Full text
PDF

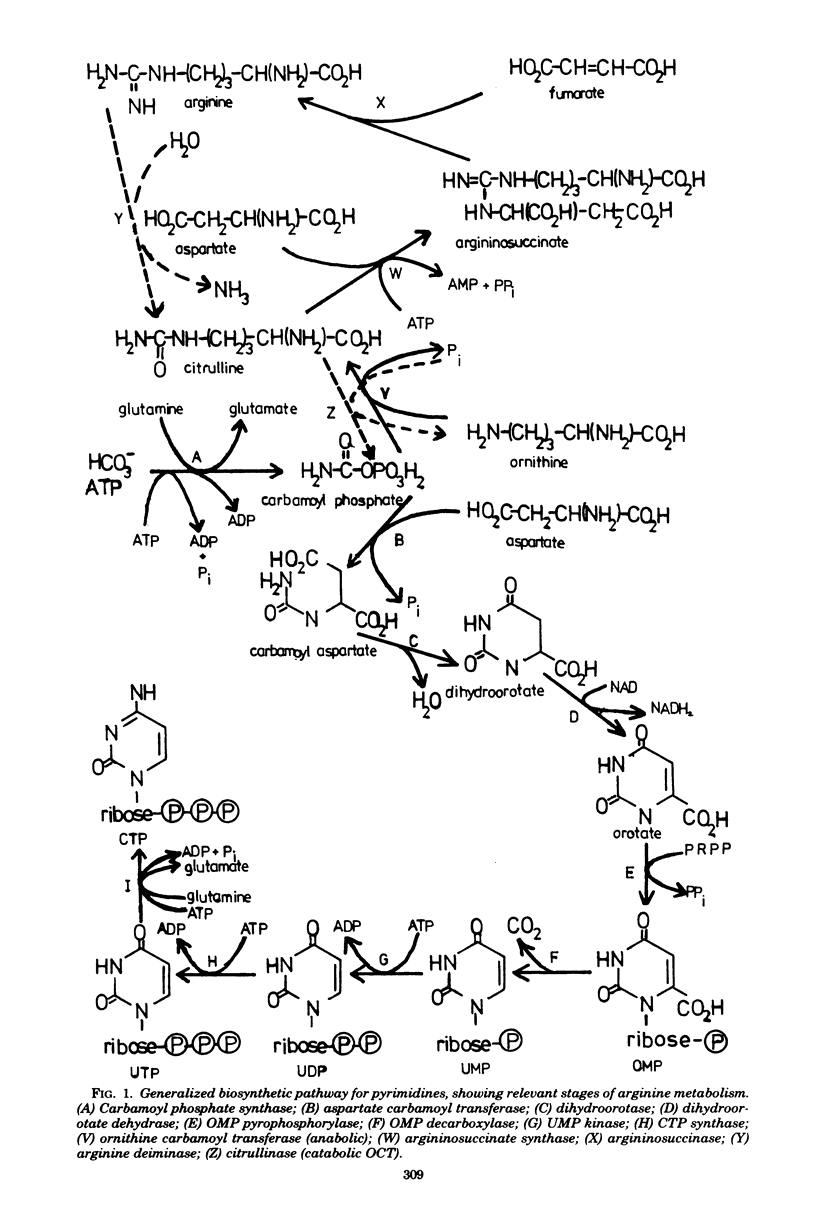


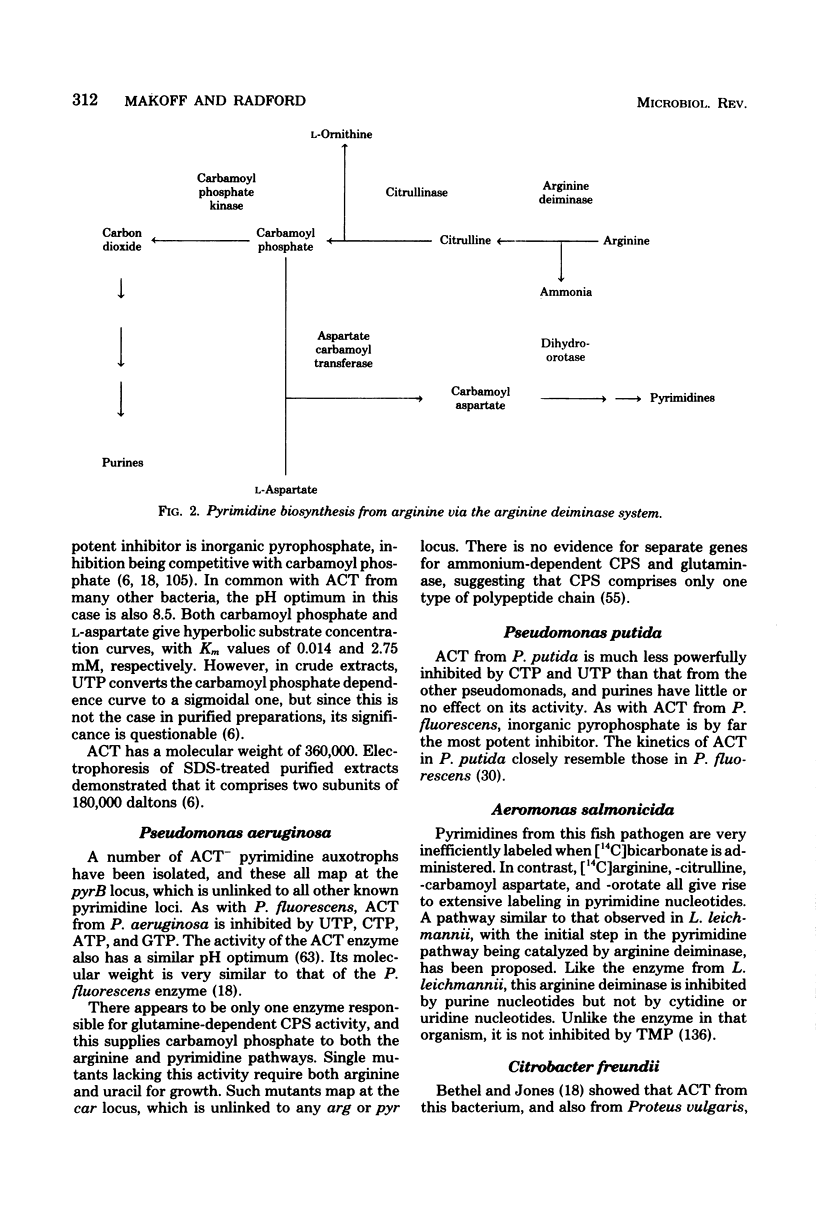









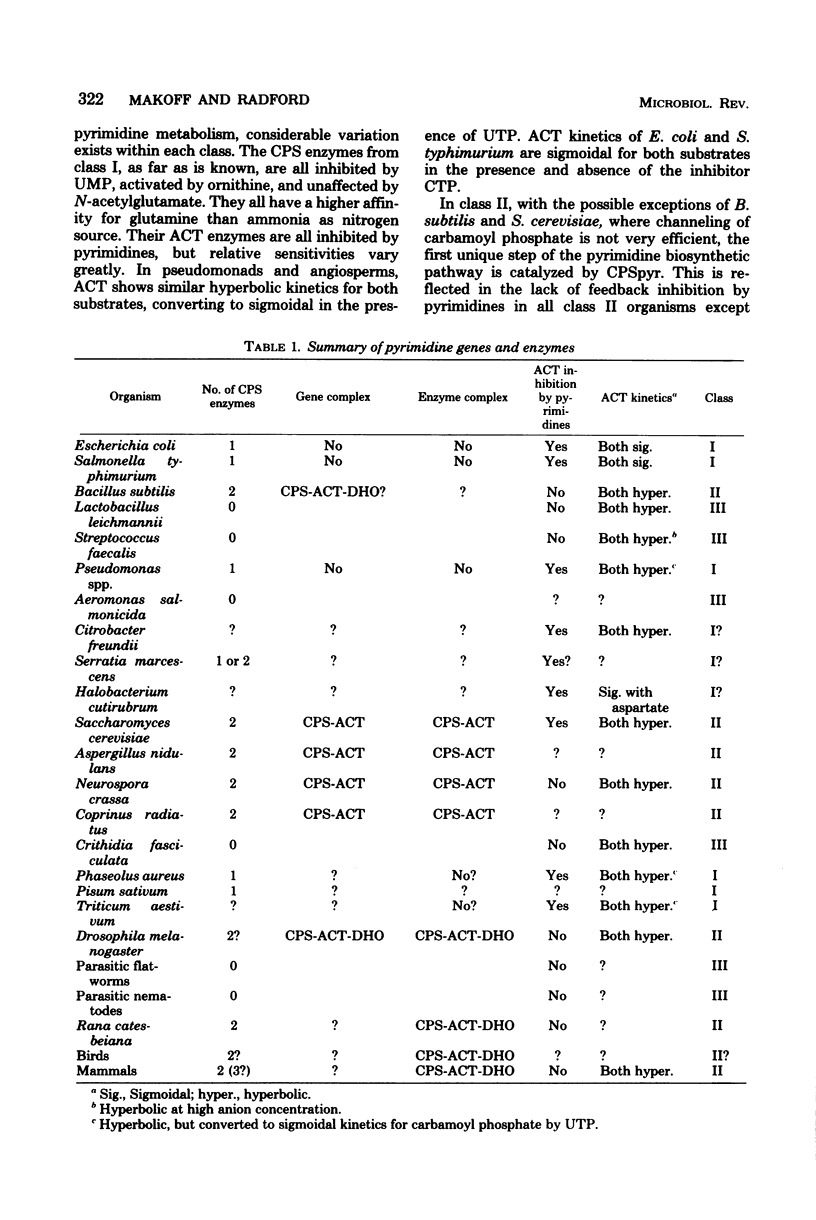






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abd-el-Al A. Arginine-auxotrophic phenotype resulting from a mutation in the pryA gene of Escherichia coli B-r. J Bacteriol. 1969 Jan;97(1):466–468. doi: 10.1128/jb.97.1.466-468.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abd-el-Al A., Ingraham J. L. Cold sensitivity and other phenotypes resulting from mutation in pyrA gene. J Biol Chem. 1969 Aug 10;244(15):4039–4045. [PubMed] [Google Scholar]
- Abd-el-Al A., Ingraham J. L. Control of carbamyl phosphate synthesis in Salmonella typhimurium. J Biol Chem. 1969 Aug 10;244(15):4033–4038. [PubMed] [Google Scholar]
- Abdelal A. T., Ingraham J. L. Carbamylphosphate synthetase from Salmonella typhimurium. Regulations, subunit composition, and function of the subunits. J Biol Chem. 1975 Jun 25;250(12):4410–4417. [PubMed] [Google Scholar]
- Achar B. S., Savithri H. S., Vaidyanathan C. S., Rao N. A. Studies on plant aspartate transcarbamylase. Purification and properties of the enzyme from mung-bean (Phaseolus aureus) seedlings. Eur J Biochem. 1974 Aug 15;47(1):15–22. doi: 10.1111/j.1432-1033.1974.tb03662.x. [DOI] [PubMed] [Google Scholar]
- Adair L. B., Jones M. E. Purification and characteristics of aspartate transcarbamylase from Pseudomonas fluorescens. J Biol Chem. 1972 Apr 25;247(8):2308–2315. [PubMed] [Google Scholar]
- Aitken D. M., Bhatti A. R., Kaplan J. G. Characterization of the aspartate carbamoyltransferase subunit obtained from a multienzyme aggregate in the pyrimidine pathway of yeast. Activity and physical properties. Biochim Biophys Acta. 1973 May 5;309(1):50–57. doi: 10.1016/0005-2744(73)90316-1. [DOI] [PubMed] [Google Scholar]
- Aitken D. M., Lue P. F., Kaplan J. G. Kinetics and reaction mechanism of the carbamylphosphate synthetase of a multienzyme aggregate from yeast. Can J Biochem. 1975 Jun;53(6):721–730. doi: 10.1139/o75-099. [DOI] [PubMed] [Google Scholar]
- Anderson P. M. Binding of allosteric effectors to carbamyl-phosphate synthetase from Escherichia coli. Biochemistry. 1977 Feb 22;16(4):587–593. doi: 10.1021/bi00623a005. [DOI] [PubMed] [Google Scholar]
- Anderson P. M. Evidence that the catalytic and regulatory functions of carbamylphosphate synthetase from Escherichia coli are not dependent on oligomer formation. Biochemistry. 1977 Feb 22;16(4):583–586. doi: 10.1021/bi00623a004. [DOI] [PubMed] [Google Scholar]
- Anderson P. M., Marvin S. V. Effect of allosteric effectors and adenosine triphosphate on the aggregation and rate of inhibition by N-ethylmaleimide of carbamyl phosphate synthetase of Escherichia coli. Biochemistry. 1970 Jan 6;9(1):171–178. doi: 10.1021/bi00803a022. [DOI] [PubMed] [Google Scholar]
- Anderson P. M., Marvin S. V. Effect of ornithine, IMP, and UMP on carbamyl phosphate synthetase from Escherichia coli. Biochem Biophys Res Commun. 1968 Sep 30;32(6):928–934. doi: 10.1016/0006-291x(68)90116-2. [DOI] [PubMed] [Google Scholar]
- Anderson P. M., Meister A. Bicarbonate-dependent cleavage of adenosine triphosphate and other reactions catalyzed by Escherichia coli carbamyl phosphate synthetase. Biochemistry. 1966 Oct;5(10):3157–3163. doi: 10.1021/bi00874a012. [DOI] [PubMed] [Google Scholar]
- Anderson P. M., Meister A. Control of Escherichia coli carbamyl phosphate synthetase by purine and pyrimidine nucleotides. Biochemistry. 1966 Oct;5(10):3164–3169. doi: 10.1021/bi00874a013. [DOI] [PubMed] [Google Scholar]
- BOWERS M. D., GRISOLIA S. Biosynthesis of carbamyl aspartate in pigeon and rat tissues. Comp Biochem Physiol. 1962 Jan;5:1–16. doi: 10.1016/0010-406x(62)90137-8. [DOI] [PubMed] [Google Scholar]
- BROWN G. J., Jr, COHEN P. P. Comparative biochemistry of urea synthesis. 3. Activities of urea-cycle enzymes in various higher and lower vertebrates. Biochem J. 1960 Apr;75:82–91. doi: 10.1042/bj0750082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barry E. G., Perkins D. D. Position of linkage group V markers in chromosome 2 of Neurospora crassa. J Hered. 1969 May-Jun;60(3):120–125. doi: 10.1093/oxfordjournals.jhered.a107952. [DOI] [PubMed] [Google Scholar]
