Abstract
The purpose of this study was to compare the dietary habits, nutrient intake, bone mineral density (BMD) and bone metabolism in Korean male collegians as related to smoking situation. One hundred sixty one young adult males at the age of 20-26 participated in this study. The subjects were divided into four groups: non smoker (n=42), light smoker (n=34), moderate smoker (n=49) and heavy smoker (n=36). The anthropometric characteristics, smoking situations, dietary habits and nutrient intakes were observed. Bone status of the calcaneus was measured by using quantitative ultrasound (QUS). Bone metabolism markers including serum alkaline phosphatase activity (ALP) and N-mid osteocalcin (OC) were analyzed. There were no significant differences in height, weight, BMI, energy and calcium intake among the four groups. Iron intake of moderate and heavy smoker was significantly lower than that of light smoker. Heavy smokers consumed significantly lower vitamin C than moderate smokers, and their coffee consumption and lifetime alcohol consumption were significantly highest among the 4 groups. QUS parameters and serum ALP were not significantly different among the four groups. Serum OC levels were significantly lower in heavy and non smoker group compared to the moderate smoker group. In conclusion, heavy smokers in young male collegians had undesirable lifestyle and dietary habits, like as high consumption of coffee and alcohol, and low intake of Fe and vitamin C. Although, there was no significant difference in their current bone status from the other groups, these undesirable factors with heavy smoking may affect their bone health in the long term.
Keywords: Smoking, bone status, osteocalcin, male collegian
Introduction
Osteoporosis is a metabolic disorder of the fracture-developing condition by the reduction of the bone mass (Spencer & Krammer, 1986). It was thought to be a common disorder in women in the past, but it is recognized as a main disease in men as the average life expectancy continues to increase. The bone loss in men also becomes a risk factor for developing fracture as in women. Melton et al. (1998) and Legrand et al. (1999) reported that osteoporotic men had almost 2~2.7 times the fracture risk than men with normal bone mineral density.
Osteoporosis is affected by numerous factors, including age, dietary factors, lack of exercise, menopause, underweight, excessive alcohol consumption, cigarette smoking, excessive caffeine consumption and so on, which are thought to contribute negatively to bone health (Ilich & Kerstetter, 2000). Especially cigarette smoking is negatively associated with Ca and vitamin D metabolism (Kim et al., 2003; Kwak et al., 2000). And smoking significantly reduced femoral bone mineral density in healthy postmenopausal women (Hansen et al., 1991). According to a study of meta-analysis by Law and Hackshaw (1997), there was no significant difference in hip fracture rates of the subjects at the age of 50 between smokers and nonsmokers, while those for smokers in their 60s' were increased compared to that of nonsmoker counterpart. Seeman et al. (1983) indicated that smokers were likely to have vertebrae fractures by about 2.3 times osteoporosis-related than non smokers. For that reason, smoking is thought to affect the bone health negatively.
Also Midgette et al. (1993) reported that smokers ingested less healthier diets and more caffeine and alcohol. Caffeine and alcohol are risk factors for developing osteoporosis (Grazio et al., 2005; Sakamoto et al., 2001). A study by Dallongeville et al. (1998) showed that smokers ingested less vitamin C, vitamin E, Ca, and Mg good for bone health than non smokers, resulting in adverse effects on the bone health.
Statistics indicate that 52.3% of Korean men at 19 years or older smokes; among them 55.6% are 19 to 29 year olds (Korean Ministry of Health and Welfare, 2006). Smoking in the age of 19 to 29 years may influence health over the course of their whole lifetimes, although it is not until after a long time that health trouble typically occurs. In the chronic disease like osteoporosis, prevention is more important than treatment. So it seems necessary to study the effect of smoking on the bone and nutrition status.
The purpose of this study was to investigate the relationship between food habits, nutrient intake, bone status and the smoking situation in Korean healthy male collegians. For this study, 161 healthy male collegians were recruited and divided into four groups as related to their current smoking situation. And the data on their nutrient intake, dietary habits and lifestyle were ascertained. Also, their bone status using ultrasound techniques and blood bone metabolism markers were assessed.
