Abstract
Objective
Human embryonic stem cells (hESCs) offer a sustainable source of endothelial cells for therapeutic vascularization and tissue engineering, but current techniques for generating these cells remain inefficient. We endeavored to induce and isolate functional endothelial cells from differentiating hESCs.
Methods and Results
To enhance endothelial cell differentiation above a baseline of ∼2% in embryoid body (EB) spontaneous differentiation, three alternate culture conditions were compared. Vascular endothelial growth factor (VEGF) treatment of EBs showed the best induction, with markedly increased expression of endothelial cell proteins CD31, VE-Cadherin, and von Willebrand Factor, but not the hematopoietic cell marker CD45. CD31 expression peaked around days 10-14. Continuous VEGF treatment resulted in a four- to five-fold enrichment of CD31+ cells but did not increase endothelial proliferation rates, suggesting a primary effect on differentiation. CD31+ cells purified from differentiating EBs upregulated ICAM-1 and VCAM-1 in response to TNFα, confirming their ability to function as endothelial cells. These cells also expressed multiple endothelial genes and formed lumenized vessels when seeded onto porous poly(2-hydroxyethyl methacrylate) scaffolds and implanted in vivo subcutaneously in athymic rats. Collagen gel constructs containing hESC-derived endothelial cells and implanted into infarcted nude rat hearts formed robust networks of patent vessels filled with host blood cells.
Conclusions
VEGF induces functional endothelial cells from hESCs independent of endothelial cell proliferation. These enrichment methods increase endothelial cell yield, enabling applications for revascularization as well as basic studies of human endothelial biology. We demonstrate the ability of hESC-derived endothelial cells to facilitate vascularization of tissue-engineered implants.
Keywords: human embryonic stem cells, endothelial cells, VEGF, tissue engineering, angiogenesis
A persisting challenge to the application of cell-based therapies is the sourcing of specific cells of interest. Since many mature tissues cannot be rebuilt using adult cells derived from biopsies, human embryonic stem cells (hESCs) could be instrumental in regenerative tissue engineering. Their immense proliferative and differentiation potential could provide extensive banks of cells – in quantity as well as type – for therapeutic applications.
Natural and engineered tissues more than ∼200 μm thick require suitable vascular support in order to survive and function properly. While growth of host vessels into tissue engineering scaffolds has been achieved via controlled release of angiogenic molecules, this strategy requires many days to produce mature vessels. Further, host-derived angiogenesis may be compromised by conditions that reduce angiogenesis such as diabetes and radiation therapy1. As angiogenesis is directed by a series of cytokines in a precise temporal sequence, adding one or even two cytokines may lead to incomplete blood vessel formation. Providing scaffolds with exogenous vascular cells prior to implantation may increase both the speed and extent of vascularization of engineered tissues. Before this approach can be successful, it will be critical to develop reliable methods to generate sufficient endothelial cells.
Differentiation of hESCs in embryoid bodies treated with serum proceeds in a complex and largely uncontrolled manner. Methods to guide hESC differentiation into a specific lineage would therefore provide a higher yield of cells of interest without increasing the overall culture size. In the present study we describe induction of endothelium from hESCs using vascular endothelial growth factor (VEGF). We also demonstrate the ability of hESC-derived endothelial cells to facilitate angiogenesis in vivo. Enhancing endothelial differentiation with VEGF thus provides an efficient cell source for tissue engineering and cell transplantation applications.
Methods
Additional details can be found online at http://atvb.ahajournals.org.
Human Embryonic Stem Cell Culture and Differentiation
Human ESCs, H7 line passage 37-92 and H1 line passage 45-60 (WiCell, Madison, WI), were grown in feeder-free conditions as previously described2-5. To initiate differentiation, confluent cultures of undifferentiated hESCs were dissociated into small clumps and placed into ultra low-attachment plates in the presence of 20% fetal bovine serum to induce the formation of embryoid bodies (EBs)3, 6. Alternative differentiation protocols of OP9 coculture7 and EGM-2MV differentiation8 were carried out as described previously. Briefly, for OP9 coculture, single-cell dispersed hESCs were added to a monolayer of OP9 cells and fed with αMEM + 10% FBS. For the EGM-2MV culture method, hESCs were seeded onto a MEF feeder layer and then grown in EGM-2MV medium.
Vascular Endothelial Growth Factor
Recombinant human vascular endothelial growth factor 165 (VEGF) was generously donated by Genentech (South San Francisco, CA). It was stored at 4°C in 5 mM succinate buffer containing 275 mM trehalose, 0.01% Tween-20, pH 5.0 at 5 mg/ml. An intermediate stock was prepared by diluting the 5 mg/ml stock to 0.1 mg/ml in a 5 mM succinate buffer, pH 5.0, with 0.1% BSA. VEGF was added to hEB medium at a final concentration of 50 ng/ml unless otherwise noted.
Western Blots
Protein lysates were analyzed by Western blotting for CD31 (1:1000; Dako, Carpinteria, CA), von Willebrand Factor (vWF, 1:100; Dako), CD45 (1:1000; Dako), VE-Cadherin (1:500; R&D Systems, Minneapolis, MN) and β-actin (1:1000; Cell Signaling Technology, Danvers, MA).
FACS Analysis, Purification, and Expansion
VEGF-induced and control embryoid bodies were dispersed into single cells (Blendzyme IV (Roche Applied Science), 37°C, 45-60 min.) and stained with fluorescently conjugated antibodies for flow cytometry. The antibodies used included CD31-PE (BD Biosciences), VE-Cadherin (FITC-conjugated; Serotec, Raleigh, NC; unconjugated followed by Alexa Fluor 488-conjugated secondary, R&D Systems; Alexa Fluor 488-conjugated monoclonal, eBioscience), CD45 (allophycocyanin (APC)-conjugated, eBioscience) c-kit (FITC-conjugated, Chemicon, Temecula, CA; APC-conjugated, R&D Systems), CD14 (FITC-conjugated, BD Biosciences), CD34 (PE-conjugated, BD Biosciences), KDR (PE-conjugated, R&D Systems), CD115 (PE-conjugated, eBioscience) and CD133 (APC-conjugated, Miltenyi Biotec, Auburn, CA). Stained cells were analyzed on FC500 or FC500MPL flow cytometry analyzers (Beckman Coulter, Fullerton, CA). HUVECs served as positive control for endothelial cell antigens and the U937 human pro-monocyte cell line was used as positive control for hematopoietic cell antigens.
CD31-positive and CD31-negative cells were collected with a sterile cell sorter9 (FACS Vantage, BD Biosciences) and propagated in EGM or EGM-2 (Lonza, Basel, Switzerland) or hEB medium6, respectively.
Proliferation Studies
Embryoid bodies were grown in the presence or absence of VEGF for 4 or 14 days, pulsed for 24 hours with the thymidine analogue 5-bromo-2-deoxyuridine (BrdU, 10μM; Roche Applied Science, Indianapolis, IN), and embedded in paraffin for histological analysis. Sections were double stained for CD31 (1:20; Dako) and BrdU (1:40; Roche Applied Science) and proliferating endothelial cells (CD31+/BrdU+) were quantified in blinded fashion.
TNFα stimulation
Monolayers of cells (HUVECs and CD31+ cells) were serum-starved overnight and stimulated with 0.1 – 10 ng/ml TNFα for four hours. RNA was collected and probed for ICAM-1, VCAM-1, CD31, and GAPDH transcripts via quantitative RT-PCR.
Matrigel tubule-forming assay
Matrigel (BD Biosciences) was diluted 1:2 with EBM-2 on ice and then added to 24-well plates and allowed to gel in a thin layer at 37°C. Cells (HUVECs and CD31+ cells; 25,000 per well) were immediately seeded onto the gels and photographed after 5, 10, 24, 48, and 72 hours.
