Abstract
Background
Human studies have suggested an important relationship between ethanol sensitivity and risk of alcoholism. These studies have led some to hypothesize that a low initial sensitivity to ethanol’s depressant effects and/or an elevated response to ethanol’s stimulant effects may represent important risk factors associated with the development of abusive drinking behavior. Unfortunately, elucidating neurobiological mechanisms that may underlie these relationships between ethanol sensitivity and ethanol drinking have been hampered by difficulties in modeling some of these interactions in animals. In this study, we re-examined some of these relationships in an outbred strain of rats using continuous access two-bottle choice drinking and a limited access operant procedure that engenders pharmacologically relevant levels of ethanol intake and permits the discrete assessment of appetitive and consummatory measures of ethanol drinking behavior.
Methods
Twenty-three male Long-Evans rats were habituated to a locomotor activity box and then tested for their response to a stimulant (0.5 g/kg) and depressant (1.5 g/kg) ethanol dose. Rats were then trained to complete a lever pressing requirement to gain access to 10% ethanol for 20 min. sessions conducted five days/week for five weeks. Appetitive behavior was assessed after 2.5 and 4.5 weeks using 20 min. extinction trials in which ethanol was not presented and lever responses were recorded. Home-cage ethanol preference was also assessed prior to, and immediately following, the five week self-administration regimen using a continuous access, two-bottle choice procedure.
Results
A significant increase in home-cage ethanol preference was observed following the self-administration procedure however neither measure of ethanol preference correlated with average daily ethanol intake during the operant self-administration sessions or with initial sensitivity to ethanol’s stimulant or depressant effects. Notably, a significant negative correlation was observed between sensitivity to ethanol’s locomotor depressant effect and daily intake during the operant self-administration sessions. No significant relationships were noted between sensitivity to ethanol’s locomotor effects and extinction responding.
Conclusions
The results of these studies suggest that the well-established relationship between a low level of response to ethanol and increased ethanol consumption reported in human studies can be observed in an outbred rodent strain using a limited access operant self-administration procedure, but not with home-cage ethanol drinking.
INTRODUCTION
A number of human studies have reported an important relationship between initial sensitivity to ethanol and subsequent vulnerability for an alcohol use disorder (AUD). Although the nature of this relationship has been somewhat controversial, in general most studies have suggested that a higher initial sensitivity to the stimulant or euphoric effects of ethanol and/or a lower response to ethanol’s depressant effects may represent significant risk factors for the development of an AUD.
For example, several studies have shown that a lower response to a range of behavioral and physiological measures of intoxication (e.g. subjective assessment, body sway, cortisol response) is associated with an increased risk of the development of alcohol-related problems later in life (Erblich and Earleywine, 1999; Pollock, 1992; Schuckit, 1994; Schuckit and Smith, 1996). Notably, although this relationship is most evident in individuals with a family history of alcoholism, it has also been observed in family history negative subjects (Schuckit, 1994; Schuckit and Smith, 1996). Other studies have demonstrated that family history positive individuals (Newlin and Thomson, 1990; Newlin and Thomson, 1999) or heavy/binge drinkers (Holdstock et al., 2000; King et al., 2002) may exhibit greater euphoric or stimulant effects of ethanol, particularly at low doses or on the rising phase of the blood ethanol curve. Collectively, these studies suggest that behavioral and physiological measures of alcohol sensitivity may represent endophenotypes associated with increased risk of alcoholism.
Although there has been considerable interest in identifying the specific neurobiological mechanisms that may underlie the relationship between individual differences in response to ethanol and ethanol drinking behaviors, these efforts have been hampered, to some extent, by difficulties in modeling some of these relationships in animals. For example, an early correlative analysis in eight inbred lines of rats reported a significant negative relationship between ethanol preference and initial sensitivity to ethanol-mediated loss of righting reflex (LORR)(Spuhler and Deitrich, 1984), however a similar relationship was not observed in a study of four outbred rat strains which assessed initial sensitivity to ethanol-induced hypothermia and motor impairment (Khanna et al., 1990).
More recently, many studies have examined the relationship between initial sensitivity and ethanol drinking using lines of rats and mice that were originally bred for differences in one of these behaviors. In general, the results of these studies do not consistently reflect the relationships between initial sensitivity and ethanol drinking that have been reported in human studies. For example, FAST and SLOW mice are lines that were selectively bred for differences in locomotor stimulant response to an i.p. injection of ethanol (1.5–2.0 g/kg)(Crabbe et al., 1987; Phillips et al., 1991). FAST mice are much more sensitive to the locomotor stimulant effects of ethanol than SLOW mice, and based on the human literature, would be expected to drink more. Although in one study FAST mice drank more ethanol in a single bottle paradigm and displayed higher ethanol preference ratios than SLOW mice (Risinger et al., 1994), another study reported no line differences using an operant ethanol self-administration procedure (# trials completed, blood ethanol levels) (Sanchez et al., 1996). Long Sleep (LS) and Short Sleep (SS) mice were selected for differences in loss of righting reflex in response to a soporific dose of ethanol (Erwin et al., 1976). While, based on human studies, SS mice would be expected to readily consume ethanol, both lines exhibited relatively low levels of ethanol intake in home-cage two-bottle preference tests (Collins, 1981) and LS mice actually consumed greater amounts of ethanol in a limited-access operant paradigm (Elmer et al., 1990). Similar mixed results were also observed in Low (LAS) and High Alcohol Sensitive (HAS) lines of rats that were selected based on criteria similar to those used for SS and LS mice. Although LAS rats are almost four times less sensitive to ethanol-induced sleep time than HAS rats, these selected lines did not exhibit any differences in initial measures of home-cage ethanol preference or intake. Interestingly, as predicted by the human literature, LAS rats did consume more ethanol in an operant procedure. However their intake in the operant procedure was no different from that of standard outbred strains and was significantly lower than intake levels observed in a line of rats selected for high alcohol intake (Files et al., 1996).
Similar experimental approaches have been used to examine ethanol sensitivity in lines of rats and mice bred for differences in home-cage ethanol preference (Colombo et al., 2000; Le and Kiianmaa, 1988; Murphy et al., 2002; Tampier and Mardones, 1999) or intake in a limited-access procedure (Shram et al., 2004). Again, several of these studies have not observed the relationships between behavioral response to ethanol and ethanol drinking behavior noted in human studies. For example, Sardinian alcohol-preferring rats were selected based on high and low home-cage ethanol preference (Colombo et al., 2000). Although sP rats consume > 6 g/kg/day and sNP rats less than 0.5 g/kg/day, sP rats were significantly more sensitive to the depressant effects of ethanol (LORR, ataxia). Notably, sP rats do appear to be more sensitive to the locomotor stimulant effect of ethanol (Agabio et al., 2001).
Interestingly, one line of alcohol-preferring rats does exhibit a relationship between initial sensitivity and intake similar to that observed in human studies. Alcohol-preferring P rats and non-preferring nP rats, also selectively bred on the basis of home-cage preference (Murphy et al., 2002), have been extensively studied and P rats have been validated as a rodent model of alcoholism. For example, P rats voluntarily consume ethanol for its pharmacological effects, develop tolerance and dependence following long-term self-administration, and exhibit a dramatically elevated willingness to work for ethanol in an operant paradigm (reviewed in Murphy et al., 2002). Notably, P rats exhibit greater sensitivity to the low dose stimulatory effects of ethanol than nP rats (Waller et al., 1986) and are also less sensitive to the depressant effects of moderate to high doses of ethanol across a range of measures including loss of righting reflex (Kurtz et al., 1996), impairment on a jump test (Lumeng et al., 1982) or oscillating bar (Suwaki et al., 2001), and hypothermia (Stewart et al., 1992).
Taken together, the result of animal studies provide somewhat mixed evidence in support of the relationships between initial ethanol sensitivity and drinking behavior that have been reported in human studies. There are, however, numerous genetic, environmental, and experimental factors that could potentially account for the observed disparities between the human and rodent studies noted above. One important factor may relate to the models of ethanol drinking that have commonly been employed in animal studies. With the exception of rodent lines that have been selectively bred for high ethanol preference, most strains of rats do not voluntarily consume pharmacologically relevant doses of ethanol, particularly in continuous access, home-cage preference tests. Thus, it may be difficult to observe a relationship between initial ethanol sensitivity and ethanol intake in a drinking paradigm in which subjects do not necessarily consume ethanol for its post-ingestional effects. Notably, alcohol preferring P rats, which do voluntarily consume pharmacologically meaningful doses of ethanol, also seem to most closely recapitulate the relationships between initial sensitivity and intake observed in humans (Murphy et al., 2002).