Subjects and Methods
Subjects and study design
The 483 male collegians aged 20-26 y were recruited from the Gyeonggi-do area in Korea through posters and announcement notices. All subjects completed a questionnaire upon study entry. 161 subjects were selected and divided into 4 groups according to their smoking situations: non smoker (n=42), light smoker (n=34), moderate smoker (n=49) and heavy smoker (n=36). The heavy smoker group was composed of subjects who smoked over 20 cigarettes a day. The moderate smoker group was composed of subjects who smoked 10~20 cigarettes a day. The light smoker group was composed of subjects who smoked under 10 cigarettes a day. This grouping was referred to previous studies (Webb & Carey, 2008). Anthropometric characteristics of the subjects were observed. The quantitative ultrasound was used for bone mineral density (BMD) of calcaneus and bone metabolism markers including serum alkaline phosphatase activity (ALP) and N-mid osteocalcin (OC) were analyzed.
Anthropometric measurements
Height and weight was measured by using an electrical digital scale (DS-102, JENIX, Korea) in the standing position with light clothes. Body mass index (BMI) was calculated from the measured weight and height measurements as weight/height2 (kg/m2).
Questionnaire interview
All volunteers completed a questionnaire conducted by the investigators. The questionnaire included the participant's age, cigarette smoking history, alcohol drinking history, physical activity, and food habits like as frequency of meal, milk consumption and carbonated beverage consumption (Grainge et al., 1998). Questions about cigarette smoking situations included whether they had smoked or not, the number of cigarettes smoked a day, and the duration of smoking history. Also smoking status was expressed as pack-years (The sum of the number of cigarettes smoked per day in each year of life up until the time of the scan, divided by 20, the number of cigarettes in a packet). Questions about their alcohol consumption included whether they consumed alcohol or not, frequencies of alcohol drinking, amounts of alcohol consumed on each, and the duration of drinking history. Food intakes were surveyed by using 3 days 24-hr recall method under the guidance of investigators. Food models, measuring cups, household glasses, bowl and spoons familiar to the respondents were used to help the subjects recall the portion sizes. Energy and nutrient intakes were calculated by using the CAN-Pro 2.0 (The Korean Nutrition Society, 2002).
Measurement of bone status
Bone status of the calcaneus was measured by using quantitative ultrasound (Sahara, Hologic, USA). The measurements obtained included the broadband ultrasound attenuation (BUA), speed of sound (SOS), and QUI (Quantitative ultrasound index) from which bone mineral density (BMD) and T-scores estimations were computed. The assessment of bone status using quantitative ultrasound (QUS) techniques may offer a possible alternative to the central dual-energy X-ray absorptiometry (DXA) assessment, because QUS is radiation free, relatively cheep and easily transportable (Gonnelli et al., 2005). Some studies have reported that QUS parameters at the calcaneus can discriminate male patients with fracture from control subjects (Pluskiewicz & Drozdzowska, 1999).
Blood bone metabolism markers analysis
Ten milliliters of blood from each subject were collected using evacuated tubes. After blood samples were left at room temperature for about 30 minutes, they were centrifuged for 15 minutes at 3,000 rpm for serum alkaline phosphatase (Autoanalyzer, Toshiba, Japan) and N-mid osteocalcin (Osteo-RIACT, CIS Bio International, France) analysis.
Statistical analysis
The data were given numerically as means with standard deviations. ANOVA analysis (One-Way Analysis of Variance) and Duncan's multiple range tests were carried out to identify any significant differences among the 4 groups. Chi-square tests were used to test significance on the distribution rate within the groups. Data analysis was conducted using statistical software package for Windows (SAS version 8.01, SAS institute, USA).
