Full text
PDF


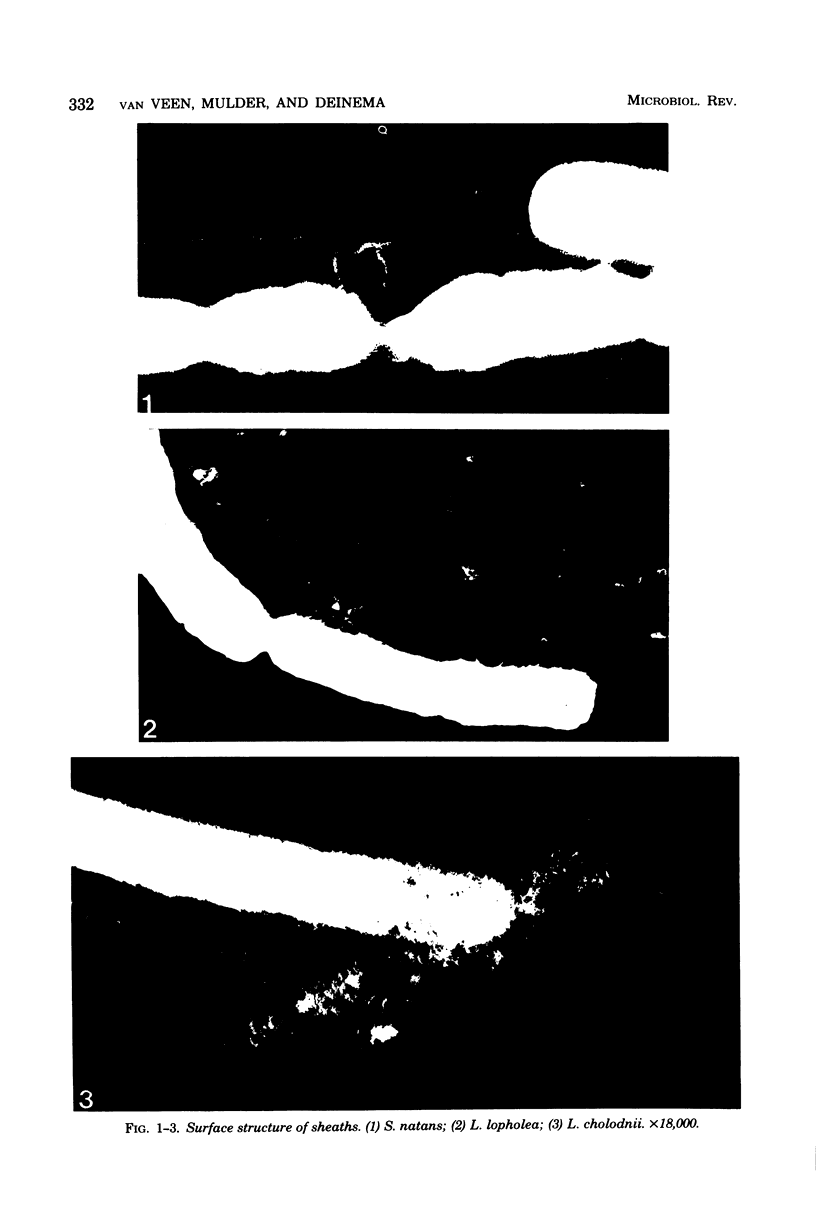




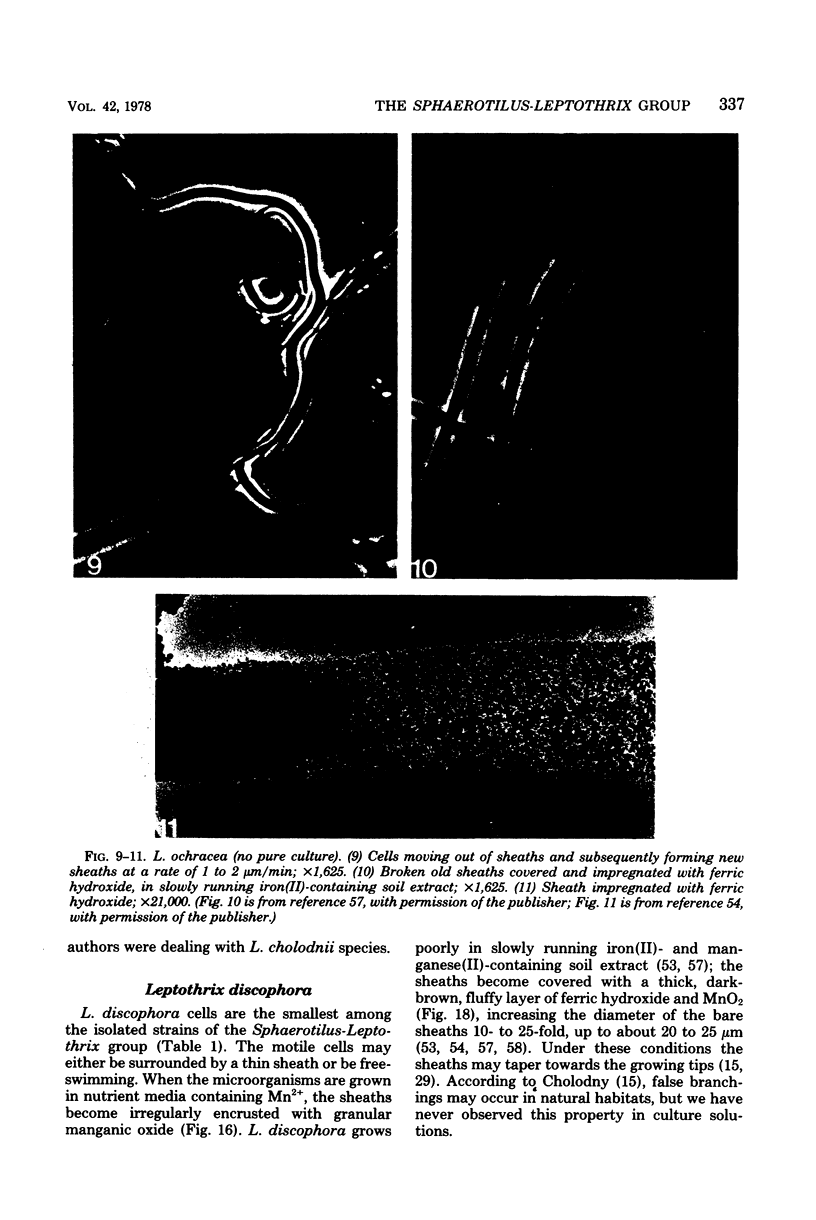
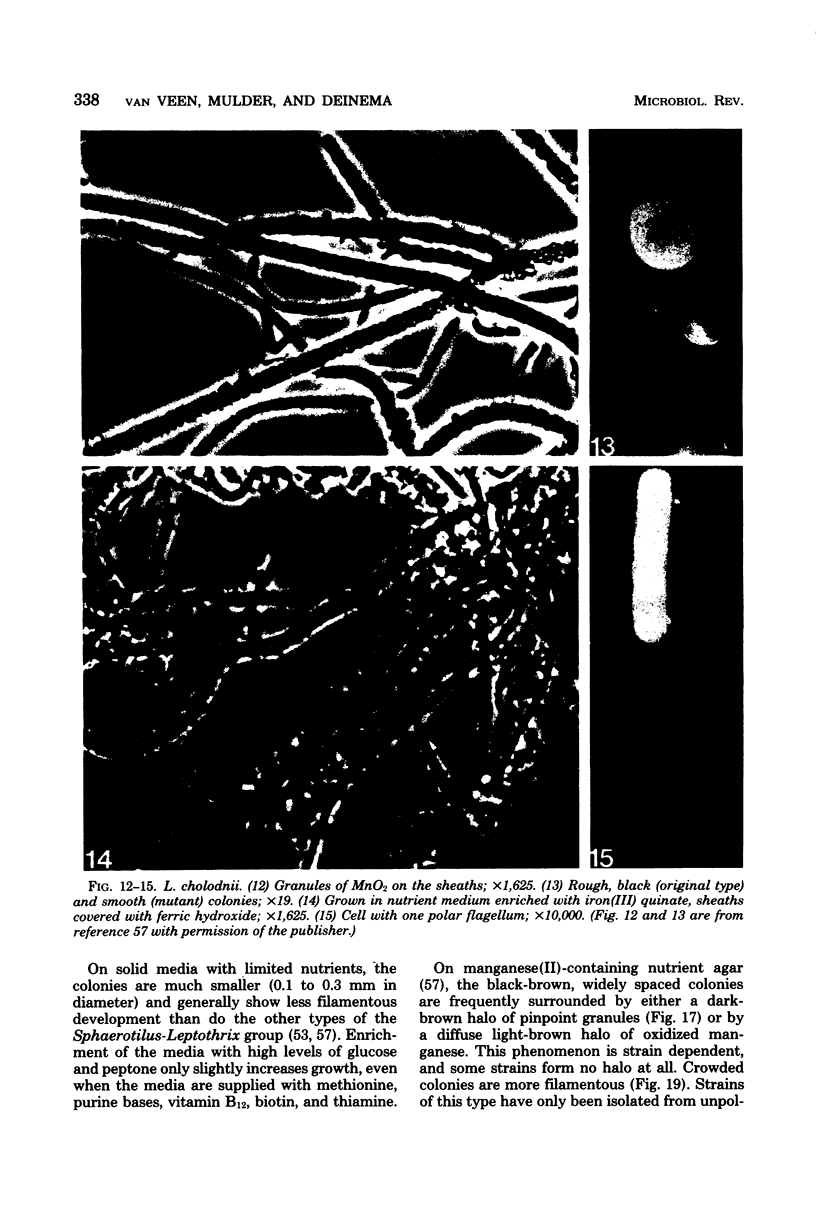
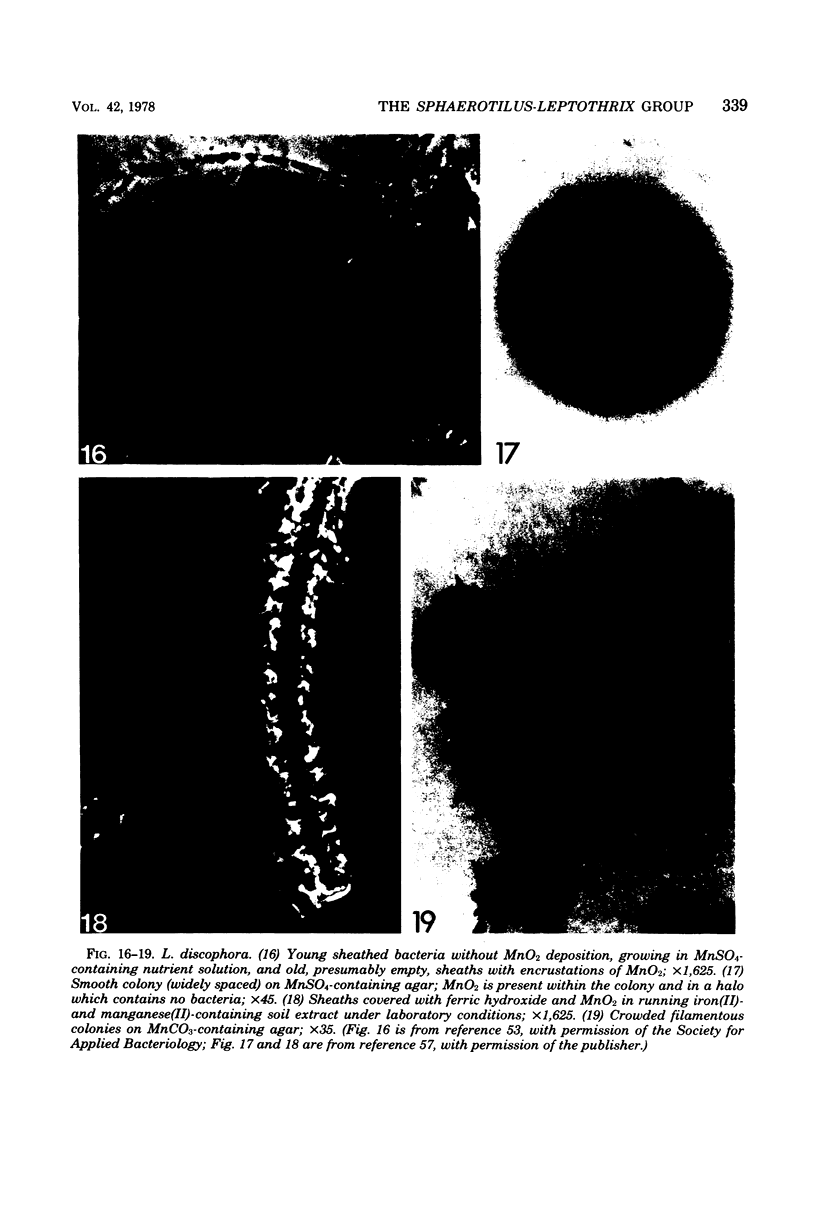














Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ali S. H., Stokes J. L. Stimulation of heterotrophic and autotrophic growth of Sphaerotilus discophorus by manganous ions. Antonie Van Leeuwenhoek. 1971;37(4):519–528. doi: 10.1007/BF02218522. [DOI] [PubMed] [Google Scholar]
- Armbruster E. H. Improved technique for isolation and identification of Sphaerotilus. Appl Microbiol. 1969 Feb;17(2):320–321. doi: 10.1128/am.17.2.320-321.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BECK J. V. A ferrous-ion-oxidizing bacterium. I. Isolation and some general physiological characteristics. J Bacteriol. 1960 Apr;79:502–509. doi: 10.1128/jb.79.4.502-509.1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bott T. L., Brock T. D. Growth rate of Sphaerotilus in a thermally polluted environment. Appl Microbiol. 1970 Jan;19(1):100–102. doi: 10.1128/am.19.1.100-102.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brantner H. Untersuchungen zur biologischen Eisen- und Manganoxydation. Zentralbl Bakteriol Parasitenkd Infektionskr Hyg. 1970;124(5):412–426. [PubMed] [Google Scholar]
- COLMER A. R., TEMPLE K. L., HINKLE M. E. An iron-oxidizing bacterium from the acid drainage of some bituminous coal mines. J Bacteriol. 1950 Mar;59(3):317–328. doi: 10.1128/jb.59.3.317-328.1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crombach W. H., van Veen W. L., van der Vlies A. W., Bots W. C. DNA base composition of some sheathed bacteria. Antonie Van Leeuwenhoek. 1974;40(2):217–220. doi: 10.1007/BF00394379. [DOI] [PubMed] [Google Scholar]
