Abstract
We have previously reported that when mixed with copper, 8-hydroxyquinoline (8-OHQ) and its analog clioquinol (CQ) inhibited the proteasomal activity and proliferation in cultured human cancer cells. CQ treatment of high copper-containing human tumor xenografts also caused cancer suppression, associated with proteasome inhibition in vivo. However, the nature of copper dependence of these events has not been elucidated experimentally. In the current study, by using chemical probe molecules that mimic structures of 8-OHQ and CQ, but have no copper binding capability, we dissected the complex cellular processes elicited by 8-OHQ-Cu or CQ-Cu mixture and revealed that copper-binding to 8-OHQ or CQ is required for transportation of copper complex into human breast cancer cells and the consequent proteasome-inhibitory, growth-suppressive and apoptosis-inducing activities. In contrast, the non-copper-binding analogs of 8-OHQ or CQ blocked the very first step – copper binding in this chain of events mediated by 8-OHQ-Cu or CQ-Cu.
Keywords: Copper-dependence, clioquinol, breast cancer, chemical probe, chemical biology
INTRODUCTION
Copper is an essential cofactor for many enzymes and a key one-electron-transfer reaction donor involved in reactive oxygen species (ROS) generation. Therefore, the concentration of copper in organisms is tightly regulated [1, 2]. However, copper concentration is elevated in cancerous tissues of breast, prostate, lung, and brain [3–6]. The detailed molecular mechanisms for this cancer-associated copper elevation are not clear. Regardless of that, the vital role of copper in angiogenesis, a process critical for tumor growth, has been well documented [7–9]. Because the copper level elevation is cancer-specific, copper targeting agents can be developed as more selective anti-cancer therapeutics [10]. Strong copper chelator tetrathiomolybdate showed the effect to stabilization of kidney cancer [11, 12]. However, cancer kept advancing in patients before the copper concentration was significantly lowered suggesting that just passively eliminating free copper is not enough.
The ubiquitin-proteasome pathway is essential for cell cycle, apoptosis, angiogenesis and differentiation [13, 14]. This pathway contributes to the pathological state of several human diseases including cancer, in which some regulatory proteins are either stabilized due to decreased degradation or lost due to accelerated degradation [15]. The 20S proteasome, the proteolytic core of 26S proteasome complex, contains multiple peptidase activities, including the chymotrypsin (CT)-like, trypsin-like and peptidylglutamyl peptide hydrolyzing (PGPH)-like activities [16]. It has been reported that all three types of activities contributed significantly to protein breakdown and their relative importance varied widely with the substrate [17]. However, only the inhibition of the CT-like but not other proteasomal activities is a strong stimulus that induces apoptosis [18, 19].
8-hydroxyquinoline is a monoprotic bidentate chelating agent. Previous studies have shown that 8-hydroxyquinoline and some of its derivatives exhibited substantial cytotoxic activity against cancer cells[20, 21]. The bis-8-hydroxyquinoline substituted benzylamines derivatives are more efficient than the mono-hydroxyquinoline analogues in their antitumor activities[22] and in the inhibition of the precipitation of Aβ-peptides induced by Cu2+ and Zn2+ in the Alzheimer disease [23]. Others reported that 8-hydroxyquinoline are more active than their substituted quinoline analogues in differentiation-inducing effect in the MCF-7 human breast cancer cell[24].
We and others have previously reported that both 8-OHQ, CQ and some other analogues could bind copper in solution [25–30]. The X-ray crystal structures of copper complexes or structural models have also been reported [31–33]. These ligand-copper mixtures inhibited the proteasome and superoxide dismutase-1 activities, resulting in proliferation suppression and apoptosis induction in cultured cancer cells and that CQ also inhibited tumor growth in a xenograft mice model [27, 29]. It was found that CQ can act therapeutically by changing the distribution of copper or facilitating copper uptake in yeast cells [34]. Another report showed that CQ inhibited the proteasome and induced cell death primarily through a copper-dependent mechanism, displaying preclinical activity in leukemia and myeloma [35]. These results suggested that the chelation of copper or changes in cellular copper may be required for the anti-cancer activities of 8-OHQ and CQ although this speculation has not been proven through experiments.
However, copper-independent growth-inhibitory activities were also reported, such as its ability to up-regulate PI3K and inhibit p53 activity [36]. Since copper ions are always present in cells, these observations could not completely exclude the possibility of CQ copper complex formation. In order to clarify the role of copper chelating in the anti-cancer activities of 8-OHQ and CQ, we investigated the enzymatic and cellular activities of their close analogs (named 2, 3, 5 and 6) that lacked copper-binding abilities. We found that in contrast to 8-OHQ and CQ that could enhance the cellular copper uptake, their non-copper-binding derivatives failed to do so. Furthermore, different from 8-OHQ and CQ, the non-copper-binding compounds 2, 3, 5, and 6 could neither inhibit the CT-like activity of the proteasome in human breast cancer cell cultures nor induce cancer cell death. Our results demonstrated that copper binding and cell-uptake of copper are required for the proteasome-inhibitory and growth suppressive activities of 8-OHQ and CQ in human breast cancer cells.
RESULTS
Synthesis and characterization of 8-OHQ and CQ analogues
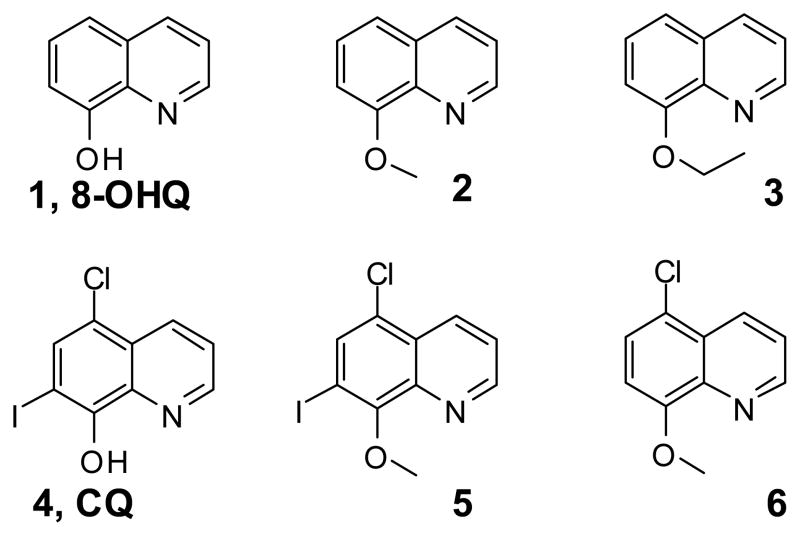
The main hypothesis of this investigation is that we can test the role of copper-binding by 8-OHQ and CQ in their proteasome and cancer cell inhibition by investigating their analogs that maintain the similar molecular structure without copper-binding capability. For this purpose, we synthesized and/or acquired compounds 1–6 (Figure 1). Compound 1 is 8-OHQ and 2 and 3 have similar structure as 1, but their 8-hydroxyl group, which is crucial for copper chelating [37], is blocked. Compound 5 and 6 are mimics of CQ (4) without copper-binding capability. These compounds were purified by chromatographic methods and their structures and purities were confirmed by LC/MS and 1H NMR methods.