Results
General characteristics
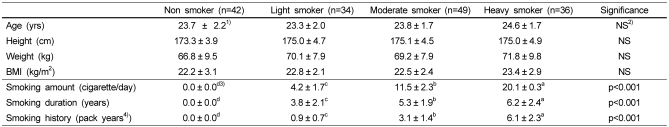
The mean age of the subjects was 23.7 in non smoker group, 23.3 in light smoker group, 23.8 in moderate smoker group, and 24.6 in heavy smoker group. No significant differences were found in height, weight and BMI among the groups (Table 1). The average number of smoked cigarettes of the subjects was 4.2 in light smoker group, 11.5 in moderate smoker group, and 20.1 in heavy smoker group.
Table 1.
General characteristics of subjects
1)Mean ± SD
2)Not Significant
3)Values with different superscripts within a row are significantly different at a=0.05 as determined by Duncan's multiple range test.
4)The sum of the number of cigarettes smoked per day in each year of life up until the time of the scan, divided by 20, the number of cigarettes in a packet
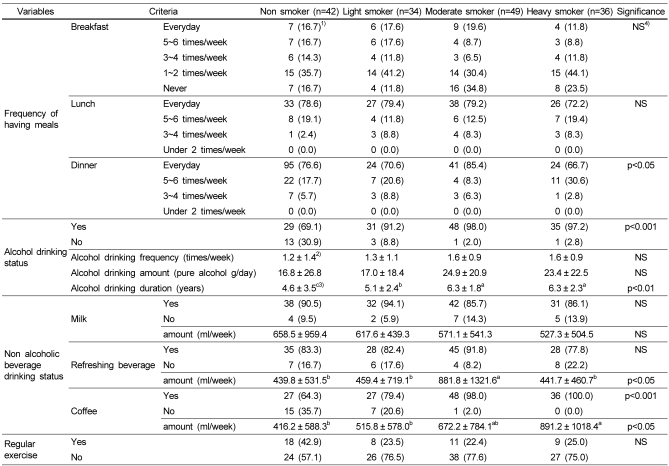
Life styles and dietary habits
There was no significant difference in frequencies of having breakfast and lunch among the 4 groups (Table 3). However, frequency of having dinner in heavy smoker group was significantly higher than that in the other groups (p<0.05). The proportion of drinking alcohol in smoker groups was over 90%, and it was significantly higher than that of non smoker group (p<0.001) Drinking history was long in order: moderate and heavy smoker, light smoker and non smoker (p<0.01). The amount of drinking refreshing beverage in moderate smoker was higher than the other groups (p<0.05). The proportion of the subjects who drink coffee were higher in moderate (98.0%) and heavy smoker (100.0%) group than that in light (79.4%) and non smoker (64.3%) group (p<0.001). And the mean amount of coffee drunk in heavy smoker group was significantly higher than in non and moderate smoker group (p<0.05). There was no significance difference in exercise and milk consumption status among the 4 groups.
Table 3.
Nutrient mean intakes as related to smoking situation
1)Mean ± SD
2)Not significant
3)Values with different superscripts within a row are significantly different at a=0.05 as determined by Duncan's multiple range test
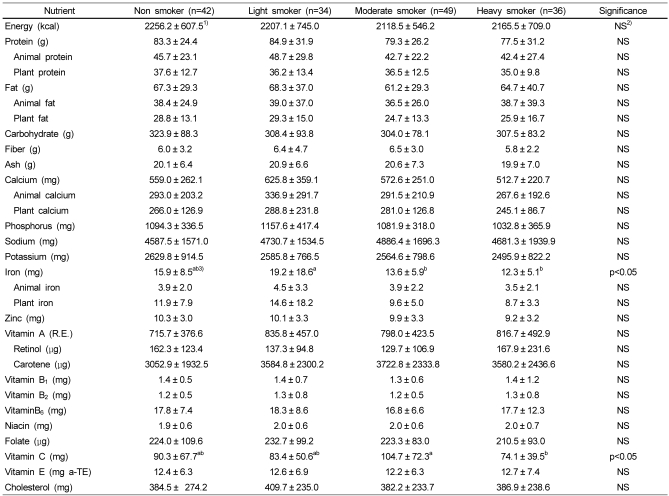
Nutrient intakes
Nutrient intakes of each group are shown in Tables 3 and 4. No significant difference was found in the intakes of nutrients except vitamin C and iron among the four groups. Iron intake of moderate and heavy smoker group was significantly lower than that of light smoker group (p<0.05). The vitamin C intake in heavy smoker was significantly lower than that in moderate smokers (p<0.05).