- DONDERO N. C., PHILLIPS R. A., HEUKELEKIAN H. Isolation and preservation of cultures of Sphaerotilus. Appl Microbiol. 1961 May;9:219–227. doi: 10.1128/am.9.3.219-227.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DONDERO N. C. Sphaerotilus, its nature and economic significance. Adv Appl Microbiol. 1961;3:77–107. doi: 10.1016/s0065-2164(08)70507-0. [DOI] [PubMed] [Google Scholar]
- Dawes E. A., Senior P. J. The role and regulation of energy reserve polymers in micro-organisms. Adv Microb Physiol. 1973;10:135–266. doi: 10.1016/s0065-2911(08)60088-0. [DOI] [PubMed] [Google Scholar]
- Deinema M. H., Henstra S., von Elgg E. W. Structural and physiological characteristics of some sheathed bacteria. Antonie Van Leeuwenhoek. 1977;43(1):19–29. doi: 10.1007/BF02316206. [DOI] [PubMed] [Google Scholar]
- Dias F. F., Dondero N. A., Finstein M. S. Attached growth of Sphaerotilus and mixed populations in a continuous-flow apparatus. Appl Microbiol. 1968 Aug;16(8):1191–1199. doi: 10.1128/am.16.8.1191-1199.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dias F. F., Okrend H., Dondero N. C. Calcium nutrition of Sphaerotilus growing in a continuous-flow apparatus. Appl Microbiol. 1968 Sep;16(9):1364–1369. doi: 10.1128/am.16.9.1364-1369.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dondero N. C. The Sphaerotilus-Leptothrix group. Annu Rev Microbiol. 1975;29:407–428. doi: 10.1146/annurev.mi.29.100175.002203. [DOI] [PubMed] [Google Scholar]
- Ehrlich H. L. Bacteriology of Manganese Nodules: I. Bacterial Action on Manganese in Nodule Enrichments. Appl Microbiol. 1963 Jan;11(1):15–19. doi: 10.1128/am.11.1.15-19.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GAUDY E., WOLFE R. S. Composition of an extracellular poly-saccharide produced by Sphaerotilus natans. Appl Microbiol. 