Abstract
Purpose
The purpose of this study was to determine whether cholesterol, the host cell receptor for pneumolysin of Streptococcus pneumoniae, could effectively treat pneumococcal keratitis.
Methods
New Zealand White rabbits were intrastromally injected with 105 colony-forming units (CFUs) of S. pneumoniae D39. Corneas were treated with topical drops of 1% cholesterol every 2 hours beginning 25 hours after infection and were examined by slit lamp microscopy 24, 36, and 48 hours after infection. Rabbits were killed, and CFUs were recovered from the corneas after the final slit lamp examination (SLE). Minimal inhibitory concentration (MIC) assays of cholesterol against bacteria were performed. Specific inhibition of pneumolysin by cholesterol in the rabbit cornea was tested by intrastromal injection of pneumolysin with or without cholesterol and was compared with cholesterol inhibition of pneumolysin in vitro using hemolysis assays with rabbit erythrocytes.
Results
Corneas treated with cholesterol had significantly lower SLE scores 48 hours after infection than corneas treated with vehicle (P = 0.0015). Treated corneas also had significantly less log10 CFUs than vehicle-treated corneas (P = 0.0006). Cholesterol at a 1% concentration was bactericidal to bacteria in vitro, and lower concentrations of cholesterol were partially inhibitory in a concentration-dependent manner. Cholesterol also specifically inhibited 1 μg pneumolysin in vivo and up to 50 ng pneumolysin in vitro.
Conclusions
Topical cholesterol is an effective treatment for S. pneumoniae keratitis. Cholesterol not only inhibits pneumolysin, it is also bactericidal.
Bacterial infections of the cornea occur in approximately 25,000 people in the United States each year and in a variable number of people worldwide. Streptococcus pneumoniae often occurs as one of the top three species causing bacterial keratitis.1–16 Antibiotics are routinely used to treat ocular pneumococcal infections; however, this bacterium is developing resistance to antibiotics.17,18 Identification of the ocular virulence factors of this organism and development of new therapies to treat pneumococcal keratitis could prove useful for the prevention of loss of vision.
One of the key virulence factors of S. pneumoniae is the toxin pneumolysin, which belongs to the family of pore-forming cholesterol-dependent cytolysins (CDCs) including perfrin-golysin, streptolysin, and listeriolysin.19 The binding of cholesterol and the ability to form pores in host cell membranes are major characteristics of the members of this group of toxins.20–23 In addition to its cytolytic function, pneumolysin activates host complement, resulting in inflammation mediated by polymorphonuclear leukocytes (PMNs). The cytolytic and complement-activating functions of pneumolysin are associated with different domains of the toxin.24–27
Pneumolysin is important for the inflammation associated with pneumococcal keratitis. Johnson et al.28 produced a pneumolysin-deficient strain of S. pneumoniae and showed that this strain was defective in causing the full effects of keratitis compared with the parent strain. Restoration of the gene for pneumolysin allowed this strain to recover its full virulence.29 The major clinical difference between the parent or rescued strain and the pneumolysin-deficient strain was the degree of inflammation, especially polymorphonuclear leukocyte (PMN) infiltration into the cornea. When the complement activation domain of the pneumolysin gene was deleted from S. pneumoniae, corneal virulence was also significantly reduced, but to a lesser extent than when the entire gene was deleted.30 These results suggested that the complement activation domain of pneumolysin contributed to corneal virulence, though this domain was not fully responsible for the damage caused by the toxin.
Binding of pneumolysin by exogenous cholesterol, the target site for pneumolysin binding, has been shown to inhibit the cytolytic function of the toxin.31,32 If the activity of pneumolysin is important in keratitis, then cholesterol could have therapeutic value by providing an excess of toxin-binding sites and inhibiting the binding of pneumolysin to host cells. In this study, soluble cholesterol was used as a topical treatment for S. pneumoniae keratitis to determine whether this treatment could inhibit the effects of pneumolysin in the cornea and thus could ameliorate the damage associated with pneumococcal keratitis.
Methods
Bacterial Strains and Growth Conditions
S. pneumoniae D39 (Item 6302; American Type Culture Collection, Manassas, VA) was cultured on blood agar base (Oxoid; Remel, Inc., Lenexa, KS) containing 5% sheep blood (Hemostat Laboratories, Dixon, CA) at 37°C and routinely grown in Todd Hewitt broth supplemented with 0.5% yeast extract (THY; BD Biosciences, Sparks, MD) at 37°C, unless otherwise indicated. Subcultures of bacteria were grown to concentrations of 108 colony-forming units (CFUs)/mL and were diluted in THY broth to achieve an inoculum of 105 CFUs/10 μL for intrastromal injections. Tenfold dilutions of the inoculum were cultured in triplicate on 5% sheep blood agar to verify the number of CFUs.