- Bernhardt S. A., Davis R. H. Carbamoyl phosphate compartmentation in Neurospora: histochemical localization of aspartate and ornithine transcarbamoylases. Proc Natl Acad Sci U S A. 1972 Jul;69(7):1868–1872. doi: 10.1073/pnas.69.7.1868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bethell M. R., Jones M. E. Molecular size and feedback-regulation characteristics of bacterial asartate transcarbamulases. Arch Biochem Biophys. 1969 Nov;134(2):352–365. doi: 10.1016/0003-9861(69)90294-x. [DOI] [PubMed] [Google Scholar]
- Bethell M. R., Smith K. E., White J. S., Jones M. E. Carbamyl phosphate: an allosteric substrate for aspartate transcarbamylase of Escherichia coli. Proc Natl Acad Sci U S A. 1968 Aug;60(4):1442–1449. doi: 10.1073/pnas.60.4.1442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brabson J. S., Switzer R. L. Purification and properties of Bacillus subtilis aspartate transcarbamylase. J Biol Chem. 1975 Nov 25;250(22):8664–8669. [PubMed] [Google Scholar]
- Bresnick E., Mossé H. Aspartate carbamoyltransferase from rat liver. Biochem J. 1966 Oct;101(1):63–69. doi: 10.1042/bj1010063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broman K., Stalon V., Wiame J. M. The duplication of arginine catabolism and the meaning of the two ornithine carbamoyltransferases in Bacillus licheniformis. Biochem Biophys Res Commun. 1975 Sep 16;66(2):821–827. doi: 10.1016/0006-291x(75)90583-5. [DOI] [PubMed] [Google Scholar]
- CURCI M. R., DONACHI W. D. AN ATTEMPT TO FIND PYRIMIDINE INHIBITORS OF A MAMMALIAN ASPARTATE CARBAMOYLTRANSFERASE. Biochim Biophys Acta. 1964 May 4;85:338–341. doi: 10.1016/0926-6569(64)90256-1. [DOI] [PubMed] [Google Scholar]
- Chang T. Y., Jones M. E. Aspartate transcarbamylase from Streptococcus faecalis. Purification, properties, and nature of an allosteric activator site. Biochemistry. 1974 Feb 12;13(4):629–638. doi: 10.1021/bi00701a001. [DOI] [PubMed] [Google Scholar]
- Cohlberg J. A., Pigiet V. P., Jr, Schachman H. K. Structure and arrangement of the regulatory subunits in aspartate transcarbamylase. Biochemistry. 1972 Aug 29;11(18):3396–3411. doi: 10.1021/bi00768a013. [DOI] [PubMed] [Google Scholar]
- Coleman M. S., Jones M. E. Aspartate transcarbamylases of Citrobacter freundii. Biochemistry. 1971 Aug 31;10(18):3390–3396. doi: 10.1021/bi00794a012. [DOI] [PubMed] [Google Scholar]
- Coleman P. F., Suttle D. P., Stark G. R. Purification from hamster cells of the multifunctional protein that initiates de novo synthesis of pyrimidine nucleotides. J Biol Chem. 1977 Sep 25;252(18):6379–6385. [PubMed] [Google Scholar]
- Condon S., Collins J. K., O'donovan G. A. Regulation of arginine and pyrimidine biosynthesis in Pseudomonas putida. J Gen Microbiol. 1976 Feb;92(2):375–383. doi: 10.1099/00221287-92-2-375. [DOI] [PubMed] [Google Scholar]
- Cybis J., Davis R. H. Organization and control in the arginine biosynthetic pathway of Neurospora. J Bacteriol. 1975 Jul;123(1):196–202. doi: 10.1128/jb.123.1.196-202.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAVIS R. H. A mutant form of ornithine transcarbamylase found in a strain of Neurospora carrying a pyrimidine-proline suppressor gene. Arch Biochem Biophys. 1962 Apr;97:185–191. doi: 10.1016/0003-9861(62)90063-2. [DOI] [PubMed] [Google Scholar]
- DAVIS R. H. Consequences of a suppressor gene effective with pyrimidine and proline mutants of Neurospora. Genetics. 1962 Mar;47:351–360. doi: 10.1093/genetics/47.3.351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAVIS R. H., WOODWARD V. W. The relationship between gene suppression and aspartate transcarbamylase activity in pyr-3 mutants of Neurospora. Genetics. 1962 Aug;47:1075–1083. doi: 10.1093/genetics/47.8.1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DONACHIE W. D. THE REGULATION OF PYRIMIDINE BIOSYNTHESIS IN NEUROSPORA CRASSA. I. END-PRODUCT INHIBITION AND REPRESSION OF ASPARTATE CARBAMOYLTRANSFERASE. Biochim Biophys Acta. 1964 Feb 10;82:284–292. doi: 10.1016/0304-4165(64)90299-5. [DOI] [PubMed] [Google Scholar]
- Davis R. H. AN ENZYMATIC DIFFERENCE AMONG PYR-3 MUTANTS OF NEUROSPORA CRASSA. Proc Natl Acad Sci U S A. 1960 May;46(5):677–682. doi: 10.1073/pnas.46.5.677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis R. H. Carbamyl phosphate synthesis in Neurospora crassa. II. Genetics, metabolic position, and regulation of arginine-specific carbamyl phosphokinase. Biochim Biophys Acta. 1965 Aug 24;107(1):54–68. doi: 10.1016/0304-4165(65)90388-0. [DOI] [PubMed] [Google Scholar]
