Abstract
Neuronal nicotinic acetylcholinic receptors (nAChR) are promising targets for the development of novel analgesics. Nicotine and other nAChR-agonists produce profound analgesia in rodent models of acute and persistent pain. However, significant side-effects are of concern. Nornicotine (N-desmethyl-nicotine) appears to activate different nAChR subtypes, has a better pharmacokinetic profile, and produces less toxicity than nicotine. Little is known about its analgesic properties. In the present study, the S(−)- and R(+)- enantiomers of nornicotine were characterized with regard to analgesia and side-effects profile. Efficacy was demonstrated in rat models of pain where central sensitization is involved: i.e. the chronic constriction nerve injury model of peripheral neuropathy and the formalin model of tonic inflammatory pain. The desirable (analgesic) properties resided predominantly in the S(−)- rather than the R(+)- enantiomer. In contrast, undesirable effects (motor in-coordination, reduced locomotor activity, ataxia) were more pronounced with the R(+)- enantiomer. This is an interesting finding, which may suggest separation of toxicity from analgesia by utilization of S(−)-enantiomer of nornicotine. Maximum analgesic effectiveness without significant side-effects was achieved when S(−)-nornicotine (sub-analgesic dose) was combined with a low-dose of the μ-opioid, morphine. These preclinical data suggest that S(−)-nornicotine may be of value, either alone or in combination with an opioid, for treatment of a broad-spectrum of pain (i.e. nociceptive, neuropathic, mixed pain).
Keywords: S(−)-nornicotine, R(+)-nornicotine, nornicotine-antinociception, nornicotine-antihyperalgesia, nornicotine-side effects, nornicotine-morphine combination therapy
1. Introduction
Pain is a serious and costly public health problem. In many patients, including those with both chronic pain conditions of malignant (cancer-related) and nonmalignant origin, pain is inadequately managed with currently available analgesic drugs. These drugs include opioids, non-steroidal anti-inflammatory agents (NSAID’s), and various adjuvant agents (antidepressants, anticonvulsants) initially approved for other uses besides pain. Given the need for more effective and/or less toxic pain therapies, a great deal of emphasis has been placed on identifying novel molecular targets that could form the basis for new analgesics. One of the promising targets is the neuronal nicotinic acetylcholine receptor (nAChR) [Arneric et al., 2007; Decker et al., 2004; Decker and Meyer 1999; Flores 2000; Holladay et al., 1997; Lippiello et al., 2007; Lloyd and Williams 2000; Meyer, 2006; Meyer et al., 2000; Vincler 2005; Williams et al., 1999, reviews]. Nicotinic receptor-mediated analgesia appears to involve multiple neuronal pathways. The specific role of a variety of nAChR subunits (α2-α10, β2-β4) has not been fully elucidated. Efforts to this point have mainly focused on the α4β2 subtype (the most abundant CNS subtype), which is thought to be involved in antinociception via activation of multiple descending inhibitory pathways [Decker et al., 2004; Vincler, 2005, reviews]. The efficacy of agents acting at α4β2 nAChR (e.g. nicotine, epibatidine, ABT-594) has been well-established across a range of preclinical models of pain [Aceto et al., 1983; Bannon et al., 1998a; 1998b; Boyce et al., 2000; Curzon et al., 1998; Damaj et al., 1998; Decker et al., 1998; Gilbert et al., 2001; Kesingland et al., 2000; Rao et al., 1996; Sahley and Berntson, 1979; Tripathi et al., 1982]. Nevertheless, significant toxicity (i.e. motor, cardiovascular, respiratory, and gastrointestinal effects) make such compounds less desirable as analgesic drugs. Therefore, the key issue for successful development of drugs from this class of agents appears to depend upon separation of efficacy from toxicity. In this regard, the targeting of other central and/or peripheral nAChRs subtypes (i.e. α7, α9α10) may hold promise [Lippiello et al., 2007].
Nornicotine, the N-desmethyl derivative of nicotine, which is detectable in the brain but not in the periphery after systemic administration of nicotine in rats [Crooks and Dwoskin, 1997; Crooks et al., 1997; Ghosheh et al., 2001], may be a viable candidate to explore as a potential novel nAChR-agonist analgesic drug. Nornicotine appears to differ from nicotine in several aspects. First, nornicotine has lower affinity than nicotine for [3H]nicotine-sensitive sites in brain [Ki (nM) = 25 vs. 1.4 for (±)-nornicotine vs. (−)-nicotine (Damaj et al., 1998) and 47 vs. 1 for S(−)-nornicotine vs. S(−)-nicotine (Xu et al., 2001)]. Second, in contrast to (−)-nicotine, (±)-nornicotine has greater selectivity for the α7 nAChR homomer (which is present on macrophages and spinal cord microglia, and is thought to play an important role in inflammation [Wang et al 2003; Waldburger et al., 2008]) compared to the α4β2 nAChR subtype (which is considered to be involved in the central analgesic action of nicotinic agents) [the EC50 (μmol/L) values for activation of nAChR subunits expressed in Xenopus oocytes equal to 17.4±4.9 (α7) vs. 375±262 (α4β2) for (±)-nornicotine and 13.2±2.6 (α7) vs. 2.5±0.6 (α4β2) for (−)-nicotine (Papke et al., 2007)]. Third, (±)- nornicotine may possess less toxicity relative to (−)-nicotine, based on reduced selectivity for the ganglionic-like α3β4 nAChR subtype [EC50 = 614±136 vs. 87±4 μmol/L (Papke et al., 2007)]. Fourth, (±)-nornicotine was less potent (~10-fold) compared to (−)-nicotine in reinforcing effect [Bardo et al. 1999]; decreasing (−)-nicotine self-administration [Green et al., 2000], locomotor activity [Dwoskin et al., 1999], schedule-controlled operant responding, and cardiovascular effects [Risner et al., 1988]. Similar to S(−)-nicotine, S(−)-nornicotine desensitized nicotinic receptor-stimulated dopamine release from rat striatum but with lower (~12-fold) potency [Dwoskin et al., 2001]. S(−)-Nicotine produced transient hypoactivity followed by dose-related hyperactivity whereas rebound hyperactivity was not observed with S(−)-nornicotine in rats [Dwoskin et al., 1999]. In addition, S(−)-nornicotine was less potent (~3-fold) than S(−)nicotine in partial substitution for amphetamine in a drug discrimination paradigm [Bardo et al., 1997]. Fifth, (±)-nornicotine has a more favorable pharmacokinetic profile compared to (−)nicotine [e.g. a longer half-life in a brain (166 vs. 52 min); a greater brain accumulation upon repeated administration (10- vs. 2-fold)] in rats [Ghosheh et al., 1999; 2001]. The plasma half-life also was longer for (±)-nornicotine (8 h) than for (−)-nicotine (1 h) in humans [Kyermaten et al., 1990]. Sixth, although limited data has shown that (±)-nornicotine was less potent than (−)-nicotine against thermal nociception (ED50 = 56 vs. 8 μmol/kg SC, 146 vs. 74 μmol/rat IT; the tail-flick test) in mice [Damaj et al., 1998] little is known about its effectiveness in chronic pain (e.g. neuropathic and persistent inflammatory pain).
Nornicotine exists in the S(−)- and the R(+)-enatiomeric forms, which appear to differ with regard to their neurochemical and behavioral profile. For example, R(+)-nornicotine was more potent (~ 2-fold) than S(−)-nornicotine in decreasing S(−)-nicotine self-administration [Stairs et al., 2007] and stimulation of dopamine release from the reward-relevant nucleus accumbens [Green et al., 2001]. In contrast, S(−)-nornicotine was more effective (~2-fold) than R(+)- nornicotine in evoking dopamine release from rat striatal slices (thought to involve α3β2 nAChR subunit) [Teng et al., 1997]. Hyperactivity was observed after repeated administration of S(−)-nornicotine but not after R(−)-nornicotine in rats [Dwoskin et al., 1999]. In addition, although nornicotine enantiomers produced similar increases in blood pressure and heart rate after acute administration, tolerance to this effect developed only with repeated doses of the R(+)- enantiomer [Stairs et al., 2007]. Interestingly, in contrast to (−)-nicotine, which was more potent (~10-fold) than (+)-nicotine in displacing [3H]nicotine binding from rat brain membranes, nornicotine was not steroselective in this assay [Ki (nM) = 5.35±2.03 vs. 63.2±5.7 and 675±171 vs. 423±82 (Zhang and Norberg, 1993); 14±0.4 vs. 102±5 and 227±7 vs. 202±7 (Copeland et al., 1991) for the S(−)- vs. the R(+)- enantiomers of nicotine and nornicotine]. In addition, unlike nicotine, nornicotine demonstrated a lack of steroselectivity for (−)-[3H]nicotine binding sites in M10 cells (though to express the recombinant α4β2 nAChR subtype) [Ki (nM) = 3.23±0.69 vs. 81.0±8.5 for (−)- vs. (+)-nicotine and 34.3±2.4 vs. 17.3±0.2 for S(−)- vs. R(+)-nornicotine (Zhang et al., 1998)]. Little is known about selectivity of nornicotine enantiomers for other nAChR subunits. The above data suggest that it may be possible to separate the desirable (analgesic) effects from toxicity; i.e. the side-effects being more related to one nornicotine enantiomer compared to the other.
Chronic pain treatment typically involves a multimodal approach requiring combination of drugs from different classes (with differing mechanisms) in order to produce adequate pain relief [Gilron and Max, 2005; Kalso 2005, reviews]. Combination therapy can result in an enhancement of overall analgesic efficacy with a decreased risk of toxicity. There is evidence of separate but interacting mechanism(s) of pain modulation by nicotinic and opioid systems, and thus, combining nicotinic agents and opioids may likely results in a synergistic (supra-additive) effect. (−)-Nicotine has been shown to enhance morphine analgesia in rodent models of pain [Haghparast et al., 2008; Suh et al., 1996a; 1996b; Zarrindast et al., 1996; 1997]; however, issues related to toxicity, narrow therapeutic index, and abuse liability may hamper its clinical use. Overall, (±)- and/or S(−)-nornicotine appear to have a better safety index than (−)-nicotine in rodents [Bardo et al., 1997; 1999; Dwoskin et al., 1999; Green et al., 2000; 2001; Risner, 1988] and thus, is likely a more suitable candidate for combination with opioids. The fact that the enantiomers of nornicotine exhibit a different pharmacology profile at nAChRs also opens up the possibility that the one enantiomer may interact with an opioid to a greater extent than the other enantiomer, thereby requiring reduced amounts of the individual drugs for pain management and reducing side-effects. Precedent for this concept exists with the optical isomers of NMDA-receptor antagonists such as norketamine [Holtman et al., 2008]. This effect is particularly evident with opioids acting primarily at μ-opioid receptors, which are the majority of clinically effective opioids.
