Abstract
During the course of oncogenesis and tumor progression, cancer cells constitutively upregulate signaling pathways relevant to cell proliferation and survival as a strategy to overcome genomic instability and acquire resistance phenotype to chemotherapeutic agents. In light of this clinical and molecular heterogeneity of human cancers, it is desirable to concomitantly target these genetic abnormalities by using an agent with pleiotropic mode of action. Indole-3-carbinol and its metabolite 3,3’-diindoylmethane (DIM) target multiple aspects of cancer cell cycle regulation and survival including Akt-NFκB signaling, caspase activation, cyclin-dependent kinase activities, estrogen metabolism, estrogen receptor signaling, endoplasmic reticulum stress, and BRCA gene expression. This broad spectrum of antitumor activities in conjunction with low toxicity underscores the translational value of indole-3-carbinol and its metabolites in cancer prevention/therapy. Furthermore, novel antitumor agents with overlapping underlying mechanisms have emerged via structural optimization of indole-3-carbinol and DIM, which may provide considerable therapeutic advantages over the parental compounds with respect to chemical stability and anti-tumor potency. Together, these agents might foster new strategies for cancer prevention and therapy.
Keywords: indole-3-carbinol; 3,3’-diindoylmethane; Akt-NFκB signaling; nuclear receptor signaling; endoplasmic reticulum stress; BRCA gene expression
1. Introduction
Epidemiological and dietary studies have provided a link between high dietary intake of cruciferous vegetables and lowered cancer risks (1–4). Considerable evidence attributes this chemopreventive effect to the antitumor activity of a common phytochemical, indole-3-carbinol, and its metabolic products. These indole derivatives have been shown to suppress the proliferation of various cancer cell lines at the concentration range of 50 – 100 µM, including those of breast (5–8), colon (9–11), prostate (12–14), and endometrium (15), by targeting a wide spectrum of signaling pathways governing hormonal homeostasis, cell cycle progression, and cell proliferation and survival [reviews:(16–20)]. Moreover, indole-3-carbinol inhibited spontaneous or chemical-induced tumorigenesis in mammary gland, liver, lung, cervix, and gastrointestinal tract in different animal model studies (21–27). These preclinical findings demonstrate the translational value of indole-3-carbinol in cancer prevention and therapy [review: (3)], which has led to its human trials in cervical dysplasia (28), breast cancer (29, 30), vulvar intraepithelial neoplasia (31), and recurrent respiratory papillomatosis (32).
From a mechanistic perspective, the in vivo efficacy of indole-3-carbinol arises from a dynamic interaction between its metabolic disposition and pleiotropic modes of action, which could be pharmacologically exploited to foster new strategies or to develop novel chemopreventive/therapeutic agents. Thus, this minireview addresses the multifaceted aspects of the chemical biology of indole-3-carbinol by giving an overview of the following subjects: (A) metabolic transformation of indole-3-carbinol and its pharmacological relevance, (B) pleiotropic effects of indole-3-carbinol on multiple signaling targets, and (C) pharmacological exploitation of indole-3-carbinol and DIM to develop novel antitumor agents.
2. Metabolic transformation of indole-3-carbinol and its pharmacological relevance
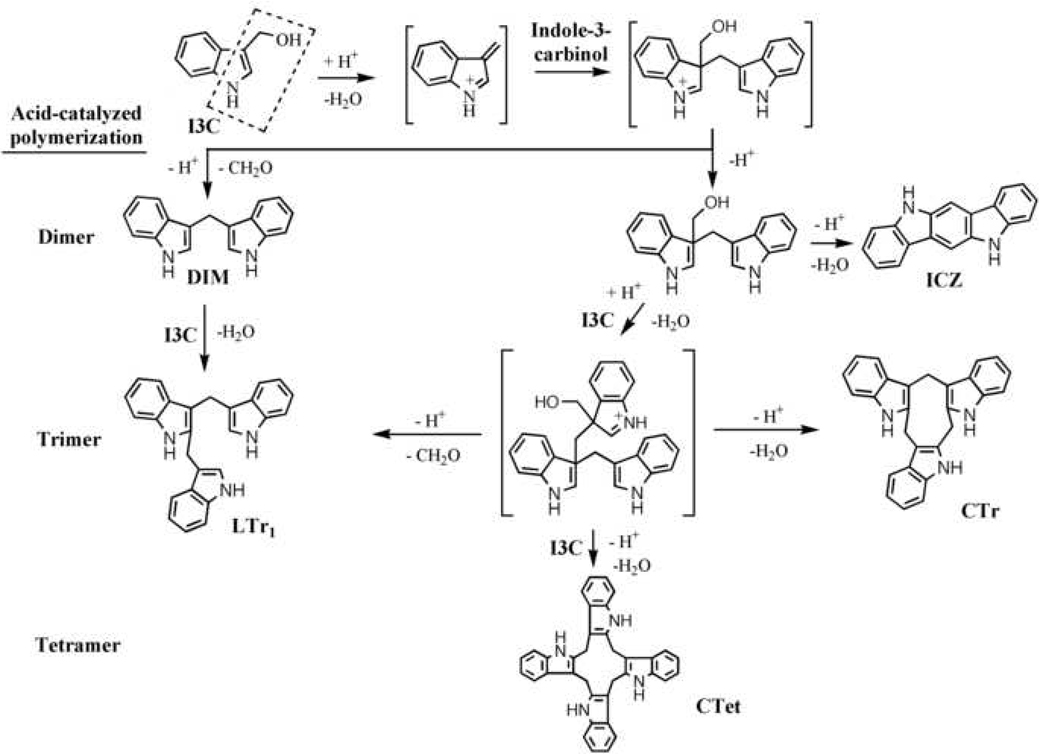
The intrinsic instability of indole-3-carbinol in acidic milieu arises from the vinyl hemiaminal moiety of the indole ring (Fig. 1, enclosed by dashed line) (33). This unique structural feature underlies the high susceptibility of indole-3-carbinol to acid-catalyzed dehydration and condensation to generate a complex series of oligomeric products in vivo (Fig. 1), including DIM (3,3’-diindoylmethane), ICZ (indolo[3,2b]-carbazole), LTr1 (a linear trimer), CTr (a cyclic trimer), and CTet (a cyclic tetramer) (34–37).
Fig. 1.
Metabolic transformation of indole-3-carbinol.
As each of these major metabolites exhibits pharmacological activities (Table 1), the observed chemopreventive effect of indole-3-carbinol in vivo might, at least in part, be attributable to these acid condensation products. Among them, DIM induces apoptosis and cell cycle arrest in cancer cells through signaling mechanisms analogous to those of indole-3-carbinol (14, 38–46), while the functions of ICZ, LTr1, and CTr are associated with nuclear receptors such as the aryl hydrocarbon receptor (AhR) and/or estrogen receptor (ER) (47, 48). It is noteworthy that the tetrameric product CTet suppressed breast cancer growth by inhibiting the expression of cyclin-dependent kinase (CDK)6 and other cell-cycle regulatory proteins, with fivefold higher potency than indole-3-carbinol (49).
Table 1.
