Abstract
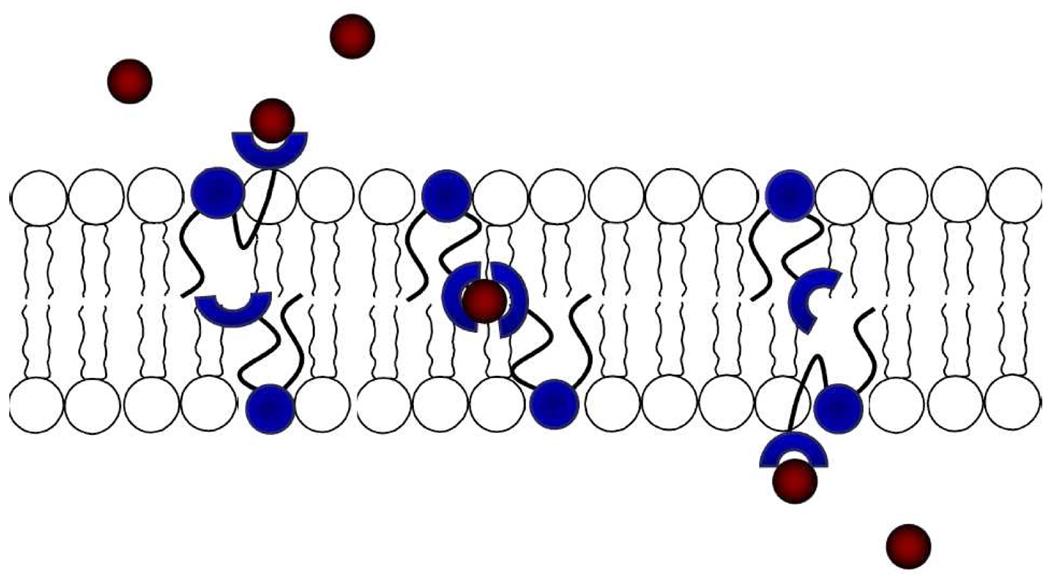
A new type of synthetic membrane transporter is described and shown to operate in vesicles by a relay mechanism. The transporter structure is a phosphatidylcholine derivative with a urea group appended to the end of its sn-2 acyl chain. The urea can bind a chloride ion at the membrane surface via hydrogen bonds and then relay it through the bilayer interior to an acceptor molecule located in the opposite membrane leaflet. Three phosphatidylcholine derivatives were studied and transport rates increased with transporter affinity for chloride. The results of various controls studies are consistent with an anion counter transport process using a relay mechanism and a kinetically active aggregate of two or four transporter molecules. Transport is inhibited if the transporter resides in only one leaflet of the membrane, if the bilayer is too thick, and if the counter anion is sulfate dianion. The expected favorable formulation properties of these amphiphilic compounds should facilitate efforts to transform them into tools for biomedical research and perhaps as therapeutic agents.
Synthetic bilayer membrane transporters are usually classified mechanistically as mobile carriers or as ion channels.1 A mobile carrier associates with a target ion to form a discrete supramolecular complex that diffuses across the membrane; whereas, an ion channel is a relatively immobile structure that spans the bilayer and allows a continuous flow of ions.2 In recent years there has been increased effort to design synthetic membrane transport systems for anions, especially Cl−.3 One of the long-term goals of this work is to create transporter replacement therapies that can alleviate the symptoms of diseases caused by diminished levels of endogenous Cl− transport (e.g., cystic fibrosis).4 