Rabbit Corneal Infections and Treatment with Cholesterol
New Zealand White rabbits were used in these studies and were maintained in accordance with the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research. The Institutional Animal Care and Use Committee of the University of Mississippi Medical Center approved the animal protocol. Rabbits were anesthetized by subcutaneous injections of a mixture of xylazine (100 mg/mL; Butler Company, Columbus, OH) and ketamine hydrochloride (100 mg/mL; Fort Dodge Animal Health, Fort Dodge, IA). Proparacaine hydrochloride (Falcon Pharmaceuticals, Fort Worth, TX) was topically applied to each eye before intrastromal injection. S. pneumoniae D39 (105 CFUs/10 μL) was injected intrastromally. Topical treatment of rabbit eyes was initiated at 25 hours and continued at 2-hour intervals up to 47 hours after infection. Treatment consisted of one drop of a solution of 1% soluble cholesterol (40 mg pure cholesterol/g, as determined by HPLC, complexed with methyl-β-cyclodextrin; Sigma-Aldrich, St. Louis, MO) and 20% glycerol in PBS in each of 11 eyes and one drop of a solution of 20% glycerol in PBS (control solution) in each of 10 eyes.
Two observers, masked as to the identity of the rabbit groups, used a biomicroscope (Topcon; Koaku Kikai K.K., Tokyo, Japan) to perform slit lamp examination (SLE) of infected rabbit corneas 24, 36, and 48 hours after infection. Seven ocular parameters were graded: injection, chemosis, iritis, fibrin, hypopyon, corneal edema, and corneal infiltrate.28 Each parameter was given a grade from 0 (normal) to 4 (very severe). Grades were totaled for each eye, and an average was calculated for the two observers, resulting in an overall score for each eye ranging from 0 (normal) to a theoretical maximum of 28. Scores were expressed as the mean ± SEM.
Forty-eight hours after infection, the rabbits were killed by intravenous overdose of sodium pentobarbital (100 mg/mL; Sigma-Aldrich). Corneas were removed, dissected, and homogenized in sterile PBS using a tissue homogenizer (IKA Works, Inc., Wilmington, NC). Aliquots of corneal homogenates were serially diluted and plated in triplicate on blood agar for 48 hours at 37°C. Colonies were counted, and bacterial CFUs for the corneas were determined and expressed in base 10 logarithm ± SEM. These experiments were performed twice, yielding similar results, and the data from the two experiments were combined (n = 11 corneas for cholesterol-treated rabbits; n = 10 corneas for PBS-treated control rabbits).
Cholesterol Inhibition of S. pneumoniae In Vitro
Cholesterol was tested for possible inhibition of S. pneumoniae D39 using the broth macrodilution method of the Clinical and Laboratory Standards Institute.33 S. pneumoniae colonies were isolated on 5% sheep blood agar and resuspended in cation-adjusted Mueller-Hinton broth containing 5% lysed horse blood to a McFarland turbidity standard of 0.5. Bacteria were adjusted to 1 × 106 CFU/mL and were incubated for 20 hours at 37°C with an equal volume of PBS, PBS containing 20% glycerol, or 2-fold dilutions of cholesterol in PBS containing 20% glycerol. Absorbance at 625 nm was determined for each sample, and 10-fold dilutions of each sample were plated in triplicate on 5% sheep blood agar. Three separate experiments yielded similar results.
Injection of Pneumolysin into Rabbit Corneas
Recombinant pneumolysin was purified as described previously,34 and its purity was confirmed by SDS-PAGE. After systemic anesthesia with ketamine and xylazine and topical anesthesia with proparacaine, pneumolysin in PBS containing 20% glycerol (1 μg, 500 ng, 100 ng, 50 ng, 10 ng, 5 ng, and 0 ng) or pneumolysin mixed 1:1 (vol/vol) with 2% cholesterol in PBS containing 20% glycerol (final concentration of 1% cholesterol) was injected into rabbit corneas. Corneas were examined periodically for 48 hours by two observers, corneal erosions were stained with fluorescein and measured, and photographs were taken with the aid of a slit lamp microscope.
Neutralization of Pneumolysin In Vitro
Neutralization of pneumolysin by cholesterol was determined by a hemolysis assay performed in triplicate. Fifty microliters of pneumolysin in PBS at a concentration of 500 ng, 100 ng, 50 ng, 10 ng, 5 ng, or 0 ng were mixed and incubated with 100 μL PBS containing 20% glycerol or 100 μL of 2% cholesterol in PBS containing 20% glycerol in round-bottom wells of 96-well plates. Rabbit erythrocytes were washed and suspended to 5% with PBS, and then 50 μL suspension was added to each well. The plates were incubated for 30 minutes at 37°C and then placed at 4°C until intact erythrocytes settled into the bottoms of the wells. Supernatants were placed into wells of flat-bottom, 96-well plates, and the absorbance was taken at 450 nm. Erythrocytes incubated with PBS containing 20% glycerol and erythrocytes incubated with 2% cholesterol in PBS containing 20% glycerol served as negative controls and as background subtraction for the appropriate wells. Erythrocytes incubated with 0.5% saponin and PBS containing 20% glycerol served as a 100% lysis control.
