Abstract
Outer hair cells boost auditory performance in mammals. This amplification relies on an expansive array of intramembranous molecular motors, identified as prestin, that drive somatic electromotility. By measuring nonlinear capacitance, the electrical signature of electromotility, we are able to assess prestin's conformational state and interrogate the effectiveness of anions on prestin's activity. We find that the affinity of anions depends on the state of prestin that we set with a variety of perturbations (in membrane tension, temperature, and voltage), and that movement into the expanded state reduces the affinity of prestin for anions. These data signify that anions work allosterically on prestin. Consequently, anions are released from prestin's binding site during expansion, i.e., during hyperpolarization. This is at odds with the extrinsic voltage sensor model, which suggests that prestin-bound intracellular anions are propelled deep into the membrane. Furthermore, we hypothesize that prestin's susceptibility to many biophysical forces, and notably its piezoelectric nature, may reflect anion interactions with the motor.
Introduction
Allosteric modulation of protein function is ubiquitous, denoting the control of a protein's conformational state (tensed versus relaxed) and activity by perturbation (via ligand or nonligand mechanisms) of sites distal to the protein's active site (1–4). Monod et al. (3) originally developed a concerted allosteric model to describe the effects of oxygen binding on hemoglobin, but this model has also been shown to be useful for understanding other proteins, including intramembranous proteins. For example, modeling the modulation of Maxi-K channels by voltage and Ca2+ has benefited from allosteric theory (5,6). One interesting consequence of allostery is the coupling of the conformational state and ligand-binding affinity, a phenomenon that forms the basis of state-dependent block of ion channels (7).
The conformational state of the outer hair cell (OHC) motor protein, prestin, is primarily driven by voltage. Changes in the conformational state of the molecule can be gleaned from the voltage dependence of its nonlinear charge movement or its equivalent bell-shaped nonlinear capacitance (NLC) (8, 9). Indeed, the voltage at peak capacitance (Vpkcm) or, equivalently, the half-maximal charge movement (Vh), is a sensitive indicator of the distribution of motors in the compact or expanded state, according to two-state models of OHC motor activity (10,11). However, the distribution is not fixed; rather, it depends on a host of external influences. Prolonged negative holding potentials (prepulse) (12), positive membrane tension (13–16), and increasing temperature (17,18) can each significantly shift Vpkcm rightward, in the depolarizing direction, in a multiexponential, time-dependent manner (19), indicating a redistribution of compact motors into the expanded state.
Recent observations have established an important role for anions in controlling prestin and OHC function (20–24). Charge movement in prestin is modulated by Cl− ions with a K1/2 of ∼6 mM (21,22). We have argued that the binding of anions works allosterically to modify prestin activity (20,23–26). Here we capitalize on prestin's sensitivity to membrane tension, temperature, and voltage to show that prestin's binding affinity for the anions Cl− and salicylate is dependent on motor conformation, confirming an allosteric interaction.
Materials and Methods
General preparation and procedure
Hartley albino guinea pigs were overdosed with halothane. The temporal bones were excised and the cochleae were dissected in a calcium-free (no chelator) solution and then treated with enzyme (0.5 mg/mL Dispase I, 10–12 min). Individual OHCs were isolated by gentle trituration. The suspension of cells was then transferred to a petri dish for the cells to settle.
The base extracellular solution contained (in mM) NaCl 132, CaCl2 2, MgCl2 2, and Hepes 10. The final solutions were adjusted to ∼300 mOsm with D-glucose (except for the solutions used for the osmotic challenge experiment, which were adjusted to ∼280 mOsm) and pH 7.2–7.3 with NaOH. The intracellular solution was the same as the extracellular solution except for the addition of 10 mM EGTA. Other Cl− levels were achieved by replacing Cl− with gluconate. Salicylate (10 mM) was added to the base extracellular solution (∼300 mOsm, pH 7.2–7.3) and subsequently diluted into concentrations of 0.1, 1, 10, 100, 1000, and 10,000 μM. Continuous local perfusion was achieved with the use of a custom-made Y-tube. Perfusions of salicylate either as a concentration series or at a single dose were performed with and without positive pressure in the patch pipette (increased tension at ∼0.7 kPa). Pipette pressure was monitored via a pressure monitor (PM015R; World Precision Instruments, Sarasota, FL). Whole-cell NLC (see below) recordings were made after the pressure stabilized.