In addition, recent studies have demonstrated that there are distinct appetitive and consummatory elements of ethanol-drinking related behavior (Samson et al., 2001; Samson et al., 1999; Samson et al., 1998b) which are not readily separable in some operant self-administration models. While both of these measures may potentially represent behaviors associated with increased risk of excessive ethanol drinking, these measures do not correlate with each other (Samson et al., 2001) and can be readily separated by pharmacological manipulations (Czachowski, 2005). Thus, it is possible that initial ethanol sensitivity may correlate better with appetitive or consummatory measures of drinking behavior in animal models. However, to date, no studies have examined the relationship between initial sensitivity to ethanol and these two ethanol drinking-related measures.
Finally, while the majority of animal studies that have sought to examine the relationship between initial sensitivity and ethanol intake have focused on group differences between inbred or selectively bred rodent lines, the human studies that first reported these relationships reflect the characterization of individual differences in genetically heterogenous populations. To our knowledge, no studies have examined the relationship between initial sensitivity and ethanol drinking behavior by assessing individual differences in these measures in an outbred population of rodents.
To that end, in this study we sought to re-examine the relationship between initial sensitivity to ethanol’s locomotor stimulant and depressant effects and drinking-related measures using outbred Long-Evans rats and a limited-access operant ethanol self-administration procedure in which many subjects consume pharmacologically meaningful doses of ethanol and which procedurally separates appetitive and consummatory measures of ethanol drinking. The relationship between initial sensitivity to ethanol’s locomotor effects and home-cage ethanol preference was also examined.
METHODS
Animals
The subjects were 23 male Long-Evans rats (Harlan Sprague Dawley, Inc., Indianapolis, IN) weighing between 250–275 grams at the start of the study. Rats were singly housed and had ad libitum access to food and water, except as indicated below. Rats were maintained on a 12-hr light/dark cycle (lights on 6:00 AM to 6:00 PM), and animal care procedures were in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals and approved by the Institutional Animal Care and Use Committee.
Initial Sensitivity to Locomotor Effects of Ethanol
After one week of handling to adapt to the laboratory housing conditions, response to the locomotor stimulant and depressant effect of ethanol were measured using two identical locomotor activity boxes (Omnitech Digiscan Animal Activity Monitors, model-RXYZCM, Columbus, OH). Rats were first acclimated to the locomotor activity boxes for three days (30 min. sessions). Then, over the next three days, a within-subject design was used to test the locomotor stimulant and depressant effect of ethanol in each animal. This was done to maximize the number of animals available for subsequent correlational analyses of the relationship between measures of initial locomotor sensitivity to ethanol and ethanol drinking-related behaviors. Therefore, initial ethanol sensitivity in this study was operationally defined as the locomotor response to a low and high dose of ethanol assessed prior to an extended period of voluntary ethanol self-administration. The order of testing was the same for all subjects (Day 1: Saline, Day 2: 0.5 g/kg, Day 3: 1.5 g/kg) to minimize the development of rapid behavioral tolerance (Khanna et al., 1996). Subjects were placed in the activity boxes immediately after injections and locomotor activity was recorded for 30 min. in 5 min. bins.
Ethanol Preference Tests
Following the assessment of ethanol’s locomotor effects, all subjects were given a three day exposure to 10% ethanol in the home cage as the only available solution. Following the forced ethanol exposure, a home-cage, two-bottle choice procedure was performed for 10 days to assess the preference ratio for 10% ethanol relative to water and home-cage ethanol intake (g/kg). Both of these procedures have been frequently used in similar rodent studies as these prior experiences with ethanol drinking facilitate the initiation of operant ethanol self-administration (Czachowski et al., 2001; Czachowski et al., 2003; Files et al., 1997; Nadal et al., 2002; Samson et al., 2003). The two-bottle preference test was also repeated after 5 weeks of operant ethanol self-administration to assess the effect of an operant self-administration regimen on home-cage ethanol drinking measures (see below).
Operant Ethanol Self-administration
Following the completion of the first two-bottle choice experiment, subjects were trained to consume 10% ethanol as a reinforcer in operant sessions. All operant sessions were conducted in modular operant chambers housed in sound-attenuating enclosures (MED Associates, St. Albans, VT). The operant chambers had a retractable lever, a retractable sipper tube, and a house light. Each chamber was also equipped with a fan for ventilation and added masking sounds. The operant chambers were interfaced to a PC for experimental control and data acquisition using MED-PC software (MED Associates, St. Albans, VT).
Operant training began using one 2-h session in the operant chamber in which all subjects were shaped by reinforcing successive approximations to a lever press. During these sessions, the reinforcer was 10% sucrose (10S) and each lever press was followed by a 2 min. presentation of the sipper tube. These sessions were followed by three 1-h sessions using 10S and a 30 sec. sipper tube access following each response. Rats were fluid restricted during these training sessions to facilitate the acquisition of the lever press task however water was available ad libitum in the home cage throughout the remainder of the study and ad libitum food was always available in the home cage. Daily sessions were then conducted 5 days/week. Subjects were placed in the chamber with the houselight off and the lever and sipper tube retracted. The illumination of the house light and extension of the lever marked the start of each session. During the next 15 sessions a modified sucrose-fading procedure was used to train all subjects to complete a response requirement of 30 lever presses to elicit a presentation of the sipper tube for 20 minutes. Across these sessions, the lever press requirement was gradually increased from 1 to 30 and the composition of the reinforcer solution was modified by decreasing the sucrose concentration and increasing the ethanol concentration until rats were stably completing the response requirement for 10E. After the training sessions, subjects were maintained on 10E for two weeks to ensure stable drinking patterns. Following this period, two extinction trials were performed at Week 3 and Week 5 to assess “seeking” or “appetitive” behavior. These sessions were identical to the daily drinking sessions except that the 10E reinforcer solution was not presented and the total number of lever presses was recorded over a 20 minute period.
Statistics
The overall effect 0.5 and 1.5 g/kg ethanol on locomotor activity during the 30 minute sessions was assessed using a two-way repeated measures ANOVA with time and dose as factors. Post hoc analysis of the effect of the two doses at each 5 minute time bin during the 30 minute sessions was performed using Bonferroni t-tests. The stimulant effect of 0.5 g/kg ethanol at the 5 minute time bin and the depressant effect of 1.5 g/kg ethanol across the 30 minute locomotor sessions, measured as the percent change in activity relative to saline, were also analyzed using one-sample t tests against a theoretical mean of zero. Group comparisons from the ethanol preference and operant self-administration sessions were performed using a Student’s unpaired t-test. The relationships between locomotor activity measures and ethanol drinking-related behaviors were assessed using Pearson’s correlation test. The significance level for all statistical analyses was set at p < 0.05.
RESULTS
Initial Sensitivity to the Locomotor Stimulant and Depressant Effects of Ethanol
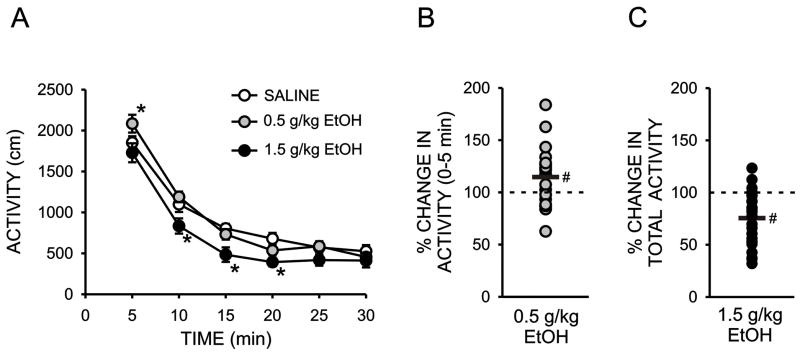
The overall goal of this study was to examine the possible relationship between the locomotor stimulant and depressant effects of ethanol and ethanol drinking-related behaviors. To that end, we first assessed the locomotor effects of a low (0.5 g/kg) and high (1.5 g/kg) dose of ethanol prior to the initiation of ethanol drinking using 30 minute sessions. A two-way repeated measures ANOVA performed on the effect of ethanol on locomotor activity within five minute bins across the 30 minute sessions revealed a significant overall effect of ethanol (F2,44 = 12.70; p < 0.001) and time (F5,220 = 186.64; p < 0.001) as well as a significant ethanol X time interaction (F10,220 = 2.27; p < 0.02). Post-hoc analyses, using Bonferroni t-tests to control for multiple comparisons, revealed a significant depressant effect of 1.5 g/kg ethanol, relative to saline, at the 10, 15, and 20 minute time bins (Fig 1A). This analysis also revealed a statistically significant, stimulant effect of 0.5 g/kg ethanol, relative to saline, at the 5 minute time bin (Fig 1A). The depressant effect of 1.5 g/kg ethanol across the entire 30 minute locomotor sessions, expressed at the percent change in locomotor activity relative to saline, was also statistically significant (24.5 ± 5.4% inhibition, p < 0.001, one sample t-test, Fig 1C) and individual differences in this parameter were used as the measure of the initial depressant effect of ethanol in all subsequent correlational analyses. In contrast, a similar analysis indicated no significant effect of 0.5 g/kg ethanol on percent change in total locomotor activity across the 30 sessions (2.7 ± 5.0% stimulation, p > 0.05, one sample t-test, data not shown). This result is consistent with other studies reporting little or no locomotor stimulant effects of low doses of ethanol in rats (Frye and Breese, 1981; Linakis and Cunningham, 1979). However, as illustrated in Figures 1A and 1B, a modest but statistically significant stimulant effect of 0.5 g/kg ethanol was observed during the first 5 minutes of the session (14.6 ± 5.8% stimulation, p < 0.05, one sample t-test) and large individual differences were observed at this time point (range: −37% to +84% change in locomotor activity, relative to saline). Thus, for all subsequent correlational analyses, the % change in locomotor activity in the first five minutes following 0.5 g/kg ethanol was used as the measure of the locomotor stimulant effect if ethanol.
