Abstract
Background
Administration of cardiac progenitor cells (CPCs) 4 h after reperfusion ameliorates LV function in rats with acute myocardial infarction (MI). Clinically, however, this approach is not feasible because expansion of autologous CPCs after acute MI requires several weeks. Therefore, we determined whether CPCs are beneficial in the more clinically relevant setting of an old MI (scar).
Methods and Results
One month after coronary occlusion/reperfusion, rats received vehicle or EGFP-labeled CPCs intracoronarily. Thirty-five days later, CPC-treated rats exhibited more viable myocardium in the risk region, less fibrosis in the noninfarcted region, and improved LV function. EGFPpos cells expressing cardiomyocyte, endothelial, and vascular smooth muscle cell markers were observed only in 7/17 treated rats and occupied only 2.6% and 1.1% of risk and noninfarcted regions, respectively. Transplantation of CPCs was associated with increased proliferation and expression of cardiac proteins by endogenous CPCs.
Conclusions
Intracoronary administration of CPCs in the setting of an old MI produces beneficial structural and functional effects. Although exogenous CPCs can differentiate into new cardiac cells, this mechanism is not sufficient to explain the benefits, suggesting paracrine effects; among these, our data identify activation of endogenous CPCs. This is the first report that CPCs are beneficial in the setting of an old MI when given intracoronarily – the most widely applicable therapeutic approach in patients. Furthermore, this is the first evidence that exogenous CPC administration activates endogenous CPCs. These results open new therapeutic applications for use of autologous CPCs in patients with old MI and chronic ischemic cardiomyopathy.
Keywords: myocardial regeneration, cardiac progenitor cells, cardiac function, myocardial infarction, reperfusion, stem cells
INTRODUCTION
Cell-based therapies have the potential to alleviate left ventricular (LV) dysfunction and remodeling following acute myocardial infarction (MI) in experimental animal models1 as well as in humans.2–3 Among the various cells used, c-kitpos cardiac progenitor cells (CPCs) are attractive because they normally reside in the heart and presumably are responsible for replenishing the pool of cardiac myocytes and coronary vessels under normal conditions.4–6 The practical utility of CPCs is further supported by the fact that these cells can be isolated from small fragments of cardiac tissue and expanded for subsequent autologous administration.5–6
Transplantation of autologous or syngeneic CPCs has been found to be effective in limiting LV dysfunction and remodeling in rodent models of acute MI, both in the setting of a permanent coronary occlusion4–6 and in that of a transient occlusion followed by reperfusion.7 Clinically, however, this approach would be problematic because administration of autologous CPCs to patients with acute MI would necessitate an endomyocardial biopsy followed by isolation and expansion of c-kitpos cells from the tissue fragment, all of which would require weeks, so that CPC transplantation would have to be postponed until after the acute phase of the infarct. By the time a sufficient number of autologous CPCs would be ready for therapeutic use, the inflammatory response to acute injury would have abated and a scar would have formed. At this chronic stage, the local myocardial environment is very different from that of an acute MI because the expression of the cytokines and adhesion molecules that are upregulated during the acute phase and play an important role in the chemotaxis, homing, and differentiation of stem cells8–11 is absent or markedly diminished. For example, CPCs are known to express CXCR-4, the receptor for stromal-derived factor-1 (SDF-1),7 a cytokine that plays a pivotal role in orchestrating the chemotaxis, homing, and differentiation of various progenitor/stem cells.8, 10–11 The expression of SDF-1 in the myocardium increases markedly after ischemia/reperfusion, peaking at 1 d, but then resolves by 7 d.8, 10–11 The migration, survival, and differentiation of CPCs are also modulated by hepatocyte growth factor (HGF) and insulin-like growth factor-1 (IGF-1),5–6, 12–13 both of which are upregulated shortly after ischemia/reperfusion but subsequently decline.14–15 In view of the marked downregulation of SDF-1, HGF, IGF-1, and other signals in the setting of a mature scar, it is unclear whether transplanting CPCs at this stage would still be effective.
In a recent study of rats with subacute MI (20-day-old infarcts), CPC administration was found to result in tissue regeneration and LV functional improvement. 6 In this investigation, however, as well as in most previous studies,4–5 CPCs were injected directly into the myocardium, an approach that would be impractical for widespread use in patients. Furthermore, this study6 was performed in the setting of a permanent coronary occlusion, which differs importantly from the contemporary clinical situation, in which the occluded artery is usually recanalized.16 Recently, Johnston et al.17 have reported salubrious effects of intracoronary infusion of cardiosphere-derived cells (CDCs) in pigs with old MI; however, studies with intracoronary infusion of a selected population of c-kitpos cells (CPCs) have not been reported. Accordingly, the goal of the present investigation was to determine whether administration of CPCs is effective in regenerating cardiac tissue and alleviating postinfarction LV remodeling and dysfunction when these cells are infused intracoronarily (the most broadly applicable clinical strategy) in the setting of an old MI (scar) produced by a temporary coronary occlusion followed by reperfusion. To directly compare the results of this investigation with those of our previous study in the setting of an acute MI,7 we utilized a similar rat model and the same dose of CPCs as in that study.7
METHODS
Detailed methodology is provided in the online supplement.
RESULTS
Exclusions and Gross Measurements
Exclusions are detailed in the online supplement. Intracoronary injection of CPCs resulted in uniform distribution of these cells throughout the LV (supplemental Fig. 9).
As expected, both of the groups subjected to infarction exhibited LV hypertrophy and dilatation as compared with normal rats (Table 1). There were no significant differences between the vehicle- and CPC-treated groups with respect to body weight, LV weight, LV length, and LV volume (determined directly at postmortem examination), although LV volume tended to be lower in CPC-treated rats (−12%, P=NS).
Table 1.