Figure 1.
Structures of six 8-hydroxyquinoline analogues.
Binding constant of Cu-ligand determined by UV-vis titration
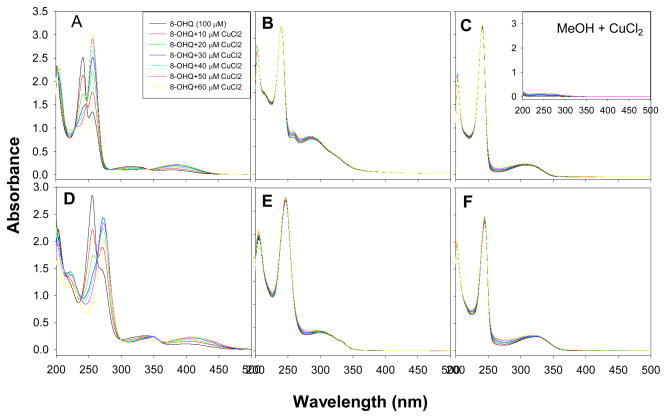
Both 8-OHQ and CQ bind to copper and form complexes. The UV absorption maxima of 8-OHQ and CQ at ~250 nm were shifted to ~275 nm upon copper binding (Figure 2A&D) indicating the formation of copper complex. Previous reports indicated that they were 2:1 (ligand:copper) complexes and CQ-Cu has a stability constant of 2×1010 [37, 38]. However, compounds 2, 3, 5, and 6 could not form complexes with copper (Figure 2B, C, E, F) in the physiologically achievable concentration range.
Figure 2. UV-vis titration of the binding between CQ (A), 8-OHQ (D), or 8-hydroxyquinoline analogs (B,C,E,F) with CuCl2 in methanol solution.
The 0.1 mM methanol solution of each compound was titrated with different amount of CuCl2 methanol solution (2.5 mM).
8-OHQ and CQ, but not their non-copper-binding analogs, can facilitate transport of copper into cells
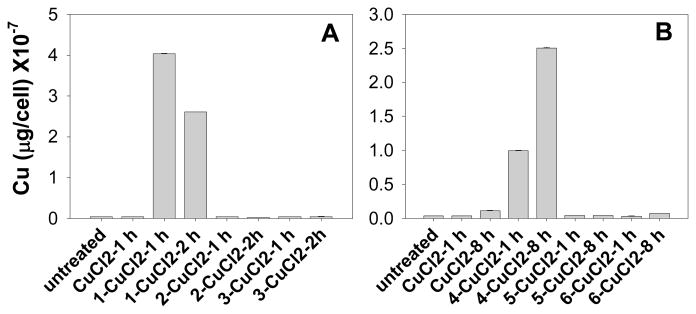
To investigate the relationship between the amount of cellular copper and the copper binding ability of 1–6, we determined the amount of cellular copper after incubation with each compound-copper mixture. Human breast cancer cell lines MCF10dcis.com (DCIS) cells were treated with 10 μM of each mixture for 1, 2 or 8 h, and the cells were collected and dissolved in nitric acid for measurement of the copper content by ICP-OES. The effects of compound-copper mixture on cellular copper accumulations are shown in Figure 3. The amount of copper in untreated DCIS cells is 0.03 ×10−7 μg/cell. When cells were treated with the mixture of 1-, 2- and 3-CuCl2 at 10.0 μM for 1 h, the levels of cellular copper were found to be 4.04 ×10−7, 0.03 ×10−7, and 0.04 ×10−7 μg/cell, respectively (Figure 3A), corresponding to at least 100-fold difference in the ability to accumulate cellular copper between 8-OHQ and its inactive analogs 2- and 3. Similar results were obtained for CQ and its inactive analogs. The accumulation of copper in DCIS cells treated for 8 hrs with 4 (CQ)-, 5- and 6-CuCl2 were 2.50 ×10−7, 0.05 ×10−7, and 0.07 ×10−7 μg/cell respectively (Figure 3B), representing a 40–50-fold difference. These data showed a close correlation between the copper-bonding ability of compounds and the cell-uptake of copper.
Figure 3. Effect of compound-copper mixtures on cellular copper uptake in human breast cancer DCIS cells.
Cells were treated with 10 μM compound-copper mixtures for indicated time periods. Then cells were collected and the copper accumulations in cells were quantitatively measured by ICP-OES.
To study whether failure of the copper-mixtures of inactive analogs of 8-OHQ and CQ to accumulate cellular copper content (Figure 3) is due to failure of these analogs to enter cells, we performed parallel artificial membrane permeability assay (PAMPA) and CaCo-2 membrane permeability assay and evaluated the membrane permeability of 1–6. We found that the permeability values of all these six compounds in these different assays are highly comparable (Table 1). Therefore, failure of these non-copper-binding analogs of 8-OHQ and CQ to accumulate cellular copper should be due to their inability to bind to copper and to transport copper into cells.
Table 1.
CaCo-2 and PAMPA Permeability Assay Results
| CaCo2 Papp A/B (nm/s) | Caco2 Papp B/A (nm/s) | CaCo2 Efflux Ratio | PAMPA Pe (nm/s) | |
|---|---|---|---|---|
| 1 | 380±148 | 290±11 | 0.8 | 213±16 |
| 2 | 483±26 | 299±7 | 0.6 | 682±109 |
| 3 | 207±146 | 94±25 | 0.5 | 726±248 |
| 4 | 473±61 | 315±22 | 0.7 | 334±31 |
| 5 | 215±31 | 125±16 | 0.6 | 779±54 |
| 6 | 604±24 | 413±27 | 0.7 | 308±49 |
We have realized that a ligand alone could also have an effect on copper detoxification. Therefore, the effect of 8-OHQ and CQ as well as their non-copper-binding analogs on copper detoxification should also be studied in the future.