Figure 1.
Initial sensitivity to the locomotor stimulant and depressant effects of ethanol. A. Horizontal locomotor activity (counts/5 min) during five minute bins across 30 minute test sessions immediately following an i.p. injection of saline, 0.5, or 1.5 g/kg ethanol. B. Scatterplot of individual differences in the percent change in locomotor activity, relative to saline treatment, during the first five minutes following administration of 0.5 g/kg ethanol. C. Scatterplot of individual differences in the percent change in locomotor activity, relative to saline treatment, during the 30 minutes following administration of 1.5 g/kg ethanol. (*, significant difference relative to saline, Bonferroni t-test, P < 0.05; # significant difference relative to a theoretical mean of zero, one-sample t test, P < 0.05).
Appetitive and Consummatory Drinking Behavior
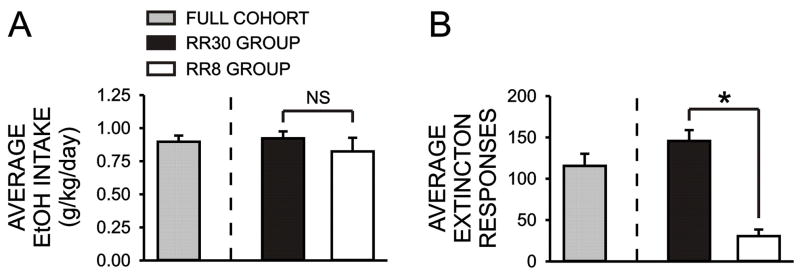
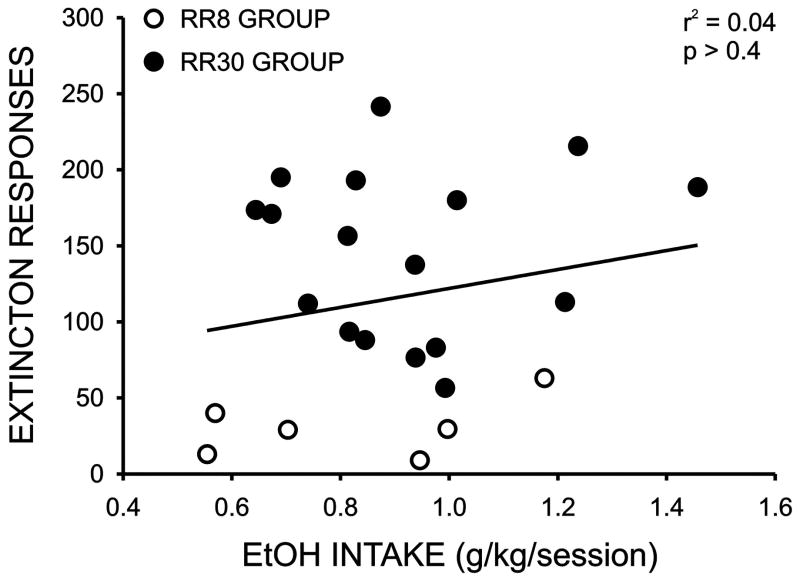
Over the course of training subjects to a response requirement of 30, six subjects failed to exhibit stable daily responding when the response requirement was increased beyond 10–20. Since the purpose of this study was to examine the possible relationship between initial sensitivity to ethanol and appetitive and consummatory measures of ethanol drinking, we did not want to exclude subjects that would likely go on to exhibit the lowest levels of appetitive behavior. Since the remainder of the subjects readily acquired the RR30 protocol, we maintained those subjects at that level and used a response requirement of eight (RR8) for the remaining six rats. In order to obtain a measure of consummatory behavior for subsequent correlational analyses, we measured average daily ethanol intake for each session during a two week baseline period following the completion of operant training. As illustrated in Figure 2A, average daily ethanol intake for all subjects was similar to that observed in previous studies using this drinking paradigm (0.90 ± 0.05 g/kg/session) (Samson et al., 2001; Samson et al., 2003) and, importantly, no differences in daily intake were observed between the RR30 and RR8 groups (RR30: 0.92 ± 0.05 g/kg/session; RR8: 0.82 ± 0.05 g/kg/session, p > 0.05). To obtain a measure of appetitive behavior for the correlational analyses, we took the average of two extinction trials conducted during week 3 and week 5. On these trials, subjects were places in the operant chambers and lever presses were counted for a twenty minute period however ethanol was never presented. Overall group extinction responding was also similar to that observed in previous studies using a similar measure of appetitive behavior (115.6 ± 14.7 responses/session) (Samson et al., 2001; Samson et al., 2003) and, not surprisingly, rats in the RR30 group exhibited significantly higher extinction responding (RR30: 145.6 ± 13.3 responses/session; RR8: 30.6 ± 8.0 responses/session, p < 0.001). As illustrated in Figure 3 there were large individual differences in measures of both ethanol consumption and extinction responding and no correlation was observed between ethanol intake and extinction responding for the full cohort (Figure 3) or when the RR30 and RR8 groups were analyzed separately (data not shown).
Figure 2.
Bar graph illustrating mean (± SEM) baseline ethanol intake/daily session across the first two weeks of the study (A) and mean (± SEM) number of appetitive responses during the two extinction sessions (B) for the full cohort of rats (gray bars) as well as for the subgroups maintained on daily response requirements of 30 lever presses (RR30, black bars) and 8 lever presses (RR8, white bars). *, p < 0.05, unpaired Students t test; NS, not significant.
Figure 3.
Plot of the mean number of responses on extinction sessions 1 and 2 versus average ethanol intake/daily session across the first two weeks of the study. The line indicates the linear regression for the full cohort. Regression analysis of the two response requirement subgroups also revealed no significant relationship between extinction responding and ethanol intake (RR30: r2 = 0.002, p > 0.05; RR8: r2 = 0.02, p > 0.05).
Relationship Between Home-Cage Ethanol Drinking Measures and Limited-Access Operant Ethanol Self-Administration
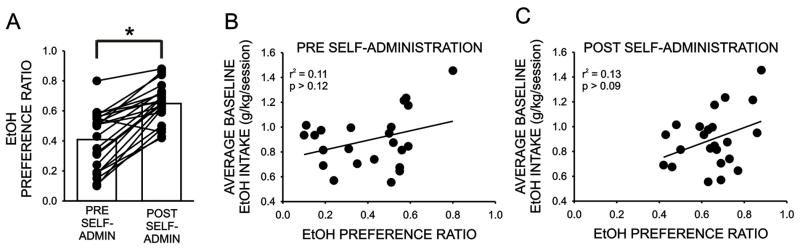
Home-cage ethanol drinking measures (preference ratio, 10% ethanol vs. water; daily ethanol intake (g/kg)) were assessed prior to, and immediately, following the ethanol self-administration regimen. As illustrated in Figure 4A, the ethanol preference ratio was initially 0.41 ± 0.04 and increased significantly following five weeks of daily operant ethanol self-administration (to 0.65 ± 0.03; P < 0.0001). Notably, no correlation was observed between ethanol intake during the limited access operant sessions and either measure of ethanol preference ratio (Figure 4A,B). Thus, although home-cage ethanol preference ratio was increased following five weeks of daily ethanol self-administration in the operant chambers, this increase in preference did not result in a strengthening of the relationship between these two measures of ethanol drinking.
Figure 4.