The field of anion transport is still in its early stages with most published studies focusing on fundamental transport studies using model bilayer membranes. In terms of transporter designs, nearly all have been highly lipophilic compounds that partition strongly and non-selectively into any membrane.5 However, next-generation designs must begin to address the requirements for pharmaceutical success, including the following formulation features: (a) acceptable solubility in physiological solution, (b) appropriate cell targeting and subsequent membrane partitioning, (c) lengthy residence time in the apical plasma membrane of target cells. Suitably designed amphiphilic transporters are likely to exhibit these desirable properties, however, it is quite challenging to design amphiphilic transporters that operate by carrier or ion channel mechanisms. This quandary has prompted us to design a new type of membrane transporter that operates by a relay mechanism.6
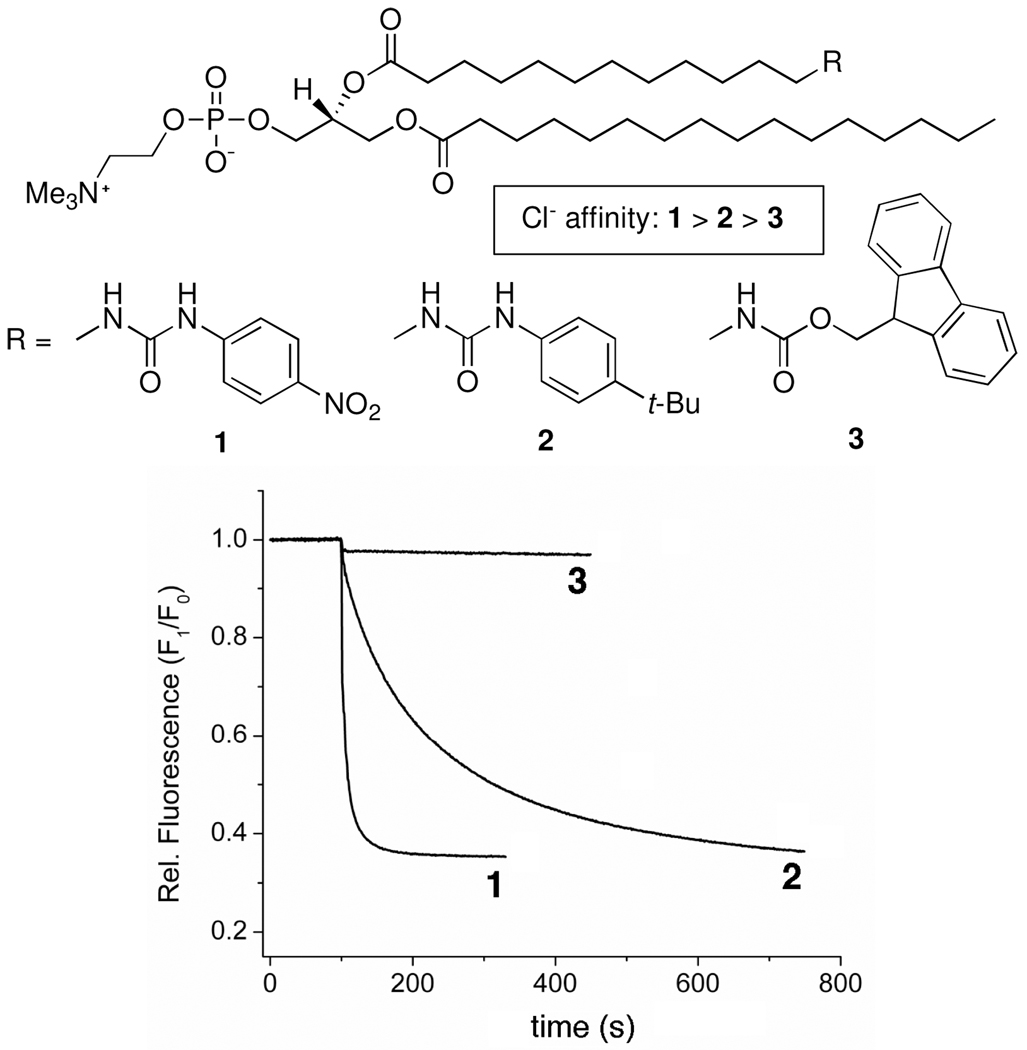
A generalized picture of the relay transport process is shown in Scheme 1. The transporter structure is a phospholipid derivative with an ionophore appended to the end of its sn-2 acyl chain.7 The ionophore can bind an ion at the membrane surface and then relay it through the bilayer interior to an acceptor molecule located in the opposite leaflet.8 In this initial study, the ionophore is a simple urea group that can associate with Cl− via hydrogen bonds.9 The phosphatidylcholine derivatives 1–3 were synthesized in a few steps and high yield using established procedures.10 Transporter 1 contains a 4-nitrophenylurea group with a relatively high affinity for Cl−, transporter 2 contains a weaker binding 4-tert-butylphenylurea group, and carbamate derivative 3 is a control structure with very weak Cl- affinity.11
Scheme 1.
