Abstract
Paradoxical low flow, low gradient, severe aortic stenosis (AS) despite preserved ejection fraction is a recently described clinical entity whereby patients with severe AS on the basis of aortic valve area have a lower than expected gradient in relation to generally accepted values. This mode of presentation of severe AS is relatively frequent (up to 35% of cases) and such patients have a cluster of findings, indicating that they are at a more advanced stage of their disease and have a poorer prognosis if treated medically rather than surgically. Yet, a majority of these patients do not undergo surgery likely due to the fact that the reduced gradient is conducive to an underestimation of the severity of the disease and/or of symptoms. The purpose of this article is to review and further analyse the distinguishing characteristics of this entity and to present its implications with regards to currently accepted guidelines for AS severity.
Keywords: Aortic valve stenosis, Severity, Low-gradient, Echocardiography, Hypertension
Introduction
According to current ACC/AHA guidelines,1 cut-off values for Doppler-Echocardiographic measurements of severe aortic stenosis (AS) are defined as follows: aortic valve area (AVA) <1.0 cm2 and/or indexed for body surface area <0.6 cm2/m2, mean gradient > 40 mmHg and peak velocity > 4.0 m/s (corresponding to a peak gradient of 64 mmHg). It is not clear in the guidelines if these criteria are mutually inclusive or exclusive but, as also stated in the guidelines, ‘When stenosis is severe and cardiac output is normal, the mean transvalvular pressure gradient is generally greater than 40 mmHg.’ Hence, when LV function is normal, clinicians expect to see a high gradient in patients with severe AS as well as consistency between the values proposed by the guidelines. And indeed, typical reaction for the echocardiographer faced with a lower than expected gradient in a patient with severe AS on the basis of AVA would be to question the validity of the valve area calculation, since this value is derived from multiple measurements and thus more prone to error than gradient measurements.
Notwithstanding these considerations, it had long been our clinical observation that many patients with severe AS on the basis of AVA calculation indeed have unequivocally low gradients (e.g. mean gradient < 30 mmHg) despite a preserved LV ejection fraction (i.e. EF > 50%). Hence, we hypothesized that the lower gradients were likely due to a decrease in transvalvular flow and, in a recent study of 512 consecutive patients with severe AS (indexed AVA <0.6 cm2/m2) and preserved EF (>50%), we found that 35% had paradoxically low flows (PLF) [i.e. stroke volume index (SVi) <35 mL/m2].2 The same patients were also observed to have a cluster of findings suggesting that they were at more advanced stage of their disease and had a worse prognosis if treated medically rather than surgically. In the same context, Minners et al.3 reported a series of 2427 consecutive patients with preserved LV function and AVA <2.0 cm2 and found that, in reference to the guidelines, 30% had the inconsistent finding of an AVA <1.0 cm2 but a mean gradient <40 mmHg. On the basis of a previous observation by Carabello (Table 1),4 these authors also emphasized that, based on the Gorlin formula and assuming normal cardiac output, an AVA of 1.0 cm2 yields a gradient of 26 mmHg and that an AVA ≤0.81 cm2 is necessary to yield a gradient ≥40 mmHg, thus suggesting that it is the guidelines per se that are inherently inconsistent. In a further comment, Jander5 observed that low flow was thus not a necessary prerequisite for inconsistently low gradients and suggested an adjustment of the AVA cut-off value for severe stenosis to 0.8 cm2. The same author also hypothesized that many patients with low flow may nonetheless have gradients >40 mmHg.
Table 1.
Relation of the aortic valve area to the gradient
| Aortic valve area (cm2) | Mean gradient (mmHg) |
|---|---|
| 4 | 1.7 |
| 3 | 2.9 |
| 2 | 6.6 |
| 1 | 26 |
| 0.9 | 32 |
| 0.8 | 41 |
| 0.7 | 53 |
| 0.6 | 73 |
| 0.5 | 105 |
Because of our previous demonstration2 that patients with PLF severe AS despite preserved EF are often misdiagnosed and have a poor prognosis if not operated, the present article purports to review the distinguishing characteristics of this entity in order to optimize its diagnosis and treatment and improve survival. Given the questions raised by Jander5 in relation to the interpretation of data in individual patients, we also proceeded to re-analyse our previous data within the context of the cut-off values proposed by the guidelines.1 In this as well as in our previous2 paper, the term ‘paradoxical’ does not refer to intrinsic physiology but rather to the frequent misconception by clinicians that patients with AS and normal EF necessarily have normal flow (NF).