The purpose of the present study was three-fold: First, to determine if the S(−)- and the R(+)- enantiomers of nornicotine have efficacy in well-established rodent models of acute (thermal nociception) and chronic pain, including peripheral neuropathy (chronic constriction nerve injury, CCI) and tonic inflammatory pain (the formalin test). Second, to determine to what extent the analgesic activities of S(−)- and/or R(+)-nornicotine are separated from their side-effects (i.e. motor in-coordination, changes in locomotor activity, ataxia). Third, to determine whether an enhanced analgesia and reduced side-effects profile is produced when S(−)-nornicotine is combined with the μ-opioid agonist, morphine.
2. Materials and methods
2.1. Animals
Male Sprague-Dawley rats (~90 days, ~350 g; Harlan, Indianapolis, IN) were housed individually in transparent cages with a sawdust-covered floor in a humidity- and temperature-controlled facility (a 12 h alternative light/dark circle) with free access to standard laboratory chow and tap water. All experiments were conducted during the light phase of the cycle (0800–1700). Rats were handled and trained in the test situation before initiation of the procedure. Body weights were determined on the day of experimentation. At the end of the experiment, rats were euthanized with pentobarbital sodium (150 mg/kg, intraperitoneal, IP). A cross-over paradigm was used within an experiment (if possible) to minimize the number of rats. All experiments were performed according to a protocol approved by the University of Kentucky Animal Care and Use Committee (IACUC) and were carried out in compliance with the National Institutes of Health Guide for Care and Use of Laboratory Animals (Publication No.85-23, revised 1985).
2.2. Drugs
S(−)- and R(+)-nornicotine fumarate salts were supplied by Yaupon Therapeutics, Inc. (Radnor, PA). Morphine sulfate was purchased from Mallincrodt (St. Louis, MI). Drugs were dissolved in saline and given either by the parenteral (IP, 1ml/kg), neuroaxial (intrathecal, IT, 10 μl), or oral (PO, 1 ml/kg, with help of a gavage feeding needle after overnight fasting) routes. Doses refer to salt forms. Saline served as control (1 ml/kg).
2.3. Chronic constriction nerve injury (CCI) model neuropathic pain
Unilateral peripheral mononeuropathy was produced according to the method described by Bennett and Xie (1988). Briefly, under pentobarbital anesthesia (40 mg/kg, IP), ligation of the sciatic nerve and sham surgery were performed on the left and right hind paws, respectively. Proximal to the sciatic trifurcation, the nerve (about 7 mm) was freed from adhering tissue and four loose ligatures were tied around the nerve (1 mm apart) using 4.0 chromic catgut, barely constricting the diameter of the nerve. In sham surgery, the right sciatic nerve was exposed using the same procedure, but the nerve was not ligated. The incision was closed in layers with 3.0 silk threads. Rats showed a mild eversion of the affected paw and a mild degree of foot drop. No severe motor impairment was observed. Enhanced sensitivity to noxious stimuli (hyperalgesia) and enhanced sensitivity to initially non-noxious stimuli (allodynia) developed within 7 days after CCI.
The presence of mechanical hyperalgesia in CCI rats was assessed by the paw pressure technique (Randall and Selitto, 1957) utilizing the Basile Analgesia Meter (UGO Basile, Italy). Increasing pressure (32g/s) was applied to the dorsal side of the paw with vocalization used as the end point (vocalization threshold, VT, g) prior to (pre-CCI baseline) and on days 7, 9, 11, and 14 after surgery, when the abnormal pain behavior was at a stable maximum. The nerve-injured and sham-operated paws were tested in each rat. Responses (VT) were assessed prior to (post-CCI baseline) and at several time points (5–120 min) after administration of S(−)-nornicotine (0.1–20 mg/kg IP; 5–60 mg/kg PO) or R(+)-nornicotine (5–15 mg/kg IP) (randomized doses, 48 h intervals). A cut-off at 300 g was used to prevent tissue damage.
The presence of tactile allodynia was characterized on days 7–16 of recovery from CCI surgery, according to the method of Chaplan et al., (1994). Each rat was confined under a Plexiglas box on a raised platform of wire mesh. After a short habituation period (5 min), von Frey filaments (Stoelting, Wooddale, IL) with incremental stiffness (0.4–15 g) were presented to the ventral surface of the injured and sham operated paw and held there for 6–8 s. A positive response was noted by sharp paw withdrawal or flinching (paw withdrawal threshold, PWT, g). If no response was elicited by the initially selected filament, a stronger stimulus was presented (cut-off = 15 g). Both the CCI and sham-operated paws were tested. The withdrawal thresholds (PWT) were assessed prior to drug treatment (baseline) and at fixed time points (5–120 min) after administration (randomized doses) of S(−)-nornicotine (0.01–10 mg/kg IP) or R(+)-nornicotine (0.1–10 mg/kg IP). The 50% threshold was calculated using the up down method (Dixon, 1980).
2.4. Formalin model of persistent inflammatory pain
The formalin test was performed as previously described by Wheeler-Aceto and Cowan (1991). Briefly, 50 μl of 5% formalin was injected subcutaneously (SC) into the dorsal surface of the left hind paw and the incidences of spontaneous flinches, defined as quick shakes of the injected paw, were counted continuously in 5 min intervals for 60 min. S(−)-Nornicotine (0.5–20 mg/kg IP), R(+)-nornicotine (0.5–10 mg/kg IP), or saline (control) were administered 15 min prior to the SC formalin injection. Each rat was used in only one trial.
2.5. Thermal model of acute nociceptive pain
Responsiveness to thermal noxious stimuli was assessed by the thermal plantar test (Hargreaves et al., 1988), using the IITC Paw Stimulator Analgesia Meter (Life Science, Woodland Hills, CA) in freely moving intact rats. Briefly, radiant heat (60% intensity) was positioned under the glass floor (the temperature maintained at 30° ± 0.1° C) directly beneath the plantar hind paw. Latency to paw withdrawal (PWL, s) from the heat source was measured prior to (PWL baseline, determined twice, 15 min apart) and at fixed time points (5–180 min) after administration of S(−)-nornicotine (10–40 mg/kg IP; randomized doses, weekly intervals). Both feet were tested, and mean values for the response were calculated based on the two scores. A cut-off at 10 s prevented tissue damage.
2.6. Motor coordination (rotarod)
The motor effect of nornicotine was assessed in the rotarod test using a Rat Rota Rod apparatus (Ugo Basile, Comeno, Italy). Each rat was trained to run on the rotarod at a constant speed (10 rpm) until it could remain there for 180 s without falling (for two consecutive days). After that, latency to fall (s) was determined prior to and at fixed time points (5–120 min) after administration of S(−)-nornicotine (2.5–20 mg/kg IP) or R(+)-nornicotine (1–15 mg/kg IP). Doses were randomized and administered in 48 h intervals. Cut of time was equal to 180 s.
2.7. Locomotor activity
Spontaneous locomotor activity was determined using the Opto-Varimex infrared photocell-based activity monitor (Columbus Instrument, Columbus, OH). Horizontal (ambulation) and vertical (rearing) activities were recorded during 5 min sessions prior to (baseline) and following (5–120 min) administration of S(−)-nornicotine (2.5–20 mg/kg IP) or R(+)-nornicotine (1–15 mg/kg IP).
2.8. Ataxia and activity level
Ataxia and overall activity were also assessed by direct observation using the following rating scale: Ataxia: coordinated movement (0), loss of balance when rearing (1), falling to side with attempted walking (2), inability to walk (3); Activity level: normal activity (0), 25% of time stationary (−1), 75% of time stationary (−2), 100% of time stationary (−3). Rats were observed (5 min sessions) prior to (baseline) and at fixed time points (5–120 min) after administration of S(−)-nornicotine (2.5–20 mg/kg IP) or R(+)-nornicotine (1–15 mg/kg IP). Saline was used as control.
2.9. S(−)-Nornicotine and morphine in combination
Three series of experiments were conducted to characterize the effects of S(−)-nornicotine and morphine in combination in rodent models of acute thermal nociception (the tail-flick test) and peripheral neuropathy (CCI). First, antinociception was determined following IP administration of morphine (3 mg/kg), S(−)-nornicotine (2.5 mg/kg), and R(+)-nornicotine (2.5 mg/kg alone and S(−)- or R(+)-nornicotine (2.5 mg/kg) in combination with morphine (3 mg/kg). Responsiveness to thermal noxious stimulation was assessed by the tail-flick test using a Tail Flick Analgesia Meter (Life Science). Tail-flick latency (TFL, s) was measured by recording the time from the onset of heat stimulus to the tail to withdrawal of the tail from the heat source prior to drug administration (baseline TFL ≈ 2–3 s) and at fixed time points (5–60 min) thereafter. A cut-off time of 10 s prevented tissue damage. Second, rats were subjected to chronic catheterization of the spinal subarachnoid space, according to the method of Yaksh and Rudy (1976). Thereafter, morphine (0.5–30 μg) and S(−)-nornicotine (10–100 μg) each separately, and morphine (0.5 μg) in combination with S(−)-nornicotine (10–100 μg) were administered by the intrathecal route (IT). The responsiveness (TFL, s) was measured at several time points (0–120 min). Third, various doses of S(−)-nornicotine (0.01–0.5 mg/kg IP; 0.1–7.5 mg/kg PO) in combination with a fixed dose of morphine (3 mg/kg IP; 5 mg/kg PO) were administered by the IP and the PO routes in CCI rats. Morphine (3–7.5 mg/kg IP; 5–20 mg/kg/PO) alone was tested as control. Responsiveness (VT, g) was assessed by the paw pressure test. Finally, the motor effects of morphine (3 mg/kg IP; 5 mg/kg PO) in combination with S(−)-nornicotine (3 mg/kg IP; 7.5 mg/kg PO) were tested on the rotarod.