8-OHQ and CQ, but not their non-copper-binding analogs, can inhibit breast cancer cell growth
We have reported previously that a mixture of 8-OHQ or CQ with copper selectively inhibited proliferation and induced apoptosis in malignant breast cells [28, 32]. To investigate the relationship between anti-proliferative activity of compounds 1–6 and their copper-binding property and capability to enhance cell-uptake of copper, we studied effects of compound/CuCl2 mixtures on cell growth.
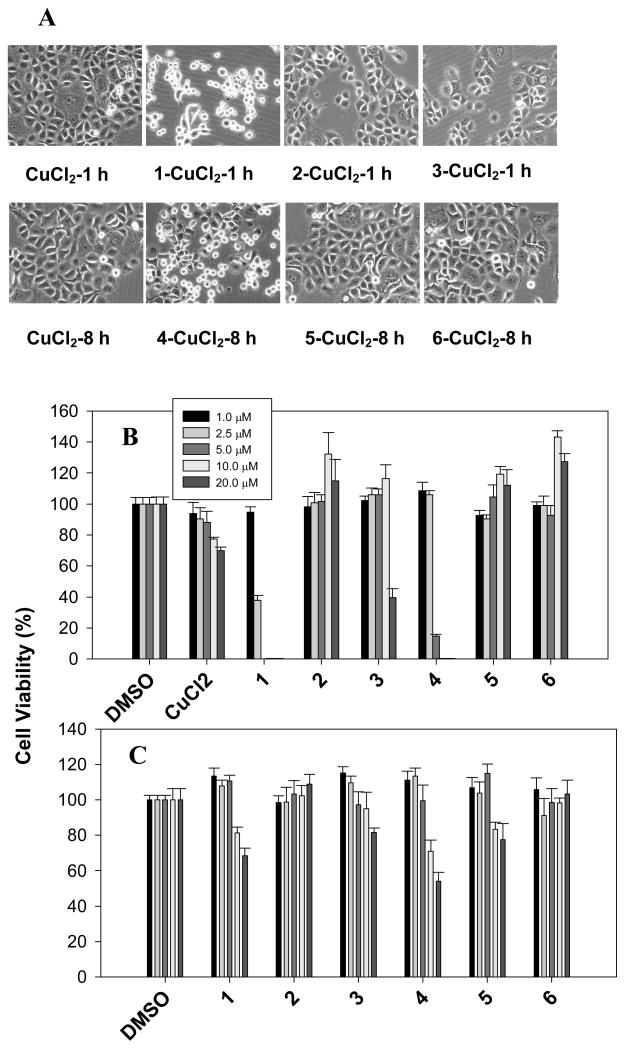
Human breast cancer DCIS cells were treated with active 8-OHQ and CQ as well as their non-copper-binding counterparts (2, 3, 5, 6), followed by observing cellular morphological changes and performance of MTT assay. After even 1 hr treatment, 8-OHQ or 1 but not its analogs (2, 3) caused the cells rounded up and detached (Figure 4A). Similarly an 8 hrs treatment with CQ or 4 but not its inactive analogs (5, 6) caused the apoptotic morphological changes (Figure 4A). Furthermore, the cellular morphological changes were correlated well with the growth-inhibitory activities of these copper-compound mixtures. When DCIS cells were treated with various concentrations of each mixture for 24 h, copper-containing mixtures of 2, 3, 5 and 6 had much less growth-inhibitory effect in contrast to that of 1- and 4-CuCl2 (Figure 4B). The 2-CuCl2 had no effect on cell proliferation at all the concentrations tested, 3-Cu could inhibit the cell proliferation by ~60% at 20 μM, while 1-Cu caused ~60% inhibition even at 2.5 μM (Figure 4B). Similarly, copper mixtures of 5 and 6 could not inhibit cell growth even at the highest concentration used (20 μM) (Figure 4B). In contrast, 4-Cu caused ~85% inhibition even at 5.0 μM (Figure 4B). Compound alone, including 8-OHQ and CQ, showed much less or no toxicity under the same conditions (Figure 4C). Therefore, these data showed that copper-dependent inhibition on cell proliferation by active compounds is related to their copper-binding capability.
Figure 4. The anti-proliferative effect of the compound-copper mixtures.
Cell morphological changes in the DCIS cells treated with 10 μM compound-copper mixtures for indicated time periods (A). Cell viabilities were measured after 24 hrs treatment with compound-CuCl2 (B) or compound alone (C) at different concentrations. After the medium was removed, viable cells were analyzed by MTT method. Data (mean±SD, n=4) are expressed as a percentage of viable cells in DMSO treatment.
8-OHQ- and CQ-Cu, but not mixture of their analogues and Cu, could induce cancer cell death in a concentration- and time-dependent manner
Compounds 1 and 4 have some toxicity in DCIS cells. However, their toxicity was greatly enhanced when 1-Cu or 4-Cu was used. Such copper-dependence was studied using both morphological examination and a cell proliferation assay (Figure 4B). We then investigated whether this was due to apoptotic cell death. DCIS cells were treated with 1–6/CuCl2 mixtures at various concentrations for 12 h. After treatment, cell morphology was observed under the microscopy (Figure 5A&B). Treatment of DCIS cells with 1-CuCl2 and 4-CuCl2 mixture resulted in a time- and concentration-dependent cytotoxicity (Figure 5A&B and SUPPLEMENTAL DATA), while 2–, 5-CuCl2 mixtures and compound alone did not affect cell growth (Figure 5A&B). After treatment with 1- and 4-CuCl2, the cell death-related, calpain-mediated PARP cleavage fragment p65 appeared in a time-dependent manner (Figure 5C)[39]. In contrast, 2- and 5-CuCl2 mixture has no obvious activity on induction of cell death (Figure 5A–C and SUPPLEMENTAL DATA).
Figure 5. Dose-dependent induction of cell death in DCIS cells by 8-OHQ, CQ and their analogs.
Cell morphological changes in the DCIS cells treated with compound 1, 2, 4 and 5, CuCl2, or compound-Cu mixture at increasing concentrations for 12 hrs (A, B). Time-dependent PARP cleavage after treatment with CuCl2 mixtures of 1, 2, 4 and 5 is also shown (C).