Pharmacological effects of indole-3-carbinol metabolites in cancer cells
| Metabolite | Cellular responses | Signaling targets | Ref. | |
|---|---|---|---|---|
| DIM |
|
↓ | Akt phosphorylation | (14, 38 – 46, 50, 51) |
| NF-κB signaling | ||||
| Survivin expression | ||||
| Bcl-2 expression | ||||
| Cdc25A expression | ||||
| CDK 6 expression | ||||
| AR expression | ||||
| ERα signaling | ||||
| ↑ | p21WAF1 expression | |||
| p27kip1 expression | ||||
| DR5 expression | ||||
| AhR signaling | ||||
| ICZ | Activation of AhR and consequent activation of gene expression of Phase I and II enzymes | ↑ | AhR signaling | (47, 48) |
| LTr1 |
|
↓ | ER signaling | (52) |
| ↑ | AhR signaling | |||
| CTr | A potent agonist of (ER)α | ↑ | ER signaling | (53) |
| CTet | Cell cycle arrest | ↓ | CDK 6 expression | (49) |
| ↑ | p27kip1 expression | |||
(↓, downregulation; ↑, upregulation)
3. Pleiotropic effects of indole-3-carbinol on multiple signaling targets
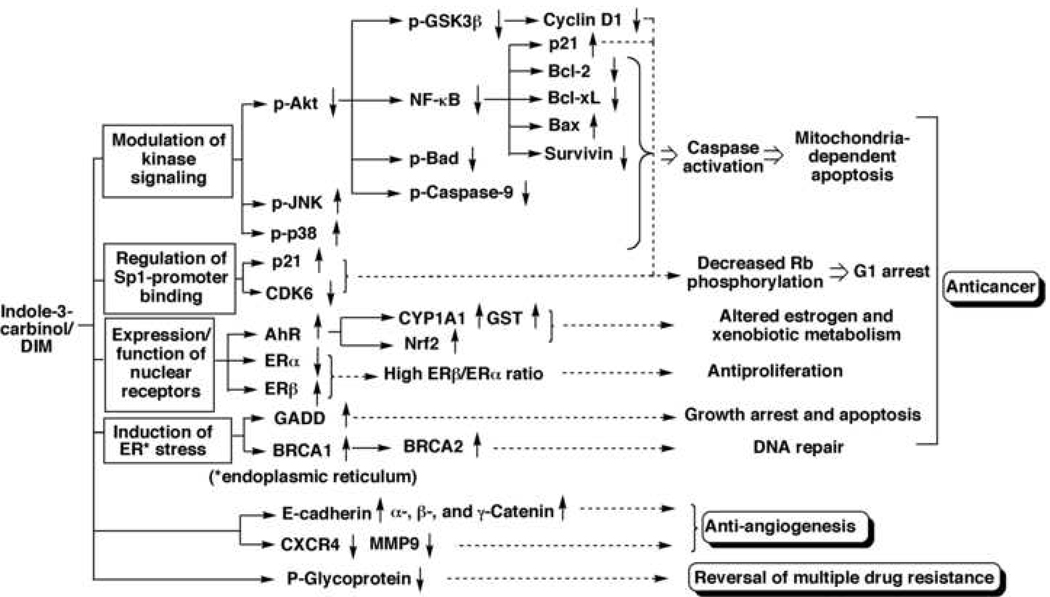
Substantial evidence indicates that the antitumor effect of indole-3-carbinol is attributable to its ability to target a plethora of signaling pathways governing apoptosis, cell cycle progression, hormonal homeostasis, DNA repair, angiogenesis, and multiple drug resistance [reviews: (16–20)]. Moreover, indole-3-carbinol proves to be an effective chemopreventive agent against estrogen-responsive cancers such as breast and cervical cancers, in part, because it functions as a negative regulator of estrogen by inhibiting ERα signaling (54, 55) and altering cytochrome P450-mediated estrogen metabolism (56). It is important to note that the pharmacological responses of indole-3-carbinol in various cellular events might be contributed, in part, by its major metabolite, DIM as these two indole derivatives exhibit a high degree of similarity in their mode of action. Consequently, this section is aimed at providing an overview of the causative relationship between individual signaling targets and various indole-3-carbinol-induced cellular responses (Fig. 2).
Fig. 2.
An overview of the signaling pathways targeted by indole-3-carbinol and DIM.
3.1. Apoptosis Induction
Mounting evidence indicates that inactivation of Akt and its downstream effector, the nuclear transcription factor NF-κB, plays a pivotal role in the proapoptotic action of indole-3-carbinol/DIM in tumor cells (6, 12, 57, 58). It is well documented that Akt promotes cell survival by stimulating NF-κB signaling through IκBα kinase in conjunction with the phosphorylating deactivation of several proapoptotic proteins including glycogen synthase kinase (GSK)3β, Bad, Forkhead transcription factors, and caspase-9, thereby constituting an important target for cancer therapy [recent reviews: (59–64)]. NF-κB acts as an important survival factor in cancer cells by mediating the transcription of a series of antiapoptotic genes, including: Bcl-2, Bcl-xL, survivin, p53, and p21 (65). In addition, indole-3-carbinol has been shown to activate the stress-induced MAP kinases p38 and c-jun N-terminal kinase (JNK) in prostate cancer cells (66), and to inhibit constitutively active STAT3, a transcription factor, in pancreatic carcinoma cells (67). Together, the concerted effects on these proapoptotic components underlie the ability of indole-3-carbinol/DIM to induce mitochondria-dependent apoptosis in tumor cells.
3.2. Cell cycle arrest
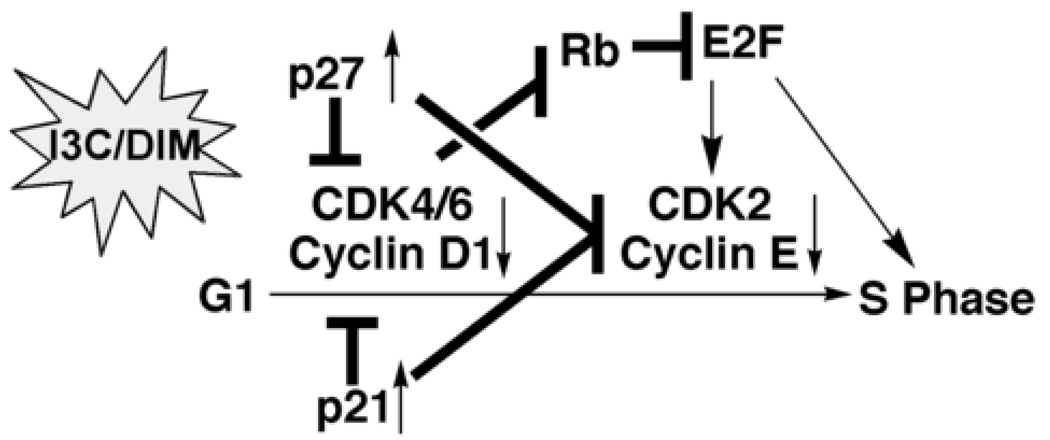
Indole-3-carbinol and DIM exhibit the ability to cause G1 arrest in breast and prostate cancer cells (68, 69). This cell cycle arrest involves the upregulation of the CDK inhibitors p21WAF1 and p27kip1, and the concurrent downregulation of cyclin D1, cyclin E, and CDKs 2, 4, and 6, which is, in part, attributable to the effect of indole-3-carbinol/DIM on regulating Sp1-promoter binding activity (50, 70, 71). In addition, indole-3-carbinol was reported to inhibit CDK2 kinase activity in MCF-7 cells through selective alterations in cyclin E composition, size distribution, and subcellular localization of the CDK2 protein complex (72). Together, inhibition of CDK4/6/cyclin D1 and CDK2/cyclin E activities led to decreased Rb phosphorylation, which causes the Rb protein to bind to the E2F transcription factor. This E2F sequestration blocks the transcription of S phase genes, resulting in G1 arrest (Fig. 3).
Fig. 3.
Effects of indole-3-carbinol/DIM on G1 cell cycle arrest.
3.3. Modulation of the functional /expression status of nuclear receptors
3.3.1. Aryl hydrocarbon receptor (AhR)
AhR is a transcription factor that can be activated by several types of aromatic compounds such as dioxins and flavonoids. Indole-3-carbinol, DIM, and ICZ have been reported to bind and activate AhR with varying potencies (73, 74). In addition, indole-3-carbinol has been reported to increase AhR expression in MCF-7 cells (75). AhR activation plays an important role in the chemopreventive effect of indole-3-carbinol and its metabolites. Specifically, AhR upregulates the gene expression of the Phase I enzyme CYP1A1 and the Phase II enzymes glutathione S-transferase and oxidoreductases in prostate and breast cancer cells (42, 75) and in rat liver (76, 77). These xenobiotic-metabolizing enzymes are involved in inhibiting the activation of chemical carcinogens. In addition, CYP1A1 mediates the 2-hydroxylation of estrone, one of the two major competing hydroxylation pathways of estrone metabolism, leading to increased levels of 2-hydroxyestrone (78). Relative to 16-hydroxyestrone, which is linked to stimulation of estrogen and DNA damage in mammary epithelial cells, 2-hydroxyestrone competes with estradiol for estrogen receptor binding, thereby abrogating the proliferative effect of estradiol (79). Consequently, I3C may act as a phytoestrogen by inducing a competing metabolic pathway that increases production of 2-hydroxyesterone and thus reduces the substrate available for production of 16-hydroxyesterone (80). Together, the ability of indole-3-carbinol to facilitate the metabolism of genotoxic agents, either environmental or endogenous, underlies its effect on cancer prevention.
