Abstract
Purpose
To evaluate the safety, pharmacokinetics and determine the recommended dose of the selective apoptotic antineoplastic drug, OSI-461 administered on a twice-daily regimen to patients with advanced solid malignancies.
Methods
In this phase I trial, 33 patients were treated with OSI-461 doses ranging from 400 to 1,200 mg given twice daily in 4-week cycles. Pharmacokinetic studies were performed to characterize the plasma disposition of OSI-461 and the effect of food intake on OSI-461 absorption. Secondary biomarker studies were performed to assess the biologic activity of OSI-461 including the measurement of pGSK-3β, a PKG substrate, and pharmacogenetic studies to identify polymorphisms of CYP3A that influence drug metabolism and of ABCG2, involved in drug resistance.
Results
Thirty-three patients were treated with 86 courses of OSI-461. The dose-limiting toxicities were grade 3 abdominal pain, found in one patient at the 1,000 mg BID fed dose level and all patients at the 1,200 mg BID fed dose level. There was also one episode each of grade 3 fatigue and grade 3 constipation at the 1,000 and 1,200 mg BID fed dose levels, respectively. Other common toxicities included mild to moderate fatigue, nausea, anorexia and mild elevation in bilirubin. Pharmacokinetic studies of OSI-461 revealed approximately a twofold increase in AUC0–24 when OSI-461 was administered with food. An increase in pGSK-3β post-dose was seen in the majority of patients and was greater at higher dose levels. No patients exhibited CYP3A4 polymorphisms, while 100% of patients were found to have the CYP3A5*3/CYP3A5*3 polymorphism. Two known polymorphisms of the ABCG2 gene, G34 → A34 and C421 → A421, occurred at frequencies of 11.76 and 29%, respectively.
Conclusions
Toxicity and pharmacodynamic data show that the recommended oral dose of OSI-461 is 800 mg twice daily administered with food. The drug appears to be well-tolerated, and overall bioavailability appears to be markedly increased when the drug is administered with food. These results support further disease-directed evaluations of OSI-461 at a dose of 800 mg BID in combination with other chemotherapeutic agents.
Keywords: Phase I, OSI-461, SAAND, Pharmacokinetics, Developmental therapeutics, Pharmacogenomics
Introduction
OSI-461 (Z-5-fluoro-2-methyl-(4-pyridylidene)-3-(N-benzyl)-indenylacetamide hydrochloride) is the second agent of a new class of drugs termed selective apoptotic antineoplastic drugs (SAANDs). OSI-461 induces apoptosis of tumor cells by inhibiting cyclic GMP (cGMP) phosphodiesterases (PDE) which leads to increased cGMP and activation of cGMP-dependent protein kinase G (PKG) and not through cyclooxygenase 1 or 2 inhibition [19, 28, 37, 41]. The cyclic nucleotide second messenger, cGMP, has been shown to regulate a wide variety of processes in various tissues of the body, including promoting apoptosis in certain cells [3, 20, 30]. cGMP mediates most of its intracellular effects through the activation of specific cGMP-dependent protein kinases (PKG) [7]. Members of the PDE family act as regulatory switches by catalyzing the hydrolysis of cyclic nucleotides to inactive 5′ nucleotides, terminating the action of agents which use cGMP as second-messengers to initiate cellular responses [12]. Activation of PKG has been associated with inhibition of PDE2 and PDE5, a decrease in β-catenin levels and increased expression of 15-lipoxygenase-1 (15-LOX-1) [4, 19, 37].
Evidence indicates that PDE inhibition of cGMP is involved in the malignant processes. Prior studies show that cancer cells in the bladder, colon, breast, and lung share increased amounts and activity of PDE5 [24, 25, 31, 40], and that suppression of PDE5 promotes apoptosis and inhibits growth in human colonic HT29 cancer cells [45]. Selective inhibitors of PDE5 appear to maintain a persistent increase in cGMP as opposed to a transient increase when PDE5 activity is present. This concept is further supported by a study of the first SAAND, exisulind, which induced apoptosis and inhibited cell proliferation in colonic tumor cell lines via a cellular signal transduction that resulted in persistent increases in cGMP and down-stream targets [37].
OSI-461 has approximately 100 times greater affinity for cGMP PDE than exisulind [37], and as a result, OSI-461 selectively induces apoptosis in a wide variety of epithelial and non-epithelial-derived tumor cell lines at micromolar concentrations while sparing normal cells. OSI-461 showed growth inhibitory effects in colon, lung, and prostate cancer cell lines with growth 50% inhibitory concentration (GIC50) values of 1–2 µm [2, 3]. Similar inhibition with OSI-461 was observed using human leukemia cell lines (CCRF-CEM, K562, Molt-4), a myeloma cell line (RPMI8226), a pancreatic tumor cell line (PAN-1), and an ovarian tumor cell line (OVCAR-3) [37, 41].
OSI-461 also demonstrated in vivo inhibition of tumor growth in implanted human A549 non-small cell lung cancer cells in rats treated with OSI-461 10, 25, 50, 100 mg/kg or docetaxel 2.5 mg/kg and compared to untreated controls [40]. Affinity purified antibody-labeled sections revealed that these cells had increased levels of PDE1 and PDE5. A549 adenocarcinomas implanted in rats treated with OSI-461 exhibited a dose-dependent decrease in the proliferation rate of all tumors as compared to controls and at higher doses when compared to docetaxel-treated groups [40]. In this study, OSI-461 also increased the ratio of non-metaphase to metaphase mitotic cells. In vivo A549 cells exposed to OSI-461 displayed a decreased ability to form a functional bipolar mitotic spindle. This data correlated with an observed increase within the G2/M stage of the cell cycle of A549 cells, breast (MDA-MB 453) and histocytic lymphoma (U947) cell lines treated with OSI-461 for 24 h [41]. In SW480 colon cancer cells lines at doses twice that of the IC50, OSI-461 was able to induce M phase cell cycle arrest independent of PKG activation resulting in depolymerization of microtubules, inhibition of spindle formation and induction of multinucleated cells [43]. In addition, modulation of key mechanisms involved in the malignant transformation has been seen in with single-agent use in LNCaP prostate cells and in combination with acyclic retinoid in HepG2 hepatoma cells [18, 29].
In preclinical toxicology studies, OSI-461 appeared to cause a dose-related increase in liver and thyroid weights (data on file at OSI Pharmaceuticals). In repeated administration to rats and dogs for 28 days, trace follicular cell hypertrophy with increased numbers of mitotic figures in the thyroid gland and mild centrilobar hypertrophy in the liver suggested a mild stimulation of microsomal enzymes, resulting in increased metabolism of thyroid hormones, and thus thyroid stimulation. No serious toxicities were observed in rats, mice, or dogs with longer treatment. In a single dose trial of OSI-461 performed in healthy volunteers, there were no severe adverse events [1]. The most frequently occurring events included headache, abdominal pain, rash, and somnolence. In a phase 1 trial of 21 patients with solid tumors, therapy was well tolerated without any serious drug-related adverse events [35]. Two patients experienced asymptomatic AST/ALT elevation. Three patients exhibited disease stability after two cycles of treatment.
Preclinical pharmacologic studies revealed that OSI-461 is extensively protein bound and rapidly absorbed, distributed, and eliminated following oral administration to rats and dogs. Absorption of OSI-461 occurs in two phases; first from initial absorption in the upper gastrointestinal tract and second from absorption in the lower gut or from enterohepatic circulation. Peak plasma concentration of the drug occurred within 0.25–1 h following oral dosing. The elimination half-life (t1/2) ranged from 2.37 to 4.5 h. OSI-461 appears to be metabolized by cytochrome (CYP) P450 enzymes in vitro. OSI-461 is metabolized into a major metabolite and two minor metabolites by cytochrome P450 enzymes of all species. The major metabolite is an inactive N-dealkylated (de-benzylated) product (OSI-487734AA) while one minor metabolite is identified as an active mono-hydroxylated product (OSI-486553AA). The other minor metabolite is an inactive N-oxide metabolite of OSI-461 (OSIP486511AA). In human microsomes, formation of the metabolite, OSIP487734AA, was correlated with CYP3A4/5 activity and preclinical studies suggest that it can inhibit or induce specific isoforms suggesting potential for drug–drug interactions in human trials (data on file at OSI Pharmaceuticals). Finally, when OSI-461 was administered in fed dogs, an 11-fold median increase in OSI-461 AUC and a 16-fold increase in Cmax were observed, indicating that oral bioavailability may be enhanced with food intake.
The decision to pursue the clinical development of OSI-461 was based on the agent’s novel mechanism of action as an inhibitor of cyclic GMP PDE resulting in PKG activation as well as the selective apoptotic activity in neoplastic cells without altering normal differentiated cell functions. The principal objectives of this phase I and pharmacologic study of OSI-461 were to: (1) characterize the toxicities of OSI-461 administered orally in two divided daily doses in patients with advanced solid malignancies, (2) determine the recommended dose for subsequent clinical trials, (3) characterize the pharmacokinetic (PK) behavior of OSI-461, (4) to evaluate the effect of food on the relative bioavailability of OSI-461, (5) evaluate selected biomarkers and pharmacogenetic markers of drug effect and metabolism, and (6) seek preliminary evidence of antitumor activity in patients with advanced malignancies.
