Abstract
Combining triflic acid promoted glycosylations of trichloroacetimidates with reductive opening of benzylidene acetals with triflic acid and triethylsilane as one-pot procedures provides easy access to a wide range of di- and branched trisaccharides.
Protein- and lipid-bound saccharides are ubiquitous in biological systems involved in many important molecular processes such as fertilization, embryogenesis, neuronal development, hormone activities, and the proliferation of cells and their organization into specific tissues.1,2 These interactions are also important in health science and are involved in the invasion and attachment of pathogens, inflammation, metastasis, blood group immunology, and xenotransplantation.3-5 A major obstacle to advances in glycobiology is the lack of pure and structurally well-defined carbohydrates and glycoconjugates. These compounds are often found in low concentrations and in micro-heterogeneous forms, greatly complicating their isolation and characterization. In many cases, well-defined oligosaccharides can only be obtained by organic synthesis.6-9
Although the methods for oligosaccharide synthesis have improved considerably during the past decade, the construction of complex carbohydrates remains a significant challenge due to the combined demands of elaborate procedures for glycosyl donor and acceptor preparation and the requirements of regio- and stereoselectivity in glycoside bond formation. To streamline the preparation of complex oligosaccharides, one-pot multi-step approaches for selective monosaccharide protection and oligosaccharide assembly are being pursued, which do not require intermediate work-up and purification step and hence speed-up the process of chemical synthesis considerably.10,11 For example, the observation that acetal formation, regioselective reductive opening of arylidene acetals, reductive etherification and acetylation can be catalyzed by triflic acid (TfOH) or trimethylsilyl triflate (TMSOTf) made it possible to program these reactions in tandem by sequential addition of reagents to give easy access to a wide variety of selective protected monosaccharide building blocks. Furthermore, many laboratories have demonstrated that chemoselective, orthogonal and iterative glycosylation strategies, which exploit differential reactivities of anomeric leaving groups, allow several selected glycosyl donors to react in a specific order resulting in a single oligosaccharide product.12-17
To further streamline the process of oligosaccharide assembly, we report here a strategy whereby a regioselective opening of a benzylidene acetal and glycosylations are combined in a one-pot multi-step synthetic procedure. The attraction of the approach is that it makes it possible to assemble branched oligosaccharides by a one-pot procedure; a task that cannot readily be accomplished by chemoselective, orthogonal and iterative glycosylations. In this respect, differential reactivities of hydroxyls have been exploited for the synthesis of branched oligosaccharides by one-pot procedures18-24 but the scope of this approach is limited because of the need of exceptional high regioselectivities. Trichloroacetimidates were selected as glycosyl donors because their activation requires only catalytic TfOH or TMSOTf.25 Furthermore, TfOH combined with triethylsilane (Et3SiH) was employed for the opening of a 4,6-O-benzylidene acetals26,27 because these conditions provide in general high regioselectivies and excellent yields, and furthermore it was anticipated that these reaction conditions would be compatible with TfOH mediated glycosylations of trichloroacetimidates.
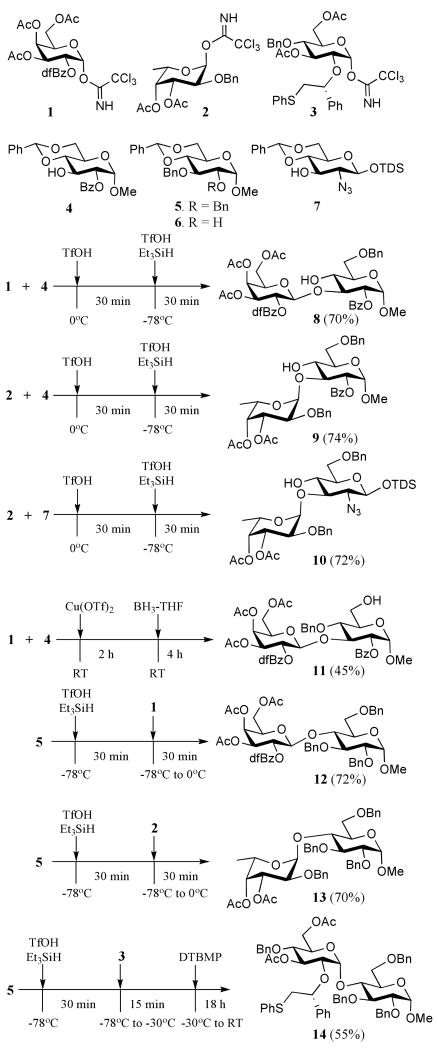
Thus, a mixture of trichloroacetimidate 128 and benzylidene acetal protected glycosyl acceptor 429 in dichloromethane at 0 °C was treated with a catalytic amount of TfOH. After a reaction time of 30 min., TLC and MALDI-MS analysis indicated consumption of the starting material and the formation of the expected disaccharide. The reaction mixture was cooled to -78 °C followed by addition of TfOH (1.8 eq.) and Et3SiH (2.0 eq.). After stirring for 30 minutes at -78 °C, the reaction was quenched by the addition of triethylamine and methanol, and purification by silica gel column chromatography gave disaccharide 8 in a yield of 70%. As expected, only a β-galactoside was formed due to the presence of a 2,5-difluorobenzyl group30 at C-2 of the galactosyl donor 1.28 This protecting group is an excellent neighboring group participant and unlike benzoyl and pivaloyl esters can be cleaved under mild conditions using a catalytic amount of sodium methoxide in methanol.