Distinguishing features of paradoxically low flow patients
Patients with PLF severe AS despite a preserved EF are identified as those presenting with an indexed AVA <0.6 cm2/m2, an EF > 50%, and a SVi <35 mL/m2. Both AVA and indexed AVA are proposed in the ACC/AHA guidelines (see Table 4 of Bonow et al.1) as valid indicators of AS severity. The rationale for using indexed AVA is particularly relevant in this situation. Indeed, using non-indexed values, patients with a small body surface area (BSA) and a low gradient due to a lower albeit normal cardiac index could otherwise have been misclassified as having PLF severe AS when in fact, relative to BSA, flow is normal and the AS is only moderate. Notwithstanding these considerations, compared with patients with NF, patients with PLF have markedly lower gradients to the extent that in our study, 55% had a mean gradient <30 mmHg.2 Apart from these findings, other distinguishing features of PLF patients when compared with NF patients are: (i) a higher level of global LV haemodynamic load reflected by higher valvulo-arterial impedance (Zva); (ii) smaller and relatively thicker ventricles; (iii) lower values for LV mid-wall radius shortening consistent with more pronounced intrinsic myocardial dysfunction, and (iv) a tendency to have a lower LVEF although remaining within the normal range. Overall, these findings are consistent with a greater and probably more long standing increase in LV haemodynamic load resulting in more pronounced concentric LV remodelling, a smaller LV cavity size and a decrease in intrinsic myocardial function. This observation is further confirmed by the fact that overall, these patients have a significantly poorer prognosis than patients with NF; as well, their prognosis is also much poorer if treated medically rather than surgically.2 Nonetheless, the proportion of these patients referred for surgery is much lower than in their counterparts with the more classical NF high gradient pattern of AS. Although symptomatic status was not available, this result strongly suggests that, due to the lower gradients, the condition may often be misdiagnosed, which leads to underestimation or neglect of symptoms and inappropriate delay of aortic valve replacement (AVR).
Corroborating studies
The SEAS trial was a large randomized prospective study examining the effects of a combined simvastatin and ezetemide treatment vs. placebo in 1873 asymptomatic patients with moderate AS on the basis of peak transvalvular velocity. In a recent sub-study,6 these investigators specifically analysed their data for the presence of low flow severe AS and using parameters analogous to those reported in the study of Hachicha et al.,2 reported a prevalence of 28% (100 patients) among the 359 patients found to have severe AS. Moreover, these patients had a significantly increased global LV load, smaller LV volumes, more concentric remodelling, and decreased mid-wall shortening compared with NF patients; mean gradient was <30 mmHg in 56% of the patients. Overall, these findings are most consistent with the data of Hachicha et al.2 and confirm that this is not a rare occurrence and indeed represents a more advanced stage of the disease. Very similar results are also reported by Barasch et al. who found a prevalence of 22% in 215 patients and also observed that a mean gradient <30 mmHg was associated with an almost 50% lower referral to surgery and a higher mortality rate.
Physiopathology of paradoxically low flow aortic stenosis despite preserved ejection fraction
The more pronounced concentric LV remodelling and smaller LV cavity size found in PLF patients is akin to a restrictive physiology. Indeed, the decrease in stroke volume is primarily due to deficient ventricular filling in relation with the smaller cavity size rather than deficient ventricular emptying. Nonetheless, in many patients, the problem is further compounded by the presence of intrinsic myocardial dysfunction causing the EF, albeit normal, to be lower (i.e. 50–60%) than expected in similar situations. Indeed, patients with severe AS and LV concentric remodelling often tend to have relatively higher EF's than normal (e.g. >70%) to compensate for the insufficiency in ventricular filling.7 The latter observation illustrates that a normal LVEF should not be construed as being equivalent to normal LV flow output and that it may indeed remain normal despite a significant reduction in intrinsic myocardial shortening. As well, it emphasizes that the evaluation of LV function should be more comprehensive and includes parameters (e.g. mid-wall radius shortening, longitudinal shortening, myocardial strain rate) going beyond the characterization of LV emptying.
Contribution of vascular component to increased global left ventricular haemodynamic load
The most frequent cause of AS nowadays is degenerative AS and there is an increasing body of evidence, suggesting that it is an active process akin to atherosclerosis.8,9 Thus, patients with degenerative AS often have other manifestations of atherosclerosis including a decrease in systemic arterial compliance due to increased rigidity of the arterial wall, which in the presence of normal LV stroke volume, translates into the presence of concomitant systolic hypertension. As well, such patients may also have alterations in LV function which might not only be due to AS but also to hypertension or associated coronary artery disease and in varying proportions depending on the severity of each entity. In this context, it should be emphasized that the pathophysiology of adverse outcomes in AS is essentially due to an imbalance between the increase in LV haemodynamic load and the capacity of the left ventricle to overcome this increase in load both at rest and during exercise. Hence, if the disease is limited to the valve, severity can be described in relatively simple terms such as AVA and gradients. However, if the disease is not limited to the valve but also involves the vascular system, systemic arterial compliance and systemic vascular resistance should also be taken into consideration.
In a recent study of 208 consecutive patients with at least moderate AS, systemic arterial compliance was reduced in 41% of subjects and had an additive effect to the extent that patients with the combination of decreased arterial compliance and moderate AS had levels of global LV haemodynamic load as elevated as patients with severe AS and normal compliance.10 These findings emphasize that the LV in degenerative AS is often facing a double load, i.e. valvular plus vascular. In this context, it was recently proposed to quantify global LV haemodynamic load by calculating Zva, which is obtained by dividing estimated LV systolic pressure by the SVi. Conceptually, this parameter represents the cost in mmHg for each millilitre of blood indexed for body surface area pumped by the left ventricle. Zva can easily be estimated during Doppler-echocardiography by adding the mean aortic gradient to the peripheral LV systolic pressure measured by sphygmomanometry and dividing the result by SVi and it has been shown to be superior to the standard indices of AS severity in predicting LV dysfunction and patient outcome.2,10 Zva might be viewed as an oversimplification from a fundamental standpoint but is, nonetheless, the best available parameter to routinely quantify overall haemodynamic load in the clinical situation.11
It is also interesting to note that PLF patients have markedly higher values for Zva than NF patients and that this increase is largely due to a more pronounced decrease in systemic arterial compliance than to an increase in AS severity. Since the increase in LV wall thickness in hypertension not only represents physiological hypertrophy in response to the increased load but may also involve neuro-hormonal mechanisms leading to interstitial fibrosis, one might speculate that this factor might contribute to the more pronounced LV concentric remodelling seen in these patients; as well, it might also contribute to a greater decrease in intrinsic myocardial function.
