Abstract
Alzheimer’s disease (AD) is the most prevalent form of neurodegeneration; however, therapies to prevent or treat AD are inadequate. Amyloid-beta (Aβ) protein accrues in cortical senile plaques, one of the key neuropathological hallmarks of AD, and is elevated in brains of early onset AD patients in a small number of families that bear certain genetic mutations, further implicating its role in this devastating neurological disease. In addition, soluble Aβ oligomers have been shown to be detrimental to neuronal function. Therapeutic strategies aimed at lowering cerebral Aβ levels are currently under development. One strategy is to immunize AD patients with Aβ peptides so that they will generate antibodies that bind to Aβ protein and enhance its clearance. As of 1999, Aβ immunotherapy, either through active immunization with Aβ peptides or through passive transfer of Aβ-specific antibodies, has been shown to reduce cerebral Aβ levels and improve cognitive deficits in AD mouse models and lower plaque load in nonhuman primates. However, a Phase II clinical trial of active immunization using full-length human Aβ1-42 peptide and a strong Th1-biased adjuvant, QS-21, ended prematurely in 2002 because of the onset of meningoencephalitis in ~6% of the AD patients enrolled in the study. It is possible that T cell recognition of the human full-length Aβ peptide as a self-protein may have induced an adverse autoimmune response in these patients. Although only ~20% of immunized patients generated anti-Aβ titers, responders showed some general slowing of cognitive decline. Focal cortical regions devoid of Aβ plaques were observed in brain tissues of several immunized patients who have since come to autopsy. In order to avoid a deleterious immune response, passive Aβ immunotherapy is under investigation by administering monthly intravenous injections of humanized Aβ monoclonal antibodies to AD patients. However, a safe and effective active Aβ vaccine would be more cost-effective and more readily available to a larger AD population. We have developed several novel short Aβ immunogens that target the Aβ N-terminus containing a strong B cell epitope while avoiding the Aβ mid-region and C-terminus containing T cell epitopes. These immunogens include dendrimeric Aβ1-15 (16 copies of Aβ1-15 on a lysine antigen tree), 2xAβ1-15 (a tandem repeat of two lysine-linked Aβ1-15 peptides), and 2xAβ1-15 with the addition of a three amino acid RGD motif (R-2xAβ1-15). Intranasal immunization with our short Aβ fragment immunogens and a mucosal adjuvant, mutant Escherichia coli heat-labile enterotoxin LT(R192G), resulted in reduced cerebral Aβ levels, plaque deposition, and gliosis, as well as increased plasma Aβ levels and improved cognition in a transgenic mouse model of AD. Preclinical trials in nonhuman primates, and human clinical trials using similar Aβ immunogens, are now underway. Aβ immunotherapy looks promising but must be made safer and more effective at generating antibody titers in the elderly. It is hoped that these novel immunogens will enhance Aβ antibody generation across a broad population and avoid the adverse events seen in the earlier clinical trial.
Keywords: amyloid-beta, vaccine, Alzheimer’s disease, immunotherapy, T cells, B cells, adjuvant, nonhuman primates, transgenic mice
Introduction
Alzheimer’s disease (AD) is the most common form of dementia, affecting ~26 million people worldwide. Currently, there are no effective treatments and no known means to prevent this devastating neurological disease. While the major clinical symptoms include progressive memory loss, personality changes, language problems, and confusion, it is believed that the onset of two major pathological hallmarks of AD, extracellular amyloid-beta (Aβ) plaques, and intracellular neurofibrillary tangles containing hyperphosphorylated tau, precedes clinical symptoms by years. In addition, to plaques and tangles, AD brain is characterized by gliosis, inflammation, neuritic dystrophy, neuron loss, and changes in neurotransmitter levels (Dickson, 1997; Hardy and Selkoe, 2002). Aβ protein, a 40- to 42-amino acid protein, is generated by proteolytic cleavage from the beta-amyloid precursor protein (β APP) by beta-secretase at its amino-terminus and by gamma-secretase at its carboxyterminus (Wolfe, 2006). Aβ is now a major therapeutic target for AD because genetic mutations in APP and pre-senilin proteins 1 and 2 (PS1 and PS2), part of the gamma-secretase complex, are associated with AD in a small number of families; Aβ is deposited early in plaques and blood vessels in the brain; and Aβ oligomers and fibrillar aggregates are toxic to neurons (Selkoe, 2001; Klein, 2002; Walsh and Selkoe, 2004). Current therapeutic strategies aim to lower production of Aβ by inhibiting or modulating beta- or gamma-secretase, prevent formation of Aβ aggregates and/or dissolve preformed aggregates, and enhance clearance of Aβ from the brain. Aβ immunotherapy, one of the strategies under intense investigation, uses anti-Aβ antibodies by either active or passive vaccination to reduce Aβ deposition in the brain and enhance Aβ clearance.
Aβ immunotherapy in rodents, monkeys, and humans
Preclinical studies in rodents
In the mid-1990s, anti-Aβ antibodies were shown to dissolve Aβ aggregates and prevent monomers from aggregating in vitro (Solomon et al., 1996, 1997). In 1999, it was demonstrated for the first time that active vaccination with Aβ1-42 synthetic peptide resulted in anti-Aβ antibody generation and a significant reduction in plaque burden in the brains of APP transgenic mice in vivo (Schenk et al., 1999). Subsequently, we and many other groups confirmed and extended these studies in multiple transgenic AD mouse models using a variety of adjuvants and routes of administration, demonstrating that active Aβ immunization prevented or reduced plaque deposition (if given early enough) (Lemere et al., 2000; Weiner et al., 2000; Das et al., 2001; Sigurdsson et al., 2001; McLaurin et al., 2002), increased peripheral Aβ in blood (Lemere et al., 2003), and prevented or improved cognitive deficits (Janus et al., 2000; Morgan et al., 2000). The resulting antibodies recognized B cell epitopes within the first 15 amino acids of Aβ (Lemere et al., 2000, 2001; Town et al., 2001; McLaurin et al., 2002; Cribbs et al., 2003; Seabrook et al., 2004). T cell epitopes were mapped to the Aβ mid-region and carboxy-terminus (Monsonego et al., 2001; Cribbs et al., 2003).
