Abstract
Objective
To evaluate patient characteristics and fresh IVF cycle parameters that influence success of sibling frozen-thawed embryo transfer (FET) cycles.
Design
Retrospective study.
Setting
Academic infertility practice.
Patient(s)
Infertile women undergoing FET cycles utilizing embryos cryopreserved on day 3 post-insemination following an initial fresh IVF cycle.
Intervention(s)
90 FET cycles.
Main Outcome Measure(s)
Clinical pregnancy (CP).
Result(s)
The likelihood of CP following FET was significantly higher in women who had achieved CP in the preceding fresh IVF cycle (71.4% vs. 40.6%). Multivariable logistic regression analysis confirmed that patients achieving CP following the fresh IVF cycle were more likely to achieve CP following FET (OR= 5.5, 95% CI 1.2 to 25.3) after adjusting for age, number and cleavage status of embryos transferred. Additionally, higher serum levels of progesterone on the day of hCG administration emerged as predictive of CP after FET at a statistically significant level.
Conclusion(s)
The outcome of the fresh ET cycle is the foremost predictor of CP after FET of the sibling embryos. Relationship between serum P on the day of hCG administration in the fresh cycle and the outcome of subsequent FET is noteworthy and merits further investigation.
Keywords: Frozen-thawed embryos, progesterone, in vitro fertilization
INTRODUCTION
Twenty five years after the first human pregnancy conceived by a frozen-thawed embryo, frozen embryo transfer (FET) has become an integral part of assisted reproductive technologies (1). Although cryopreservation of surplus embryos allows couples to increase the odds of pregnancy resulting from a single attempt at controlled ovarian hyperstimulation (COH), the “take-home” baby rate from FET cycles remains considerably lower than that of the fresh embryo transfer cycles. In 2005, for women younger than 35 years of age in USA, the liveborn rate per initiated cycle for fresh and frozen embryos were 43.4 and 31.7 percent, respectively (2).
A multitude of patient and embryo characteristics are recognized to influence the outcome of FET cycles, including female partner’s age, ovarian reserve status, the underlying etiology for infertility, the quality and developmental cleavage state of the embryos at the time of cryopreservation, cryo-protocol (i.e. slow freeze versus vitrification), post-thaw resumption of embryo cleavage, and the outcome of the fresh IVF cycle (3–6). The need to identify additional predictors of success of IVF cycles is especially pressing, given the notion that single embryo transfer may yield acceptable pregnancy rates while reducing the risk of multiple gestations and their associated morbidities (7, 8). Although serum levels of sex steroids, specifically estradiol (E2) and progesterone (P) during COH have been studied as predictors of success of the fresh embryo transfer (9, 10), prognostic values of serum hormonal parameters during the fresh IVF cycle on the outcome of a subsequent FET cycle are not well understood. Our group has previously shown that most pregnancies after FET occur in women who had values of serum progesterone between 1–2 ng/ml during the fresh cycle, whereas extremes of serum progesterone levels were associated with poor pregnancy outcome (11). The aim of the current study was to evaluate patient and fresh IVF cycle parameters that may impact on the outcome of the FET cycle.
MATERIALS AND METHODS
Patients
Approval for the study was obtained from the institutional review board of the Montefiore Medical Center. All FET cycles from January 2002 to December 2006 were identified. Analyses were limited to FET cycles utilizing embryos cryopreserved on day 3 post insemination and for which complete information was available for the original fresh embryo transfer cycle (N=90). Cycles utilizing donor oocytes and where fresh embryo transfer or a subsequent FET was not achieved were excluded.