A. Home-cage ethanol preference ratio assessed prior to, and following, the completion of the limited-access ethanol self-administration regimen. Bars illustrate the mean ethanol preference ratios for the full cohort and the lines indicate the change in preference for each subject. *, p < 0.05, paired Students t test. B. Average baseline ethanol intake/session versus home-cage ethanol preference ratio assessed prior to the initiation of the limited access sessions. C. Average baseline ethanol intake versus home-cage ethanol preference ratio assessed following the five week limited access sessions.
Home-cage ethanol intake (g/kg/day) also increased following the self-administration procedure (from 2.7 ± 0.3 to 3.6 ± 0.2 g/kg/day). Although the relationship between this measure and intake during the operant sessions was somewhat stronger than that observed with preference, it too did not strengthen after self-administration and was not statistically significant (pre self-administration: r2 = 0.15, P > 0.05; post self-administration: r2 = 0.15, P > 0.05).
Finally, we also examined the relationship between ethanol intake during the three day forced exposure procedure, where ethanol was the only drinking solution available (used to facilitate the initiation of operant ethanol self-administration; Czachowski et al., 2001; Nadal et al, 2002) and measures of voluntary ethanol drinking during the home-cage and operant procedures. No relationship was detected between ethanol intake during the forced exposure and intake during the operant self-administration procedure or home-cage drinking measures (preference ratio or intake) assessed after the operant self-administration regimen. However, a significant positive correlation was observed between ethanol intake in the forced exposure procedure and the measures of home-cage ethanol drinking assessed immediately following this procedure, prior to operant ethanol self-administration (preference ratio, r2 = 0.20, P < 0.03; ethanol intake, r2 = 0.22, P < 0.02).
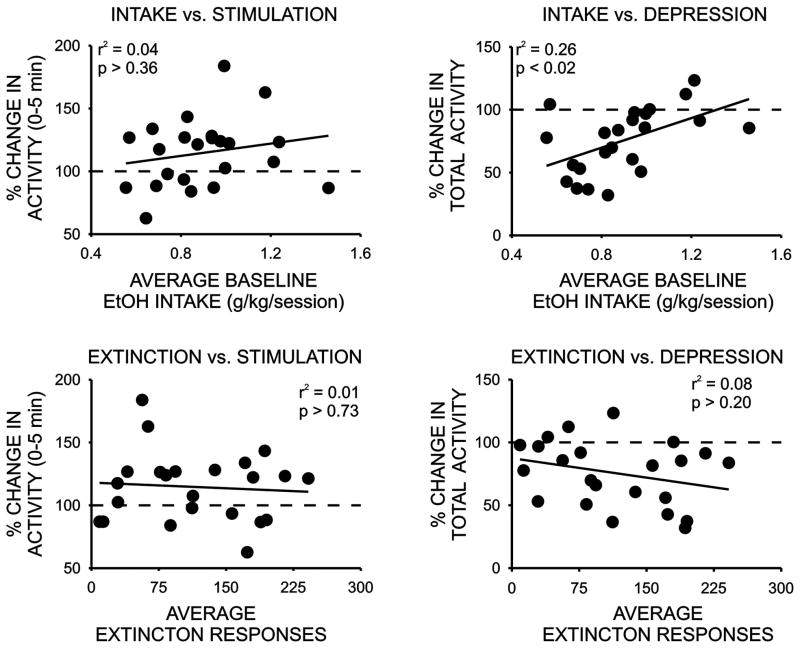
Relationship Between Initial Sensitivity to Ethanol’s Locomotor Effects and Ethanol Drinking-Related Behaviors
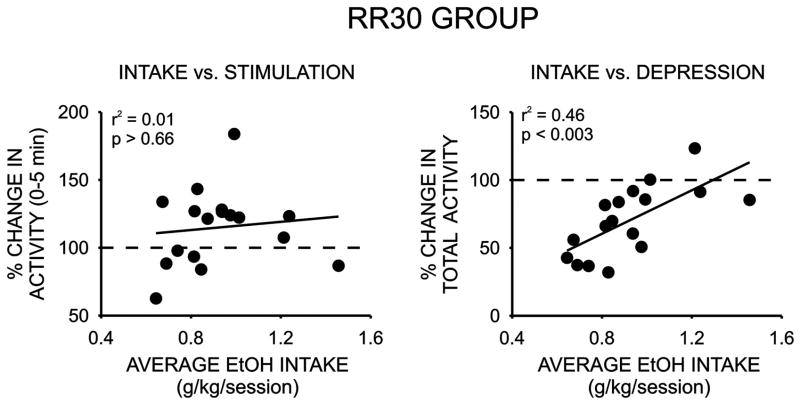
We next examined the relationship between initial sensitivity to the locomotor stimulant and depressant effects of ethanol and appetitive and consummatory measures of ethanol drinking behavior. As noted above, only a modest stimulant effect was detected in response to the low dose of ethanol and this effect was only significant during the initial five minutes of the activity sessions. Although large individual differences in locomotor stimulant response to ethanol were observed, no significant correlations between this measure and either ethanol intake or extinction responding were observed (Figure 5, Table 1). We next examined possible relationships between the locomotor depressant response to a 1.5 g/kg dose of ethanol and drinking-related behaviors. No significant correlation was observed between the depressant effect of ethanol and extinction responding however a significant relationship was detected between the locomotor depressant effect of ethanol and ethanol intake during the operant sessions (Figure 5, Table 1). Rats that exhibited the largest decrease in locomotor activity in response to 1.5 g/kg ethanol consumed the lowest amounts of ethanol during the operant sessions and rats that were least sensitive to ethanol’s depressant effect exhibited the highest levels of ethanol intake during the limited-accees operant drinking sessions. Notably, when this relationship was examined only in the subjects that successfully completed operant training on a response requirement of 30 (RR30), the strength of this correlation doubled. Finally, we also determined if initial sensitivity to the locomotor stimulant or depressant effects of ethanol was related to home-cage ethanol drinking measures. As summarized in Table 1, no significant correlations were noted between measures of home-cage ethanol drinking, assessed either before or after operant self-administration regimen, and either the locomotor stimulant or depressant effects of ethanol.
Figure 5.
Relationship between the initial locomotor stimulant (% change in activity in the first five minutes following an i.p. injection of 0.5 g/kg ethanol) or depressant (% change in activity over 30 minutes following an i.p. injection of 1.5 g/kg ethanol) effect of ethanol and average daily ethanol intake/session or extinction responding in the limited access procedure.
Table 1.
Correlation between ethanol drinking-related behaviors, assessed using either a limited-access operant self-administration protocol or a two-bottle home-cage preference test, administered prior to (pre-SA), or following (post-SA) the self-administration regimen, and the initial locomotor stimulant (0.5 g/kg ethanol, i.p.) or depressant (1.5 g/kg ethanol, i.p.) effect of ethanol. Operant drinking-related measures included ethanol intake (g/kg/session) and extinction responses. Home-cages measures included ethanol preference ratio and daily ethanol intake (g/kg). The number of subjects used for all correlational analyses was 23 except for those involving subjects on a response requirement of 30 (RR30) (N = 17) or a response requirement of 8 (RR8)(N = 6).
| Measures | r2 | P |
|---|---|---|
| Operant intake vs. locomotor stimulation | 0.04 | NS |
| Operant intake vs. locomotor depression | 0.26 | < 0.01* |
| Extinction responses vs. locomotor stimulation | 0.01 | NS |
| Extinction responses vs. locomotor depression | 0.08 | NS |
| RR30 operant intake vs. locomotor stimulation (N = 17) | 0.01 | NS |
| RR30 operant intake vs. locomotor depression (N=17) | 0.46 | < .003* |
| RR8 operant intake vs. locomotor stimulation (N = 6) | 0.18 | NS |
| RR8 operant intake vs. locomotor depression (N = 6) | 0.27 | NS |
| Home-cage intake (ratio) - pre SA vs. locomotor stimulation | 0.12 | NS |
| Home-cage intake (g/kg) - pre SA vs locomotor stimulation | 0.12 | NS |
| Home-cage intake (ratio) - pre SA vs. locomotor depression | 0.05 | NS |
| Home-cage intake (g/kg) - pre SA vs locomotor depression | 0.05 | NS |
| Home-cage intake (ratio) - post SA vs. locomotor stimulation | 0.12 | NS |
| Home-cage intake (g/kg) - post SA vs locomotor stimulation | 0.11 | NS |
| Home-cage intake (ratio) - post SA vs. locomotor depression | 0.09 | NS |
| Home-cage intake (g/kg) - post SA vs locomotor depression | 0.08 | NS |
DISCUSSION
There are several key findings to emerge from this study. First, although a high dose of ethanol produced a robust depressant effect on locomotor activity, only modest locomotor stimulation was observed following injection of a low dose of ethanol. Second, large individual differences in appetitive and consummatory measures of ethanol drinking were observed and daily ethanol intake did not differ between the two groups of rats maintained on low and high daily response requirements. Moreover, as previously reported (Samson et al., 2001; Samson and Czachowski, 2003), no correlation was observed between these appetitive and consummatory measures of ethanol drinking in either cohort. Third, home-cage ethanol drinking measures (preference ratio and daily ethanol intake) increased significantly following the self-administration regimen, however neither set of home-cage ethanol drinking measures correlated with daily intake during the limited-access self-administration sessions or with initial sensitivity to ethanol’s locomotor stimulant or depressant effects. Finally, a significant correlation was observed between initial sensitivity to the locomotor depressant effect of ethanol and ethanol drinking in a limited-access operant procedure which engenders pharmacologically relevant levels of ethanol intake and the direction of this correlation was similar to that reported in human studies relating low initial sensitivity to ethanol with excessive ethanol drinking behavior (Eng et al., 2005; Schuckit, 1994; Schuckit and Smith, 1996; Schuckit and Smith, 2006).