3.3.2. Estrogen receptor (ER)
Indole-3-carbinol is a negative regulator of ERα signaling in human tumor cells (54). In addition to altering estrogen metabolism through CYP1A1, indole-3-carbinol and its metabolites also affect ER signaling through two different mechanisms. First, indole-3-carbinol and DIM could bind to and inhibit the activity of ER (24, 51), which diminished estradiol-mediated cellular and biochemical effects in estrogen-sensitive cells and tissues (81). Consequently, indole-3-carbinol could cooperate with tamoxifen to inhibit breast cancer proliferation (82). Second, indole-3-carbinol and DIM could suppress ERα expression in breast cancer cells (55, 83), which might be attributable to the ability of these indole derivatives to bind to the nuclear AhR (55). Moreover, indole-3-carbinol was reported to increase the binding of ERβ to the estrogen response element, resulting in a significantly higher ERβ/ERα ratio that is associated with an antiproliferative status in human breast cancer cells (83).
3.4. Endoplasmic reticulum stress
Evidence indicates that indole-3-carbinol and DIM induced endoplasmic reticulum stress responses in cancer cells via unfolded protein response pathways, which led to the induction of a series of stress-related proteins including a series of GADD (Growth Arrest in response to DNA Damage) proteins (54, 84, 85). These endoplasmic reticulum stress-related proteins negatively regulate cell growth, leading to cell cycle arrest and apoptosis (86). For example, GADD153/CHOP, a nuclear transcription factor, represses the Bcl-2 promoter, and sensitizes mitochondria to the proapoptotic effects of BH3-only proteins (87). In addition, GADD153 enhances the expression of death receptor (DR)5, which underlies the effect of indole-3-carbinol on augmenting TRAIL-induced apoptosis in LNCaP prostate cancer cells (88). It is also noteworthy that there exists a mechanistic link between indole-3-carbinol/DIM-induced endoplasmic reticulum stress and upregulation of the expression of the tumor suppressor genes BRCA1 and BRCA2 in prostate and breast cancer cells (89). BRCA1 and BRCA2 genes represent main genetic elements associated with hereditary susceptibility to breast and ovarian cancer, and loss of these genes contributes to oncogenesis and tumor progression. The proteins encoded by the two genes play an important role in maintaining genomic stability by participating in DNA repair (90, 91).
3.5. Tumor invasion and angiogenesis
Indole-3-carbinol has been reported to inhibit the migration and invasion of breast cancer cells by modulating the expression of a series of signaling proteins associated with invasion and migration, including E-cadherin, α, β, and γ catenin, BRCA1 (92, 93), the chemokine receptor CXCR4, and the matrix metalloproteinase (MMP)-9 (94). Moreover, indole-3-carbinol has been shown to inhibit the growth and phorbol ester-activated tube formation of endothelial cells, accompanied by decreased vascular endothelial growth factor (VEGF), increased interleukin-8 (IL-8) secretion, and decreased activities of MMP-2 and MMP-9 (95).
3.6. Reversal of multiple drug resistance (MDR)
Indole-3-carbinol has been reported to reverse the multiple-drug resistant phenotype of human leukemia cells and murine melanoma cells to doxorubicin and vinca alkaloids by down-regulating the expression of the MDR-1 gene product p-glycoprotein (96–98). Moreover, the ability of dietary administration of indole-3-carbinol to sensitize MDR tumors to chemotherapeutic drugs has also been demonstrated in vivo (98). Together, these findings indicate that indole-3-carbinol may be effective as a dietary adjuvant in the treatment of MDR cancers.
4. Chemo- and radiosensitizing effects of indole-3-carbinol/DIM
Considering the pleiotropic effects on multiple signaling targets relevant to cell survival, indole-3-carbinol/DIM exhibit the ability to sensitize cancer cells to apoptosis induced by various anticancer agents and radiation. As summarized in Table 2, indole-3-carbinol and various therapeutic agents work through different signal transduction pathways in a synergistic manner to suppress cell viability of various types of cancer cells.
Table 2.
Chemo-/radio-sensitizing effects of indole-3-carbinol and DIM
| Treatment | Cell model | Proposed mechanism (ref) |
|---|---|---|
| Indole-3-carbinol | ||
| Cisplatin | Cervical cancer cells | Downregulation of Bcl-2 expression (99) |
| LNCaP prostate cancer cells | Inhibition of Akt/NF-κB signaling (20) | |
| Tamoxifen | MCF-7 breast cancer cells | Downregulation of CDK6 expression (82) |
| TRAIL | LNCaP cells (TRAIL-resistant) | Induction of death receptor (DR)4 and DR5 expression (88) |
| Ultraviolet B | Melanoma cells | Downregulation of Bcl-2 expression (100) |
| DIM | ||
| Taxotere | MDA-MB-231 breast cancer cells | Inactivation of Akt/NF-κB signaling (45) |
| Paclitaxel | Her2/Neu breast cancer cells | Downregulation of Bcl-2 expression and Her2/Neu activation (101) |
For example, indole-3-carbinol was shown to be an effective sensitizer of TRAIL treatment against TRAIL-resistant LNCaP prostate cancer cells by up-regulating the expression of two TRAIL death receptors, DR4 and DR5 (88). In addition, treatment of MCF-7 cells with indole-3-carbinol and tamoxifen synergistically ablated expression of the phosphorylated retinoblastoma protein (Rb), an endogenous substrate for the G1 cyclin-dependent kinases (CDKs), through specific down-regulation of the expression of CDK6 (82).
5. Pharmacological exploitation of indole-3-carbinol and DIM to develop novel antitumor agents
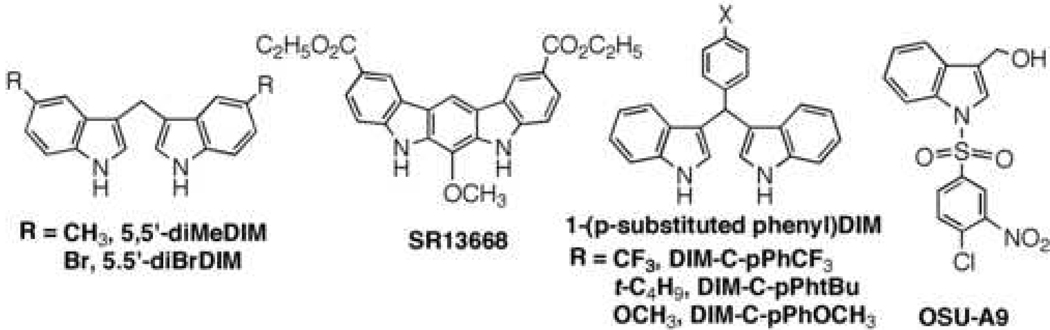
From a mechanistic perspective, the ability of indole-3-carbinol/DIM to target a broad spectrum of signaling pathways underlies their antitumor effect against a variety of cancer cells with different genetic and cellular abnormalities. However, indole-3-carbinol and DIM exhibit low - moderate potencies in suppressing tumor cell proliferation in vitro, and suffer from metabolic instability and/or unpredictable pharmacokinetic properties in vivo (29). Consequently, structural modifications of indole-3-carbinol/DIM to develop novel indole derivatives with improved potency have been the focus of many recent investigations. This medicinal chemistry effort has led to several different classes of novel agents with distinct pharmacological activities (Fig. 4).