Patients and methods
Patient selection
Patients with histologically documented solid malignancies for whom no effective therapy existed were eligible for this study. Other relevant eligibility criteria included: (1) age at least 18 years; (2) Eastern Cooperative Oncology Group (ECOG) performance status (PS) of ≤2; (3) no chemotherapy or investigational agents within 4 weeks of initiating study therapy; (4) adequate hematopoietic function (absolute neutrophil count ≥ 1,500/µL, platelet count ≥ 100,000/µL), hepatic function (total bilirubin within normal limits (1.0 mg/dL); AST and ALT concentrations ≤2.5 times the institutional upper limit of normal (ULN), and renal function (serum creatinine ≤ 2.0 mg/dL); (5) expected remaining life span ≥ 3 months; (6) no peripheral neuropathy > grade 1; (7) no coexisting medical condition or medication which would interfere with the conduct of the study; (8) no uncontrolled or symptomatic brain metastases; and (9) a negative serum pregnancy test in all women of childbearing age prior to study entry. Informed consent was obtained according to federal and institutional guidelines. The study was approved by the local Institutional Review Board.
Drug administration
This study was divided into two parts. In part I (fasted) drug was administered under fasting conditions and in part II (fed) drug was administered under both a fasting state and fed state. The starting daily dose of OSI-461 was 600 mg BID in the fasted portion and 400 mg BID in fed portion of the study. Initial doses were based on prior OSI-461 phase I studies in humans where a maximum tolerated dose (MTD) was not reached at doses up to 400 mg BID [35]. The dose escalation scheme proceeded using a modified Fibonacci plan, with at least three patients treated at each dose level. Patients in the fasted part were treated at dose levels of 600, 800 and 1,000 mg BID, whereas patients in the fed part were treated at dose levels of 400, 800, 1,000 and 1,200 mg BID. The dose escalation did not occur until the last patient in each cohort was observed for one cycle (4 weeks) of OSI-461 therapy. In the absence of a DLT, escalation to the next level was allowed. No intrapatient dose escalations were permitted. The occurrence of one DLT prompted expansion of that dose level to a maximum of six patients. The MTD in the fasted cohort was defined as one dose level below the dose that induced DLTs in greater than one-third of new patients. The recommended dose in fed cohort was the dose level at which less than 33% of patients experienced a DLT. DLT was defined as grade 3 or 4 non-hematologic toxicity (excluding inadequately treated nausea, diarrhea, and/or vomiting), grade 4 neutropenia, platelets < 25,000, or a treatment delay of 14 days or greater because of drug-related toxicity. Toxicities were graded according the National Cancer Institute’s Common Terminology Criteria, version 2.0. For patients with transaminases ≥2.5 times the ULN but ≤5 times the ULN, the dose of OSI-461 was reduced by 50%. The study drug was withheld if the total bilirubin was greater than the ULN or the transaminases were greater than five times the ULN. Therapy could be reinstituted after discussion with the sponsor at a 50% reduction if the transaminases and bilirubin returned to normal. Dose modifications for peripheral neuropathy included a 50% dose reduction for grade 2 and for grade 3 OSI-461 was withheld until resolution to grade 1 or less than reinstituted at a 50% dose reduction. For any other treatment-related grades 3 or 4 toxicity study drug was held until resolution to grade 1 or less and restarted at a 50% dose reduction after discussion with the sponsor.
In fasted portion of the study, OSI-461 administration began on Day 1 of Cycle 1 under fasting conditions. Patients in fed portion of the study were administered one dose of OSI-461 3 days prior (Day-2) to starting Cycle 1 in a fasting state. Patients were instructed to fast for at least 8 h prior to the scheduled dose and were not allowed any food until at least 2 h after the dose. On Day 1 of Cycle 1, patients were provided with a high-fat, high-calorie meal (approximately 50% fat, 30% carbohydrates, 15% protein) within one half-hour of the scheduled dosing time. Standard meal plans were developed by a nutritionist and meals were provided by the institution to control the calorie and fat content. Example meal options included: (1) nonvegetarian: croissant, with two eggs, cheese, and sausage with 6 ounces of juice totaling 890 calories, made up of 54 g fat, 35 g protein, and 61 g carbohydrate; and (2) vegetarian: croissant with 1 packet of cream cheese, 2 eggs scrambled with 1 ounce cheese, 8 ounces of whole milk, 4 ounces of juice totaling 910 calories, made up of 55 g fat, 33 g protein, and 67 g carbohydrate. All patients were asked to take the study medication with food for the remainder of the study and kept logs of morning and evening meal and dosing times.
OSI-461 was supplied by OSI Pharmaceuticals, Inc (Boulder, CO) in gelatin capsules containing 100 mg of OSI-461 plus excipients. Supplies of the study drug were stored in a dry place, at controlled room temperatures (20–25°C), and protected from environmental extremes. OSI-461 was taken orally with eight ounces of water every 12 h.
Pretreatment and follow-up studies
Before each treatment cycle, interval histories, physical examinations, concomitant medication histories, assessments of ECOG PS, and routine laboratory studies were performed. Routine laboratory studies included a complete blood count with platelets and differential WBC count, standard chemistry panel (including electrolytes, blood urea nitrogen, creatinine, and glucose), and a liver panel (including lactate dehydrogenase, alkaline phosphatase, total bilirubin, ALT, and AST). Bi-weekly evaluations included an interval history, physical examination, concomitant medication history, assessment of ECOG PS, and routine laboratory studies.
A formal assessment of disease was performed prior to treatment and then after every other cycle using RECIST criteria [36]. A complete response was defined as the disappearance of all target lesions. Patients were allowed to continue treatment in the absence of disease progression or intolerable toxicity.
Pharmacokinetic sampling and assay
To study the PKs of OSI-461, whole blood samples were obtained by venipuncture at designated time intervals. On Days 1 and 29 of fasted cohort and Days-2, 1 and 29 of fed cohort of the first treatment cycle, samples were collected 15 min prior to OSI-461 dosing and then 15, 30, 60, and 90 min after dosing and 2, 4, 6, 8, 12 (fed cohort only), and 24 h after dosing. Trough levels were collected prior to the morning dose of OSI-461 on Day 15. Seven milliliters of blood was drawn to obtain at least 2 mL of plasma. Whole blood samples were centrifuged within 15 min of collection for 10–20 min, at 2,000–3,000 rpm and 4°C. Plasma was removed from the cell fraction and frozen at −70 ± 10°C until shipment for analysis.
Plasma was assayed for OSI-461 by liquid chromatographic/tandem mass spectrometry (HPLC/MS-MS) by the Midwest Research Institute (Kansas City, MO). Analytic standards were provided by OSI Pharmaceuticals, Inc. The lower limit of quantitation for OSI-461 was 4 ng/mL and the extraction efficiency of SPE was 74.2%. Before quantitation, the samples were thawed and centrifuged at 2,600 rpm for approximately 5 min, 150 µL of plasma was transferred into a microcentrifuge tube, and 100 µL of internal standard (OSIP486821; 5-fluoro-2-methylindene-3-(N-benzyl) acetamide) was added to each tube and vortexed for approximately 10 s. Waters Oasis 1 cc (30 mg) SPE cartridges (Waters, Bedford, MA) were conditioned with 1 mL of methanol (HPLC grade, Burdick and Jackson, Muskegon, MI) followed by 1 mL of high-purity water. The prepared plasma samples were loaded onto the cartridges and washed with 1 mL of high-purity water. The samples were then eluted with 1 mL of acetonitrile (HPLC grade, Burdick and Jackson, Muskegon, MI) and the eluent was collected and evaporated under a stream of nitrogen at approximately 35°C. The samples were then reconstituted with 150 µL of water:acetonitrile (50:50) for analysis.
The chromatographic system consisted of a Waters 2690 liquid chromatograph (Waters Corp, Milford, PA), a 5 µm, 2.0 × 50 mm YMC Basic column (Waters, Bedford, MA), and a 10 × 2 mm YMC Basic guard column (Waters, Bedford, MA). The two mobile phases consisted of 1.9% ethanol (AAPER, Shelbyville, KY) with 5 mM ammonium formate buffer (pH 3.5) and 90% acetonitrile, 5 mM ammonium formate buffer (pH 3.5). The injection volume was 10 µL, and the flow rate was 0.250 mL/min. The Micromass Quattro II (Waters Corp., Milford, PA) mass spectrometer was used with a capillary voltage of 3.5 KV, and a source block temperature of 130°C. The dessolvation temperature was 400°C and the nitrogen pressure was set at 120 pounds per square inch. The capillary voltage, cone voltage, extractor voltage, collision energy, and other parameters were adjusted to optimize the signal intensity by the analyst. Data was collected using positive electrospray ionization in the multiple reaction monitoring mode. OSI-461 was monitored using m/z transition 385 to 251 and OSIP486821 using m/z transition 296 to 163.