Experiments were performed at room temperature and at 32–35°C. To study the effect of temperature, bath solutions at room temperature were exchanged with preheated bath solutions to increase the temperature around the OHC of interest. A temperature probe (model DP26-TC-AP; Omega Engineering) was placed in the bath near the recorded OHC to document the temperature rise. Recordings of NLC were made when the temperature rose ∼10°C. A Nikon Eclipse E600-FN microscope with a 40× water immersion lens was used to observe cells during electrical measurements. Digital images were taken with a Hamamatsu image processor.
Patch-clamp and stimulus protocols
OHCs were recorded under a whole-cell, patch-clamp configuration. An Axon 200B amplifier was used for data collection. NLC was measured using a continuous high-resolution (2.56 ms sampling) two-sine stimulus protocol (10 mV peak at both 390.6 and 781.2 Hz) superimposed on a 200 ms voltage ramp from −180 to +200 mV (12,27). All recordings and analyses were performed using jClamp (SciSoft, Ridgefield, CT; www.scisoftco.com). Capacitance data were fit to the first derivative of a two-state Boltzmann function (8):
where Qmax is the maximum nonlinear charge moved; Vpkcm or Vh is the voltage at peak capacitance or, equivalently, at half-maximum charge transfer; Vm is the membrane potential; z is the valence; Clin is the linear membrane capacitance; e is the electron charge; k is Boltzmann's constant; and T is the absolute temperature. Qsp, the specific density of charge movement is defined as Qmax/(Clin − 6.5 pF); 6.5 pF corresponds to the area in OHCs devoid of prestin NLC (28). Peak NLC was calculated by subtracting the linear capacitance (Clin) from the peak amplitude (Cmpk). We used Clin determined from the fit to each individual NLC trace. Paired t-tests were used and reported as the mean ± SE. Dose response curves were fitted in SigmaPlot (Systat Software) using a four-parameter Hill equation. For the Cl− dose response curve, the low-concentration end point was fixed to 25% of the maximum charge because we previously determined this asymptote using a full range of Cl concentrations (22).
Results
Membrane tension alters the IC50 of salicylate on NLC
Salicylate is known to block NLC in OHCs via intracellular interactions with the motor (29,30). Recently, it was shown that salicylate competes with Cl− for prestin's anion-binding site(s) (20,21). We assessed salicylate's relative affinity (competition with Cl−) by noting the degree of reduction in NLC with intracellular Cl− fixed at 10 mM, the normal resting level of chloride (20). Fig. 1, A and B, demonstrate that with a fixed extracellular salicylate perfusion of 0.1 mM, tension reduced the effectiveness of the salicylate insult (intra- and extracellular solutions both contained 10 mM Cl). The reduction of nonlinear peak capacitance was 11.22 ± 0.68 pF for collapsed cells and 7.44 ± 1.15 pF for pressurized (∼0.7 kPa) cells (n = 4, P < 0.01, paired t-test). In percentage terms, this corresponds to reductions of 22.08% and 16.57%, respectively. Since the perfusion is done extracellularly, it is possible that the manipulation of membrane tension can change membrane properties such that salicylate entry into the cells will be different under the two conditions. To rule out this possibility, we introduced 0.1 mM salicylate intracellularly via the patch pipette, and obtained comparable significant results. Indeed, even with equal concentrations of salicylate intra- and extracellularly, similar results were obtained. The use of an osmotic challenge (∼280 mOsm) produced comparable results (data not shown).
Figure 1.
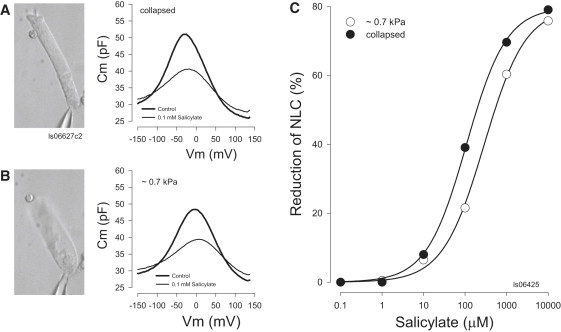
Competition between salicylate and chloride for prestin's anion-binding site is dependent on membrane tension. (A) Image of patch-clamped OHC under collapsed conditions and associated NLC traces obtained before (control) and after perfusion with 0.1 mM salicylate. Cell diameter is 10 μm. (B) Corresponding image (note the reduced length and increased diameter of cell) and NLC traces for the same cell after an increase in pipette pressure (0.7 kPa). The averaged reduction of nonlinear peak capacitance due to salicylate perfusion is 11.22 + 0.68 for collapsed cells and 7.44 + 1.15 pF (n = 4, mean ± SE, P < 0.01, paired t-test). (C) Plot from another cell of the dose response curve for salicylate before and after membrane tension delivery. Averaged results show a shift in IC50 from 144.77 ± 61.33 to 550.05 ± 221.49 (mean ± SE).