Fig. 4.
Structurally optimized indole-3-carbinol and DIM derivatives.
5.1. Ring-substituted DIMs
Symmetrical disubstitutions at the 5,5’ positions of DIM with methyl and bromo groups gave rise to two structural variants, 5,5’-diMeDIM and 5,5’-diBrDIM, respectively (Fig. 4) (102, 103). Despite structural similarity, these two ring-substituted DIM derivatives inhibited breast cancer cell growth through different mechanisms, indicating a subtle structure-activity relationship. While 5,5’-diMeDIM represents a selective AhR inhibitor (102), 5,5’-diBrDIM is a mitochondrial poison that induced cell death by decreasing mitochondrial membrane potential (103).
5.2. SR13668, an Akt inhibitor
Structural modifications after fusing the two indoyl moieties of DIM generated a novel class of antitumor agents, of which SR13668 represents an optimal agent (104). Unlike other DIM derivatives that are agonists of nuclear receptors, SR13668-mediated antitumor effect was facilitated by blocking growth factor-stimulated Akt phosphorylation. However, the mode of action of SR13668 in inhibiting Akt activation is distinct from that of many other Akt inhibitors, i.e., it does not target the ATP binding site.
5.3. 1-(p-Substituted phenyl)DIMs (C-DIMs)
Substitutions of a proton with bulky aromatic substituents on the methylene group of DIM alter the activity of resulting compounds, i.e., C-DIMs, in interacting with various types of nuclear receptors. It is noteworthy that these C-DIMs are no longer AhR agonists, but could activate peroxisomal proliferator-activated receptor (PPAR)γ and/or Nur77 (also known as nerve growth factor (NGF)I-Bα) [PPARγ C-DIMs: (105, 106) Nur77 C-DIMs: (107, 108)]. For example, of the three representative C-DIM derivatives depicted in Fig. 4, DIM-C-pPhtBu and DIM-C-pPhOCH3 are PPARγ-specific and Nur77-specific agonists, respectively, while C-pPhCF3 transactivates both PPARγ and Nur77. Transactivation of these nuclear receptors activates downstream responses including cell cycle arrest and induction of cell death pathways including caspase activation and poly(ADP-ribose)polymerase (PARP) cleavage.
5.4. OSU-A9, a multi-targeted agent
OSU-A9 was developed by using indole-3-carbinol as a scaffold via N-substitution of the vinyl hemiaminal function with a benzenesulfonyl moiety (66). This modification not only improves the acid stability, but also results in a 100-fold increase in apoptosis-inducing potency as compared to its parent compound. Equally important, OSU-A9 retains the pleiotropic mechanisms of indole-3-carbinol in mediating cell cycle arrest and apoptosis induction in cancer cells. Despite this broad spectrum of pharmacological activities, nonmalignant cells were less sensitive to the antiproliferative effect of OSU-A9 relative to cancer cell lines. Moreover, OSU-A9 has been shown to suppress prostate tumor growth in vivo without causing overt toxicity, underlying its potential use in cancer prevention and/or therapy.
Acknowledgments
The authors acknowledge support from National Cancer Institute grant (CA112250), William R. Hearst Foundation, Prostate Cancer Foundation, and the Lucius A. Wing Endowed Chair Fund at the Ohio State University (to C.-S. Chen), and National Science Council (NSC-93018P and NSC-94-2314B-039-033) and China Medical University (CMU95–171 and CMU 95–276) in Taiwan (to J.-R. Weng).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Cohen JH, Kristal AR, Stanford JL. Fruit and vegetable intakes and prostate cancer risk. J Natl Cancer Inst. 2000;92:61–68. doi: 10.1093/jnci/92.1.61. [DOI] [PubMed] [Google Scholar]
- 2.Higdon JV, Delage B, Williams DE, Dashwood RH. Cruciferous vegetables and human cancer risk: epidemiologic evidence and mechanistic basis. Pharmacol Res. 2007;55:224–236. doi: 10.1016/j.phrs.2007.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Minich DM, Bland JS. A review of the clinical efficacy and safety of cruciferous vegetable phytochemicals. Nutr Rev. 2007;65:259–267. doi: 10.1301/nr.2007.jun.259-267. [DOI] [PubMed] [Google Scholar]
- 4.Verhoeven DT, Goldbohm RA, van Poppel G, Verhagen H, van den Brandt PA. Epidemiological studies on brassica vegetables and cancer risk. Cancer Epidemiol Biomarkers Prev. 1996;5:733–748. [PubMed] [Google Scholar]
- 5.Hong C, Firestone GL, Bjeldanes LF. Bcl-2 family-mediated apoptotic effects of 3,3'-diindolylmethane (DIM) in human breast cancer cells. Biochem Pharmacol. 2002;63:1085–1097. doi: 10.1016/s0006-2952(02)00856-0. [DOI] [PubMed] [Google Scholar]
- 6.Howells LM, Gallacher-Horley B, Houghton CE, Manson MM, Hudson EA. Indole-3-carbinol inhibits protein kinase B/Akt and induces apoptosis in the human breast tumor cell line MDA MB468 but not in the nontumorigenic HBL100 line. Mol Cancer Ther. 2002;1:1161–1172. [PubMed] [Google Scholar]
- 7.Katdare M, Osborne MP, Telang NT. Inhibition of aberrant proliferation and induction of apoptosis in pre-neoplastic human mammary epithelial cells by natural phytochemicals. Oncol Rep. 1998;5:311–315. doi: 10.3892/or.5.2.311. [DOI] [PubMed] [Google Scholar]
- 8.Rahman KM, Aranha O, Sarkar FH. Indole-3-carbinol (I3C) induces apoptosis in tumorigenic but not in nontumorigenic breast epithelial cells. Nutr Cancer. 2003;45:101–112. doi: 10.1207/S15327914NC4501_12. [DOI] [PubMed] [Google Scholar]
- 9.Frydoonfar HR, McGrath DR, Spigelman AD. Inhibition of proliferation of a colon cancer cell line by indole-3-carbinol. Colorectal Dis. 2002;4:205–207. doi: 10.1046/j.1463-1318.2002.00346.x. [DOI] [PubMed] [Google Scholar]
- 10.Hudson EA, Howells LM, Gallacher-Horley B, Fox LH, Gescher A, Manson MM. Growth-inhibitory effects of the chemopreventive agent indole-3- carbinol are increased in combination with the polyamine putrescine in the SW480 colon tumour cell line. BMC Cancer. 2003;3:2. doi: 10.1186/1471-2407-3-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zheng Q, Hirose Y, Yoshimi N, Murakami A, Koshimizu K, Ohigashi H, et al. Further investigation of the modifying effect of various chemopreventive agents on apoptosis and cell proliferation in human colon cancer cells. J Cancer Res Clin Oncol. 2002;128:539–546. doi: 10.1007/s00432-002-0373-y. [DOI] [PubMed] [Google Scholar]
- 12.Chinni SR, Sarkar FH. Akt inactivation is a key event in indole-3-carbinol-induced apoptosis in PC-3 cells. Clin Cancer Res. 2002;8:1228–1236. [PubMed] [Google Scholar]