Pharmacokinetic analysis
Noncompartmental analysis was used to determine the PK parameters of OSI-461. Individual OSI-461 plasma concentrations were analyzed by model-independent methods using the program WinNonlin 5.0.1 (Pharsight Corporation, Mountain View, CA). Area under the concentration–time curve (AUC) from time 0 to the time of the final quantifiable sample was calculated using the linear trapezoidal method. The AUC was extrapolated to infinity (AUCinf) by dividing the last measured plasma concentration by the terminal rate constant (λz), which was determined from the slope of the terminal phase of the plasma concentration–time curve. Systemic clearance (Cl/F) was determined by dividing the dose by AUCinf. The volume of distribution at steady state (Vss) was calculated using standard noncompartmental methods [8]. Maximum plasma concentrations (Cmax) were the observed values. For the analysis, below the limit of quanitation (BLOQ) values prior to Cmax were recorded as “0”. All pre-dose sample times were recorded as “0” time for the first dose. The geometric mean was calculated by taking the log of the PK value, averaging the log-scale values, and then exponentiating (inverse-log) the log-scale mean to get the final geometric mean for that specific parameter.
OSI-461 PK parameters were summarized using descriptive statistics. The relationships between the OSI-461 dose and both Cmax and AUC were assessed by univariate linear regression. A paired t test was used to compare between days and a univariate analysis of variance was used to determine the relationship between (1) OSI-461 PK parameters and dose, (2) OSI-461 exposure and toxicity, and (3) estimated clearance and toxicity. When the means of more than two groups were being compared, the Tukey–Kramer test was used to determine which means were significantly different. A significance level of 0.05 was set for all analysis. Statistical analysis was performed using the JMP version 3.1 statistical software program (SAS Institute, Cary, NC).
Biomarker and pharmacogenetic sampling
The biologic activity of OSI-461 was assessed by looking at secondary biomarker studies. To measure the activity of OSI-461, the PKG substrate, glycogen synthase kinase-3β (GSK-3β) was evaluated. Phosphorylated GSK-3β is a biomarker for cGMP activity. Plasma samples for the pGSK-3β biomarker assay were obtained from all patients on Days 1 and 29 of the first treatment cycle. The biological activity of OSI-461 was assayed through the analysis of surrogate biomarkers, present in peripheral circulating blood cells.
In fasted part of the study, samples were collected 15 min prior to OSI-461 dosing and then 2, 8, and 24 h after dosing. Seven milliliters of blood was drawn into a green top tube and WBCs were immediately separated by standard Ficoll gradient centrifugation. The resulting WBCs were then washed twice in PBS and collected by centrifugation. The WBC pellet was immediately frozen and stored at −70°C until shipment.
In fed part of the study, samples were collected 15 min prior to OSI-461 dosing and then 1, 2, 4, 6, and 24 h after dosing. Different collection procedures were followed for this part of the study due to difficulties in obtaining interpretable pGSK-3β results with the method utilized in the fasted part of the study. Five milliliters of blood was drawn into a plastic syringe containing 1 mL of ACD (85 mM trisodium citrate, 65 mM citric acid, 100 mM glucose), mixed, and dispersed into a 15 mL polypropylene tube. The blood was then centrifuged at approximately 1,200 rpm for 10 min at 20°C within 30 min of collection. The plateletrich plasma was then carefully removed to about ¼ inch above the red cell layer, and placed in a 15 mL polypropylene tube and re-centrifuged for 5 min at approximately 2,700 rpm at 20°C. The samples were immediately frozen, stored at −80°C, and later shipped to OSI Pharmaceuticals, Inc. for proteomic biomarker analysis of pGSK-3β. Changes in GSK-3β phosphorylation were measured by SDS–polyacrylamide electrophoresis and immunostaining with rabbit anti-(phosphor-Ser9)-GSK-3β (Cell Signaling, Boston, MA) antibody. A ratio with actin was determined and samples were evaluated for increased phosphorylation.
The CYP3A family plays a significant role in metabolism resulting in the elimination of numerous drugs within the liver and variability in CYP3A dependent drug clearance suggests that genetic mutations may be involved [15]. To assess CYP3A polymorphisms that influence drug metabolism, CYP3A4 and CYP3A5 single nucleotide polymorphisms (SNPs) were analyzed. Additionally, the ABCG2 gene encodes for the breast cancer resistance protein, BCRP and has been associated with chemotherapy drug resistance [6]. Mutations in this gene confer lower protein expression leading to lower drug resistance [10, 14]. Pharmacogenetic samples were obtained on Day 1 at baseline and 1 h post-dose, from all patients in fed cohort who signed a written amendment to the informed consent. Samples were processed and analyzed for the presence of CYP3A4 and CYP3A5 and ABCG2 SNPs involved in OSI-461 metabolism and drug resistance. Genomic DNA was extracted from patient whole blood using a combination of protocols from both QIAamp® DNA Blood Maxi and Mini kits (Qiagen, Valencia, CA). Isolated DNA was then amplified by Touchdown PCR to create enough templates for sequencing. PCR reactions were carried out in a total volume of 25 µL using 100 ng DNA, 0.10–0.25 µM concentration of both the forward and reverse primers, 1× PCR buffer II, 3.0 mM MgCl2, 0.2 µM concentrations of each dNTP, and 0.625 U of AmpliTaq Gold DNA polymerase® (Applied Biosystems, Foster City, CA). Thermal cycling conditions consisted of an initial denaturation of 94°C for 15 min, 40 cycles of 1 min at 94°C, 1 min at 59–54°C and 1 min at 72°C, followed by a final extension of 7 min at 72°C. PCR was performed by Mastercycler EP® (Eppendorf, Westbury, NY). Without further purification, PCR amplicons, along with a single primer, were sent to the Sequencing Core Laboratory at the University of Colorado Cancer Center for DNA sequence analysis.
Results
Thirty-three patients received 86 total courses of OSI-461 at doses ranging from 400 to 1,200 mg BID. All 33 patients were evaluable for toxicity and response. Patient characteristics are listed in Table 1. All 33 patients had previously received chemotherapy, radiotherapy, or both treatment modalities. The treatment dose levels, as well as the number of patients and courses administered as a function of the study cohort (fasted or fed) are listed in Table 2. The median number of courses administered per patient was 2 (range 1–8).
Table 1.
Patient characteristics (no. of patients = 33)
| Fasted | Fed | |
|---|---|---|
| Sex (male/female) | 6/5 | 12/10 |
| Age (years) | ||
| Median | 64 | 63 |
| Range | 43–78 | 35–83 |
| ECOG performance status | ||
| 0 | 3 | 3 |
| 1 | 7 | 18 |
| 2 | 1 | 1 |
| Previous treatment | ||
| Chemotherapy only | 5 | 13 |
| Radiation only | – | 1 |
| Chemotherapy and radiotherapy | 6 | 8 |
| Tumor type | ||
| Colorectal | 5 | 2 |
| Renal | 1 | 3 |
| Melanoma | 1 | 3 |
| Pancreatic | 3 | – |
| Breast | – | 3 |
| Prostate | 1 | 2 |
| Gallbladder | – | 2 |
| Othera | – | 7 |
Hepatocellular, non-small cell lung, ovarian, GIST, thymoma, carcinoid, spindle-cell sarcoma, and bladder
Table 2.
Dose levels, number of patients entered, and DLTs
| Dose level (mg BID) |
No. of patients |
Total no. of cycles |
Range per patient (cycles) |
No. patients with DLT |
|---|---|---|---|---|
| Fasted | ||||
| 600 | 3 | 8 | 2–4 | 0 |
| 800 | 5 | 11 | 1–4 | 0 |
| 1,000 | 3 | 11 | 1–8 | 0 |
| Total | 11 | 30 | 1–8 | 0 |
| Fed | ||||
| 400 | 4 | 13 | 1–8 | 0 |
| 800 | 7 | 21 | 1–8 | 0 |
| 1,000 | 8 | 18 | 1–4 | 2 |
| 1,200 | 3 | 4 | 1–2 | 3 |
| Total | 22 | 56 | 1–8 | 5 |
| Study total | 33 | 86 | 1–8 | 5 |
Toxicities
Thirty patients (91%) experienced adverse events considered related to OSI-461. The most common related adverse events were fatigue (14 patients, 42%), followed by nausea (10 patients, 30%) and anorexia (9 patients, 27%). No grade 4 treatment-related events and eight grade 3 treatment-related events, including abdominal pain, constipation, fatigue, transaminitis, nausea, vomiting and hypoalbuminemia, were reported during the study. Thirty-two patients (97%) experienced at least one adverse event regardless of causality while on study (Table 3). The most common adverse events were fatigue (16 patients, 48%), followed by nausea (11 patients, 33%) and anorexia (10 patients, 30%). Of those adverse events that occurred regardless of causality, 5 (15%) were grade 4 (zero related to study drug) and 11 (33%) were grade 3 (8 related to study drug). A total of seven patients required dose reduction while on study.
Table 3.