Methylcellulose assay
hESC-derived CD31+ cells (103) were mixed with methylcellulose containing IL-1, IL-3, IL-6, G-CSF, GM-CSF, KL at 10 ng/ml and erythropoietin at 3 U/ml10. Positive control long-term-culture hematopoietic progenitor cells were included to ensure the colony forming ability of the assay. Cultures were observed for 6 days and colony formation was recorded for each cell type.
Collagen/Geltrex Constructs
CD31+ cells were mixed with human mesenchymal stem cells at a ratio of 2:1. Three million cells were gently combined with 100 μl of cold pre-gel mixture (1.25 mg/ml Collagen Type I (Invitrogen), 25 mM NaOH, 11% v/v basement membrane extract (Geltrex, Invitrogen) and 57% medium (EGM-2, Lonza)) and allowed to form a tethered gel for 1 hour at room temperature using the Flexcell Tissue Train culture plates (Flexcell International, Hillsborough, NC). The constructs were then allowed to float freely in culture medium and were cultured for 5 days in EGM-2 in static culture under standard tissue culture conditions.
All animal experiments were approved by the University of Washington Animal Care and Use Committee and were in accordance with federal guidelines for the care and use of laboratory animals. Male athymic (nude) rats (rnu/rnu; Charles River, Charles River, MA) underwent myocardial ischemia/reperfusion as previously described5. Seven weeks after injury, the cultured cell/gel constructs were sutured directly onto the epicardium across the infarcted region. Rats were given subcutaneous cyclosporine A (0.75 mg/day; Wako Pure Chemicals, Osaka, Japan) from days -1 to 7. Hearts were collected on day 7 and fixed and processed for histology as described above.
Porous Poly(2-hydroxyethyl methacrylate) Scaffolds
Porous poly(2-hydroxyethyl methacrylate) (polyHEMA) scaffolds were prepared by a sphere-templating process as described11, 12, with one significant modification. The scaffolds used here were created with uncrosslinked poly(methyl methacrylate) beads that were sieved through fine screens to produce sharply defined size fractions. The scaffolds made from these beads had precisely controlled 60 μm spherical pores interconnected by pore throats 24-30 μm in diameter. The hydroxyl groups on the porous material surface were chemically activated with carbonyl di-imidazole and then covalently coupled to type I collagen to create a cell-adhesive substrate13. Scaffolds were 5 mm in diameter and 400-700 μm thick.
hESC-derived endothelial cells and endothelial-depleted cells (106 cells per scaffold) were seeded by centrifugation and cultured for 2 days in vitro before implantation.
Scaffold Implantation
Cell-scaffold constructs were rinsed in EBM and kept briefly on ice prior to implantation. Athymic nude rats (Harlan, Indianapolis, IN) were anesthetized with isoflurane (initially 5% isoflurane in an induction chamber followed by 1.5-2% isoflurane in 98% O2 at a flow of 2 L/min via a nose cone). Two small dorsal subcutaneous pockets were made near each hindlimb. One scaffold was placed into each pocket and the incision was closed with a 4-0 non-absorbable suture. Rats recovered in room air in a warm chamber.
Ten days following implantation, the rats were sacrificed by pentobarbital overdose. Cell-scaffold constructs were removed from the subcutaneous space with some surrounding tissue, fixed in methyl Carnoy's fixative (60% methanol, 30% chloroform, 10% glacial acetic acid), and processed for histology.
Immunohistochemistry
hESC-derived endothelial cells were stained for CD31 (Dako, Carpinteria, CA), eNOS (BD Biosciences), smooth muscle alpha-actin (Dako), Ulex europaeus agglutinin-1 lectin (Ulex; Vector Laboratories, Burlingame, CA), VE-Cadherin (R&D Systems), and vWF (Dako) as described previously14, 15. Antibody/lectin positive cells were stained with species-specific secondary antibodies, visualized with 3,3′-diaminobenzidine (DAB; Sigma-Aldrich, St. Louis, MO) and counterstained with hematoxylin. In addition, cells were incubated with 10 μg/ml DiI-AcLDL or AlexaFluor594-conjugated AcLDL (Invitrogen, Carlsbad, CA) for 4 hours and counterstained with Hoechst 33342 (Sigma-Aldrich). HUVECs served as positive controls for all endothelial stains and assays.
Sections of the cell/gel construct-implanted hearts were stained with CD31 (1:10; Dako) followed by an AlexaFluor488-conjugated goat anti-mouse secondary antibody (1:100, Invitrogen). Red blood cells were visible due to innate autofluorescence and did not require additional staining. Nuclei were counterstained with Hoescht 33342, and slides were coverslipped with Vectashield (Vector Labs). Slides were imaged on an Axio Observer inverted microscope (Carl Zeiss MicroImaging, Thornwood, NY) and captured with an Axiocam MRm camera (Carl Zeiss MicroImaging).
Confocal microscopy
Fluorescent images of immunohistochemistry stains were obtained on a Zeiss LSM META confocal microscope (Carl Zeiss MicroImaging).
Results
VEGF Induces Endothelium from hESCs
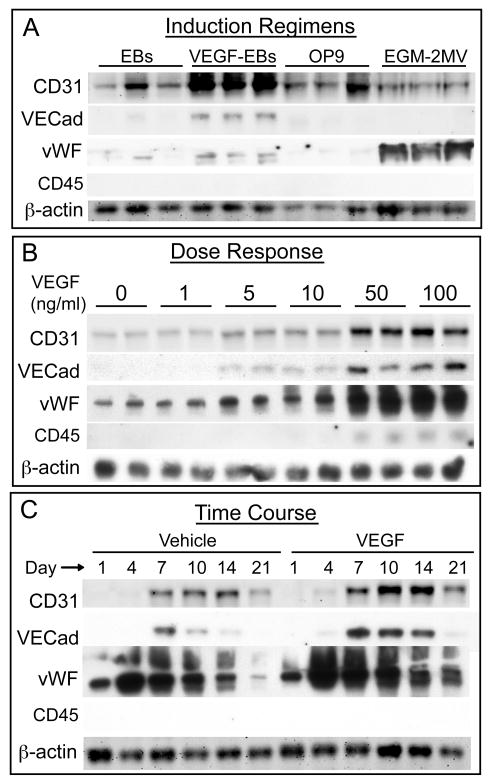
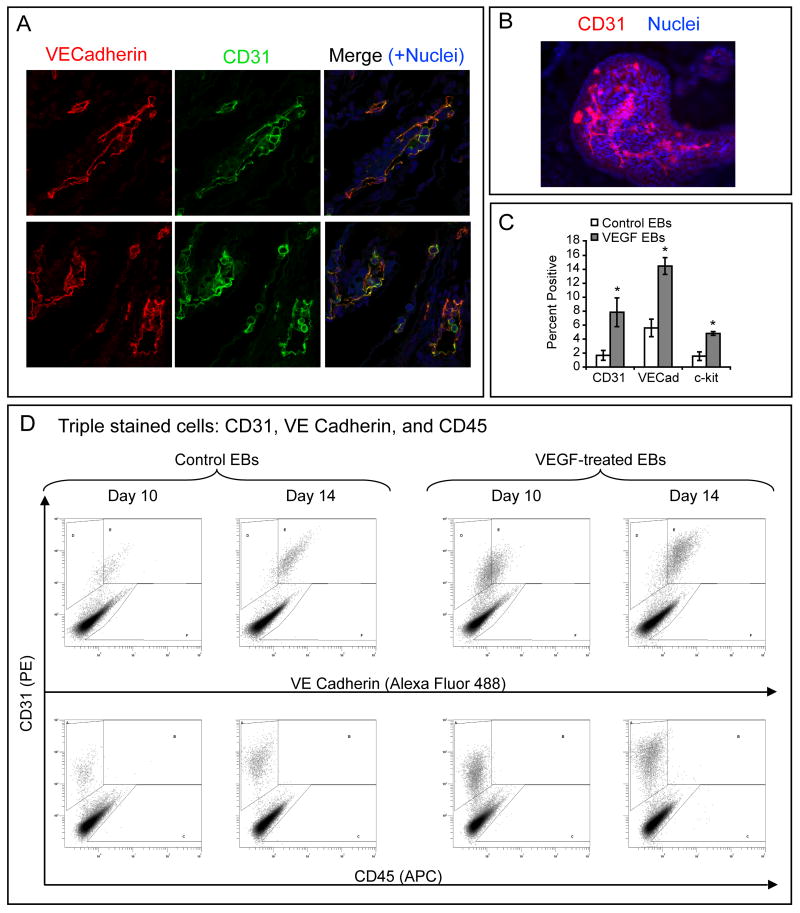
Human ESCs (H7 line, passage 44) were grown under four different culture conditions. Two groups were allowed to grow as embryoid bodies (EBs) in media containing 20% FBS, one of which was given 50 ng/ml VEGF every 2-3 days. A third group of cells was grown in coculture with OP9 stromal cells. The last group of cells was grown in EGM-2MV medium (Figure 1). Protein was collected after 14 days and immunoblotted for CD31, VE-Cadherin, von Willebrand Factor (vWF), CD45, and β-actin expression (Figure 1A and Supplemental Figure I A; for supplemental figures, please see http://atvb.ahajournals.org). The control EBs had a moderate level of CD31 expression, no VE-Cadherin expression, a small amount of vWF expression, and no CD45 expression. EBs treated with VEGF showed a marked increase in CD31, VE-Cadherin, and vWF, but still had no CD45 expression. OP9 co-cultures had a moderate amount of CD31 expression but little to no VE-Cadherin or vWF expression, and no CD45 expression. EGM-2MV treated cultures had CD31 levels similar to control EBs, and no VE-Cadherin or CD45 expression. Interestingly, hESCs differentiated in EGM-2MV had very high levels of vWF compared to EBs or OP9 cocultures. Immunofluorescence of EBs showed that CD31 illuminates vessel-like structures within the EB and co-localizes with VE-Cadherin (Figure 2A-B).