1962 May;10:200–205. doi: 10.1128/am.10.3.200-205.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GAUDY E., WOLFE R. S. Factors affecting filamentous growth of Sphaerotilus natans. Appl Microbiol. 1961 Nov;9:580–584. doi: 10.1128/am.9.6.580-584.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOHNL G. Ernährungs- und stoffwechselphysiologische Untersuchungen an Sphaerotilus natans. Arch Mikrobiol. 1955;23(3):207–250. [PubMed] [Google Scholar]
- Hajj H., Makemson J. Determination of growth of Sphaerotilus discophorus in the presence of manganese. Appl Environ Microbiol. 1976 Nov;32(5):699–702. doi: 10.1128/aem.32.5.699-702.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoeniger J. F., Tauschel H. D., Stokes J. L. The fine structure of Sphaerotilus natans. Can J Microbiol. 1973 Mar;19(3):309–313. doi: 10.1139/m73-051. [DOI] [PubMed] [Google Scholar]
- Ivler D. Aids in classification and characterization of Neisseria species. J Gen Microbiol. 1971 Dec;69(3):x–x. [PubMed] [Google Scholar]
- JOHNSON A. H., STOKES J. L. EFFECT OF AMINO ACIDS ON GROWTH OF SPHAEROTILUS DISCOPHORUS. Antonie Van Leeuwenhoek. 1965;31:165–174. doi: 10.1007/BF02045887. [DOI] [PubMed] [Google Scholar]
- Johnson A. H., Stokes J. L. Managanese oxidation by Sphaerotilus discophorus. J Bacteriol. 1966 Apr;91(4):1543–1547. doi: 10.1128/jb.91.4.1543-1547.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KUCERA S., WOLFE R. S. A selective enrichment method for Gallionella ferruginea. J Bacteriol. 1957 Sep;74(3):344–349. doi: 10.1128/jb.74.3.344-349.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewin R. A. Flexithrix dorotheae gen. et sp. nov. (flexibacterales); and suggestions for reclassifying sheathed bacteria. Can J Microbiol. 1970 Jun;16(6):511–515. [PubMed] [Google Scholar]