OSI-461 adverse events occurring in ≥10% of patients regardless of causality
| Dose | Fasted | Fed | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 600 (N = 3) | 800 (N = 5) | 1,000 (N = 3) | 400 (N = 4) | 800 (N = 7) | 1,000 (N = 8) | 1,200 (N = 3) | ||||||||
| Toxicity grade | Any | 3–4 | Any | 3–4 | Any | 3–4 | Any | 3–4 | Any | 3–4 | Any | 3–4 | Any | 3–4 |
| Fatigue | 3 | – | 3 | – | 3 | – | 1 | – | 3 | – | 3 | 1 | – | – |
| Nausea | 2 | 1 | 1 | – | 1 | – | 1 | – | 1 | – | 2 | – | 3 | – |
| Anorexia | 1 | – | 1 | – | 1 | – | – | – | 1 | – | 4 | – | 2 | – |
| Abdominal pain | – | – | – | – | - | – | – | – | 2 | – | 3 | 2 | 3 | 3 |
| Constipation | 2 | – | – | – | 1 | – | 1 | – | 1 | – | 3 | – | 1 | 1 |
| Elevated LFTs | 2 | – | 1 | – | 2 | – | 2 | 1 | 3 | – | 4 | 1 | 2 | – |
| Elevated Bilirubin | – | – | – | – | 1 | – | 1 | – | 3 | – | 3 | 2 | 1 | 1 |
Toxicity and dose reductions were more commonly seen in patients in the fed state. Minimal toxicity was reported up to 800 mg BID, but DLTs occurred at the 1,000 and 1,200 mg BID dose levels when OSI-461 was administered with food. The most severe toxicity related to OSI-461 in this study was grade 3 abdominal pain. Three out of three patients in the 1,200 mg BID fed cohort experienced grade 3 abdominal pain, with all episodes occurring in the first cycle. One patient also experienced grade 3 constipation at the same time as the abdominal pain, and discontinued the study due to these events. The other two patients had their dose reduced per protocol to 600 mg BID and both had a recurrence of grade 3 abdominal pain upon rechallenge. Two patients out of eight in the 1,000 mg BID fed cohort experienced grade 3 toxicity, one patient with abdominal pain and one patient with fatigue. Both grade 3 toxicities resolved and did not recur with rechallenge at 500 mg BID. No patients in the 800 mg BID fed dose cohort experienced any drug-related grade 3 adverse events.
Regarding hepatotoxicity, there were two episodes of grade 3 transaminitis in a single patient that resulted in study drug interruption at the 400 mg BID dose fed cohort. The correlation between the patient’s transaminitis and OSI-461 administration was unclear given that the patient had a diagnosis of hepatocellular cancer and transient transaminitis prior to enrolling in the study. Thus these elevations were determined not to meet the DLT criteria. The dose was not reduced and patient continued on study until disease progression. Three out of seven patients (43%) enrolled in the 800 mg BID fed cohort were dose reduced secondary to grade 1 total bilirubin elevation. One of the three patients had cholangiocarcinoma and another patient had liver metastases. Both of these patients had a prior history of transient hyperbilirubinemia which was thought to be secondary to their disease. Overall, five patients (15%) experienced a two grade or more worsening in total bilirubin, two patients (6%) experienced a two grade worsening in AST, and one patient experienced a two grade or more worsening in ALT. Mild grade 1 elevations of bilirubin were seen in 27% of patients. There were no significant hematologic or renal toxicities noted throughout all courses administered to patients.
Pharmacokinetic studies
Plasma sampling for PK studies in the fasted portion of the study was performed in three subjects each in the 600 and 1,000 mg BID cohorts, and four patients in the 800 mg BID cohort who received an oral dose of OSI-461 under fasting conditions. In the fed portion of the study four, seven, eight and three patients were enrolled in the 400, 800, 1,000 and 1,200 mg BID cohorts. Each of these subjects received an oral dose of OSI-461 under fasted conditions three days prior (Day-2) to starting Cycle 1 and on Day 1 with a high-fat meal. For all subsequent doses patients were instructed to take the dose with food.
Pharmacokinetic parameters for 10 of the 11 subjects treated under fasting conditions are reported in Table 4, Part a. Median Cmax and AUC values were similar between Day 1 and Day 29 for all dose levels. Median steady state trough plasma concentrations were also similar between each dose level on Day 15 and Day 29 of the study. The geometric mean ratio for Cmax on Day 29 versus Day 1 was 1.26 (90% CI 76.7–206), indicating a lack of OSI-461 accumulation with repeated dosing. As a result, it appears that in the fasting state neither the peak plasma concentrations nor systemic exposure of OSI-461 increased with dose.
Table 4.
Median Pharmacokinetic Parameters for OSI-461
| (a) Fasted | |||
|---|---|---|---|
| Parameter | 600 mg BID (N = 3) | 800 mg BID (N = 4) | 1000 mg BID (N = 3) |
| Cycle 1, Day 1 | |||
| Cmax (µg/mL) | 1.09 (0.780–5.34) | 0.571 (0.491–3.90) | 0.784 (0.775–1.79) |
| AUC0–24 (h µg/mL) | 8.21 (4.47–41.2) | 7.66 (3.15–23.9) | 9.18 (7.18–20.4) |
| Cycle 1, Day 29 | |||
| Cmax (µg/mL) | 1.96 (0.358–2.79) | 1.60 (0.589–1.79) | 1.43 (1.03–1.83) |
| AUC0-tau (h µg/mL) | 12.0 (1.95–16.3) | 9.43 (4.8–14) | 11.2 (6.22–16.2) |
| (b) Fed | ||||
|---|---|---|---|---|
| Parameter | 400 mg BID (N = 4) | 800 mg BID (N = 7) | 1,000 mg BID (N = 8) | 1,200 mg BID (N = 3) |
| Cycle 1, Day-2 (fasted) | ||||
| Cmax (µg/mL) | 0.902 (0.369–2.15) | 0.770 (0.355–5.49) | 1.14 (0.434–4.15) | 0.666 (0.799–4.54) |
| AUC0–24 (h µg/mL) | 8.12 (2.39–13.2) | 4.45 (2.21–44.8) | 10.7 (4.92–51.6) | 5.98 (3.21–31.5) |
| Cycle 1, Day 1 (fed) | ||||
| Cmax (µg/mL) | 1.77 (0.799–2.51) | 2.37 (0.558–6.54) | 1.88 (0.546–3.2) | 0.838 (0.487–1.9) |
| AUC0–24 (h µg/mL) | 15.3 (9.01–33.7) | 17.9 (5.48–74.6) | 20.9 (6.62–23.6) | 6.94 (5.51–19.3) |
| Cycle 1, Day 29 | ||||
| Cmax (µg/mL) | 1.34 (1.1–7.11) | 1.88 (1.63–2.13) | 3.21 (2.17–5.53) | NAa |
| AUC0-tau (h µg/mL) | 11.3 (8.26–71.3) | 13.2 (12.3–14) | 30.7 (10.9–35) | NAa |
NA: data not available as patients did not continue treatment at this dose up to Day 29 due to dose-limiting toxicity
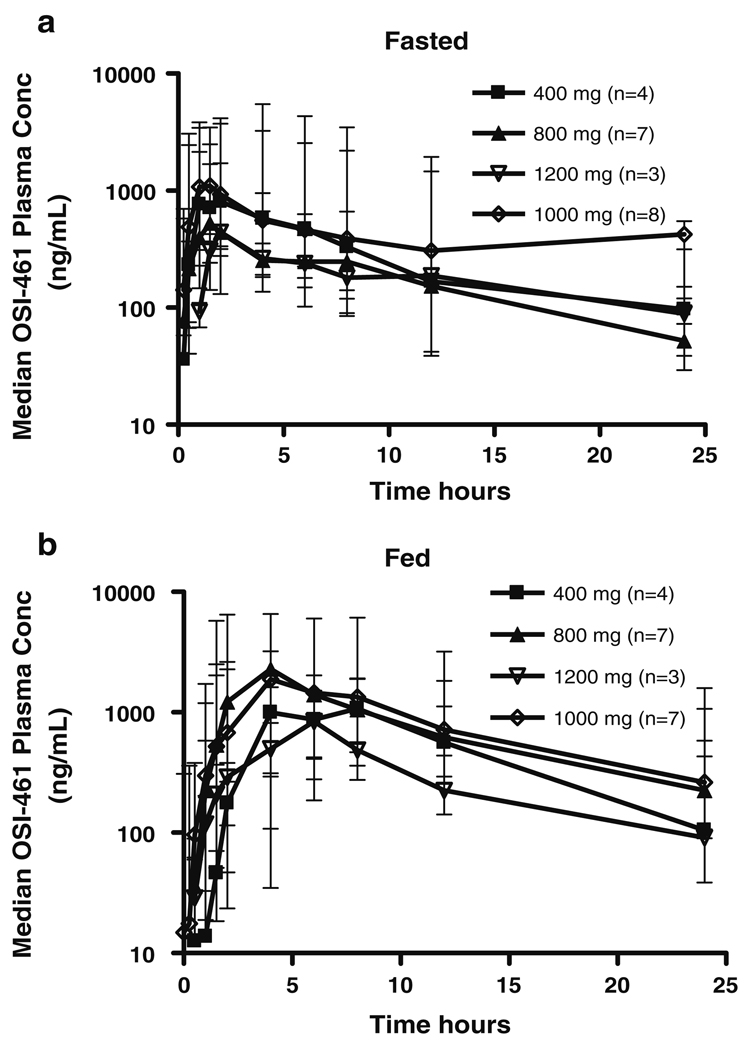
Subsequent to the initial fasted cohort, a PK analysis in dogs revealed a significant increase in the exposure of OSI-461 in the fed state. Therefore the study protocol was amended to assess exposure to OSI-461 in both the fasted and fed state. Pharmacokinetic parameters for the 22 subjects in the fed portion of the study are reported in Table 4, Part b. Median Cmax and AUC values between Day-2 (fasted) and Day 1 (fed) increased for all dose levels when OSI-461 was administered with food. Median plasma concentration–time curves are shown in Fig. 1. The geometric mean fed/fasted dose normalized AUC0–24 ratio for all subjects was 1.91 (90% CI 144–253). There was approximately a twofold increase in AUC0–24 when OSI-461 was administered with food. In the fed state, across all dose levels, the geometric mean ratio of Cmax on Day 29 versus Day 1 was 1.13 (90% CI 73.8–174), indicating a lack of OSI-461 accumulation with repeated dosing.