With regards to the clinical presentation of patients with AS and a decrease in systemic arterial compliance, the following considerations also need to be emphasized. (i) The signs of AS severity can be masked by the presence of concomitant hypertension, particularly if associated with a significant decrease in systemic arterial compliance; indeed, decreased compliance tends to abolish the peak-to-peak gradient as recorded during catheterization and may also modify other signs of AS severity.12,13 (ii) Peripheral blood pressure and systemic arterial compliance are important considerations with regards to total haemodynamic load in these patients; these parameters should thus become an integral part of their evaluation, the practical implication being that blood pressure should routinely be measured at the time of the echocardiogram in every patient with AS. (iii) Because compliance and impedance are not constants but vary with the level of blood pressure and given that the signs of AS severity may be partially masked by hypertension, evaluations of AS severity should ideally be performed when blood pressure control is optimal to the extent that the examination should be deferred or repeated if the patient is hypertensive at the time of the first evaluation. (iv) Progression of valvulo-arterial disease may eventually result in a pseudo-normalization of blood pressure due to a reduction in stroke volume. In our patients with PLF,2 average blood pressure was 131/74 mmHg despite a severe decrease in arterial compliance. Pseudo-normalization of blood pressure is thus a frequent phenomenon in these patients and a normal blood pressure reading should not be construed to be equivalent with a normal vascular load. (v) Pathophysiology of low flow in these patients is essentially due to concentric remodelling and diastolic dysfunction in relation to restrictive LV filling and in some patients, it may be difficult to precisely delineate the relative contributions of AS and hypertension to the changes in ventricular geometry and function. This consideration may become relevant with regards to treatment in patients with marked decrease in systemic arterial compliance and borderline criteria for severe AS and it cannot be excluded that some of these patients may even have ‘pseudo-severe AS’ due to low flow. In such cases, dobutamine stress echocardiography and other diagnostic modalities such as quantification of valve calcification by multidetector computed tomography might prove useful although there is at present very little experience with regards to their utilization in this context.
Illustrative case
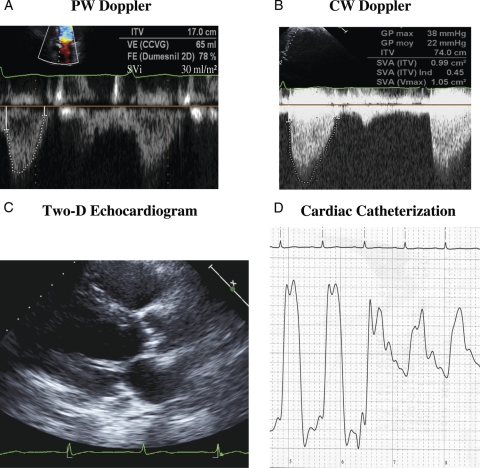
Figure 1 presents the case of a 57 y.o. male with a systolic murmur and typical class III/IV angina for more than 1 year. The two-dimensional echocardiogram shows a heavily calcified aortic valve, small LV cavity (38 mL/m2) and marked LV concentric remodelling (wall thickness to radius ratio = 0.56). Maximal and mean gradients by Doppler are 38 and 22 mmHg, respectively, whereas AVA is 0.99 cm2, indexed AVA 0.45 cm2/m2, SVi 30 mL/m2, Zva 5.73 mmHg/mL/m2, and EF 72%. On the basis of these results, the patient was identified as a PLF AS and referred to surgery. Cardiac catheterization prior to AVR showed the following results: LV systolic pressure 175 mmHg, LV end-diastolic pressure 35 mmHg, aortic pressure 150/76, peak-to-peak gradient 25 mmHg, AVA 0.95 cm2, indexed AVA 0.43 cm2/m2; coronary angiography revealed a 25% stenosis on the distal LAD. A heavily calcified stenotic valve was found at operation, AVR was performed and post-operative course was uneventful with no recurrence of symptoms. It should be emphasized that patient had been seen 1 year earlier with similar symptoms and findings but had been dismissed on medical treatment because of underestimation of AS severity due to the low gradients.
Figure 1.
Illustrative case of patient with PLF AS. (A) Pulse wave (PW) Doppler tracing in LV outflow tract; ITV, time velocity integral; VE, stroke volume; FE, ejection fraction; SVi, stroke volume index. (B) Continuous wave (CW) Doppler tracing through aortic valve; GP, pressure gradient; SVA, aortic valve area; SVAind, aortic valve area indexed for body surface area. (C) Two-dimensional echocardiogram in the parasternal long-axis view in diastole showing heavily calcified valve and small LV with concentric hypertrophy. (D) Pullback pressure tracing from LV to aorta; see text for details.