- Davis R. H. Metabolite distribution in cells. Science. 1972 Nov 24;178(4063):835–840. doi: 10.1126/science.178.4063.835. [DOI] [PubMed] [Google Scholar]
- Denis-Duphil M., Lacroute F. Fine structure of the ura 2 locus in Saccharomyces cervisiae. I. In vivo complementation studies. Mol Gen Genet. 1971;112(4):354–364. doi: 10.1007/BF00334436. [DOI] [PubMed] [Google Scholar]
- Elliott K. R., Tipton K. F. Kinetic studies of bovine liver carbamoyl phosphate synthetase. Biochem J. 1974 Sep;141(3):807–816. doi: 10.1042/bj1410807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans D. R., Warren S. G., Edwards B. F., McMurray C. H., Bethge P. H., Wiley D. C., Lipscomb W. N. Aqueous central cavity in aspartate transcarbamylase from Escherichia coli. Science. 1973 Feb 16;179(4074):683–685. doi: 10.1126/science.179.4074.683. [DOI] [PubMed] [Google Scholar]
- Falk D. R., Nash D. Sex-linked auxotrophic and putative auxotrophic mutants of Drosophila melanogaster. Genetics. 1974 Apr;76(4):755–766. doi: 10.1093/genetics/76.4.755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foley R., Poon J., Anderson P. M. Characterization of the reactive sulfhydryl groups in carbamyl phosphate synthetase of Escherichia coli. Biochemistry. 1971 Nov 23;10(24):4562–4569. doi: 10.1021/bi00800a034. [DOI] [PubMed] [Google Scholar]
- Forman H. J., Waddell R., Hamilton P. B., Grisolia S. Activation of carbamoyl phosphate synthase by N-acetyl-L-aspartate. Biochem J. 1974 Oct;143(1):63–66. doi: 10.1042/bj1430063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GERHART J. C., PARDEE A. B. The enzymology of control by feedback inhibition. J Biol Chem. 1962 Mar;237:891–896. [PubMed] [Google Scholar]
- Gans M., Masson M. Structure fine du locus ur-l chez Coprinus radiatus. Mol Gen Genet. 1969 Oct 13;105(2):164–181. doi: 10.1007/BF00445686. [DOI] [PubMed] [Google Scholar]
- Gerhart J. C., Holoubek H. The purification of aspartate transcarbamylase of Escherichia coli and separation of its protein subunits. J Biol Chem. 1967 Jun 25;242(12):2886–2892. [PubMed] [Google Scholar]
- Gerhart J. C., Schachman H. K. Allosteric interactions in aspartate transcarbamylase. II. Evidence for different conformational states of the protein in the presence and absence of specific ligands. Biochemistry. 1968 Feb;7(2):538–552. doi: 10.1021/bi00842a600. [DOI] [PubMed] [Google Scholar]
- Gerhart J. C., Schachman H. K. Distinct subunits for the regulation and catalytic activity of aspartate transcarbamylase. Biochemistry. 1965 Jun;4(6):1054–1062. doi: 10.1021/bi00882a012. [DOI] [PubMed] [Google Scholar]
- Haas D., Holloway B. W., Schamböck A., Leisinger T. The genetic organization of arginine biosynthesis in Pseudomonas aeruginosa. Mol Gen Genet. 1977 Jul 7;154(1):7–22. doi: 10.1007/BF00265571. [DOI] [PubMed] [Google Scholar]
- Hager S. E., Jones M. E. A glutamine-dependent enzyme for the synthesis of carbamyl phosphate for pyrimidine biosynthesis in fetal rat liver. J Biol Chem. 1967 Dec 25;242(24):5674–5680. [PubMed] [Google Scholar]
- Hager S. E., Jones M. E. Initial steps in pyrimidine synthesis in Ehrlich ascites carcinoma in vitro. II. The synthesis of carbamyl phosphate by a soluble, glutamine-dependent carbamyl phosphate synthetase. J Biol Chem. 1967 Dec 25;242(24):5667–5673. [PubMed] [Google Scholar]
- Hill J. M., Woodward V. W. Genetic control of aspartate transcarbamylase by the pyr-3 locus of Neurospora crassa. Arch Biochem Biophys. 1968 Apr;125(1):1–12. doi: 10.1016/0003-9861(68)90631-0. [DOI] [PubMed] [Google Scholar]
- Hirsch M. L. Séparation des enzymes jouant un rôle dans la compartimentation entre les chaînes de biosyntheèse de l'uracile et de l'arginine chez le Coprinus radiatus. C R Acad Sci Hebd Seances Acad Sci D. 1968 Oct 28;267(18):1473–1476. [PubMed] [Google Scholar]
- Hoogenraad N. J., Levine R. L., Kretchmer N. Copurification of carbamoyl phosphate synthetase and aspartate transcarbamoylase from mouse spleen. Biochem Biophys Res Commun. 1971 Aug 20;44(4):981–988. doi: 10.1016/0006-291x(71)90808-4. [DOI] [PubMed] [Google Scholar]