Passive immunization was investigated by directly injecting Aβ monoclonal antibodies into AD transgenic mice, thereby bypassing a cellular immune response. Intraperitoneal injections of Aβ monoclonal antibodies decreased cerebral Aβ levels (Bard et al., 2000), increased peripheral Aβ levels in blood (DeMattos et al., 2001), and improved behavior (Kotilinek et al., 2002; Wilcock et al., 2004). In very old APP transgenic mice, passive Aβ vaccination improved cognitive performance within days, without reducing plaque burden, suggesting that removal of soluble oligomers may be enough to rescue cognition (Dodart et al., 2002). Direct application of Aβ monoclonal antibodies demonstrated plaque clearance and enhancement of microglial phagocytosis of Aβ using multiphoton imaging (Bacskai et al., 2001). Intrahippocampal injection of an Aβ monoclonal antibody into 3xTg-AD mice that develop both plaques and tangles cleared extracellular and intracellular Aβ as well as early tau aggregates but not hyperphosphorylated tau, a late-stage pathogenic marker (Oddo et al., 2004). Microhemorrhage was observed in very old APP transgenic mice with abundant vascular amyloid, although the mice still showed some cognitive benefit from the vaccine (Pfeifer et al., 2002; Wilcock et al., 2004; Racke et al., 2005). In addition, both active and passive Aβ vaccination was shown to protect against Aβ oligomer-induced long-term-potentiation (LTP) deficits in rats (Klyubin et al., 2005).
Preclinical studies in nonhuman primates
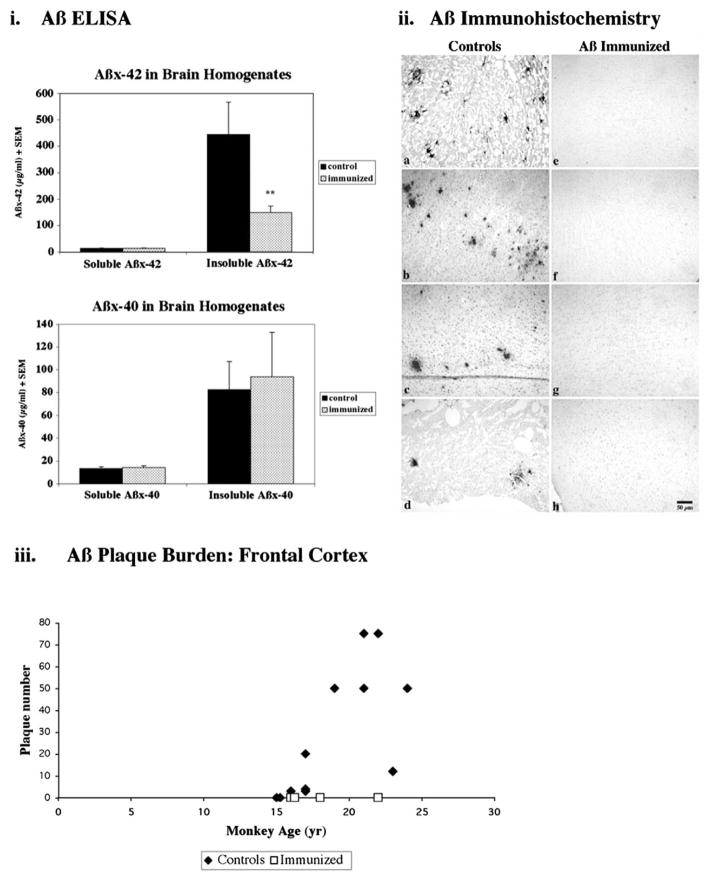
Small Aβ immunization studies have been undertaken in nonhuman primate monkeys. For example, Gandy et al. (2004) (including our lab) immunized two 15-year-old rhesus monkeys with full-length Aβ peptide and adjuvant, resulting in anti-Aβ antibody generation and increased Aβ levels in blood. However, cerebral Aβ levels were unchanged, possibly because the animals were too young for plaque deposition in the brain and/or the trial was too short to show an effect. In another trial, we immunized four Caribbean vervets (African green monkeys from St. Kitts in the Eastern Caribbean) 16–25 years of age with full-length Aβ and complete and incomplete Freund’s adjuvant (Lemere et al., 2004). Vervets naturally develop cerebral Aβ plaque pathology and vascular amyloid with aging, similar to other nonhuman primates. The immunized vervets generated anti-Aβ antibodies that recognized Aβ1-7 and bound monomeric, oligomeric, and fibrillar Aβ but not APP or APP C-terminal fragments. Plaque deposition was absent while insoluble Aβ42 levels were significantly reduced in the brains of the 4 immunized vervets compared to 13 age-matched archived brain samples, as shown in Fig. 1. Plasma Aβ was elevated in the immunized animals. No adverse events were observed.
Fig. 1.
Cerebral Aβ levels. (i) Aβ ELISA was used to detect differences in soluble and insoluble Aβ levels in brain homogenates. No differences were observed between immunized (dotted) and control (solid) vervet soluble Aβx-42 or Aβx-40 cerebral levels. However, insoluble Aβx-42 was reduced 66% ( p < 0.035, 2-tailed Alternate Welch’s T test) in the 4 immunized vervets compared to 13 aged age-matched controls. Insoluble Aβx-40 was much less abundant and was no significantly different between the two treatment groups. (ii) Aβ42 immunohistochemistry using Mab 21F12 on paraffin frontal sections revealed plaque labeling in 11 of 13 age-matched control vervets (a, 22 years; b, 21 years; c, 23 years; d, 17 years). Aβ IR plaques were not detected in the frontal cortex of any of the four immunized vervets (e, 22 years; f, 18 years; g, 16 years; h, 16 years); five additional cortical regions per immunized vervet were also devoid of plaque labeling. Scale bar, 50 microns. (iii) Aβ deposition into cerebral plaques was quantified by visually counting the number of Aβ42 (Mab 21F12-immunoreactive) plaques occupying four 4x fields (~2.4 × 3 mm) in the frontal cortex from each of the 4 immunized (open squares) and 13 control (solid diamonds) vervets. Although the two youngest controls (15 years each) did not show any Aβ plaque labeling, all of the older animals (aged 16–24 years) showed some plaque labeling. Adapted from Lemere et al. (2004) with permission from the American Society for Investigative Pathology.