Patient characteristics for the original fresh IVF and subsequent FET cycles were evaluated, including age, body mass index (BMI), day 3 FSH level, and etiology for infertility. Due to inter-cycle variability in ovarian reserve markers and the suboptimal outcomes following IVF in women who demonstrate normalization in FSH levels in subsequent cycles following an initial documented elevation (12), for patients who had undergone prior assessments, the highest reported FSH level was used to reflect ovarian reserve status. Cycle characteristics evaluated for the fresh cycles included serum level of E2 (pg/mL) and P (ng/mL) on the day of and the day following triggering of ovulation with human chorionic gonadotropin (hCG), type of oocyte insemination (IVF or intracytoplasmic sperm injection-ICSI), number of egg retrieved (total and metaphase 2, i.e. mature eggs), total number of embryos available on day 3, number of embryos cryopreserved and the outcome of the fresh cycle (i.e. clinical pregnancy [CP] defined as gestational sac on transvaginal sonogram). Cycle characteristics for the FET cycle included the number of frozen-thawed embryos transferred in the FET cycle, average blastomere number for the available and transferred embryos, post-thaw blastomere survival (%), embryo fragmentation (%) and endometrial thickness (mm). The outcome of interest was CP following FET defined as presence of an intrauterine sac on transvaginal ultrasound.
Protocol for Controlled Ovarian Hyperstimulation
Standard protocols were followed to achieve COH for the fresh IVF cycle, followed by ultrasound guided transvaginal retrieval of oocytes and insemination with or without ICSI as indicated (13). Evaluation for fertilization was performed at 12–20 hours post-insemination and the presence of two pronuclei confirmed normal fertilization. All embryos demonstrating evidence of abnormal fertilization (three or more pronuclei, or arrested one pronucleus) were considered inappropriate for fresh transfer or cryopreservation and were subsequently discarded. Embryos were randomly pooled and cultured in groups of 2–4 and cultured (day 0–3) at 6% CO2 and 37°C in a dual culture system under oil overlay, in either QHTF Medium with 10% HSA (Sage Biopharma, Trumbull, CT) or G1v3 with 10% HSA (Vitrolife, Gothenburg, Sweden). On day 3 post-insemination, embryos were evaluated for fresh ET; selection for fresh transfer from either culture milieu was based on the most optimal cleavage and the best morphological parameters using standard criteria. The surplus embryos were chosen for cryopreservation based on an appropriate cleavage rate (at least 6 to 8 cells on day 3 post-insemination), <20% fragmentation, regularly shaped blastomeres, and an intact zona pellucida. Briefly, the embryos were dehydrated using a cleavage-stage embryo freeze kit (Sage Biopharma, Trumbull, CT) containing 1.5 M propanediol with HSA followed by 1.5 M PROH/0.1 M sucrose with HSA. The embryos were loaded into cryostraws using a standard slow freeze protocol with manual seeding at −70°C followed by cooling to −30°C at a rate of −0.3°C /min followed by plunging into liquid nitrogen.
On the day of FET, the embryos were rapidly thawed and sequentially rehydrated according to protocol using the cleavage-stage embryo thaw kit (Sage Biopharma, Trumbull, CT) containing 0.5 M sucrose with HSA and 0.2 M sucrose with HSA. Following assessment of thawed embryos for blastomere survival, assisted hatching was performed on all frozen-thawed embryos using the dilute acid tyrode technique (14) before ET. Embryo transfers were performed using an Echotip catheter (COOK Ob/Gyn, Spencer, IN). FET were performed in programmed artificial cycles (GnRH agonist, supplementation with intramuscular E2 valerate and P) following standard protocol. Luteal support, provided with intramuscular injections of P in oil, was continued until documentation of the fetal heart, and subsequently tapered off.
Blood samples and Hormone Measurement
Fresh IVF cycles were monitored by serial follicular tracking by transvaginal ultrasound and assessments of serum E2 and P levels. Both E2 and P levels on the day of as well as on the day following hCG administration were regarded as independent variables of interest for this study. Samples were analyzed using the Elecsys 1010 immunoanalyzer (Roche, Indianapolis, IN). The serum P assay has a sensitivity of 0.03 ng/mL, with intra- and inter-assay variation coefficients of 2.7% and 5.5%, respectively. The E2 assay has a sensitivity of 5 pg/mL, with intra- and inter-assay variation coefficients of 5.7% and 6.2%, respectively.