- 13.Frydoonfar HR, McGrath DR, Spigelman AD. The effect of indole-3- carbinol and sulforaphane on a prostate cancer cell line. ANZ J Surg. 2003;73:154–156. doi: 10.1046/j.1445-2197.2003.02652.x. [DOI] [PubMed] [Google Scholar]
- 14.Nachshon-Kedmi M, Yannai S, Haj A, Fares FA. Indole-3-carbinol and 3,3'- diindolylmethane induce apoptosis in human prostate cancer cells. Food Chem Toxicol. 2003;41:745–752. doi: 10.1016/s0278-6915(03)00004-8. [DOI] [PubMed] [Google Scholar]
- 15.Leong H, Firestone GL, Bjeldanes LF. Cytostatic effects of 3,3'- diindolylmethane in human endometrial cancer cells result from an estrogen receptor-mediated increase in transforming growth factor-alpha expression. Carcinogenesis. 2001;22:1809–1817. doi: 10.1093/carcin/22.11.1809. [DOI] [PubMed] [Google Scholar]
- 16.Aggarwal BB, Ichikawa H. Molecular targets and anticancer potential of indole-3-carbinol and its derivatives. Cell Cycle. 2005;4:1201–1215. doi: 10.4161/cc.4.9.1993. [DOI] [PubMed] [Google Scholar]
- 17.Aggarwal BB, Shishodia S. Molecular targets of dietary agents for prevention and therapy of cancer. Biochem Pharmacol. 2006;71:1397–1421. doi: 10.1016/j.bcp.2006.02.009. [DOI] [PubMed] [Google Scholar]
- 18.Kim YS, Milner JA. Targets for indole-3-carbinol in cancer prevention. J Nutr Biochem. 2005;16:65–73. doi: 10.1016/j.jnutbio.2004.10.007. [DOI] [PubMed] [Google Scholar]
- 19.Rogan EG. The natural chemopreventive compound indole-3-carbinol: state of the science. In Vivo. 2006;20:221–228. [PubMed] [Google Scholar]
- 20.Sarkar FH, Li Y. Indole-3-carbinol and prostate cancer. J Nutr. 2004;134:3493S–3498S. doi: 10.1093/jn/134.12.3493S. [DOI] [PubMed] [Google Scholar]
- 21.Bradlow HL, Michnovicz J, Telang NT, Osborne MP. Effects of dietary indole-3-carbinol on estradiol metabolism and spontaneous mammary tumors in mice. Carcinogenesis. 1991;12:1571–1574. doi: 10.1093/carcin/12.9.1571. [DOI] [PubMed] [Google Scholar]
- 22.Grubbs CJ, Steele VE, Casebolt T, Juliana MM, Eto I, Whitaker LM, et al. Chemoprevention of chemically-induced mammary carcinogenesis by indole-3- carbinol. Anticancer Res. 1995;15:709–716. [PubMed] [Google Scholar]
- 23.He YH, Friesen MD, Ruch RJ, Schut HA. Indole-3-carbinol as a chemopreventive agent in 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine (PhIP) carcinogenesis: inhibition of PhIP-DNA adduct formation, acceleration of PhIP metabolism, and induction of cytochrome P450 in female F344 rats. Food Chem Toxicol. 2000;38:15–23. doi: 10.1016/s0278-6915(99)00117-9. [DOI] [PubMed] [Google Scholar]
- 24.Jin L, Qi M, Chen DZ, Anderson A, Yang GY, Arbeit JM, et al. Indole-3- carbinol prevents cervical cancer in human papilloma virus type 16 (HPV16) transgenic mice. Cancer Res. 1999;59:3991–3997. [PubMed] [Google Scholar]
- 25.Kojima T, Tanaka T, Mori H. Chemoprevention of spontaneous endometrial cancer in female Donryu rats by dietary indole-3-carbinol. Cancer Res. 1994;54:1446–1449. [PubMed] [Google Scholar]
- 26.Oganesian A, Hendricks JD, Williams DE. Long term dietary indole-3- carbinol inhibits diethylnitrosamine-initiated hepatocarcinogenesis in the infant mouse model. Cancer Lett. 1997;118:87–94. doi: 10.1016/s0304-3835(97)00235-8. [DOI] [PubMed] [Google Scholar]
- 27.Yu Z, Mahadevan B, Lohr CV, Fischer KA, Louderback MA, Krueger SK, et al. Indole-3-carbinol in the maternal diet provides chemoprotection for the fetus against transplacental carcinogenesis by the polycyclic aromatic hydrocarbon dibenzo[a,l]pyrene. Carcinogenesis. 2006;27:2116–2123. doi: 10.1093/carcin/bgl072. [DOI] [PubMed] [Google Scholar]
- 28.Bell MC, Crowley-Nowick P, Bradlow HL, Sepkovic DW, Schmidt-Grimminger D, Howell P, et al. Placebo-controlled trial of indole-3-carbinol in the treatment of CIN. Gynecol Oncol. 2000;78:123–129. doi: 10.1006/gyno.2000.5847. [DOI] [PubMed] [Google Scholar]
- 29.Reed GA, Arneson DW, Putnam WC, Smith HJ, Gray JC, Sullivan DK, et al. Single-dose and multiple-dose administration of indole-3-carbinol to women: pharmacokinetics based on 3,3'-diindolylmethane. Cancer Epidemiol Biomarkers Prev. 2006;15:2477–2481. doi: 10.1158/1055-9965.EPI-06-0396. [DOI] [PubMed] [Google Scholar]
- 30.Reed GA, Peterson KS, Smith HJ, Gray JC, Sullivan DK, Mayo MS, et al. A phase I study of indole-3-carbinol in women: tolerability and effects. Cancer Epidemiol Biomarkers Prev. 2005;14:1953–1960. doi: 10.1158/1055-9965.EPI-05-0121. [DOI] [PubMed] [Google Scholar]
- 31.Naik R, Nixon S, Lopes A, Godfrey K, Hatem MH, Monaghan JM. A randomized phase II trial of indole-3-carbinol in the treatment of vulvar intraepithelial neoplasia. Int J Gynecol Cancer. 2006;16:786–790. doi: 10.1111/j.1525-1438.2006.00386.x. [DOI] [PubMed] [Google Scholar]
- 32.Rosen CA, Bryson PC. Indole-3-carbinol for recurrent respiratory papillomatosis: long-term results. J Voice. 2004;18:248–253. doi: 10.1016/j.jvoice.2003.05.005. [DOI] [PubMed] [Google Scholar]
- 33.Grose KR, Bjeldanes LF. Oligomerization of indole-3-carbinol in aqueous acid. Chem Res Toxicol. 1992;5:188–193. doi: 10.1021/tx00026a007. [DOI] [PubMed] [Google Scholar]
- 34.Anderton MJ, Jukes R, Lamb JH, Manson MM, Gescher A, Steward WP, et al. Liquid chromatographic assay for the simultaneous determination of indole- 3-carbinol and its acid condensation products in plasma. J Chromatogr B Analyt Technol Biomed Life Sci. 2003;787:281–291. doi: 10.1016/s1570-0232(02)00923-6. [DOI] [PubMed] [Google Scholar]
- 35.Anderton MJ, Manson MM, Verschoyle RD, Gescher A, Lamb JH, Farmer PB, et al. Pharmacokinetics and tissue disposition of indole-3-carbinol and its acid condensation products after oral administration to mice. Clin Cancer Res. 2004;10:5233–5241. doi: 10.1158/1078-0432.CCR-04-0163. [DOI] [PubMed] [Google Scholar]
- 36.De Kruif CA, Marsman JW, Venekamp JC, Falke HE, Noordhoek J, Blaauboer BJ, et al. Structure elucidation of acid reaction products of indole-3- carbinol: detection in vivo and enzyme induction in vitro. Chem Biol Interact. 1991;80:303–315. doi: 10.1016/0009-2797(91)90090-t. [DOI] [PubMed] [Google Scholar]
- 37.Stresser DM, Williams DE, Griffin DA, Bailey GS. Mechanisms of tumor modulation by indole-3-carbinol. Disposition and excretion in male Fischer 344 rats. Drug Metab Dispos. 1995;23:965–975. [PubMed] [Google Scholar]