Relay mechanism for dimeric transporter aggregate.
The ability of compounds 1–3 to transport Cl− into vesicles was measured using a standard fluorescence quenching assay.12 Briefly, compounds 1–3 were pre-incorporated into separate samples of unilamellar vesicles composed of 1-palmitoyl-2-oleoylphosphatidylcholine (POPC):cholesterol (7:3 molar ratio, diameter 200 nm) and encapsulating the chloride sensitive fluorescent probe lucigenin. Addition of NaCl to the vesicle dispersions induces Cl− influx and quenching of the lucigenin fluorescence. The traces in Figure 1 clearly show that transporter 1 is superior to 2, whereas control 3 is essentially inactive. This trend of stronger anionophore producing enhanced transport has been reported before with a separate class of mobile carriers for Cl− that also utilize urea groups.13
Figure 1.
Fluorescence quenching due to Cl− influx. At 100 s, an aliquot of NaCl (25 mM final conc.) was added to vesicles encapsulating the chloride sensitive probe, lucigenin (1 mM) and NaNO3 (225 mM), T = 25 °C. The vesicle membranes were composed of POPC:cholesterol (7:3) and either 1, 2, or 3 (5 mol %).
In order to elucidate the transport mechanism, a series of additional experiments were conducted with the most effective transporter, 1. The first experiment demonstrated that replacing the intravesicle NaNO3 with an isomolar concentration of Na2SO4 produced a greatly diminished rate of Cl− influx (see Figure S1 in the SI). This is consistent with an anion exchange process; that is, significant Cl− influx can only occur if there is a corresponding counter anion efflux, which is greatly diminished with the heavily solvated SO42–.3