Table 4.
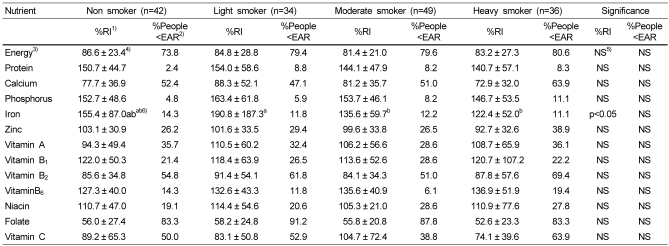
Percentage of recommended intake (RI) and proportion of subjects who consumed under estimated average requirements as related to smoking situation
1)Percent of Recommended Intake (RI)
2)Percent of people whose intakes do not meet Estimated Average Requirements (EAR)
3)Percent of Estimated Energy Requirements (EER) corresponds to the EAR for the other nutrients is suggested for energy
4)Mean ± SD
5)Not significant
6)Values with different superscripts within a row are significantly different at a=0.05 as determined by Duncan's multiple range test
Quantitative ultrasound parameters and blood bone metabolism parameters
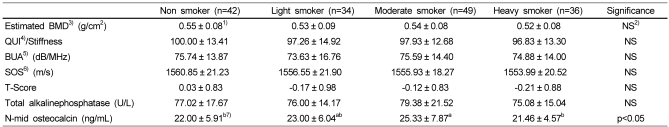
Quantitative ultrasound (QUS) parameters of the calcaneus among the four groups are shown in Table 5. QUS parameters of the calcaneus were not significantly different among the four groups. However, serum osteocalcin levels were significantly lower in heavy and non smoker group compared to the moderate smoker group (p<0.05). Serum ALP tended to be lower in heavy smoker than the other groups, although there were no statistical differences.
Table 5.
Bone mineral density and serum bone metabolism markers as related to smoking situation
1)Mean ± SD
2)Not significant
3)Bone Mineral Density
4)Quantitative Ultrasound Index
5)Broadband Ultrasound Attenuation
6)Speed Of Sound
7)Values with different superscripts within a row are significantly different at a=0.05 as determined by Duncan's multiple range test
Discussion
Dietary habit and smoking
The current study result shows that the subject in heavy smoking group drinks more caffeine and alcohol than the other groups. In other words, heavy smokers appear to have undesirable lifestyle for bone health. This result is similar to some studies by Field et al. (2005), Saules et al. (2004), and Szule et al. (2002) that smokers consumed more alcohol as well as more frequently and they also drink more caffeine than nonsmokers.
Hoidrup et al. (1999) reported that the subjects with the higher alcohol consumption had the higher rates of hip fracture. Chronic alcohol consumption results in the deficiency of nutrients such as Ca, Mg, and Zn, which are essential for bone health, metabolic troubles such as abnormal vitamin D metabolism and functional deficiency of PTH, and the induction of direct toxicity on osteoblasts (Ilich & Kerstetter, 2000).
Caffeine also may have a deleterious effect on bone health. Massey and Whiting (1993) reported that high caffeine intake resulted in increased Ca excretion and decreased Ca absorption. It was suggested that excessive caffeine intake might decrease bone mineral (Medras et al., 2000), and showed a negative association between caffeine intake and bone mineral density (Rubin et al., 1999).