Acute sensitivity to the locomotor stimulant effects of ethanol and other drugs of abuse has been postulated to reflect the positive reinforcing properties of these drugs (Di Chiara and Imperato, 1988; Katner and Weiss, 2001) and, as reviewed in the Introduction, some human studies have suggested that increased sensitivity to the stimulatory effects of ethanol may be associated with greater risk of abusive drinking (Newlin and Thomson, 1999; Holdstock et al., 2000; King et al., 2002). Although locomotor stimulant effects of ethanol can be reliably observed in mice, these measures do not consistently correlate with ethanol drinking (Risinger et al., 1994; Sanchez et al., 1996). In addition, few studies have reported locomotor stimulant effects of ethanol in rats, despite the fact that ethanol can clearly function as a reinforcer in this species (Koob, 2003; Samson and Czachowski, 2003). Our finding that administration of a low dose of ethanol resulted in a modest, albeit significant, increase in locomotor activity in the first five minutes following ethanol administration is similar to that observed in a few other studies that have reported significant locomotor effects of ethanol in rats (Hall et al., 1998; Waller et al., 1986). Although large individual differences in this response were noted, no correlations were observed between initial sensitivity to the locomotor stimulant effect of ethanol and any drinking-related measures. If locomotor stimulation is related to the europhic effects of ethanol, we might have predicted a positive relationship between this measure and extinction responding in the operant procedure, as extinction responding is also thought to represent a measure of ethanol’s reinforcing saliency (Samson and Czachowski, 2003). However no such relationship was observed. Thus, given that ethanol functioned as a reinforcer in this study and that initial sensitivity to the locomotor stimulant effect of ethanol did not correlate with any ethanol drinking-related behaviors, our data do not lend support to the idea that ethanol-mediated locomotor stimulation represents a sensitive measure of ethanol’s reinforcing effects, at least in Long-Evans rats. It should, however, be emphasized that the modest and transient nature of the locomotor stimulant response to ethanol may have limited our ability to detect any relationships between ethanol’s stimulant effects and ethanol drinking measures.
The correlational analyses between the continuous home-cage and limited-access measures of ethanol drinking revealed several interesting findings. Although home-cage preference ratio and ethanol intake increased significantly following the five week self-administration procedure, no correlations were observed between any home-cage ethanol drinking measures and ethanol intake in the operant procedure. Therefore, at least in an outbred population of rats, initial home-cage ethanol preference is not predictive of ethanol intake in a limited-access paradigm where subjects voluntarily consume pharmacologically relevant doses of ethanol. Moreover, even though a history of operant ethanol self-administration increased home-cage ethanol drinking, this increase did not strengthen the relationship between home-cage drinking and consumption in the limited-access operant procedure. This finding is in good agreement with a similar analysis conducted by Samson and colleagues on the drinking patterns of 234 rats, collected over ten years, which also found no significant relationship between home-cage ethanol intake or preference and operant ethanol drinking in a limited-access paradigm (Samson and Czachowski, 2003). These findings contrast with the results of studies with lines of rats selected for high and low home-cage ethanol preference as ethanol-preferring lines generally exhibit relatively high ethanol intake in operant procedures (Czachowski and Samson, 2002; Ritz et al., 1994a; Ritz et al., 1994b; Samson et al., 1998a). However, even in these studies, some exceptions were noted. For example, AA rats, bred for high ethanol preference, do not exhibit high intake in an operant procedure. In addition, NP rats, which display very low ethanol intake and preference in the home cage, consume similar amounts of ethanol in a limited-access operant procedure as some lines of ethanol-preferring rats (Samson et al., 1998a). Therefore, although the genes selected for high ethanol preference and intake in continuous home-cage drinking procedures clearly contribute to increased intake in operant limited-access paradigms, there must also be distinct genetic influences that underlie these complex behaviors.
We also noted a significant correlation between daily ethanol intake during the three-day single bottle procedure, where 10% ethanol was the only solution available, and daily ethanol intake and preference ratio in the initial home-cage two-bottle choice drinking test. Forced exposure to ethanol using a single bottle procedure has been shown to increase ethanol preference and intake in two bottle procedures and is commonly used to facilitate ethanol acceptance and training in operant procedures similar to those used in this study (e.g. Czachowski et al., 2001; Nadal et al., 2002). Our data reveal that initial acceptance of ethanol in the single bottle procedure does correlate with ethanol intake when rats are subsequently given a choice between ethanol and water, suggesting that some common factor drives ethanol intake in these two procedures. However this relationship appears to be transient and specific for initial home-cage drinking, as intake in the single bottle procedure did not correlate with ethanol drinking measures in the operant chambers or with home-cage intake assessed after the operant self-administration sessions.
Our data also support and extend previous studies demonstrating that, even within a given drinking procedure, appetitive and consummatory measures of ethanol drinking behavior are not correlated. The absence of a significant relationship between measures of ethanol intake and appetitive or seeking measures has previously been reported in studies where all subjects were trained to complete the same daily response requirement to gain access to ethanol (Samson et al., 2001; Samson and Czachowski, 2003). Our data extend this observation to cohorts of rats maintained on low and high daily response requirements. Thus, even though one group of subjects in this study clearly exhibited a low level of ethanol seeking or appetitive behavior, evidenced by an inability to increase their daily lever press response requirement beyond eight, no difference in average daily ethanol intake was observed between these subjects and rats that readily responded on a response requirement of thirty and no correlation between ethanol intake and extinction responding was observed in either cohort. These findings, coupled with evidence that appetitive and consummatory measures of ethanol drinking behavior can be differentially influenced by a variety of pharmacological treatments (Czachowski, 2005; Czachowski et al., 2001; Czachowski et al., 2006; Czachowski et al., 2002), suggest that these two elements of ethanol drinking behavior involve distinct physiological mechanisms.
The most notable finding from this study is the observation that initial sensitivity to the locomotor depressant effect of ethanol correlated with ethanol intake in the limited-access operant procedure. As reviewed in the Introduction, although human studies have consistently noted that a low response to a broad range of ethanol’s depressant effects represents an important risk factor for the development of an AUD (Erblich and Earleywine, 1999; Pollock, 1992; Schuckit, 1994; Schuckit and Smith, 1996), this kind of relationship has not been consistently observed in in rodent studies (e.g. Colombo et al., 2000; Sanchez et al., 1996). Interestingly, the inbred P line of ethanol-preferring rats, which voluntarily consume pharmacologically relevant doses of ethanol in the home cage, and represent one of the more extensively characterized and validated rodent models of alcoholism (Waller et al., 2002), do recapitulate this relationship (Kurtz et al., 1996; Lumeng et al., 1982; Suwaki et al., 2001). For example, relative to the non-preferring NP rats, P rats are less sensitive to the effects of ethanol on the loss of righting reflex test (Kurtz et al., 1996), impairment on a jump test (Lumeng et al., 1982), the oscillating bar (Suwaki et al., 2001), and ethanol-induced hypothermia (Stewart et al., 1992).
It is therefore noteworthy that we observed the negative relationship between sensitivity to ethanol’s locomotor depressant effect and ethanol intake in the operant procedure, where subjects consume pharmacologically relevant levels of ethanol in short, 20 minute sessions, but not with continuous access home-cage drinking measures, where intake levels are comparably lower. In outbred rodent strains, ethanol drinking during standard home-cage procedures is generally spread out into small bouts that are often associated with eating (Files et al., 1994; Samson et al., 1996; Samson and Czachowski, 2003). This pattern of intake may not result in blood levels necessary to produce significant behavioral measures of intoxication. Thus, other factors, such as taste and satiety, may play a greater role in regulating intake in these procedures rather than ethanol’s post-ingestive effects (Samson and Czachowski, 2003).