- MULDER E. G., VAN VEENW INVESTIGATIONS ON THE SPHAEROTILUSLEPTOTHRIX GROUP. Antonie Van Leeuwenhoek. 1963;29:121–153. doi: 10.1007/BF02046045. [DOI] [PubMed] [Google Scholar]
- Mandel M., Johnson A., Stokes J. L. Deoxyribonucleic acid base composition of Sphaerotilus natans and Sphaerotilus discophorus. J Bacteriol. 1966 Apr;91(4):1657–1658. doi: 10.1128/jb.91.4.1657-1658.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OKREND H., DONDERO N. C. REQUIREMENT OF SPHAEROTILUS FOR CYANOCOBALAMIN. J Bacteriol. 1964 Feb;87:286–292. doi: 10.1128/jb.87.2.286-292.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osaki S., Johnson D. A., Frieden E. The possible significance of the ferrous oxidase activity of ceruloplasmin in normal human serum. J Biol Chem. 1966 Jun 25;241(12):2746–2751. [PubMed] [Google Scholar]
- Osaki S. Kinetic studies of ferrous ion oxidation with crystalline human ferroxidase (ceruloplasmin). J Biol Chem. 1966 Nov 10;241(21):5053–5059. [PubMed] [Google Scholar]
- PRAVE P. Untersuchungen über die Stoffwechselphysiologie des Eisenbakteriums Leptothrix ochracea Kutzing. Arch Mikrobiol. 1957;27(1):33–62. [PubMed] [Google Scholar]
- Petitprez M., Leclerc H. Les baéries du group Sphaerotilus-Leptothrix. Ann Inst Pasteur Lille. 1969;20:115–140. [PubMed] [Google Scholar]
- RAZZELL W. E., TRUSELL P. C. ISOLATION AND PROPERTIES OF AN IRON-OXIDIZING THIOBACILLUS. J Bacteriol. 1963 Mar;85:595–603. doi: 10.1128/jb.85.3.595-603.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROMANO A. H., PELOQUIN J. P. COMPOSITION OF THE SHEATH OF SPHAEROTILUS NATANS. J Bacteriol. 