Fig. 1.
Median plasma OSI-461 concentration versus time curves
There was no increase in the median fasted (Day-2) AUC0–24 when the dose was increased from 400 to 1,200 mg BID. On Day 1 in the fed state, an increase in the Cmax of OSI-461 was seen up to the 800 mg BID dose and an increase in AUC up to the 1,000 mg BID dose and maintained throughout the first cycle. However, the magnitude of the food effect was not sustained from 1,000 to 1,200 mg BID on Day 1 and was not able to be determined at the end of the first cycle due to dose reductions as a result of DLTs in the 1,200 mg cohort. Despite the food effect on bioavailability, the median systemic exposure (AUC0–24) at 1,200 mg BID remained below that observed in the 400 mg BID cohort.
Biomarker studies
All 33 subjects were assessed for pGSK-3β measured prior to the dose of OSI-461 and after. The results for the first 7 patients were not interpretable; therefore biomarker data is only available for 26 subjects. A positive response was considered to be a twofold increase in the ratio of pGSK-3β to actin. The response was measured at two or more time points.
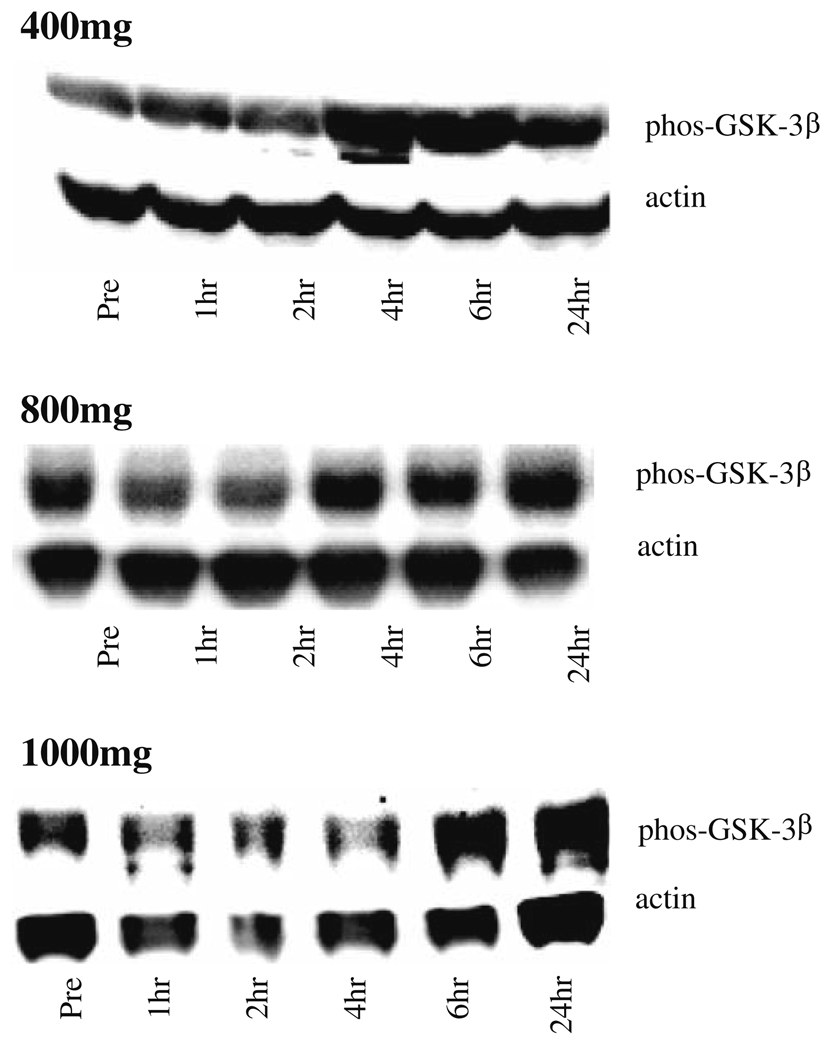
Although the numbers are too small to establish statistical significance, the majority of patients in fed cohort showed increased pGSK-3β post-dose (Fig. 2), which suggests a biological response to OSI-461. The magnitude of the maximum phosphorylation seen was not dose-dependent. As seen in Table 5, the greatest number of patients responded in the 1,000 mg BID fed cohort (6/7 evaluable patients), with smaller numbers in the 400 mg BID fed cohort (3/4 evaluable patients) and the 800 mg BID fed cohort (4/7 evaluable patients).
Fig. 2.
OSI-461 related increase in pGSK-3β in the fed cohort. a Representative western blots for each dose level. b No results for 1,200 mg dose level due to inability to accurately measure actin signal in samples
Table 5.
pGSK-3β response per patient by dose of OSI-461
Positive response defined as ≥1 time point with pGSK-3β signal ≥ twofold signal increase over predose
No results due to inability to accurately measure actin signal in samples
Pharmacogenetic studies
To assess CYP3A polymorphisms that influence drug metabolism, CYP3A4 and CYP3A5 SNPs were analyzed in 17 subjects who signed a written amendment to the informed consent. Alterations of CYP3A4 have not been directly linked to CYP3A activity but are associated with certain cancers and may protect DNA against DNA damaging agents [16]. No CYP3A4 polymorphisms were found for any patient in this study. In contrast, 100% of patients analyzed were found to have the CYP3A5*3/CYP3A5*3 or G/G polymorphism.
The ABCG2 gene is part of the ATP-binding cassette membrane transporter family and has been tied to resistance to anticancer drugs [6, 21, 23]. In this study, the two known mutations related to low expression of BCRP and low drug resistance, G34 → A34 and C421 → A421 polymorphisms occurred at frequencies of 11.76 and 29%, respectively, with no cases of a double mutation in the 17 subjects analyzed as seen in Table 6. Of the 15 Caucasian subjects the G34 → A34 and C421 → A421 polymorphisms occurred at a frequency of 6.76% (1 subject) and 33.33% (5 subjects), respectively. One African–American subject had neither mutation and one Asian subject was homozygous for G34 → A34 and had no C421 → A421 polymorphism.
Table 6.
Detection of heterozygous and homozygous ABCG2 gene
| Pt # | ABCG-2 #34a | ABCG-2 #421b | Racec | ||
|---|---|---|---|---|---|
| Val12Met mutation | Sequencing result | Gln141Lys mutation | Sequencing result | ||
| 01015 | No | Wild-type: G/G | No | Wild-type: C/C | White |
| 01016 | No | Wild-type: G/G | Yes | Heterozygote: C/A | White |
| 01017 | Yes | Homozygote: A/A | No | Wild-type: C/C | Asian |
| 01018 | No | Wild-type: G/G | Yes | Heterozygote: C/A | White |
| 01019 | No | Wild-type: G/G | No | Wild-type: C/C | White |
| 01020 | Yes | Heterozygote: A/G | No | Wild-type: C/C | White |
| 01021 | No | Wild-type: G/G | Yes | Heterozygote: C/A | White |
| 01022 | No | Wild-type: G/G | No | Wild-type: C/C | Black |
| 01023 | No | Wild-type: G/G | Yes | Heterozygote: C/A | White |
| 01024 | No | Wild-type: G/G | No | Wild-type: C/C | White |
| 01026 | No | Wild-type: G/G | No | Wild-type: C/C | White |
| 01027 | No | Wild-type: G/G | No | Wild-type: C/C | White |
| 01028 | No | Wild-type: G/G | No | Wild-type: C/C | White |
| 01029 | No | Wild-type: G/G | No | Wild-type: C/C | White |
| 01031 | No | Wild-type: G/G | Yes | Homozygote: A/A | White |
| 01032 | No | Wild-type: G/G | No | Wild-type: C/C | White |
| 01033 | No | Wild-type: G/G | No | Wild-type: C/C | White |
For ABCG-2 #34, the G → A mutation results in similar or somewhat lower protein expression, and increased drug resistance, as compared to wild-type BRCP
For ABCG-2 #421, the C → A mutation results in markedly decreased protein expression, and low level drug resistance, as compared to wild-type BRCP
For ABCG-2 #34 the of the A mutation frequency in Caucasians is 4.7%; and for ABCG-2 #421 the A mutation frequency in Caucasians is 25.9%
Antitumor activity
There were no objective responses seen within the study, but 8 of the 33 patients (24%) achieved stable disease as their best response to OSI-461. One patient with metastatic pancreatic cancer 1,000 mg BID fasting cohort who had previously undergone radiation and chemotherapy with gemcitabine and fluorouracil maintained stable disease for eight cycles of OSI-461 with no related serious adverse events. Another patient with metastatic hepatocellular carcinoma in the 400 mg BID fed who had been previously treated also maintained stable disease for eight cycles. Both patients were eventually found to have progressive disease and were taken off study.