2.10. Data presentation and statistics
All data were normalized for pre-injection baseline. Areas under the time curves (AUC0-t) were calculated for normalized data by the trapezoidal rule. The percent of maximum possible effect (%MPE) was calculated as follows: 1) Antihyperalgesia (the paw-pressure test): %MPE (at peak time, tmax) = (VT − post-CCI baseline/preCCI baseline − post-CCI baseline)*100; pre- CCI baseline ≈ 230 g, post-CCI baseline ≈ 100 g; 2) Antiallodynia (the von Frey test): %MPE (tmax) = (PWT − baseline/15 − baseline)*100; cut-off = 15 g; 3) Blockade of flinching (the formalin test): %MPE = (AUCsaline − AUCdrug /AUCsaline)*100; the AUCdrug and AUCsaline are areas under the curves, 0–20 min (the 1st phase) or 20–60 min (the 2nd phase), for drug and saline, respectively); 4) Antinociception (the thermal plantar test): %MPE (tmax) = (PWL − baseline/10 − baseline)*100; cut-off = 10 s; 5) Antinociception (the tail-flick test): %MPE (tmax) = (TFL − baseline/10 − baseline)*100; cut-off = 10 s; 6) Motor in-coordination (the rotarod test): %MPE (tmax) = (180 − latency to fall/180)*100; cut off = 180 s. Dose-response curves were generated for %MPE as a function of log dose. The effective doses for a 50% maximum possible effect (ED50) and 95% confidence limits (95% CL) were calculated using the method of Tallarida and Murray (1987). All data are the mean ± SEM for (n) rats. Statistical analyses were performed using linear regression, one- and two-way analysis of variance (ANOVA), post-hoc Student Newman Keuls (SNK) and t test. The level of significance was P ≤ 0.05.
3. Results
3.1. The antihyperalgesic and antiallodynic effects of nornicotine enantiomers (CCI model)
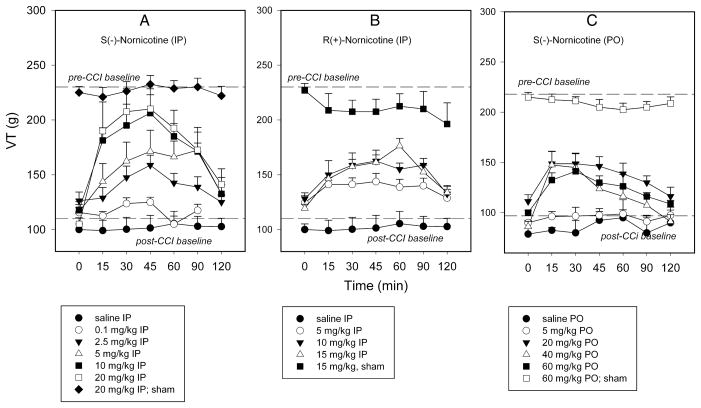
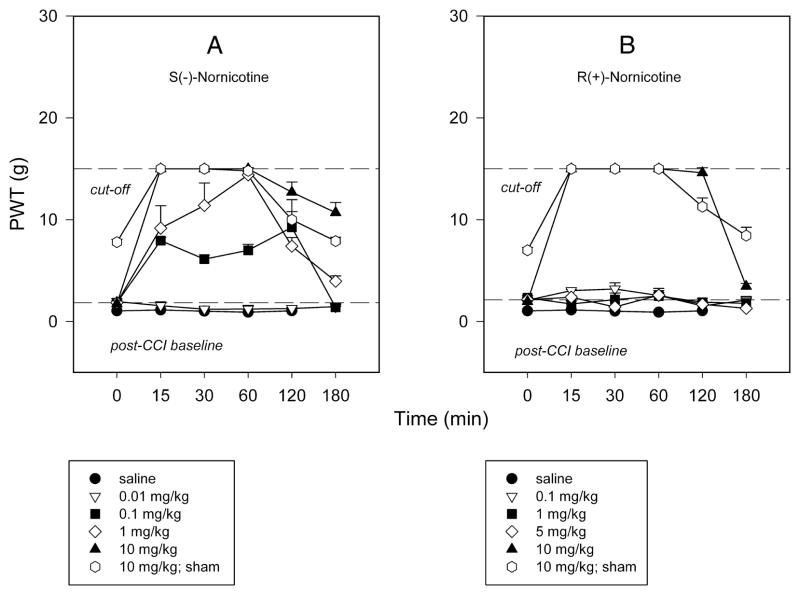
Chronic constriction injury of the sciatic nerve (CCI) resulted in long-lasting pain behaviors characterized by both mechanical hyperalgesia and tactile allodynia in control rats. This was demonstrated respectively by: 1) A decrease in vocalization threshold (VT, g) in response to a noxious stimulus compared to pre-surgical values (pre-CCI baseline VT = 230.6 ± 4.78 g; post-CCI baseline VT = 105 ± 5.8 g; post-sham baseline VT = 225 ± 5.4 g), and 2) A decrease in paw withdrawal threshold (PWT, g) in response to a normally non-noxious mechanical stimulus in the nerve-injured paw (PWT = 1.7 ± 0.29 g) compared to sham-operated paw (PWT = 7.8 ± 0.27 g). No significant changes in VT and PWT were observed after administration of saline (see FIG 1 and 2).
FIG 1.
Time course of responsiveness to mechanical noxious stimuli (vocalization threshold, VT, g) in nerve-injured paw (chronic constriction nerve injury, CCI) and sham-operated paw after intraperitoneal (IP) administration: S(−)-nornicotine [Panel A]; R(+)-nornicotine [Panel B] and oral (PO) administration: S(−)-nornicotine [Panel C]. Saline (IP, PO) served as control. Mean ± SEM (n = 8 rats).
FIG 2.
Time course of responsiveness to non-noxious mechanical stimuli (50% von Frey paw withdrawal threshold, PWT, g) in nerve-injured paw (chronic constriction nerve injury, CCI) and sham-operated paw after intraperitoneal (IP) administration: S(−)-nornicotine [Panel A]; R(+)-nornicotine [Panel B]. Saline served as control. Mean ± SEM (n = 4 rats).
The antihyperalgesic and antiallodynic effects of S(−)- and R(+)-nornicotine were characterized in the CCI model. Both S(−)- and R(+)-nornicotine (IP) reversed, in a dose-related manner (F5,43 = 9.9, P<0.0001 and F3,27 = 8.2; P<0.001, respectively), mechanical hyperalgesia in the nerve-injured (CCI) paw [FIG 1A,B]. The effects produced by S(−)-nornicotine (5, 10, 20 mg/kg) and R(+)-nornicotine (10, 15 mg/kg) were different from the effect of saline (P<0.05; post-hoc SNK test). The antihyperalgesic effect was approximately 3-fold greater for the S(−)- enantiomer compared to the R(−)-enantiomer, as revealed by the estimation of ED50 values (95% CL) of 5.2 (3.1–8.5) and 15.5 (11.2–21.3) mg/kg IP, respectively. In addition, the S(−)-enantiomer was more effective than the R(−)- enantiomer (baseline-normalized data) at comparable doses (5 mg/kg: VTtmax = 71.25±16.3 vs. 32.5±6.9 g; 10 mg/kg: VTtmax = 105.6±16.6 vs. 46.9±5.3 g, P<0.05 and 0.0025, respectively; t-test). This also was found for the AUC0–120min values. Almost complete reversal of hyperalgesia (%MPE ≈ 85%) was obtained with the highest dose of S(−)-nornicotine tested (20 mg/kg, IP) [FIG 1A]. The antihyperalgesic effect of a dose of R(+)- nornicotine greater than 15 mg/kg could not be accurately assessed due to toxicity (sedation). The effectiveness of oral S(−)-nornicotine was apparent, but limited (%MPE ≈ 40% at 20–60 mg/ kg PO) in the CCI model [FIG 1C]. Neither S(−)- or R(+)-nornicotine had significant effects on the sham-operated paw at the highest doses tested [see FIG 1A,B,C]. The present data also demonstrated that S(−)-nornicotine (IP) reversed, in a dose-related manner (F4,19 = 376.8; P<0.0001), tactile allodynia in the nerve-injured paw with an ED50 (95% CL) of 0.15 (0.06–0.35) mg/kg IP. Of note, S(−)-nornicotine (0.01–10 mg/kg) produced significantly greater effect compared to saline (P<0.05; post-hoc SNK) [Fig 2A]. This effect was seen only at the high dose of R(+)-nornicotine (10 mg/kg) tested [FIG 2B].
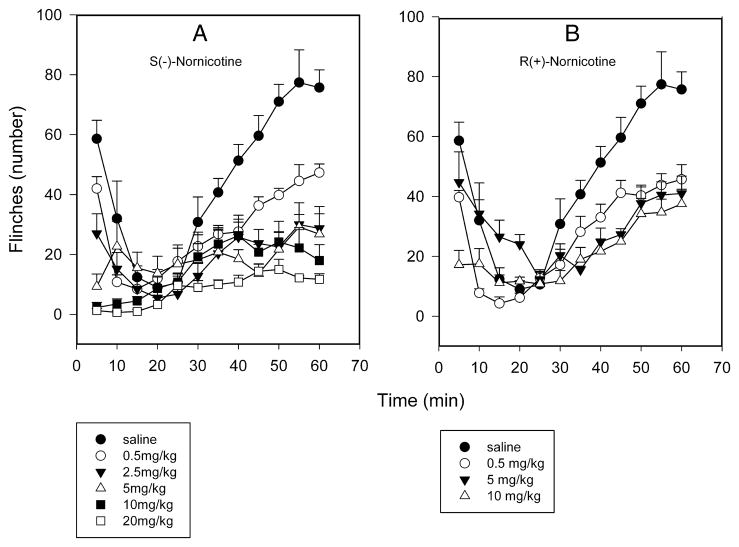
3.2. Effect of nornicotine enantiomers on formalin-induced flinching (formalin test)
Formalin injection (SC) into the paw caused a typical biphasic flinching behavior in control rats consisting of an early phase (0–10 min) and a late phase (20–60 min), which are thought to represent nociception and central sensitization, respectively [FIG 3A,B]. S(−)-Nornicotine inhibited, in a dose-related fashion, formalin-induced flinching behaviors in both phases of the formalin test (F5,36 = 5.9, P<0.0005 and F5,36 = 21.6, P<0.0001, respectively) with ED50 values (95% CL) of similar magnitude: 1.8 (0.7–4.9) and 1.5 (0.6–3.6) mg/kg IP for the 1st and the 2nd phase, respectively [FIG 3A]. R(+)-Nornicotine (F3,24 = 302.3, P<0.0001) was approximately 4-fold less effective in this paradigm as evidenced by an ED50 value (95% CL) of 5.5 (1.9, 16.1) mg/kg IP for the late phase (ED50 was not determinable for the early phase because of lack of a significant dose-response effect) [FIG 3B]. The effects of both S(−)- (0.5–20 mg/kg) and R(+)-nornicotine (0.5–10 mg/kg) were significantly different from the effect of saline (P<0.5; post-hoc SNK). Of note, the differences between enantiomers were less pronounced when compared at the same doses (0.5, 5 or 10 mg/kg).