Statistical Analysis
Data were analyzed using the statistical analysis system (SAS) for computers (SAS Institute, Cary, NC). Clinical scores were analyzed using nonparametric one-way analysis of variance. Bacterial loads were analyzed using the general linear models procedure of least squares means. P < 0.05 was considered significant.
Results
Treatment of Experimental Pneumococcal Keratitis
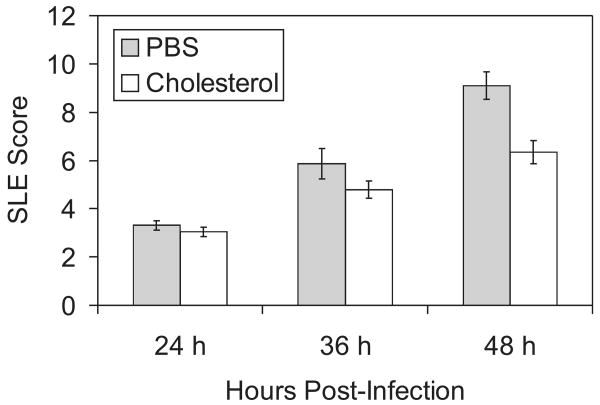
Cholesterol was tested as a treatment for experimental pneumococcal keratitis. Rabbit eyes that received topical cholesterol drops after intrastromal injections of S. pneumoniae had significantly lower SLE scores 48 hours after infection than eyes that received drops of the control solution (Fig. 1; P = 0.0015). Control eyes exhibited a larger amount of corneal infiltrate and opacity than treated eyes (Fig. 2). The parameters contributing to the significant difference in SLE scores between control eyes and treated eyes were presence of hypopyon (average 3-fold difference), fibrin (2.3-fold difference), stromal edema (1.9-fold difference), iritis (1.5-fold difference), and stromal infiltrate (1.5-fold difference). Control eyes had an average SLE score of approximately 9 at 48 hours after infection, though the theoretical maximum is 28; this result was attributed to some of the parameters being graded on a percentage coverage scale. For example, the hypopyon shown in Figure 2B (left panel) covers approximately 1/16 of the eye, so the parameter grade for the hypopyon was assigned 0.25 out of a possible 4.0. Cholesterol-treated corneas also had significantly lower log CFU compared with control eyes (2.64 ± 0.50 vs. 5.35 ± 0.42; P = 0.0006). Administration of topical drops of cholesterol to uninfected eyes had no effect (not shown).
Figure 1.
SLE scores of infected corneas treated with cholesterol or placebo. Rabbit corneas, infected with S. pneumoniae and then treated with either PBS (control) or 1% cholesterol in PBS, were examined by SLE. Corneas were examined 24, 36, and 48 hours after infection. Corneas treated with cholesterol had significantly lower SLE scores than control corneas 48 hours after infection. Data are mean ± SEM.
Figure 2.
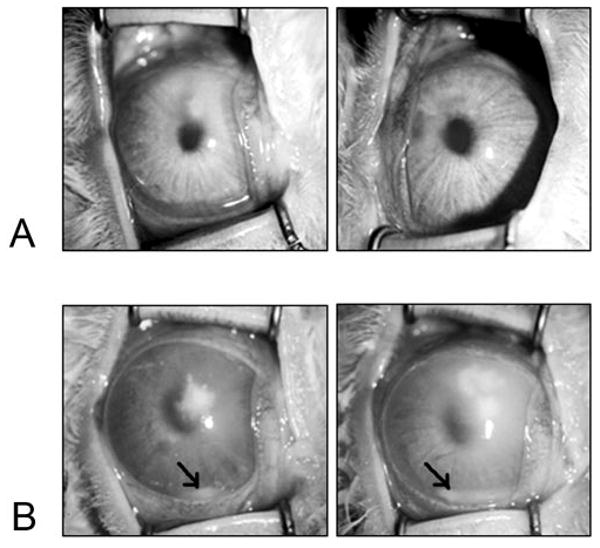
Infected eyes after treatment with cholesterol or placebo. Rabbit corneas were photographed 48 hours after infection. Corneas were treated with 1% cholesterol in PBS (A) or PBS alone as a control (B). Control corneas had more infiltrate and showed hypopyon formation (arrows).