Body weight and gross measurements.
| Group | Initial Body Weight (g) | Final Body Weight (g) | LV Weight (g) | LV Weight/Body Weight (%) | LV Length (mm) | LV Volume (μl) |
|---|---|---|---|---|---|---|
| Normal (n=4) | 171.5±3.8 | 202.9±4.5 | 0.44±0.03 | 0.22±0.01 | 8.9±0.9 | 100.7±6.8 |
| Vehicle (n=15) | 174.7±1.6 | 201.8±2.9 | 0.96±0.03† | 0.48±0.02† | 10.9±0.2† | 166.7±8.9* |
| CSC-treated (n=17) | 173.5±1.7 | 202.7±2.7 | 0.93±0.04† | 0.46±0.02† | 10.5±0.2* | 146.7±11.9 |
Data are mean ± standard error (SEM).
P<0.05,
P<0.01 vs. normal
Echocardiographic and Hemodynamic Measurements
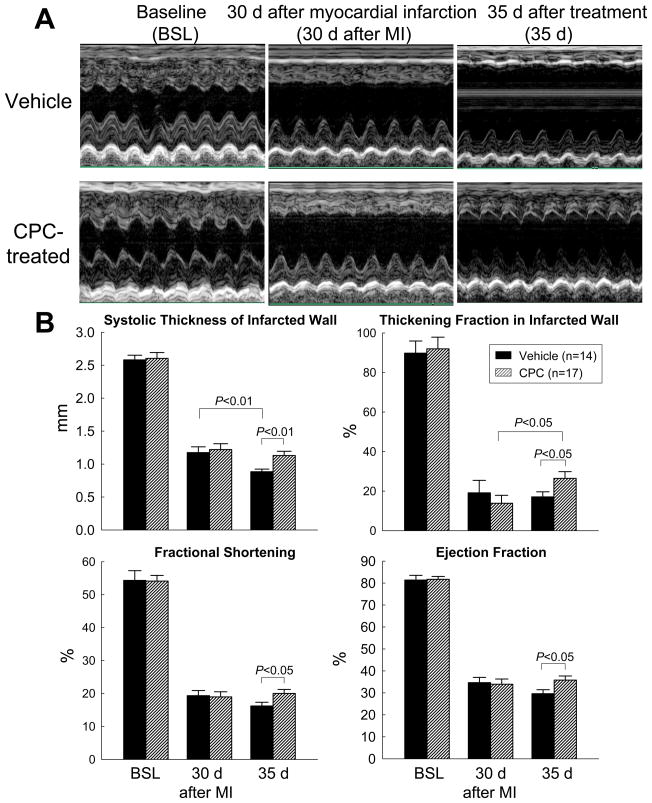
At the time of treatment (30 days after MI), thickening fraction (ThF) in the infarcted (anterior) LV wall, LV fractional shortening (FS), and LV ejection fraction (EF) were markedly decreased vis-a-vis baseline (Figs. 1A and 1B, supplemental Table 2), indicating the development of severe post-MI dysfunction; all echocardiographic parameters were virtually indistinguishable between the vehicle-treated and the CPC-treated group, demonstrating that the degree of regional and global LV functional deterioration at the time of treatment was similar. As expected, in the vehicle group the infarcted wall ThF, FS, and EF continued to deteriorate during the ensuing 35 days; in contrast, in the CPC-treated group the infarcted wall ThF increased significantly, and FS and EF remained stable (Fig. 1B). As a consequence, at 35 days all three parameters were significantly higher in the CPC-treated than in the vehicle group (Fig. 1B). Thus, transplantation of CPCs improved both regional and global LV function.
Figure 1. Echocardiographic assessment of LV function.
(A) Representative M-mode echocardiographic images from a vehicle-treated and a CPC-treated rat recorded at baseline (BSL), at 30 d after MI (before vehicle or CPC treatment) (MI), and at 35 d after treatment (35 d). (B) Quantitative echocardiographic parameters including systolic thickness of the anterior (infarcted) wall, thickening fraction in the anterior (infarcted) wall, LV fractional shortening, and LV ejection fraction. Data are means ± SEM.
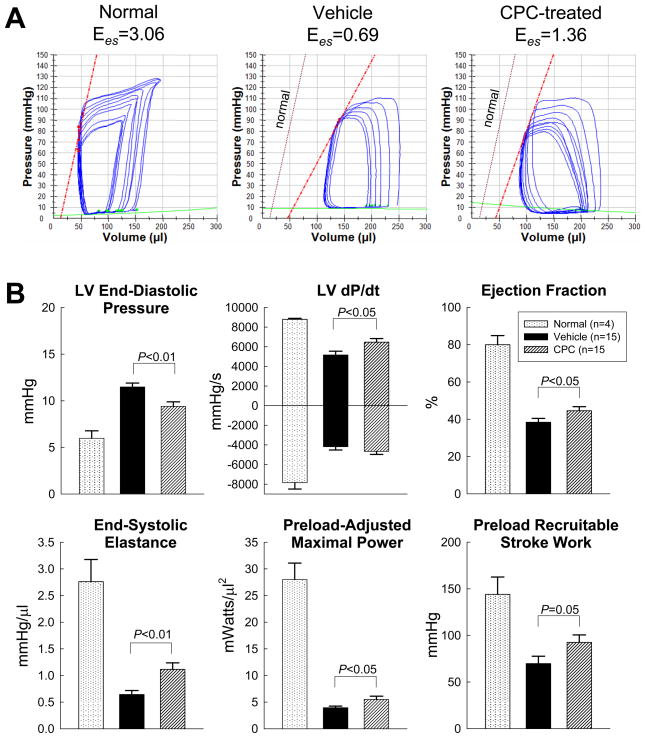
The hemodynamic studies performed just prior to euthanasia (35 days after treatment) led to similar conclusions. Compared with normal, age-matched rats not subjected to surgery, all LV functional parameters were markedly depressed in both groups of infarcted rats, but the deterioration was less in the treated than in the vehicle group (Fig. 2, supplemental Table 3). This was the case not only for load-dependent (LV end-diastolic pressure [LVEDP], LV dP/dtmax, and LVEF), but also for load-independent indices of LV systolic function (end-systolic elastance [Ees], preload-activated maximal power [PAMP], and preload-recruitable stroke work [PRSW]) (Fig. 2).