Statistical Analysis
CP following FET was regarded as the outcome of interest. Associations between demographic and clinical characteristics of the patients according to the outcome of FET cycles were assessed using student t test (for data demonstrating a Gaussian distribution) or Mann-Whitney –U test (for skewed data) for continuous variables and chi square or Fisher’s exact tests for categorical variables, as appropriate. Multivariable logistic regression models were specified to evaluate associations between CP following FET and variables of interest. Logistic regression was conducted using model-building strategies described by Hosmer and Lemeshow (15). In view of skewed distribution, we decided to categorize P and tertiles were compute as low, middle and high. Areas under the receiver operator curves (ROC) were calculated to determine the predictive accuracy of serum hormone levels during COH on the probability of pregnancy after FET.
Because of the relatively small number of the outcome of interest, CP (n = 21), a propensity score analysis was used to adjust for covariates of importance without unduly burdening the statistical models (16). Briefly, a propensity score was derived from a separate multivariable model (linear or logistic, as appropriate) incorporating the adjustment covariates of interest. It was then used as a single adjustment variable (summarizing the included covariates) in the logistic regression models that were used to determine an association between the FET outcome and the independent variables of interest (outcome of the fresh cycle and serum progesterone level on the day of hCG). The strength of association between CP after FET and the independent variables are presented as odds ratio with 95% confidence interval (CI). All statistical tests used a two-tailed alpha of 0.05. Analyses were performed using STATA 9.2 (StataCorp LP, College Station, TX).
RESULTS
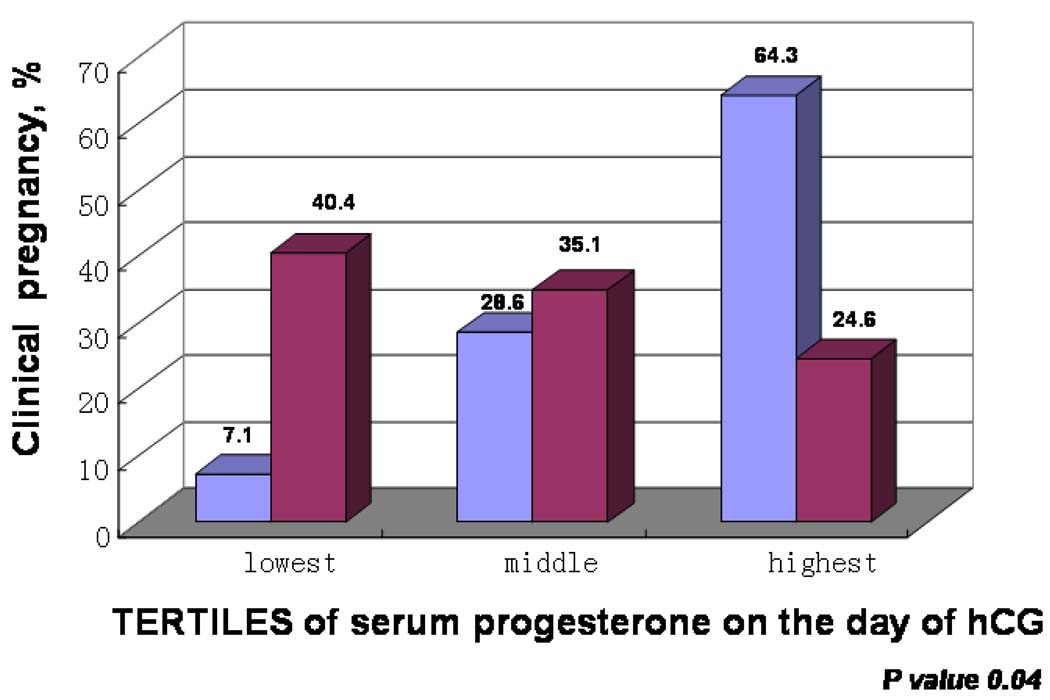
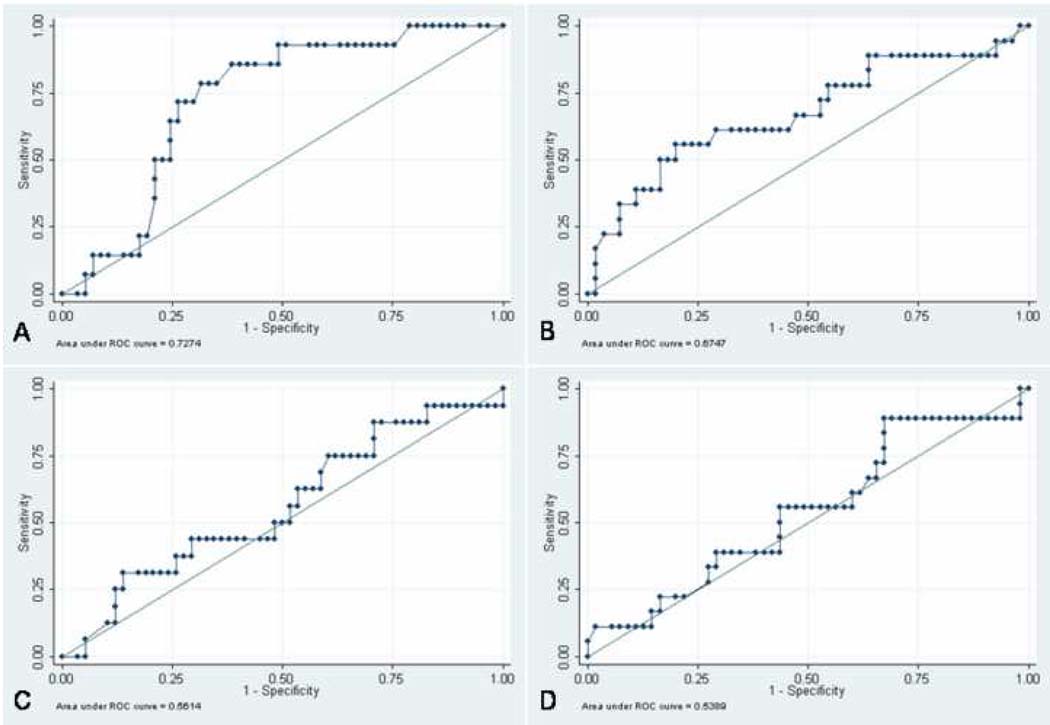