Tension effects were explored in greater detail in another set of experiments to measure the IC50 of salicylate. A series of salicylate concentrations (0.1–10,000 μM) were applied to OHCs first under collapsed conditions and then, after complete washout (assessed by the return of NLC to pretreatment conditions), under pressurized conditions (∼0.7 kPa). Fig. 1 C illustrates the shift in the dose response curve obtained for a single OHC. Data from four cells (with pipette solutions containing 10 mM Cl−) yielded a shift of IC50 from 144.77 ± 61.33 to 550.05 ± 221.49 mM. This represents an ∼4-fold reduction in effectiveness, indicating an equivalent reduction in the affinity of salicylate for prestin. For cells with 140 mM Cl− in and out, the shift for IC50s for salicylate dose response curves was from 333.89 ± 46.81 to 563.77 ± 27.99 mM (n = 3). The smaller shift at higher chloride levels is consistent with our previous finding that salicylate is more effective at lower chloride levels (20).
Membrane voltage prepulse and temperature alter salicylate's effect on NLC
The data obtained so far indicate that increasing membrane tension, which tends to redistribute the motors into the expanded state, reduces the affinity of salicylate for prestin's anion-binding site. If the conformation of prestin were responsible for this effect, we would expect any method we used to redistribute prestin into the expanded state to work similarly. Fig. 2 A confirms that by heating the cells, we diminished the ability of salicylate to reduce NLC. The average reductions of nonlinear peak capacitance for 1 mM salicylate treatment were 9.9 ± 1.00 pF at room temperature and 5.58 ± 0.73 pF after an ∼10°C increase in bath temperature (n = 4, P < 0.05, paired t-test). Intermediate temperatures between room temperature and +10°C were proportionally effective. The effectiveness of salicylate insult (reduction of NLC) fell at a rate of 0.366 pF/°C with increases in temperature (see Fig. S1 in the Supporting Material).
Figure 2.
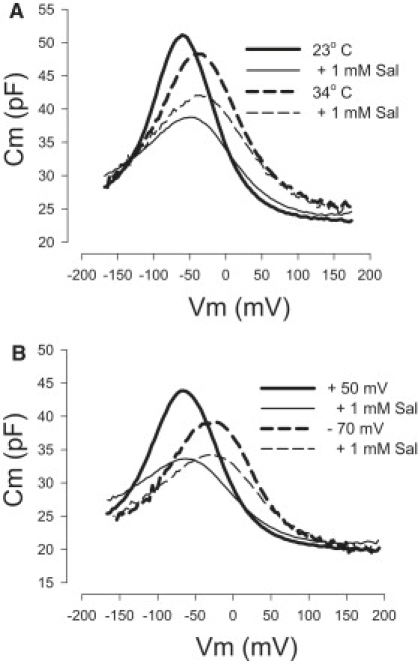
Competition between salicylate and chloride for prestin's anion-binding site is dependent on temperature and holding voltage. (A) A representative OHC was whole-cell patched with 140 mM Cl− in and out, and perfused with 1 mM salicylate first at 23°C (solid lines) and then at 34°C (dashed lines). The reduction of NLC after salicylate perfusion (thin lines) is less under the high-temperature condition. Average reductions of nonlinear peak capacitance for salicylate treatment are 9.9 ± 1.00 pF (mean ± SE) vs. 5.58 ± 0.73 pF (mean ± SE) (n = 4, P < 0.05, paired t-test). (B) A representative OHC was whole-cell patched and perfused with 1 mM salicylate at room temperature. The OHC was held at either −70 mV (dashed lines) or + 50 mV (solid lines). The reduction of NLC after salicylate perfusion (thin lines) is less at the −70 mV holding potential. The average reduction of nonlinear peak capacitance for salicylate treatment is 10.50 ± 0.16 (mean ± SE) at positive holding potentials and 6.41 ± 1.05 (mean ± SE) at negative holding potentials (n = 4, P < 0.005, paired t-test).