- 38.Abdelrahim M, Newman K, Vanderlaag K, Samudio I, Safe S. 3,3'- diindolylmethane (DIM) and its derivatives induce apoptosis in pancreatic cancer cells through endoplasmic reticulum stress-dependent upregulation of DR5. Carcinogenesis. 2006;27:717–728. doi: 10.1093/carcin/bgi270. [DOI] [PubMed] [Google Scholar]
- 39.Bhuiyan MM, Li Y, Banerjee S, Ahmed F, Wang Z, Ali S, et al. Downregulation of androgen receptor by 3,3'-diindolylmethane contributes to inhibition of cell proliferation and induction of apoptosis in both hormone-sensitive LNCaP and insensitive C4-2B prostate cancer cells. Cancer Res. 2006;66:10064–10072. doi: 10.1158/0008-5472.CAN-06-2011. [DOI] [PubMed] [Google Scholar]
- 40.Garikapaty VP, Ashok BT, Tadi K, Mittelman A, Tiwari RK. 3,3'- Diindolylmethane downregulates pro-survival pathway in hormone independent prostate cancer. Biochem Biophys Res Commun. 2006;340:718–725. doi: 10.1016/j.bbrc.2005.12.059. [DOI] [PubMed] [Google Scholar]
- 41.Kong D, Li Y, Wang Z, Banerjee S, Sarkar FH. Inhibition of angiogenesis and invasion by 3,3'-diindolylmethane is mediated by the nuclear factor-kappaB downstream target genes MMP-9 and uPA that regulated bioavailability of vascular endothelial growth factor in prostate cancer. Cancer Res. 2007;67:3310–3319. doi: 10.1158/0008-5472.CAN-06-4277. [DOI] [PubMed] [Google Scholar]
- 42.Li Y, Li X, Sarkar FH. Gene expression profiles of I3C- and DIM-treated PC3 human prostate cancer cells determined by cDNA microarray analysis. J Nutr. 2003;133:1011–1019. doi: 10.1093/jn/133.4.1011. [DOI] [PubMed] [Google Scholar]
- 43.Li Y, Wang Z, Kong D, Murthy S, Dou QP, Sheng S, et al. Regulation of FOXO3a/beta-catenin/GSK-3beta signaling by 3,3'-diindolylmethane contributes to inhibition of cell proliferation and induction of apoptosis in prostate cancer cells. J Biol Chem. 2007;282:21542–21550. doi: 10.1074/jbc.M701978200. [DOI] [PubMed] [Google Scholar]
- 44.Nachshon-Kedmi M, Yannai S, Fares FA. Induction of apoptosis in human prostate cancer cell line, PC3, by 3,3'-diindolylmethane through the mitochondrial pathway. Br J Cancer. 2004;91:1358–1363. doi: 10.1038/sj.bjc.6602145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rahman KW, Ali S, Aboukameel A, Sarkar SH, Wang Z, Philip PA, et al. Inactivation of NF-{kappa}B by 3,3'-diindolylmethane contributes to increased apoptosis induced by chemotherapeutic agent in breast cancer cells. Mol Cancer Ther. 2007;6:2757–2765. doi: 10.1158/1535-7163.MCT-07-0336. [DOI] [PubMed] [Google Scholar]
- 46.Rahman KW, Li Y, Wang Z, Sarkar SH, Sarkar FH. Gene expression profiling revealed survivin as a target of 3,3'-diindolylmethane-induced cell growth inhibition and apoptosis in breast cancer cells. Cancer Res. 2006;66:4952–4960. doi: 10.1158/0008-5472.CAN-05-3918. [DOI] [PubMed] [Google Scholar]
- 47.Herrmann S, Seidelin M, Bisgaard HC, Vang O. Indolo[3,2-b]carbazole inhibits gap junctional intercellular communication in rat primary hepatocytes and acts as a potential tumor promoter. Carcinogenesis. 2002;23:1861–1868. doi: 10.1093/carcin/23.11.1861. [DOI] [PubMed] [Google Scholar]
- 48.Liu H, Wormke M, Safe SH, Bjeldanes LF. Indolo[3,2-b]carbazole: a dietary-derived factor that exhibits both antiestrogenic and estrogenic activity. J Natl Cancer Inst. 1994;86:1758–1765. doi: 10.1093/jnci/86.23.1758. [DOI] [PubMed] [Google Scholar]
- 49.Brandi G, Paiardini M, Cervasi B, Fiorucci C, Filippone P, De Marco C, et al. A new indole-3-carbinol tetrameric derivative inhibits cyclin-dependent kinase 6 expression, and induces G1 cell cycle arrest in both estrogen-dependent and estrogen-independent breast cancer cell lines. Cancer Res. 2003;63:4028–4036. [PubMed] [Google Scholar]
- 50.Firestone GL, Bjeldanes LF. Indole-3-carbinol and 3-3'-diindolylmethane antiproliferative signaling pathways control cell-cycle gene transcription in human breast cancer cells by regulating promoter-Sp1 transcription factor interactions. J Nutr. 2003;133:2448S–2455S. doi: 10.1093/jn/133.7.2448S. [DOI] [PubMed] [Google Scholar]
- 51.Mulvey L, Chandrasekaran A, Liu K, Lombardi S, Wang XP, Auborn KJ, et al. Interplay of genes regulated by estrogen and diindolylmethane in breast cancer cell lines. Mol Med. 2007;13:69–78. doi: 10.2119/2006-00038.Mulvey. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chang YC, Riby J, Chang GH, Peng BC, Firestone G, Bjeldanes LF. Cytostatic and antiestrogenic effects of 2-(indol-3-ylmethyl)-3,3'-diindolylmethane, a major in vivo product of dietary indole-3-carbinol. Biochem Pharmacol. 1999;58:825–834. doi: 10.1016/s0006-2952(99)00165-3. [DOI] [PubMed] [Google Scholar]
- 53.Riby JE, Feng C, Chang YC, Schaldach CM, Firestone GL, Bjeldanes LF. The major cyclic trimeric product of indole-3-carbinol is a strong agonist of the estrogen receptor signaling pathway. Biochemistry. 2000;39:910–918. doi: 10.1021/bi9919706. [DOI] [PubMed] [Google Scholar]
- 54.Auborn KJ, Fan S, Rosen EM, Goodwin L, Chandraskaren A, Williams DE, et al. Indole-3-carbinol is a negative regulator of estrogen. J Nutr. 2003;133:2470S–2475S. doi: 10.1093/jn/133.7.2470s. [DOI] [PubMed] [Google Scholar]
- 55.Wang TT, Milner MJ, Milner JA, Kim YS. Estrogen receptor alpha as a target for indole-3-carbinol. J Nutr Biochem. 2006;17:659–664. doi: 10.1016/j.jnutbio.2005.10.012. [DOI] [PubMed] [Google Scholar]
- 56.Parkin DR, Malejka-Giganti D. Differences in the hepatic P450-dependent metabolism of estrogen and tamoxifen in response to treatment of rats with 3,3'- diindolylmethane and its parent compound indole-3-carbinol. Cancer Detect Prev. 2004;28:72–79. doi: 10.1016/j.cdp.2003.11.006. [DOI] [PubMed] [Google Scholar]
- 57.Rahman KW, Sarkar FH. Inhibition of nuclear translocation of nuclear factor-{kappa}B contributes to 3,3'-diindolylmethane-induced apoptosis in breast cancer cells. Cancer Res. 2005;65:364–371. [PubMed] [Google Scholar]
- 58.Takada Y, Andreeff M, Aggarwal BB. Indole-3-carbinol suppresses NF-kappaB and IkappaBalpha kinase activation, causing inhibition of expression of NF-kappaB-regulated antiapoptotic and metastatic gene products and enhancement of apoptosis in myeloid and leukemia cells. Blood. 2005;106:641–649. doi: 10.1182/blood-2004-12-4589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bellacosa A, Kumar CC, Di Cristofano A, Testa JR. Activation of AKT kinases in cancer: implications for therapeutic targeting. Adv Cancer Res. 2005;94:29–86. doi: 10.1016/S0065-230X(05)94002-5. [DOI] [PubMed] [Google Scholar]