Clinical Aβ immunotherapy trials in humans
The first clinical trial of Aβ immunotherapy, sponsored by ELAN/Wyeth, involved an active vaccine, AN1792, that contained Aβ1-42 synthetic peptide and a strong, T helper 1 (Th1)-biased adjuvant, QS-21. Although the Phase I and IIa trials were deemed safe, the multisite Phase IIb trial was halted in 2002 due to the occurrence of meningo-encephalitis in ~6% (18/300) of AD patients worldwide (Schenk, 2002). The cause of these adverse events is unknown, but many have speculated that they may have been due to an autoimmune-like T cell response to Aβ, a self-antigen (Orgogozo et al., 2003; Gilman et al., 2005). Other possibilities include reformulation of the vaccine in polysorbate 80 and a strong pro-inflammatory Th1 response due to the adjuvant, QS-21. Only 19% of the immunized patients generated an Aβ antibody response, perhaps due to the limited dosing (1–3 inoculations). Importantly, the occurrence of meningoencephalitis was independent of antibody response. The Aβ antibodies generated by AN1792 were shown to bind to the amino-terminus of Aβ (Lee et al., 2005) and to human Aβ plaques and vascular amyloid but not soluble Aβ42 (Hock et al., 2002). Tau levels in CSF were lower in antibody responders (Gilman et al., 2005). Transient cortical shrinkage was observed by MRI in these same patients, although brain volumes resumed baseline levels after ~1 year following vaccination (Fox et al., 2005). In most of the patients who have come to autopsy since the initiation of the trial, plaque deposition was focally and regionally reduced (Nicoll et al., 2003, 2006; Ferrer et al., 2004; Masliah et al., 2005; Holmes et al., 2008). Some slowing of cognitive decline has been observed in antibody responders (Gilman et al., 2005; Hock et al., 2003; International Conference on Alzheimer’s Disease, Chicago, IL, 2008). However, two patients from an earlier Phase I AN1792 trial who came to autopsy several years later, generated Aβ antibodies, had very few plaques (suggestive of plaque clearance, and yet had severe dementia (Holmes et al., 2008). A possible explanation for this finding may be that at the time of vaccination, the patients may have had substantial cerebral pathology, including neuron loss and neuritic plaques, which could not be reversed by the removal of Aβ plaques. Thus, the need for early intervention, possibly before the onset of symptoms, is critical in moving forward with Aβ-lowering treatments.
Several passive Aβ immunization trials are currently underway. The ELAN/Wyeth Phase II clinical trial results were reported at the 11th International Conference on Alzheimer’s Disease (ICAD) in Chicago in July 2008. Intravenous administration of a humanized monoclonal antibody, Bapineuzimab, recognizing the amino-terminus of Aβ showed a nonsignificant trend for cognitive stabilization in mild-to-moderate AD patients. Post hoc analysis demonstrated significant cognitive benefits in multiple tests in Apo E4 noncarriers but only a trend in Apo E4 carriers, possibly due to accelerated pathogenesis in E4 carriers (ICAD, 2008). A Phase III trial is currently underway. Eli Lilly is currently testing a humanized monoclonal antibody that recognizes the mid-region of Aβ, and binds soluble Aβ protein.
Possible mechanisms of Aβ clearance via immunotherapy
Several mechanisms have been proposed based on in vitro and in vivo studies. First, Aβ antibodies may prevent Aβ aggregation and/or dissolve preformed Aβ aggregates (Solomon et al., 1996, 1997). Second, upon binding of the Aβ antibodies to Aβ, the Fc portion of the antibodies may bind the Fc receptor on microglia, inducing phagocytosis of Aβ (Bard et al., 2000). This would require that Aβ antibodies cross the blood-brain barrier (BBB) and bind Aβ within the central nervous system (CNS). While some evidence has been reported to support this mechanism, two studies have demonstrated that Fc-mediated phagocytosis is not required for immunotherapy-induced Aβ clearance, as Fab fragments of Aβ antibodies (missing the Fc region) cleared Aβ when applied to the surface of APP transgenic mouse brain (Bacskai et al., 2002), and Aβ vaccination in APP transgenic mice lacking the FcR lowered cerebral Aβ (Das et al., 2003). A third mechanism proposes that the presence of Aβ antibodies in the periphery (i.e., blood) causes a shift in the gradient of Aβ transport across the BBB resulting in an increase in efflux from the brain to blood (DeMattos et al., 2001; Lemere et al., 2003). A fourth mechanism proposes that certain antibodies bind Aβ oligomers, thereby neutralizing the toxic effects of this Aβ species on synapses (Klyubin et al., 2005). These mechanisms are not mutually exclusive and may overlap under certain conditions. In addition, the mechanism of action may be disease state dependent. For example, a prevention vaccine may not require that the antibodies cross the BBB to induce Aβ phagocytosis, whereas a therapeutic vaccine (once plaque deposition is well underway) would likely benefit from the transport of Aβ antibodies into the CNS.