Discussion
OSI-461 is a SAAND that induces apoptosis through inhibition of cGMP PDE 2 and 5 thus activating PKG which leads to the down-regulation of β-catenin and activation of JNK as well as cyclin D1 [17, 19, 34, 37]. Independent of PKG activation, OSI-461 also appears to induce M phase cell cycle arrest by depolymerization of microtubules and inhibition of mitotic spindle formation [28, 41, 43]. OSI-461 exhibits a high affinity for PDE 2 and 5 compared to other drugs in this class thus resulting in greater selectivity for tumor cells [37]. Preclinical studies using OSI-461 have shown antitumor activity against a broad range of both solid and hematologic human cancer cell lines as both a single agent and in combination with other potential anticancer therapies [19, 28, 29, 37, 41]. This phase I and pharmacologic study was designed to evaluate the safety and toxicology profile of OSI-461 as well as determine the effect of food on the PKs of OSI-461 in patients with advanced malignancies.
Results of this study determined the principal DLT of OSI-461 to be grade 3 abdominal pain, primarily noted at the 1,200 mg BID fed dose level and recurred upon re-challenge in two patients despite a 50% dose reduction. Expansion at the 1,000 mg BID fed dose level resulted in one out of eight patients also experiencing grade 3 abdominal pain. In addition, one patient in the same cohort developed grade 3 fatigue. Other grade 3 toxicities seen during the study included constipation, transaminitis, nausea, vomiting and hypoalbuminemia. Anorexia, fatigue, nausea and transaminitis were the most common drug-related toxicities. No treatment-related grade 4 toxicities were noted during any part of the trial and no DLTs were noted in the fasted cohort of patients. As a result, 800 mg BID administered with food was declared the recommended phase II dose, as none of the seven patients enrolled at that dose level developed intolerable toxicity.
Mild gastrointestinal toxicities such as abdominal pain have been reported with OSI-461 but severe toxicities have not been seen (data on file at OSI Pharmaceuticals) [1, 35]. This finding is not unexpected as it is a common toxicity associated with other sulindac derivatives such as exisulind. Mild to moderate GI toxicities such has nausea, vomiting, anorexia, diarrhea and constipation have been commonly described in a number of clinical studies with exisulind alone or in combination with other anticancer agents in patients with FAP, prostate and lung cancer [9, 11, 13, 22, 26, 27, 33, 39, 42]. In most exisulind studies toxicities were noted at all dose levels but primarily occurred at higher doses. More specifically, mild to moderate abdominal pain has been associated with exisulind, with 13–15% of patients reported to have had reversible grade 3 toxicity [13, 33, 39, 42]. No relationship between abdominal pain and transaminase elevations associated with OSI-461 was seen. Of concern, fed patients at the 1,200 mg BID dose level were taking 24 pills daily (OSI-461 100 mg capsules). PK results were examined to determine if the DLTs were due to increased drug exposure or the large number of capsules ingested at this dose level. Based on the findings it appears that the DLTs observed in the 1,200 mg BID cohort (grade 3 abdominal pain, grade 3 constipation) are most likely a result of local effects of either the drug or the formulation excipients on the gastrointestinal tract and not the result of increased systemic exposure.
Metabolism of OSI-461 occurs in liver and preclinical studies were concerning for hepatotoxicity with repeated dosing at higher doses. In this study, significant hepatotoxicity was not of concern as transaminase and bilirubin elevations were predominantly mild to moderate and reversible in nature. Four of the six patients who experienced a grade 2 or more increase in bilirubin or liver enzyme elevations also had some form of liver or gallbladder cancer pathology and had transient elevations in either their transaminases and/or total bilirubin prior to study enrollment. There did not appear to be a significant amount of hepatotoxicity noted in patients enrolled in this study who had no known liver disease.
A prior study of OSI-461 demonstrated that Cmax and AUC values increased in an approximate linear fashion as the dose was increased from 100 to 400 mg BID with a significant amount of interpatient variability [35]. This was further substantiated by the fact that patients in fasted portion of this study who received OSI-461 showed substantial interpatient variability and no increase in peak plasma concentrations or systemic exposure with increasing doses. As the study was ongoing a PK study in dogs revealed an 11-fold median increase in OSI-461 AUC and a 16-fold increase in Cmax when OSI-461 was administered with food, suggesting that administration in the fed state may increase the overall bioavailability and improve the intrapatient variability of OSI-461 in patients. Thus the study was amended to evaluate the PKs in both the fasting and fed states. It is a known PK principal that food can alter the rate and/or the extent of absorption of drug. After comparing the AUC and Cmax in patients treated under the fed portion of this study, there was approximately a twofold increase in the AUC0–24 when OSI-461 was administered with food. Potential mechanisms for increased drug absorption as a result of food intake include the enhancement of drug solubility and a decrease in first-pass effect. The addition of a high-fat meal results in increased bile secretion capable of increasing drug solubility, increased lymphatic uptake resulting in decreased first-pass effect, slowed gastric emptying which prolongs the retention of the drug and increased intestinal motility [32]. Other anticancer agents whose bioavailability are increased with food include exemestane (1.6-fold higher), danazol (threefold higher), and retinods, such as fenretinide (3.4-fold higher) [2, 5, 38]. The effect of food on the relationship of PK parameters and pharmacodynamic response seen with OSI-461 potentially explains the increased gastrointestinal toxicity and the increases in pGSK-3β seen predominantly at higher dose levels. Food intake can minimize the variability of drug absorption. In this study the degree of the food effect seen at 400, 800 and 1,000 mg BID was not sustained in a linear fashion through the 1,200 mg BID dose level, suggesting that there was actually less exposure at higher dose levels, particularly in the fed state. Theoretically this inconsistency may be explained by chemical, pharmaceutical and metabolic properties of a drug. The degree by which the administered dose is solubilized by food and how these forms are presented to the absorption sites may lead to differences in PK variability [32]. The exact mechanism for decreased OSI-461 exposure is unknown, but may be a result of saturation of drug dissolution in gastrointestinal fluids. Nevertheless, there was a clear increase in the systemic exposure throughout all dose levels in the fed state when compared to the fasted state. A change in formulation may be able to overcome the PK challenges seen with OSI-461 and improve the drugs overall safety and efficacy.
Phospho-GSK-3β was chosen as a pharmacodynamic biomarker for OSI-461 because it is a substrate for the cyclic GMP-dependent protein kinase, PKG. The pharmacodynamic effect of OSI-461-mediated PKG activation is increased phosphorylation of PKG substrates, including pGSK-3β. The numbers of patients with a positive response increased as doses of OSI-461 were escalated showing a drug-related pharmacodynamic response with the greatest response near the MTD. These results suggest that increased pGSK-3β, a biomarker of drug effect, can be evaluated in surrogate tissue, and the predicted drug effect can be demonstrated.
Metabolism of OSI-461 is correlated with CYP3A activity and variability of this enzyme dependent drug clearance suggests that genetic mutations may be involved. A significant relationship between mean plasma concentrations of exisulind and grade 2 or greater toxicities has been observed. It was suggested that this association may be related to the biliary excretion of the drug and the hepatobiliary transport system. In response it has been proposed that future studies of exisulind and other structurally related analogs include an assessment of polymorphisms involved with this process [42]. As such, no CYP3A4 polymorphisms were found in any patient in this study and all patients analyzed were found to have the CYP3A5*3/CYP3A5*3 polymorphism, which is associated with a lack of protein expression that results in decreased metabolism of CYP3A substrates and may be present in up to 30% of Caucasians [15]. No relationship between these polymorphisms and the PK of OSI-461 was observed. Therefore subjects in this study should not have increased metabolism of OSI-461 or increased resistance to the drug.
Differences in gene and protein expression of the ABCG2 gene can be seen between resistant and wild-type parental cell lines and naturally occurring SNPs can alter the function and expression of ABCG2 [23]. Two known polymorphisms, G34 → A34 and C421 → A421, are found in Caucasians at a frequency of 4.7 and 25.0%, respectively [44]. Both mutations result in lower expression of BCRP and lower drug resistance. The occurrence of these two polymorphisms in our study patient population is consistent with the published mutation distribution among ethnic groups and there was no relationship between these polymorphisms and the PK of OSI-461.
In this phase I study, no objective responses were observed, although one patient with pancreatic adenocarcinoma in the 1,000 mg BID fasting cohort and one patient with hepatocellular carcinoma in the 400 mg BID fed cohort each achieved stable disease for eight cycles. Potential reasons for a lack of response may in part due to the PK variability of the drug, extensive prior therapy received by the patients and the wide range of tumor types studied. Stabilization of disease has also been noted in other phase I and II studies with OSI-461 in a variety of tumor types (colorectal, breast, CLL, renal cell, prostate, squamous cell of the parotid). Response has been seen in a single patient with CLL and chemical and radiological responses have been seen in patients with prostate cancer (data on file at OSI Pharmaceuticals) [35]
In summary, the results of this phase I and pharmacologic study demonstrate that OSI-461 administered with food in a twice-daily oral regimen results in a relatively mild toxicity profile and maintains sufficient plasma concentrations associated with preclinical activity. The dose recommended for subsequent disease-directed studies is 800 mg BID. Given the minimal antitumor activity observed in single-agent administration future studies of OSI-461 in combination with other mechanism based or chemotherapeutic agents should be explored.