FIG 3.
Time course of formalin-induced flinching behavior (number of flinches; the 1st and 2nd phases) after intraperitoneal (IP) administration: S(−)-nornicotine [Panel A]; R(+)-nornicotine [Panel B]. Saline served as control. Mean ± SEM (n = 6 rats/dose).
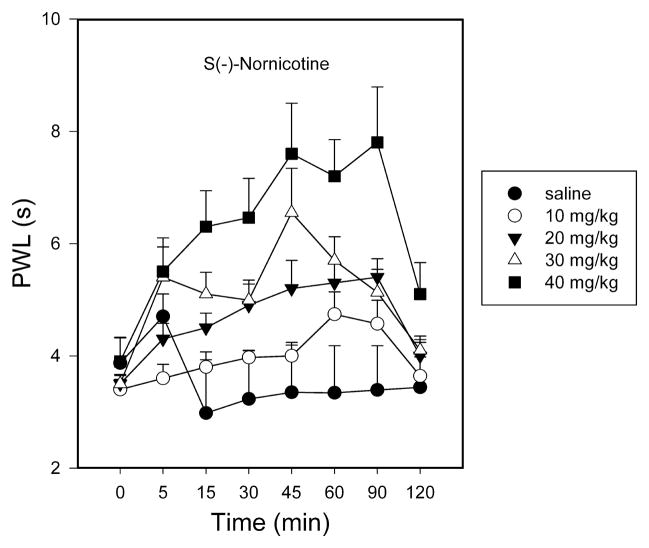
3.3. Antinociceptive effect of S(−)-nornicotine (thermal plantar test)
S(−)-Nornicotine (10–40 mg/kg, IP) caused a dose-dependent increase in latency to paw withdrawal (F3,31 = 4.2, P<0.025) with an ED50 value (95% CL) of 29.3 (21.5, 40.6) mg/kg IP in a rat model of acute pain (thermal plantar test) [FIG 4]. The antinociceptive effect of S(−)-nornicotine was modest compared to its actions against hyperalgesia, allodynia, or formalin-induced nociceptive behaviors (see data above). High doses of S(−)-nornicotine (> 40 mg/kg IP) could not be tested due to toxicity. R(+)-Nornicotine was not tested in this paradigm because of the expected toxicity of these high doses.
FIG 4.
Time course of responsiveness to thermal noxious stimuli (paw withdrawal latency, PWL, s) after intraperitoneal (IP) administration: S(−)-nornicotine. Saline served as control. Mean ± SEM (n = 8 rats).
3.4. Side-effects of nornicotine enantiomers
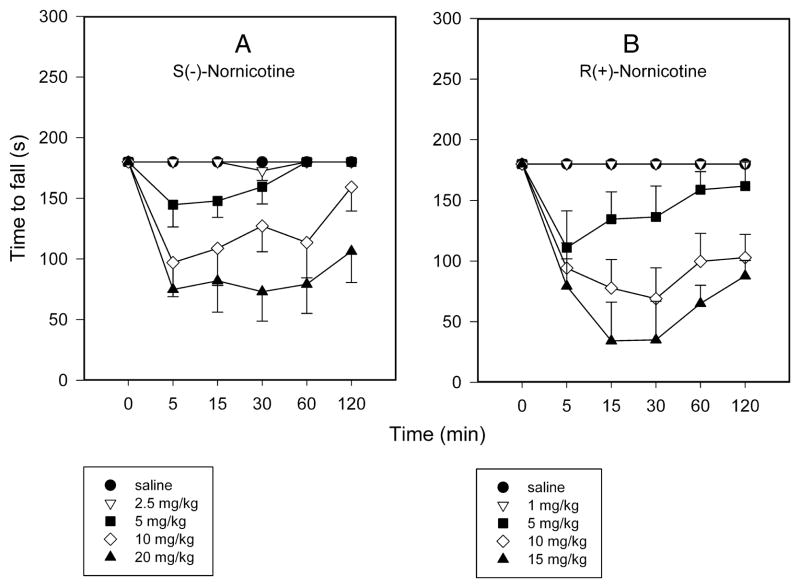
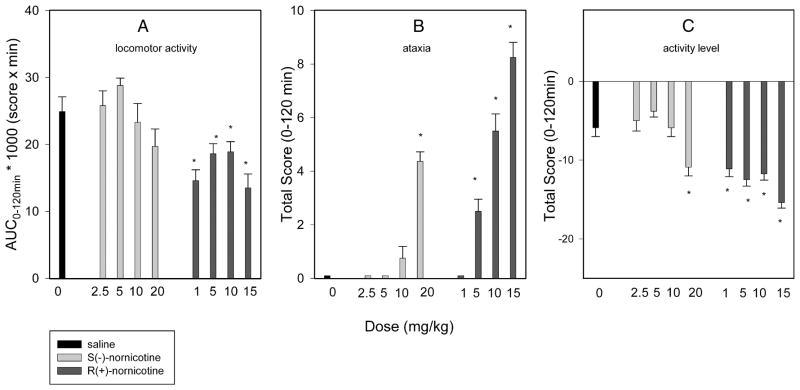
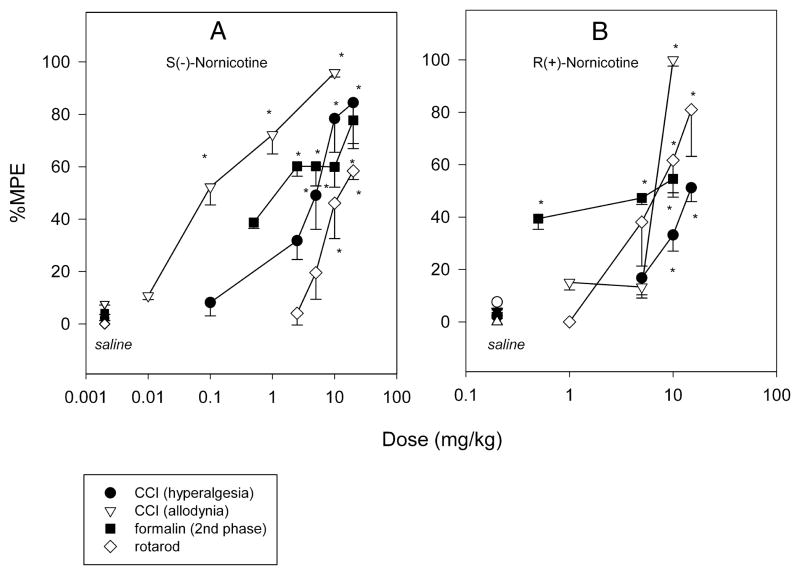
The S(−)- and the R(+)- enantiomers of nornicotine caused a dose-related effect on rotarod performance in intact rats (F4,29 = 12.6 and 9.9, P<0.0001, respectively) [FIG 5A,B]. However, R(+)-nornicotine was approximately 2-fold more potent than S(−)-nornicotine in this test, as demonstrated by the ED50 values (95% CL) of 6.9 (5.9–10.6) and 13.6 (9.2–19.9) mg/kg IP, respectively. R(+)-Nornicotine also caused a significant decrease in locomotor activity compared to the saline control (P = 0.013; Kruskal-Wallis 1-way RM ANOVA on ranks), whereas this effect was not statistically significant for S(−)-nornicotine [FIG 6A]. In addition, ataxia and change in activity levels were assessed within the dose range of analgesic activity (CCI, formalin). Both S(−)- and R(+)-nornicotine produced a dose-related ataxia [FIG 6B], and also decreased, in a dose-related fashion, overall activity levels [FIG 6C]. Once again, the undesirable side-effects were more pronounced with the R(+)- rather than the S(−)- enantiomer. Importantly, the present data showed that a dose of S(−)-nornicotine which caused an undesirable side-effect (e.g. motor in-coordination, rotarod test) was higher than a dose that produced a desirable analgesic effect [FIG 7A]. Of note, this separation on dose (ED50 rotarod / ED50 analgesia) was more pronounced for tactile allodynia (~ 90) compared to mechanical hyperalgesia (~3) or formalin-induced behaviors (~9). In contrast, the analgesic and motor effects occurred within a similar dose range of R(+)-nornicotine [FIG 7B]. In addition, although not specifically tested in the present study, other potentially dose-limiting toxicities (i.e. sedation, labored breathing) were observed at a lower dose of R(+)-nicotine compared to S(−)-nornicotine (>15 and 40 mg/kg IP, respectively).
FIG 5.
Time course of the motor effect (latency to fall, s) in rotarod performance test after intraperitoneal (IP) administration: S(−)-nornicotine [Panel A]; R(+)-nornicotine [Panel B]. Saline served as control. Mean ± SEM (n = 6 rats).
FIG 6.
Dose-response curves for: [Panel A] locomotor activity (AUC0–120min is area under the curve, 0–120 min, for a drug or saline); [Panel B] ataxia (total score, 0–120 min); [Panel C] activity level (total score, 0–120 min) after intraperitoneal (IP) administration: S(−)-nornicotine, R(+)-nornicotine, saline (control). Mean ± SEM (n = 8 rats). * Significantly different from saline (P<0.05; post-hoc SNK).