Novel short Aβ immunogens for active vaccination
Although the first clinical trial for an active Aβ vaccine ended prematurely due to adverse events, preclinical and, to some degree, clinical studies indicate potential for this therapeutic strategy to prevent AD or stop it in early stages. A modified active vaccine would be less costly to prepare than humanized monoclonal antibodies, would require fewer doctor visits, and would be more feasible than passive immunization for the large population of individuals with or at risk for AD. The effects of an active vaccine would be longer lasting than passive administration of anti-Aβ antibodies whose half-life is typically around 30 days. However, the cellular immune response to an active vaccine may be difficult to stop, should problems arise, once it is underway. Thus, going forward, many labs, including our own, have sought to develop second-generation active Aβ vaccines that target the B cell epitope in the Aβ amino-terminus and avoid an Aβ-specific T cell response directed at the mid-region or carboxyl-terminus of Aβ. Examples of three such vaccines being tested in our lab are described below. A more complete listing of novel immunogens under investigation for active Aβ immunization may be found in our recent review article (Lemere et al., 2007).
In an attempt to design a vaccine that would generate strong Aβ titers while avoiding an adverse T cell-mediated response, we first constructed a dendrimeric immunogen, dAβ1-15, consisting of 16 copies of Aβ1-15 peptide on a branched lysine tree (Seabrook et al., 2006). Weekly intranasal vaccination with dAβ1-15 with a mucosal adjuvant, mutant Escherichia coli heat-labile enterotoxin LT(R192G) (Dickinson and Clements, 1995), in J20 hAPPFAD transgenic mice (Mucke et al., 2000) 6–12 months of age resulted in a robust, predominantly Th2-biased (IgG2b) anti-Aβ antibody response and a significant reduction in cerebral plaque burden. Splenocytes from the immunized mice recognized and proliferated upon incubation with dAβ1-15 but only minimally upon incubation with full-length Aβ, providing evidence that this immunogen avoided an Aβ-specific cellular immune response.
Next, we tested two novel short Aβ immunogens by synthesizing two linear tandem-repeat copies of Aβ1-15 linked by two lysine residues, with or without an RGD motif at the amino-terminus of the peptide (Maier et al., 2006). RGD has been shown previously to immunogenicity of other antigens, such as Streptococcus mutans epitope (Yano et al., 2003), and may act as an adjuvant. In our study, intranasal immunization of wild-type mice (B6D2F1) with 2xAβ1-15 and R-2xAβ1-15 (with the RDG motif) and adjuvant LT(R192G) led to a strong humoral response, that is, high levels of anti-Aβ antibodies, primarily of Th2-biased IgG1 and IgG2b antibodies. Splenocytes from the immunized animals recognized and proliferated upon incubation with 2xAβ1-15 or R-2xAβ1-15 but not with full-length Aβ, suggesting that these vaccines avoided an Aβ-specific cellular immune response. The addition of the RGD motif did not provide sufficient adjuvant activity to induce a strong antibody response.
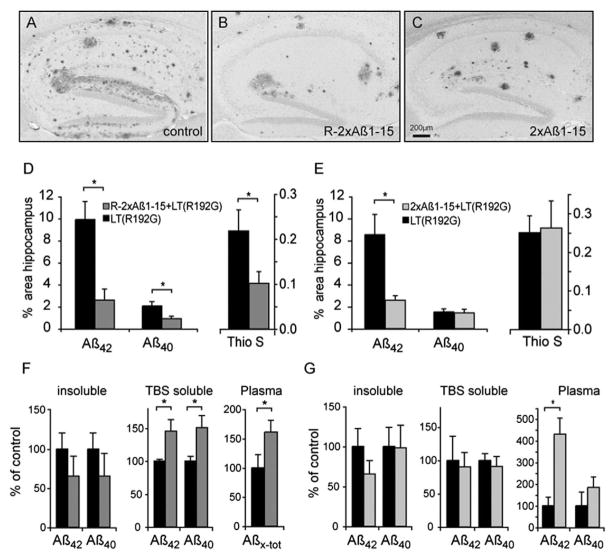
Each vaccine was tested in J20 hAPPFAD transgenic mice at an age when the mice first begin to show cerebral Aβ deposition. Weekly intranasal vaccination with R-2xAβ1-15 and adjuvant LT(R192G) in mice 6–12 months of age resulted in strong Aβ antibody production in the absence of an Aβ-specific cellular immune response. Aβ42- and Aβ40-immunoreactive plaques and thioflavin S fibrillar amyloid deposits were significantly reduced in the hippocampi of immunized mice (Fig. 2A–D), indicating an effect on both diffuse and compacted plaques. Insoluble Aβ levels were nonsignificantly reduced while soluble Aβ40 and Aβ42 were increased in brain homogenates, and Aβ levels were elevated in plasma (Fig. 2F). Weekly immunization with 2xAβ1-15 of J20 transgenic mice 4.5–12 months of age also resulted in high anti-Aβ titers but preferentially cleared Aβ42-immunoreactive diffuse plaques and not Aβ40-immunoreactive or compacted amyloid plaques (Fig. 2A–C and E). While there was a trend for reduced insoluble Aβ42, soluble Aβ levels were unchanged and plasma Aβ42 was elevated (Fig. 2G).
Fig. 2.