Figure 2. Hemodynamic assessment of LV function at 35 d after treatment.
(A) Representative pressure-volume loops from a normal, a vehicle-treated, and a CPC-treated rat recorded during preload manipulation by a brief period of inferior vena cava occlusion. (B) Quantitative analysis of hemodynamic variables including LV end-diastolic pressure, dP/dt, ejection fraction, end-systolic elastance (Ees), preload-adjusted maximal power, and preload-recruitable stroke work. Data are means ± SEM.
Thus, two independent methods of functional assessment (echocardiography and hemodynamic studies) consistently demonstrated that intracoronary CPC infusion improved LV systolic performance.
Morphometric and Histologic Analysis
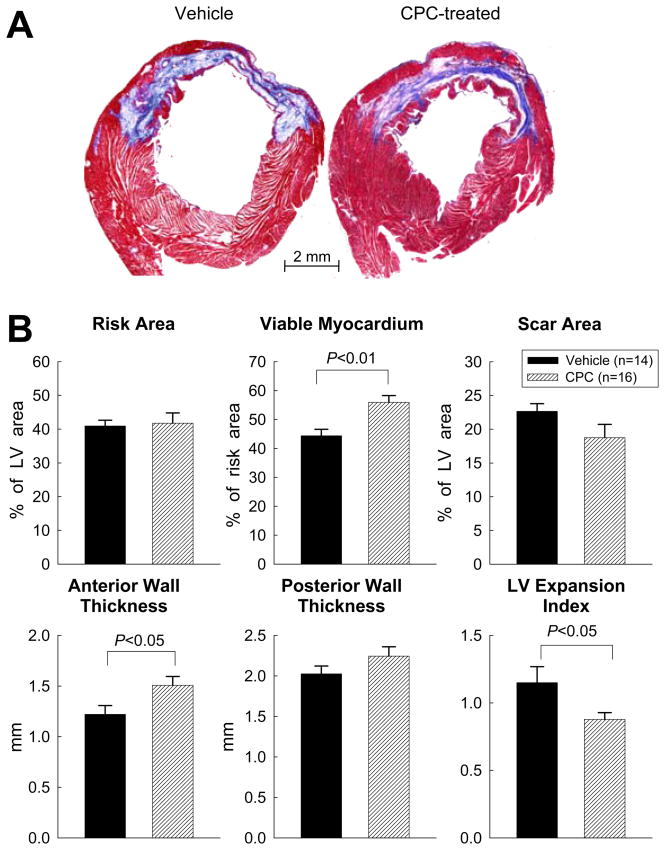
As illustrated in Fig. 3A, the amount of viable tissue within the risk region was greater in CPC-treated vs. vehicle-treated rats. In each heart, a detailed quantitative analysis was performed on two sections (one from each of two mid-ventricular slices) (supplemental Fig. 10); the results are summarized in supplemental Table 4. Although the circumferential extent of the scar and the size of the risk region were indistinguishable between the two groups, the amount of viable myocardium within the risk region was 25% greater (P<0.01) and the thickness of the anterior (infarcted) wall 24% greater (P<0.05) in CPC-treated than in vehicle-treated rats (Fig. 3B, supplemental Table 4), suggesting that the infusion of CPCs resulted in formation of new myocardium in the infarcted region. The LV expansion index was also reduced (P<0.05) in the CPC-treated group (Fig. 3B). The hemodynamic parameters at the time of euthanasia were significantly related to the amount of viable myocardium in the risk region (r = 0.44–0.69 [P<0.05–0.01]; supplemental Fig. 11), suggesting that the functional improvement noted in CPC-treated rats was accounted for, in part, by the greater proportion of viable tissue in the risk region.
Figure 3. Morphometric analysis.
(A) Representative Masson’s trichrome-stained myocardial sections from a vehicle-treated and a CPC-treated rat. Scar tissue and viable myocardium are identified in blue and red, respectively. (B) Quantitative analysis of LV morphometric parameters (for definition, see supplemental Fig. 11). Data are means ± SEM.
Although in the risk region the median myocyte cross-sectional area was slightly smaller (P<0.05) in the CPC-treated vs. the vehicle-treated group, no difference was observed in the noninfarcted region (supplemental Fig. 12B and C). These data are consistent with the gross measurements (vide supra) and indicate that transplantation of CPCs did not affect hypertrophy in the noninfarcted region, although a small effect was noted in the risk region.