1963 Aug;86:252–258. doi: 10.1128/jb.86.2.252-258.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROUF M. A., STOKES J. L. Isolation and identification of the sudanophilic granules of Sphaerotilus natans. J Bacteriol. 1962 Feb;83:343–347. doi: 10.1128/jb.83.2.343-347.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROUF M. A., STOKES J. L. MORPHOLOGY, NUTRITION AND PHYSIOLOGY OF SPHAEROTILUS DISCOPHORUS. Arch Mikrobiol. 1964 Aug 17;49:132–149. doi: 10.1007/BF00422137. [DOI] [PubMed] [Google Scholar]
- Rae I. C., Edwards J. F., Davis N. Utilization of iron gallate and other organic iron complexes by bacteria from water supplies. Appl Microbiol. 1973 Jun;25(6):991–995. doi: 10.1128/am.25.6.991-995.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Righelato R. C., Pirt S. J. Improved control of organism concentration in continuous culture of filamentous micro-organisms. J Appl Bacteriol. 1967 Apr;30(1):246–250. doi: 10.1111/j.1365-2672.1967.tb00295.x. [DOI] [PubMed] [Google Scholar]
- Rogers S. R., Anderson J. J. Measurement of growth and iron deposition in Sphaerotilus discophorus. J Bacteriol. 1976 Apr;126(1):257–263. doi: 10.1128/jb.126.1.257-263.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers S. R., Anderson J. J. Role of iron deposition in Sphaerotilus discophorus. J Bacteriol. 1976 Apr;126(1):264–271. doi: 10.1128/jb.126.1.264-271.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHLEGEL H. G., GOTTSCHALK G., VON BARTHA R. Formation and utilization of poly-beta-hydroxybutyric acid by Knallgas bacteria (Hydrogenomonas). Nature. 1961 Jul 29;191:463–465. doi: 10.1038/191463a0. [DOI] [PubMed] [Google Scholar]