Figure 1. Induction of Endothelium with VEGF.
A-C, CD31, VE-Cadherin, vWF, and CD45 Western blots. A, Induction regimens tested for endothelial cell differentiation (14 days) showed that VEGF treatment of EBs resulted in higher levels of CD31, VE-Cadherin, and vWF protein than standard EBs or OP9 coculture. EGM-2MV treatment resulted in high levels of vWF expression but low levels of CD31 and VE Cadherin expression. No expression of CD45 was seen under any condition. B, VEGF dose response shows CD31, VE Cadherin, and vWF protein levels increased with increasing concentrations of VEGF from 1-50 ng/ml. The effect of dose plateaued between 50-100 ng/ml. C, VEGF-treated (50 ng/ml) and vehicle-treated EBs were harvested from days 1-21. CD31 expression peaked at day 10-14 in both control and VEGF-treated EBs, VE Cadherin expression peaked at about day 7, and vWF peaked earlier, around day 4. VEGF treatment resulted in higher levels of CD31, VE-Cadherin, and vWF at all time points from day 4 of differentiation onwards. CD45 was not detected at any time point.
Figure 2. Endothelial cell induction in EBs by treatment with VEGF.
A, Immunofluorescence of VEGF-treated EBs shows that endothelial cell markers CD31 and VE-Cadherin co-localize in vascular structures. B, Control EBs were stained for CD31 (red) and nuclei were counterstained with Hoechst 33342 (blue). CD31+ structures can be seen within the EB that have the morphology of branching vessels. C, Quantitative analysis of three independent experiments demonstrates induction of endothelial cells by VEGF. Comparison of control EBs vs. VEGF-treated EBs was statistically significant for each marker (*, p<0.05). Data presented as mean ± SEM. D, Triple-stain FACS density plots for day 10 and day 14 control and VEGF-treated EBs shows that CD31 and VE-Cadherin were coexpressed but CD45 was undetectable at all times and all conditions.
Analysis of transcripts for CD31, VE-Cadherin, KDR, and cytokeratins 8 and 18 by quantitative RT-PCR revealed that VEGF treatment of EBs markedly induced the expression of CD31 and VE-Cadherin while having no significant effect on the overall levels of KDR or cytokeratin transcripts (Supplemental Figure II).
Dose Response and Time Course of VEGF Treatment
To determine the optimal dose of VEGF, EBs were grown in 0-100 ng/ml VEGF for 14 days. Western blotting for CD31, VE-Cadherin, vWF, and CD45 revealed that increasing the VEGF dose to 50 ng/ml had a positive effect on the expression of CD31, VE-Cadherin, and vWF, and that this effect reached a plateau beyond 50 ng/ml (Figure 1B). CD45 expression remained low throughout, with vanishingly small amounts detected at 50 and 100 ng/ml VEGF. To establish the time course of endothelial cell differentiation, EBs grown in the presence of 50 ng/ml VEGF or vehicle were harvested from days 1-21 of differentiation. Protein samples were again probed for CD31, VE-Cadherin, vWF, and CD45 (Figure 1C). CD31 expression was absent in day 1 EBs, barely detectable in day 4 EBs treated with VEGF but not controls, and increased in both control and VEGF-treated EBs through day 14, with a decrease in both groups at day 21. The decrease at day 21 is likely due to proliferation of non-endothelial cell types in the cultures. The overall level of CD31 in VEGF-treated EBs was higher than control EBs at all time points in three independent experiments. The pattern of expression of VE-Cadherin followed a similar trend: VEGF treatment resulted in higher and more sustained levels of VE-Cadherin expression in EBs, with the highest level seen in day 7 VEGF-treated EBs. vWF expression was seen in day 1 EBs for both control and VEGF treatment. In both groups of EBs, vWF expression rose rapidly at 4 days and then stayed elevated through day 10 before declining at days 14 and 21. Expression of vWF in VEGF-treated EBs was higher than control EBs from days 4 to 21. CD45 expression was not detected at any time in either control or VEGF-treated EBs.
VEGF Treatment Induces Endothelial and Stem Cell Markers
FACS analysis was performed to evaluate the distribution of cells expressing endothelial (CD31, VE-Cadherin) and hematopoietic cell markers (CD45, c-kit). FACS analysis showed that VEGF-treated EBs had more CD31-positive (7.9% ± 2.1%, VEGF; 1.7% ± 0.7%, vehicle), VE-Cadherin-positive (15% ± 1.2%, VEGF; 5.6% ± 1.3%, vehicle) and c-kit-positive (4.8% ± 1.3%, VEGF; 1.6% ± 0.6%, vehicle) cells compared to control EBs (p<0.05 for all markers) (Figure 2C). c-kit, a stem cell marker found on hematopoietic stem cells and endothelial progenitors16, was not co-expressed on the CD31+ cells to any appreciable extent (data not shown). The stem cell marker CD133, though highly expressed in both control and VEGF-treated cells, was not co-expressed with CD31 (data not shown). Cells from VEGF-treated and control EBs triply stained with CD31, VE-Cadherin, and CD45 showed that VEGF treatment increased the number of CD31+/VE-Cadherin+ cells at days 10 and 14 of differentiation (Figure 2D). There were no detectable CD45+ cells in either condition. Importantly, VEGF treatment also increased the percentage of CD31+ and VE-Cadherin+ cells in the H1 hESC line (Supplemental Figure III), suggesting these results are not line-specific.