In contrast, Samson and colleagues have demonstrated that the same 20 minute, operant procedure employed in this study does engender significant blood ethanol levels. In a cohort of rats drinking slightly less ethanol than that consumed by subjects in this study (0.69 vs. 0.90 g/kg/session), they reported blood ethanol levels in excess of 60 mg% immediately following the 20 minute operant sessions (Czachowski et al., 2002). These blood levels are within the range associated with a variety of behavioral measures of intoxication (Colombo, 1997; Deitrich and Harris, 1996). Taken together, these findings suggest that the relationship between low initial sensitivity to ethanol’s depressant effects and elevated ethanol consumption may be better modeled in rodents using drinking paradigms where the majority of subjects voluntarily consume ethanol at, or near, levels that may be associated with this drug’s CNS depressant effects.
It should also be noted that the measures of locomotor depression in this study may not reflect true initial sensitivity to ethanol as these measures were made following a prior assessment of locomotor response to a low dose of ethanol (see Methods). This fixed dosing order was used to maximize the number of subjects available for subsequent correlational analyses with ethanol drinking measures while reducing the possible influence of rapid behavioral tolerance, which becomes more pronounced at higher ethanol doses (Khanna et al, 1996). While this design differs from that employed in most other studies that have examined the relationship between initial ethanol sensitivity and ethanol drinking (e.g. Colombo et al, 2000; Shram et al, 2004), it seems unlikely that prior exposure to a single low dose of ethanol influenced our finding of a significant correlation between the level of response to a locomotor depressant dose of ethanol and ethanol intake in the operant procedure. It is also worth noting that the behavioral assessments of ethanol sensitivity employed in the initial human studies that established a relationship between a low level of response to ethanol and risk of alcoholism were carried out on young men with at least some prior drinking history (Schuckit et al, 1994; Schuckit et al, 1996). Moreover, it has also been shown that the quantity or frequency of alcohol drinking in non-alcoholic men at the time that initial sensitivity to ethanol is assessed does not have a major impact on the subsequent relationship between the level of response to alcohol and future risk of alcoholism (Schuckit and Smith, 1997).
Although a significant relationship was observed between initial sensitivity to ethanol-mediated locomotor depression and ethanol intake in the operant procedure, no correlations were detected between ethanol-mediated locomotor stimulation or depression and appetitive or seeking behavior (i.e. extinction responding). However, the strength of the correlation between initial sensitivity to ethanol’s depressant effect and ethanol intake was almost twice as high when examined only in the cohort of rats maintained on the higher daily response requirement. This finding may suggest that, although initial sensitivity to ethanol’s locomotor depressant effects does not directly correlate with appetitive or seeking behavior, it appears to account for more of the variance in ethanol drinking in subjects that exhibit higher levels of seeking behavior. In other words, although rats that exhibit low and high motivation to drink (i.e. appetitive behavior) do not differ in the amount of ethanol that they consume, these data suggest that intake in these cohorts may be regulated by distinct genetic or physiological processes.
Given that a low behavioral response to ethanol has emerged as one of the most consistent phenotypic predictors of heavy drinking and alcoholism, there is considerable interest in elucidating the neurobiological mechanisms that may be responsible for this relationship. Ethanol’s behavioral effects are mediated by interactions between this drug and a variety of neurotransmitter systems that mediate and modulate synaptic transmission (e.g. GABA, glutamate, DA. 5-HT, etc…; Gass and Olive, 2008; Gonzales et al., 2004; Weiner and Valenzuela, 2006). Alterations in genes that influence how ethanol interacts with any of this diverse array of neurotransmitter systems could potentially contribute to a “low ethanol sensitivity” phenotype. Although there are likely to be many genes that give rise to this complex phenotype, recent human and animal studies have identified a couple of potential candidates. Several lines of evidence suggest that a polymorphism in the gene encoding the serotonin transporter on chromosome 17 may be associated with a lower behavioral response to ethanol and increased risk of heavy drinking. The presence of the L allele of this gene results in faster serotonin uptake rates and lower serotonin levels (Mayfield et al., 2008). Importantly, the L allele is associated with decreased sensitivity to the ataxic and sedating effects of ethanol in humans (Hinckers et al., 2006; Hu et al., 2005; Schuckit et al., 1999) and non-human primates (Barr et al., 2003) and the presence of this allele has also been correlated with higher levels of ethanol intake in adolescents (Hinckers et al., 2006) and increased risk of alcoholism (Hu et al., 2005; Schuckit et al., 1999). There is also some evidence that a polymorphism in a subunit of the GABAA receptor expressed in the cerebellum may also contribute to this relationship. A proline to serine substitution at amino acid residue 385 in the GABAA receptor α6 subunit was associated with a low response to ethanol and increased risk of alcoholism, particularly when also expressed along with the L allele of the serotonin transporter (Hu et al., 2005; Mayfield et al., 2008). Although these are intriguing findings, additional studies will be needed to establish a causal relationship between these and other genetic polymorphisms and the relationship between ethanol sensitivity and alcoholism.
In summary, the results of this study demonstrate that, although home-cage ethanol preference is significantly increased after a history of operant ethanol self-administration, continuous access, home-cage drinking measures do not predict appetitive or consummatory measures of ethanol drinking in an operant procedure that engenders pharmacologically relevant levels of ethanol intake. In addition, the relationship between a lower initial sensitivity to ethanol’s locomotor depressant effects and higher ethanol intake, observed in human studies, can also be seen in an outbred rodent strain using a limited-access operant procedure but not with home-cage ethanol drinking measures. Collectively, these data suggest that post-ingestive effects may contribute to ethanol intake in the operant sipper tube procedure and that this model may prove useful in identifying the physiological mechanisms that may underlie the relationship between initial ethanol sensitivity and ethanol drinking.
Figure 6.
Relationship between locomotor stimulant and depressant effects of ethanol and average daily ethanol intake/session for the subgroup of rats maintained on the daily response requirement of 30 lever presses.
Acknowledgments
This work was supported by National Institutes of Health Grants AA 13960, 16643, and 10756.
References
- Agabio R, Carai MA, Lobina C, Pani M, Reali R, Vacca G, Gessa GL, Colombo G. Alcohol stimulates motor activity in selectively bred Sardinian alcohol-preferring (sP), but not in Sardinian alcohol-nonpreferring (sNP), rats. Alcohol. 2001;23(2):123–6. doi: 10.1016/s0741-8329(00)00144-0. [DOI] [PubMed] [Google Scholar]
- Barr CS, Newman TK, Becker ML, Champoux M, Lesch KP, Suomi SJ, Goldman D, Higley JD. Serotonin transporter gene variation is associated with alcohol sensitivity in rhesus macaques exposed to early-life stress. Alcohol Clin Exp Res. 2003;27(5):812–7. doi: 10.1097/01.ALC.0000067976.62827.ED. [DOI] [PubMed] [Google Scholar]