- Hutson J. Y., Downing M. Pyrimidine biosynthesis in Lactobacillus leichmannii. J Bacteriol. 1968 Oct;96(4):1249–1254. doi: 10.1128/jb.96.4.1249-1254.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isaac J. H., Holloway B. W. Control of pyrimidine biosynthesis in Pseudomonas aeruginosa. J Bacteriol. 1968 Nov;96(5):1732–1741. doi: 10.1128/jb.96.5.1732-1741.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Issaly I. M., Issaly A. S., Reissig J. L. Carbamyl phosphate biosynthesis in Bacillus subtilis. Biochim Biophys Acta. 1970 Mar 18;198(3):482–494. doi: 10.1016/0005-2744(70)90126-9. [DOI] [PubMed] [Google Scholar]
- Ito K., Nakanishi S., Terada M., Tatibana M. Control of pyrimidine biosynthesis in mammalian tissues. II. Glutamine-utilizing carbamoyl phosphate synthetase of various experimental tumors: distribution, purification and characterization. Biochim Biophys Acta. 1970 Dec 16;220(3):477–490. doi: 10.1016/0005-2744(70)90279-2. [DOI] [PubMed] [Google Scholar]
- Ito K., Uchino H. Control of pyrimidine biosynthesis in human lymphocytes. Simultaneous increase in activities of glutamine-utilizing carbamyl phosphate synthetase and aspartate transcarbamylase in phytohemagglutinin-stimulated human peripheral lymphocytes and their enzyme co-purification. J Biol Chem. 1973 Jan 25;248(2):389–392. [PubMed] [Google Scholar]
- Jarry B., Falk D. Functional diversity within the rudimentary locus of Drosophila melanogaster. Mol Gen Genet. 1974;135(2):113–122. doi: 10.1007/BF00264779. [DOI] [PubMed] [Google Scholar]
- Jarry B. Isolation of a multifunctional complex containing the first three enzymes of pyrimidine biosynthesis in drosophila melanogaster. FEBS Lett. 1976 Nov;70(1):71–75. doi: 10.1016/0014-5793(76)80728-4. [DOI] [PubMed] [Google Scholar]
- Kaplan J. G., Duphil M., Lacroute F. A study of the aspartate transcarbamylase activity of yeast. Arch Biochem Biophys. 1967 Mar;119(1):541–551. doi: 10.1016/0003-9861(67)90489-4. [DOI] [PubMed] [Google Scholar]
- Kaplan J. G., Lacroute F., Messmer I. On the loss of feedback inhibition of yeast aspartate transcarbamylase during derepression of pyrimidine biosynthesis. Arch Biochem Biophys. 1969 Feb;129(2):539–544. doi: 10.1016/0003-9861(69)90212-4. [DOI] [PubMed] [Google Scholar]
- Kaplan J. G., Messmer I. The combined effects of temperature and dilution on the activity and feedback inhibition of yeast aspartate transcarbamylase. Can J Biochem. 1969 Apr;47(4):477–479. doi: 10.1139/o69-074. [DOI] [PubMed] [Google Scholar]
- Kent R. J., Lin R. L., Sallach H. J., Cohen P. P. Reversible dissociation of a carbamoyl phosphate synthase-aspartate transcarbamoylase-dihydroorotase complex from ovarian eggs of Rana catesbeiana: effect of uridine triphosphate and other modifiers. Proc Natl Acad Sci U S A. 1975 May;72(5):1712–1716. doi: 10.1073/pnas.72.5.1712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerson L. A., Appel S. H. Kinetic studies on rat liver carbamyl phosphate synthetase. J Biol Chem. 1968 Aug 25;243(16):4279–4285. [PubMed] [Google Scholar]
- Kidder G. W., Davis J. S., Cousens K. Citrulline utilization in Crithidia. Biochem Biophys Res Commun. 1966 Aug 12;24(3):365–369. doi: 10.1016/0006-291x(66)90165-3. [DOI] [PubMed] [Google Scholar]
- Kidder G. W., Dewey V. C., Nolan L. L. Enzymes of the orotate biosynthetic pathway in Crithidia fasciculata. Can J Biochem. 1976 Jan;54(1):32–41. doi: 10.1139/o76-006. [DOI] [PubMed] [Google Scholar]
- Knight D. M., Jones E. E. Regulation of Escherichia coli ornithine transcarbamylase by orotate. J Biol Chem. 1977 Sep 10;252(17):5928–5930. [PubMed] [Google Scholar]
- Koskimies O., Oliver I., Hurwitz R., Kretchmer N. Aspartate transcarbamoylase: distribution in various tissues and identification of three molecular forms. Biochem Biophys Res Commun. 1971 Mar 19;42(6):1162–1168. doi: 10.1016/0006-291x(71)90027-1. [DOI] [PubMed] [Google Scholar]
- Kurelec B. Aspartate transcarbamylase in some parasitic platyhelminths. Comp Biochem Physiol B. 1974 Jan 15;47(1):33–40. doi: 10.1016/0305-0491(74)90088-1. [DOI] [PubMed] [Google Scholar]
- Kurelec B. Initial steps in the de novo pyrimidine biosynthesis in Ascaris suum. J Parasitol. 1973 Dec;59(6):1006–1011. [PubMed] [Google Scholar]
- Kurelec B. Lack of carbamyl phosphate synthesis in some parasitic platyhelminths. Comp Biochem Physiol B. 1972 Dec 15;43(4):769–780. doi: 10.1016/0305-0491(72)90223-4. [DOI] [PubMed] [Google Scholar]