Neuropathological and biochemical analysis of R-2xAβ1-15 (B, D) or 2x-Aβ1-15 (C, E) immunized animals compared to their corresponding group of adjuvant-treated control hAPPFAD animals (A, D, E). (A–C) Sections representing the median Aβ plaque load are shown for each group (A, B, C). (D and E) Quantitative image analysis of Aβ42- and Aβ40-specific immunoreactive and thioflavin S-positive plaque load. Aβ42-, Aβ40-, and thioflavin S-positive areas were significantly reduced in the hippocampus after immunization with R-2xAβ1-15+LT(R192G) (D, p < 0.05, MWU). 2xAβ1-15+LT(R192G)-immunized mice showed a significant reduction of Aβ42-specific immunoreactivity (E, p < 0.05, MWU) compared to vehicle-treated controls. (F and G) Insoluble (guanidinium-soluble) brain Aβ, TBS-soluble brain Aβ, and plasma Aβ levels were analyzed by capture ELISA [absolute values of controls in R-2xAβ1-15 immunization experiment (F) were plasma Aβx–tot 0.03±0.01 pmol/ml, Aβ42 insoluble 2052±417 pmol/g, Aβ40 insoluble 485±100 pmol/g, Aβ42 TBS soluble 1.6±0.1 pmol/g, and Aβ40 TBS soluble 0.3±0.1 pmol/g; and in the 2xAβ1-15 immunization experiment (G, measured in a different ELISA run), Aβ40 plasma 0.9±0.6 pmol/ml, Aβ42 plasma 0.3±0.1 pmol/ml, Aβ42 insoluble 3590±800 pmol/g, Aβ40 insoluble 132±32.5 pmol/g, Aβ42 TBS soluble 2.1±0.8 pmol/g, and Aβ40 TBS soluble 4.6±0.5 pmol/g]. Asterisk indicates a significant difference (MWU, p < 0.05). Adapted from Maier et al. (2006) with permission from the Society for Neuroscience.
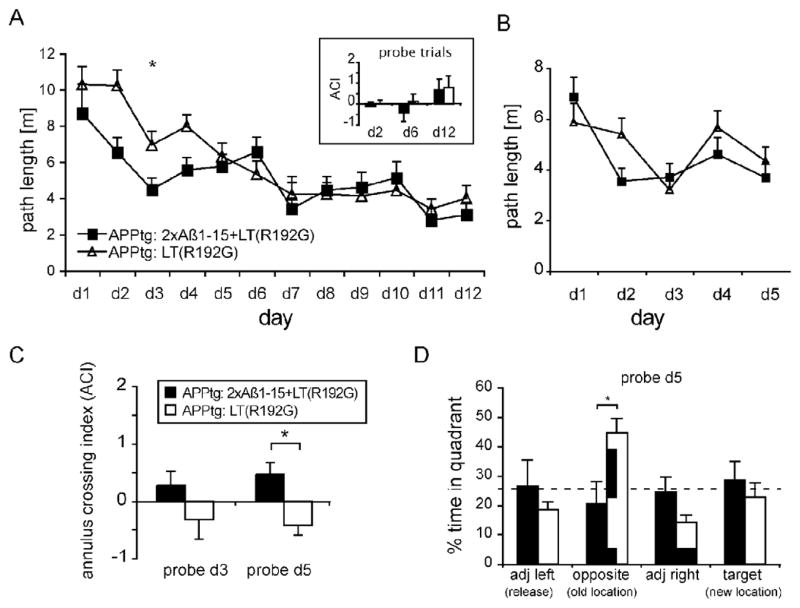
J20 mice immunized with 2xAβ-15 were subjected to cognitive evaluation using a reference memory test, the Morris water maze (MWM). After training, immunized mice were able to find the platform in the pool more efficiently than their vehicle control counterparts, indicating faster learning acquisition (Fig. 3A), and had better spatial memory retention of the platform location when the platform was hidden (Fig. 3C and D). In general, the mice with the highest anti-Aβ titers performed the best in the MWM tasks.
Fig. 3.
The effect of immunization with 2xAβ1-15+LT(R192G) was assessed in a reference memory version of the Morris water maze (MWM). (A) 2xAβ1-15+LT(R192G)-immunized hAPPFAD mice (n = 6) showed significantly faster learning acquisition during the first four training sessions of a 12-day test as compared to adjuvant-only-treated control hAPPFAD animals (n = 7; p < 0.05, day 1 to day 5). Both cohorts of mice showed comparable spatial memory as evaluated by the annulus crossing index (ACI, defined as average frequency of swims over the platform site in the target quadrant minus average of swims over sites in other quadrants of the pool) at the end of training (inset, legend see C). (B) During the learning reversal task, 2xAβ1-15+LT(R192G)-immunized hAPPFAD mice showed a trend of faster initial acquisition of the new platform location as compared to control hAPPFAD mice during the first three training sessions (p = 0.09, day 1 to day 3). During the reversal stage (day 4 and 5), both cohorts of mice showed a comparable response to platform displacement. (C) ACI during probe trials administered 1 h after training on day 3 and day 5 in the platform reversal task. 2xAβ1-15+LT(R192G)-immunized mice (black bars) show a positive ACI for the platform location on day 3 or day 5, whereas control hAPPFAD mice (gray bars) show a significantly lower and negative ACI. (D) Quadrant dwell time of probe trial on day 5 indicates that control hAPPFAD mice (gray bars) persevered with their search in the original, previous location of the pool. Asterisks indicate significant results ( p < 0.05). Adapted from Maier et al. (2006) with permission from the Society for Neuroscience.
Conclusions
Aβ immunotherapy has been shown to lower cerebral Aβ levels, especially if given prior to or in the early stages of pathology, in AD-like transgenic mouse models, nonhuman primates, and human AD patients. Thus far, human clinical trials with active Aβ immunization have shown limited efficacy and resulted in adverse effects in 6% of immunized patients (AN1792 trial), possibly due to T cell-mediated inflammation in the brain. Passive immunotherapy trials involving the direct administration of humanized monoclonal antibodies should avoid T cell-mediated side effects but are costly and less feasible for a very large population. Several human trials are underway currently. Active Aβ immunotherapy remains under investigation, as it would be less costly and would provide a long-lasting immune response, thereby reducing travel to the doctor’s office. Many active Aβ vaccines now target the amino-terminus of Aβ to generate a strong humoral response and avoid an Aβ-specific T cell response, thought to account for the adverse effects in the AN1792 trial. As more and more preclinical and clinical data are collected, it appears that Aβ immunotherapy, like other Aβ-lowering therapies, may have its best efficacy if given before or in the early stages of cognitive decline, prior to massive neuritic dystrophy and neuronal loss. Thus, identifying those at risk and in the earliest stages of the disease, through genetics, biomarkers, and/or imaging, is crucial to early intervention.