The relay mechanism in Scheme 1 implies that the transporter must reside in both leaflets of the bilayer. This condition was met in the initial experiment which employed vesicles with 1 pre-incorporated in the membrane. However, no Cl− transport was observed when the experiment was repeated with one variation, external addition of 1 to preformed vesicles (see Figure S2 in the SI for details). In this case, the polar lipid 1 inserts into the outer leaflet of the vesicle membrane (confirmed by UV absorption) and does not migrate to the inner leaflet. To be effective, transporter 1 needs to populate both sides of the bilayer membrane.
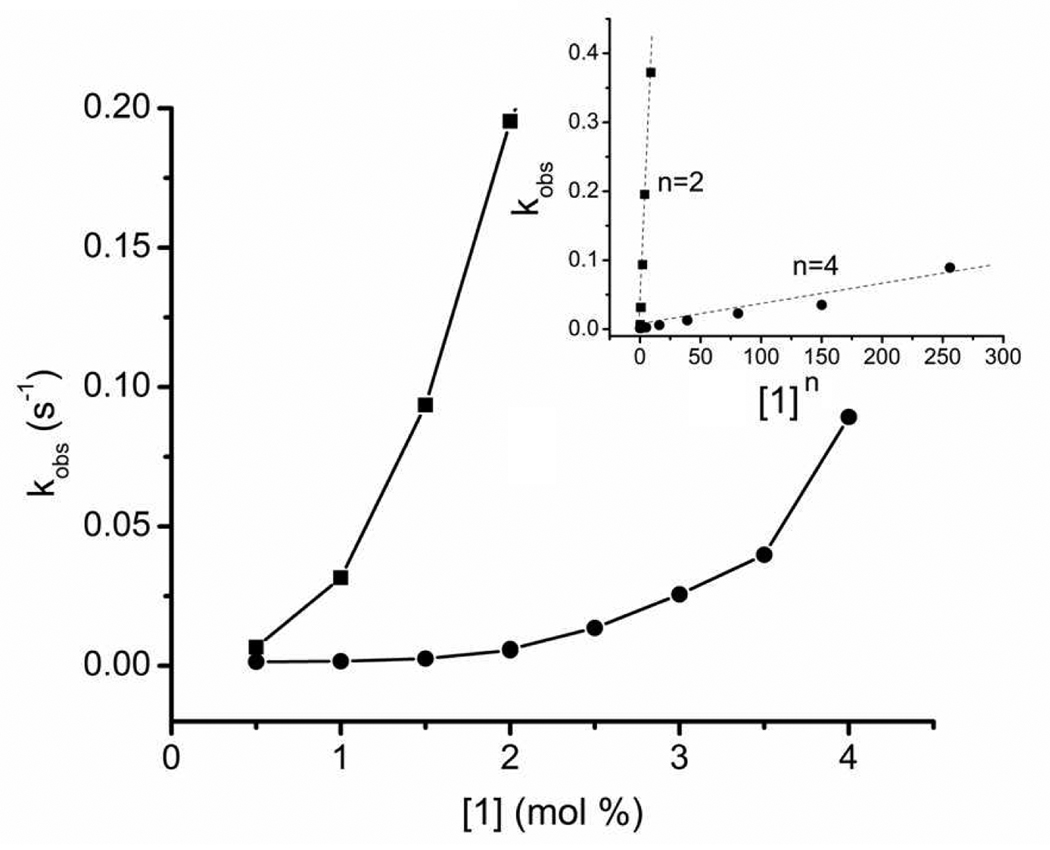
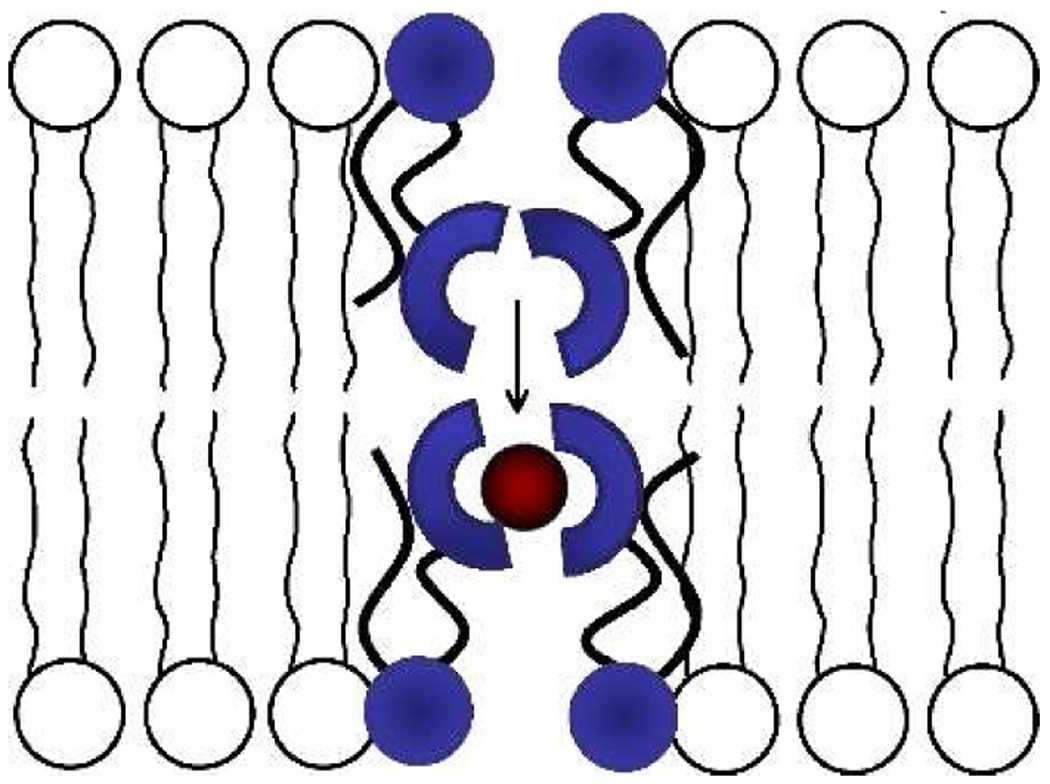
The dependence of observed Cl− influx rate constants (kobs) on transporter concentration was determined in two vesicle systems with membranes of different compositions and thickness (i.e., 1,2-dimyristoylphosphatidylcholine (DMPC):cholesterol (7:3) and the thicker POPC:cholesterol (7:3)). In both cases, the curves were non-linear (Figure 2) indicating that transport is mediated by kinetically active aggregates of 1. Furthermore, linear relationships are obtained for the two membrane compositions when kobs is plotted against [1]n, where n = 2 and 4, respectively.14 Thus, the transporter aggregate number is two for the DMPC:cholesterol membrane which is consistent with the slightly overlapped tail-to-tail dimer shown in Scheme 1. An aggregation of four in the thicker POPC:cholesterol membrane membrane suggests that a pair of transporters are in each leaflet as shown in Scheme 2. An increased transporter aggregation number in thicker membranes has been seen before with self-assembled pore systems.15
Figure 2.