- Lacroute F. Regulation of pyrimidine biosynthesis in Saccharomyces cerevisiae. J Bacteriol. 1968 Mar;95(3):824–832. doi: 10.1128/jb.95.3.824-832.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lan S. J., Sallach H. J., Cohen P. P. The enzymatic steps of pyrimidine biosynthesis in the unfertilized frog egg. Biochemistry. 1969 Sep;8(9):3673–3680. doi: 10.1021/bi00837a027. [DOI] [PubMed] [Google Scholar]
- Liebl V., Kaplan J. G., Kushner D. J. Regulation of a salt-dependent enzyme: the aspartate transcarbamylase of an extreme halophile. Can J Biochem. 1969 Dec;47(12):1095–1097. doi: 10.1139/o69-175. [DOI] [PubMed] [Google Scholar]
- Lue P. F., Kaplan J. G. Aggregation states of a regulatory enzyme complex catalyzing the early steps of pyrimidine biosynthesis in bakers' yeast. Can J Biochem. 1971 Apr;49(4):403–411. doi: 10.1139/o71-059. [DOI] [PubMed] [Google Scholar]
- Lue P. F., Kaplan J. G. Heat-induced disaggregation of a multifunctional enzyme complex catalyzing the first steps in pyrimidine biosynthesis in bakers' yeast. Can J Biochem. 1970 Feb;48(2):155–159. doi: 10.1139/o70-024. [DOI] [PubMed] [Google Scholar]
- Lue P. F., Kaplan J. G. Metabolic compartmentation at the molecular level: the function of a multienzyme aggregate in the pyrimidine pathway of yeast. Biochim Biophys Acta. 1970 Dec 16;220(3):365–372. doi: 10.1016/0005-2744(70)90268-8. [DOI] [PubMed] [Google Scholar]
- Lue P. F., Kaplan J. G. The aspartate transcarbamylase and carbamoyl phosphate synthetase of yeast: a multi-functional enzyme complex. Biochem Biophys Res Commun. 1969 Feb 21;34(4):426–433. doi: 10.1016/0006-291x(69)90399-4. [DOI] [PubMed] [Google Scholar]
- Makoff A. J. Characterization of the aspartate carbamoyltransferase fragment generated by protease action on the pyrimidine-3 gene product of Neurospora crassa. Biochim Biophys Acta. 1977 Dec 8;485(2):330–335. doi: 10.1016/0005-2744(77)90168-1. [DOI] [PubMed] [Google Scholar]
- Makoff A. J., Radford A. Dissociation of an enzyme complex from Neurospora crassa. Evidence for a protease. Biochim Biophys Acta. 1977 Dec 8;485(2):314–329. doi: 10.1016/0005-2744(77)90167-x. [DOI] [PubMed] [Google Scholar]
- Makoff A. J., Radford A. Glutamine utilization in both the arginine-specific and pyrimidine-specific carbamoyl phosphate synthase enzymes of eurospora crassa. Mol Gen Genet. 1976 Dec 8;149(2):175–178. doi: 10.1007/BF00332886. [DOI] [PubMed] [Google Scholar]
- Makoff A. J., Radford A. The location of the feedback-specific region with the pyrimidine-3 locus of Neurospora crassa. Mol Gen Genet. 1976 Aug 2;146(3):247–251. doi: 10.1007/BF00701247. [DOI] [PubMed] [Google Scholar]
- Matthews S. L., Anderson P. M. Evidence for the presence of two nonidentical subunits in carbamyl phosphate synthetase of Escherichia coli. Biochemistry. 1972 Mar 28;11(7):1176–1183. doi: 10.1021/bi00757a010. [DOI] [PubMed] [Google Scholar]
- Mergeay M., Gigot D., Beckmann J., Glansdorff N., Piérard A. Physiology and genetics of carbamoylphosphate synthesis in Escherichia coli K12. Mol Gen Genet. 1974;133(4):299–316. doi: 10.1007/BF00332706. [DOI] [PubMed] [Google Scholar]
- Mori M., Ishida H., Tatibana M. Aggregation states and catalytic properties of the multienzyme complex catalyzing the initial steps of pyrimidine biosynthesis in rat liver. Biochemistry. 1975 Jun 17;14(12):2622–2630. doi: 10.1021/bi00683a010. [DOI] [PubMed] [Google Scholar]
- Mori M., Tatibana M. Dissociation by elastase digestion of enzyme complex catalyzing the initial steps of pyrimidine biosynthesis in rat liver. Biochem Biophys Res Commun. 1973 Oct 15;54(4):1525–1531. doi: 10.1016/0006-291x(73)91159-5. [DOI] [PubMed] [Google Scholar]
- Mori M., Tatibana M. Purification of homogeneous glutamine-dependent carbamyl phosphate synthetase from ascites hepatoma cells as a complex with aspartate transcarbamylase and dihydroorotase. J Biochem. 1975 Jul;78(1):239–242. [PubMed] [Google Scholar]
- Mylyk O. M. Heterokaryon incompatibility genes in Neurospora crassa detected using duplication-producing chromosome rearrangements. Genetics. 1975 May;80(1):107–124. doi: 10.1093/genetics/80.1.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NEUMANN J., JONES M. E. END-PRODUCT INHIBITION OF ASPARTATE TRANSCARBAMYLASE IN VARIOUS SPECIES. Arch Biochem Biophys. 1964 Mar;104:438–447. doi: 10.1016/0003-9861(64)90487-4. [DOI] [PubMed] [Google Scholar]
- Natale P. J., Tremblay G. C. On the availability of intramitochondrial carbamoylphosphate for the extramitochondrial biosynthesis of pyrimidines. Biochem Biophys Res Commun. 1969 Oct 22;37(3):512–517. doi: 10.1016/0006-291x(69)90945-0. [DOI] [PubMed] [Google Scholar]