In the next set of experiments, the sequence of reactions was repeated, however, in this case the fucosyl donor 2 31 was employed instead of 1. Fortunately, disaccharide 9 was isolated in an overall yield of 74% as a single regio- and stereoisomer demonstrating that the methodology is compatible with acid sensitive fucosides. It was also found that fucosylation of 2-azido-containing glycosyl acceptor 732 followed by a regioselective opening of the benzylidene acetal using standard conditions of the resulting disaccharide led to the facile formation of disaccharide 10.
It has been established that the regioselective ring opening of 4,6-O-benzylidene acetals can be reversed to provide saccharides with a free C-6 hydroxyl by employing Cu(OTf)2 in combination with borane33 instead of TfOH and Et3SiH. Furthermore, it was anticipated that trichloroacetimidates can be activated by Cu(OTf)2 and thus it may be possible to glycosylate the C-3 hydroxyl of 4 followed by a regioselective opening of the benzylidene acetal of the resulting product to give a disaccharide having a C-6 hydroxyl. Indeed, a Cu(OTf)2 (0.15 eq.) mediated glycosylation of glucosyl donor 1 with acceptor 4 gave, after a reaction time of 2h at room temperature, a disaccharide, which was treated with borane-THF complex to provide after stirring for an additional 4 h at ambient temperature, disaccharide 11 in a yield of 45%. In our efforts to improve the reaction yield, it was found that prolonged reaction times led to decomposition and formation of by-products.
Next, attention was focused on a reaction sequence whereby a regioselective benzylidene acetal opening is followed by glycosylation. Thus, Et3SiH and TfOH were added to a cooled (-78 °C) solution of compound 5 in dichloromethane and after a reaction time of 30 min, glycosyl donor 1 or 2 was added followed by gradually raising the temperature to 0 °C over a period of 30 min. Standard work-up and purification by silica gel column chromatography gave regio- and stereo-isomerically pure disaccharides 12 and 13, respectively, in yields of 72 and 70%.
Recently, we demonstrated that glycosylations with glycosyl donors modified at C-2 with a (S)-(phenylthiomethyl)benzyl moiety give exclusively α-anomeric selectivity due to neighboring group participation resulting in an intermediate trans-fused 1,2-sulfonium ion.34,35 To explore the possibility of a one-pot procedure involving benzylidine acetal opening and glycosylation with such a donor, compound 536 was treated with TfOH and Et3SiH followed by subsequent addition of 334 and DTBMP to give 14 as only the α-anomer.
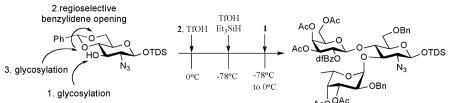
Next, we explored the possibility of synthesizing branched trisaccharides by a reaction sequence involving glycosylation, reductive benzylidene acetal opening followed by another glycosylation (Scheme 2). Thus, a mixture of trichloroacetimidate 2 and benzylidene protected glycosyl acceptor 4 in dichloromethane at 0 °C was treated with a catalytic amount of TfOH. After a reaction time of 30 min., the reaction mixture was cooled to -78 °C followed by addition of TfOH (1.8 eq.) and Et3SiH (2.0 eq.) and after a further reaction time of 30 min, galactosyl donor 1 was added and the reaction mixture was allowed to warm to 0 °C over a period of 30 min, to give after standard work-up and purification by silica gel column chromatography, trisaccharide 15 in a yield of 63%. A similar procedure gave trisaccharide 16 by a glycosylation of 1 with 4, followed by reductive opening of the benzylidene acetal and glycosylation of the resulting acceptor with fucosyl donor 2. Furthermore, a TfOH promoted glycosylation of 2-azido-2-deoxyglucoside 7 with 2 followed by benzylidene acetal opening with TfOH/Et3SiH gave a disaccharide acceptor, which was glycosylated with galactosyl donor 1 to give protected Lewisx trisaccharide 17 in an excellent yield of 67%. Removal of anomeric TDS protecting group of 17 followed by conversion of the resulting lactol into leaving group provides an opportunity to prepare more complex oligosaccharides. Finally, it was demonstrated that the methodology can also be employed for the preparation of 2,4-branched trisaccharides by glycosylation of acceptor 637 with fucosyl donor 2 to give a 2,4-linked disaccharide, which was subjected to Et3SiH/TfOH to regioselectively open the benzylidene to afford a glycosyl acceptor having a C-4 hydroxyl. Addition of galactosyl donor 1 to the latter compound followed by standard work-up and purification by silica gel column chromatography gave trisaccharide 18 in 60% yield.