Additionally, Fig. 2 B shows that holding at negative voltages, which redistributes prestin into the expanded state, diminishes the ability of salicylate to reduce NLC. The average reduction of nonlinear peak capacitance for 1 mM salicylate treatment was 10.50 ± 0.16 pF at ∼ +55 mV holding potential and 6.41 ± 1.05 pF at ∼ −75 mV holding potential (n = 4, P < 0.005, paired t-test). Intermediate holding voltages between +55 and −75 mV were proportionally effective. The effectiveness of salicylate insult (reduction of NLC) fell at a rate of 0.029 pF/mV with hyperpolarization (see Fig. S1).
Membrane tension reduces chloride's binding affinity for prestin
Finally, we confirmed the generality and physiological significance of our results by testing the effects of membrane tension on Cl-anion affinity. In this series of experiments, the chloride concentration was set to the same level intra- and extracellularly, and the effects of membrane tension on NLC were determined for a range of chloride ion concentrations (1–140 mM). NLC was measured first under collapsed conditions and then under pressurized conditions (∼0.7 kPa). Fig. 3 A illustrates the average shift (n = 4–5) in the dose response curve. The fitted sigmoidal (Hill) functions indicate a K1/2 of 1.68 mM under collapsed conditions, and 4.74 mM under pressurized conditions. The shift of Vpkcm due to ∼0.7 kPa pressurization is ∼20 mV to the right regardless of chloride concentration (Fig. 3 B), indicating that the results are not due to chloride-dependent changes in the susceptibility to membrane tension.
Figure 3.
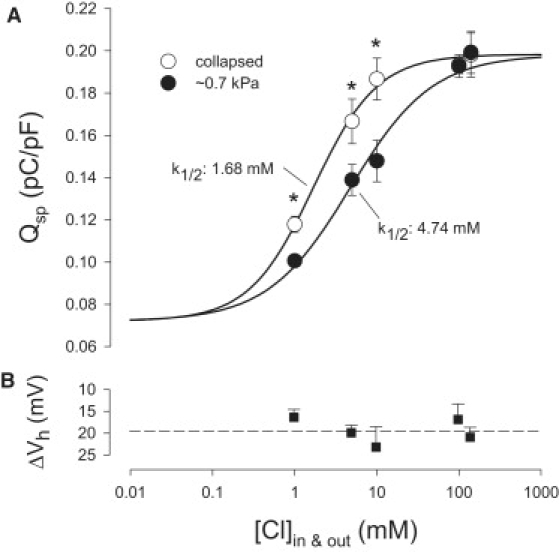
Chloride binding to prestin is state-dependent. OHCs were whole-cell patched and NLC was recorded. Chloride concentration was set to the same level intra- and extracellularly, and the effects of membrane tension on NLC were determined for a range of chloride ion concentrations (1–140 mM). NLC was measured first under collapsed conditions and then under pressurized conditions (∼0.7 kPa). OHC NLC was fitted (Eq. 1) to determine Vh and total nonlinear charge moved (Qmax), with Qmax normalized to cell surface area (Qsp) for comparison across cells. (A) OHC Qsp is Cl−-dependent, with ∼25% of the charge insensitive to chloride as demonstrated previously (22). Changing prestin's state by application of positive membrane tension shifts the dose response curve to the right. Paired t-test significance (∗, P < 0.05; n = 4–5) is noted in the figure. The fitted sigmoidal (Hill) functions indicate a K1/2 of 1.68 mM under collapsed conditions and 4.74 mM under pressurized conditions. (B) The shift of Vh due to ∼0.7 kPa pressurization is ∼20 mV to the right regardless of chloride concentration, indicating no chloride-dependent changes in the susceptibility to membrane tension.
It is important to note that with each type of manipulation available to us, we had to limit the perturbation magnitude to maintain our cell recordings during the lengthy protocols; therefore, we obtained only a limited interrogation of the changes in binding affinity that might be realized by a complete shift between compact and expanded states.
Discussion
The OHC motor is voltage-dependent (31) and possesses a voltage sensor that controls its conformational state (compact/expanded) at rates in excess of 75 kHz (10,11,32). The voltage dependence of the sensor charge movement is shallow, with a Boltzmann slope factor of ∼29 mV, and thus very large voltage changes (>200 mV) are required to fully redistribute motors between its two conformations (compact and expanded) (8, 9). The midpoint voltage (Vpkcm or Vh) of this operational range can be modified by several manipulations, including membrane tension, prior (prepulse) holding voltage, and temperature. The effect of tension on Vpkcm is on the order of a 20 mV rightward (i.e., depolarizing) shift per kPa of intracellular pressure, with the cell being able to withstand only a few kilopascals before it ruptures (15,16). Changes in the holding potential can shift Vpkcm tens of millivolts, with the shift being opposite to the prepulse polarity and having maximum effectiveness near normal resting potentials (12,19). Increases in temperature cause a 20 mV rightward shift per 10°C (17,18).
