimitsu R, Watase T, Busch DH, Pamer EG, Kuwano H, Yoshikai Y. Overexpression of IL-15 in vivo increases antigen-driven memory CD8+ T cells following a microbe exposure. J Immunol. 2002;168:1198–1203. doi: 10.4049/jimmunol.168.3.1198. [DOI] [PubMed] [Google Scholar]