Interpretation of results within the context of guidelines
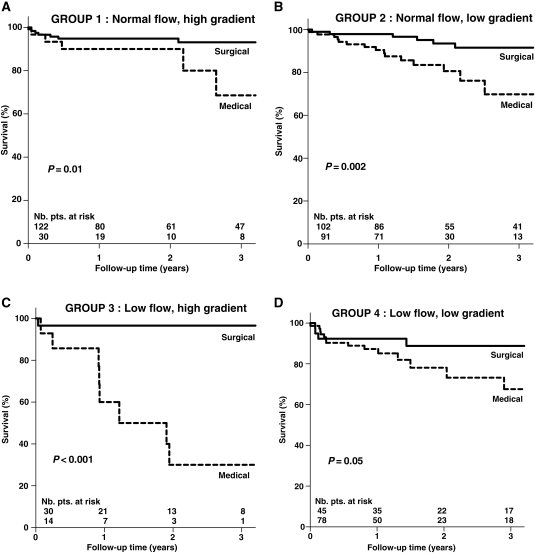
In view of the important questions raised by Jander5 and their potential implications with regards to prognosis and treatment, we elected to further analyse our previously reported data2 within the context of the cut-off values proposed by the guidelines.1 Hence, our 512 consecutive patients with severe AS (indexed AVA ≤ 0.6 cm2/m2) and preserved EF (>50%) were subdivided into four subgroups based on the presence of normal or paradoxical low flow (i.e. SVi more than or ≤35 mL/m2) and high or low gradient (i.e. mean gradient more than or ≤40 mmHg). Figure 2 is the result of this analysis. Of interest is the fact that finally only 38% of patients (i.e. those represented by Groups 1 ‘NF, high gradient’ and 3 ‘low flow, high gradient’) are fully consistent with the criteria proposed by the guidelines with regards to indexed AVA and mean gradient, whereas 62% (i.e. Groups 2 ‘NF, low gradient’ and 4 ‘low flow, low gradient’) had a lower than expected gradient i.e. <40 mmHg. As well, indications for operation appear to be much more driven by the presence of a high gradient than a low AVA. Indeed, AVR was performed in 80 and 68% of patients in Groups 1 and 3, respectively, whereas only 53 and 36% of patients underwent operation in Groups 2 and 4, respectively. Overall, the patients treated surgically were significantly younger (68 ± 12 vs. 74 ± 14 y.o., P < 0.001) and comprised a higher proportion of females (62 vs. 50%, P < 0.006) than the patients treated medically, whereas there were no significant differences between the two groups for LVEF or the presence of hypertension, coronary artery disease, or diabetes. Figure 3 provides survival curves in each subgroup as a function of treatment: medical vs. surgical and shows that in each case, prognosis is significantly better if operated than if treated medically. The results in Group 2 ‘NF, low gradient’ are of particular interest since, in relation to guidelines, these patients can be deemed to have an inconsistently low gradient that is not due to low flow but rather to the previously mentioned inherent inconsistency contained in the guidelines, i.e. that in a patient with NF, an AVA of 1.0 cm2, or 0.6 cm2/m2 will theoretically yield a mean gradient of 26 mmHg rather than 40 mmHg.3 For this reason, it has been hypothesized that these patients might not have truly severe AS and that it might thus be appropriate to re-adjust the cut-off value for severe AS to 0.80 cm2 rather than 1.0 cm2.3,5 The present results, however, show that although the degree of AS severity is less in these patients than in the other groups, their prognosis is nonetheless significantly worse if treated medically rather than surgically. Similar results have recently been observed by the Mayo clinic group (M. Enriquez-Sarano, personal communication) whereby, regardless of gradient, patients with an AVA ≤1.0 cm2 have a significantly worse prognosis if not operated. At the other end of the spectrum are the patients in Group 3 ‘low flow, high gradient’ who have much more severe stenosis to the extent that despite low flow, their gradients are >40 mmHg. Their tendency to have a better survival if treated surgically than the patients in Group 4 who have less severe stenosis is intriguing but might indicate better cardiac reserve to the extent that they have been able to survive up to that level of severity. Indirectly, the higher peri-operative mortality observed in the Group 4 patients (Figure 3D) could also be consistent with this hypothesis, i.e. that the latter patients have less myocardial reserve than the Group 3 patients (Figure 3C).
Figure 2.
Characterization of 512 consecutive patients with severe AS (AVAi <0.6 cm2/m2) and preserved ejection fraction (>0.50) based on for SVi > or ≤35 mL/m2 and mean pressure gradient > or ≤40 mmHg (data taken from Hachicha et al.2). AVA, aortic valve area; AVR, aortic valve replacement; LVEDD, left ventricular end-diastolic diameter; LVDEVI, left ventricular end-diastolic volume index; SVi, stroke volume index; Zva, valvulo-arterial impedance.
Figure 3.
Overall survival in the four groups of patients (Figure 2) as a function of the type of treatment: medical vs. surgical (data taken from Hachicha et al.2).