- Collins AC. A review of research using Short-sleep and Long-sleep mice. In: McClearn RADGE, Erwin VG, editors. Development of Animal Models as Pharmacogenetic Tools. Vol. 6. National Institute on Alcohol and Alcoholism, Department of Health Education and Welfare Publication No. (ADM) 78–847; Washington, D.C: 1981. pp. 161–170. [Google Scholar]
- Colombo G. ESBRA-Nordmann 1996 Award Lecture: ethanol drinking behaviour in Sardinian alcohol-preferring rats. Alcohol Alcohol. 1997;32(4):443–53. doi: 10.1093/oxfordjournals.alcalc.a008279. [DOI] [PubMed] [Google Scholar]
- Colombo G, Agabio R, Carai MA, Lobina C, Pani M, Reali R, Vacca G, Gessa GL. Different sensitivity to ethanol in alcohol-preferring sP and -nonpreferring sNP rats. Alcoholism: Clinical & Experimental Research. 2000;24(11):1603–8. [PubMed] [Google Scholar]
- Crabbe JC, Young ER, Deutsch CM, Tam BR, Kosobud A. Mice genetically selected for differences in open-field activity after ethanol. Pharmacol Biochem Behav. 1987;27(3):577–81. doi: 10.1016/0091-3057(87)90371-6. [DOI] [PubMed] [Google Scholar]
- Czachowski CL. Manipulations of serotonin function in the nucleus accumbens core produce differential effects on ethanol and sucrose seeking and intake. Alcohol Clin Exp Res. 2005;29(7):1146–55. doi: 10.1097/01.alc.0000171944.50381.86. [DOI] [PubMed] [Google Scholar]
- Czachowski CL, Chappell AM, Samson HH. Effects of raclopride in the nucleus accumbens on ethanol seeking and consumption. Alcohol Clin Exp Res. 2001;25(10):1431–40. doi: 10.1097/00000374-200110000-00005. [DOI] [PubMed] [Google Scholar]
- Czachowski CL, Legg BH, Samson HH. Assessment of sucrose and ethanol reinforcement: the across-session breakpoint procedure. Physiology & Behavior. 2003;78(1):51–9. doi: 10.1016/s0031-9384(02)00963-0. [DOI] [PubMed] [Google Scholar]
- Czachowski CL, Legg BH, Stansfield KH. Ethanol and sucrose seeking and consumption following repeated administration of the GABA(B) agonist baclofen in rats. Alcohol Clin Exp Res. 2006;30(5):812–8. doi: 10.1111/j.1530-0277.2006.00094.x. [DOI] [PubMed] [Google Scholar]
- Czachowski CL, Samson HH. Ethanol- and sucrose-reinforced appetitive and consummatory responding in HAD1, HAD2, and P rats. Alcohol Clin Exp Res. 2002;26(11):1653–61. doi: 10.1097/01.ALC.0000036284.74513.A5. [DOI] [PubMed] [Google Scholar]
- Czachowski CL, Santini LA, Legg BH, Samson HH. Separate measures of ethanol seeking and drinking in the rat: effects of remoxipride. Alcohol. 2002;28(1):39–46. doi: 10.1016/s0741-8329(02)00236-7. [DOI] [PubMed] [Google Scholar]
- Deitrich RA, Harris RA. How much alcohol should I use in my experiments? Alcoholism: Clinical & Experimental Research. 1996;20(1):1–2. doi: 10.1111/j.1530-0277.1996.tb01033.x. [DOI] [PubMed] [Google Scholar]
- Di Chiara G, Imperato A. Drugs abused by humans preferentially increase synaptic dopamine concentrations in the mesolimbic system of freely moving rats. Proc Natl Acad Sci U S A. 1988;85(14):5274–8. doi: 10.1073/pnas.85.14.5274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elmer GI, Meisch RA, Goldberg SR, George FR. Ethanol self-administration in long sleep and short sleep mice indicates reinforcement is not inversely related to neurosensitivity. J Pharmacol Exp Ther. 1990;254(3):1054–62. [PubMed] [Google Scholar]
- Eng MY, Schuckit MA, Smith TL. The level of response to alcohol in daughters of alcoholics and controls. Drug Alcohol Depend. 2005;79(1):83–93. doi: 10.1016/j.drugalcdep.2005.01.002. [DOI] [PubMed] [Google Scholar]
- Erblich J, Earleywine M. Children of alcoholics exhibit attenuated cognitive impairment during an ethanol challenge. Alcohol Clin Exp Res. 1999;23(3):476–82. [PubMed] [Google Scholar]
- Erwin VG, Heston WD, McClearn GE, Deitrich RA. Effect of hypnotics on mice genetically selected for sensitivity to ethanol. Pharmacol Biochem Behav. 1976;4(6):679–83. doi: 10.1016/0091-3057(76)90219-7. [DOI] [PubMed] [Google Scholar]
- Files FJ, Denning CE, Hyytia P, Kiianmaa K, Samson HH. Ethanol-reinforced responding by AA and ANA rats following the sucrose-substitution initiation procedure. Alcohol Clin Exp Res. 1997;21(4):749–53. [PubMed] [Google Scholar]
- Files FJ, Lewis RS, Samson HH. Effects of continuous versus limited access to ethanol on ethanol self-administration. Alcohol. 1994;11(6):523–31. doi: 10.1016/0741-8329(94)90079-5. [DOI] [PubMed] [Google Scholar]
- Files FJ, Samson HH, Brice GT, Deitrich RA, Draski LJ. Initiation of ethanol self-administration by the sucrose-substitution method with HAS and LAS rats. Alcoholism: Clinical & Experimental Research. 1996;20(4):677–81. doi: 10.1111/j.1530-0277.1996.tb01671.x. [DOI] [PubMed] [Google Scholar]
- Frye GD, Breese GR. An evaluation of the locomotor stimulating action of ethanol in rats and mice. Psychopharmacology (Berl) 1981;75(4):372–9. doi: 10.1007/BF00435856. [DOI] [PubMed] [Google Scholar]
- Gass JT, Olive MF. Glutamatergic substrates of drug addiction and alcoholism. Biochem Pharmacol. 2008;75(1):218–65. doi: 10.1016/j.bcp.2007.06.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzales RA, Job MO, Doyon WM. The role of mesolimbic dopamine in the development and maintenance of ethanol reinforcement. Pharmacol Ther. 2004;103(2):121–46. doi: 10.1016/j.pharmthera.2004.06.002. [DOI] [PubMed] [Google Scholar]
- Hall FS, Huang S, Fong GW, Pert A, Linnoila M. Effects of isolation-rearing on locomotion, anxiety and responses to ethanol in Fawn Hooded and Wistar rats. Psychopharmacology. 1998;139(3):203–9. doi: 10.1007/s002130050705. [DOI] [PubMed] [Google Scholar]
- Hinckers AS, Laucht M, Schmidt MH, Mann KF, Schumann G, Schuckit MA, Heinz A. Low level of response to alcohol as associated with serotonin transporter genotype and high alcohol intake in adolescents. Biol Psychiatry. 2006;60(3):282–7. doi: 10.1016/j.biopsych.2005.12.009. [DOI] [PubMed] [Google Scholar]
- Holdstock L, King AC, de Wit H. Subjective and objective responses to ethanol in moderate/heavy and light social drinkers. Alcohol Clin Exp Res. 2000;24(6):789–94. [PubMed] [Google Scholar]
- Hu X, Oroszi G, Chun J, Smith TL, Goldman D, Schuckit MA. An expanded evaluation of the relationship of four alleles to the level of response to alcohol and the alcoholism risk. Alcohol Clin Exp Res. 2005;29(1):8–16. doi: 10.1097/01.alc.0000150008.68473.62. [DOI] [PubMed] [Google Scholar]
- Katner SN, Weiss F. Neurochemical characteristics associated with ethanol preference in selected alcohol-preferring and -nonpreferring rats: a quantitative microdialysis study. Alcohol Clin Exp Res. 2001;25(2):198–205. [PubMed] [Google Scholar]
- Khanna JM, Chau A, Shah G. Characterization of the Phenomenon of rapid tolerance to ethanol. Alcohol. 1996;13(6):621–8. doi: 10.1016/s0741-8329(96)00083-3. [DOI] [PubMed] [Google Scholar]
- Khanna JM, Kalant H, Shah G, Sharma H. Comparison of sensitivity and alcohol consumption in four outbred strains of rats. Alcohol. 1990;7(5):429–34. doi: 10.1016/0741-8329(90)90027-a. [DOI] [PubMed] [Google Scholar]
- King AC, Houle T, de Wit H, Holdstock L, Schuster A. Biphasic alcohol response differs in heavy versus light drinkers. Alcohol Clin Exp Res. 2002;26(6):827–35. [PubMed] [Google Scholar]
- Koob GF. Alcoholism: allostasis and beyond. Alcohol Clin Exp Res. 2003;27(2):232–43. doi: 10.1097/01.ALC.0000057122.36127.C2. [DOI] [PubMed] [Google Scholar]
- Kurtz DL, Stewart RB, Zweifel M, Li TK, Froehlich JC. Genetic differences in tolerance and sensitization to the sedative/hypnotic effects of alcohol. Pharmacol Biochem Behav. 1996;53(3):585–91. doi: 10.1016/0091-3057(95)02055-1. [DOI] [PubMed] [Google Scholar]
- Le AD, Kiianmaa K. Characteristics of ethanol tolerance in alcohol drinking (AA) and alcohol avoiding (ANA) rats. Psychopharmacology. 1988;94(4):479–83. doi: 10.1007/BF00212841. [DOI] [PubMed] [Google Scholar]