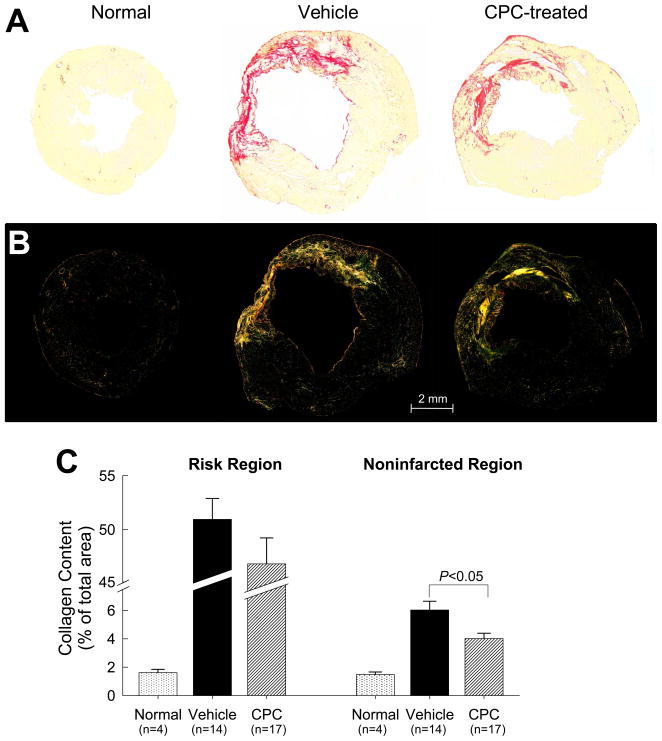
In both groups, collagen content in the noninfarcted region (posterior wall) was increased compared with normal hearts, but the increase was significantly less (−33%, P<0.01) in CPC-treated rats (Fig. 4C). This reduced collagen deposition in the noninfarcted myocardium may have contributed, at least partially, to the functional benefits of CPC therapy.
Figure 4. Myocardial collagen content.
Representative microscopic images of a normal, a vehicle-treated, and a CPC-treated heart obtained using regular (A) and polarized (B) light. (C) Collagen content expressed as percent of total area in the scarred and noninfarcted region. LV sections were stained with picrosirius red and collagen content was quantitated under polarized light. Data are means ± SEM.
Detection of Transplanted CPCs and Their Progeny
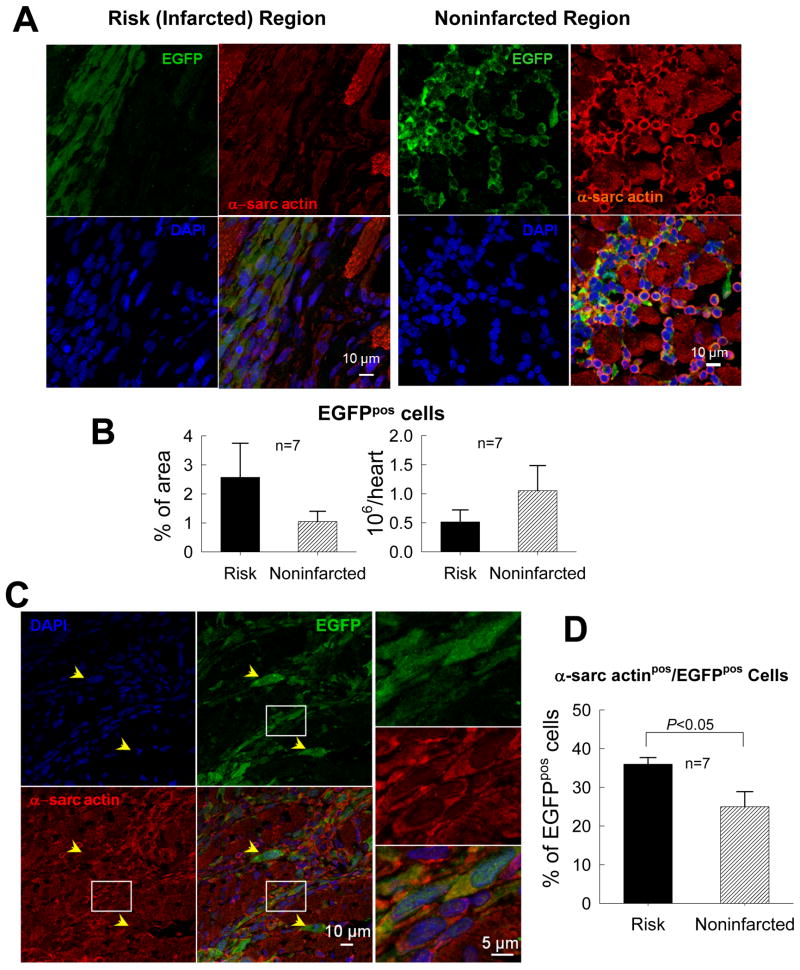
Surprisingly, despite careful analysis of four LV slices per heart, performed on 7–10 histologic sections in each slice at 40–80 μm intervals, EGFPpos cells were found in only seven of the 17 CPC-treated rats. In these seven hearts, EGFPpos cells were relatively rare, accounting for only 2.6 ± 1.1% of the area of the risk region and 1.1 ± 0.4% of the noninfarcted region (Fig. 5). These areas of EGFP positivity were calculated to correspond to 0.51 ± 0.21 × 106 EGFPpos cells/heart in the risk region and 1.05 ± 0.43 × 106 EGFPpos cells/heart in the noninfarcted region (Fig. 5B). Real time PCR detection of the EGFP cDNA marker in transplanted CPC genomic DNA essentially confirmed the immunohistochemical findings (online supplement; supplemental Fig. 13A). Thus, at 35 days after intracoronary infusion of CPCs, the transplanted cells (or their progeny) were present in a minority of the hearts and, in those, they occupied a small fraction of the myocardium.
Figure 5. Myocardial content and differentiation of transplanted CPCs.
(A) Representative confocal microscopic images from a CPC-treated rat showing presence of transplanted CPCs in the risk (infarcted) and noninfarcted regions, as evinced from immunoreactivity for EGFP (green). Some EGFPpos cells also express α-sarcomeric actin (red). (B) Quantitation of EGFPpos cells in the risk and noninfarcted region, expressed as percent EGFPpos myocardial area and as total calculated number of EGFPpos cells/heart. The number of EGFPpos cells/heart was estimated by multiplying the number of EGFPpos cells per unit area by the estimated number of EGFPpos cells present through the thickness of each slice in which EGFPpos cells were found (estimated 100 cells per 2-mm thickness of slice). (C) Representative confocal microscopic images showing colocalization of EGFP and α-sarcomeric actin in several cells in the border zone (the area in the white box is magnified in the three panels on the right). Yellow arrows indicate EGFPpos cells that do not expressα-sarcomeric actin. (D) Quantitative analysis of α-sarcomeric actinpos/EGFPpos cells. Data are means ± SEM.