- 60.Chen YL, Law PY, Loh HH. Inhibition of PI3K/Akt signaling: an emerging paradigm for targeted cancer therapy. Curr Med Chem Anticancer Agents. 2005;5:575–589. doi: 10.2174/156801105774574649. [DOI] [PubMed] [Google Scholar]
- 61.Cheng JQ, Lindsley CW, Cheng GZ, Yang H, Nicosia SV. The Akt/PKB pathway: molecular target for cancer drug discovery. Oncogene. 2005;24:7482–7492. doi: 10.1038/sj.onc.1209088. [DOI] [PubMed] [Google Scholar]
- 62.Hennessy BT, Smith DL, Ram PT, Lu Y, Mills GB. Exploiting the PI3K/AKT pathway for cancer drug discovery. Nat Rev Drug Discov. 2005;4:988–1004. doi: 10.1038/nrd1902. [DOI] [PubMed] [Google Scholar]
- 63.Kim D, Cheng GZ, Lindsley CW, Yang H, Cheng JQ. Targeting the phosphatidylinositol-3 kinase/Akt pathway for the treatment of cancer. Curr Opin Investig Drugs. 2005;6:1250–1258. [PubMed] [Google Scholar]
- 64.Yoeli-Lerner M, Toker A. Akt/PKB signaling in cancer: a function in cell motility and invasion. Cell Cycle. 2006;5:603–605. doi: 10.4161/cc.5.6.2561. [DOI] [PubMed] [Google Scholar]
- 65.Aggarwal BB. Nuclear factor-kappaB: the enemy within. Cancer Cell. 2004;6:203–208. doi: 10.1016/j.ccr.2004.09.003. [DOI] [PubMed] [Google Scholar]
- 66.Weng JR, Tsai CH, Kulp SK, Wang D, Lin CH, Yang HC, et al. A potent indole-3-carbinol derived antitumor agent with pleiotropic effects on multiple signaling pathways in prostate cancer cells. Cancer Res. 2007;67:7815–7824. doi: 10.1158/0008-5472.CAN-07-0794. [DOI] [PubMed] [Google Scholar]
- 67.Lian JP, Word B, Taylor S, Hammons GJ, Lyn-Cook BD. Modulation of the constitutive activated STAT3 transcription factor in pancreatic cancer prevention: effects of indole-3-carbinol (I3C) and genistein. Anticancer Res. 2004;24:133–137. [PubMed] [Google Scholar]
- 68.Chinni SR, Li Y, Upadhyay S, Koppolu PK, Sarkar FH. Indole-3- carbinol (I3C) induced cell growth inhibition, G1 cell cycle arrest and apoptosis in prostate cancer cells. Oncogene. 2001;20:2927–2936. doi: 10.1038/sj.onc.1204365. [DOI] [PubMed] [Google Scholar]
- 69.Cover CM, Hsieh SJ, Tran SH, Hallden G, Kim GS, Bjeldanes LF, et al. Indole-3-carbinol inhibits the expression of cyclin-dependent kinase-6 and induces a G1 cell cycle arrest of human breast cancer cells independent of estrogen receptor signaling. J Biol Chem. 1998;273:3838–3847. doi: 10.1074/jbc.273.7.3838. [DOI] [PubMed] [Google Scholar]
- 70.Cram EJ, Liu BD, Bjeldanes LF, Firestone GL. Indole-3-carbinol inhibits CDK6 expression in human MCF-7 breast cancer cells by disrupting Sp1 transcription factor interactions with a composite element in the CDK6 gene promoter. J Biol Chem. 2001;276:22332–22340. doi: 10.1074/jbc.M010539200. [DOI] [PubMed] [Google Scholar]
- 71.Hong C, Kim HA, Firestone GL, Bjeldanes LF. 3,3'-Diindolylmethane (DIM) induces a G(1) cell cycle arrest in human breast cancer cells that is accompanied by Sp1-mediated activation of p21(WAF1/CIP1) expression. Carcinogenesis. 2002;23:1297–1305. doi: 10.1093/carcin/23.8.1297. [DOI] [PubMed] [Google Scholar]
- 72.Garcia HH, Brar GA, Nguyen DH, Bjeldanes LF, Firestone GL. Indole-3-carbinol (I3C) inhibits cyclin-dependent kinase-2 function in human breast cancer cells by regulating the size distribution, associated cyclin E forms, and subcellular localization of the CDK2 protein complex. J Biol Chem. 2005;280:8756–8764. doi: 10.1074/jbc.M407957200. [DOI] [PubMed] [Google Scholar]
- 73.Bjeldanes LF, Kim JY, Grose KR, Bartholomew JC, Bradfield CA. Aromatic hydrocarbon responsiveness-receptor agonists generated from indole-3- carbinol in vitro and in vivo: comparisons with 2,3,7,8-tetrachlorodibenzo-p-dioxin. Proc Natl Acad Sci U S A. 1991;88:9543–9547. doi: 10.1073/pnas.88.21.9543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kohle C, Bock KW. Coordinate regulation of Phase I and II xenobiotic metabolisms by the Ah receptor and Nrf2. Biochem Pharmacol. 2007;73:1853–1862. doi: 10.1016/j.bcp.2007.01.009. [DOI] [PubMed] [Google Scholar]
- 75.Ociepa-Zawal M, Rubis B, Lacinski M, Trzeciak WH. The effect of indole- 3-carbinol on the expression of CYP1A1, CYP1B1 and AhR genes and proliferation of MCF-7 cells. Acta Biochim Pol. 2007;54:113–117. [PubMed] [Google Scholar]
- 76.Dashwood RH. Indole-3-carbinol: anticarcinogen or tumor promoter in brassica vegetables? Chem Biol Interact. 1998;110:1–5. doi: 10.1016/s0009-2797(97)00115-4. [DOI] [PubMed] [Google Scholar]
- 77.Larsen-Su SA, Williams DE. Transplacental exposure to indole-3-carbinol induces sex-specific expression of CYP1A1 and CYP1B1 in the liver of Fischer 344 neonatal rats. Toxicol Sci. 2001;64:162–168. doi: 10.1093/toxsci/64.2.162. [DOI] [PubMed] [Google Scholar]
- 78.Bradlow HL, Sepkovic DW, Telang NT, Osborne MP. Multifunctional aspects of the action of indole-3-carbinol as an antitumor agent. Ann N Y Acad Sci. 1999;889:204–213. doi: 10.1111/j.1749-6632.1999.tb08736.x. [DOI] [PubMed] [Google Scholar]
- 79.Newfield L, Goldsmith A, Bradlow HL, Auborn K. Estrogen metabolism and human papillomavirus-induced tumors of the larynx: chemo-prophylaxis with indole-3-carbinol. Anticancer Res. 1993;13:337–341. [PubMed] [Google Scholar]
- 80.Greenwald P. Clinical trials in cancer prevention: current results and perspectives for the future. J Nutr. 2004;134:3507S–3512S. doi: 10.1093/jn/134.12.3507S. [DOI] [PubMed] [Google Scholar]
- 81.Ashok BT, Chen YG, Liu X, Garikapaty VP, Seplowitz R, Tschorn J, et al. Multiple molecular targets of indole-3-carbinol, a chemopreventive anti-estrogen in breast cancer. Eur J Cancer Prev. 2002;11 Suppl 2:S86–S93. [PubMed] [Google Scholar]
- 82.Cover CM, Hsieh SJ, Cram EJ, Hong C, Riby JE, Bjeldanes LF, et al. Indole-3-carbinol and tamoxifen cooperate to arrest the cell cycle of MCF-7 human breast cancer cells. Cancer Res. 1999;59:1244–1251. [PubMed] [Google Scholar]
- 83.Sundar SN, Kerekatte V, Equinozio CN, Doan VB, Bjeldanes LF, Firestone GL. Indole-3-carbinol selectively uncouples expression and activity of estrogen receptor subtypes in human breast cancer cells. Mol Endocrinol. 2006;20:3070–3082. doi: 10.1210/me.2005-0263. [DOI] [PubMed] [Google Scholar]
- 84.Carter TH, Liu K, Ralph W, Jr, Chen D, Qi M, Fan S, et al. Diindolylmethane alters gene expression in human keratinocytes in vitro. J Nutr. 2002;132:3314–3324. doi: 10.1093/jn/132.11.3314. [DOI] [PubMed] [Google Scholar]