Nutrients intake and smoking
Lloveras et al. (2001) reported that male smokers had fewer intakes of dairy products and fruits than nonsmokers. Gamber et al. (1995) and Dyer et al. (2003) also reported that smokers had less Ca intake and lack of exercise. In this study, heavy smokers ingested significantly less vitamin C than the moderate smoker and less Fe than the light smoker groups. Vitamin C and Fe play an important role for bone formation. Vitamin C is involved in the synthesis of collagen and the regulation of osteoblast differentiation. Fe also acts as a catalytic cofactor in collage maturation (Prockop, 1971). In the study by the Prynne et al. (2006), vitamin C showed a positive association with bone mineral status at several sites in boys aged 16 year to 18 year. That is, the boys achieved a greater increment of 0.003 (whole body) to 0.005 (femoral neck) g/cm2 on the increase of vitamin C intake. Medeiros et al. (1997) reported that Fe-deficient growing rats had less strength for bone fracture than Fe-adequate rats. In the study of Angus et al. (1988), Fe intake showed a positive association with BMD of thigh, even if Ca intake did not show a significant association with BMD. Like this, smoking may be harmful for bone health, because nutrient intake can be influenced by smoking. For that reason, low Vitamin C and Fe intakes of heavy smokers in our study seemed to have negative influences on the bone metabolism.
Smoking and bone health in male collegian
Smoking is known to act complexly on the bone health in the nutrition and bone metabolism. De Vernejoul et al. (1983) and Ramp et al. (1991) reported that toxic materials resulted from cigarette smoke, such as nicotine, Pb, Cd etc., might act negatively on the osteoblast and the collagen formation. Supervia et al. (2006) and Benson and Shulman (2005) reported that smokers had significantly lower BMD than nonsmokers and smoking increased bone resorption and altered the sex hormone metabolism. Lorentzon et al. (2007) also reported that BMD measurements of the lumbar vertebra in male smokers were significantly lower than those in nonsmokers. When Vogel et al. (1997) was assessed in 1303 male Japanese-Americans, BMD of heel bone (cancellous) and lumba (trabecula) in non smokers were significantly higher than those of current and past smokers. Ortego-Ceneno et al. (1997) also provided evidence that heavy smokers (more than 20 cigarettes/day) had significantly lower BMD in all skeletal sites than non smokers for healthy males at the age of 20 to 45 years. In our study, however bone mineral density was not significantly different among the groups as related to smoking situation. Thus, the disagreement of our results may be due to the characteristics of our study population, young adult males who have a relatively short history of cigarette smoking. Actually, the average smoking duration of smokers was 3.8~6.2 years. In addition, our study only examined 1 bone site in the subjects. Further studies are therefore warranted to include various aged subjects to investigate the effects on BMD of smoking as well as measurement of several bone sites in men.
Some studies of smoking on osteocalcin have shown mixed effects. Laroche et al. (1994) reported that daily smoking resulted in a significant decrease in osteocalcin levels compared to nonsmoking subjects. In contrast to this study, Ortego-Centeno et al. (1997) found that there was no significant difference between smoking and serum osteocalcin depending on smoking in male subjects aged 27-28 years.
In this current study, although serum osteocalcin levels in heavy smoker group were significantly lower than those in moderate smoker group, they were not significantly different from any other groups. Under the present conditions with some limitations as mentioned earlier, the conclusion may not be mentioned in the same breath. However, BMD by ultrasound and serum osteocalcin tend to be low in the male collegian subjects who smoke about one pack of cigarettes daily and have smoked for 5 years or more.
In conclusion, heavy smokers in young male collegians had undesirable lifestyles and dietary habits such as high consumption of coffee and alcohol, and low intake of Fe and vitamin C. Although, there was no significant difference in their current BMD from the other groups, these undesirable factors with heavy smoking may affect their bone health in the long term. Therefore, in addition to antismoking education, intensive individualized education for smokers on desirable dietary habits and balanced nutrient intake appears to be necessary to have beneficial effects on bone health.
Table 2.
Dietary habits and life styles of subjects
1)n (%)
2)Mean ± SD
3)Values with different superscripts within a row are significantly different at a=0.05 as determined by Duncan's multiple range test.