FIG 7.
Dose-response curves for S(−)-nornicotine [Panel A] and R(+)-nornicotine [Panel B]: mechanical hyperalgesia (chronic constriction nerve injury model, CCI; n = 8); tactile allodynia (CCI; n = 4); formalin-induced flinching (the 2nd phase in the formalin test; n = 6); motor coordination (rotarod; n =6). Saline served as control. Percent maximum possible effect (%MPE) was calculated as described in the Methods section. Data are mean ± SEM (n rats). * Significantly different from saline (P<0.05; post-hoc SNK).
3.5. Interaction between S(−)-nornicotine and morphine
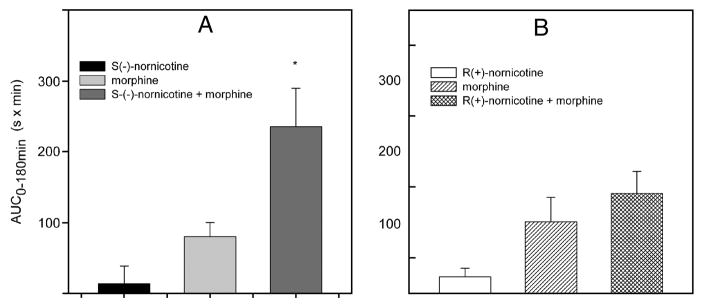
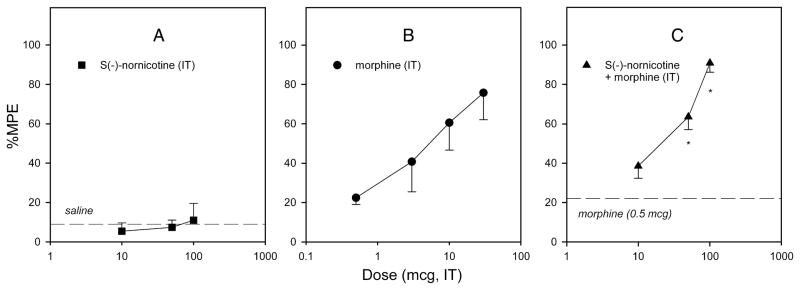
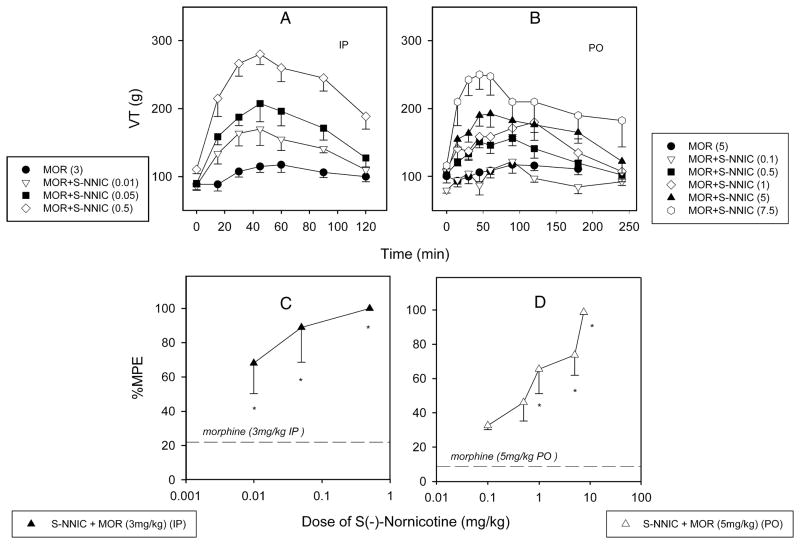
S(−)-Nornicotine in combination with morphine was tested in various pain models [acute nociception (the tail-flick test) and peripheral neuropathy (CCI)], and by differing routes of administration [parenteral (IP), oral (PO), and intrathecal (IT)]. Initially, we demonstrated that S(−)-nornicotine, at a dose that had no antinociceptive activity on its own (2.5 mg/kg), enhanced morphine (3 mg/kg) antinociception in intact rats (IP, the tail-flick test) [FIG 8A]. This was less pronounced when a combination of morphine and R(+)-nornicotine was utilized [FIG 8B]. Next, S(−)-nornicotine and morphine alone and in combination were assessed after IT administration in the tail-flick test. S(−)-Nornicotine alone did not produce antinociception in the dose range tested (10–100 μg IT) [FIG 9A], whereas morphine alone produced a dose-related antinociception with an ED50 value (95% CL) of 4.7 (1.9–11.4) μg IT in this paradigm [FIG 9B]. Interestingly, a low-dose of morphine (0.5 μg ≈ 0.1 × ED50) enhanced effectiveness of S(−)-nornicotine, at doses exhibiting no antinociceptive activity of their own, at the spinal level [FIG 9C]. The maximum effect (%MPE ≈ 100%) was achieved when morphine (0.5 μg, IT) was combined with S(−)-nornicotine (100 μg, IT). Finally, activity of this drug combination was tested after IP and PO administration in a CCI model. Once again, S(−)-nornicotine, at doses that had exhibited no significant activity on their own, produced a dose-related antihyperalgesia in a presence of a low-dose of morphine (F3,31 = 17.6 and F5,39 = 7.6, P<0.0001 for IP and PO, respectively) [FIG 10A,B]. Maximum efficacy (%MPE = 100%) was achieved when a sub-analgesic dose of S(−)-nornicotine (0.5 mg/kg IP or 7.5 mg/kg PO) was paired with a low-dose of morphine (3 mg/kg IP or 5 mg/kg PO) [FIG 10C,D]. Morphine alone produced dose-related effects in a CCI model after IP and PO administration (F3,27 = 46.5 and 24.7, P<0.0001, respectively) with the ED50 values (95% CL) of 4.1 (3.6–4.6) mg/kg IP and 10.5 (7.5–14.6) mg/kg PO, respectively. Importantly, no motor effect was noted during combination drug therapy at the maximum antihyperalgesic doses tested: i.e. morphine (3 mg IP or 5 mg/kg PO) and S(−)-nornicotine (3 mg/ kg IP or 7.5 mg/kg PO). This was evident by undisrupted performance in the rotarod test; rats were able to stay on the drum until the 180 s cut-off time.
FIG 8.
Responsiveness to thermal noxious stimuli (the tail-flick test) after intraperitoneal (IP) administration: S(−)-nornicotine (2.5 mg/kg), morphine (3 mg/kg), S(−)-nornicotine (2.5 mg/kg) - morphine (3 mg/kg) combination [Panel A]; R(+)-nornicotine (2.5 mg/kg), morphine (3 mg/kg), R(+)-nornicotine (2.5 mg/kg) - morphine (3 mg/kg) combination [Panel B]. The AUC0–180min values are areas under the curves (0–180 min). Mean ± SEM (n = 8 rats). * Significantly different from morphine alone (P<0.05; t-test).
FIG 9.
Dose-response curves for the antinociceptive effects (the tail-flick test) after intrathecal (IT) administration: S(−)-nornicotine (10–100 μg) [Panel A]; morphine (0.5–30 μg) [Panel B]; S(−)-nornicotine (10–100 μg) in combination with a fixed dose of morphine (0.5 μg) [Panel C]. Percent maximum possible effect (%MPE) was calculated as described in the Methods section. Dashed lines represent the effects of saline and morphine (0.5 μg) alone, respectively. Mean ± SEM (n = 6–8 rats). * Significantly different from morphine alone (P<0.05; post-hoc SNK test).
FIG 10.
Time courses of responsiveness to mechanical noxious stimuli (vocalization threshold, VT, g) in nerve-injured paw (chronic constriction nerve injury model, CCI): S(−)-nornicotine (S- NNIC); morphine (MOR); S(−)-nornicotine and morphine combination after intraperitoneal (IP) [Panel A] and oral (PO) [panel B] administration. Dose-response curves for the antihyperalgesic effects: S(−)-nornicotine (0.01–0.5 mg/kg, IP) in combination with morphine (3 mg/kg, IP) [Panel C]; S(−)-nornicotine (0.1–7.5 mg/kg, PO) in combination with morphine (5 mg/kg, PO) [panel D]. Percent maximum possible effect (%MPE) was calculated as described in the Methods section. Dashed lines are effects of morphine alone (3 mg/kg IP; 5 mg/kg PO). Mean ± SEM (n = 8 rats). * Significantly different from morphine alone (P<0.05; post-hoc SNK).
4. Discussion
The S(−)- and R(+)- enantiomers of nornicotine (the N-desmethyl derivative of nicotine that also binds to nAChRs) were characterized with regard to analgesia and side-effect profile in rats. First, nornicotine enantiomers efficacy was shown in two well-established models of pain where central sensitization is involved: i.e. the chronic constriction nerve injury model of peripheral neuropathy (CCI), and the formalin model of tonic inflammatory pain (the paw formalin test). Second, the stereoselectivity of nornicotine enantiomers was demonstrated with regard to both desirable and undesirable effects. The analgesic properties resided more predominantly in the S(−)- than the R(+)-enantiomer. In contrast, the side-effects were more pronounced with the R(+)- rather than the S(−)-enantiomer. This is a useful observation, which may suggest a better separation of toxicity from analgesia for S(−)-nornicotine than R(+)-nornicotine. Third, effectiveness of S(−)-nornicotine and morphine combination drug therapy was demonstrated in preclinical models of nociceptive and neuropathic pain. Together, data from the present study suggest that S(−)-nornicotine may be of value, either alone and/or in combination with a μ-opioid agonist (i.e. morphine), as a novel analgesic therapy.
The initial objective of the present research was to assess the analgesic efficacy of nornicotine enantiomers in rat models of pain. This was accomplished by demonstrating the ability of nornicotine to alleviate pain sensitization occurring as the result of an injury to nerve or tissue. Specifically, the S(−)- and R(+)- enantiomeric forms of nornicotine attenuated, in a dose-related manner, an enhanced sensitivity to a noxious (mechanical hyperalgesia) and previously non-noxious (tactile allodynia) stimulus in the CCI model of peripheral neuropathy. In addition, the nornicotine enantiomers inhibited both the early and the late nociceptive responses in a formalin model of persistent inflammatory pain. These findings with nornicotine enantiomers are consistent with the effects of other nicotinic agents, such as (−)-nicotine, epibatidine, and ABT-594 in rodent models of persistent pain [Bannon et al., 1998a; 1998b; Curzon et al., 1998; Gilbert et al., 2001; Kesingland et al., 2000].