Table 1 describes the patient and cycle characteristics by FET cycle (CP versus not pregnant). Patients with a pregnancy following fresh ET had a higher CP rate following FET (71.4% vs. 40.6%, respectively, p=0.01). Additionally, number of oocytes fertilized and the total number of embryos available on day#3 were positively associated with the likelihood for CP following FET. Serum P, both the day of and post hCG, was significantly higher in patients achieving CP following FET (Figure 1). Using ROC analyses, P level on the day of hCG was identified as the best available hormonal predictor of FET success (Figure 2).
Table 1.
Patient and Cycle Characteristics by FET outcome
| Clinical pregnancy after FET, n=21 |
Not pregnant after FET, n=69 |
P value | ||
|---|---|---|---|---|
| Fresh | Age, yrs | 32.7±3.7 | 33.6±4.3 | 0.43a |
| cycle | FSH, mIU/ml | 6.6±2.1 | 6.3±1.9 | 0.58a |
| E2 on the day of hCG, pg/mL | 2543(2106–3989) | 2555(1665–3354) | 0.45b | |
| E2 post hCG, pg/mL | 3258(2546–4300) | 3019(3076–4300) | 0.62b | |
| P on the day of hCG, ng/mL | 1.5 (1.1–1.7) | 0.8 (0.6–1.3) | <0.01b d | |
| P post hCG, ng/mL | 7.2 (4.2–9.9) | 4.7 (2.7–6.5) | 0.03b d | |
| ICSI | 10 (47.6) | 35 (50.7) | 0.80c | |
| Oocytes retrieved | 18.3±8.0 | 16.6±8.3 | 0.45a | |
| Mature oocytes | 15.7±7.0 | 13.7 ± 6.2 | 0.25a | |
| Oocytes fertilized | 12.5±5.1 | 10.3±4.0 | 0.05a | |
| Embryos transferred | 2.6±0.5 | 2.8±0.8 | 0.51a | |
| Blastomeres per embryo transferred | 8.1±0.5 | 8.1±0.8 | 0.90a | |
| Embryos available on Day 3 | 10.5±3.7 | 8.6±3.0 | 0.02a d | |
| Clinical pregnancy following fresh ET | 15 (71.4) | 28 (40.6) | 0.01c d | |
| FET | Age, yrs | 35.0±3.7 | 34.8±4.4 | 0.83a |
| cycle | Endometrial thickness, mm | 9.1 ±2.6 | 8.5±3.4 | 0.55a |
| Post-thaw blastomere survival, % | 82.6±12.3 | 77.1±22.1 | 0.31a | |
| Embryos transferred | 3.0±0.6 | 2.8±0.8 | 0.38a | |
| Blastomeres/ embryo transferred | 6.7±1.9 | 5.9±1.6 | 0.08a |
Note: Continuous data are presented as means ± SD (if normally distributed) or as median (interquartile range) if skewed; categorical data are presented as n (%)
Student Test.;
Mann-Whitney;
chi square;
Denotes statistical significance
Figure 1. Outcome of FET by Serum Progesterone on the Day of hCG.