Cholesterol Inhibition of S. pneumoniae In Vitro
The possible growth inhibition of S. pneumoniae by cholesterol was examined to determine whether the significant difference in CFU recovered from infected rabbit corneas could have resulted from the inhibition of bacterial growth by the topical cholesterol treatments (Table 1). Optical density measurements after minimal inhibitory concentration assay were confusing in that the optical densities of the bacteria incubated with the two lowest concentrations of cholesterol were lower than the optical densities of bacteria incubated with higher concentrations of cholesterol or with no cholesterol. These optical density measurements suggested that small amounts of cholesterol inhibited bacterial growth, whereas high concentrations of cholesterol had little or no effect. To resolve these unexpected results, the minimal inhibitory concentration assay was repeated, and the bacteria were serially diluted and plated in triplicate. Colony counts of the resultant CFUs showed that the cholesterol was bactericidal in a concentration-dependent manner; that is, as the concentration of cholesterol increased, the CFUs of the bacteria decreased (Table 1). Glycerol was also tested for possible effects because it was present in all the cholesterol formulations for this study. The CFUs of the bacteria incubated in broth containing 20% glycerol in PBS were similar to the CFUs of bacteria incubated in broth containing PBS alone, indicating that glycerol had no effect on bacterial growth.
Table 1.
Effect of Cholesterol on S. pneumoniae In Vitro
| Sample Incubated with Bacteria | CFU/mL* |
|---|---|
| PBS | 2.86 × 107 |
| PBS + 20% glycerol | 4.99 × 107 |
| 2% cholesterol | 140 |
| 1% cholesterol | 0 |
| 0.5% cholesterol | 6.93 × 102 |
| 0.25% cholesterol | 1.96 × 103 |
| 0.125% cholesterol | 1.29 × 104 |
| 0.063% cholesterol | 3.33 × 104 |
Cholesterol was prepared in PBS containing 20% glycerol.
CFU/mL data are from 1 of 3 representative experiments.
Specific Inhibition of Pneumolysin by Cholesterol In Vivo
To test whether the ability of topical cholesterol to significantly reduce the clinical symptoms of pneumococcal keratitis could be attributed to specific inhibition of pneumolysin, purified pneumolysin in the presence or absence of cholesterol was intrastromally injected into rabbit corneas. At concentrations of 500 ng or lower, pneumolysin had no effect on the corneas (data not shown). At a concentration of 1 μg, however, pneumolysin caused corneal epithelial erosions beginning between 2 and 5 hours and continuing until 24 hours after infection (Figs. 3A, 3B). The erosions in the corneas receiving pneumolysin began to heal 24 hours after injection, as evidenced by a re-epithelialization of the cornea and diffuse fluorescein staining (Figs. 3A, 3B). Pneumolysin mixed with cholesterol did not cause erosions until 24 hours after injection (Figs. 3C, 3D). Pneumolysin alone caused corneal erosions that were 6 mm in diameter, whereas pneumolysin mixed with cholesterol caused corneal erosions 4 mm in diameter. All corneas were fully healed by 48 hours after injection. Corneas injected with cholesterol alone became slightly hazy at the site of injection; this haze faded with time (48 hours), and no other changes in the corneas were observed.
Figure 3.
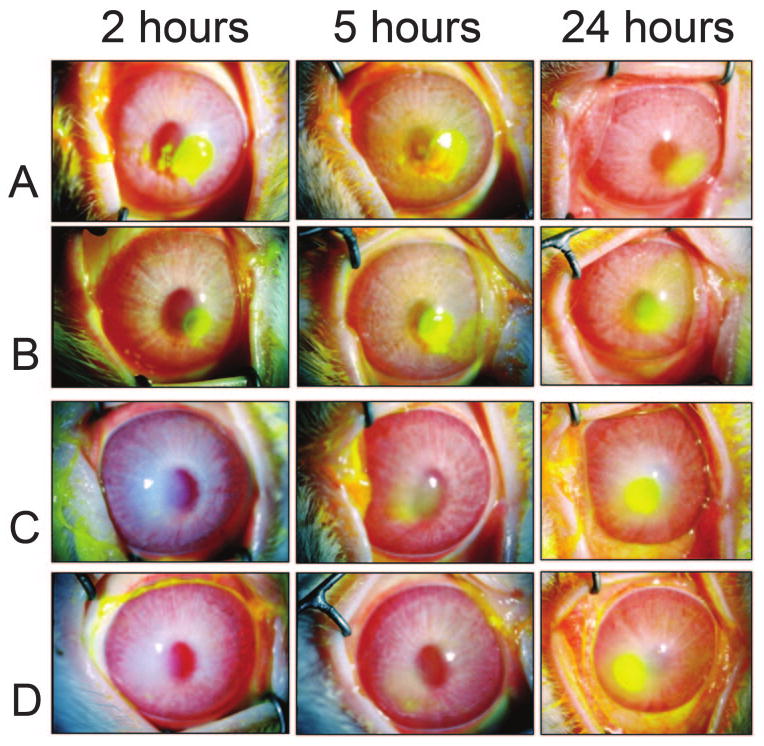
Cholesterol inhibition of erosions caused by pneumolysin. Rabbit corneas were injected with pneumolysin (A, B) or pneumolysin mixed with cholesterol (C, D). The presence of corneal erosions is highlighted by fluorescein staining, which is readily apparent 2 and 5 hours after injection in the corneas injected with pneumolysin (A, B) and 24 hours after injection in the corneas injected with pneumolysin mixed with cholesterol (C, D).