In this work, we exploited prestin's sensitivity to these biophysical forces to test the hypothesis that anions work allosterically by interrogating the effect of the motor conformational state on anion affinity. Although we were only able to interrogate conformation-dependent binding affinity over a limited population shift between compact and expanded states (see Materials and Methods), each perturbation we made to shift the state dependence of prestin motors from the compact to the expanded state reduced the affinity of the tested anion for the motor. We reason that prestin's anion-binding site, which has been tentatively identified in prestin as homologous to the Cl-binding motif conserved in ClC channels (GXXXP (26,33,34)), is altered during induced conformational change to modify binding affinity for anions. We especially note a parallel in the reciprocal nature of the voltage dependence and tension dependence of prestin (piezoelectricity) and the reciprocal nature of anion binding and the conformational state of prestin (allostery). The piezoelectric nature of prestin was first identified by Iwasa (13), who found that membrane tension can cause a shift in the voltage dependence of OHC NLC; thus, tension can induce displacement currents in the OHC (14,35). This linkage between deformation and electrical activity is greater in the OHC than in piezoelectric crystals (36), and has been the focus of models that aim to enhance the frequency-dependent effects of electromotility (37). We conjecture that the piezoelectric nature of prestin may ultimately reside in the allosteric action of anions. Furthermore, we hypothesize that the sensitivity of prestin to the range of biophysical perturbations observed to influence the state of the motor may involve anion interactions with the motor. To be sure, the shift magnitudes from membrane tension, temperature, and prepulse are dwarfed by that observed when physiologically tolerable chloride concentrations are changed within the OHC: at the K1/2 for the Cl-dependent Vpkcm shift (4.8 mM, close to the resting intracellular [Cl−] (20)), the shift is ∼−8 mV/mM (22). Thus, we would predict that other perturbations that affect the prestin state, such as changes in the membrane lipid environment of OHCs (38,39) or prestin-transfected cells (40), result from alterations of anion-binding affinity.
The competitiveness of salicylate with chloride for prestin's anion-binding site results from a higher affinity for salicylate over chloride (21). If we assume that chloride and salicylate binding affinity can be manipulated independently, then the reduced binding affinity of chloride by our manipulations could have increased salicylate's effectiveness. This was not the case, and we must conclude that our manipulations affect the binding site in a manner that influences binding of all anions. Thus, the effects of salicylate are direct, and not a result of displacing chloride. This is in line with the variable effects of different species of anions on NLC (25).
In summary, all perturbations that caused Vpkcm to shift in the depolarizing direction, i.e., that caused prestin motors to move into the expanded state, reduced prestin's anion-binding affinity. These observations have direct implications for the mechanism of voltage sensing in prestin (Fig. 4). Oliver et al.'s (21) popular model of prestin as a dysfunctional anion transporter, in which an intracellular chloride anion serves as extrinsic voltage sensor, predicts that truncated transport of chloride from its intracellular binding site on prestin to an extracellularly directed intramembranous site within the protein evokes prestin's expanded state. On the contrary, our data necessarily indicate that intracellularly bound anions are released (due to reduced affinity) upon motor expansion, and thus conflict with their model. We maintain that anion binding is associated with motor contraction and OHC shortening, in line with our previous observations that increasing Cl− intracellularly boosts the number of motors residing in the contracted state (22,23). Of interest, an analysis of a variety of models, including those resulting from intrinsic sensing, extrinsic sensing, and a combination of transporting and sensing mechanisms, found that predicted movements of chloride during transport are associated with the contraction of prestin (41). We believe these fine points of contention with the extrinsic voltage sensor model can be used to argue for a general mechanism of allostery that portrays prestin as a protein of normal character that does not require unusual modes (or models) of action. To be sure, we determined that the sensor charge movement in prestin relies on particular charged residues, as is the case in other voltage-dependent proteins (42), and that prestin's voltage-dependent charge movement can be isolated from its anion transport (26). These data do not imply that models that detect (incorporate) composite charge movement contributions from anions and intrinsic residues are faulty in their assessment of NLC (41,43), as we fully acknowledge that any charged species moving through the membrane field can contribute to NLC. We simply want to point out that the assignment of charge components to effect electromotility or not is not a simple process, and it could be that the movement of a transported anion through prestin (and its resultant displacement current) is unrelated to the mechanism that drives the voltage-dependent mechanical activity of the protein. Thus, the only thing that is absolutely clear to us is that NLC is modulated by anions. Consequently, our new findings and our previous observations that also conflict with the extrinsic voltage sensor model—namely, that 1), changes in the intracellular anion concentration and species shift the voltage dependence of NLC (Vpkcm); 2), the anion valence does not correlate with the voltage sensor valence; 3), prestin successfully transports anions; 4), the intrinsic charge contributes to prestin's voltage sensing; and 5), single-site mutations can separate voltage sensing and transport (23,25,26)—lead us to conclude that anions work through an allosteric action on prestin.