- Natale P. J., Tremblay G. C. Studies on the availability of intramitochondrial carbamoylphosphate for utilization in extramitochondrial reactions in rat liver. Arch Biochem Biophys. 1974 Jun;162(2):357–368. doi: 10.1016/0003-9861(74)90193-3. [DOI] [PubMed] [Google Scholar]
- Nelbach M. E., Pigiet V. P., Jr, Gerhart J. C., Schachman H. K. A role for zinc in the quaternary structure of aspartate transcarbamylase from Escherichia coli. Biochemistry. 1972 Feb 1;11(3):315–327. doi: 10.1021/bi00753a002. [DOI] [PubMed] [Google Scholar]
- Norberg P., Kaplan J. G., Kushner D. J. Kinetics and regulation of the salt-dependent aspartate transcarbamylase of Halobacterium cutirubrum. J Bacteriol. 1973 Feb;113(2):680–686. doi: 10.1128/jb.113.2.680-686.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norby S. A specific nutritional requirement for pyrimidines in rudimentary mutants of Drosophila melanogaster. Hereditas. 1970;66(2):205–214. doi: 10.1111/j.1601-5223.1970.tb02346.x. [DOI] [PubMed] [Google Scholar]
- Norby S. The biochemical genetics of rudimentary mutants of Drosophila melanogaster. I. Aspartate carbamoyltransferase levels in complementing and non-complementing strains. Hereditas. 1973;73(1):11–16. [PubMed] [Google Scholar]
- O'Donovan G. A., Gerhart J. C. Isolation and partial characterization of regulatory mutants of the pyrimidine pathway in Salmonella typhimurium. J Bacteriol. 1972 Mar;109(3):1085–1096. doi: 10.1128/jb.109.3.1085-1096.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Donovan G. A., Holoubek H., Gerhart J. C. Regulatory properties of intergeneric hybrids of aspartate transcarbamylase. Nat New Biol. 1972 Aug 30;238(87):264–266. doi: 10.1038/newbio238264a0. [DOI] [PubMed] [Google Scholar]
- O'Donovan G. A., Neuhard J. Pyrimidine metabolism in microorganisms. Bacteriol Rev. 1970 Sep;34(3):278–343. doi: 10.1128/br.34.3.278-343.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Neal D., Naylor A. W. Purine and pyrimidine nucleotide inhibition of carbamyl phosphate synthetase from pea seedlings. Biochem Biophys Res Commun. 1968 May 10;31(3):322–327. doi: 10.1016/0006-291x(68)90478-6. [DOI] [PubMed] [Google Scholar]
- O'Neal T. D., Naylor A. W. Partial purification and properties of carbamoyl phosphate synthetase of Alaska pea (Pisum sativum L. cultivar Alaska). Biochem J. 1969 Jun;113(2):271–279. doi: 10.1042/bj1130271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'neal T. D., Naylor A. W. Some regulatory properties of pea leaf carbamoyl phosphate synthetase. Plant Physiol. 1976 Jan;57(1):23–28. doi: 10.1104/pp.57.1.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ong B. L., Jackson J. F. Aspartate transcarbamoylase from Phaseolus aureus. Partial purification and properties. Biochem J. 1972 Sep;129(3):571–581. doi: 10.1042/bj1290571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ong B. L., Jackson J. F. Pyrimidine nucleotide biosynthesis in Phaseolus aureus. Enzymic aspects of the control of carbamoyl phosphate synthesis and utilization. Biochem J. 1972 Sep;129(3):583–593. doi: 10.1042/bj1290583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer L. M., Cove D. J. Pyrimidine biosynthesis in Aspergillus nidulans: isolation and preliminary characterisation of auxotrophic mutants. Mol Gen Genet. 1975 Jun 19;138(3):243–255. doi: 10.1007/BF00269351. [DOI] [PubMed] [Google Scholar]
- Palmer L. M., Scazzocchio C., Cove D. J. Pyrimidine biosynthesis in Aspergillus nidulans. Isolation and characterisation of mutants resistant to fluoropyrimidines. Mol Gen Genet. 1975 Sep 29;140(2):165–173. doi: 10.1007/BF00329784. [DOI] [PubMed] [Google Scholar]
- Piérard A., Glansdorff N., Mergeay M., Wiame J. M. Control of the biosynthesis of carbamoyl phosphate in Escherichia coli. J Mol Biol. 1965 Nov;14(1):23–36. doi: 10.1016/s0022-2836(65)80226-1. [DOI] [PubMed] [Google Scholar]
- Potvin B. W., Kelleher R. J., Jr, Gooder H. Pyrimidine biosynthetic pathway of Baccillus subtilis. J Bacteriol. 1975 Aug;123(2):604–615. doi: 10.1128/jb.123.2.604-615.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potvin B., Gooder H. Carbamyl phosphate synthesis in Bacillus subtilis. Biochem Genet. 1975 Feb;13(1-2):125–143. doi: 10.1007/BF00486011. [DOI] [PubMed] [Google Scholar]