Blue bars – clinical pregnancy after FET
Purple bars – unsuccessful FET
Figure 2. Predictors of FET Success: Area Under the Receiver-operating Curve (AUC) for Serum Hormone Levels During COH.
A) Serum P on the day of hCG: AUC=0.73 (95% CI= 0.60 to 0.86),
B) post-hCG P: AUC= 0.67 (95% CI= 0.52 to 0.83),
C) E2 on the day of hCG: AUC= 0.56 (95% CI = 0.40 to 0.72),
D) post-hCG E2 :AUC= 0.54 (95% CI = 0.38 to 0.7).
The difference between these areas and the reference area of 0.5 was significant for serum P on the day of hCG (p= 0.0005) and serum P post hCG (p=0.03)
Multivariate logistic regression analysis demonstrated that success of the fresh cycle and P level on the day of hCG were independent and positive predictors of CP following FET (Table 2). In patients who achieved a CP following fresh embryo transfer, a subsequent FET cycle utilizing sibling embryos conferred a 5 times increased chance of achieving CP (OR=5.5 and 95% CI 1.2–25.3) after adjustment for age, number of frozen embryos transferred and mean number of blastomeres in the transferred embryos. Those FET cycles that followed fresh IVF cycles with serum P levels in the highest tertile were 20 times more likely to result in CP compared to fresh cycles with P levels in the lowest tertile (Table 2). Additionally, there were no differences noted in age, FSH levels, BMI, number of eggs retrieved, or the outcome of the fresh IVF cycle across the tertiles of P on the day of hCG (data not shown).
Table 2.
Likelihood of clinical pregnancy (presented as odds ratio ± 95% confidence interval) after FET, by specified determinant
| Determinant | Unadjusted OR (95% CI) |
P value |
Adjusted ORa (95% CI) |
P value |
|---|---|---|---|---|
| Success of fresh embryo transfer | 3.7 (1.3−10.6) | 0.02 | 5.5 (1.2−25.3) | 0.03 |
| P on the day of hCG (middle tertile)c | 4.6 (0.5−44.6) | 0.19 | 7.2 (0.6−82.2) | 0.11 |
| P on the day of hCG (highest tertile)c | 14.8 (1.7−129.6)b | 0.02 | 20.8 (1.5−283.5) | 0.02b |
Propensity score analysis, adjusting for age, number of frozen ET and mean number of blastomers of transferred embryos
Statistically significant
Compared to the lowest tertile of P on the day of hCG
DISCUSSION
Cryopreservation of human embryos in assisted reproduction allows couples to increase the odds of achieving pregnancy after a single attempt at ovarian hyperstimulation. Although the goal of any ART cycle should be singleton pregnancy and many factors influencing the outcome of FET cycles are known, the rate of multiple births after FET remains high. In 2005, out of all U.S. ART cycles that used frozen non-donor embryos and resulted in live births, approximately 23 per cent produced more than one infant (2). Thus, the need to improve our knowledge in predicting success of FET is pressing, especially given the drive for single-embryo transfers.