Duration of VEGF Treatment
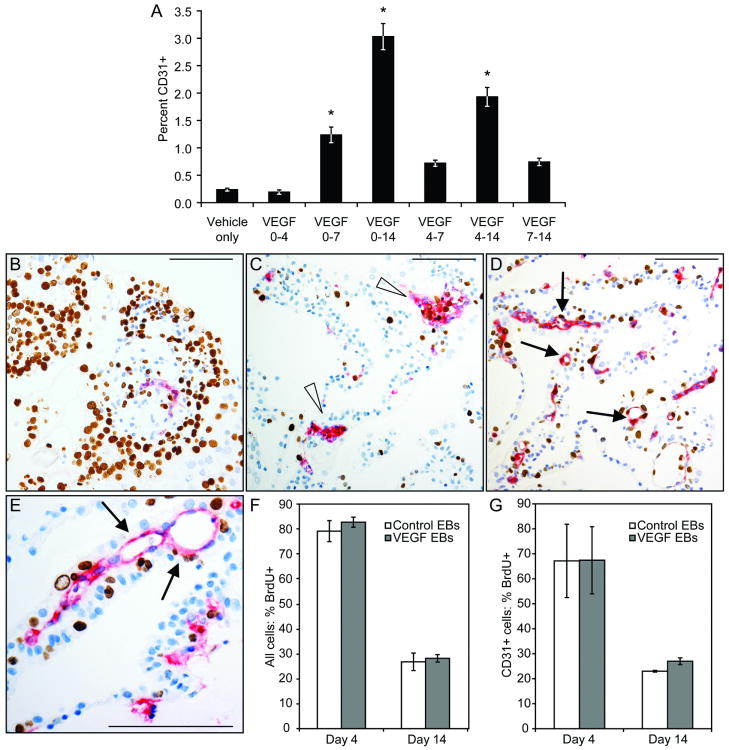
To determine whether VEGF was needed for the duration of differentiation or for only a short window of time, EBs were treated with VEGF for various intervals. After 14 days EBs were dissociated and CD31 expression was assessed via flow cytometry. As shown in Figure 3A, EBs that received VEGF for only a portion of the 14 day differentiation had fewer CD31+ cells than those that received VEGF throughout. VEGF treatment from days 0-4 did not increase the percentage of CD31+ cells. Treatment from days 0-7, 4-7, and 7-14 resulted in a modest increase in the proportion of CD31+ cells. Ten days of treatment, from days 4-14, produced an intermediate number of CD31+ cells. These data indicate that increasing the duration of VEGF treatment has a positive effect on the number of CD31+ cells that arise. The lower absolute value of the percentage of CD31+ cells in this experiment, relative to the experiments shown in Figure 2C, demonstrates the inherent variability of the embryoid body system.
Figure 3. Endothelial cell induction dynamics.
A, VEGF was administered to EBs for intervals ranging throughout the entire treatment period as shown. Cells were analyzed by FACS for CD31 expression; increasing duration of VEGF increased levels of CD31+ cells. *, p<0.01 vs. vehicle-treated EBs. B-G, Proliferation in VEGF-treated EBs. EBs were grown for 4 or 14 days and pulsed for 24 hours with BrdU. B-E, CD31 (red) and BrdU (brown) stains were quantified to determine proliferation rates. B, Day 4 EB section from control group. C, Day 14 EB section from control group. Note the clusters of CD31+ cells and lack of vessel lumens (arrowheads). D, Day 14 EB section from VEGF-treated group. Many CD31+ vessel-like structures with lumens can be seen (arrows). E, Vessel-like structures in Day 14 VEGF-treated EBs (arrows). F, Global proliferation rates in control and VEGF-treated EBs did not differ; proliferation at 14 days was markedly lower than at 4 days. G, BrdU incorporation rates in CD31+ cells were similar for VEGF-treated EBs and control EBs at both day 4 and day 14. Scale bars, 100 μm.
VEGF Does Not Affect Endothelial Proliferation in EBs
To examine whether VEGF-induced enrichment is due to enhanced proliferation of CD31+ endothelial cells, EBs were labeled with BrdU for 24 hours at 4 and 14 days and stained for CD31 and BrdU (Figure 3B-3E). Global BrdU incorporation rates of Day 4 EBs for both vehicle- and VEGF-treated cultures were remarkably high, with 80% ± 4.2% of cells in vehicle-treated EBs and 83% ± 2.0% of cells in VEGF-treated EBs containing BrdU+ nuclei (Figure 3B and 3F; p>0.05). Day 14 cultures had a much lower overall proliferative index, with 27% ± 3.5% of cells in vehicle-treated EBs and 28% ± 1.5% of cells in VEGF-treated EBs containing BrdU+ nuclei (Figure 3C-3E, 3F; p>0.05).
Examination of the proliferation rates in CD31+ cells for vehicle- and VEGF-treated EBs at day 4 and 14 of differentiation revealed similar results. At day 4, CD31+ cells in vehicle- and VEGF-treated EBs were 67% ± 15% and 67% ± 14% BrdU+, respectively. At day 14, the CD31+ cells in VEGF- and vehicle-treated cultures had similar proliferative indexes: 27% ± 1.3% BrdU+ in VEGF EBs and 23% ± 0.4% BrdU+ in vehicle EBs (Figure 3G; p>0.05). The similar rates of DNA synthesis for CD31+ cells and the entire cell population indicate that there is no preferential endothelial cell proliferation in either treatment group at 4 or 14 days. Identical analysis using vWF as the marker for endothelial cells also showed no difference in proliferation rates (data not shown). These data suggest that preferential proliferation of endothelial cells does not play a role in the increase of endothelial cells in VEGF-treated EBs.
Histological analyses also showed that treatment of EBs with VEGF resulted in many CD31+ vessel-like structures (arrows, Figure 3D, 3E), whereas CD31+ cells in control EBs were typically found clustered together with scant vascular organization (arrowheads, Figure 3C). This observation suggests development of endothelial networks in EBs treated with VEGF and is consistent with a role for VEGF as a morphogen in differentiating EBs.
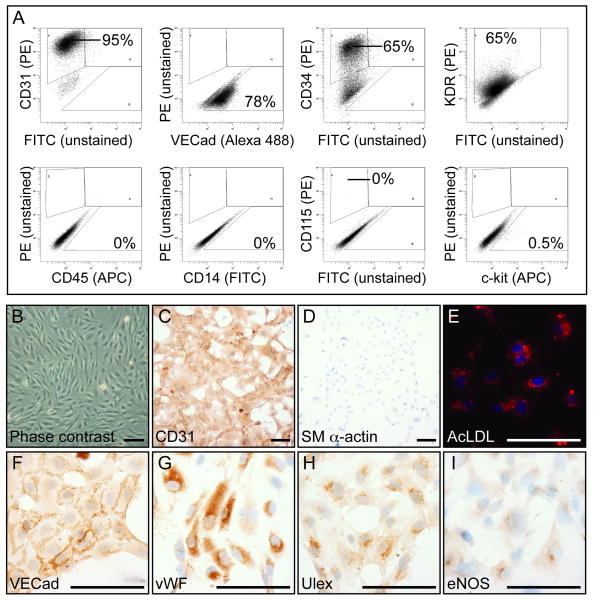
Selection and Characterization of hESC-derived Endothelial Cells
FACS-sorted CD31+ hESCs were expanded in vitro and characterized by flow cytometry and immunocytochemistry. As shown in Figure 4A and Supplemental Figure II B, CD31+ cells (95%) were positive for VE-Cadherin (78%), CD34 (65%), and KDR (65%). They were also stained with a panel of hematopoietic antibodies and were found to be negative for CD45, CD14, and CD115 (Figure 4A and Supplemental Figure I C-E). c-kit, a marker found on hematopoietic stem cells, labeled 0.5% of the CD31+ cells.
Figure 4. hESC-derived CD31+ cells express markers of mature endothelium and do not express hematopoietic cell markers.
A, CD31+ isolated cells from VEGF-treated EBs were sorted, expanded, and stained for various markers by flow cytometry. These cells remained 95% CD31+ and were 78% VE-Cadherin+, 65% CD34+, 65% KDR+, and 0.5% c-kit+, but were CD45, CD14, and CD115 negative. B, Phase contrast shows CD31+ cells have typical endothelial cobblestone morphology. C-I, Immunocytochemistry shows that the CD31+ cells stained positively (DAB, brown color) for CD31, VE-Cadherin, vWF, Ulex, and eNOS, but were negative for smooth muscle alpha actin. CD31+ cells also took up AcLDL, as demonstrated by the red color in the cytoplasm. Scale bars, 100 μm.