Specific Inhibition of Pneumolysin by Cholesterol In Vitro
The potency of the specific cholesterol formulation used in this study was quantified in vitro. Pneumolysin preparations at concentrations of 10 ng or lower did not lyse rabbit erythrocytes, but concentrations of 50 ng and greater caused at least 81% lysis (Fig. 4). Cholesterol at a concentration of 1% inhibited the hemolytic activity of 50 ng pneumolysin by 92% (6.43% hemolysis vs. 81.17% hemolysis) and 100 ng pneumolysin by 42% (47.50% hemolysis vs. 82.13% hemolysis). Cholesterol did not inhibit 500 ng pneumolysin at this concentration (Fig. 4).
Figure 4.
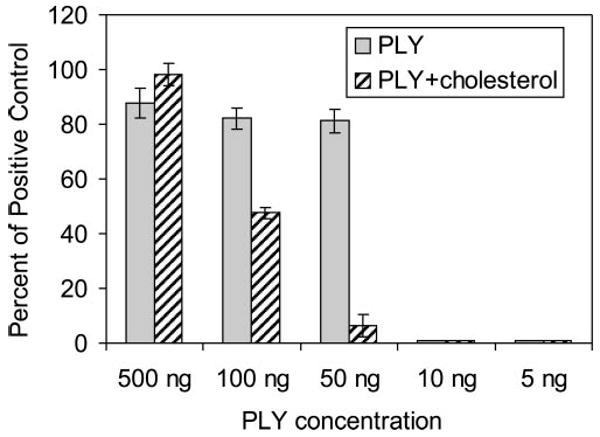
Cholesterol inhibition of hemolysis by pneumolysin. The hemolytic activity of pneumolysin (PLY) was inhibited by cholesterol. Different concentrations of pneumolysin, or pneumolysin mixed with 1% cholesterol, were incubated with rabbit erythrocytes. Lysis was measured by spectrophotometric analysis of supernatants after incubation and was compared with a saponin control that represented 100% lysis. Data are mean ± SD.
Discussion
A novel treatment for S. pneumoniae keratitis, based on bactericidal activity and the specific inhibition of the major ocular toxin pneumolysin by its cellular receptor (cholesterol), has been introduced in the present study. The binding of pneumolysin to cholesterol35 and the ability of exogenous cholesterol to inhibit the hemolytic activity of pneumolysin31,32 have long been known. The actual application of this knowledge in the treatment of pneumococcal disease, however, has not been reported until now. Cholesterol was able to inhibit corneal epithelial erosion formation by pneumolysin, alleviate the clinical symptoms of S. pneumoniae keratitis, and kill S. pneumoniae in the cornea.
Cholesterol not only targeted pneumolysin, it also targeted the bacteria by killing them. This effect was shown in vivo by a significant decrease in CFUs in the corneas that were treated with cholesterol and in vitro by a minimal inhibitory concentration assay. Minimal inhibitory concentration assays of cholesterol against S. pneumoniae should be performed with caution because the optical density measurements were not representative of actual CFUs (Table 1). It is recommended that cultures be plated for actual CFU counts after the assay.
The reduction in SLE scores by treatment with cholesterol was statistically significant. Considering the specificity of cholesterol for pneumolysin, however, the reduction would be expected to be more dramatic than it actually was. One explanation for the lack of dramatic reduction could be that the cholesterol did not inhibit the complement activation activity of pneumolysin. Alternatively, S. pneumoniae virulence factors other than pneumolysin could be responsible for some ocular disease parameters. Another possibility is that the cholesterol formulation used in this study was complexed with methyl-β-cyclodextrin, a cholesterol synthesis inhibitor, to make it soluble (Sigma-Aldrich). The presence of a cholesterol synthesis inhibitor could decrease the in vivo cellular production of cholesterol in the cornea, offsetting the benefits of treatment with exogenous cholesterol. It is expected that the use of a purer cholesterol formulation for treatment would cause a more dramatic reduction in SLE score, provided the cholesterol could be suspended in a feasible form for treatment purposes.
The inhibition of pneumolysin by cholesterol in the cornea (Fig. 3) was different from the in vitro inhibition (Fig. 4). First, nanogram concentrations of pneumolysin were not sufficient to cause corneal epithelial erosions; 1 μg was the concentration shown to cause erosions. In the hemolysis assay, however, nanogram concentrations of the same pneumolysin preparation were able to cause hemolysis of rabbit red blood cells. Second, 1% cholesterol was able to inhibit erosion formation by 1 μg pneumolysin for up to 24 hours in vivo, whereas 1% cholesterol was incapable of inhibiting the hemolytic activity of 500 ng pneumolysin in vitro. The differences could have been the result of possible differences in the host cells (corneal cells vs. red blood cells). In vivo and in vitro results, however, showed that cholesterol specifically inhibited pneumolysin.