Figure 4.
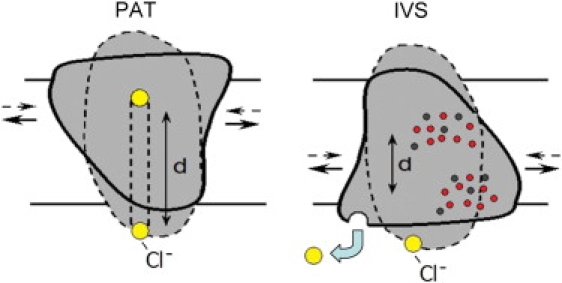
Two models representing the events after anion binding to prestin. The left panel depicts the extrinsic voltage sensor model (21), where Cl− binds and enters the defunct transporter, which in turn triggers an extension of prestin. The right panel depicts the intrinsic voltage sensor model (23–25), where Cl− binding to prestin allosterically promotes the contracted state, but voltage sensing results from movement of intrinsically charged amino acid residues (red: positive residues; black: negative residues) (26). Our new data confirm that during expansion, anions are released from prestin. (In both panels, d is the perpendicular distance traversed across the membrane field by the voltage sensor.)
Supporting Material
A figure is available at http://www.biophysj.org/biophysj/supplemental/S0006-3495(09)01669-5.
Supporting Material
Acknowledgments
We thank Fred Sigworth for his thoughts on allostery.
This work was supported by grants from the National Institute on Deafness and Other Communication Disorders (DC00273 to J.S.S.) and Oshe grant for Surgical Research at Yale University School of Medicine (to L.S.).
References
- 1.Gunasekaran K., Ma B., Nussinov R. Is allostery an intrinsic property of all dynamic proteins? Proteins. 2004;57:433–443. doi: 10.1002/prot.20232. [DOI] [PubMed] [Google Scholar]
- 2.Goodey N.M., Benkovic S.J. Allosteric regulation and catalysis emerge via a common route. Nat. Chem. Biol. 2008;4:474–482. doi: 10.1038/nchembio.98. [DOI] [PubMed] [Google Scholar]
- 3.Monod J., Wyman J., Changeux J.P. On the nature of allosteric transitions: a plausible model. J. Mol. Biol. 1965;12:88–118. doi: 10.1016/s0022-2836(65)80285-6. [DOI] [PubMed] [Google Scholar]
- 4.Koshland D.E., Némethy G., Filmer D. Comparison of experimental binding data and theoretical models in proteins containing subunits. Biochemistry. 1966;5:365–385. doi: 10.1021/bi00865a047. [DOI] [PubMed] [Google Scholar]
- 5.Cui J., Aldrich R.W. Allosteric linkage between voltage and Ca(2+)-dependent activation of BK-type mslo1 K(+) channels. Biochemistry. 2000;39:15612–15619. doi: 10.1021/bi001509+. [DOI] [PubMed] [Google Scholar]
- 6.Rothberg B.S. Allosteric modulation of ion channels: the case of Maxi-K. Sci. STKE. 2004;2004:e16. doi: 10.1126/stke.2272004pe16. [DOI] [PubMed] [Google Scholar]
- 7.Ragsdale D.S., McPhee J.C., Catterall W.A. Molecular determinants of state-dependent block of Na+ channels by local anesthetics. Science. 1994;265:1724–1728. doi: 10.1126/science.8085162. [DOI] [PubMed] [Google Scholar]
- 8.Santos-Sacchi J. Reversible inhibition of voltage-dependent outer hair cell motility and capacitance. J. Neurosci. 1991;11:3096–3110. doi: 10.1523/JNEUROSCI.11-10-03096.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ashmore J.F. Forward and reverse transduction in the mammalian cochlea. Neurosci. Res. Suppl. 1990;12:S39–S50. doi: 10.1016/0921-8696(90)90007-p. [DOI] [PubMed] [Google Scholar]
- 10.Santos-Sacchi J. Harmonics of outer hair cell motility. Biophys. J. 1993;65:2217–2227. doi: 10.1016/S0006-3495(93)81247-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Iwasa K.H. A membrane motor model for the fast motility of the outer hair cell. J. Acoust. Soc. Am. 1994;96:2216–2224. doi: 10.1121/1.410094. [DOI] [PubMed] [Google Scholar]