Scheme 2.
One-pot Synthesis of Trisaccharides by Glycosylations and Benzylidene Acetal Opening
In conclusion, it has been demonstrated that one pot-procedures involving reductive opening of benyzlidene acetals and glycosylations can give easy access to a wide range of di- and trisaccharides. It is to be expected that the utility of the methodology can be further extended by combining acid catalyzed reductive etherifications and acetylations with glycosylations. Also the use of a thioglycoside as the initial acceptor may provide an opportunity to prepare more complex structures by employing the resulting thioglycoside product as a glycosyl donor.
Supplementary Material
Scheme 1.
One-pot Synthesis of Disaccharides by Glycosylation and Benzylidene Acetal Opening
Acknowledgments
This research was supported by a grant from the NIH NIGM (R01GM065248).
Footnotes
Supporting Information Available Experimental procedures and 1H and 13C NMR spectra. This information is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.Kobata A. Eur J Biochem. 1992;209:483–501. doi: 10.1111/j.1432-1033.1992.tb17313.x. [DOI] [PubMed] [Google Scholar]
- 2.Varki A. Cell. 2006;126:841–845. doi: 10.1016/j.cell.2006.08.022. [DOI] [PubMed] [Google Scholar]
- 3.Dube DH, Bertozzi CR. Nat Rev Drug Discov. 2005;4:477–488. doi: 10.1038/nrd1751. [DOI] [PubMed] [Google Scholar]
- 4.Walsh G, Jefferis R. Nature Biotech. 2006;24:1241–1252. doi: 10.1038/nbt1252. [DOI] [PubMed] [Google Scholar]
- 5.Opdenakker G, Rudd PM, Ponting CP, Dwek RA. Faseb Journal. 1993;7:1330–1337. doi: 10.1096/fasebj.7.14.8224606. [DOI] [PubMed] [Google Scholar]
- 6.Davis BG. J Chem Soc Perkin Trans. 1999;1:3215–3237. [Google Scholar]
- 7.Demchenko AV. Synlett. 2003:1225–1240. [Google Scholar]
- 8.Boons GJ. Contemp Org Synth. 1996;3:173–200. [Google Scholar]
- 9.Seeberger PH, Werz DB. Nature. 2007;446:1046–51. doi: 10.1038/nature05819. [DOI] [PubMed] [Google Scholar]
- 10.Francais A, Urban D, Beau JM. Angew Chem Int Ed. 2007;46:8662–8665. doi: 10.1002/anie.200703437. [DOI] [PubMed] [Google Scholar]
- 11.Wang CC, Lee JC, Luo SY, Kulkarni SS, Huang YW, Lee CC, Chang KL, Hung SC. Nature. 2007;446:896–899. doi: 10.1038/nature05730. [DOI] [PubMed] [Google Scholar]
- 12.Boons GJ. Tetrahedron. 1996;52:1095–1121. [Google Scholar]
- 13.Codee JDC, Litjens R, van den Bos LJ, Overkleeft HS, van der Marel GA. Chem Soc Rev. 2005;34:769–782. doi: 10.1039/b417138c. [DOI] [PubMed] [Google Scholar]
- 14.Wang YH, Ye XS, Zhang LH. Org Biomol Chem. 2007;5:2189–2200. doi: 10.1039/b704586g. [DOI] [PubMed] [Google Scholar]
- 15.Wang YH, Zhang LH, Ye XS. Combinatorial Chemistry & High Throughput Screening. 2006;9:63–75. doi: 10.2174/138620706775213912. [DOI] [PubMed] [Google Scholar]
- 16.Douglas NL, Ley SV, Lucking U, Warriner SL. J Chem Soc Perkin Trans. 1998;1:51–65. [Google Scholar]
- 17.Koeller KM, Wong CH. Chem Rev. 2000;100:4465–4493. doi: 10.1021/cr990297n. [DOI] [PubMed] [Google Scholar]