- 85.Sun S, Han J, Ralph WM, Jr, Chandrasekaran A, Liu K, Auborn KJ, et al. Endoplasmic reticulum stress as a correlate of cytotoxicity in human tumor cells exposed to diindolylmethane in vitro. Cell Stress Chaperones. 2004;9:76–87. doi: 10.1379/CSC-2R.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Marciniak SJ, Ron D. Endoplasmic reticulum stress signaling in disease. Physiol Rev. 2006;86:1133–1149. doi: 10.1152/physrev.00015.2006. [DOI] [PubMed] [Google Scholar]
- 87.Barone MV, Crozat A, Tabaee A, Philipson L, Ron D. CHOP (GADD153) and its oncogenic variant, TLS-CHOP, have opposing effects on the induction of G1/S arrest. Genes Dev. 1994;8:453–464. doi: 10.1101/gad.8.4.453. [DOI] [PubMed] [Google Scholar]
- 88.Jeon KI, Rih JK, Kim HJ, Lee YJ, Cho CH, Goldberg ID, et al. Pretreatment of indole-3-carbinol augments TRAIL-induced apoptosis in a prostate cancer cell line. LNCaP, FEBS Lett. 2003;544:246–251. doi: 10.1016/s0014-5793(03)00473-3. [DOI] [PubMed] [Google Scholar]
- 89.Fan S, Meng Q, Auborn K, Carter T, Rosen EM. BRCA1 and BRCA2 as molecular targets for phytochemicals indole-3-carbinol and genistein in breast and prostate cancer cells. Br J Cancer. 2006;94:407–426. doi: 10.1038/sj.bjc.6602935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Scully R. Role of BRCA gene dysfunction in breast and ovarian cancer predisposition. Breast Cancer Res. 2000;2:324–330. doi: 10.1186/bcr76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Tutt A, Ashworth A. The relationship between the roles of BRCA genes in DNA repair and cancer predisposition. Trends Mol Med. 2002;8:571–576. doi: 10.1016/s1471-4914(02)02434-6. [DOI] [PubMed] [Google Scholar]
- 92.Meng Q, Goldberg ID, Rosen EM, Fan S. Inhibitory effects of Indole-3- carbinol on invasion and migration in human breast cancer cells. Breast Cancer Res Treat. 2000;63:147–152. doi: 10.1023/a:1006495824158. [DOI] [PubMed] [Google Scholar]
- 93.Meng Q, Qi M, Chen DZ, Yuan R, Goldberg ID, Rosen EM, et al. Suppression of breast cancer invasion and migration by indole-3-carbinol: associated with up-regulation of BRCA1 and E-cadherin/catenin complexes. J Mol Med. 2000;78:155–165. doi: 10.1007/s001090000088. [DOI] [PubMed] [Google Scholar]
- 94.Rahman KM, Sarkar FH, Banerjee S, Wang Z, Liao DJ, Hong X, et al. Therapeutic intervention of experimental breast cancer bone metastasis by indole-3- carbinol in SCID-human mouse model. Mol Cancer Ther. 2006;5:2747–2756. doi: 10.1158/1535-7163.MCT-06-0221. [DOI] [PubMed] [Google Scholar]
- 95.Wu HT, Lin SH, Chen YH. Inhibition of cell proliferation and in vitro markers of angiogenesis by indole-3-carbinol, a major indole metabolite present in cruciferous vegetables. J Agric Food Chem. 2005;53:5164–5169. doi: 10.1021/jf050034w. [DOI] [PubMed] [Google Scholar]
- 96.Arora A, Seth K, Kalra N, Shukla Y. Modulation of P-glycoprotein-mediated multidrug resistance in K562 leukemic cells by indole-3-carbinol. Toxicol Appl Pharmacol. 2005;202:237–243. doi: 10.1016/j.taap.2004.06.017. [DOI] [PubMed] [Google Scholar]
- 97.Arora A, Shukla Y. Modulation of vinca-alkaloid induced P-glycoprotein expression by indole-3-carbinol. Cancer Lett. 2003;189:167–173. doi: 10.1016/s0304-3835(02)00550-5. [DOI] [PubMed] [Google Scholar]
- 98.Christensen JG, LeBlanc GA. Reversal of multidrug resistance in vivo by dietary administration of the phytochemical indole-3-carbinol. Cancer Res. 1996;56:574–581. [PubMed] [Google Scholar]
- 99.Chen D, Carter TH, Auborn KJ. Apoptosis in cervical cancer cells: implications for adjunct anti-estrogen therapy for cervical cancer. Anticancer Res. 2004;24:2649–2656. [PubMed] [Google Scholar]
- 100.Kim DS, Jeong YM, Moon SI, Kim SY, Kwon SB, Park ES, et al. Indole-3-carbinol enhances ultraviolet B-induced apoptosis by sensitizing human melanoma cells. Cell Mol Life Sci. 2006;63:2661–2668. doi: 10.1007/s00018-006-6306-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.McGuire KP, Ngoubilly N, Neavyn M, Lanza-Jacoby S. 3,3'-diindolylmethane and paclitaxel act synergistically to promote apoptosis in HER2/Neu human breast cancer cells. J Surg Res. 2006;132:208–213. doi: 10.1016/j.jss.2006.02.008. [DOI] [PubMed] [Google Scholar]
- 102.McDougal A, Gupta MS, Morrow D, Ramamoorthy K, Lee JE, Safe SH. Methyl-substituted diindolylmethanes as inhibitors of estrogen-induced growth of T47D cells and mammary tumors in rats. Breast Cancer Res Treat. 2001;66:147–157. doi: 10.1023/a:1010608000074. [DOI] [PubMed] [Google Scholar]
- 103.Vanderlaag K, Samudio I, Burghardt R, Barhoumi R, Safe S. Inhibition of breast cancer cell growth and induction of cell death by 1,1-bis(3'-indolyl)methane (DIM) and 5,5'-dibromoDIM. Cancer Lett. 2006;236:198–212. doi: 10.1016/j.canlet.2005.05.036. [DOI] [PubMed] [Google Scholar]
- 104.Chao WR, Yean D, Amin K, Green C, Jong L. Computer-aided rational drug design: a novel agent (SR13668) designed to mimic the unique anticancer mechanisms of dietary indole-3-carbinol to block Akt signaling. J Med Chem. 2007;50:3412–3415. doi: 10.1021/jm070040e. [DOI] [PubMed] [Google Scholar]
- 105.Kassouf W, Chintharlapalli S, Abdelrahim M, Nelkin G, Safe S, Kamat AM. Inhibition of bladder tumor growth by 1,1-bis(3'-indolyl)-1-(p-substitutedphenyl) methanes: a new class of peroxisome proliferator-activated receptor gamma agonists. Cancer Res. 2006;66:412–418. doi: 10.1158/0008-5472.CAN-05-2755. [DOI] [PubMed] [Google Scholar]
- 106.Qin C, Morrow D, Stewart J, Spencer K, Porter W, Smith R., 3rd A new class of peroxisome proliferator-activated receptor gamma (PPARgamma) agonists that inhibit growth of breast cancer cells: 1,1-Bis(3'-indolyl)-1-(p-substituted phenyl)methanes. Mol Cancer Ther. 2004;3:247–260. [PubMed] [Google Scholar]
- 107.Chintharlapalli S, Burghardt R, Papineni S, Ramaiah S, Yoon K, Safe S. Activation of Nur77 by selected 1,1-Bis(3'-indolyl)-1-(p-substituted phenyl)methanes induces apoptosis through nuclear pathways. J Biol Chem. 2005;280:24903–24914. doi: 10.1074/jbc.M500107200. [DOI] [PubMed] [Google Scholar]
- 108.Cho SD, Yoon K, Chintharlapalli S, Abdelrahim M, Lei P, Hamilton S, et al. Nur77 agonists induce proapoptotic genes and responses in colon cancer cells through nuclear receptor-dependent and nuclear receptor-independent pathways. Cancer Res. 2007;67:674–683. doi: 10.1158/0008-5472.CAN-06-2907. [DOI] [PubMed] [Google Scholar]