Acknowledgments
This work was supported in part by NIH/NIA RO1 AG20159 (C.A.L.), and the generosity of the Brunozzi family.
References
- Bacskai B, Kajdasz S, McLellan M, Games D, Seubert P, Schenk D, et al. Non-Fc-mediated mechanisms are involved in clearance of amyloid-β in vivo by immunotherapy. The Journal of Neuroscience. 2002;22:7873–7878. doi: 10.1523/JNEUROSCI.22-18-07873.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bacskai BJ, Kajdasz ST, Christie RH, Carter C, Games D, Seubert P, et al. Imaging of amyloid-β deposits in brains of living mice permits direct observation of clearance of plaques with immunotherapy. Nature Medicine. 2001;7:369–372. doi: 10.1038/85525. [DOI] [PubMed] [Google Scholar]
- Bard F, Cannon C, Barbour R, Burke RL, Games D, Grajeda H, et al. Peripherally administered antibodies against amyloid beta-peptide enter the central nervous system and reduce pathology in a mouse model of Alzheimer disease. Nature Medicine. 2000;6:916–919. doi: 10.1038/78682. [DOI] [PubMed] [Google Scholar]
- Cribbs DH, Ghochikyan A, Vasilevko V, Tran M, Petrushina I, Sadzikava N, et al. Adjuvant-dependent modulation of Th1 and Th2 responses to immunization with beta-amyloid. International Immunology. 2003;15:505–514. doi: 10.1093/intimm/dxg049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das P, Howard V, Loosbrock N, Dickson D, Murphy MP, Golde TE. Amyloid-beta immunization effectively reduces amyloid deposition in FcRgamma−/− knock-out mice. The Journal of Neuroscience. 2003;23:8532–8538. doi: 10.1523/JNEUROSCI.23-24-08532.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das P, Murphy M, Younkin L, Younkin S, Golde T. Reduced effectiveness of Aβ1-42 immunization in APP transgenic mice with significant amyloid deposition. Neurobiology of Aging. 2001;22:721–727. doi: 10.1016/s0197-4580(01)00245-7. [DOI] [PubMed] [Google Scholar]
- DeMattos R, Bales K, Cummins D, Dodart JC, Paul S, Holtzman D. Peripheral anti-Aβ antibody alters CNS and plasma clearance and decreases brain Aβ burden in a mouse model of Alzheimer’s disease. Proceedings of the National Academy of Sciences of the United States of America. 2001;98:8850–8855. doi: 10.1073/pnas.151261398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickinson BL, Clements JD. Dissociation of Escherichia coli heat-labile enterotoxin adjuvanticity from ADP-ribosyltransferase activity. Infection and Immunity. 1995;63:1617–1623. doi: 10.1128/iai.63.5.1617-1623.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickson DW. The pathogenesis of senile plaques. Journal of Neuropathology and Experimental Neurology. 1997;56:321–339. doi: 10.1097/00005072-199704000-00001. [DOI] [PubMed] [Google Scholar]
- Dodart JC, Bales K, Gannon K, Greene S, DeMattos R, Mathis C, et al. Immunization reverses memory deficits without reducing brain Aβ burden in Alzheimer’s disease model. Nature Neuroscience. 2002;5:452–457. doi: 10.1038/nn842. [DOI] [PubMed] [Google Scholar]
- Ferrer I, Boada Rovira M, Sanchez Guerra ML, Rey MJ, Costa-Jussa F. Neuropathology and pathogenesis of encephalitis following amyloid-beta immunization in Alzheimer’s disease. Brain Pathology. 2004;14:11–20. doi: 10.1111/j.1750-3639.2004.tb00493.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox NC, Black RS, Gilman S, Rossor MN, Griffith SG, Jenkins L, et al. Effects of A{beta} immunization (AN1792) on MRI measures of cerebral volume in Alzheimer disease. Neurology. 2005;64:1563–1572. doi: 10.1212/01.WNL.0000159743.08996.99. [DOI] [PubMed] [Google Scholar]
- Gandy S, DeMattos RB, Lemere CA, Heppner FL, Leverone J, Aguzzi A, et al. Alzheimer’s Abeta vaccination of rhesus monkeys (Macaca mulatta) Mechanisms of Ageing and Development. 2004;125:149–151. doi: 10.1016/j.mad.2003.12.002. [DOI] [PubMed] [Google Scholar]
- Gilman S, Koller M, Black RS, Jenkins L, Griffith SG, Fox NC, et al. Clinical effects of A{beta} immunization (AN1792) in patients with AD in an interrupted trial. Neurology. 2005;64:1553–1562. doi: 10.1212/01.WNL.0000159740.16984.3C. [DOI] [PubMed] [Google Scholar]
- Hardy J, Selkoe DJ. The amyloid hypothesis of Alzheimer’s disease: progress and problems on the road to therapeutics. Science. 2002;297:353–356. doi: 10.1126/science.1072994. [DOI] [PubMed] [Google Scholar]
- Hock C, Konietzko U, Papassotiropoulos A, Wollmer A, Streffer J, von Rotz RC, et al. Generation of antibodies for beta-amyloid by vaccination of patients with Alzheimer disease. Nature Medicine. 2002;8:1270–1275. doi: 10.1038/nm783. [DOI] [PubMed] [Google Scholar]
- Hock C, Konietzko U, Streffer JR, Tracy J, Signorell A, Muller-Tillmanns B, et al. Antibodies against beta-amyloid slow cognitive decline in Alzheimer’s disease. Neuron. 2003;38:547–554. doi: 10.1016/s0896-6273(03)00294-0. [DOI] [PubMed] [Google Scholar]
- Holmes C, Boche D, Wilkinson D, Yadegarfar G, Hopkins V, Bayer A, et al. Long-term effects of Aβ 42 immunisation in Alzheimer’s disease: follow-up of a randomised, placebo-controlled phase I trial. Lancet. 2008;372:216–223. doi: 10.1016/S0140-6736(08)61075-2. [DOI] [PubMed] [Google Scholar]