The number of events per group is too small to allow separate multivariate analysis in each subgroup. Hence, we elected to perform a multivariate Cox proportional hazard analysis in the whole cohort which includes the variables that were significantly different between the medically and surgically treated patients (i.e. age, gender, gradient), the variables that were predictive of mortality on univariate analysis (i.e. age, LVEF, Zva, treatment: medical vs. surgical) and the variables that were the main distinctive features between the four groups (i.e. SVi and gradient). After adjustment for these seven variables, AVR did indeed remain associated with a powerful protective effect [HR: 0.29 (95% CI: 0.15–0.54), P < 0.0001]. In the same line, these results are also to a large extent supported by the aforementioned observations at the Mayo Clinic and the results of Barasch et al.14 who showed that, in patients with an indexed AVA <0.6 cm2/m2, the presence of a mean gradient <30 mmHg is associated with almost 50% lower referral to surgery and a two-fold increase in mortality compared with patients with higher gradients. As well, Pai et al.15 recently reported that patients with severe AS on the basis of AVA, low gradient (<30 mmHg), and preserved LVEF had significantly better survival when treated surgically than when treated medically (5 year survival 90% vs. 20%, P < 0.0001). Overall, these findings confirm that the present AVA and indexed AVA cut-off values for severe stenosis appear to be adequate regardless of gradients and that, unfortunately, due to the lower gradients, this condition is often misdiagnosed, which leads to underestimation or neglect of symptoms and inappropriate delay of AVR. Hence, this entity of low flow low gradient AS despite preserved EF is particularly important to recognize, so that we do not deny surgery to a symptomatic patient with a low pressure gradient.
Comprehensive evaluation of aortic stenosis severity
Table 2 is a list of the various parameters which in our opinion should be measured during a comprehensive Doppler-echocardiographic examination for AS. In particular, peripheral blood pressure should be recorded in every patient, and systemic arterial compliance and valvulo-arterial impedance routinely calculated. This will add little time to the examination, since the only additional measurement to be performed is peripheral blood pressure which should ideally be performed at the same time as the LV stroke volume and transvalvular gradient measurements. Such parameters provide essential information in patients with discrepant measurements. In this context, it should be reiterated that the classical markers of AS severity may be blunted in the presence of hypertension, whereas blood pressure may be pseudo-normalized in patients with high haemodynamic load and low stroke volume.2,10,12,16 Moreover, the discovery of high impedance (i.e. >4.5 mmHg/mL/m2) might provide an explanation for otherwise unexplained symptoms in patients with the combination of moderate AS and decreased arterial compliance (i.e. <0.6 mL/mmHg/m2); such a combination was found in 24% of patients by Briand et al.10 and the impacts on LV function were as pronounced as those observed in patients with the combination of severe AS and normal arterial compliance. Although beyond the scope of the present topic, it should be mentioned that the energy loss index is a parameter akin to the indexed AVA but taking into account pressure recovery;17 from a practical standpoint, it is most useful in patients with a small aortic diameter (<30 mm) where the recorded gradients and AVA may significantly overestimate AS severity.18,19 Cut-off value for severe AS using the energy loss index is <0.55 cm2 m2.17
Table 2.
Comprehensive Doppler-echocardiographic examination of aortic stenosis
| Quantification of valvular obstruction |
| Maximal velocity |
| Mean gradient |
| Aortic valve area |
| Indexed aortic valve area |
| Energy loss index |
| Quantification of vascular load |
| Peripheral blood pressure |
| Systemic arterial compliance |
| Systemic vascular resistance |
| Quantification of global LV haemodynamic load |
| Valvulo-arterial impedance |
| Quantification of LV geometry |
| LV end-diastolic internal diameter |
| LV end-diastolic volume index |
| Relative wall thickness |
| Quantification of LV systolic function |
| LVOT stroke volume index |
| Cardiac index |
| Ejection fraction by Simpson method |
| Ejection fraction by Dumesnil method |
| Mid-wall fractional shortening |
The main pitfall associated with the echocardiographic diagnosis of paradoxical low flow and/or low gradient AS is an error in the calculation of the stroke volume. Indeed, this measurement is included in the calculation of many parameters including AVA, systemic arterial compliance, and valvulo-arterial impedance; moreover, it is derived from two separate measurements [left ventricular outflow tract (LVOT) diameter and LVOT time–velocity integral] having each their potential for error. Hence, we would suggest the following verifications be done when confronted with a possible diagnosis of PLF AS: (i) measurements for LVOT diameter and LVOT time–velocity integral as well as transvalvular velocities should be re-checked for accuracy; (ii) the EF calculated by the Dumesnil method20 is obtained by dividing the LVOT stroke volume by the LV end-diastolic volume calculated from the LV end-diastolic diameter using the Teicholz formula; hence, a result consistent with that obtained by other means of calculating EF (e.g. Simpson method, Quinones, eyeball, etc.) is indirect and independent confirmation that the measure for stroke volume is valid, whereas a value lower than expected can suggest that the LVOT stroke volume is underestimated due to an error in measurement; an important caveat is, however, that a low EF by the Dumesnil method can also be encountered in patients with significant mitral regurgitation, because a significant proportion of the total LV stroke volume is ejected through the mitral valve rather than the aortic valve. (iii) LV geometry measurements should be reviewed with the expectation of finding a small LV cavity (LV end-diastolic internal diameter <50 mm and/or LV end-diastolic volume index <60 mL/m2) and a noticeable increase in relative wall thickness ratio (i.e. >0.45). In patients having persistent ambiguities or discrepancies on their echocardiograms, cardiac magnetic resonance imaging may also be used to validate LV geometry and stroke volume measurements. As a footnote, it should be noted that, in Hachicha et al.,2 the stroke volume measured by Doppler in the LVOT was validated by independent calculations made using Simpson's method, whereas the SEAS trial sub-study utilized yet another method i.e. the stroke volume using the Teicholz correction of the cube formula and that both studies reported a similarly high prevalence for PLF AS. Hence, it is very unlikely that the prevalence of this syndrome has been overestimated due to underestimation in stroke volume measurements.