4) Not significant
References
- 1.Angus RM, Sambrook PN, Pocock NA, Eisman JA. Dietary intake and bone mineral density. Bone Miner. 1988;4:265–277. [PubMed] [Google Scholar]
- 2.Benson BW, Shulman JD. Inclusion of tobacco exposure as a predictive factor for decreased bone mineral content. Nicotine Tob Res. 2005;7:719–724. doi: 10.1080/14622200500259119. [DOI] [PubMed] [Google Scholar]
- 3.Dallongeville J, Marécaux N, Fruchart JC, Amouyel P. Cigarette smoking is associated with unhealthy patterns of nutrient intake: a meta-analysis. J Nutr. 1998;128:1450–1457. doi: 10.1093/jn/128.9.1450. [DOI] [PubMed] [Google Scholar]
- 4.De Vernejoul MC, Bielakoff J, Herve M, Gueris J, Hott M, Modrowski D, Kuntz D, Miravet L, Ryckewaert A. Evidence for defective osteoblastic function. A role for alcohol and tobacco consumption in osteoporosis in middle-aged men. Clin Orthop Relat Res. 1983;179:107–115. [PubMed] [Google Scholar]
- 5.Dyer AR, Elliott P, Stamler J, Chan Q, Ueshima H, Zhou BF INTERMAP Research Group. Dietary intake in male and female smokers, ex-smokers, and never smokers: the INTERMAP study. J Hum Hypertens. 2003;17:641–654. doi: 10.1038/sj.jhh.1001607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Field M, Mogg K, Bradley BP. Alcohol increases cognitive biases for smoking cues in smokers. Psychopharmacology. 2005;180:63–72. doi: 10.1007/s00213-005-2251-1. [DOI] [PubMed] [Google Scholar]
- 7.Gambert SR, Schultz BM, Hamdy RC. Osteoporosis. Clinical features, prevention, and treatment. Endocrinol Metab Clin North Am. 1995;24:317–371. [PubMed] [Google Scholar]
- 8.Gonnelli S, Cepollaro C, Gennari L, Montagnani A, Caffarelli C, Merlotti D, Rossi S, Cadirni A, Nuti R. Quantitative ultrasound and dual-energy X-ray absorptiometry in the prediction of fragility fracture in men. Osteoporos Int. 2005;16:963–968. doi: 10.1007/s00198-004-1771-6. [DOI] [PubMed] [Google Scholar]
- 9.Grainge MJ, Coupland CA, Cliffe SJ, Chilvers CE, Hosking DJ The Nottingham EPIC Study Group. Cigarette smoking, alcohol and caffeine consumption, and bone mineral density in postmenopausal women. Osteoporos Int. 1998;8:355–363. doi: 10.1007/s001980050075. [DOI] [PubMed] [Google Scholar]
- 10.Grazio S, Korsic M, Jajic I. Effects of smoking and alcohol consumption on vertebral deformity in the elderly-an epidemiological study. Coll Antropol. 2005;29:567–572. [PubMed] [Google Scholar]
- 11.Hansen MA, Overgaard K, Riis BJ, Christiansen C. Potential risk factors for development of postmenopausal osteoporosis-examined over a 12-year period. Osteoporos Int. 1991;1:95–102. doi: 10.1007/BF01880450. [DOI] [PubMed] [Google Scholar]
- 12.Hoidrup S, Grønbaek M, Gottschau A, Lauritzen JB, Schroll M. Alcohol intake, beverage preference, and risk of hip fracture in men and women. Copenhagen Centre for Prospective Population Studies. Am J Epidemiol. 1999;149:993–1001. doi: 10.1093/oxfordjournals.aje.a009760. [DOI] [PubMed] [Google Scholar]
- 13.Ilich JZ, Kerstetter JE. Nutrition in bone health revisited: a story beyond calcium. J Am Coll Nutr. 2000;19:715–737. doi: 10.1080/07315724.2000.10718070. [DOI] [PubMed] [Google Scholar]