- Janus C, Pearson J, McLaurin J, Mathews PM, Jiang Y, Schmidt SD, et al. A beta peptide immunization reduces behavioural impairment and plaques in a model of Alzheimer’s disease. Nature. 2000;408:979–982. doi: 10.1038/35050110. [DOI] [PubMed] [Google Scholar]
- Klein W. Abeta toxicity in Alzheimer’s disease: globular oligomers (ADDLs) as new vaccine and drug targets. Neurochemistry International. 2002;41:345–352. doi: 10.1016/s0197-0186(02)00050-5. [DOI] [PubMed] [Google Scholar]
- Klyubin I, Walsh DM, Lemere CA, Cullen WK, Shankar GM, Betts V, et al. Amyloid beta protein immunotherapy neutralizes Abeta oligomers that disrupt synaptic plasticity in vivo. Nature Medicine. 2005;11:556–561. doi: 10.1038/nm1234. [DOI] [PubMed] [Google Scholar]
- Kotilinek LA, Bacskai B, Westerman M, Kawarabayashi T, Younkin L, Hyman BT, et al. Reversible memory loss in a transgenic model of Alzheimer’s disease. The Journal of Neuroscience. 2002;22:6331–6335. doi: 10.1523/JNEUROSCI.22-15-06331.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee M, Bard F, Johnson-Wood K, Lee C, Hu K, Griffith SG, et al. Abeta42 immunization in Alzheimer’s disease generates Abeta N-terminal antibodies. Annals of Neurology. 2005;58:430–435. doi: 10.1002/ana.20592. [DOI] [PubMed] [Google Scholar]
- Lemere CA, Beierschmitt A, Iglesias M, Spooner ET, Bloom JK, Leverone JF, et al. Alzheimer’s disease abeta vaccine reduces central nervous system abeta levels in a non-human primate, the Caribbean vervet. The American Journal of Pathology. 2004;165:283–297. doi: 10.1016/s0002-9440(10)63296-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemere CA, Maier M, Peng Y, Jiang L, Seabrook TJ. Novel Aβ immunogens: is shorter better? Current Alzheimer Research. 2007;4:427–436. doi: 10.2174/156720507781788800. [DOI] [PubMed] [Google Scholar]
- Lemere CA, Maron R, Selkoe DJ, Weiner HL. Nasal vaccination with beta-amyloid peptide for the treatment of Alzheimer’s disease. DNA and Cell Biology. 2001;20:705–711. doi: 10.1089/10445490152717569. [DOI] [PubMed] [Google Scholar]
- Lemere CA, Maron R, Spooner ET, Grenfell TJ, Mori C, Desai R, et al. Nasal A beta treatment induces anti-A beta antibody production and decreases cerebral amyloid burden in PD-APP mice. Annals of the New York Academy of Sciences. 2000;920:328–331. doi: 10.1111/j.1749-6632.2000.tb06943.x. [DOI] [PubMed] [Google Scholar]
- Lemere CA, Spooner ET, LaFrancois J, Malester B, Mori C, Leverone JF, et al. Evidence for peripheral clearance of cerebral Abeta protein following chronic, active Abeta immunization in PSAPP mice. Neurobiology of Disease. 2003;14:10–18. doi: 10.1016/s0969-9961(03)00044-5. [DOI] [PubMed] [Google Scholar]
- Maier M, Seabrook TJ, Lazo ND, Jiang L, Das P, Janus C, et al. Short amyloid-β (Aβ) immunogens reduce cerebral Aβ load and learning deficits in an Alzheimer’s disease mouse model in the absence of an Aβ-specific cellular immune response. The Journal of Neuroscience. 2006;26:4717–4728. doi: 10.1523/JNEUROSCI.0381-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masliah E, Hansen L, Adame A, Crews L, Bard F, Lee C, et al. Abeta vaccination effects on plaque pathology in the absence of encephalitis in Alzheimer disease. Neurology. 2005;64:129–131. doi: 10.1212/01.WNL.0000148590.39911.DF. [DOI] [PubMed] [Google Scholar]
- McLaurin J, Cecal R, Kierstead M, Tian X, Phinney A, Manea M, et al. Therapeutically effective antibodies against amyloid-beta peptide target amyloid-beta residues 4–10 and inhibit cytotoxicity and fibrillogenesis. Nature Medicine. 2002;8:1263–1269. doi: 10.1038/nm790. [DOI] [PubMed] [Google Scholar]
- Monsonego A, Maron R, Zota V, Selkoe D, Weiner H. Immune hyporesponsiveness to amyloid-β peptide in amyloid precursor protein transgenic mice: implications for the pathogenesis and treatment of Alzheimer’s disease. Proceedings of the National Academy of Sciences of the United States of America. 2001;98:10273–10278. doi: 10.1073/pnas.191118298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan D, Diamond DM, Gottschall PE, Ugen KE, Dickey C, Hardy J, et al. Abeta peptide vaccination prevents memory loss in an animal model of Alzheimer’s disease. Nature. 2000;408:982–985. doi: 10.1038/35050116. [DOI] [PubMed] [Google Scholar]
- Mucke L, Masliah E, Yu GQ, Mallory M, Rockenstein EM, Tatsuno G, et al. High-level neuronal expression of Aβ1-42 in wild-type human amyloid protein precursor transgenic mice: synaptotoxicity without plaque formation. The Journal of Neuroscience. 2000;20:4050–4058. doi: 10.1523/JNEUROSCI.20-11-04050.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicoll JA, Wilkinson D, Holmes C, Steart P, Markham H, Weller RO. Neuropathology of human Alzheimer disease after immunization with amyloid-beta peptide: a case report. Nature Medicine. 2003;9:448–452. doi: 10.1038/nm840. [DOI] [PubMed] [Google Scholar]