To further confirm the cells' identity and demonstrate the cellular morphology, CD31+ cells were grown in chamber slides and stained for immunocytochemistry. The cells took on a cobblestone pattern in cell culture characteristic of endothelial cells (Figure 4B). They were also found to be positive for the endothelial-specific markers CD31, VE-Cadherin, vWF, Ulex, and endothelial nitric oxide synthase. hESC-derived endothelial cells took up Acetylated LDL in culture, indicating further their endothelial phenotype and function (Figure 4E). CD31+ cells were found to be negative for smooth muscle alpha actin, a marker of smooth muscle cells and myofibroblasts (Figure 4B and Supplemental Figure I B). These results demonstrate that FACS-based sorting can be used to purify and expand endothelial cells from EBs.
hESC-Derived CD31+ Cells Upregulate ICAM-1 and VCAM-1 in Response to TNFα, Form Tubes on Matrigel, and Do Not Form Hematopoietic Cell Colonies in vitro
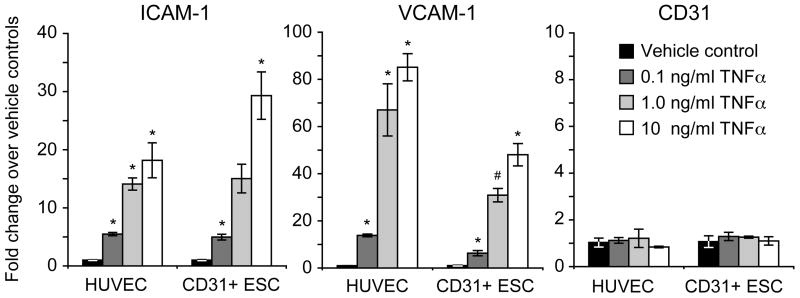
Following stimulation with 0.1, 1, or 10 ng/ml of the inflammatory cytokine TNFα for four hours, hESC-derived CD31+ cells and HUVECs both upregulated the inflammatory cell adhesion molecules ICAM-1 and VCAM-1, whereas CD31 expression remained the same for all treatments (Figure 5 and Supplemental Figure IV).
Figure 5. hESC-derived endothelial cells upregulate appropriate adhesion markers in response to TNFα.
CD31+ cells isolated from VEGF-treated EBs were expanded in vitro and stimulated with TNFα for 4 hours. ICAM-1 and VCAM-1 expression was upregulated in a dose-dependent manner similar to HUVEC controls. CD31 expression did not change upon TNFα stimulation. *, p<0.05 vs. vehicle control; #, p=0.06 vs. vehicle control
hESC-derived CD31+ cells and HUVECs were plated onto Matrigel and examined by phase contrast microscopy for 72 hours. Both cell types rapidly assembled into networks of tubular structures, forming robust networks by 10 hours of culture. The networks formed by each cell type subsequently degraded to varying degrees by 72 hours (Supplemental Figure V B).
hESC-derived CD31+ cells were grown in methylcellulose to test for hematopoietic progenitor colony-forming cells, as previously described10. After 6 days of culture, positive control bone marrow cells had formed hematopoietic colonies while hESC-derived CD31+ cells were adherent to the surface of the culture dish but had no visible hematopoietic colonies (Supplemental Figure V A).
hESC-Derived Endothelial Cells Form Robust Perfused Vascular Networks in vivo
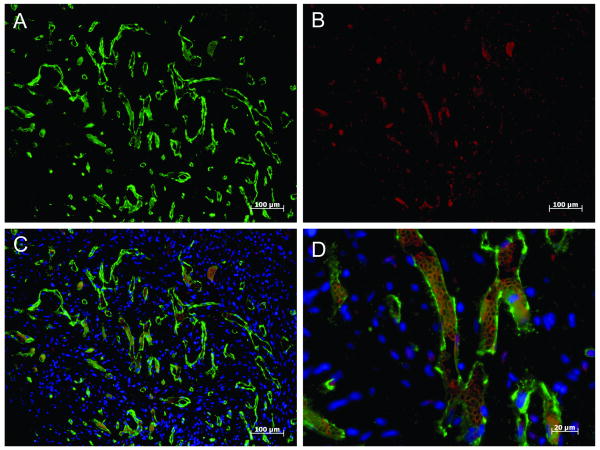
In order to determine whether FACS-sorted CD31+ hESCs expanded in vitro were able to form a functional vascular network once delivered in vivo, hESC-derived endothelial seeded collagen gel constructs were implanted in seven-week-old ischemia/reperfusion infarcted hearts5. As shown in Figure 6A, an extensive vascular network positive for human CD31 was generated throughout each construct. Furthermore, the presence of autofluorescent red blood cells within the human CD31+ vessel structures clearly demonstrates that these are functional human vessels that have anastamosed with the host vasculature (Figure 6B-D).
Figure 6. hESC-derived endothelial form robust perfused vascular networks in vivo.
Human-specific CD31 staining (A) and unstained host red blood cell autofluorescence (B) combine to show an extensive network of human blood vessels conducting host blood throughout the construct after 1 week of implantation (C). High magnification of a region with human vessels containing rat red blood cells (D). A-C, 10× objective; D, 40× objective.
Vessel Formation on polyHEMA Tissue Engineering Scaffolds
Unique polyHEMA tissue engineering scaffolds12 were seeded with hESC-derived endothelial cells and hESC-derived endothelial cell-depleted cells in vitro (Supplemental Figure VI). In situ hybridization for human pan-centromeric sequences confirmed that every cell stained positively with this probe on pre-implantation cell/scaffold constructs, establishing it as a reliable way to track these cells in vivo following implantation (Supplemental Figure VI C, VI F).
After 10 days in vivo, human cells were readily detected by human pan centromeric probe in situ hybridization on all scaffolds (Supplemental Figure VII G-H). In the hESC-derived endothelial cell-seeded scaffolds, CD31+/Ulex+ lumen-containing human vessels were seen around the edges of the scaffolds (Supplemental Figure VII E: Ulex; Supplemental Figure VII G: CD31). No human vessels were seen in the scaffolds seeded with the endothelial cell-depleted population (Supplemental Figure VII H). The human vessels were distributed exclusively within 100-150 μm of the edges. In contrast, CD31-negative cells (for both the hESC-derived endothelial cell and endothelial cell depleted population-seeded scaffolds) were found both at the periphery as well as at the center of the scaffolds (Supplemental Figure VII H).
Discussion
To induce the differentiation of endothelial cells, we compared several protocols, based on published ESC literature and the tenets of embryonic development. Of the four methods tested, VEGF treatment of EBs resulted in the highest expression of the endothelial cell-surface markers CD31 (PECAM1) and VE-Cadherin without inducing any detectable levels of hematopoietic cells, as determined by CD45 staining. VEGF treatment for 14 days at 50 ng/ml resulted in a 4.7-fold increase in the number of differentiating endothelial cells. Proliferation analysis revealed no differences in DNA synthesis rates of CD31+ cells in vehicle- and VEGF-treated EBs, suggesting that VEGF plays a critical role in inducing endothelial cell differentiation or enhanced survival. Furthermore, CD31+ selected cells formed blood-filled vessels in the infarcted heart and neovessel-like structures when seeded onto polyHEMA scaffolds. Thus treatment with VEGF followed by purification can facilitate the efficient generation of hESC-derived endothelial cells for tissue engineering and cell grafting applications as well as basic vascular biology applications.