Differentiation of Transplanted CPCs
A significant fraction of the transplanted EGFPpos cells expressed cardiac specific proteins, i.e., α-sarcomeric actin, MHC, α-actinin, and TnI (Fig. 5C and supplemental Figs. 14 and 15), suggesting differentiation toward a cardiomyocytic lineage; for example, 36.0 ± 1.7% of total EGFPpos cells in the risk region and 25.0 ± 3.9% in the noninfarcted region were positive for α-sarcomeric actin (Fig. 5D). However, since most of the EGFPpos/α-sarcomeric actinpos cells were small and did not exhibit the typical morphology and sarcomeric structure of mature cardiac myocytes (Fig. 5C), it remains unclear whether these cells were genuine differentiating myocytes. In addition, microvessels containing EGFPpos cells that expressed vWF, PECAM, or α-SMA were found in the risk region (supplemental Figs. 16, 17, and 18), suggesting differentiation into the endothelial and vascular smooth muscle lineages. These observations are compatible with a possible cardiac and vascular specification of transplanted CPCs resulting in formation of immature myocytes and new coronary vessels.
Relationship Between Salubrious Effects of CPC Infusion and Presence of Transplanted CPCs at 35 Days
When the seven CPC-treated hearts that exhibited EGFPpos cells and the 10 hearts that displayed no EGFP positivity were examined separately (supplemental Fig. 19), the amount of viable myocardium in the risk region was significantly increased in both subsets relative to vehicle-treated hearts (supplemental Fig. 19A). The anterior LV wall thickness was significantly increased only in the EGFPneg hearts, and the LV expansion index was similar between the two subgroups. Echocardiographic (supplemental Fig. 19B) and hemodynamic (supplemental Fig. 19C) analysis revealed no consistent pattern favoring one subgroup over the other; both EGFPpos and EGFPneg hearts exhibited evidence of functional improvement. Together, these data suggest that the beneficial effects of exogenous CPCs do not depend on the presence of these cells, or their progeny, at 35 days after infusion.
Cellular Proliferation After Transplantation
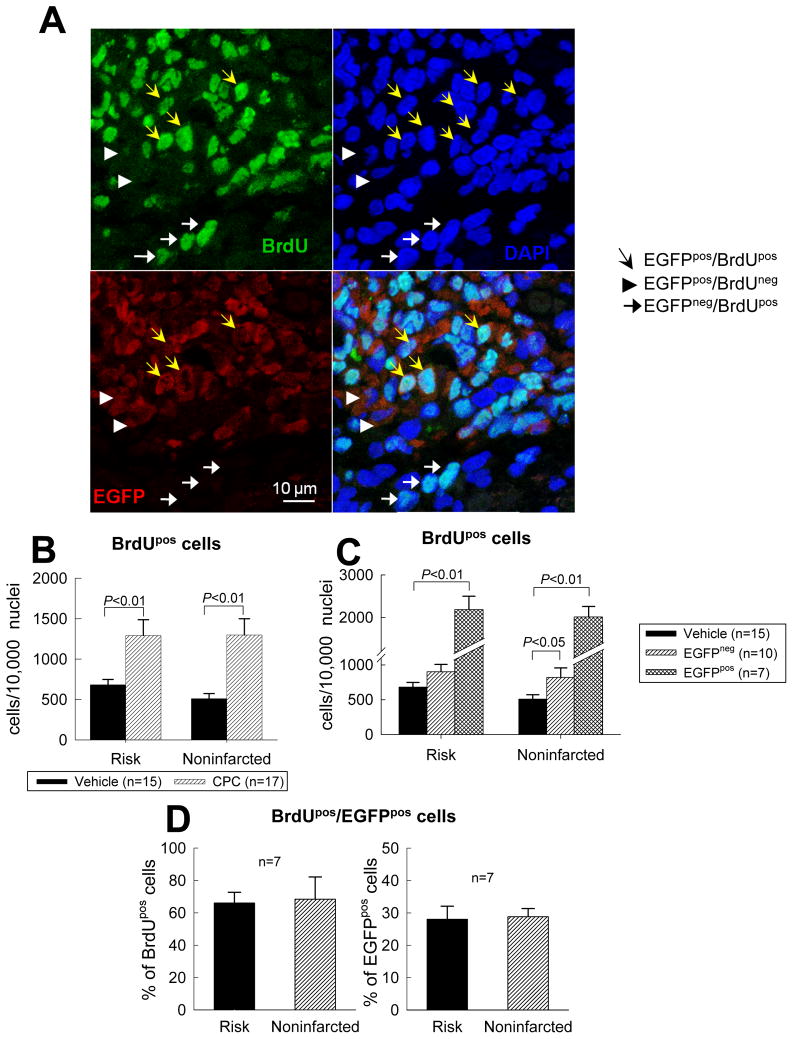
Tissue BrdU incorporation (BrdU was given in the last 2 weeks of life) was significantly greater in CPC-treated hearts relative to vehicle-treated hearts (Fig. 6B), both in the seven CPC-treated rats that exhibited EGFPpos cells and in the 10 CPC-treated hearts that did not (Fig. 6C). The higher content of BrdUpos cells in CPC-treated hearts was generalized, being equally apparent in the risk and noninfarcted regions (Figs. 6B and 6C). In the seven CPC-treated hearts that exhibited EGFPpos cells, most of the BrdU incorporation was accounted for by EGFPpos cells: approximately two thirds of the BrdUpos cells expressed EGFP and, conversely, approximately one third of the EGFPpos cells were also positive for BrdU (Fig. 6D). The analysis of Ki67pos cells (presented in supplemental Fig. 20) yielded similar findings. Taken together, the BrdU and Ki67 data indicate that administration of CPCs was associated with increased cellular proliferative activity in the heart and that although most of this activity was accounted for by the transplanted CPCs, other cells were also involved.