- 14.Kim SK, Yeon BY, Choi MK. Comparison of nutrient intakes and serum mineral levels between smoker and non-smokers. The Korean Journal of Nutrition. 2003;36:635–645. [Google Scholar]
- 15.Korean ministry of health and welfare. Report on 2005 national health and nutrition survey: Health Behavior Survey. Seoul. Republic of Korea: Kukjingihoek; 2006. p. 113. [Google Scholar]
- 16.Kwak CS, Lee JW, Hyun WJ. The effect of smoking and alcohol drinking on nutritional status and eating habits in adults males. Korean Journal of Community Nutrition. 2000;5:161–171. [Google Scholar]
- 17.Laroche M, Lasne Y, Felez A, Moulinier L, Bon E, Cantagrel A, Léophonte P, Mazières B. Osteocalcin and smoking. Rev Rhum Ed Fr. 1994;61:433–436. [PubMed] [Google Scholar]
- 18.Law MR, Hackshaw AK. A meta-analysis of cigarette smoking, bone mineral density and risk of hip fracture: recognition of a major effect. BMJ. 1997;315:841–846. doi: 10.1136/bmj.315.7112.841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Legrand E, Chappard D, Pascaretti C, Duquenne M, Rondeau C, Simon Y, Rohmer V, Basle MF, Audran M. Bone mineral density and vertebral fractures in men. Osteoporos Int. 1999;10:265–270. doi: 10.1007/s001980050225. [DOI] [PubMed] [Google Scholar]
- 20.Lloveras G, Ribas Barba L, Ramon JM, Serra Majem L, Román Viñas B. Food consumption and nutrient intake in relation to smoking. Med Clin (Barc) 2001;116:129–132. [PubMed] [Google Scholar]
- 21.Lorentzon M, Mellström D, Haug E, Ohlsson C. Smoking is associated with lower bone mineral density and reduced cortical thickness in young men. J Clin Endocrinol Metab. 2007;92:497–503. doi: 10.1210/jc.2006-1294. [DOI] [PubMed] [Google Scholar]
- 22.Massey LK, Whiting SJ. Caffeine, urinary calcium, calcium metabolism and bone. J Nutr. 1993;123:1611–1614. doi: 10.1093/jn/123.9.1611. [DOI] [PubMed] [Google Scholar]
- 23.Medeiros DM, Ilich JZ, Ireton J, Matkovic V, Shiry L, Wildman R. Femurs from rats fed diets deficient in copper or iron have decreased mechanical strength and altered mineral composition. Journal of Trace Elements in Experimental Medicine. 1997;10:197–203. [Google Scholar]
- 24.Medras M, Jankowska EA, Rogucka E. The effect of smoking tobacco and drinking of alcohol and coffee on bone mineral density of healthy men 40 years of age. Pol Arch Med Wewn. 2000;103:187–193. [PubMed] [Google Scholar]
- 25.Melton LJ, 3rd, Atkinson EJ, O'Connor MK, O'Fallon WM, Riggs BL. Bone density and fracture risk in men. J Bone Miner Res. 1998;13:1915–1923. doi: 10.1359/jbmr.1998.13.12.1915. [DOI] [PubMed] [Google Scholar]
- 26.Midgette AS, Baron JA, Rohan TE. Do cigarette smokers have diets that increase their risks of coronary heart disease and cancer? Am J Epidemiol. 1993;137:521–529. doi: 10.1093/oxfordjournals.aje.a116705. [DOI] [PubMed] [Google Scholar]
- 27.Ortego-Centeno N, Munoz-Torres M, Jodar E, Hernandez-Quero J, Jurado-Duce A, de la Higuera Torres-Puchol J. Effect of tobacco consumption on bone mineral density in healthy young males. Calcif Tissue Int. 1997;60:496–500. doi: 10.1007/s002239900270. [DOI] [PubMed] [Google Scholar]