Proteasome inhibition before cell death by 8-OHQ-copper or CQ-copper mixture, but not their analogues
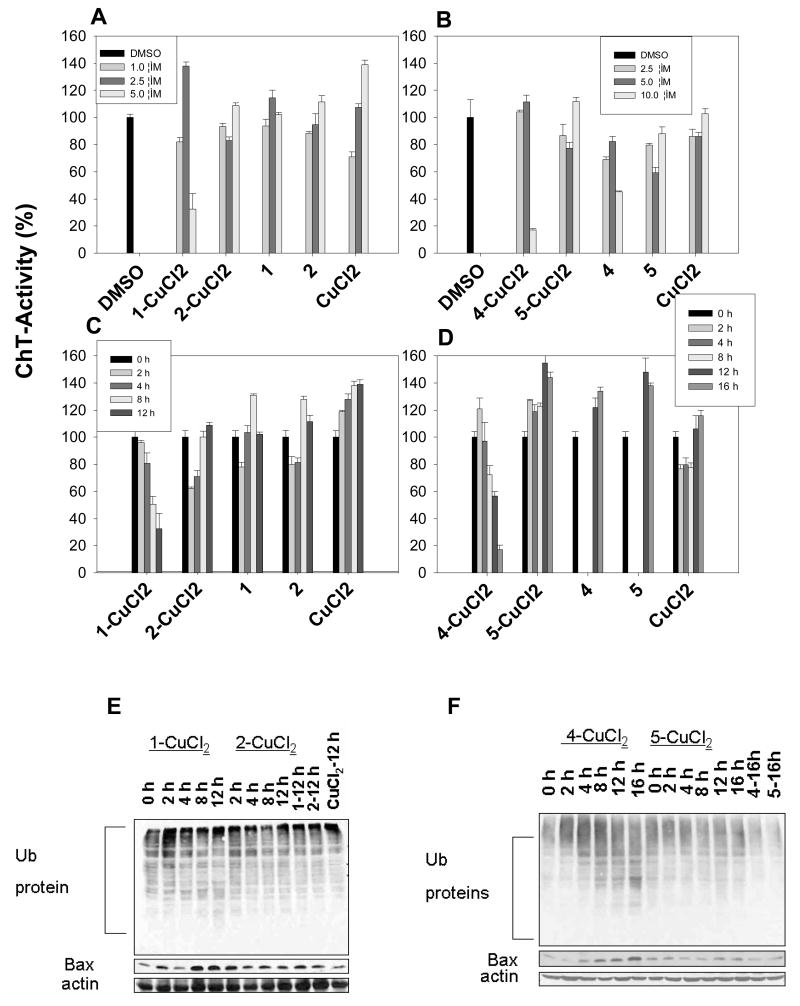
To ascertain the requirement of copper binding for proteasome inhibition activity of compound 1 and 4, we performed a time-dependent study. DCIS cells were treated with the copper mixtures of 1 vs. 2 and 4 vs. 5 for various times. Cells were harvested and lysed. Proteasome inhibition was measured by levels of the chymotrypsin-like activity, ubiquitinated proteins, and the proteasome target protein Bax.
The results from the activity assay showed that 1-CuCl2 mixture inhibited the CT-like activity in a concentration- and time-dependent manner (Figure 6A–D). We detected a ~20% inhibition of the proteasomal CT-like activity by 1-CuCl2 mixture after 4 hrs of treatment, ~50% inhibition after 8 h, and ~67% inhibition after 12 hrs (Figure 6C). In contrast, 2-CuCl2 did not show obvious inhibition on proteasomal CT-like activity. Similarly, a ~28% inhibition of the proteasomal CT-like activity by 4-CuCl2 mixture after 8 hrs of treatment, ~44% inhibition after 12 h, and ~83% inhibition after 16 hrs were observed (Figure 6D). Compound 5-CuCl2 mixture did not inhibit proteasomal CT-like activity. Consistent with decreased proteasomal activity, ubquititinated proteins and Bax also accumulated in a time-dependent manner by mixtures of 1-CuCl2 or 4-CuCl2, but not 2-CuCl2 or 5-CuCl2 (Figure 6E&F). As to cells treated with compound 1 (8-OHQ)-copper mixture, both CT-like and the Trypsin-like activity (data not shown) were inhibited leading to the accumulation of Ub-protein. When cells were treated with compound 4 (CQ)-copper mixture, they were not inhibited in the same way (data not shown). This might explain the apparent discrepancy in the Ub-protein accumulation (Figure 6E&F). Alternatively, these copper complexes might have multiple targets, including 20S and 19S proteasomes, the JAMM domain of the 19S caps in the 26S proteasome could be another possible target for these complexes and further studies are needed to assess this possibility [40].
Figure 6. Inhibition of proteasomal chymotrypsin-like activity by 1-, 2-, 4-, and 5-CuCl2 mixtures.
DCIS cells were treated with either solvent alone (DMSO) or with 1-, 2-, 4- and 5-CuCl2 mixtures at various concentrations (A, B) or for different time period (C, D), followed by harvesting cells to measure the proteasomal chymotrypsin-like activity (A–D), accumulation of ubiquitinated proteins, and Bax protein (E, F).
Taken together, our data strongly suggest that tumor cellular proteasome inhibition and growth suppression by 1 (8-hydroxyquinoline) and 4 (clioquinol) requires their capabilities to bind copper and transport copper into cells.
DISCUSSION
Copper is a crucial cofactor for a variety of cellular processes such as respiration, iron homeostasis, antioxidant defense, angiogenesis, and immune responses. Now it has become very clear that the cellular and organ levels of copper ions are tightly regulated [1]. Many tumor tissues have the tendency to accumulate high concentrations of copper and copper is required in tumor development because of its role in angiogenesis. Therefore, the idea to convert cancer-promoting copper into cancer-inhibiting drug is very attractive.
The ubiquitin-proteasome pathway plays a critical role in degradation of proteins that regulate cell cycle progression, proliferation, apoptosis and angiogenesis [13, 14]. We have previously found that copper-binding compounds such as 8-OHQ and CQ, when mixed with copper, could inhibit the proteasome activity and induce apoptosis in cancer cells [28, 29, 32, 33]. Although this copper-dependent anti-cancer activity has been repeatedly reported, the nature of such dependence has not been experimentally elucidated. Several papers also reported copper-independent cancer cell growth inhibition, adding controversies and complications into this research area. In this investigation, we applied small chemical probe molecules to dissect the complex chemical and biological processes in order to gain thorough understanding of the copper-dependent proteasome inhibition, cell death induction, and the inhibition of cancer cell proliferation by 8-OHQ and CQ.