Nornicotine stereochemistry appears to be an important factor since the S(−)-enantiomer showed greater analgesic potency (approximately 3-fold) than the R(+)-enantiomer in all pain models tested. These findings are not consistent with in vitro data showing a lack of stereoselectivity [Ki = 34.3±2.4 vs. 17.3±0.2 nM for S(−)- vs. R(+)-nornicotine] in displacement of [3H]nicotine from the binding sites in the rat brain membranes (thought to involve the α4β2 nAChR subtype) [Zhang et al., 1998]. Although the α4β2 nAChR subtype plays an important role in the antinociceptive effects of nicotinic agents in acute nociception assays (e.g. tail-flick, hot-plate) the involvement of other subtype(s) cannot be excluded (e.g. α7) [Damaj et al., 2000; Damaj et al., 2007]. In addition, emerging evidence indicates that expression of several nAChR subtypes, localized centrally and/or in periphery (e.g. α5, α7, α9, α10), may be altered following nerve injury and/or inflammation [Vincler and Eisenach, 2004; Vincler et al., 2006; Yang et al., 2004]. In this regard, it is interesting to note that enantioselectivity of nornicotine appears to vary across rodent models of pain used with differences being more pronounced in a CCI model of peripheral neuropathy than in a formalin model of persistent inflammatory pain.
The next objective of the present study was to determine if differences in stereochemistry allows for separation of the desirable (analgesic) and undesirable (toxicity) properties of nornicotine enantiomers. Several side-effects, including motor dysfunction (rotarod), decreased locomotor activity, and ataxia were more prominent in the R(+)- than in the S(−)-nornicotine. Previous data also showed that R(+)-nornicotine was more potent than S(−)-nornicotine with regard to duration of cardiovascular effects (45 and 20 min, respectively) in dogs [Risner et al., 1988], dopamine release from the reward-relevant nucleus accumbens (~2-fold) [Green et al., 2001], and in decreasing self-administration of S(−)-nicotine (~2-fold) in rats [Stairs et al., 2007]. These findings suggest that one enantiomer of nornicotine may elicit less toxicity during an equivalent analgesic response than the other enantiomer. Indeed, S(−)-nornicotine had an expanded therapeutic index compared to R(+)-nornicotine when the motor and analgesic effects were taken into account. Specifically, S(−)-nornicotine reversed hypersensitivity (hyperalgesia, allodynia), as well as inhibited initiation and propagation of formalin-induced nociceptive behaviors at lower doses than those needed to disrupt motor functioning (rotarod). In contrast, R(+)-nornicotine displayed no separation between analgesic efficacy and motor dysfunction in rats. In addition, other side-effects, which were not specifically tested in this study (e.g. sedation, increased respiratory rate), were not obvious with S(−)-nornicotine at maximum analgesic dose. This was not the case with R(+)-nornicotine. In this regard, nornicotine differs from nicotine where the (−)-isomer is clearly more potent than the (+)-isomer in both antinociception (30-fold, tail-flick test), motor in-coordination (15-fold, rotarod), and decrease in spontaneous activity (6-fold, open-field activity) [Martin et al., 1983]. In addition, the greater antinociceptive potency of (−)-nicotine relative to (+)-nicotine is consistent with the difference (25-fold) in their affinities at the [3H]nicotine sensitive brain sites [Zhang et al., 1998].
It is appealing to speculate that the diversity of nAChRs may likely allow for better separation between the beneficial (analgesic) and side-effects liabilities for nicotinic agent(s) with improved nAChR subtype selectivity. For example, an argument has been made that such a separation by dose (rotarod/ antinociception) demonstrated with ABT-594 but not with epibatidine [Bannon et al., 1998a; 1998b; Boyce et al., 2000; Kesingland et al., 2000; Sulivan et al., 1994] is due the fact that in contrast to epibatidine, ABT-594 has greater selectivity for neuronal α4β2 nAChRs subtype (implicated in antinociceptive activity) than for α3β4 subtype expressed in autonomic ganglia (responsible for various side-effects) [Decker et al., 2004; Flores, 2000; Lloyd and Williams, 2000, reviews]. In this regard, S(−)-nicotine was found to be relatively efficacious at both α4β2 and α3β4 nAChRs, while in contrast, (±)-nornicotine was less potent at these nicotinic receptor subtypes [Papke et al., 2007]. On another note, α-bungarotoxin-sensitive α7-type nAChR appears to be very responsive to either (±)-nornicotine or S(−)-nicotine [Papke et al., 2007]. This is an interesting observation, since the homomeric α7 nAChR, which is present on macrophages and spinal cord microglia, is believed to be mainly implicated in inflammatory processes [Wang et al., 2003; Waldburger et al., 2008]. Although, nornicotine is not steroselective at the α4β2 nAChR subtype little is known about the other subtypes (e.g. α7, α3β4, α1β1δγ). In this regard, precedence exists for ABT-594 (R-isomer) and A-98593 (S-isomer), which showed no streoselectivity for binding at the α4β2 nAChR subtypes but the R-enantiomer was more potent than the S-enantiomer both at the brain α7 and the neuromuscular α1β1δγ nAChR subtypes [Donnelly-Roberts et al., 1998]. Clear differences between S(−)- and R(+)-nornicotine in a battery of persistent pain and behavioral assays may suggest that they likely modulate diverse nAChR function in a streoselective fashion. Thus, S(−)-nornicotine may offer potential advantages over R(+)- and/or (±)-nornicotine in term of analgesic properties versus the undesirable actions.
Our final objective was to determine if combination drug therapy, consisting of nornicotine enantiomers and a μ-opioid (morphine), could be of value for enhancing analgesic efficacy with a reduction in toxicity. Several interesting findings were noted: First, nornicotine enantiomers interacted with morphine in a stereoselective fashion. The S(−)-enantiomer significantly enhanced morphine (a low-dose) antinociception in the tail-flick assay, while the R(+)-enantiomer was not effective in this paradigm. Of note, S(−)-nornicotine alone was effective against acute thermal nociception (thermal plantar test) only within the similar dose range that caused marked in-coordination (rotarod). This undesirable side-effect was not observed with S(−)-nornicotine and morphine combination therapy. Second, the spinal cord appears to be an important site of action in these effects, as evidenced by enhancement of the antinociceptive effect of S(−)-nornicotine by morphine after the neuroaxial (IT) route of administration in rats. Third, morphine potentiated the effect of S(−)-nornicotine, in a dose range that exhibited no significant activity against mechanical hyperalgesia, (via both parenteral and oral routes), in a CCI model of peripheral neuropathy. This is an important finding, since the decreased effectiveness of μ-opioids in neuropathic pain states is an important clinical problem [Ballantine and Mao, 2003]. Again, the maximum antihyperalgesic effect was achieved without the toxicity typically seen with the higher doses of these drugs alone. Whether S(−)-nornicotine in combination with morphine (and other μ-opiods) produces a greater than additive effect (i.e. a synergistic effect) on analgesia will need to be tested with isobolographic analysis. Although a body of evidence suggests the involvement of separate, but interacting cholinergic mechanisms and opioid mechanisms in analgesia, the mechanism of the interaction between nicotinic agents and opioids is yet to be determined. Previous studies have shown that (−)-nicotine enhances and prolongs morphine antinociception at the spinal and supraspinal levels in a tail-flick test in mice [Haghparast et al., 2008; Suh et al., 1996a; 1996b; Zarrindast et al., 1996]. In addition, the sparing effect of (−)-nicotine (intranasal, transdermal) on postoperative consumption of morphine has been demonstrated in a clinical setting [Flood and Daniel, 2004; Habib et al., 2008]. Results of the present study, showing that morphine enhanced the antinociceptive effect of S(−)-nornicotine (spinal, systemic, oral), provide additional support for the concept of combining a nicotinic agent and an opioid in pain management. From a clinical standpoint, this novel combination drug therapy may be of value in managing pain with different etiologies (i.e. nociceptive, neuropathic, mixed pain states), thereby the enhancing analgesic efficacy and reducing the toxicity usually observed with a higher dose of each drug alone.