Acknowledgments
Dr. O’Bryant and Dr. Lieu contributed equally to the writing of this manuscript. This study was supported by a grant from OSI Pharmaceuticals, Inc. (Boulder, CO, USA).
Abbreviations
- SAAND
Selective apoptotic antineoplastic drug
Contributor Information
C. L. O’Bryant, University of Colorado Cancer Center, Aurora, CO, USA
C. H. Lieu, University of Colorado Cancer Center, Aurora, CO, USA
S. Leong, University of Colorado Cancer Center, Aurora, CO, USA
R. Boinpally, OSI Pharmaceuticals, Inc., Boulder, CO, USA
M. Basche, University of Colorado Cancer Center, Aurora, CO, USA
L. Gore, University of Colorado Cancer Center, Aurora, CO, USA
K. Leonardi, University of Colorado Cancer Center, Aurora, CO, USA
M. K. Schultz, University of Colorado Cancer Center, Aurora, CO, USA
S. Hariharan, University of Colorado Cancer Center, Aurora, CO, USA
L. Chow, University of Colorado Cancer Center, Aurora, CO, USA
S. Diab, University of Colorado Cancer Center, Aurora, CO, USA
A. Gibbs, OSI Pharmaceuticals, Inc., Boulder, CO, USA
S. G. Eckhardt, University of Colorado Cancer Center, Aurora, CO, USA
References
- 1.Alila H, Finn TS, Sperl G, Worrells L, Lobacki J, Purvis J, Thompson WJ, Pamukcu R, Menander K. A pharmacokinetic and safety study of a selective apoptotic antineoplastic drug (SAAND), CP-461 in healthy volunteers. Proc Am Soc Clin Oncol. 2000 Abstract 817. [Google Scholar]
- 2.Charman WN, Rogge MC, Boddy AW, et al. Effect of food and a monoglyceride emulsion formulation on danazol bioavailability. J Clin Pharmacol. 1993;33:381–386. doi: 10.1002/j.1552-4604.1993.tb04673.x. [DOI] [PubMed] [Google Scholar]
- 3.Chiche JD, Schlutsmeyer SM, Bloch DB, et al. Adenovirus-mediated gene transfer of cGMP-dependent protein kinase increases the sensitivity of cultured vascular smooth muscle cells to the antiproliferative and pro-apoptotic effects of nitric oxide/cGMP. J Biol Chem. 1998;273:34263–34271. doi: 10.1074/jbc.273.51.34263. [DOI] [PubMed] [Google Scholar]
- 4.Deguchi A, Xing SW, Shureiqi I, Yang P, Newman RA, Lippman SM, Feinmark SJ, Oehlen B, Weinstein IB. Activation of protein kinase G up-regulates expression of 15-lipoxygenase-1 in human colon cancer cells. Cancer Res. 2005;65:8442–8447. doi: 10.1158/0008-5472.CAN-05-1109. [DOI] [PubMed] [Google Scholar]
- 5.Doose DR, Minn FL, Stellar S, et al. Effects of meals and meal composition on the bioavailability of fenretinide. J Clin Pharmacol. 1992;32:1089–1095. [PubMed] [Google Scholar]
- 6.Doyle LA, Yang W, Abruzzo LV, Krogmann T, Gao Y, Rishi AK, et al. A multidrug resistance transporter from human MCF-7 breast cancer cells. Proc Natl Acad Sci USA. 1998;95:15665–15670. doi: 10.1073/pnas.95.26.15665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Francis SH, Corbin JD. Cyclic nucleotide-dependent protein kinases: intracellular receptors for cAMP and cGMP action. Crit Rev Clin Lab Sci. 1999;36:275–328. doi: 10.1080/10408369991239213. [DOI] [PubMed] [Google Scholar]
- 8.Gibaldi M, Perrier D. Pharmacokinetics. 2nd edn. New York: Marcel Dekker; 1982. pp. 409–417. [Google Scholar]
- 9.Hoang T, Kim K, Merchant J, Traynor AM, McGovern J, Oettel KR, Sanchez FA, Ahuja HG, Hensing TA, Larson M, Schiller JH. Phase I/II study of gemcitabine and exisulind as second-line therapy in patients with advanced non-small cell lung cancer. J Thorac Oncol. 2006;1(3):218–225. doi: 10.1016/s1556-0864(15)31571-9. [DOI] [PubMed] [Google Scholar]
- 10.Imai Y, Nakane M, Kage K, Tsukahara S, Ishikawa E, Tsuruo T, Miki Y, Sugimoto Y. C421A polymorphism in the human breast cancer resistance protein gene is associated with low expression of Q141 K protein and low-level drug resistance. Mol Cancer Ther Jun. 2002;1(8):611–616. [PubMed] [Google Scholar]
- 11.Jones SF, Kuhn JG, Greco FA, Raefsky EL, Hainsworth JD, Dickson NR, Thompson DS, Willcutt NT, White MB, Burris HA., 3rd A phase I/II study of exisulind in combination with docetaxel/carboplatin in patients with metastatic non-small-cell lung cancer. Clin Lung Cancer. 2005;6(6):361–366. doi: 10.3816/clc.2005.n.016. [DOI] [PubMed] [Google Scholar]
- 12.Juilfs DM, et al. Cyclic GMP as substrate and regulator of cyclic nucleotide phosphodiesterases (PDEs) Rev Physiol Biochem Pharmacol. 1999;135:67–104. doi: 10.1007/BFb0033670. [DOI] [PubMed] [Google Scholar]
- 13.Kelly K, Mikhaeel N, Dempsey J, et al. A phase I study of exisulind in previously treated patients with lung cancer. Lung Cancer. 2000;29 suppl 1:76. [Google Scholar]
- 14.Kobayashi D, Ieiri I, Hirota T, Takane H, Maegawa S, Kigawa J, Suzuki H, Nanba E, Oshimura M, Terakawa N, Otsubo K, Mine K, Sugiyama Y. Functional assessment of ABCG2 (BCRP) gene polymorphisms to protein expression in human placenta. Drug Metab Dispos. 2005;33(1):94–101. doi: 10.1124/dmd.104.001628. [DOI] [PubMed] [Google Scholar]
- 15.Kuehl P, Zhang J, Lin Y, Lamba J, Assem M, Schuetz J, Watkins PB, Daly A, Wrighton SA, Hall SD, Maurel P, Relling M, Brimer C, Yasuda K, Venkataramanan R, Strom S, Thummel K, Boguski MS, Schuetz E. Sequence diversity in CYP3A promoters and characterization of the genetic basis of polymorphic CYP3A5 expression. Nat Genet. 2001;27(4):383–391. doi: 10.1038/86882. [DOI] [PubMed] [Google Scholar]
- 16.Lamba JK, Lin YS, Schuetz EG, Thummel KE. Genetic contribution to variable human CYP3A-mediated metabolism. Adv Drug Deliv Rev. 2002;54(10):1271–1294. doi: 10.1016/s0169-409x(02)00066-2. [DOI] [PubMed] [Google Scholar]
- 17.Li H, Liu L, David ML, Whitehead CM, Chen M, Fetter JR, Sperl GJ, Pamukcu R, Thompson WJ. Pro-apoptotic actions of exisulind and CP461 in SW480 colon tumor cells involve beta-catenin and cyclin D1 down-regulation. Biochem Pharmacol. 2002;64(9):1325–1336. doi: 10.1016/s0006-2952(02)01345-x. [DOI] [PubMed] [Google Scholar]
- 18.Lim JT, Piazza GA, Pamukcu R, Thompson WJ, Weinstein IB. Exisulind and related compounds inhibit expression and function of the androgen receptor in human prostate cancer cells. Clin Cancer Res. 2003;9:4972–4982. [PubMed] [Google Scholar]
- 19.Liu L, Li H, Underwood T, Lloyd M, David M, Sperl G, Pamukcu R, Thompson WJ. Cyclic GMP-dependent protein kinase activation and induction by exisulind and CP461 in colon tumor cells. J Pharmacol Exp Ther. 2001;299(2):583–592. [PubMed] [Google Scholar]
- 20.Loweth AC, Williams GT, Scarpello JH, Morgan NG. Evidence for the involvement of cGMP and protein kinase G in nitric oxide-induced apoptosis in the pancreatic B-cell line, HIT-T15. FEBS Lett. 1997;400:285–288. doi: 10.1016/s0014-5793(96)01392-0. [DOI] [PubMed] [Google Scholar]
- 21.Maliepaard M, van Gastelen MA, de Jong LA, Pluim D, van Waardenburg RC, Ruevekamp-Holmers MC, Floot BG, Schellens JH. Overexpression of the BCRP/MXR/ABCP gene in a topotecan-selected ovarian tumor cell line. Cancer Res. 1999;59:4559–4563. [PubMed] [Google Scholar]