- Powers S. G., Griffith O. W., Meister A. Inhibition of carbamyl phosphate synthetase by P1, P5-di(adenosine 5')-pentaphosphate: evidence for two ATP binding sites. J Biol Chem. 1977 May 25;252(10):3558–3560. [PubMed] [Google Scholar]
- Prescott L. M., Jones M. E. Streptococcus faecalis aspartate transcarbamylase. I. Purification and general properties. Biochemistry. 1970 Sep 15;9(19):3783–3794. doi: 10.1021/bi00821a018. [DOI] [PubMed] [Google Scholar]
- Pringle J. R. Methods for avoiding proteolytic artefacts in studies of enzymes and other proteins from yeasts. Methods Cell Biol. 1975;12:149–184. doi: 10.1016/s0091-679x(08)60956-5. [DOI] [PubMed] [Google Scholar]
- REISSIG J. L. Spectrum of forward mutants in the pyr-3 region of Neurospora. J Gen Microbiol. 1963 Feb;30:327–337. doi: 10.1099/00221287-30-2-327. [DOI] [PubMed] [Google Scholar]
- Radford A. Change in complementation patterns and enzyme activity of pyrimidine-3 mutants of Neurospora crassa by induced reversion. Mutat Res. 1971 May;12(1):57–64. doi: 10.1016/0027-5107(71)90072-8. [DOI] [PubMed] [Google Scholar]
- Radford A. Information from ICR-170-induced mutations on the structure of the pyrimidine-3 locus in Neurospora. Mutat Res. 1969 Nov-Dec;8(3):537–544. doi: 10.1016/0027-5107(69)90071-2. [DOI] [PubMed] [Google Scholar]
- Radford A. Polarised complementation at the pyrimidine-3 locus of Neurospora. Mol Gen Genet. 1969 Jul 3;104(3):288–294. doi: 10.1007/BF02539292. [DOI] [PubMed] [Google Scholar]
- Radford A. Pyrimidine-requiring suppressor mutations of arginine-3 in Neurospora and their bearing on the structure of the pyrimidine-3 locus. Mol Gen Genet. 1970;107(2):97–106. doi: 10.1007/BF00333625. [DOI] [PubMed] [Google Scholar]
- Rawls J. M., Fristrom J. W. A complex genetic locus that controls of the first three steps of pyrimidine biosynthesis in Drosophila. Nature. 1975 Jun 26;255(5511):738–740. doi: 10.1038/255738a0. [DOI] [PubMed] [Google Scholar]
- Rochovansky O. Pyrimidine biosynthesis in the chick. Arch Biochem Biophys. 1970 Jun;138(2):574–581. doi: 10.1016/0003-9861(70)90383-8. [DOI] [PubMed] [Google Scholar]
- Rosenbusch J. P., Weber K. Localization of the zinc binding site of aspartate transcarbamoylase in the regulatory subunit. Proc Natl Acad Sci U S A. 1971 May;68(5):1019–1023. doi: 10.1073/pnas.68.5.1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenbusch J. P., Weber K. Subunit structure of aspartate transcarbamylase from Escherichia coli. J Biol Chem. 1971 Mar 25;246(6):1644–1657. [PubMed] [Google Scholar]
- Shoaf W. T., Jones M. E. Initial steps in pyrimidine synthesis in Ehrlich ascites carcinoma. Biochem Biophys Res Commun. 1971 Nov 5;45(3):796–802. doi: 10.1016/0006-291x(71)90487-6. [DOI] [PubMed] [Google Scholar]
- Shoaf W. T., Jones M. E. Uridylic acid synthesis in Ehrlich ascites carcinoma. Properties, subcellular distribution, and nature of enzyme complexes of the six biosynthetic enzymes. Biochemistry. 1973 Oct 9;12(21):4039–4051. doi: 10.1021/bi00745a004. [DOI] [PubMed] [Google Scholar]
- Stanier R. Y., Palleroni N. J., Doudoroff M. The aerobic pseudomonads: a taxonomic study. J Gen Microbiol. 1966 May;43(2):159–271. doi: 10.1099/00221287-43-2-159. [DOI] [PubMed] [Google Scholar]
- Syvanen J. M., Roth J. R. Structural genes for catalytic and regulatory subunits of aspartate transcarbamylase. J Mol Biol. 1973 May 25;76(3):363–378. doi: 10.1016/0022-2836(73)90510-x. [DOI] [PubMed] [Google Scholar]
- Syvanen J. M., Roth J. R. Structural genes for ornithine transcarbamylase in Salmonella typhimurium and Escherichia coli K-12. J Bacteriol. 1972 Apr;110(1):66–70. doi: 10.1128/jb.110.1.66-70.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Söderholm G., Schwartz M., Nørby S. Aspartate caramoyltransferase from wild type and rudimentary Drosophila melanogaster. FEBS Lett. 1975 May 1;53(2):148–153. doi: 10.1016/0014-5793(75)80007-x. [DOI] [PubMed] [Google Scholar]
- TAMIR H., RATNER S. ENZYMES OF ARGININE METABOLISM IN CHICKS. Arch Biochem Biophys. 1963 Aug;102:249–258. doi: 10.1016/0003-9861(63)90178-4. [DOI] [PubMed] [Google Scholar]
- Tatibana M., Ito K. Control of pyrimidine biosynthesis in mammalian tissues. I. Partial purification and characterization of glutamine-utilizing carbamyl phosphate synthetase of mouse spleen and its tissue distribution. J Biol Chem. 1969 Oct 10;244(19):5403–5413. [PubMed] [Google Scholar]