Finally, it should be emphasized that less active elderly patients may have a tendency to minimize their symptoms and that this sentiment may even be re-enforced by their treating physician when he or she is confronted with a relatively low gradient on the echocardiogram. In such patients, recent studies have shown that exercise testing is both safe and useful in confirming or infirming the presence of symptoms.21,22 Moreover, such evaluations may also be helpful in convincing both patient and physician that the situation is more serious than previously thought. As well, recent studies also show that measurements of plasma brain natriuretic peptides23,24 and quantification of valve calcification by multidetector computed tomography25,26 may also be useful in confirming AS severity.
Clinical implications
The main implication of these considerations is that the diagnosis of severe AS should be based on results for AVA and indexed AVA rather than on gradients. Pending further confirmation and given the presently available outcome data, present cut-off values for these variables also appear to be adequate. Hence, notwithstanding contraindications due to co-morbidities or refusal of the patient, which is not infrequent in elderly patients, the appropriate treatment in a symptomatic patient with an AVA <1.0 cm2 and/or an indexed AVA <0.6 cm2/m2 is definitely to proceed with an AVR. In patients with low flow AS and preserved EF, the aortic annulus may be smaller than expected due to the marked concentric remodelling and the smaller LV cavity and the prevention of valve prosthesis-patient mismatch may thus become more challenging. As well, the restrictive physiology observed in these patients may possibly predispose to hypotension and low output failure after coming off extra-corporeal circulation as well as to higher peri-operative mortality.27,28 Notwithstanding these considerations, the results nonetheless clearly show that these patients have a much better prognosis if treated surgically than medically.2,15 Moreover, the rapidly evolving field of percutaneous valve implantation will probably become an attractive alternative in patients considered to be at higher risk with regards to prosthesis-patient mismatch and/or peri-operative mortality as well as in more elderly patients reluctant to undergo open heart surgery.29,30
Unanswered questions include definition of the optimal medical treatment in patients with concomitant systolic hypertension due to decreased systemic arterial compliance. Indeed, such treatment may often be of limited efficacy given that the peripheral systolic pressure may remain high despite an adequate pharmacological treatment and a normal or low diastolic pressure (e.g. 160/60 mmHg). In this context, the cases of patients with the combination of moderate AS and decreased systemic arterial compliance who remain symptomatic despite optimal medical treatment are particularly challenging. Indeed, it might eventually be found that some of these patients might actually benefit from operation although not meeting the currently accepted criteria for AS severity, the rationale being that their total LV haemodynamic load is markedly increased and that any significant decrease might contribute to improve their prognosis and well being. In contemplating the surgical option, indications that the operation is likely to result in significant benefit should, however, be sought by estimating for instance the relative contributions of the valvular and vascular loads to the increased haemodynamic load. In this context, particular care should also be taken to avoid prosthesis–patient mismatch in order to obtain the optimal decrease in valvular load.31
Funding
This work is supported by a research grant (MOP # 57445) from the Canadian Institutes of Health Research, Ottawa, Ontario, Canada. P.P. holds the Canada Research Chair in Valvular Heart Diseases, Canadian Institutes of Health Research. Funding to pay the Open Access publication charges for this article was provided by the Québec Heart Institute Foundation.
Conflict of interest: none declared.
Acknowledgements
The authors would like to thank Dr Zeineb Hachicha for her help in the analysis of data and preparation of figures.