Prior to the commencement of this study, several groups had described methods of differentiating ESCs into endothelium, but none had specifically addressed the induction of human ESC-derived endothelial cells. By coculturing hESCs with OP9 macrophage colony stimulating factor-deficient mouse stromal cells, Vodyanik et al. demonstrated the efficient differentiation of hematopoietic lineages from hESCs7. Despite the close linkage between the hematopoietic and endothelial cell lineages during development, we found this method was inefficient for endothelial differentiation (Figure 1B). One recent report indicates that sorting OP9 co-cultured hESCs for KDR (VEGF receptor-2) +/Tra1-60- cells followed by treatment with VEGF may be necessary for effective endothelial cell differentiation17. In another study, rhesus monkey ESCs grown in a medium optimized for endothelial cells (EGM-2MV)8 uniformly expressed many endothelial cell markers after 30 days. When we tested the effect of EGM-2MV medium on hESCs, we found that it effectively induced cells expressing vWF but not CD31, VE-Cadherin, or CD45 (Figure 1A). In contrast, we found that cell purification followed by culture in endothelial growth medium was required to generate significantly homogeneous endothelial cell cultures. Further studies focused on methods that increased the number of CD31+ cells, because vWF is intracellular and thus not amenable to cell sorting. Based on our time course studies (Figure 1C) we propose that vWF expression may mark a more immature population of human endothelium than does CD31. In contrast VE-Cadherin likely identifies a more mature population than CD31, as the former was not expressed until day 7 of differentiation (Figure 1C). Lastly we posited that addition of VEGF to EBs would augment the differentiation of endothelial cells. We found that VEGF treatment resulted in a robust increase the proportion of CD31+/VE-Cadherin+/CD45- endothelial cells in our EB cultures (Figure 1, 2C-D).
VEGF is a well-established mitogen, survival factor, and differentiation factor for endothelial cells18. It is absolutely required for vascular development, as VEGF-null mice die early in development due to lack of blood vessels19, 20. Formation of endothelium in mouse EBs was successfully induced by the addition of VEGF21, but similar results with human EBs have not been reported. Indeed, recent studies have indicated that VEGF plays an important role in the development of the hemangioblast from both EBs22 and two-dimensional cultures23, as well as stimulating the differentiation of hESC-derived CD34+ vascular progenitor cells into endothelial cells24. Despite an early report that VEGF does not induce formation of endothelial cells in H9 human EBs9, our data demonstrate that VEGF does indeed induce endothelial formation in two hESC lines, H1 and H7.
Evidence from the differentiation of mouse ESCs into hemangioblasts indicates that VEGF is only required during a short window of time to exert its effect on the expansion of KDR+ cells25. Differentiation driven by other factors, such as Wnts26, has also demonstrated that treatment for a brief time during early differentiation can result in large differences in the mature cell population, indicating that a cell-fate decision (as opposed to proliferation or survival) was affected by the treatment protocol. To address whether a shorter exposure to VEGF would have the same effect as continuous treatment, we administered VEGF for various intervals during 14 days of differentiation (Figure 3A). In contrast with these other systems, our results indicate that there was no particular time that was especially inductive, and continuous treatment with VEGF resulted in the largest number of endothelial cells.
Three parallel mechanisms can account for the expansion of endothelial cells during EB culture under VEGF treatment. Preferential proliferation, inhibition of death, or increase in progenitor cell differentiation would all result in a larger pool of endothelial cells. The equivalent rates of DNA synthesis in control and VEGF-treated EB CD31+ cells (and in vWF+ cells; data not shown) at days 4 and 14 presented here (Figure 3G) excludes a role for enhanced endothelial proliferation. Inhibition of endothelial cell death may play a role, as VEGF is a known survival factor for endothelium18; this mechanism will be examined in future studies. Differentiation of mesodermal progenitors into endothelial cells is a likely mechanism as well. We propose that VEGF may stimulate differentiation of endothelial cells from KDR+ precursors. Because continuous VEGF treatment is superior to short windows during early and late differentiation, the inductive effect of VEGF must operate throughout the maturation of these cultures. Although KDR is expressed by multiple mesodermal lineages during development, only vascular endothelium and bone marrow-derived endothelial progenitor cells maintain expression of this marker in adult tissues. Thus, the activation of KDR by VEGF may instruct the cell to maintain expression of the receptor and thereby encourage its differentiation into vascular endothelium. Expansion of the KDR+ population prior to CD31 expression is a possible explanation. Future accounting studies of multiple mesodermal lineages will help illuminate the precise mechanisms of VEGF's role during EB differentiation.
VEGF is well recognized as a potent vasculogenic and angiogenic molecule during development and in adult tissues18. Its critical role in mesoderm development is substantiated by the presence of the VEGF receptor KDR on mesodermal precursor cells in mouse embryos and ESCs. A recent report using a KDR-LacZ reporter mouse strain indicates that KDR is expressed in many more mesodermal lineages than previously thought, including skeletal and cardiac myocyte precursors in the early embryo27. Interestingly, KDR is also expressed on undifferentiated hESCs9 and is highly expressed in differentiating EBs (Supplemental Figure II). Additionally, Keller's group has shown that KDR+ cells emerging during early mESC and hESC differentiation can become hematopoietic cells, endothelial cells, smooth muscle cells, and cardiomyocytes28-31. Administration of VEGF promotes endothelial cell differentiation in mESCs21, 29, erythroid differentiation from human ESCs32, as well as KDR+ cell expansion following mesoderm induction25. Thus appropriately timed VEGF treatment may expand these populations of cells in hESC cultures, and could provide a uniquely useful cell source for cardiac tissue engineering applications. Further investigation of the lineages induced with VEGF treatment in EBs is thus warranted.
Endothelial cell identity is established not only by expression of relevant markers but also by functional cell behavior. They form tubes on Matrigel in vitro33, upregulate ICAM-1 and VCAM-1 in response to inflammatory cytokines34, and form blood vessels when implanted in vivo35, 36. For example, Yoder et al. have shown that two populations of endothelial progenitor cells derived from blood have distinctly different phenotypes, and only endothelial colony forming cells (ECFCs), but not endothelial colony-forming units (CFU-ECs), were endothelial in nature35. The ECFCs expressed CD31, VE-Cadherin, vWF, KDR, bound Ulex, and took up AcLDL but were negative for CD14, CD45, and CD115. In contrast the CFU-ECs expressed CD14, CD45, and CD115, but had relatively lower expression of CD31, VE-Cadherin, vWF, KDR, Ulex binding, and AcLDL uptake. Our hESC-derived CD31+ isolated cells match the profile of ECFCs, and not CFU-ECs, for every marker or assay examined. Furthermore, when stimulated with the inflammatory cytokine TNFα, both hESC-derived endothelial cells and HUVEC controls upregulated the cell adhesion molecules VCAM-1 and ICAM-1, but did not upregulate CD31, as expected36 (Figure 5).
Because of the close developmental relationship between endothelium and blood, it is also important to demonstrate that putative endothelial cells do not retain hematopoietic cell markers. To test our cultures for hematopoietic elements, we examined the expression of CD45 protein (Figures 1, 2, 4), CD14, and CD115 (Figure 4) (positive controls shown in Supplemental Figure I). The expression of CD45 was vanishingly low in differentiating EBs and CD31+ cells did not express CD45 at day 10 or day 14 of differentiation (Figure 2). Isolated CD31+ cells were completely negative for CD45, CD14, and CD115 (Figure 4). Quantitative RT-PCR analysis of CD115 confirmed that CD31+ isolated cells contained no transcript for this gene (Supplemental Figure I F). Furthermore, hematopoietic progenitor cells have the ability to generate colonies in methylcellulose culture conditions. When we plated our isolated CD31+ cells into these conditions, no hematopoietic colonies formed (Supplemental Figure V).