- 18.Mong KKT, Wong CH. Angew Chem Int Ed. 2002;41:4087–4090. doi: 10.1002/1521-3773(20021104)41:21<4087::AID-ANIE4087>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 19.Takahashi T, Adachi M, Matsuda A, Doi T. Tetrahedron Lett. 2000;41:2599–2603. [Google Scholar]
- 20.Tanaka H, Adachi M, Takahashi T. Chem Eur J. 2005;11:849–862. doi: 10.1002/chem.200400840. [DOI] [PubMed] [Google Scholar]
- 21.Valverde S, Garcia M, Gomez AM, Lopez JC. Chem Comm. 2000:813–814. [Google Scholar]
- 22.Yamada H, Kato T, Takahashi T. Tetrahedron Lett. 1999;40:4581–4584. [Google Scholar]
- 23.Yamada H, Harada T, Takakhashi T. J Am Chem Soc. 1994;116:7919–7920. [Google Scholar]
- 24.Yamada H, Harada T, Miyazaki H, Takahashi T. Tetrahedron Lett. 1994;35:3979–3982. [Google Scholar]
- 25.Jung KH, Muller M, Schmidt RR. Chem Rev. 2000;100:4423–4442. doi: 10.1021/cr990307k. [DOI] [PubMed] [Google Scholar]
- 26.Wang CC, Lee JC, Luo SY, Fan HF, Pai CL, Yang WC, Lu LD, Hung SC. Angew Chem Int Ed. 2002;41:2360–2362. doi: 10.1002/1521-3773(20020703)41:13<2360::AID-ANIE2360>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 27.Sakagami M, Hamana H. Tetrahedron Lett. 2000;41:5547–5551. [Google Scholar]
- 28.Cato D, Buskas T, Boons GJ. J Carbohydr Chem. 2005;24:503–516. [Google Scholar]
- 29.Kim S, Chang H, Kim WJ. J Org Chem. 1985;50:1751–1752. [Google Scholar]
- 30.Sjolin P, Kihlberg J. J Org Chem. 2001;66:2957–2965. doi: 10.1021/jo001584q. [DOI] [PubMed] [Google Scholar]
- 31.Rencurosi A, Lay L, Russo G, Caneva E, Poletti L. J Org Chem. 2005;70:7765–7768. doi: 10.1021/jo050704x. [DOI] [PubMed] [Google Scholar]
- 32.Muller T, Hummel G, Schmidt RR. Liebigs Ann Chem. 1994:325–329. [Google Scholar]
- 33.Shie CR, Tzeng ZH, Kulkarni SS, Uang BJ, Hsu CY, Hung SC. Angew Chem Int Ed. 2005;44:1665–1668. doi: 10.1002/anie.200462172. [DOI] [PubMed] [Google Scholar]
- 34.Kim JH, Yang H, Park J, Boons GJ. J Am Chem Soc. 2005;127:12090–12097. doi: 10.1021/ja052548h. [DOI] [PubMed] [Google Scholar]
- 35.Kim JH, Yang H, Boons GJ. Angew Chem Int Ed. 2005;44:947–949. doi: 10.1002/anie.200461745. [DOI] [PubMed] [Google Scholar]
- 36.Ogawa T, Katano K, Matsui M. Tetrahedron. 1980;36:2727–2733. [Google Scholar]
- 37.Methyl 4,6-O-benzylidene-3-O-benzyl-α-D-glucopyranoside (6): To a solution of methyl 4,6-benzylidene-α-D-glucopyranoside (50 mg, 0.18 mmol) in DCM (1.0 mL) was added benzaldehyde (22 μL, 0.21 mmol) and triethylsilane (34 μL, 0.21 mmol), and the mixture was stirred under an atmosphere of argon for 30 min. The mixture was cooled (-78° C) and TfOH (0.3 eq) was added. The reaction mixture was quenched after 30 min. with pyridine (50 μL), diluted with DCM (5 mL), and washed with sat. aq. NaHCO3 (3 mL) and brine (3 mL). The organic layer was dried (MgSO4), concentrated in vacuo and purified by silical gel column chromatography (hexane/ethylacetate, 4/1, v/v) to give 6 as a white solid in 50% yield.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.