The chemical probe molecules 2, 3, 5, and 6 maintain the overall structural skeletons as 8-OHQ or 1 and CQ or 4, but do not have the copper-binding capability. They provide ideal tool molecules to only investigate the role of copper-binding in this complicated cellular process triggered by 8-OHQ and CQ without altering molecular interactions with numerous cellular components that 8-OHQ and CQ have.
The first finding is that the strong copper-binding capabilities by 8-OHQ and CQ in combination with their superb membrane permeability transported a large amount of copper into cells, most likely still in the form of 8-OHQ-Cu or CQ-Cu complexes. Analogs that did not bind copper lost such activity although analog molecules themselves were expected to still accumulate inside cells for their excellent cell membrane permeability (Table 1). In the presence of 8-OHQ and CQ, the intracellular copper level increased by 135-fold and 50 fold respectively, while 2, 3, 5 and 6 did not change the accumulation of intracellular copper. It is known that copper intake is mediated by membrane transporter proteins such as Ctr1p, Ctr3p and others and such intake is well controlled [1, 41]. Our unpublished work on the direct effect of synthesized 8-OHQ–copper complex (Cu [8-OHQ]2) showed that the copper complexes have anti-proliferative effects comparable to their copper mixtures. The proteasome-inhibitory effects were strictly attributed to the complexes with copper. Ligand alone was active against the tumor cells only when grown in copper-enriched medium, where an increased level of cellular copper was available for reacting with the ligand [32]. Incubation of cells with copper only elevated the cellular level of copper slightly due to the tight regulation of copper influx by cells. It seemed that the copper transport by 8-OHQ and CQ was independent of transporter proteins. This elevation of cellular copper may produce significant consequences inside cells.
One of the consequences is the inhibition of human breast cancer cell proliferation by 8-OHQ-Cu or CQ-Cu. In contrast, copper mixtures of compounds 2, 3, 5, and 6 did not inhibit cancer cell proliferation. The net accumulation of compounds 2, 3, 5, and 6 to a similar level as 8-OHQ and CQ were expected due to their similar membrane permeability and low efflux ratio (Table 1). Our data strongly suggest that the inhibition of cancer cell proliferation requires both the copper binding and cell-uptake of copper complex. The active inhibitor form is most likely 8-OHQ-Cu or CQ-Cu complexes.
The inhibition of proliferation of human breast cancer cells by 8-OHQ-Cu and CQ-Cu was not due to the passive copper-binding. Actually, copper mixtures of 8-OHQ and CQ inhibited proteasomal CT-like activity and induced cell death. Losing the capability to bind copper and to cause cellular copper accumulation of analogs 2, 3, 5, and 6 is associated with their failure to inhibit CT-like activity and to induce cell death. Comparing the time courses for 8-OHQ-Cu- or CQ-Cu-induced inhibitions of proteasome and DCIS cell proliferation, we found that both processes reached the maximum effect at 8–12 hrs.
Using an array of chemical probe molecules that mimic structures of 8-OHQ and CQ, but have no copper-binding capability, we revealed that the copper-binding by 8-OHQ and CQ and the enhancement of cellular copper complex accumulation are prerequisite for their activities to inhibit cancer cell proliferation. Our studies dissected the actions of 8-OHQ and CQ into several steps. The process starts with the copper-binding by 8-OHQ or CQ and then an overwhelming copper transport into cells that generates a 50–135 fold increase in cellular copper (most likely copper complex) accumulation. These copper complexes should then inhibit proteasomal CT-like activity and induce cell death leading to the inhibition of proliferation of cancer cells. The inactive analogs blocked the very first step in this cancer cell inhibition process.
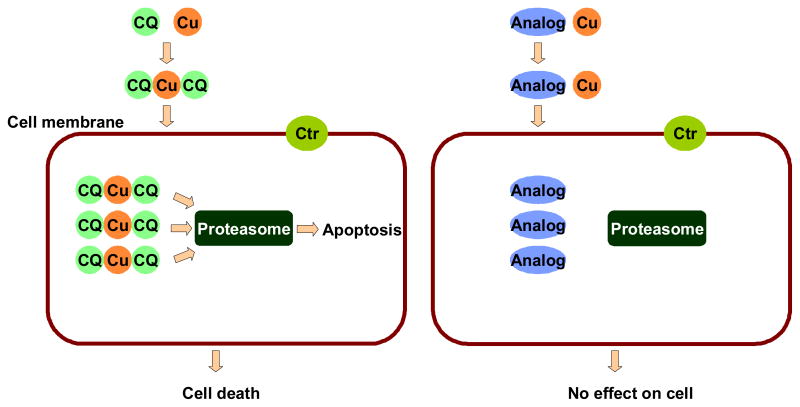
Both compounds and their copper complexes can enter cells and accumulate inside cells due to their excellent membrane permeability and low efflux ratio (Table 1). After CuCl2 treatment for two days, the addition of 8-OHQ analogs did not cause any cancer cell inhibitory activity compared to the strong inhibition when 8-OHQ-CuCl2 mixture was added. In the former case, the cellular copper concentration is rather limited and the addition of 8-OHQ analogs only induced the cellular accumulation of inactive 8-OHQ analogs (due to their high membrane permeability). However, in the latter case, the significant cellular accumulation of 8-OHQ-Cu or CQ-Cu was possible. It is likely that only the high cellular level of their copper complexes mediated inhibition of proteasome activity and cell proliferation (Figure 7).
Figure 7.
Working model for the anti-cancer action of 8-OHQ and CQ (shown as example) and the blockade of such action by non-copper binding analogs.
In summary, 8-OHQ and CQ are bioactive copper chelators. When interacted with copper salt or cellular copper, they inhibited proliferation of cancer cells in vitro and tumor growth in vivo. However, controversy has been developed due to reports of copper-independent cell proliferation suppression by these molecules [36]. Using small chemical probe molecules that mimic 8-OHQ and CQ structures, but have no copper binding capability, we dissected the complex molecular interactions network and tested the hypothesis that copper binding and transportation of copper into cells are prerequisites for their cancer cell growth-inhibitory activities. Without copper binding, analogs penetrated cell membrane and accumulated inside cell in the same way as 8-OHQ- or CQ-Cu. These non-copper-binding molecules failed to transport copper into cells. Consequently, the inhibition of proteasomal CT-like activity and cell growht as well as the induction of cell death, as demonstrated by 8-OHQ-Cu and CQ-Cu, were also impaired. The discovery that copper-binding and transportation are the structural and functional requirements for 8-OHQ’s and CQ’s biological activities to inhibit cancer cell proliferation demonstrates the utility of small chemical probe molecules in the study of complex cellular processes.