Acknowledgments
This work was supported by NIH/NIDA (DA 022091) and Yaupon Therapeutics Inc. The authors would like to thank Marhaba Hojahmat, Ph.D. (Yaupon Therapeutics, Inc.) for providing the nornicotine enantiomers and Katelyn Etscheidt (Dept. of Anesthesiology) for technical help.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Arneric SP, Holladay M, Williams M. Neuronal nicotinic receptors: A perspective on two decades ofd drug discovery research. Biochem Pharmacol. 2007;74:1092–1101. doi: 10.1016/j.bcp.2007.06.033. [DOI] [PubMed] [Google Scholar]
- Aceto MD, Awaya H, Martin BR, May EL. Antinociceptive action of nicotine and its methiodide derivatives in mice and rats. Br J Pharmacol. 1983;79:869–876. doi: 10.1111/j.1476-5381.1983.tb10531.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballantyne J, Mao J. Opioid therapy for chronic pain. N Engl J Med. 2003;349:1943–1953. doi: 10.1056/NEJMra025411. [DOI] [PubMed] [Google Scholar]
- Bannon AW, Decker MW, Curzon P, Buckley MJ, Kim DJ, Radek RJ, Lynch JK, Wasicak JT, Lin NH, Arnold WH, Holladay MW, Williams M, Arneric SP. ABT-594 [(R)-5-(2-azetidinylmethoxy)-2-chloropyridine]: A novel, orally effective antinociceptive agent acting via neuronal nicotinic acetylcholine receptors: II. In vivo characterization. J Phamacol Exp Ther. 1998a;285:787–794. [PubMed] [Google Scholar]
- Bannon AW, Decker MW, Holladay MW, Curzon P, Donnelly-Roberts D, Puttfarcken PS, Bitner RS, Diaz A, Dickenson AH, Porsolt RD, Williams M, Arneric SP. Broad-spectrum, non-opioid analgesic activity by selective modulation of neuronal nicotinic acetylcholine receptors. Science. 1998b;279:77–81. doi: 10.1126/science.279.5347.77. [DOI] [PubMed] [Google Scholar]
- Bardo MT, Bevins RA, Klebaur JE, Crooks PA, Dwoskin LP. (−)-Nornicotine partially substitutes for (+)-amphetamine in a drug discrimination paradigm in rats. Pharmacol Biochem Behav. 1997;58:1083–1087. doi: 10.1016/s0091-3057(97)00303-1. [DOI] [PubMed] [Google Scholar]
- Bardo MT, Green TA, Crooks PA, Dwoskin LP. Nornicotine is self-administered intravenously by rats. Psychopharmacology. 1999;146:290–296. doi: 10.1007/s002130051119. [DOI] [PubMed] [Google Scholar]
- Bennett G, Xie Y. A peripheral mononeuropathy in rat that produced disorders of pain sensation like those seen in man. Pain. 1988;33:87–107. doi: 10.1016/0304-3959(88)90209-6. [DOI] [PubMed] [Google Scholar]
- Boyce S, Webb JK, Shepheard SL, Russell MG, Hill RG, Rupniak NM. Analgesic and toxic effects of ABT-594 resemble epibatidine and nicotine in rats. Pain. 2000;85:443–450. doi: 10.1016/S0304-3959(99)00303-6. [DOI] [PubMed] [Google Scholar]
- Chaplan SR, Bach FW, Pogrel JW, Chung JM, Yaksh TL. Quanitative assessment of tactile allodynia in the rat paw. J Neurosci Methods. 1994;53:55–63. doi: 10.1016/0165-0270(94)90144-9. [DOI] [PubMed] [Google Scholar]
- Copeland JR, Adem A, Jacob PJ, Nordberg A. A comparison of the binding of nicotine and nornicotine stereoisomers to nicotinic binding sites in rat brain cortex. Naunyn-Schmiedeberg’s Arch Pharmacol. 1991;343:123–127. doi: 10.1007/BF00168598. [DOI] [PubMed] [Google Scholar]
- Crooks PA, Dwoskin LP. Contribution of CNS nicotine metabolites to the neuropharmacological effects of nicotine and tobacco smoking. Biochem Pharmacol. 1997;54:743–753. doi: 10.1016/s0006-2952(97)00117-2. [DOI] [PubMed] [Google Scholar]
- Crooks PA, Li M, Dwoskin LP. Metabolites of nicotine in rat brain after peripheral nicotine administration. Drug Metabolism Disposition. 1997;25:47–54. [PubMed] [Google Scholar]
- Curzon P, Nikkel AL, Bannon AW, Arneric SP, Decker MW. Differences between the antinociceptive effects of the cholinergic channel activators A-85380 and (±)-epibatidine in rats. J Pharmacol Exp Ther. 1998;287:847–853. [PubMed] [Google Scholar]
- Damaj MI, Fei-Yin M, Dukat M, Glassco W, Glennon RA, Martin BR. Antinociceptive responses to nicotinic acetylcholine receptor ligands after systemic and intrathecal administration in mice. J Pharmacol Exp Ther. 1998;284:1058–1065. [PubMed] [Google Scholar]
- Damaj MI, Fonek C, Marks MJ, Deshpande P, Labarca C, Lester HA, Collins AC, Martin BR. Genetic approaches identify differential roles for α4 β2 nicotinic receptors in acute models of antinociception in mice. J Pharmacol Exp Ther. 2007;321:1161–1169. doi: 10.1124/jpet.106.112649. [DOI] [PubMed] [Google Scholar]
- Damaj MI, Meyer EM, Martin BR. The antinociceptive effects of α7 nicotinic agonists in an acute pain model. Neuropaharmacology. 2000;39:2785–2791. doi: 10.1016/s0028-3908(00)00139-8. [DOI] [PubMed] [Google Scholar]
- Decker MW, Bannon AW, Buckley MJ, Kim DJ, Holladay MW, Ryther KB, Lin NH, Wasicak JT, Williams M, Arneric SP. Antinociceptive effects of the novel neuronal nicotinic acetylcholine receptor agonist, ABT-594, in mice. Eur J Pharmacol. 1998;346:23–33. doi: 10.1016/s0014-2999(98)00042-9. [DOI] [PubMed] [Google Scholar]
- Decker MW, Meyer MD. Therapeutic potential of neuronal nicotinic acetylcholine receptor agonists as novel analgesics. Biochem Pharmacol. 1999;58:917–923. doi: 10.1016/s0006-2952(99)00122-7. [DOI] [PubMed] [Google Scholar]
- Decker MA, Rueter LE, Bitner RS. Nicotinic acetylcholine receptor agonists: A potential new class of analgesics. Current Topics Med Chem. 2004;4:369–385. doi: 10.2174/1568026043451447. [DOI] [PubMed] [Google Scholar]
- Dixon WJ. Efficient analysis of experimental observation. Ann Rev Pharmacol Toxicol. 1980;20:441–461. doi: 10.1146/annurev.pa.20.040180.002301. [DOI] [PubMed] [Google Scholar]
- Donnelly-Roberts DI, Puttfarcken PS, Kuntzweiler TA, Briggs CA, Anderson DJ, Campbell JE, Piattoni-Kaplan M, McKenna DG, Wasicak JT, Holladay MW, Williams M, Arneric SP. ABT-594 [(R)-5(azetidinylmethoxy)-2-chloropyridine]: A novel, orally effective analgesic acting via neuronal nicotinic acetylcholine receptors: I. In vitro characterization. J Pharm Exp Therap. 1998;285:777–786. [PubMed] [Google Scholar]
- Dwoskin LP, Crooks PA, Teng LH, Green TA, Bardo MT. Acute and chronic effects of nornicotine on locomotor activity in rats: altered response to nicotine. Psychopharmacology. 1999;145:442–445. doi: 10.1007/s002130051079. [DOI] [PubMed] [Google Scholar]
- Dwoskin LP, Teng L, Crooks PA. Nornicotine, a nicotine metabolite and tobacco alkaloid: desensitization of nicotinic receptor-stimulated dopamine release from rat straitu. Eur J Pharmacol. 2001;428:69–79. doi: 10.1016/s0014-2999(01)01283-3. [DOI] [PubMed] [Google Scholar]
- Flood P, Daniel D. Intranasal nicotine for postoperative pain treatment. Anesthesiology. 2004;1001:1417–1421. doi: 10.1097/00000542-200412000-00023. [DOI] [PubMed] [Google Scholar]
- Flores CM. The promise and pitfalls of a nicotinic cholinergic approach to pain management. Pain. 2000;88:1–6. doi: 10.1016/S0304-3959(00)00389-4. [DOI] [PubMed] [Google Scholar]
- Ghosheh O, Dwoskin LP, Li WK, Crooks PA. Residence times and half-lives of nicotine metabolites in rat brain after acute peripheral administration of [2′-14C]Nicotine. Drug Metabolism Disposition. 1999;27:1448–1455. [PubMed] [Google Scholar]
- Ghosheh O, Dwoskin LP, Miller DK, Crooks PA. Accumulation of nicotine and its metabolites in rat brain after intermittent or continous peripheral administration of [2′-14C]nicotine. Drug Metabolism Disposition. 2001;29:645–651. [PubMed] [Google Scholar]
- Gilbert SD, Clark TM, Flores CM. Antihyperalgesic activity of epibatine in the formalin model of facial pain. Pain. 2001;89:159–165. doi: 10.1016/s0304-3959(00)00358-4. [DOI] [PubMed] [Google Scholar]
- Gilron I, Max MB. Combination pharmacotherapy for neuropathic pain: current evidence and future direction. Expert Rev Neurotherapeutics. 2005;5:823–830. doi: 10.1586/14737175.5.6.823. [DOI] [PubMed] [Google Scholar]
- Green TA, Phillips SB, Crooks PA, Dwoskin LP, Bardo MT. Nornicotine pretreatement decreases intravenous nicotine self-adminsitration in rats. Psychopharmacology. 2000;152:289–294. doi: 10.1007/s002130000524. [DOI] [PubMed] [Google Scholar]
- Green TA, Crooks PA, Bardo MT, Dwoskin LP. Contributory role for nornicotine in nicotine neuropaharmacology: nornicotine-evoked [3H]-dopamine overflow from the rat nucleus accumbens slices. Biochem Pharmacol. 2001;62:1597–16003. doi: 10.1016/s0006-2952(01)00838-3. [DOI] [PubMed] [Google Scholar]
- Habib AS, White WD, Gasim MA, Saleh G, Polascik TJ, Moul JW, Gan TJ. Transdermal nicotine for analgesia after radical retropubic prostatectomy. Anesth Analg. 2008;107:999–104. doi: 10.1213/ane.0b013e31816f2616. [DOI] [PubMed] [Google Scholar]
- Haghparast A, Khani A, Naderi N, Alizadeh AM, Motamedi F. Repeated administration of nicotine attenuates the development of morphine tolerance and dependence in mice. Pharmacol Biochem Behav. 2008;88:385–361. doi: 10.1016/j.pbb.2007.09.010. [DOI] [PubMed] [Google Scholar]
- Hargreaves K, Dubner K, Brown F, Flores C, Joris J. A new and sensitive method for measuring thermal antinociception in cutaneous hyperalgesia. Pain. 1988;32:77–88. doi: 10.1016/0304-3959(88)90026-7. [DOI] [PubMed] [Google Scholar]