- Nicoll JAR, Barton E, Boche D, Neal JW, Ferrer I, Thompson P, et al. Aβ species removal after Aβ 42 immunization. Journal of Neuropathology and Experimental Neurology. 2006;65:1040–1048. doi: 10.1097/01.jnen.0000240466.10758.ce. [DOI] [PubMed] [Google Scholar]
- Oddo S, Billings L, Kesslak JP, Cribbs DH, LaFerla FM. Abeta immunotherapy leads to clearance of early, but not late, hyperphosphorylated tau aggregates via the proteasome. Neuron. 2004;43:321–332. doi: 10.1016/j.neuron.2004.07.003. [DOI] [PubMed] [Google Scholar]
- Orgogozo JM, Gilman S, Dartigues JF, Laurent B, Puel M, Kirby LC, et al. Subacute meningoencephalitis in a subset of patients with AD after Abeta42 immunization. Neurology. 2003;61:46–54. doi: 10.1212/01.wnl.0000073623.84147.a8. [DOI] [PubMed] [Google Scholar]
- Pfeifer M, Boncristiano S, Bondolfi L, Stalder A, Deller T, Staufenbiel M, et al. Cerebral hemorrhage after passive anti-Abeta immunotherapy. Science. 2002;298:1379. doi: 10.1126/science.1078259. [DOI] [PubMed] [Google Scholar]
- Racke MM, Boone LI, Hepburn DL, Parsadainian M, Bryan MT, Ness DK, et al. Exacerbation of cerebral amyloid angiopathy-associated microhemorrhage in amyloid precursor protein transgenic mice by immunotherapy is dependent on antibody recognition of deposited forms of amyloid beta. The Journal of Neuroscience. 2005;25:629–636. doi: 10.1523/JNEUROSCI.4337-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schenk D. Amyloid-β immunotherapy for Alzheimer’s disease: the end of the beginning. Nature. 2002;3:824–828. doi: 10.1038/nrn938. [DOI] [PubMed] [Google Scholar]
- Schenk D, Barbour R, Dunn W, Gordon G, Grajeda H, Guido T, et al. Immunization with amyloid-β attenuates Alzheimer-disease-like pathology in the PDAPP mouse. Nature. 1999;400:173–177. doi: 10.1038/22124. [DOI] [PubMed] [Google Scholar]
- Seabrook TJ, Iglesias M, Bloom JK, Spooner ET, Lemere CA. Differences in the immune response to long term Abeta vaccination in C57BL/6 and B6D2F1 mice. Vaccine. 2004;22:4075–4083. doi: 10.1016/j.vaccine.2004.03.061. [DOI] [PubMed] [Google Scholar]
- Seabrook TJ, Thomas K, Jiang L, Bloom J, Spooner E, Maier M, et al. Dendrimeric Aβ1-15 is an effective immunogen in wildtype and APP tg mice. Neurobiology of Aging. 2006;28:813–823. doi: 10.1016/j.neurobiolaging.2006.04.007. [DOI] [PubMed] [Google Scholar]
- Selkoe DJ. Alzheimer’s disease: genes, proteins, and therapy. Physiological Reviews. 2001;81:741–766. doi: 10.1152/physrev.2001.81.2.741. [DOI] [PubMed] [Google Scholar]
- Sigurdsson EM, Scholtzova H, Mehta PD, Frangione B, Wisniewski T. Immunization with a nontoxic/nonfibrillar amyloid-beta homologous peptide reduces Alzheimer’s disease-associated pathology in transgenic mice. The American Journal of Pathology. 2001;159:439–447. doi: 10.1016/s0002-9440(10)61715-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solomon B, Koppel R, Frenkel D, Hanan-Aharon E. Disaggregation of Alzheimer β-amyloid by site-directed mAb. Proceedings of the National Academy of Sciences of the United States of America. 1997;94:4109–4112. doi: 10.1073/pnas.94.8.4109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solomon B, Koppel R, Hanan E, Katzav T. Monoclonal antibodies inhibit in vitro fibrillar aggregation of the Alzheimer β-amyloid peptide. Proceedings of the National Academy of Sciences of the United States of America. 1996;93:452–455. doi: 10.1073/pnas.93.1.452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Town T, Tan J, Sansone N, Obregon D, Klein T, Mullan M. Characterization of murine immunoglobulin G antibodies against human amyloid-β1-42. Neuroscience Letters. 2001;307:101–104. doi: 10.1016/s0304-3940(01)01951-6. [DOI] [PubMed] [Google Scholar]
- Walsh DM, Selkoe DJ. Oligomers on the brain: the emerging role of soluble protein aggregates in neurodegeneration. Protein and Peptide Letters. 2004;11:213–228. doi: 10.2174/0929866043407174. [DOI] [PubMed] [Google Scholar]
- Weiner HL, Lemere CA, Maron R, Spooner ET, Grenfell TJ, Mori C, et al. Nasal administration of amyloid-beta peptide decreases cerebral amyloid burden in a mouse model of Alzheimer’s disease. Annals of Neurology. 2000;48:567–579. [PubMed] [Google Scholar]
- Wilcock DM, Rojiani A, Rosenthal A, Subbarao S, Freeman MJ, Gordon MN, et al. Passive immunotherapy against Abeta in aged APP-transgenic mice reverses cognitive deficits and depletes parenchymal amyloid deposits in spite of increased vascular amyloid and microhemorrhage. Journal of Neuroinflammation. 2004;1:24. doi: 10.1186/1742-2094-1-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfe M. Shutting down Alzheimer’s. Scientific American. 2006;294:72–79. doi: 10.1038/scientificamerican0506-72. [DOI] [PubMed] [Google Scholar]
- Yano A, Onozuka A, Matin K, Imai S, Hanada N, Nisizawa T. RGD motif enhances immunogenicity and adjuvanticity of peptide antigens following intranasal immunization. Vaccine. 2003;22:237–243. doi: 10.1016/s0264-410x(03)00561-9. [DOI] [PubMed] [Google Scholar]