MATERIAL AND METHODS
Materials
8-hydroxyquinoline (1), 8-ethoxyquinoline (3) 5-chloro-7-iodo-8-hydroxyquinoline (4), CuCl2, 3-[4,5-dimethyltiazol-2-yl]-2.5-diphenyltetrazolium bromide (MTT), dimethylsulfoxide (DMSO) were purchased from Sigma-Aldrich (St. Louis, MO). All the chemicals used for synthesis of 8-methoxyquinoline (2), 5-chloro-7-iodo-8-methoxyquinoline (5) and 5-chloro-8-methoxyquinoline (6) were purchased from Acros (Geel, Belgium). DMEM/F12, Horse serum, sodium bicarbonate, HEPES buffer solution, penicillin, and streptomycin were purchased from Invitrogen (Carlsbad, CA). Fluorogenic peptide substrates (Suc-LLVY-AMC) for the proteasomal CT-like activity assay were from Calbiochem (San Diego, CA). Mouse monoclonal antibody against human poly (ADP-ribose) polymerase (PARP) was purchased from Biomol International LP (Plymouth Meeting, PA). Mouse monoclonal antibodies against Bax (B-9), and ubiquitin (P4D1), goat polyclonal antibody against actin (C-11), and secondary antibodies were from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA). The water used in this study was purified by reverse osmosis on a Milli-Ro followed by ion exchange.
Instruments
UV-vis spectra were recorded on a Shimadzu-2550 UV spectrophotometer (Kyoto, Japan). Fluorescence spectra were measured on a Hitachi F-4500 spectrofluorimeter (Tokyo, Japan). Proton NMR was carried out on a Bruker AVANCE 400 nuclear magnetic resonance spectrometer (Bruker, Germany). LC-MS were performed on a Shimadzu system. A C18 column (2.0 μm, 2.0 × 50 mm) was used for the separation. The mobile phases were methanol and water both containing 0.05% methanoic acid. A linear gradient was used to increase from 25:75 v/v methanol/water to 100% methanol over 8.0 min at a flow rate of 0.3 mL/min. The UV detection was at 214 nm. Mass spectra were recorded in positive and negative ion mode using electrospray ionization. Cellular copper content was determined on a Varian 715-ES, ICP-OES (Palo Alto, CA). A Zeiss (Thornwood, NY) Axiovert 25 microscope with phase contrast was used for cellular morphology studies.
Synthesis of 2 (8-methoxyquinoline), 5 (5-chloro-7-iodo-8-hydroxyquinoline) and 6 (5-chloro-8-methoxyquinoline)
8-hydroxyquinoline (0.2 g, 1.38 mmol) was dissolved in DMF (2 ml). Solid K2CO3 (0.55 g) and iodomethane (0.26 ml) were added. The reaction mixture was stirred at room temperature for 24 hrs. Water (20 ml) was added to stop the reaction followed by acylacetate extraction, washing with water, and drying. Yield: 72%, ESI-MS: m/z 160 (M+1). 5-chloro-8-methoxyquinoline and 5-chloro-7-iodo-8-methoxyquinoline were made in the same way.
5-chloro-8-methoxyquinoline
Yield: 60 %, ESI-MS: m/z 194 (M+1)
5-chloro-7-iodo-8-methoxyquinoline
Yield: 87%, ESI-MS: m/z 320 (M+1.
Determination of Cu-ligand binding constant using UV-vis titration
Methanol solution of CuCl2 (2.5 mM) was titrated into a 3 ml methanol solution of a compound (0.1 mM) at a 10 μl increment. The UV spectra were recorded on a UV-2550 UV-Vis spectrophotometer.
Cell proliferation assay
The MTT assay was used to measure the effects of the various compounds or compound-copper mixtures on breast cancer cell proliferation. Cells were plated in a 96-well plate and grown to 70–80% confluency, followed by the addition of each compound-copper mixture at the indicated concentrations. After incubation at 37 C for 24 hrs, inhibition of cell proliferation was measured using MTT method.
Measurement of copper accumulation in the breast cancer cells
For copper uptake measurement, the DCIS cells were plated in 150 mm dishes in DMEM/F12 media (containing 5% horse bovine serum, 0.029 mol/L sodium bicarbonate, 10 mmol/L HEPES buffer solution, 100 units/mL of penicillin, and 100 μg/mL of streptomycin). Once plates were 80% confluent, 10 μM compound-CuCl2 mixture was added, and plates were incubated at 37°C in 5% CO2 for various times. After rinsing three times with ice-cold phosphate-buffered saline, cells were harvested by digesting with trypsin-EDTA scraping, followed by centrifugation for 5 min at 1400 rpm and 4°C. The resulting pellet was dissolved in 70% nitric acid at 65°C for at least 4 h. Samples were diluted to 5% nitric acid with water and the Cu content was determined using a Varian 715-ES, ICP-OES (Palo Alto, CA).
Parallel artificial membrane permeability assay (PAMPA)
This assay is to analyze permeability of various compounds on a homogeneous artificial lipid membrane. A 6 μL of 10 mM compounds solution in DMSO was applied to each well in a 96-well plate. Compounds were diluted 200 fold in buffer (pH=7.4; pION INC, Woburn, MA). A 180 μL of diluted solution was added to a donor plate (pION INC, Woburn, MA). A filter plate (acceptor plate; pION INC, Woburn, MA) containing 200 μL of acceptor sink buffer (ASB, pH=7.4; pION INC, Woburn, MA) was then placed over the donor plate. The plates were incubated at room temperature for 0.5 hr with magnetic stirring in individual well to allow the compounds to cross the membrane. Fractions were collected from both the donor plate and the acceptor plate, and concentrations were assessed by UV plate spectrometry. Sample preparation, sample analysis, and data processing are fully automated using Biomek ADME-TOX workstation and the UV-based PAMPA Evolution-96 Command Software. (Beckman Coulter, Inc.; Fullerton, CA).
Caco-2 permeability assay
High throughput Caco-2 permeability assay was performed in the 96-well transwell system. The cells were cultured in MEM containing 20% FBS in 75 cm2 flasks, 100 units/ml of penicillin, and 100 μg/ml of streptomycin and were maintained at 37 °C in a humidified incubator with an atmosphere of 5% CO2. The Caco-2 cells were seeded onto inserts of a 96-well plate at a density of 0.165×105 cells/insert and cultured in the MEM containing 10% FBS for 21 days and the integrity of the monolayer was checked by trans-membrane electric resistance measurements.