Among the prognosticators that affect success of frozen-thawed embryos are maternal age, embryo quality, embryonal development at the time of cryopreservation, and the outcome of the fresh IVF cycle (3–6, 8, 17). Embryo characteristics on day 3 of culture, such as exhibiting at least four blastomeres at FET and loss of less than two blastomeres upon thawing yield a higher take-home baby rate (5, 17). In concert with other studies (3–6), we found that clinical pregnancy after a fresh cycle was associated with an increased chance of a successful frozen cycle. To strengthen this observation, we employed multivariable logistic regression analyses that confirmed the success of the sibling fresh embryo transfer cycle as the foremost predictor of CP following FET after adjustment for factors that are recognized to influence the likelihood of success of an IVF cycle.
Our findings of a relationship between hormonal profile of the fresh IVF cycle, specifically serum P levels on the day of hCG administration, and the outcome of an ensuing FET cycle are intriguing. While many centers frequently measure serum levels of sex steroids during COH, the data on predictive value of hormonal parameters on the likelihood of pregnancy are limited. E2 levels are routinely measured during COH, yet they appear to be of little utility in predicting pregnancy (18). Serum P levels during COH have attracted attention as a possible predictor of pregnancy. While some investigators found detrimental influences of elevated P on the outcome of fresh IVF cycle (10, 19), others either failed to demonstrate such an association (9, 20), or even reported to the contrary. Indeed, elevated serum P on the day of hCG were associated with higher pregnancy rates after IVF in a cohort of women with PCOS (21).
Increasing levels of P during COH are suggested to adversely influence endometrial receptivity rather than being detrimental to the developing oocyte or embryo quality (22). In contrast to fresh IVF cycle, the potential effect of increasing P is washed out in donor egg recipients and frozen embryo transfer cycles as the patients do not undergo concurrent COH. In fact, our data on serum progesterone in the FET model is in concert with a similar observation in donor egg cycles. Higher P in egg donors during COH was reported to be a positive predictor of clinical pregnancy in the recipients (23). As such, an elevated progesterone level in the fresh cycle may represent an indicator of embryo quality embryo and granulosa cell function. In a prior study of patients undergoing FET, we described adverse implications of elevated P during COH on post thaw embryo parameters (11). In that earlier study of patients, optimal FET outcomes were observed in patients in whom P levels on day of hCG in the fresh cycle were between 1–2 ng/ml whereas extremes of serum P levels were associated with reduced success (11). Consistent with the prior observations, in the current study we did not observe a CP following FET in patients with serum P level on the day of hCG of the fresh cycle greater than 3.4 ng/ml. When taken together, these data are suggestive of both “threshold” and “ceiling” implications of P levels during COH cycles and imply that only moderately elevated serum P translates to a positive prognostic variable. Given the observed beneficial implications of P levels remote from FET on the outcome of FET cycle, it is plausible that the moderately elevated serum P levels during COH (specifically on the day of hCG) reflect qualitative aspects of the granulosa cell-oocyte units, and competence of the resulting embryos.
Our study is limited by the relatively small sample size as well as by the retrospective design. While we have attempted to adjust for parameters that are recognized to influence success of FET cycles, our conclusions are inherently affected by the limited ability to control non-identifiable factors that may have played a role in clinical decisions. Generalizability is restricted to the population of patients who had embryos cryopreserved on day 3 and, thus, may not be applicable to blastocysts recipients. While we have not observed a negative impact of elevated serum progesterone on the outcome of the fresh cycle in the current study, our patient population is not an appropriate platform for this research question. By virtue of limiting our population to patients who had enough embryos to freeze and good enough embryos to survive thawing and undergo FET, we deliberately limited this study to good prognosis patients. Despite these limitations, our findings were subject to rigorous statistical scrutiny and the observations reported held true in both univariate and multivariable analyses.