- 12.Santos-Sacchi J., Kakehata S., Takahashi S. Effects of membrane potential on the voltage dependence of motility-related charge in outer hair cells of the guinea-pig. J. Physiol. 1998;510:225–235. doi: 10.1111/j.1469-7793.1998.225bz.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Iwasa K.H. Effect of stress on the membrane capacitance of the auditory outer hair cell. Biophys. J. 1993;65:492–498. doi: 10.1016/S0006-3495(93)81053-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gale J.E., Ashmore J.F. Charge displacement induced by rapid stretch in the basolateral membrane of the guinea-pig outer hair cell. Proc. R. Soc. Lond. B. Biol. Sci. 1994;255:243–249. doi: 10.1098/rspb.1994.0035. [DOI] [PubMed] [Google Scholar]
- 15.Kakehata S., Santos-Sacchi J. Membrane tension directly shifts voltage dependence of outer hair cell motility and associated gating charge. Biophys. J. 1995;68:2190–2197. doi: 10.1016/S0006-3495(95)80401-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dong X.X., Iwasa K.H. Tension sensitivity of prestin: comparison with the membrane motor in outer hair cells. Biophys. J. 2004;86:1201–1208. doi: 10.1016/S0006-3495(04)74194-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Meltzer J., Santos-Sacchi J. Temperature dependence of non-linear capacitance in human embryonic kidney cells transfected with prestin, the outer hair cell motor protein. Neurosci. Lett. 2001;313:141–144. doi: 10.1016/s0304-3940(01)02266-2. [DOI] [PubMed] [Google Scholar]
- 18.Santos-Sacchi J., Huang G. Temperature dependence of outer hair cell nonlinear capacitance. Hear. Res. 1998;116:99–106. doi: 10.1016/s0378-5955(97)00204-9. [DOI] [PubMed] [Google Scholar]
- 19.Santos-Sacchi J., Navarrete E., Song L. Fast electromechanical amplification in the lateral membrane of the outer hair cell. Biophys. J. 2009;96:739–747. doi: 10.1016/j.bpj.2008.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Santos-Sacchi J., Song L., Nuttall A.L. Control of mammalian cochlear amplification by chloride anions. J. Neurosci. 2006;26:3992–3998. doi: 10.1523/JNEUROSCI.4548-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Oliver D., He D.Z., Fakler B. Intracellular anions as the voltage sensor of prestin, the outer hair cell motor protein. Science. 2001;292:2340–2343. doi: 10.1126/science.1060939. [DOI] [PubMed] [Google Scholar]
- 22.Song L., Seeger A., Santos-Sacchi J. On membrane motor activity and chloride flux in the outer hair cell: lessons learned from the environmental toxin tributyltin. Biophys. J. 2005;88:2350–2362. doi: 10.1529/biophysj.104.053579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rybalchenko V., Santos-Sacchi J. Cl− flux through a non-selective, stretch-sensitive conductance influences the outer hair cell motor of the guinea-pig. J. Physiol. 2003;547:873–891. doi: 10.1113/jphysiol.2002.036434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rybalchenko V., Santos-Sacchi J. Allosteric modulation of the outer hair cell motor protein prestin by chloride. In: Gummer A., editor. Biophysics of the Cochlea: From Molecules to Models. World Scientific Publishing; Singapore: 2003. pp. 116–126. [Google Scholar]
- 25.Rybalchenko V., Santos-Sacchi J. Anion control of voltage sensing by the motor protein prestin in outer hair cells. Biophys. J. 2008;95:4439–4447. doi: 10.1529/biophysj.108.134197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bai J.P., Surguchev A., Navaratnam D. Prestin's anion transport and voltage-sensing capabilities are independent. Biophys. J. 2009;96:3179–3186. doi: 10.1016/j.bpj.2008.12.3948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Santos-Sacchi J. Determination of cell capacitance using the exact empirical solution of dY/dCm and its phase angle. Biophys. J. 2004;87:714–727. doi: 10.1529/biophysj.103.033993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Santos-Sacchi J., Kakehata S., Takasaka T. Density of motility-related charge in the outer hair cell of the guinea pig is inversely related to best frequency. Neurosci. Lett. 1998;256:155–158. doi: 10.1016/s0304-3940(98)00788-5. [DOI] [PubMed] [Google Scholar]