The two functions of pneumolysin, cytolysis and complement activation, have been reported to be localized to specific domains of the toxin.24–27 However, deletion of only the complement activation domain of the pneumolysin gene causes a reduction, but not a complete disappearance, of corneal virulence, and this reduction is not as extensive as when the entire gene is deleted.30 These results suggest that the complement activation domain of pneumolysin contributes to corneal virulence, though this domain is not fully responsible for the damage caused by the toxin.
Pneumolysin activates the classical complement pathway,25,36–38 resulting in the production of proinflammatory cytokines. Interleukin (IL)-8 is a chemoattractant for neutrophils and has been shown to be upregulated in inflamed human corneas39 and is upregulated in monocytes in response to pneumolysin.40 The induction of IL-6 by pneumolysin in non-ocular model systems of S. pneumoniae infection has been observed.41 Interestingly, a mutant pneumolysin molecule with reduced cytolytic activity had decreased ability to induce IL-6, suggesting that the domain involved in cytolytic activity was involved in proinflammatory cytokine induction.41
The binding of pneumolysin to cholesterol causes a change in the secondary structure of pneumolysin22 rendering it unable to form pores in host cell membranes. Paton et al.25 showed that preincubation of pneumolysin with cholesterol did not affect the ability of pneumolysin to convert complement protein C3. Based on this information, an ideal treatment for S. pneumoniae keratitis would be cholesterol combined with an inhibitor of pneumolysin complement activation activity. Blocking complement activation is expected to result in a decrease in the inflammation that is the hallmark of pneumococcal keratitis. Use of cholesterol to treat pneumococcal keratitis has been demonstrated herein as a viable alternative or an adjunct therapy and could offer a means to cope with the increasing frequency and complexity of antibiotic resistance demonstrated by S. pneumoniae.
Acknowledgments
The authors thank William Johnson for his consultation in using the appropriate statistics for this study.
Supported by Intramural Research Support Program, University of Mississippi Medical Center (MEM).
Footnotes
Disclosure: M.E. Marquart, P; K.S. Monds, None; C.C. McCormick, None; S.N. Dixon, None; M.E. Sanders, None; J.M. Reed, None; L.S. McDaniel, None; A.R. Caballero, None; R.J. O'Callaghan, P
References
- 1.Asbell P, Stenson S. Ulcerative keratitis: survey of 30 years' laboratory experience. Arch Ophthalmol. 1982;100:77–80. doi: 10.1001/archopht.1982.01030030079005. [DOI] [PubMed] [Google Scholar]
- 2.Clinch TE, Palmon FE, Robinson MJ, Cohen EJ, Barron BA, Laibson PR. Microbial keratitis in children. Am J Ophthalmol. 1994;117:65–71. doi: 10.1016/s0002-9394(14)73016-8. [DOI] [PubMed] [Google Scholar]
- 3.Cruz OA, Sabir SM, Capo H, Alfonso E. Microbial keratitis in childhood. Ophthalmology. 1993;100:192–196. doi: 10.1016/s0161-6420(93)31671-4. [DOI] [PubMed] [Google Scholar]
- 4.Dunlop AA, Wright ED, Howlader SA, et al. Suppurative corneal ulceration in Bangladesh: a study of 142 cases examining the microbiological diagnosis, clinical and epidemiological features of bacterial and fungal keratitis. Aust N Z J Ophthalmol. 1994;22:105–110. doi: 10.1111/j.1442-9071.1994.tb00775.x. [DOI] [PubMed] [Google Scholar]
- 5.Gonawardena SA, Ranasinghe KP, Arseculeratne SN, Seimon CR, Ajello L. Survey of mycotic and bacterial keratitis in Sri Lanka. Mycopathologia. 1994;127:77–81. doi: 10.1007/BF01103062. [DOI] [PubMed] [Google Scholar]
- 6.Gudmundsson OG, Ormerod L, Kenyon KR, et al. Factors influencing predilection and outcome in bacterial keratitis. Cornea. 1989;8:115–121. [PubMed] [Google Scholar]
- 7.Hagan M, Wright E, Newman M, Dolin P, Johnson G. Causes of suppurative keratitis in Ghana. Br J Ophthalmol. 1995;79:1024–1028. doi: 10.1136/bjo.79.11.1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kunimoto DY, Sharma S, Reddy MK, et al. Microbial keratitis in children. Ophthalmology. 1998;105:252–257. doi: 10.1016/s0161-6420(98)92899-8. [DOI] [PubMed] [Google Scholar]