In summary, outcome of the fresh cycle is the single most important clinical predictor of success of the sibling frozen embryos. While this finding is consistent with the existing literature, multivariate logistic regression used in the current study allows to adjust for influences of important confounders, adding strength to our conclusions. Relationship between elevated serum P on the day of hCG administration in the fresh cycle and FET outcome is noteworthy and merits further investigation. The highly increased likelihood of pregnancy with FET after a successful fresh cycle merits strategic utilization of this information in selecting appropriate candidates for single embryo transfer.
Acknowledgments
Supported in part by T32 HD040135 (AJP), K24 HD 41978 (NS), and 5K12 RR17672 (LP)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Presented as a poster presentation at the 62nd Annual Meeting of the American Society for Reproductive Medicine, New Orleans, Louisiana, October 21–25, 2006.
The authors declare that they have no potential conflicts of interest
REFERENCES
- 1.Trounson A, Mohr L. Human pregnancy following cryopreservation, thawing and transfer of an eight-cell embryo. Nature. 1983;305:707–709. doi: 10.1038/305707a0. [DOI] [PubMed] [Google Scholar]
- 2. [accessed 7/16/2008]; www.cdc.gov/ART/ARTReports.htm.
- 3.Lin YP, Cassidenti DL, Chacon RR, Soubra SS, Rosen GF, Yee B. Successful implantation of frozen sibling embryos is influenced by the outcome of the cycle from which they were derived. Fertility & Sterility. 1995;63:262–267. doi: 10.1016/s0015-0282(16)57352-x. [DOI] [PubMed] [Google Scholar]
- 4.El-Toukhy T, Khalaf Y, Al-Darazi K, O'Mahony F, Wharf E, Taylor A, et al. Cryo-thawed embryos obtained from conception cycles have double the implantation and pregnancy potential of those from unsuccessful cycles. Human Reproduction. 2003;18:1313–1318. doi: 10.1093/humrep/deg235. [DOI] [PubMed] [Google Scholar]
- 5.Salumets A, Suikkari AM, Makinen S, Karro H, Roos A, Tuuri T, et al. Frozen embryo transfers: implications of clinical and embryological factors on the pregnancy outcome. Human Reproduction. 2006;21:2368–2374. doi: 10.1093/humrep/del151. [DOI] [PubMed] [Google Scholar]
- 6.Wang JX, Yap YY, Matthews CD. Frozen-thawed embryo transfer: influence of clinical factors on implantation rate and risk of multiple conception. Human Reproduction. 2001;16:2316–2319. doi: 10.1093/humrep/16.11.2316. [DOI] [PubMed] [Google Scholar]
- 7.Hyden-Granskog C, Unkila-Kallio L, Halttunen M, Tiitinen A. Single embryo transfer is an option in frozen embryo transfer. Human Reproduction. 2005;20:2935–2938. doi: 10.1093/humrep/dei133. [DOI] [PubMed] [Google Scholar]
- 8.Edgar DH, Archer J, McBain J, Bourne H. Embryonic factors affecting outcome from single cryopreserved embryo transfer. Reproductive Biomedicine Online. 2007;14:718–723. doi: 10.1016/s1472-6483(10)60674-8. [DOI] [PubMed] [Google Scholar]
- 9.Hofmann GE, Bentzien F, Bergh PA, Garrisi GJ, Williams MC, Guzman I, et al. Premature luteinization in controlled ovarian hyperstimulation has no adverse effect on oocyte and embryo quality. Fertility & Sterility. 1993;60:675–679. doi: 10.1016/s0015-0282(16)56221-9. [DOI] [PubMed] [Google Scholar]
- 10.Fanchin R, Hourvitz A, Olivennes F, Taieb J, Hazout A, Frydman R. Premature progesterone elevation spares blastulation but not pregnancy rates in in vitro fertilization with coculture. Fertility & Sterility. 1997;68:648–652. doi: 10.1016/s0015-0282(97)80464-5. [DOI] [PubMed] [Google Scholar]