- Holladay MW, Dart MJ, Lynch JK. Neuronal nicotinic acetylcholine receptors as targets for drug discovery. J Med Chem. 1997;40:4169–4194. doi: 10.1021/jm970377o. [DOI] [PubMed] [Google Scholar]
- Holtman JR, Jr, Crooks PA, Johnson-Hardy J, Wala EP. Interaction between morphine and norketamine enantiomers in rodent models of nociception. Pharmacol Biochem Behav. 2008;90:769–777. doi: 10.1016/j.pbb.2008.05.019. [DOI] [PubMed] [Google Scholar]
- Kalso E. Improving opioid effectiveness; from ideas to evidence. Eur J Pain. 2005;9:131–135. doi: 10.1016/j.ejpain.2004.05.007. [DOI] [PubMed] [Google Scholar]
- Kesingland AC, Gentry CT, Panesar MS, Bowes MA, Vernier JM, Cube R, Walker K, Urban L. Analgesic profile of the nicotinic acetylcholine receptor agonists, (+)-epibatidine and ABT-594 in models of persistent inflammatory and neuropathic pain. Pain. 2000;86:113–118. doi: 10.1016/s0304-3959(00)00233-5. [DOI] [PubMed] [Google Scholar]
- Kyerematen GA, Morgan M, Chattopadhyay B, deBrthizy JD, Vesell ES. Disposition of nicotine and eight metabolites in smokers and nonsmokers: identification in smokers of two metabolites that are longer lived than cotinine. Clin Pharmacol Ther. 1990;48:641–651. doi: 10.1038/clpt.1990.208. [DOI] [PubMed] [Google Scholar]
- Lippiello PM, Bencherif M, Hauser TA, Jordan KG, Letchworth SR, Mazurow AA. Nicotinic receptors as targets for therapeutic discovery. Expert Opin Drug Discov. 2007;2:1185–1203. doi: 10.1517/17460441.2.9.1185. [DOI] [PubMed] [Google Scholar]
- Lloyd GK, Williams M. Neuronal nicotinic acetylcholine receptors as novel drug targets. J Pharmacol Exp Ther. 2000;292:461–467. [PubMed] [Google Scholar]
- Martin BR, Tripathi HL, Aceto MD, May EL. Relationship of the biodisposition of the stereoisomers of nicotine in the central nervous system to their pharmacological actions. J Pharm Exp Ther. 1983;226:157–163. [PubMed] [Google Scholar]
- Meyer MD. Neuronal nicotinic acetylcholine receptors as a target for the treatment of neuropathic pain. Drug Dev Res. 2006;67:355–359. [Google Scholar]
- Meyer MD, Decker MW, Rueter LE, Anderson DJ, Dart MJ, Kim KH, Sullivan JP, Williams M. The identification of novel structural compounds classes exhibiting high affinity for neuronal nicotinic acetylcholine receptors and analgesic efficacy in preclinical models of pain. Eur J Pharmacol. 2000;393:171–177. doi: 10.1016/s0014-2999(00)00041-8. [DOI] [PubMed] [Google Scholar]
- Papke RL, Dwoskin LP, Crooks PA. The pharmacological activity of nicotine and nornicotine on nAChRs subtypes: relevance to nicotine dependence and drug discovery. J Neurochem. 2007;101:160–167. doi: 10.1111/j.1471-4159.2006.04355.x. [DOI] [PubMed] [Google Scholar]
- Randal L, Selitto J. A method for measurement of analgesic activity on inflamed tissue. Arch Int Pharmacodyn Ther. 1957;111:409–419. [PubMed] [Google Scholar]
- Rao TS, Correa LD, Reid RT, Lloyd GK. Evaluation of antinociceptive effects of neuronal nicotinic acetylcholine receptor (nAChR) ligands in the rat tail-flick assay. Neuropharmacology. 1996;35:393–405. doi: 10.1016/0028-3908(96)00013-5. [DOI] [PubMed] [Google Scholar]
- Risner ME, Cone EJ, Benowitz NL, Jacob P. Effects of the steroisomers on nicotine and nornicotine on schedule-controlled responding and physiological parameters of dogs. J Pharmacol Exp Ther. 1988;244:807–813. [PubMed] [Google Scholar]
- Sahley TL, Berntson GG. Antinociceptive effects of central and systemic administration of nicotine in the rat. Psychopharmacology. 1979;65:279–283. doi: 10.1007/BF00492216. [DOI] [PubMed] [Google Scholar]
- Stairs DJ, Neugebauer NM, Wei X, Boustany C, Hojahmat M, Cassis LA, Crooks PA, Dwoskin LP, Bardo MT. Effects of nornicotine enantiomers on intravenous S(−)-nicotine self-administration and cardiovascular function in rats. Psychopharmacology. 2007;90:145–155. doi: 10.1007/s00213-006-0610-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suh HW, Song DK, Lee KJ, Choi SR, Kim YH. Intrathecally injected nicotine enhances the antinociception induced by morphine but not β-endorphin, D-Pen2,5-enkephalin and U50,488H administered intrathecally in the mouse. Neuropeptides. 1996a;30:373–378. doi: 10.1016/s0143-4179(96)90027-x. [DOI] [PubMed] [Google Scholar]
- Suh HW, Song DK, Choi SR, Chung KM, Kim YH. Nicotine enhances morphine and β-endorphin-induced antinociception at the supraspinal level in the mouse. Neuropeptides. 1996b;30:479–484. doi: 10.1016/s0143-4179(96)90013-x. [DOI] [PubMed] [Google Scholar]
- Sullivan JP, Decker MW, Brioni JD, Donnelly-Roberts D, Anderson DJ, Bannon AW, Kang CH, Adams P, Piattoni-Kaplan M, Buckley MJ, Gopalakrishnan M, Williams M, Arneric SP. (±)- Epibatidine elicits a diversity of in vitro and in vivo effects mediated by nicotinic acetylcholine receptors. J Pharmacol Exp Ther. 1994;271:624–631. [PubMed] [Google Scholar]
- Tallarida RJ, Murray RB. Manual of pharmacological calculations with computer program. New York: Springler-Verlag; 1987. [Google Scholar]
- Teng LH, Buxton ST, Crooks PA, Dwoskin LP. Nicotinic receptor mediation of S (−)-nornicotine evoked [3H]overflow from rat striatal slices preloaded with [3H]dopamine. J Pharmacol Exp Ther. 1997;283:778–787. [PubMed] [Google Scholar]
- Tripathi HL, Martin BR, Aceto MD. Nicotine-induced antinociception in rats and mice: correlation with nicotine brain levels. J Pharmacol Exp Ther. 1982;221:91–96. [PubMed] [Google Scholar]
- Vincler M. Neuronal nicotinic receptors as targets for novel analgesics. Expert Opin Investig Drugs. 2005;14:1191–1198. doi: 10.1517/13543784.14.10.1191. [DOI] [PubMed] [Google Scholar]
- Vincler M, Eisenach JC. Plasticity of spinal nicotinic acetylcholine receptors following spinal nerve ligation. Neurosci Res. 2004;48:139–145. doi: 10.1016/j.neures.2003.10.007. [DOI] [PubMed] [Google Scholar]
- Vincler M, Wittenauer S, Parker R, Ellison M, Olivera BM, McIntosh JM. Molecular mechanism for analgesia involving specific antagonism of α9α10 nicotinic acetylcholine receptors. Proc Natl Acad Sci USA. 2006;103:17780–17784. doi: 10.1073/pnas.0608715103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Yu M, Ochani M, Amella CA, Tanovic M, Susaria S, Li JH, Wang H, Yang H, Ullea L, Al-Abed Y, Czura CJ, Tracey KJ. Nicotinic acetylcholine receptor α7 subunit is an essential regulator of inflammation. Nature. 2003;421:384–388. doi: 10.1038/nature01339. [DOI] [PubMed] [Google Scholar]
- Waldburger J-M, Boyle DL, Pavlov VA, Tracey KJ, Firestein GS. Acetylcholine regulation of synoviocyte cytokine expression by the α7 nicotinic receptors. Arthritis Rheumatism. 2008;58:3439–3449. doi: 10.1002/art.23987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheeler-Aceto H, Cowan A. Standardization of the rat paw formalin test for evaluation of analgesics. Psychopharmacology. 1991;104:33–44. doi: 10.1007/BF02244551. [DOI] [PubMed] [Google Scholar]
- Williams M, Kowaluk EA, Arneric SP. Emerging molecular approaches to pain therapy. J Med Chem. 1999;42:1481–1500. doi: 10.1021/jm9805034. [DOI] [PubMed] [Google Scholar]
- Yaksh TL, Rudy TA. Chronic catheterization of the spinal subarachnoid space. Physiol Behav. 1976;17:1031–1036. doi: 10.1016/0031-9384(76)90029-9. [DOI] [PubMed] [Google Scholar]
- Yang I, Zhang FH, Huang F, Lu YJ, Li GD, Bao L, Xiao HS, Zhang X. Peripheral nerve injury induces trans-synaptic modification of channels, nociceptors and signal pathways in rat dorsal spinal hor. Eur J Neurosci. 2004;19:871–883. doi: 10.1111/j.0953-816x.2004.03121.x. [DOI] [PubMed] [Google Scholar]
- Xiu R, Dwoskin LP, Grinevich VP, Deaciuc G, Crooks PA. neuronal nicotinic acetylcholine receptor binding affinities of boron-containing nicotinie analogs. Bioorg Med Chem Lett. 2001;11:1245–1248. doi: 10.1016/s0960-894x(01)00193-7. [DOI] [PubMed] [Google Scholar]
- Zarrindast MR, Nami AB, Farzin D. Nicotine potentiates morphine antinociception; a possible cholinergic mechanism. Eur Neuropsychopharmacol. 1996;6:127–133. doi: 10.1016/0924-977x(96)00002-8. [DOI] [PubMed] [Google Scholar]
- Zarrindast MR, Pazouki M, Nassir-Rad S. Involvement of cholinergic and opioid receptor mechanism in nicotine-induced antinociception. Pharmacol Toxicol. 1997;81:209–213. doi: 10.1111/j.1600-0773.1997.tb00048.x. [DOI] [PubMed] [Google Scholar]
- Zhang X, Gong ZH, fasth KJ, Langstrom B, Norberg A. Interaction of the nicotinic agonist (R,S)-3-pyridyl-1-methyl-2-(3-pyridyl)-azetidine (MPA) with nicotinic acetylcholine receptor subtypes expressed in cell lines and rat cortex. Neurochem International. 1998;32:435–441. doi: 10.1016/s0197-0186(97)00119-8. [DOI] [PubMed] [Google Scholar]
- Zhang X, Nordberg A. The competition of (−)[H3]nicotine binding by the enantiomers of nicotine, nornicotine and anatoxin-a in membranes and solubilized preparations of different brain regions of rat. Neunyn-Schmiedeberg’s Arch Pharmacol. 1993;348:28–34. doi: 10.1007/BF00168533. [DOI] [PubMed] [Google Scholar]