Figure 6. Proliferation of transplanted CPCs.
(A) Representative confocal microscopic images from a CPC-treated rat showing BrdU uptake in the infarcted region during the last 2 weeks of life. Yellow arrows indicate EGFPpos cells that are BrdU positive; white arrowheads EGFPpos cells that are BrdU negative; and white arrows EGFPneg cells that are BrdU positive. (B, C, and D) Quantitative analysis of BrdUpos cells in the risk and noninfarcted regions. Panel B shows the number of BrdUpos cells in vehicle-treated and CPC-treated groups; panel C shows the same data except that the CPC-treated group is subdivided into the subgroups that did or did not exhibit EGFPpos cells (in the seven hearts with EGFPpos cells, BrdUpos cells were counted in the entire region examined, both in the EGFPpos and in the EGFPneg areas). (D) Colocalization of BrdU and EGFP immunoreactivity in the seven CPC-treated hearts that exhibited EGFPpos cells. Data are means ± SEM.
Transplanted CPCs Proliferate and Activate Proliferation of Endogenous c-kitpos Cells
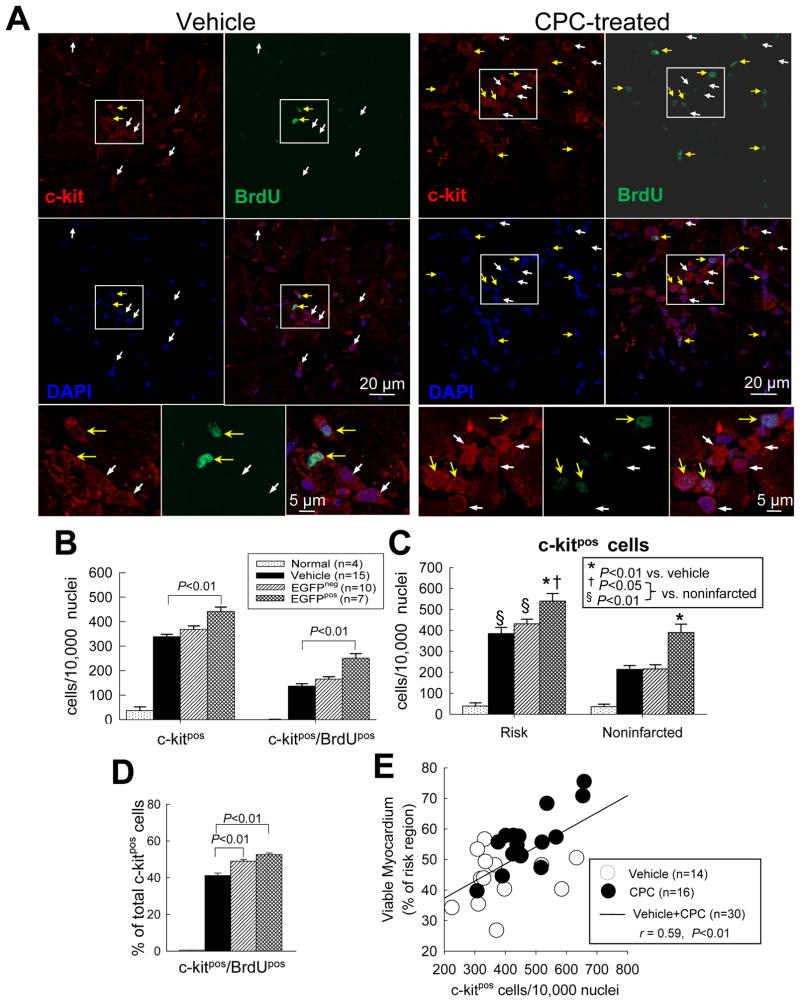
To determine whether endogenous CPCs (c-kitpos/EGFPneg cells) were among the cells activated by exogenous CPC administration, LV sections were stained with antibodies against c-kit (Fig. 7 and supplemental Fig. 21). Relative to normal hearts, in the infarcted hearts (both vehicle- and CPC-treated) there was a dramatic increase (>10-fold) in the total number of c-kitpos cells as well as in the number of c-kitpos/BrdUpos cells (i.e., new c-kitpos cells that were formed in the last two weeks of life) (Fig. 7B). The increase in c-kitpos cells was observed both in the risk region and in the noninfarcted region, although it was more pronounced in the former (Fig. 7C). Rather than absolute numbers, however, probably a better measure of c-kitpos cell proliferation is given by the percentage of the total c-kitpos cell population that were newly formed in the last two weeks of life (c-kitpos/BrdUpos cells). This percentage was essentially nil in normal hearts but averaged ~40–50% in the infarcted hearts (both vehicle- and CPC-treated) (Fig. 7D), indicating marked proliferation of endogenous CPCs in response to infarction; importantly, this percentage was significantly (P<0.01) higher in CPC-treated vs. vehicle-treated hearts, both in those with and in those without EGFPpos cells (Fig. 7D). (In the seven hearts with EGFPpos cells, this difference cannot be ascribed to the transplanted CPCs because in these hearts c-kitpos cells were measured only in areas that did not contain EGFPpos cells; in these hearts almost half of the c-kitpos cells were EGFPneg [supplemental Fig. 22].) The fact that the percentage of c-kitpos/BrdUpos cells was increased in CPC-treated hearts, even in those with no EGFPpos cells, suggests activation of endogenous CPC proliferation by the exogenous CPCs.