- 9.Liesegang TJ, Forster RK. Spectrum of microbial keratitis in South Florida. Am J Ophthalmol. 1980;90:38–47. doi: 10.1016/s0002-9394(14)75075-5. [DOI] [PubMed] [Google Scholar]
- 10.Ormerod LD, Hertzmark E, Gomez DS, Stabiner RG, Schanzlin DJ, Smith RE. Epidemiology of microbial keratitis in southern California: a multivariate analysis. Ophthalmology. 1987;94:1322–1333. doi: 10.1016/s0161-6420(87)80019-2. [DOI] [PubMed] [Google Scholar]
- 11.Parmar P, Salman A, Kalavathy CM, Jesudasan CA, Thomas PA. Pneumococcal keratitis: a clinical profile. Clin Exp Ophthalmol. 2003;31:44–47. doi: 10.1046/j.1442-9071.2003.00598.x. [DOI] [PubMed] [Google Scholar]
- 12.Schaefer F, Bruttin O, Zografos L, Guex-Crosier Y. Bacterial keratitis: a prospective clinical and microbiological study. Br J Ophthalmol. 2001;85:842–847. doi: 10.1136/bjo.85.7.842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Srinivasan M, Gonzales CA, George C, et al. Epidemiology and aetiological diagnosis of corneal ulceration in Madurai, South India. Br J Ophthalmol. 1997;81:965–971. doi: 10.1136/bjo.81.11.965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tan DT, Lee CP, Lim AS. Corneal ulcers in two institutions in Singapore: analysis of causative factors, organisms and antibiotic resistance. Ann Acad Med Singapore. 1995;24:823–829. [PubMed] [Google Scholar]
- 15.Upadhyay MP, Karmacharya PC, Koirala S, et al. Epidemiologic characteristics, predisposing factors, and etiologic diagnosis of corneal ulceration in Nepal. Am J Ophthalmol. 1991;111:92–99. doi: 10.1016/s0002-9394(14)76903-x. [DOI] [PubMed] [Google Scholar]
- 16.Williams G, Billson F, Husain R, Howlader SA, Islam N, McClellan K. Microbiological diagnosis of suppurative keratitis in Bangladesh. Br J Ophthalmol. 1987;71:315–321. doi: 10.1136/bjo.71.4.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Soriano F, Perez-Trallero E, Pallares R, et al. Streptococcus pneumoniae endophthalmitis: a study of 36 cases with special reference to antibiotic resistance and treatment options. Clin Microbiol Infect. 2006;12:519–526. doi: 10.1111/j.1469-0691.2006.01418.x. [DOI] [PubMed] [Google Scholar]
- 18.Wilkins J, Whitcher JP, Margolis TP. Penicillin-resistant Streptococcus pneumoniae keratitis. Cornea. 1996;15:99–100. [PubMed] [Google Scholar]
- 19.Tweten RK. Cholesterol-dependent cytolysins, a family of versatile pore-forming toxins. Infect Immun. 2005;73:6199–6209. doi: 10.1128/IAI.73.10.6199-6209.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Baba H, Kawamura I, Kohda C, et al. Essential role of domain 4 of pneumolysin from Streptococcus pneumoniae in cytolytic activity as determined by truncated proteins. Biochem Biophys Res Commun. 2001;281:37–44. doi: 10.1006/bbrc.2001.4297. [DOI] [PubMed] [Google Scholar]
- 21.Bonev BB, Gilbert RJ, Andrew PW, Byron O, Watts A. Structural analysis of the protein/lipid complexes associated with pore formation by the bacterial toxin pneumolysin. J Biol Chem. 2001;276:5714–5719. doi: 10.1074/jbc.M005126200. [DOI] [PubMed] [Google Scholar]
- 22.Kelly SJ, Jedrzejas MJ. Structure and molecular mechanism of a functional form of pneumolysin: a cholesterol-dependent cytolysin from Streptococcus pneumoniae. J Struct Biol. 2000;132:72–81. doi: 10.1006/jsbi.2000.4308. [DOI] [PubMed] [Google Scholar]
- 23.Palmer M, Vulicevic I, Saweljew P, Valeva A, Kehoe M, Bhakdi S. Streptolysin O: a proposed model of allosteric interaction between a pore-forming protein and its target lipid bilayer. Biochemistry. 1998;37:2378–2383. doi: 10.1021/bi9720890. [DOI] [PubMed] [Google Scholar]