References
- 1.Bonow RO, Carabello BA, Kanu C, de Leon AC, Jr, Faxon DP, Freed MD, Gaasch WH, Lytle BW, Nishimura RA, O'Gara PT, O'Rourke RA, Otto CM, Shah PM, Shanewise JS, Smith SC, Jr, Jacobs AK, Adams CD, Anderson JL, Antman EM, Faxon DP, Fuster V, Halperin JL, Hiratzka LF, Hunt SA, Lytle BW, Nishimura R, Page RL, Riegel B. ACC/AHA 2006 guidelines for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (writing committee to revise the 1998 Guidelines for the Management of Patients With Valvular Heart Disease): developed in collaboration with the Society of Cardiovascular Anesthesiologists: endorsed by the Society for Cardiovascular Angiography and Interventions and the Society of Thoracic Surgeons. Circulation. 2006;114:e84–e231. doi: 10.1161/CIRCULATIONAHA.106.176857. [DOI] [PubMed] [Google Scholar]
- 2.Hachicha Z, Dumesnil JG, Bogaty P, Pibarot P. Paradoxical low flow, low gradient severe aortic stenosis despite preserved ejection fraction is associated with higher afterload and reduced survival. Circulation. 2007;115:2856–2864. doi: 10.1161/CIRCULATIONAHA.106.668681. [DOI] [PubMed] [Google Scholar]
- 3.Minners J, Allgeier M, Gohlke-Baerwolf C, Kienzle RP, Neumann FJ, Jander N. Inconsistencies of echocardiographic criteria for the grading of aortic valve stenosis. Eur Heart J. 2008;29:1043–1048. doi: 10.1093/eurheartj/ehm543. [DOI] [PubMed] [Google Scholar]
- 4.Carabello BA. Aortic stenosis. N Engl J Med. 2002;346:677–682. doi: 10.1056/NEJMcp010846. [DOI] [PubMed] [Google Scholar]
- 5.Jander N. Low-gradient ‘severe’ aortic stenosis with preserved ejection fraction: new entity, or discrepant definitions? Eur Heart J. 2008;10:E‐11–E‐15. [Google Scholar]
- 6.Cramariuc D, Cioffi G, Rieck AE, Devereux RB, Staal EM, Ray S, Wachtell K, Gerdts E. Low-flow aortic stenosis in asymptomatic patients: valvular arterial impedance and systolic function from the SEAS substudy. J Am Coll Cardiol Img. 2009;2:390–399. doi: 10.1016/j.jcmg.2008.12.021. [DOI] [PubMed] [Google Scholar]
- 7.Dumesnil JG, Shoucri RM. Effect of the geometry of the left ventricle on the calculation of ejection fraction. Circulation. 1982;65:91–98. doi: 10.1161/01.cir.65.1.91. [DOI] [PubMed] [Google Scholar]
- 8.Freeman RV, Otto CM. Spectrum of calcific aortic valve disease: pathogenesis, disease progression, and treatment strategies. Circulation. 2005;111:3316–3326. doi: 10.1161/CIRCULATIONAHA.104.486738. [DOI] [PubMed] [Google Scholar]
- 9.O'Brien KD. Pathogenesis of calcific aortic valve disease: a disease process comes of age (and a good deal more) Arterioscler Thromb Vasc Biol. 2006;26:1721–1728. doi: 10.1161/01.ATV.0000227513.13697.ac. [DOI] [PubMed] [Google Scholar]
- 10.Briand M, Dumesnil JG, Kadem L, Tongue AG, Rieu R, Garcia D, Pibarot P. Reduced systemic arterial compliance impacts significantly on left ventricular afterload and function in aortic stenosis: implications for diagnosis and treatment. J Am Coll Cardiol. 2005;46:291–298. doi: 10.1016/j.jacc.2004.10.081. [DOI] [PubMed] [Google Scholar]
- 11.Otto CM. Valvular aortic stenosis: disease severity and timing of intervention. J Am Coll Cardiol. 2006;47:2141–2151. doi: 10.1016/j.jacc.2006.03.002. [DOI] [PubMed] [Google Scholar]
- 12.Kadem L, Dumesnil JG, Rieu R, Durand LG, Garcia D, Pibarot P. Impact of systemic hypertension on the assessment of aortic stenosis. Heart. 2005;91:354–361. doi: 10.1136/hrt.2003.030601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kadem L, Garcia D, Durand LG, Rieu R, Dumesnil JG, Pibarot P. Value and limitations of the peak-to-peak gradient for the evaluation of aortic stenosis. J Heart Valve Dis. 2006;15:609–616. [PubMed] [Google Scholar]
- 14.Barasch E, Fan D, Chukwu EO, Han J, Passick M, Petillo F, Norales A, Reichek N. Severe isolated aortic stenosis with normal left ventricular systolic function and low transvalvular gradients: pathophysiologic and prognostic insights. J Heart Valve Dis. 2008;17:81–88. [PubMed] [Google Scholar]
- 15.Pai RG, Varadarajan P, Razzouk A. Survival benefit of aortic valve replacement in patients with severe aortic stenosis with low ejection fraction and low gradient with normal ejection fraction. Ann Thorac Surg. 2008;86:1781–1789. doi: 10.1016/j.athoracsur.2008.08.008. [DOI] [PubMed] [Google Scholar]