Figure 7. Effect of CPC transplantation on c-kitpos cells.
(A) Representative confocal microscopic images from a vehicle- treated and a CPC-treated rat showing c-kitpos cells (red) and BrdUpos cells (green) in the risk region. Yellow arrows indicate c-kitpos cells that are BrdU positive; white arrows c-kitneg cells that are BrdU positive; (B) Quantitative analysis of c-kitpos cells and double positive (c-kitpos/BrdUpos) cells in normal, vehicle-treated, and CPC-treated hearts (the last group is subdivided into two subgroups, that with EGFPpos cells [EGFPpos] and that without EGFPpos cells [EGFPneg]). (C) Quantitative analysis of c-kitpos cells in risk and noninfarcted regions in normal, vehicle-treated, and CPC-treated hearts [as in (B), the last group is subdivided into EGFPpos and EGFPneg subgroups]. (D) Quantitative analysis of double positive (c-kitpos/BrdUpos) cells expressed as a percent of all c-kitpos cells. (E) Correlation between the number of c-kitpos cells and the percent of viable myocardium in the risk region (r=0.59, P<0.01). Data are means ± SEM.
A similar pattern was observed for Ki67, although in this case the percentage of c-kitpos cells that were Ki67pos was similar in the two subsets of CPC-treated hearts and in the vehicle-treated hearts (supplemental Fig. 21). (Unlike BrdU incorporation, which reflects the cumulative number of DNA replications over two weeks, Ki67 expression provides a “snapshot” of the number of cycling cells at the time of sacrifice.)
There was a significant correlation between the myocardial content of c-kitpos cells and the amount of viable myocardium in the risk region (Fig. 7E), suggesting that c-kitpos cell proliferation contributes to myocardial repair.
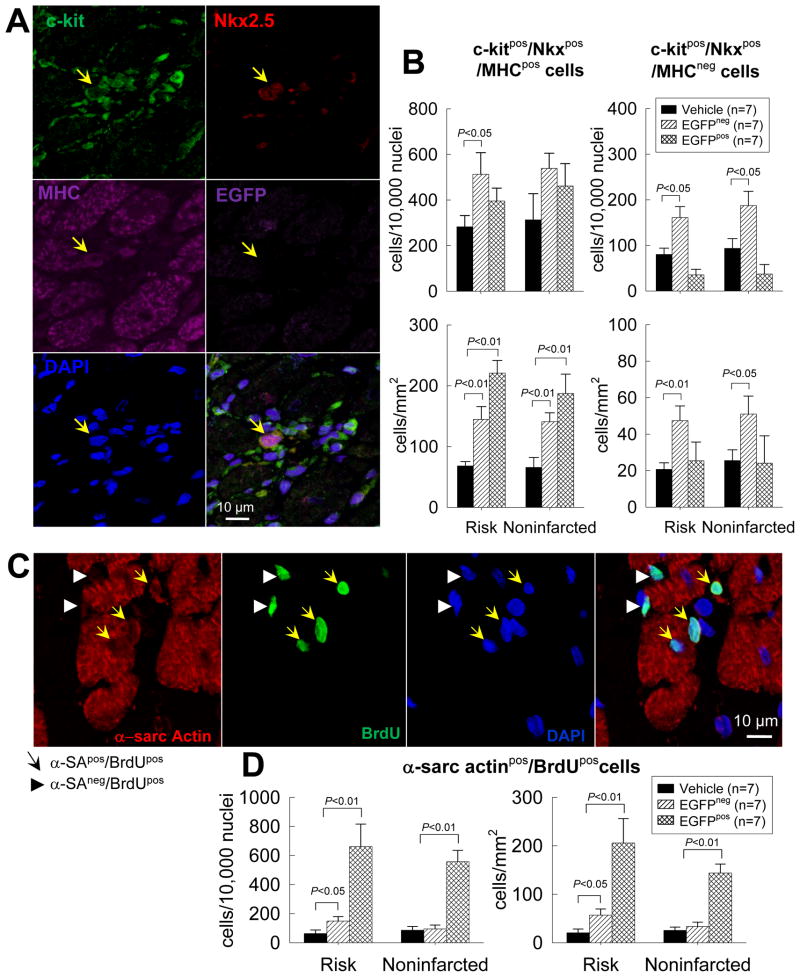
To gain insights into the effect of CPC transplantation on the commitment of endogenous CPCs to the cardiac lineage, a quantitative analysis of c-kitpos/EGFPneg cells (endogenous CPCs) expressing the cardiac transcription factor Nkx2.5 and the sarcomeric protein MHC was performed (Figs. 8A and B). The number of endogenous CPCs (c-kitpos/EGFPneg cells) that expressed Nkx2.5 and MHC was higher in CPC-treated than in vehicle-treated hearts, both in the risk and in the noninfarcted regions; the magnitude of this effect, however, varied between EGFPpos and EGFPpos hearts (Fig. 8B). Due to the presence of clusters of EGFPpos cells in CPC-treated, EGFPneg hearts, cell density was higher in these hearts than in CPC-treated, EGFPneg hearts and in control hearts; consequently, the magnitude of the increase in endogenous CPCs in EGFPpos hearts was greater when the number of cells was normalized to area (mm2) than to number of nuclei (Fig. 8B). The number of BrdUpos cells (new cells formed in the last 2 weeks of life) that expressed α-sarcomeric actin was also increased in the CPC-treated hearts (Figs. 8C and 8D), suggesting that the increase in cardiac-committed endogenous CPCs was associated with increased formation of new myocytes. However, the absolute number of BrdUpos, α-sarcomeric actinpos cells was small (ranging from 20–206 cells/mm2), and newly-formed (BrdUpos) cells with a mature myocyte phenotype and organized sarcomeric structure were extremely rare. Because BrdU was administered only in the last 2 weeks of life, definitive quantitative conclusions regarding the effect of CPC transplantation on formation of new myocytes cannot be made.