A recent report by Kennedy et al. demonstrated the induction and isolation of KDR+ cells and identified these cells as hemangioblasts, with the potential to become either hematopoietic cells or endothelial cells22. Their data showed, as does ours, that endothelial cells derived from hESCs do not express CD115 (colony stimulating factor 1 receptor, also known as c-fms), demonstrating that macrophages were not present in either cell population (Figure 4A and Supplemental Figure I F). Another report from the same group demonstrated that KDRlow/c-kitneg cells isolated from early differentiating embryoid bodies grown in the presence of growth factors including 10 ng/ml VEGF (from day 4 - 6 of differentiation) have the potential to become endothelial cells, smooth muscle cells, or cardiomyocytes31. The low proportion of CD31+ cells (4%) in these cultures increased dramatically when the cells were grown in the presence of bFGF for 10-12 days (30%). A related population of KDRhigh/c-kitpos cells isolated at the same time as the tri-potential cells expressed several markers of endothelial cell phenotype (CD31, VE-Cadherin, and KDR) without the addition of bFGF; these cells most closely approximate the population described herein.
hESC-derived ECs may have practical use in biomedical applications. Indeed, Cho et al. and Yamahara et al. have recently shown improved neovascularization of hindlimb ischemia by transplantation of hESC-derived ECs37, 38. Another application for these cells is the vascularization of scaffolds for tissue engineering and tissue repair purposes. When combined with collagen gels, our hESC-derived ECs were able to organize into vascular networks in vivo (Figure 6). Further, their ability to robustly engraft, anastamose with host vasculature, and conduct host blood confirms the endothelial identity and functionality of these cells. In addition, seeding of synthetic polyHEMA scaffolds with CD31+ cells also resulted in the formation of human vessel structures (Supplemental Figure VII). Such pre-seeding could be readily incorporated as a support technology to aid tissue engineering, thus highlighting the potential utility of hESC-derived ECs for neovascularization therapies.
Supplementary Material
Acknowledgments
The National Institutes of Health provided funding for this work (R01HL64387, R01HL61553, R01HL84642, P01HL03174, P01GM081619, and T32HL07312).
The authors would like to thank Ms. Kira Bendixen, Ms. Jennifer Deem, Dr. Sarah Fernandes, Mr. James Fugate, Mr. Paul St. Laurent, Mr. Kenny Lin, Ms. Veronica Muskheli, Mr. Mark Saiget, and Ms. Kellii Schurb for expert technical assistance. We thank Dr. Hans Reinecke for numerous helpful discussions. Mr. Fred Lewis and Ms. Michelle Black provided support for FACS sorting.
Footnotes
Nourse: VEGF induces endothelial cells from hESC
References
- 1.Peters MC, Polverini PJ, Mooney DJ. Engineering vascular networks in porous polymer matrices. J Biomed Mater Res. 2002;60:668–678. doi: 10.1002/jbm.10134. [DOI] [PubMed] [Google Scholar]
- 2.Xu C, Inokuma MS, Denham J, Golds K, Kundu P, Gold JD, Carpenter MK. Feeder-free growth of undifferentiated human embryonic stem cells. Nat Biotechnol. 2001;19:971–974. doi: 10.1038/nbt1001-971. [DOI] [PubMed] [Google Scholar]
- 3.Laflamme MA, Gold J, Xu C, Hassanipour M, Rosler E, Police S, Muskheli V, Murry CE. Formation of human myocardium in the rat heart from human embryonic stem cells. Am J Pathol. 2005;167:663–671. doi: 10.1016/S0002-9440(10)62041-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McDevitt TC, Laflamme MA, Murry CE. Proliferation of cardiomyocytes derived from human embryonic stem cells is mediated via the IGF/PI 3-kinase/Akt signaling pathway. J Mol Cell Cardiol. 2005;39:865–873. doi: 10.1016/j.yjmcc.2005.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Laflamme MA, Chen KY, Naumova AV, Muskheli V, Fugate JA, Dupras SK, Reinecke H, Xu C, Hassanipour M, Police S, O'Sullivan C, Collins L, Chen Y, Minami E, Gill EA, Ueno S, Yuan C, Gold J, Murry CE. Cardiomyocytes derived from human embryonic stem cells in pro-survival factors enhance function of infarcted rat hearts. Nat Biotechnol. 2007 doi: 10.1038/nbt1327. [DOI] [PubMed] [Google Scholar]
- 6.Xu C, Police S, Rao N, Carpenter MK. Characterization and enrichment of cardiomyocytes derived from human embryonic stem cells. Circ Res. 2002;91:501–508. doi: 10.1161/01.res.0000035254.80718.91. [DOI] [PubMed] [Google Scholar]
- 7.Vodyanik MA, Bork JA, Thomson JA, Slukvin II. Human embryonic stem cell-derived CD34+ cells: efficient production in the coculture with OP9 stromal cells and analysis of lymphohematopoietic potential. Blood. 2005;105:617–626. doi: 10.1182/blood-2004-04-1649. [DOI] [PubMed] [Google Scholar]
- 8.Kaufman DS, Lewis RL, Hanson ET, Auerbach R, Plendl J, Thomson JA. Functional endothelial cells derived from rhesus monkey embryonic stem cells. Blood. 2004;103:1325–1332. doi: 10.1182/blood-2003-03-0799. [DOI] [PubMed] [Google Scholar]
- 9.Levenberg S, Golub JS, Amit M, Itskovitz-Eldor J, Langer R. Endothelial cells derived from human embryonic stem cells. Proc Natl Acad Sci U S A. 2002;99:4391–4396. doi: 10.1073/pnas.032074999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Simmons PJ, Torok-Storb B. Identification of stromal cell precursors in human bone marrow by a novel monoclonal antibody, STRO-1. Blood. 1991;78:55–62. [PubMed] [Google Scholar]
- 11.Marshall AJ, Ratner BD. Quantitative characterization of sphere-templated porous biomaterials. Aiche Journal, Abstract. 2005;51:1221–1232. [Google Scholar]
- 12.Marshall AJ, Irvin CA, Barker T, Sage EH, Hauch KD, Ratner BD. Biomaterials with tightly controlled pore size that promote vascular in-growth. American Chemical Society Polymer Division Preprints, Abstract. 2004;45:100–101. [Google Scholar]
- 13.Bryant SJ, Cuy JL, Hauch KD, Ratner BD. Photo-patterning of porous hydrogels for tissue engineering. Biomaterials. 2007;28:2978–2986. doi: 10.1016/j.biomaterials.2006.11.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Minami E, Laflamme MA, Saffitz JE, Murry CE. Extracardiac progenitor cells repopulate most major cell types in the transplanted human heart. Circulation. 2005;112:2951–2958. doi: 10.1161/CIRCULATIONAHA.105.576017. [DOI] [PubMed] [Google Scholar]
- 15.Ismail JA, Poppa V, Kemper LE, Scatena M, Giachelli CM, Coffin JD, Murry CE. Immunohistologic labeling of murine endothelium. Cardiovasc Pathol. 2003;12:82–90. doi: 10.1016/s1054-8807(02)00166-7. [DOI] [PubMed] [Google Scholar]
- 16.Kocher AA, Schuster MD, Szabolcs MJ, Takuma S, Burkhoff D, Wang J, Homma S, Edwards NM, Itescu S. Neovascularization of ischemic myocardium by human bone-marrow-derived angioblasts prevents cardiomyocyte apoptosis, reduces remodeling and improves cardiac function. Nat Med. 2001;7:430–436. doi: 10.1038/86498. [DOI] [PubMed] [Google Scholar]