- 22.Masters GA, Li S, Dowlati A, Madajewicz S, Langer C, Schiller J, Johnson D. A phase II trial of carboplatin and gemcitabine with exisulind (IND #65, 056) in patients with advanced non-small cell lung cancer: an Eastern Cooperative Oncology Group study (E1501) J Thorac Oncol. 2006;1(7):673–678. [PubMed] [Google Scholar]
- 23.Miyake K, Mickley L, Litman T, Zhan Z, Robey R, Cristensen B, Brangi M, Greenberger L, Dean M, Fojo T, Bates SE. Molecular Cloning of cDNAs which are highly overexpressed in mitoxantrone-resistant cells: demonstration of homology to ABC transporter genes. Cancer Res. 1999;59:8–13. [PubMed] [Google Scholar]
- 24.Piazza GA, Thompson WJ, Pamukcu R, Alila HW, Whitehead CM, Liu L, Fetter JR, Gresh WE, Jr, Klein-Szanto AJ, Farnell DR, Eto I, Grubbs CJ. Exisulind, a novel proapoptotic drug, inhibits rat urinary bladder tumorgenesis. Cancer Res. 2001;61:3961–3968. [PubMed] [Google Scholar]
- 25.Piazza GA, Xu S, Klein-Szanto AJ, Ahnen DJ, Li H, Liu L, David M, Pamukcu R, Thompson WJ. Overexpression of cGMP phosphodiesterase (cGPDE) in colonic neoplasias compare to normal mucosa. Gastroenterology. 2000;118:1590. [Google Scholar]
- 26.Pusztai L, Zhen JH, Arun B, Rivera E, Whitehead C, Thompson WJ, Nealy KM, Gibbs A, Symmans WF, Esteva FJ, Booser D, Murray JL, Valero V, Smith TL, Hortobagyi GN. Phase I and II study of exisulind in combination with capecitabine in patients with metastatic breast cancer. J Clin Oncol. 2003;21(18):3454–3461. doi: 10.1200/JCO.2003.02.114. [DOI] [PubMed] [Google Scholar]
- 27.Ryan CW, Stadler WM, Vogelzang NJ. A phase I/II dose-escalation study of exisulind and docetaxel in patients with hormone-refractory prostate cancer. BJU Int. 2005;95(7):963–968. doi: 10.1111/j.1464-410X.2005.05448.x. [DOI] [PubMed] [Google Scholar]
- 28.Schmit SM, Krause LA, Gordon DG, Harwell JE, Pamukcu R, Piazza GA. Growth inhibitory activity of FGN-1 and analogues in 13 human tumor lines of various histogenesis. Proc Am Assoc Cancer Res. 1998 Abstract 1331. [Google Scholar]
- 29.Shimizu M, Suzui M, Deguchi A, Lim JT, Xiao D, Hayes JH, Papadopoulos KP, Weinstein IB. Synergistic effects of acyclic retinoid and OSI-461 on growth inhibition and gene expression in human hepatoma cells. Clin Cancer Res. 2004;10:6710–6721. doi: 10.1158/1078-0432.CCR-04-0659. [DOI] [PubMed] [Google Scholar]
- 30.Shimojo T, Hiroe M, Ishiyama S, Ito H, Nishikawa T, Marumo F. Nitric oxide induces apoptotic death of cardiomyocytes via a cyclic-GMP-dependent pathway. Exp Cell Res. 1999;247:38–47. doi: 10.1006/excr.1998.4310. [DOI] [PubMed] [Google Scholar]
- 31.Singer AL, Sherwin RP, Dunn AS, Appleman MM. Cyclic nucleotide phosphodiesterases in neoplastic and nonneoplastic human mammary tissues. Cancer Res. 1976;36:60–66. [PubMed] [Google Scholar]
- 32.Singh BN, Malhotra BK. Effects of food on the clinical pharmacokinetics of anticancer agents: underlying mechanisms and implications for oral chemotherapy. Clin Pharmacokinet. 2004;43(15):1127–1156. doi: 10.2165/00003088-200443150-00005. [DOI] [PubMed] [Google Scholar]
- 33.Sinibaldi VJ, Elza-Brown K, Schmidt J, Eisenberger MA, Rosenbaum E, Denmeade SR, Pili R, Walczak J, Baker SD, Zahurak M, Carducci MA. Phase II evaluation of docetaxel plus exisulind in patients with androgen independent prostate carcinoma. Am J Clin Oncol. 2006;29(4):395–398. doi: 10.1097/01.coc.0000225411.95479.b4. [DOI] [PubMed] [Google Scholar]
- 34.Soh JW, Mao Y, Kim MG, Pamukcu R, Li H, Piazza GA, Thompson WJ, Weinstein IB. Cyclic GMP mediates apoptosis induced by sulindac derivatives via activation of c-Jun NH2-terminal kinase 1. Clin Cancer Res. 2000;6(10):4136–4141. (erratum in: Clin Cancer Res 6(12):4967) [PubMed] [Google Scholar]
- 35.Sun W, Stevenson JP, Gallo JM, Redlinger M, Haller D, Algazy K, Giantonio B, Alila H, O’Dwyer PJ. Phase I and pharmacokinetic trial of the proapoptotic sulindac analogue CP-461 in patients with advanced cancer. Clin Cancer Res. 2002;8:3100–3104. [PubMed] [Google Scholar]
- 36.Therasse P, Arbuck S, Eisenhauer E, et al. New guidelines to evaluate the response to treatment in solid tumors: European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92:205–216. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 37.Thompson WJ, Piazza GA, Li H, Liu L, Fetter J, Zhu B, Spel G, Ahnen D, Pamukcu R. Exisulind induction of apoptosis involves guanosine 3′, 5′-cyclic monophosphate phosphodiesterase inhibition, protein kinase G activation and attenuated beta-catenin. Cancer Res. 2000;60:3338–3342. [PubMed] [Google Scholar]
- 38.Valle M, Di Salle E, Jannuzzo MG, Poggesi I, Rocchetti M, Spinelli R, Verotta D. A predictive model for exemestane pharmacokinetics/pharmacodynamics incorporating the effect of food and formulation. Br J Clin Pharmacol. 2005;59(3):355–364. doi: 10.1111/j.1365-2125.2005.02335.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.van Stolk R, Stoner G, Hayton WL, Chan K, DeYoung B, Kresty L, Kemmenoe BH, Elson P, Rybicki L, Church J, Provencher K, McLain D, Hawk E, Fryer B, Kelloff G, Ganapathi R, Budd GT. Phase I trial of exisulind (sulindac sulfone, FGN-1) as a chemopreventive agent in patients with familial adenomatous polyposis. Clin Cancer Res. 2000;6(1):78–89. [PubMed] [Google Scholar]
- 40.Whitehead CM, Earle KA, Xu S, Hartman T, Chan D, Zhao T. CP461 in an orthotopic human NSCLC rat Model involves phosphodiesterase targeting, apoptosis induction, G2/M block and antiproliferation. Proc Am Assoc Cancer Res. 2002 Abstract 4577. [Google Scholar]
- 41.Whitehead CM, Fetter J, Xu S, Hartman T, Sperl G, Pamukcu R, Thompson WJ. CP461, a pro-apoptotic inhibitor of cGMP phosphodiesterases (PDE), disrupts normal microtubule organization and bipolar spindle formation leading to a prometaphase mitotic block. Proc Am Assoc Cancer Res. 2002 Abstract 2043. [Google Scholar]
- 42.Witta SE, Gustafson DL, Pierson AS, Menter A, Holden SN, Basche M, Persky M, O’Bryant CL, Zeng C, Baron A, Long ME, Gibbs A, Kelly K, Bunn PA, Jr, Chan DC, Pallansch P, Eckhardt SG. A phase I and pharmacokinetic study of exisulind and docetaxel in patients with advanced solid tumors. Clin Cancer Res. 2004;10(21):7229–7237. doi: 10.1158/1078-0432.CCR-03-0181. [DOI] [PubMed] [Google Scholar]
- 43.Xiao D, Deguchi A, Gundersen GG, Oehlen B, Arnold L, Weinstein IB. The sulindac derivatives OSI-461, OSIP486823, and OSIP487703 arrest colon cancer cells in mitosis by causing microtubule depolymerization. Mol Cancer Ther. 2006;5(1):60–67. doi: 10.1158/1535-7163.MCT-05-0260. [DOI] [PubMed] [Google Scholar]
- 44.Zamber CP, Lamba JK, Yasuda K, Farnum J, Thummel K, Schuetz JD, Schuetz EG. Natural allelic variants of breast cancer resistance protein (BCRP) and their relationship to BCRP expression in human intestine. Pharmacogenetics. 2003;1:19–28. doi: 10.1097/00008571-200301000-00004. [DOI] [PubMed] [Google Scholar]
- 45.Zhu B, Lakshmi V, Thompson WJ, Strada S. Suppression of cyclic GMP-specific phosphodiesterase 5 promotes apoptosis and inhibits growth in HT29 cells. J Cell Biochem. 2004;94(2):336–350. doi: 10.1002/jcb.20286. [DOI] [PubMed] [Google Scholar]