- 16.Little SH, Chan KL, Burwash IG. Impact of blood pressure on the Doppler echocardiographic assessment of aortic stenosis severity. Heart. 2007;93:848–855. doi: 10.1136/hrt.2006.098392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Garcia D, Pibarot P, Dumesnil JG, Sakr F, Durand LG. Assessment of aortic valve stenosis severity: a new index based on the energy loss concept. Circulation. 2000;101:765–771. doi: 10.1161/01.cir.101.7.765. [DOI] [PubMed] [Google Scholar]
- 18.Baumgartner H, Steffenelli T, Niederberger J, Schima H, Maurer G. ‘Overestimation’ of catheter gradients by Doppler ultrasound in patients with aortic stenosis: a predictable manifestation of pressure recovery. J Am Coll Cardiol. 1999;33:1655–1661. doi: 10.1016/s0735-1097(99)00066-2. [DOI] [PubMed] [Google Scholar]
- 19.Garcia D, Dumesnil JG, Durand LG, Kadem L, Pibarot P. Discrepancies between catheter and Doppler estimates of valve effective orifice area can be predicted from the pressure recovery phenomenon: practical implications with regard to quantification of aortic stenosis severity. J Am Coll Cardiol. 2003;41:435–442. doi: 10.1016/s0735-1097(02)02764-x. [DOI] [PubMed] [Google Scholar]
- 20.Dumesnil JG, Dion D, Yvorchuk K, Davies RA, Chan K. A new, simple and accurate method for determining ejection fraction by Doppler echocardiography. Can J Cardiol. 1995;11:1007–1014. [PubMed] [Google Scholar]
- 21.Das P, Rimington H, Chambers J. Exercise testing to stratify risk in aortic stenosis. Eur Heart J. 2005;26:1309–1313. doi: 10.1093/eurheartj/ehi250. [DOI] [PubMed] [Google Scholar]
- 22.Lancellotti P, Lebois F, Simon M, Tombeux C, Chauvel C, Pierard LA. Prognostic importance of quantitative exercise Doppler echocardiography in asymptomatic valvular aortic stenosis. Circulation. 2005;112:I377–I382. doi: 10.1161/CIRCULATIONAHA.104.523274. [DOI] [PubMed] [Google Scholar]
- 23.Bergler-Klein J, Klaar U, Heger M, Rosenhek R, Mundigler G, Gabriel H, Binder T, Pacher R, Maurer G, Baumgartner H. Natriuretic peptides predict symptom-free survival and postoperative outcome in severe aortic stenosis. Circulation. 2004;109:2302–2308. doi: 10.1161/01.CIR.0000126825.50903.18. [DOI] [PubMed] [Google Scholar]
- 24.Gerber IL, Stewart RA, Legget ME, West TM, French RL, Sutton TM, Yandle TG, French JK, Richards AM, White HD. Increased plasma natriuretic peptide levels reflect symptom onset in aortic stenosis. Circulation. 2003;107:1884–1890. doi: 10.1161/01.CIR.0000060533.79248.0C. [DOI] [PubMed] [Google Scholar]
- 25.Messika-Zeitoun D, Bielak LF, Peyser PA, Sheedy PF, Turner ST, Nkomo VT, Breen JF, Maalouf J, Scott C, Tajik AJ, Enriquez-Sarano M. Aortic valve calcification: determinants and progression in the population. Arterioscler Thromb Vasc Biol. 2007;27:642–648. doi: 10.1161/01.ATV.0000255952.47980.c2. [DOI] [PubMed] [Google Scholar]
- 26.Laissy JP, Messika-Zeitoun D, Serfaty JM, Sebban V, Schouman-Claeys E, Iung B, Vahanian A. Comprehensive evaluation of preoperative patients with aortic valve stenosis: usefulness of cardiac multidetector computed tomography. Heart. 2007;93:1121–1125. doi: 10.1136/hrt.2006.107284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Orsinelli DA, Aurigemma GP, Battista S, Krendel S, Gaasch WH. Left ventricular hypertrophy and mortality after aortic valve replacement for aortic stenosis. A high risk subgroup identified by preoperative relative wall thickness. J Am Coll Cardiol. 1993;22:1679–1683. doi: 10.1016/0735-1097(93)90595-r. [DOI] [PubMed] [Google Scholar]
- 28.Duncan AI, Lowe BS, Garcia MJ, Xu M, Gillinov AM, Mihaljevic T, Koch CG. Influence of concentric left ventricular remodeling on early mortality after aortic valve replacement. Ann Thorac Surg. 2008;85:2030–2039. doi: 10.1016/j.athoracsur.2008.02.075. [DOI] [PubMed] [Google Scholar]
- 29.Webb JG, Pasupati S, Humphries K, Thompson C, Altwegg L, Moss R, Sinhal A, Carere RG, Munt B, Ricci D, Ye J, Cheung A, Lichtenstein SV. Percutaneous transarterial aortic valve replacement in selected high-risk patients with aortic stenosis. Circulation. 2007;116:755–763. doi: 10.1161/CIRCULATIONAHA.107.698258. [DOI] [PubMed] [Google Scholar]
- 30.Clavel MA, Webb JG, Pibarot P, Altwegg L, Dumont E, Thompson C, De Larochelliere R, Doyle D, Masson JB, Bergeron S, Bertrand OF, Rodes-Cabau J. Comparison of the hemodynamic performance of percutaneous and surgical bioprostheses for the treatment of severe aortic stenosis. J Am Coll Cardiol. 2009;53:1883–1891. doi: 10.1016/j.jacc.2009.01.060. [DOI] [PubMed] [Google Scholar]
- 31.Pibarot P, Dumesnil JG. Prosthesis-patient mismatch: definition, clinical impact, and prevention. Heart. 2006;92:1022–1029. doi: 10.1136/hrt.2005.067363. [DOI] [PMC free article] [PubMed] [Google Scholar]