- 29.Kakehata S., Santos-Sacchi J. Effects of salicylate and lanthanides on outer hair cell motility and associated gating charge. J. Neurosci. 1996;16:4881–4889. doi: 10.1523/JNEUROSCI.16-16-04881.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tunstall M.J., Gale J.E., Ashmore J.F. Action of salicylate on membrane capacitance of outer hair cells from the guinea-pig cochlea. J. Physiol. 1995;485:739–752. doi: 10.1113/jphysiol.1995.sp020765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Santos-Sacchi J., Dilger J.P. Whole cell currents and mechanical responses of isolated outer hair cells. Hear. Res. 1988;35:143–150. doi: 10.1016/0378-5955(88)90113-x. [DOI] [PubMed] [Google Scholar]
- 32.Frank G.,, Hemmert W., Gummer A.W. Limiting dynamics of high-frequency electromechanical transduction of outer hair cells. Proc. Natl. Acad. Sci. USA. 1999;96:4420–4425. doi: 10.1073/pnas.96.8.4420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dutzler R., Campbell E.B., MacKinnon R. Gating the selectivity filter in ClC chloride channels. Science. 2003;300:108–112. doi: 10.1126/science.1082708. [DOI] [PubMed] [Google Scholar]
- 34.Dutzler R., Campbell E.B., MacKinnon R. X-ray structure of a ClC chloride channel at 3.0 Å reveals the molecular basis of anion selectivity. Nature. 2002;415:287–294. doi: 10.1038/415287a. [DOI] [PubMed] [Google Scholar]
- 35.Takahashi S., Santos-Sacchi J. Non-uniform mapping of stress-induced, motility-related charge movement in the outer hair cell plasma membrane. Pflugers Arch. 2001;441:506–513. doi: 10.1007/s004240000455. [DOI] [PubMed] [Google Scholar]
- 36.Dong X.X., Ospeck M., Iwasa K.H. Piezoelectric reciprocal relationship of the membrane motor in the cochlear outer hair cell. Biophys. J. 2002;82:1254–1259. doi: 10.1016/S0006-3495(02)75481-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Spector A.A., Brownell W.E., Popel A.S. Effect of outer hair cell piezoelectricity on high-frequency receptor potentials. J. Acoust. Soc. Am. 2003;113:453–461. doi: 10.1121/1.1526493. [DOI] [PubMed] [Google Scholar]
- 38.Santos-Sacchi J., Wu M. Protein- and lipid-reactive agents alter outer hair cell lateral membrane motor charge movement. J. Membr. Biol. 2004;200:83–92. doi: 10.1007/s00232-004-0699-2. [DOI] [PubMed] [Google Scholar]
- 39.Rajagopalan L., Greeson J.N., Brownell W.E. Tuning of the outer hair cell motor by membrane cholesterol. J. Biol. Chem. 2007;282:36659–36670. doi: 10.1074/jbc.M705078200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sfondouris J., Rajagopalan L., Brownell W.E. Membrane composition modulates prestin-associated charge movement. J. Biol. Chem. 2008;283:22473–22481. doi: 10.1074/jbc.M803722200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Muallem D., Ashmore J. An anion antiporter model of prestin, the outer hair cell motor protein. Biophys. J. 2006;90:4035–4045. doi: 10.1529/biophysj.105.073254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bezanilla F. How membrane proteins sense voltage. Nat. Rev. Mol. Cell Biol. 2008;9:323–332. doi: 10.1038/nrm2376. [DOI] [PubMed] [Google Scholar]
- 43.Sun S.X., Farrell B., Spector A.A. Voltage and frequency dependence of prestin-associated charge transfer. J. Theor. Biol. 2009;260:137–144. doi: 10.1016/j.jtbi.2009.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