Rate constants (kobs) for Cl− influx at different concentrations of 1 in vesicles composed of DMPC:cholesterol (7:3) (ν) and POPC:cholesterol (7:3) (λ), T = 25 °C. Inset: linear relationships with [1]n, where n = 2 and 4, respectively.
Scheme 2.
Relay mechanism for transporter aggregate of four.
The final mechanistic study with 1 measured Cl− influx rates as a function of vesicle membrane thickness. Transport was monitored in vesicles composed of phospholipids with increasing acyl chain length, and thus increased membrane thickness.15,16 Figure S3 in the SI shows that increasing the acyl chain carbon number from 14 to 18 produced an incremental decrease in transport rate. Significantly, when the acyl carbon number was increased to 20 and above there was a dramatic drop to essentially zero transport. This membrane thickness threshold effect is consistent with the relay mechanism and not with the two alternatives.17 When the membrane is relatively thin, the transporters can effectively relay Cl− across the lipophilic core of the membrane as shown in Scheme 1 and Scheme 3. Once the membrane is thicker than the tail-to-tail aggregate in Scheme 2 (whose polar head groups are anchored to their respective membrane interfaces), there is a gap between the urea groups in each leaflet and the energetic barrier for Cl− relay becomes prohibitively high.
In summary, we report a new class of synthetic membrane transporters whose molecular structures are phospholipids with anionophores appended to the end of the sn-2 acyl chain. The current design uses urea groups to bind and transport Cl−, however, it should be possible to employ other molecular recognition units to produce transporters that are selective for other anions, as well as cations and neutral polar molecules. Mechanistic studies indicate that the transporters operate by a new and distinct membrane relay process. The expected favorable formulation properties of these amphiphilic compounds (e.g., as liposomes, micelles, etc) should facilitate efforts to transform them into tools for biomedical research and perhaps as therapeutic agents.
Supplementary Material
Acknowledgement
This work was supported by the NIH and the University of Notre Dame.
Footnotes
Supporting Information Available: Synthetic procedures, transport experiments and data. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.(a) Stein WD. Channels, Carriers and Pumps, An Introduction to Membrane Transport. San Diego: Academic; 1990. [Google Scholar]; (b) Smith BD, Lambert TN. Chem. Comm. 2003:2261–2268. doi: 10.1039/b303359g. [DOI] [PubMed] [Google Scholar]
- 2.Recent reviews of synthetic transporters: Gokel GW, Carasel IA. Chem. Soc. Rev. 2007;36:378–389. doi: 10.1039/b605910b. Sakai N, Mareda J, Matile S. Mol. Biosys. 2007;10:658–666. doi: 10.1039/b704684g. Fyles TM. Chem. Soc. Rev. 2007;36:335–347. doi: 10.1039/b603256g. McNally BA, Leevy WM, Smith BS. Supramol. Chem. 2007;19:29–37. doi: 10.1080/10610270600902332. Koert U, Al-Momani L, Pfeifer JR. Synthesis. 2004;8:112911–112946.
- 3. Davis AP, Sheppard DN, Smith BD. Chem. Soc. Rev. 2007:348–357. doi: 10.1039/b512651g. and references therein.
- 4.Ashcroft FM. Ion Channels and Disease. London: Academic Press; 2000. [Google Scholar]
- 5.Notable exceptions are: (a) The water soluble peptide transporters designed by Tomich and coworkers. For a leading reference, see: Shank LP, Broughman JR, Takeguchi W, Cook G, Robbins AS, Hahn L, Radke G, Iwamoto T, Schultz BD, Tomich JM. Biophys, J. 2006;90:2138–2150. doi: 10.1529/biophysj.105.070078. (b) The amphiphilic peptide transporters designed by Gokel and coworkers. For a leading reference, see: Elliott EK, Stine KJ, Gokel GW. J. Membr. Sci. 2008;321:43–50. doi: 10.1016/j.memsci.2008.01.048.