Each cultured monolayer on the 96-well plate was washed twice with HBSS/HEPES (10 mM, pH 7.4). The permeability assay was initiated by the addition of each compound solution (50 μmol/L) into inserts (apical side, A) or receivers (basolateral side, B). The Caco-2 cell monolayers were incubated for 2 hrs at 37°C. Fractions were collected from receivers (if apical to basal permeability) or inserts (if basal to apical permeability), and concentrations were assessed by UPLC/MS (Waters; Milford, MA).
The A→B (or B→A) apparent permeability coefficients (Pappa, cm/s) of each compound were calculated using the equation, Pappa=dQ/dt×1/AC0. The flux of a drug across the monolayer is dQ/dt (μmol/s). The initial drug concentration on the apical side is C0 (μmol/L). The surface area of the monolayer is A (cm2). The efflux ratio was defined by B→A/A→B.
Cell cultures and whole cell extract preparation
Human breast cancer DCIS cells were grown in DMEM/F12 supplemented with 5% (v/v) horse bovine serum, 0.029 mol/L sodium bicarbonate, 10 mmol/L HEPES buffer solution, 100 units/mL of penicillin, and 100 μg/mL of streptomycin, at 37 C in a humidified incubator with an atmosphere of 5% CO2. The whole cell extract was prepared as previously described [18]. Briefly, cells were washed twice with PBS and homogenized in a lysis buffer (50 mM Tris-HCl, pH 8.0, 150 mM NaCl, 0.5% NP40). After 20 min rocking at 4°C, the mixtures were centrifuged at 12,000 g for 15 min and the supernatants were collected as whole-cell extracts.
Proteasome activity assay
Breast cancer cells were grown to 70–80% confluency, treated as indicated, harvested, and then used for whole cell extract preparation. Proteasomal chymotrypsin-like activity was assayed as previously described [28]. Briefly, cell lysates (7.5 μg) were incubated with 40 μmol/L of fluorogenic substrate for proteasomal chymotrypsin-like activity for 2 hrs at 37 C in 100 μl of assay buffer (50 μmol/L Tris-HCl, pH 7.5). After incubation, production of hydrolyzed 7-amino-4methylcoumarin (AMC) groups was measured using a Victor3 Multilabel Counter (PerkinElmer, Boston, MA, USA). Statistical analysis was performed using SigmaPlot 8.0 software.
Western blot analysis
Breast cancer DCIS cells were treated as described above. Cell lysates (50 μg) were separated by SDS-PAGE and transferred to a nitrocellulose membrane, followed by visualization using the enhanced chemiluminescence kit (Amersham Biosciences, Piscataway, NJ), as previously described [29].
Supplementary Material
Acknowledgments
This work was supported by Shandong University, the American Lebanese Syrian Associated Charities (ALSAC) and St. Jude Children’s Research Hospital to BY, and research funds from the Karmanos Cancer Institute of Wayne State University (to QPD), the Department of Defense Breast Cancer Research Program (W81XWH-04-1-0688, DAMD17-03-1-0175, to QPD) and the National Cancer Institute (1R01CA120009, 1R21CA139386-01, to QPD).
Units & Abbreviations
- CQ
clioquinol
- 8-OHQ
8-hydroxylquinoline
- CT
chymotrypsin
- PAMPA
parallel artificial membrane permeability assay
Footnotes
Supplemental data include time-dependent morphology observations can be found with this article online.
References
- 1.Labbe S, Thiele DJ. Trends Microbiol. 1999;7:500–505. doi: 10.1016/s0966-842x(99)01638-8. [DOI] [PubMed] [Google Scholar]
- 2.Maverakis E, Fung MA, Lynch PJ, Draznin M, Michael DJ, Ruben B, Fazel N. J Am Acad Dermatol. 2007;56:116–124. doi: 10.1016/j.jaad.2006.08.015. [DOI] [PubMed] [Google Scholar]
- 3.Geraki K, Farquharson MJ, Bradley DA. Phys Med Biol. 2002;47:2327–2339. doi: 10.1088/0031-9155/47/13/310. [DOI] [PubMed] [Google Scholar]
- 4.Nayak SB, Bhat VR, Upadhyay D, Udupa SL. Indian J Physiol Pharmacol. 2003;47:108–110. [PubMed] [Google Scholar]
- 5.Diez M, Arroyo M, Cerdan FJ, Munoz M, Martin MA, Balibrea JL. Oncology. 1989;46:230–234. doi: 10.1159/000226722. [DOI] [PubMed] [Google Scholar]
- 6.Yoshida D, Ikeda Y, Nakazawa S. J Neurooncol. 1993;16:109–115. doi: 10.1007/BF01324697. [DOI] [PubMed] [Google Scholar]
- 7.Eatock MM, Schatzlein A, Kaye SB. Cancer Treat Rev. 2000;26:191–204. doi: 10.1053/ctrv.1999.0158. [DOI] [PubMed] [Google Scholar]
- 8.Fox SB, Gasparini G, Harris AL. Lancet Oncol. 2001;2:278–289. doi: 10.1016/S1470-2045(00)00323-5. [DOI] [PubMed] [Google Scholar]
- 9.Brewer GJ. Exp Biol Med (Maywood) 2001;226:665–673. doi: 10.1177/153537020222600712. [DOI] [PubMed] [Google Scholar]
- 10.Adsule S, Barve V, Chen D, Ahmed F, Dou QP, Padhye S, Sarkar FH. J Med Chem. 2006;49:7242–7246. doi: 10.1021/jm060712l. [DOI] [PubMed] [Google Scholar]
- 11.Brewer GJ, Dick RD, Grover DK, LeClaire V, Tseng M, Wicha M, Pienta K, Redman BG, Jahan T, Sondak VK, Strawderman M, LeCarpentier G, Merajver SD. Clin Cancer Res. 2000;6:1–10. [PubMed] [Google Scholar]