- Linakis JG, Cunningham CL. Effects of concentration of ethanol injected intraperitoneally on taste aversion, body temperature, and activity. Psychopharmacology (Berl) 1979;64(1):61–5. doi: 10.1007/BF00427346. [DOI] [PubMed] [Google Scholar]
- Lumeng L, Waller MB, McBride WJ, Li TK. Different sensitivities to ethanol in alcohol-preferring and -nonpreferring rats. Pharmacol Biochem Behav. 1982;16(1):125–30. doi: 10.1016/0091-3057(82)90023-5. [DOI] [PubMed] [Google Scholar]
- Mayfield RD, Harris RA, Schuckit MA. Genetic factors influencing alcohol dependence. Br J Pharmacol. 2008 doi: 10.1038/bjp.2008.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy JM, Stewart RB, Bell RL, Badia-Elder NE, Carr LG, McBride WJ, Lumeng L, Li TK. Phenotypic and genotypic characterization of the Indiana University rat lines selectively bred for high and low alcohol preference. Behavior Genetics. 2002;32(5):363–88. doi: 10.1023/a:1020266306135. [DOI] [PubMed] [Google Scholar]
- Nadal R, Armario A, Janak PH. Positive relationship between activity in a novel environment and operant ethanol self-administration in rats. Psychopharmacology. 2002;162(3):133–8. doi: 10.1007/s00213-002-1091-5. [DOI] [PubMed] [Google Scholar]
- Newlin DB, Thomson JB. Alcohol challenge with sons of alcoholics: a critical review and analysis. Psychol Bull. 1990;108(3):383–402. doi: 10.1037/0033-2909.108.3.383. [DOI] [PubMed] [Google Scholar]
- Newlin DB, Thomson JB. Chronic tolerance and sensitization to alcohol in sons of alcoholics: II. Replication and reanalysis. Exp Clin Psychopharmacol. 1999;7(3):234–43. doi: 10.1037//1064-1297.7.3.234. [DOI] [PubMed] [Google Scholar]
- Phillips TJ, Burkhart-Kasch S, Terdal ES, Crabbe JC. Response to selection for ethanol-induced locomotor activation: genetic analyses and selection response characterization. Psychopharmacology. 1991;103(4):557–66. doi: 10.1007/BF02244259. [DOI] [PubMed] [Google Scholar]
- Pollock VE. Meta-analysis of subjective sensitivity to alcohol in sons of alcoholics. Am J Psychiatry. 1992;149(11):1534–8. doi: 10.1176/ajp.149.11.1534. [DOI] [PubMed] [Google Scholar]
- Risinger FO, Malott DH, Prather LK, Niehus DR, Cunningham CL. Motivational properties of ethanol in mice selectively bred for ethanol-induced locomotor differences. Psychopharmacology (Berl) 1994;116(2):207–16. doi: 10.1007/BF02245064. [DOI] [PubMed] [Google Scholar]
- Ritz MC, Garcia JM, Protz D, George FR. Operant ethanol-reinforced behavior in P, NP, HAD, and LAD rats bred for high versus low ethanol preference. Alcohol Clin Exp Res. 1994a;18(6):1406–15. doi: 10.1111/j.1530-0277.1994.tb01443.x. [DOI] [PubMed] [Google Scholar]
- Ritz MC, Garcia JM, Protz D, Rael AM, George FR. Ethanol-reinforced behavior in P, NP, HAD and LAD rats: differential genetic regulation of reinforcement and motivation. Behav Pharmacol. 1994b;5(4 And 5):521–531. doi: 10.1097/00008877-199408000-00013. [DOI] [PubMed] [Google Scholar]
- Samson H, Files F, Brice G. Patterns of ethanol consumption in a continuous access situation: the effect of adding a sweetener to the ethanol solution. Alcohol Clin Exp Res. 1996;20(1):101–9. doi: 10.1111/j.1530-0277.1996.tb01052.x. [DOI] [PubMed] [Google Scholar]
- Samson HH, Chappell A, Czachowski C, Sharpe A. Measuring ethanol-seeking behavior: the effect of using repeated extinction trials. Alcohol. 2001;24(3):205–9. doi: 10.1016/s0741-8329(01)00157-4. [DOI] [PubMed] [Google Scholar]
- Samson HH, Czachowski CL. Behavioral measures of alcohol self-administration and intake control: rodent models. International Review of Neurobiology. 2003;54:107–43. doi: 10.1016/s0074-7742(03)54004-1. [DOI] [PubMed] [Google Scholar]
- Samson HH, Czachowski CL, Chappell A, Legg B. Measuring the appetitive strength of ethanol: use of an extinction trial procedure. Alcohol. 2003;31(1–2):77–86. doi: 10.1016/j.alcohol.2003.09.002. [DOI] [PubMed] [Google Scholar]
- Samson HH, Files FJ, Denning C, Marvin S. Comparison of alcohol-preferring and nonpreferring selectively bred rat lines. I. Ethanol initiation and limited access operant self-administration. Alcohol Clin Exp Res. 1998a;22(9):2133–46. [PubMed] [Google Scholar]
- Samson HH, Sharpe AL, Denning C. Initiation of ethanol self-administration in the rat using sucrose substitution in a sipper-tube procedure. Psychopharmacology (Berl) 1999;147(3):274–9. doi: 10.1007/s002130051167. [DOI] [PubMed] [Google Scholar]
- Samson HH, Slawecki CJ, Sharpe AL, Chappell A. Appetitive and consummatory behaviors in the control of ethanol consumption: a measure of ethanol seeking behavior. Alcohol Clin Exp Res. 1998b;22(8):1783–7. [PubMed] [Google Scholar]
- Sanchez FP, Dickenson L, George FR. Ethanol self-administration is genetically independent of locomotor stimulation in fast and slow mice. Alcohol. 1996;13(1):79–84. doi: 10.1016/0741-8329(95)02017-9. [DOI] [PubMed] [Google Scholar]
- Schuckit MA. Low level of response to alcohol as a predictor of future alcoholism. American Journal of Psychiatry. 1994;151(2):184–9. doi: 10.1176/ajp.151.2.184. [DOI] [PubMed] [Google Scholar]
- Schuckit MA, Mazzanti C, Smith TL, Ahmed U, Radel M, Iwata N, Goldman D. Selective genotyping for the role of 5-HT2A, 5-HT2C, and GABA alpha 6 receptors and the serotonin transporter in the level of response to alcohol: a pilot study. Biological Psychiatry. 1999;45(5):647–51. doi: 10.1016/s0006-3223(98)00248-0. [DOI] [PubMed] [Google Scholar]
- Schuckit MA, Smith TL. An 8-year follow-up of 450 sons of alcoholic and control subjects. Archives of General Psychiatry. 1996;53(3):202–10. doi: 10.1001/archpsyc.1996.01830030020005. [DOI] [PubMed] [Google Scholar]
- Schuckit MA, Smith TL. Assessing the risk for alcoholism among sons of alcoholics. Journal Stud Alcohol. 1997;58(2):141–45. doi: 10.15288/jsa.1997.58.141. [DOI] [PubMed] [Google Scholar]
- Schuckit MA, Smith TL. An evaluation of the level of response to alcohol, externalizing symptoms, and depressive symptoms as predictors of alcoholism. J Stud Alcohol. 2006;67(2):215–27. doi: 10.15288/jsa.2006.67.215. [DOI] [PubMed] [Google Scholar]
- Shram MJ, Bahroos M, Beleskey JI, Tampakeras M, Le AD, Tomkins DM. Motor impairing effects of ethanol and diazepam in rats selectively bred for high and low ethanol consumption in a limited-access paradigm. Alcohol Clin Exp Res. 2004;28(12):1814–21. doi: 10.1097/01.alc.0000148105.79934.14. [DOI] [PubMed] [Google Scholar]
- Spuhler K, Deitrich RA. Correlative analysis of ethanol-related phenotypes in rat inbred strains. Alcohol Clin Exp Res. 1984;8(5):480–4. doi: 10.1111/j.1530-0277.1984.tb05707.x. [DOI] [PubMed] [Google Scholar]
- Stewart RB, Kurtz DL, Zweifel M, Li TK, Froehlich JC. Differences in the hypothermic response to ethanol in rats selectively bred for oral ethanol preference and nonpreference. Psychopharmacology (Berl) 1992;106(2):169–74. doi: 10.1007/BF02801968. [DOI] [PubMed] [Google Scholar]
- Suwaki H, Kalant H, Higuchi S, Crabbe JC, Ohkuma S, Katsura M, Yoshimura M, Stewart RC, Li TK, Weiss F. Recent research on alcohol tolerance and dependence. Alcohol Clin Exp Res. 2001;25(5 Suppl ISBRA):189S–196S. doi: 10.1097/00000374-200105051-00031. [DOI] [PubMed] [Google Scholar]
- Tampier L, Mardones J. Differences in ethanol sensitivity and acute tolerance between UChA and UChB rats. Journal of Studies on Alcohol. 1999;60(2):168–71. doi: 10.15288/jsa.1999.60.168. [DOI] [PubMed] [Google Scholar]
- Waller MB, Murphy JM, McBride WJ, Lumeng L, Li TK. Effect of low dose ethanol on spontaneous motor activity in alcohol-preferring and -nonpreferring lines of rats. Pharmacol Biochem Behav. 1986;24(3):617–23. doi: 10.1016/0091-3057(86)90567-8. [DOI] [PubMed] [Google Scholar]
- Weiner JL, Valenzuela CF. Ethanol modulation of GABAergic transmission: the view from the slice. Pharmacol Ther. 2006;111(3):533–54. doi: 10.1016/j.pharmthera.2005.11.002. [DOI] [PubMed] [Google Scholar]