- SKERMAN V. B., DEMENTJEVA G., CAREY B. J. Intracellular deposition of sulfur by Sphaerotilus natans. J Bacteriol. 1957 Apr;73(4):504–512. doi: 10.1128/jb.73.4.504-512.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STOKES J. L., JOHNSON A. H. GROWTH FACTOR REQUIREMENTS OF TWO STRAINS OF SPHAEROTILUS DISCOPHORUS. Antonie Van Leeuwenhoek. 1965;31:175–180. doi: 10.1007/BF02045888. [DOI] [PubMed] [Google Scholar]
- STOKES J. L. Studies on the filamentous sheathed iron bacterium Sphaerotilus natans. J Bacteriol. 1954 Mar;67(3):278–291. doi: 10.1128/jb.67.3.278-291.1954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweisfurth R. Manganoxidierende Pilze. I. Vorkommen, Isolierungen und mikroskopische Untersuchungen. Z Allg Mikrobiol. 1971;11(5):415–430. [PubMed] [Google Scholar]
- Schweisfurth R. Manganoxydierende Pilze. II. Untersuchungen an Laboratoriumskulturen. Z Allg Mikrobiol. 1972;12(8):667–671. [PubMed] [Google Scholar]
- Stokes J. L., Parson W. L. Role of poly-beta-hydroxybutyrate in survival of Sphaerotilus discophorus during starvation. Can J Microbiol. 1968 Jul;14(7):785–789. doi: 10.1139/m68-130. [DOI] [PubMed] [Google Scholar]
- Stokes J. L., Powers M. T. Stimulation of poly-beta-hydroxybutyrate oxidation in Sphaerotilus discophorus by manganese and magnesium. Arch Mikrobiol. 1967;59(1):295–301. doi: 10.1007/BF00406343. [DOI] [PubMed] [Google Scholar]
- TEMPLE K. L., COLMER A. R. The autotrophic oxidation of iron by a new bacterium, thiobacillus ferrooxidans. J Bacteriol. 1951 Nov;62(5):605–611. doi: 10.1128/jb.62.5.605-611.1951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuovinen O. H., Kelly D. P. Biology of Thiobacillus ferrooxidans in relation to the microbiological leaching of sulphide ores. Z Allg Mikrobiol. 1972;12(4):311–346. doi: 10.1002/jobm.3630120406. [DOI] [PubMed] [Google Scholar]