- 28.Pluskiewicz W, Drozdzowska B. Ultrasound measurements at the calcaneus in men: differences between healthy and fractured persons and the influence of age and anthropometric features on ultrasound parameters. Osteoporos Int. 1999;10:47–51. doi: 10.1007/s001980050193. [DOI] [PubMed] [Google Scholar]
- 29.Prockop DJ. Role of iron in the synthesis of collagen in connective tissue. Fed Proc. 1971;30:984–990. [PubMed] [Google Scholar]
- 30.Prynne CJ, Mishra GD, O'Connell MA, Muniz G, Laskey MA, Yan L, Prentice A, Ginty F. Fruit and vegetable intakes and bone mineral status: a cross sectional study in 5 age and sex cohorts. Am J Clin Nutr. 2006;83:1420–1428. doi: 10.1093/ajcn/83.6.1420. [DOI] [PubMed] [Google Scholar]
- 31.Ramp WK, Lenz LG, Galvin RJ. Nicotine inhibits collagen synthesis and alkaline phosphatase activity, but stimulates DNA synthesis in osteoblast-like cells. Proc Soc Exp Biol Med. 1991;197:36–43. doi: 10.3181/00379727-197-43221. [DOI] [PubMed] [Google Scholar]
- 32.Rubin LA, Hawker GA, Peltekova VD, Fielding LJ, Ridout R, Cole DE. Determinants of peak bone mass: clinical and genetic analyses in a young female Canadian cohort. J Bone Miner Res. 1999;14:633–643. doi: 10.1359/jbmr.1999.14.4.633. [DOI] [PubMed] [Google Scholar]
- 33.Sakamoto W, Nishihira J, Fujie K, Iizuka T, Handa H, Ozaki M, Yukawa S. Effect of coffee consumption on bone metabolism. Bone. 2001;28:332–336. doi: 10.1016/s8756-3282(00)00444-0. [DOI] [PubMed] [Google Scholar]
- 34.Saules KK, Pomerleau CS, Snedecor SM, Mehringer AM, Shadle MB, Kurth C, Krahn DD. Relationship of onset of cigarette smoking during college to alcohol use, dieting concerns, and depressed mood: results from the Young Women's Health Survey. Addict Behav. 2004;29:893–899. doi: 10.1016/j.addbeh.2004.02.015. [DOI] [PubMed] [Google Scholar]
- 35.Seeman E, Melton LJ, 3rd, O'Fallon WM, Riggs BL. Risk factors for spinal osteoporosis in men. Am J Med. 1983;75:977–983. doi: 10.1016/0002-9343(83)90878-1. [DOI] [PubMed] [Google Scholar]
- 36.Spencer H, Kramer L. NIH Consensus Conference: osteoporosis. Factors contributing to osteoporosis. J Nutr. 1986;116:316–319. doi: 10.1093/jn/116.2.316. [DOI] [PubMed] [Google Scholar]
- 37.Supervía A, Nogués X, Enjuanes A, Vila J, Mellibovsky L, Serrano S, Aubía J, Díez-Pérez A. Effect of smoking and smoking cessation on bone mass, bone remodeling, vitamin D, PTH and sex hormones. J Musculoskelet Neuronal Interact. 2006;6:234–241. [PubMed] [Google Scholar]
- 38.Szulc P, Garnero P, Claustrat B, Marchand F, Duboeuf F, Delmas PD. Increased bone resorption in moderate smokers with low body weight: the Minos study. J Clin Endocrinol Metab. 2002;87:666–674. doi: 10.1210/jcem.87.2.8232. [DOI] [PubMed] [Google Scholar]
- 39.Vogel JM, Davis JW, Nomura A, Wasnich RD, Ross PD. The effects of smoking on bone mass and the rates of bone loss among elderly Japanese-American men. J Bone Miner Res. 1997;12:1495–1501. doi: 10.1359/jbmr.1997.12.9.1495. [DOI] [PubMed] [Google Scholar]
- 40.Webb MS, Carey MP. Tobacco smoking among low-income Black women: demographic and psychosocial correlates in a community sample. Nicotine Tob Res. 2008;10:219–229. doi: 10.1080/14622200701767845. [DOI] [PubMed] [Google Scholar]