- Tatibana M., Shigesada K. Control of pyrimidine biosynthesis in mammalian tissues. IV. Requirements of a quantitative assay of glutamine-dependent carbamyl phosphate synthetase and effect of magnesium ion as an essential activator. J Biochem. 1972 Sep;72(3):537–547. doi: 10.1093/oxfordjournals.jbchem.a129933. [DOI] [PubMed] [Google Scholar]
- Tatibana M., Shigesada K. Control of pyrimidine biosynthesis in mammalian tissues. V. Regulation of glutamine-dependent carbamyl phosphate synthetase: activation by 5-phosphoribosyl 1-pyrophosphate and inhibition by uridine triphosphate. J Biochem. 1972 Sep;72(3):549–560. doi: 10.1093/oxfordjournals.jbchem.a129934. [DOI] [PubMed] [Google Scholar]
- Tramell P. R., Campbell J. W. Carbamyl phosphate synthesis in a land snail, Strophocheilus oblongus. J Biol Chem. 1970 Dec 25;245(24):6634–6641. [PubMed] [Google Scholar]
- Trotta P. P., Burt M. E., Haschemeyer R. H., Meister A. Reversible dissociation of carbamyl phosphate synthetase into a regulated synthesis subunit and a subunit required for glutamine utilization. Proc Natl Acad Sci U S A. 1971 Oct;68(10):2599–2603. doi: 10.1073/pnas.68.10.2599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trotta P. P., Estis L. F., Meister A., Haschemeyer R. H. Self-association and allosteric properties of glutamine-dependent carbamyl phosphate synthetase. Reversible dissociation to monomeric species. J Biol Chem. 1974 Jan 25;249(2):482–489. [PubMed] [Google Scholar]
- Vekich A. J., Robinson J. L., Larson B. L. Carbamylphosphate synthetase of lactating bovine mammary tissue. Biochem Biophys Res Commun. 1973 Dec 19;55(4):1298–1304. doi: 10.1016/s0006-291x(73)80035-x. [DOI] [PubMed] [Google Scholar]
- Virden R. The molecular weights of two forms of carbamoyl phosphate synthase from rat liver. Biochem J. 1972 Apr;127(3):503–508. doi: 10.1042/bj1270503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren S. G., Edwards B. F., Evans D. R., Wiley D. C., Lipscomb W. N. Aspartate transcarbamoylase from Escherichia coli: electron density at 5.5 A resolution. Proc Natl Acad Sci U S A. 1973 Apr;70(4):1117–1121. doi: 10.1073/pnas.70.4.1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber K. New structural model of E. coli aspartate transcarbamylase and the amino-acid sequence of the regulatory polypeptide chain. Nature. 1968 Jun 22;218(5147):1116–1119. doi: 10.1038/2181116a0. [DOI] [PubMed] [Google Scholar]
- Wiley D. C., Evans D. R., Warren S. G., McMurray C. H., Edwards B. F., Franks W. A., Lipscomb W. N. The 5.5 Angstrom resolution structure of the regulatory enzyme, asparate transcarbamylase. Cold Spring Harb Symp Quant Biol. 1972;36:285–290. doi: 10.1101/sqb.1972.036.01.038. [DOI] [PubMed] [Google Scholar]
- Wiley D. C., Lipscomb W. N. Crystallographic determination of symmetry of aspartate transcarbamylase. Nature. 1968 Jun 22;218(5147):1119–1121. doi: 10.1038/2181119a0. [DOI] [PubMed] [Google Scholar]
- Williams L. G., Bernhardt S. A., Davis R. H. Evidence for two discrete carbamyl phosphate pools in Neurospora. J Biol Chem. 1971 Feb 25;246(4):973–978. [PubMed] [Google Scholar]
- Williams L. G., Bernhardt S., Davis R. H. Copurification of pyrimidine-specific carbamyl phosphate synthetase and aspartate transcarbamylase of Neurospora crassa. Biochemistry. 1970 Oct 27;9(22):4329–4335. doi: 10.1021/bi00824a013. [DOI] [PubMed] [Google Scholar]
- Williams L. G., Davis R. H. Pyrimidine-specific carbamyl phosphate synthetase in Neurospora crassa. J Bacteriol. 1970 Aug;103(2):335–341. doi: 10.1128/jb.103.2.335-341.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan Y., Demerec M. Genetic analysis of pyrimidine mutants of Salmonella typhimurium. Genetics. 1965 Sep;52(3):643–651. doi: 10.1093/genetics/52.3.643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yon R. J. Wheat-germ aspartate transcarbamoylase. Kinetic behaviour suggesting an allosteric mechanism of regulation. Biochem J. 1972 Jun;128(2):311–320. doi: 10.1042/bj1280311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yon R. J. Wheat-germ aspartate transcarbamoylase. The effects of ligands on the inactivation of the enzyme by trypsin and denaturing agents. Biochem J. 1973 Apr;131(4):699–706. doi: 10.1042/bj1310699. [DOI] [PMC free article] [PubMed] [Google Scholar]