- Tyler P. A., Marshall K. C. Microbial oxidation of manganese in hydro-electric pipelines. Antonie Van Leeuwenhoek. 1967;33(2):171–183. doi: 10.1007/BF02045548. [DOI] [PubMed] [Google Scholar]
- Tyler P. A., Marshall K. C. Pleomorphy in stalked, budding bacteria. J Bacteriol. 1967 Mar;93(3):1132–1136. doi: 10.1128/jb.93.3.1132-1136.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venosa A. D. Lysis of Sphaerotilus natans swarm cells by Bdellovibrio bacteriovorus. Appl Microbiol. 1975 May;29(5):702–705. doi: 10.1128/am.29.5.702-705.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZAVARZIN G. A. METALLOGENIUM SYMBIOTICUM. Z Allg Mikrobiol. 1964;4:390–395. [PubMed] [Google Scholar]
- Zevenhuizen L. P., Ebbink A. G. Interrelations between glycogen, poly-beta-hydroxybutyric acid and lipids during accumulation and subsequent utilization in a Pseudomonas. Antonie Van Leeuwenhoek. 1974;40(1):103–120. doi: 10.1007/BF00394558. [DOI] [PubMed] [Google Scholar]
- van Veen W. L. Bacteriology of activated sludge, in particular the filamentous bacteria. Antonie Van Leeuwenhoek. 1973;39(2):189–205. doi: 10.1007/BF02578852. [DOI] [PubMed] [Google Scholar]
- van Veen W. L. Proceedings: Netherlands Society for Microbiology meeting at Utrecht on 2 May 1973. Biological xoidation of manganese in soils. Antonie Van Leeuwenhoek. 1973 Nov;39(4):657–662. doi: 10.1007/BF02578915. [DOI] [PubMed] [Google Scholar]
- van Veen W. L., van der Kooij D., Geuze E. C., van der Vlies A. W. Investigations on the sheathed bacterium Haliscomenobacter hydrossis gen.n., sp.n., isolated from activated sludge. Antonie Van Leeuwenhoek. 1973;39(2):207–216. doi: 10.1007/BF02578853. [DOI] [PubMed] [Google Scholar]