- 17.Sone M, Itoh H, Yamahara K, Yamashita JK, Yurugi-Kobayashi T, Nonoguchi A, Suzuki Y, Chao TH, Sawada N, Fukunaga Y, Miyashita K, Park K, Oyamada N, Sawada N, Taura D, Tamura N, Kondo Y, Nito S, Suemori H, Nakatsuji N, Nishikawa S, Nakao K. Pathway for differentiation of human embryonic stem cells to vascular cell components and their potential for vascular regeneration. Arterioscler Thromb Vasc Biol. 2007;27:2127–2134. doi: 10.1161/ATVBAHA.107.143149. [DOI] [PubMed] [Google Scholar]
- 18.Olsson AK, Dimberg A, Kreuger J, Claesson-Welsh L. VEGF receptor signalling - in control of vascular function. Nat Rev Mol Cell Biol. 2006;7:359–371. doi: 10.1038/nrm1911. [DOI] [PubMed] [Google Scholar]
- 19.Carmeliet P, Ferreira V, Breier G, Pollefeyt S, Kieckens L, Gertsenstein M, Fahrig M, Vandenhoeck A, Harpal K, Eberhardt C, Declercq C, Pawling J, Moons L, Collen D, Risau W, Nagy A. Abnormal blood vessel development and lethality in embryos lacking a single VEGF allele. Nature. 1996;380:435–439. doi: 10.1038/380435a0. [DOI] [PubMed] [Google Scholar]
- 20.Ferrara N, Carver-Moore K, Chen H, Dowd M, Lu L, O'Shea KS, Powell-Braxton L, Hillan KJ, Moore MW. Heterozygous embryonic lethality induced by targeted inactivation of the VEGF gene. Nature. 1996;380:439–442. doi: 10.1038/380439a0. [DOI] [PubMed] [Google Scholar]
- 21.Vittet D, Prandini MH, Berthier R, Schweitzer A, Martin-Sisteron H, Uzan G, Dejana E. Embryonic stem cells differentiate in vitro to endothelial cells through successive maturation steps. Blood. 1996;88:3424–3431. [PubMed] [Google Scholar]
- 22.Kennedy M, D'Souza SL, Lynch-Kattman M, Schwantz S, Keller G. Development of the hemangioblast defines the onset of hematopoiesis in human ES cell differentiation cultures. Blood. 2007;109:2679–2687. doi: 10.1182/blood-2006-09-047704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang ZZ, Au P, Chen T, Shao Y, Daheron LM, Bai H, Arzigian M, Fukumura D, Jain RK, Scadden DT. Endothelial cells derived from human embryonic stem cells form durable blood vessels in vivo. Nat Biotechnol. 2007;25:317–318. doi: 10.1038/nbt1287. [DOI] [PubMed] [Google Scholar]
- 24.Ferreira LS, Gerecht S, Shieh HF, Watson N, Rupnick MA, Dallabrida SM, Vunjak-Novakovic G, Langer R. Vascular progenitor cells isolated from human embryonic stem cells give rise to endothelial and smooth muscle like cells and form vascular networks in vivo. Circ Res. 2007;101:286–294. doi: 10.1161/CIRCRESAHA.107.150201. [DOI] [PubMed] [Google Scholar]
- 25.Park C, Afrikanova I, Chung YS, Zhang WJ, Arentson E, Fong Gh G, Rosendahl A, Choi K. A hierarchical order of factors in the generation of FLK1- and SCL-expressing hematopoietic and endothelial progenitors from embryonic stem cells. Development. 2004;131:2749–2762. doi: 10.1242/dev.01130. [DOI] [PubMed] [Google Scholar]
- 26.Ueno S, Weidinger G, Osugi T, Kohn AD, Golob JL, Pabon L, Reinecke H, Moon RT, Murry CE. Biphasic role for Wnt/beta-catenin signaling in cardiac specification in zebrafish and embryonic stem cells. Proc Natl Acad Sci U S A. 2007;104:9685–9690. doi: 10.1073/pnas.0702859104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ema M, Takahashi S, Rossant J. Deletion of the selection cassette, but not cis-acting elements, in targeted Flk1-lacZ allele reveals Flk1 expression in multipotent mesodermal progenitors. Blood. 2006;107:111–117. doi: 10.1182/blood-2005-05-1970. [DOI] [PubMed] [Google Scholar]
- 28.Nishikawa SI, Nishikawa S, Hirashima M, Matsuyoshi N, Kodama H. Progressive lineage analysis by cell sorting and culture identifies FLK1+VE-cadherin+ cells at a diverging point of endothelial and hemopoietic lineages. Development. 1998;125:1747–1757. doi: 10.1242/dev.125.9.1747. [DOI] [PubMed] [Google Scholar]
- 29.Yamashita J, Itoh H, Hirashima M, Ogawa M, Nishikawa S, Yurugi T, Naito M, Nakao K. Flk1-positive cells derived from embryonic stem cells serve as vascular progenitors. Nature. 2000;408:92–96. doi: 10.1038/35040568. [DOI] [PubMed] [Google Scholar]
- 30.Kattman SJ, Huber TL, Keller GM. Multipotent flk-1+ cardiovascular progenitor cells give rise to the cardiomyocyte, endothelial, and vascular smooth muscle lineages. Dev Cell. 2006;11:723–732. doi: 10.1016/j.devcel.2006.10.002. [DOI] [PubMed] [Google Scholar]
- 31.Yang L, Soonpaa MH, Adler ED, Roepke TK, Kattman SJ, Kennedy M, Henckaerts E, Bonham K, Abbott GW, Linden RM, Field LJ, Keller GM. Human cardiovascular progenitor cells develop from a KDR+ embryonic-stem-cell-derived population. Nature. 2008;453:524–528. doi: 10.1038/nature06894. [DOI] [PubMed] [Google Scholar]
- 32.Cerdan C, Rouleau A, Bhatia M. VEGF-A165 augments erythropoietic development from human embryonic stem cells. Blood. 2004;103:2504–2512. doi: 10.1182/blood-2003-07-2563. [DOI] [PubMed] [Google Scholar]
- 33.Ingram DA, Mead LE, Tanaka H, Meade V, Fenoglio A, Mortell K, Pollok K, Ferkowicz MJ, Gilley D, Yoder MC. Identification of a novel hierarchy of endothelial progenitor cells using human peripheral and umbilical cord blood. Blood. 2004;104:2752–2760. doi: 10.1182/blood-2004-04-1396. [DOI] [PubMed] [Google Scholar]
- 34.De Caterina R, Libby P, Peng HB, Thannickal VJ, Rajavashisth TB, Gimbrone MA, Jr, Shin WS, Liao JK. Nitric oxide decreases cytokine-induced endothelial activation. Nitric oxide selectively reduces endothelial expression of adhesion molecules and proinflammatory cytokines. J Clin Invest. 1995;96:60–68. doi: 10.1172/JCI118074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yoder MC, Mead LE, Prater D, Krier TR, Mroueh KN, Li F, Krasich R, Temm CJ, Prchal JT, Ingram DA. Redefining endothelial progenitor cells via clonal analysis and hematopoietic stem/progenitor cell principals. Blood. 2007;109:1801–1809. doi: 10.1182/blood-2006-08-043471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sawa Y, Sugimoto Y, Ueki T, Ishikawa H, Sato A, Nagato T, Yoshida S. Effects of TNF-alpha on leukocyte adhesion molecule expressions in cultured human lymphatic endothelium. J Histochem Cytochem. 2007;55:721–733. doi: 10.1369/jhc.6A7171.2007. [DOI] [PubMed] [Google Scholar]
- 37.Cho SW, Moon SH, Lee SH, Kang SW, Kim J, Lim JM, Kim HS, Kim BS, Chung HM. Improvement of postnatal neovascularization by human embryonic stem cell derived endothelial-like cell transplantation in a mouse model of hindlimb ischemia. Circulation. 2007;116:2409–2419. doi: 10.1161/CIRCULATIONAHA.106.687038. [DOI] [PubMed] [Google Scholar]
- 38.Yamahara K, Sone M, Itoh H, Yamashita JK, Yurugi-Kobayashi T, Homma K, Chao TH, Miyashita K, Park K, Oyamada N, Sawada N, Taura D, Fukunaga Y, Tamura N, Nakao K. Augmentation of neovascularization [corrected] in hindlimb ischemia by combined transplantation of human embryonic stem cells-derived endothelial and mural cells. PLoS ONE. 2008;3:e1666. doi: 10.1371/journal.pone.0001666. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.