- 24.Jounblat R, Kadioglu A, Mitchell TJ, Andrew PW. Pneumococcal behavior and host responses during bronchopneumonia are affected differently by the cytolytic and complement-activating activities of pneumolysin. Infect Immun. 2003;71:1813–1819. doi: 10.1128/IAI.71.4.1813-1819.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Paton JC, Rowan-Kelly B, Ferrante A. Activation of human complement by the pneumococcal toxin pneumolysin. Infect Immun. 1984;43:1085–1087. doi: 10.1128/iai.43.3.1085-1087.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rubins JB, Charboneau D, Fasching C, et al. Distinct roles for pneumolysin's cytotoxic and complement activities in the pathogenesis of pneumococcal pneumonia. Am J Respir Crit Care Med. 1996;153:1339–1346. doi: 10.1164/ajrccm.153.4.8616564. [DOI] [PubMed] [Google Scholar]
- 27.Rubins JB, Janoff EN. Pneumolysin: a multifunctional pneumococcal virulence factor. J Lab Clin Med. 1998;131:21–27. doi: 10.1016/s0022-2143(98)90073-7. [DOI] [PubMed] [Google Scholar]
- 28.Johnson MK, Hobden JA, Hagenah M, O'Callaghan RJ, Hill JM, Chen S. The role of pneumolysin in ocular infections with Streptococcus pneumoniae. Curr Eye Res. 1990;9:1107–1114. doi: 10.3109/02713689008997584. [DOI] [PubMed] [Google Scholar]
- 29.Johnson MK, Hobden JA, O'Callaghan RJ, Hill JM. Confirmation of the role of pneumolysin in ocular infections with Streptococcus pneumoniae. Curr Eye Res. 1992;11:1221–1225. doi: 10.3109/02713689208999547. [DOI] [PubMed] [Google Scholar]
- 30.Johnson MK, Callegan MC, Engel LS, et al. Growth and virulence of a complement-activation-negative mutant of Streptococcus pneumoniae in the rabbit cornea. Curr Eye Res. 1995;14:281–284. doi: 10.3109/02713689509033527. [DOI] [PubMed] [Google Scholar]
- 31.Canvin JR, Paton JC, Boulnois GJ, Andrew PW, Mitchell TJ. Streptococcus pneumoniae produces a second haemolysin that is distinct from pneumolysin. Microb Pathogenesis. 1997;22:129–132. doi: 10.1006/mpat.1996.0098. [DOI] [PubMed] [Google Scholar]
- 32.Johnson MK, Allen JH. Ocular toxin of the pneumococcus. Am J Ophthalmol. 1971;72:175–180. doi: 10.1016/0002-9394(71)91610-2. [DOI] [PubMed] [Google Scholar]
- 33.Clinical and Laboratory Standards Institute. Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically: Approved Standard. 7th. Wayne, PA; 2006. [Google Scholar]
- 34.Thornton J, McDaniel LS. THP-1 monocytes up-regulate intercellular adhesion molecule 1 in response to pneumolysin from Streptococcus pneumoniae. Infect Immun. 2005;73:6493–6498. doi: 10.1128/IAI.73.10.6493-6498.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Johnson MK, Geoffroy C, Alouf JE. Binding of cholesterol by sulfhydryl-activated cytolysins. Infect Immun. 1980;27:97–101. doi: 10.1128/iai.27.1.97-101.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Boulnois GJ, Paton JC, Mitchell TJ, Andrew PW. Structure and function of pneumolysin, the multifunctional, thiol-activated toxin of Streptococcus pneumoniae. Mol Microbiol. 1991;5:2611–2616. doi: 10.1111/j.1365-2958.1991.tb01969.x. [DOI] [PubMed] [Google Scholar]
- 37.Mitchell TJ, Andrew PW, Saunders FK, Smith AN, Boulnois GJ. Complement activation and antibody binding by pneumolysin via a region of the toxin homologous to a human acute-phase protein. Mol Microbiol. 1991;5:1883–1888. doi: 10.1111/j.1365-2958.1991.tb00812.x. [DOI] [PubMed] [Google Scholar]
- 38.Yuste J, Botto M, Paton JC, Holden DW, Brown JS. Additive inhibition of complement deposition by pneumolysin and PspA facilitates Streptococcus pneumoniae septicemia. J Immunol. 2005;175:1813–1819. doi: 10.4049/jimmunol.175.3.1813. [DOI] [PubMed] [Google Scholar]
- 39.Spandau UH, Toksoy A, Verhaart S, Gillitzer R, Kruse FE. High expression of chemokines Gro-alpha (CXCL-1), IL-8 (CXCL-8), and MCP-1 (CCL-2) in inflamed human corneas in vivo. Arch Ophthalmol. 2003;121:825–831. doi: 10.1001/archopht.121.6.825. [DOI] [PubMed] [Google Scholar]
- 40.Rogers PD, Thornton J, Barker KS, et al. Pneumolysin-dependent and -independent gene expression identified by cDNA microarray analysis of THP-1 human mononuclear cells stimulated by Streptococcus pneumoniae. Infect Immun. 2003;71:2087–2094. doi: 10.1128/IAI.71.4.2087-2094.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rijneveld AW, van den Dobbelsteen GP, Florquin S, et al. Roles of interleukin-6 and macrophage inflammatory protein-2 in pneumolysin-induced lung inflammation in mice. J Infect Dis. 2002;185:123–126. doi: 10.1086/338008. [DOI] [PubMed] [Google Scholar]