- 12.Redman BG, Esper P, Pan Q, Dunn RL, Hussain HK, Chenevert T, Brewer GJ, Merajver SD. Clin Cancer Res. 2003;9:1666–1672. [PubMed] [Google Scholar]
- 13.Landis-Piwowar KR, Milacic V, Chen D, Yang H, Zhao Y, Chan TH, Yan B, Dou QP. Drug Resist Updat. 2006;9:263–273. doi: 10.1016/j.drup.2006.11.001. [DOI] [PubMed] [Google Scholar]
- 14.Orlowski RZ, Dees EC. Breast Cancer Res. 2003;5:1–7. doi: 10.1186/bcr460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ciechanover A. EMBO J. 1998;17:7151–7160. doi: 10.1093/emboj/17.24.7151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Seemuller E, Lupas A, Stock D, Lowe J, Huber R, Baumeister W. Science. 1995;268:579–582. doi: 10.1126/science.7725107. [DOI] [PubMed] [Google Scholar]
- 17.Kisselev AF, Callard A, Goldberg AL. J Biol Chem. 2006;281:8582–8590. doi: 10.1074/jbc.M509043200. [DOI] [PubMed] [Google Scholar]
- 18.An B, Goldfarb RH, Siman R, Dou QP. Cell Death Differ. 1998;5:1062–1075. doi: 10.1038/sj.cdd.4400436. [DOI] [PubMed] [Google Scholar]
- 19.Lopes UG, Erhardt P, Yao R, Cooper GM. J Biol Chem. 1997;272:12893–12896. doi: 10.1074/jbc.272.20.12893. [DOI] [PubMed] [Google Scholar]
- 20.Nordenberg J, Novogrodsky A, Beery E, Patia M, Wasserman L, Warshawsky A. Eur J Cancer. 1990;26:905–907. doi: 10.1016/0277-5379(90)90197-2. [DOI] [PubMed] [Google Scholar]
- 21.Shen AY, Wu SN, Chiu CT. J Pharm Pharmacol. 1999;51:543–548. doi: 10.1211/0022357991772826. [DOI] [PubMed] [Google Scholar]
- 22.Moret V, Laras Y, Cresteil T, Aubert G, Ping DQ, Di C, Barthelemy-Requin M, Beclin C, Peyrot V, Allegro D, Rolland A, De Angelis F, Gatti E, Pierre P, Pasquini L, Petrucci E, Testa U, Kraus JL. Eur J Med Chem. 2009;44:558–567. doi: 10.1016/j.ejmech.2008.03.042. [DOI] [PubMed] [Google Scholar]
- 23.Deraeve C, Boldron C, Maraval A, Mazarguil H, Gornitzka H, Vendier L, Pitie M, Meunier B. Chemistry. 2008;14:682–696. doi: 10.1002/chem.200701024. [DOI] [PubMed] [Google Scholar]
- 24.Martirosyan AR, Rahim-Bata R, Freeman AB, Clarke CD, Howard RL, Strobl JS. Biochem Pharmacol. 2004;68:1729–1738. doi: 10.1016/j.bcp.2004.05.003. [DOI] [PubMed] [Google Scholar]
- 25.Dou QP, Li B. Drug Resist Updat. 1999;2:215–223. doi: 10.1054/drup.1999.0095. [DOI] [PubMed] [Google Scholar]
- 26.Adams J. Drug Discov Today. 2003;8:307–315. doi: 10.1016/s1359-6446(03)02647-3. [DOI] [PubMed] [Google Scholar]
- 27.Ding WQ, Liu B, Vaught JL, Yamauchi H, Lind SE. Cancer Res. 2005;65:3389–3395. doi: 10.1158/0008-5472.CAN-04-3577. [DOI] [PubMed] [Google Scholar]
- 28.Daniel KG, Chen D, Orlu S, Cui QC, Miller FR, Dou QP. Breast Cancer Res. 2005;7:R897–908. doi: 10.1186/bcr1322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen D, Peng F, Cui QC, Daniel KG, Orlu S, Liu J, Dou QP. Front Biosci. 2005;10:2932–2939. doi: 10.2741/1749. [DOI] [PubMed] [Google Scholar]
- 30.Li L, Xu B. Tetrahedron. 2008;64:10986–10995. [Google Scholar]
- 31.Bevan JA, Graddon DP, McConnell JF. Nature. 1963:363. [Google Scholar]
- 32.Daniel KG, Gupta P, Harbach RH, Guida WC, Dou QP. Biochem Pharmacol. 2004;67:1139–1151. doi: 10.1016/j.bcp.2003.10.031. [DOI] [PubMed] [Google Scholar]
- 33.Chen D, Cui QC, Yang H, Barrea RA, Sarkar FH, Sheng S, Yan B, Reddy GP, Dou QP. Cancer Res. 2007;67:1636–1644. doi: 10.1158/0008-5472.CAN-06-3546. [DOI] [PubMed] [Google Scholar]
- 34.Treiber C, Simons A, Strauss M, Hafner M, Cappai R, Bayer TA, Multhaup G. J Biol Chem. 2004;279:51958–51964. doi: 10.1074/jbc.M407410200. [DOI] [PubMed] [Google Scholar]
- 35.Mao X, Li X, Sprangers R, Wang X, Venugopal A, Wood T, Zhang Y, Kuntz DA, Coe E, Trudel S, Rose D, Batey RA, Kay LE, Schimmer AD. Leukemia. 2009;23:585–590. doi: 10.1038/leu.2008.232. [DOI] [PubMed] [Google Scholar]
- 36.Filiz G, Caragounis A, Bica L, Du T, Masters CL, Crouch PJ, White AR. Int J Biochem Cell Biol. 2008;40:1030–1042. doi: 10.1016/j.biocel.2007.11.003. [DOI] [PubMed] [Google Scholar]
- 37.Vaira MD, Bazzicalupi C, Orioli P, Messori L, Bruni B, Zatta P. Inorganic Chemistry. 2004;43:3795–3797. doi: 10.1021/ic0494051. [DOI] [PubMed] [Google Scholar]
- 38.Ferrada E, Arancibia V, Loeb B, Norambuena E, Olea-Azar C, Huidobro-Toro JP. Neurotoxicology. 2007;28:445–449. doi: 10.1016/j.neuro.2007.02.004. [DOI] [PubMed] [Google Scholar]
- 39.Pink JJ, Wuerzberger-Davis S, Tagliarino C, Planchon SM, Yang X, Froelich CJ, Boothman DA. Exp Cell Res. 2000;255:144–155. doi: 10.1006/excr.1999.4790. [DOI] [PubMed] [Google Scholar]
- 40.Cvek B, Milacic V, Taraba J, Dou QP. J Med Chem. 2008;51:6256–6258. doi: 10.1021/jm8007807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Harris ED. Annu Rev Nutr. 2000;20:291–310. doi: 10.1146/annurev.nutr.20.1.291. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.