- 6.For studies of relay transport processes in much thicker synthetic plasticized membranes, see: Riggs JA, Smith BD. J. Am. Chem. Soc. 1997;119:2765–2766. White KM, Duggan PJ, Sheahan SL, Tyndall EM, Smith BD. J. Membr. Sci. 2001;194:165–175.
- 7.For membrane active phospholipids with simple acyl chain modifications, see: Menger FM, Aikens P. Angew. Chem. Int. Ed. 1992;31:898–900. Menger FM, Galloway AL, Chlebowski ME, Wu S. J. Am. Chem. Soc. 2006;128:14034–14035. doi: 10.1021/ja065702o.
- 8.It is well established that a modestly polar group appended to the end of a phospholipids acyl chain undergoes substantially dynamic movement between the lipophilic membrane interior and the polar membrane surface. Huster D, Muller P, Arnold K, Herrmann Biophys. J. 2001;80:822–831. doi: 10.1016/S0006-3495(01)76061-4. Loura LMS, Ramalho JPP. Biochim. Biophys. Acta – Biomembranes. 2007;1768:467–478. doi: 10.1016/j.bbamem.2006.10.011. Menger FM, Keiper JS, Caran KL. J. Am. Chem. Soc. 2002;124:11842–11843. doi: 10.1021/ja020838h.
- 9.McNally BA, Koulov AV, Lambert TN, Smith BD, Joos J-B, Sisson AL, Clare JP, Sgarlata V, Judd LW, Magro G, Davis AP. Chem.-Eur. J. 2008;14:9599–9606. doi: 10.1002/chem.200801163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.(a) Roseto R, Hajdu J. Tetrahedron Lett. 2005;46:2941–2944. [Google Scholar]; (b) Lampkins AJ, O’Neil EJ, Smith BD. J. Org. Chem. 2008;73:6053–6058. doi: 10.1021/jo8011336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.The Cl− association constant for N-(4-nitrophenyl)-N’-octyl urea in water saturated chloroform is ~8,000 M−1 and ~5,000 M−1 for N-(4-tert-butylphenyl)-N’-octyl urea as determined by the method in reference 9.
- 12.McNally BA, Koulov AV, Smith BD, Joos JB, Davis AP. Chem. Comm. 2005:1087–1089. doi: 10.1039/b414589e. [DOI] [PubMed] [Google Scholar]
- 13.Koulov AV, Lambert TN, Shukla R, Jain M, Boon JM, Smith BD, Li HY, Sheppard DN, Joos JB, Clare JP, Davis AP. Angew, Chem. Int. Ed. 2003;42:4931–4933. doi: 10.1002/anie.200351957. [DOI] [PubMed] [Google Scholar]
- 14.Otto S, Osifchin M, Regen SL. J. Am. Chem. Soc. 1999;121:7276–7277. [Google Scholar]
- 15.DiGiogio AF, Otto S, Bandyopadhyaya P, Regen SL. J. Am. Chem. Soc. 2000;122:11029–11030. [Google Scholar]
- 16.Lewis BA, Engelman DM. J. Mol. Biol. 1983;166:211–217. doi: 10.1016/s0022-2836(83)80007-2. [DOI] [PubMed] [Google Scholar]
- 17.Membrane thickness studies with a classic mobile carrier like valinomycin report a moderate ten fold decrease in transport rate as the acyl chain carbon number is increased from 16 to 22 ( Benz R, Frohlich O, Läuger P. Biochim. Biophys. Acta. 1977;464:465–481. doi: 10.1016/0005-2736(77)90023-2. ). With channel transport processes there is a bell-shaped relationship with membrane thickness; optimal transport is observed when the membrane thickness matches the length of the channel structure ( Weber ME, Schlesinger PH, Gokel GW. J. Am. Chem. Soc. 2005;127:636–642. doi: 10.1021/ja044936+.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.