- 11.Pal L, Kovacs P, Witt B, Jindal S, Santoro N, Barad D. Postthaw blastomere survival is predictive of the success of frozen-thawed embryo transfer cycles. Fertility & Sterility. 2004;82:821–826. doi: 10.1016/j.fertnstert.2004.02.136. [DOI] [PubMed] [Google Scholar]
- 12.Kwee J, Schats R, McDonnell J, Lambalk CB, Schoemaker J. Intercycle variability of ovarian reserve tests: results of a prospective randomized study. Human Reproduction. 2004;19:590–595. doi: 10.1093/humrep/deh119. [DOI] [PubMed] [Google Scholar]
- 13.Devroey P, Van Steirteghem A. A review of ten years experience of ICSI. Human Reproduction Update. 2004;10:19–28. doi: 10.1093/humupd/dmh004. [DOI] [PubMed] [Google Scholar]
- 14.Gabrielsen A, Agerholm I, Toft B, Hald F, Petersen K, Aagaard J, et al. Assisted hatching improves implantation rates on cryopreserved-thawed embryos. A randomized prospective study. Human Reproduction. 2004;19:2258–2262. doi: 10.1093/humrep/deh434. [DOI] [PubMed] [Google Scholar]
- 15.Hosmer DW, Lemeshow S. Applied Logistic Regression. New York: Wiley; 2000. [Google Scholar]
- 16.Kurth T, Walker AM, Glynn RJ, Chan KA, Gaziano JM, Berger K, et al. Results of multivariable logistic regression, propensity matching, propensity adjustment, and propensity-based weighting under conditions of nonuniform effect. American Journal of Epidemiology. 2006;163:262–270. doi: 10.1093/aje/kwj047. [DOI] [PubMed] [Google Scholar]
- 17.Tang R, Catt J, Howlett D. Towards defining parameters for a successful single embryo transfer in frozen cycles. Human Reproduction. 2006;21:1179–1183. doi: 10.1093/humrep/dei490. [DOI] [PubMed] [Google Scholar]
- 18.Chiasson MD, Bates GW, Robinson RD, Arthur NJ, Propst AM. Measuring estradiol levels after human chorionic gonadotropin administration for in vitro fertilization is not clinically useful. Fertility & Sterility. 2007;87:448–450. doi: 10.1016/j.fertnstert.2006.06.024. [DOI] [PubMed] [Google Scholar]
- 19.Silverberg KM, Burns WN, Olive DL, Riehl RM, Schenken RS. Serum progesterone levels predict success of in vitro fertilization/embryo transfer in patients stimulated with leuprolide acetate and human menopausal gonadotropins. Journal of Clinical Endocrinology & Metabolism. 1991;73:797–803. doi: 10.1210/jcem-73-4-797. [DOI] [PubMed] [Google Scholar]
- 20.Hofmann GE, Khoury J, Johnson CA, Thie J, Scott RT., Jr. Premature luteinization during controlled ovarian hyperstimulation for in vitro fertilization-embryo transfer has no impact on pregnancy outcome. Fertility & Sterility. 1996;66:980–986. doi: 10.1016/s0015-0282(16)58693-2. [DOI] [PubMed] [Google Scholar]
- 21.Doldi N, Marsiglio E, Destefani A, Gessi A, Merati G, Ferrari A. Elevated serum progesterone on the day of HCG administration in IVF is associated with a higher pregnancy rate in polycystic ovary syndrome. Human Reproduction. 1999;14:601–605. doi: 10.1093/humrep/14.3.601. [DOI] [PubMed] [Google Scholar]
- 22.Shulman A, Ghetler Y, Beyth Y, Ben-Nun I. The significance of an early (premature) rise of plasma progesterone in in vitro fertilization cycles induced by a "long protocol" of gonadotropin releasing hormone analogue and human menopausal gonadotropins. Journal of Assisted Reproduction & Genetics. 1996;13:207–211. doi: 10.1007/BF02065937. [DOI] [PubMed] [Google Scholar]
- 23.Legro RS, Ary BA, Paulson RJ, Stanczyk FZ, Sauer MV. Premature luteinization as detected by elevated serum progesterone is associated with a higher pregnancy rate in donor oocyte in-vitro fertilization. Human Reproduction. 1993;8:1506–1511. doi: 10.1093/oxfordjournals.humrep.a138288. [DOI] [PubMed] [Google Scholar]