Figure 8. Cardiac commitment of endogenous CPCs after trasplantation of exogenous CPCs.
The commitment of endogenous CPCs (c-kitpos/EGFPneg cells) to cardiac lineage was assessed by quantitating endogenous CPCs that expressed Nkx2.5 and MHC in vehicle- and CPC-treated hearts. New myocytes formed in the last 2 weeks of life were identified by measuring α-sarcomeric actinpos and BrdUpos cells. (A) Representative confocal microscopic image of a c-kitpos/EGFPneg/Nkx2.5pos/MHCpos cell obtained in serial sections of a CPC-treated heart. (B) Quantitative analysis of c-kitpos/EGFPneg/Nkx2.5pos/MHCpos cells in the risk and noninfarcted regions of vehicle- and CPC-treated hearts (the CPC-treated group is subdivided into two subgroups, one with EGFPpos cells [EGFPpos] and one without EGFPpos cells [EGFPneg]). The number of c-kitpos/EGFPneg/Nkx2.5pos/MHCpos cells is normalized to both number of cells (104 nuclei, upper panels) and area (mm2, lower panels). Because cell density in CPC-treated, EGFPpos hearts was higher (due to presence of clusters of EGFPpos cells), the magnitude of the increase in endogenous CPCs in CPC-treated, EGFPpos hearts was greater when the number of cells was normalized to area (mm2) than to number of nuclei. (C) Representative confocal microscopic image of α-sarcomeric actinpos/BrdUpos cells in a CPC-treated heart. (D) Quantitative analysis of the number of α-sarcomeric actinpos/BrdUpos cells in the risk and noninfarcted regions of vehicle- and CPC-treated hearts (subdivided into EGFPneg and EGFPpos subgroups). Data are means ± SEM.
DISCUSSION
We have previously found that in rats with acute MI, intracoronary administration of syngeneic CPCs early (4 h) after reperfusion was effective in regenerating cardiac tissue and ameliorating LV remodeling and dysfunction.7 Clinically, however, the application of this paradigm to patients with acute MI would be problematic because expansion of autologous CPCs from endomyocardial biopsies requires weeks. By the time autologous CPCs are ready for therapy, the infarct is a scar and inflammation has resolved. It is unclear whether infusion of CPCs would be beneficial at this stage, i.e., in the setting of a scar, in which the expression of growth factors and adhesion molecules is markedly diminished or even absent. The present study was undertaken to address this important translational issue. We used a transient coronary occlusion followed by reperfusion (as opposed to a permanent coronary occlusion) because, in current practice, most patients with acute MI are reperfused, either spontaneously or iatrogenically. A model of a 30-d old reperfused MI was selected because, by this time, the acute inflammatory response in the rat has resolved and the formation of the scar is complete.18
Our salient findings can be summarized as follows: (i) intracoronary infusion of syngeneic CPCs at 30 days after MI resulted, 35 days later, in improved LV structure, as manifested by a greater content of viable myocardium in the risk region, increased thickness of the infarcted LV wall, reduced fibrosis in the noninfarcted region, and attenuated LV expansion index; (ii) these salubrious effects were associated with improved regional and global LV function, as demonstrated by two independent methods (echocardiography and hemodynamic studies) utilizing both load-dependent and load-independent indices; (iii) in contrast, transplantation of CPCs did not alleviate LV dilation; (iv) the improvement in global LV function can be accounted for, in part, by the increased amount of viable tissue in the infarcted region; (v) although transplanted CPCs gave rise to new cells expressing markers specific for cardiac myocytes, endothelial cells, and vascular smooth muscle cells, this phenomenon cannot entirely explain the improvement in LV function because it was inconsistent (occurring only in a minority of the treated animals) and of small magnitude; (vi) transplantation of exogenous CPCs promoted proliferation of endogenous cells, including endogenous CPCs, both in the risk and in the noninfarcted regions; (vii) transplantation of exogenous CPCs was also associated with an increase in the number of endogenous CPCs that exhibited apparent cardiogenic commitment, both in the risk and in the noninfarcted regions.
Taken together, these results demonstrate that intracoronary administration of CPCs in the setting of a myocardial scar produces beneficial structural and functional effects within a relatively short timeframe (35 days) and that the observed benefits cannot be entirely accounted for by direct differentiation of exogenous CPCs into new cardiac cells (although such differentiation can occur), suggesting that paracrine mechanisms are likely to play an important role. Our quantitative analysis of cardiac -committed c-kitpos cells supports the concept that one such paracrine mechanism is the activation of endogenous CPCs. Previous studies4–7 have demonstrated salutary effects of CPCs when these cells were given immediately after MI, either intramyocardially4–6 or intracoronarily.7 However, to our knowledge, this is the first report that CPCs are beneficial in the setting of an old MI when given by the intracoronary route – the most widely applicable therapeutic approach in patients. Furthermore, this is the first evidence that exogenous CPC administration activates endogenous CPCs.19
An elegant study by Johnston et al.17 has recently appeared in which pigs with a 4-week-old MI were given a different form of cell therapy, autologous CDCs produced from endomyocardial biopsies.17 In this study, selective intracoronary infusion of CDCs into the territory of myocardium subjected to ischemia/reperfusion 4-weeks earlier resulted, 8 weeks later, in engraftment, formation of mature cardiac cells, reduction in relative infarct size, and improvement in LV remodeling and hemodynamic function. Despite the use of different cells, our results agree with these findings.
Effect of CPCs on Cardiac Structure and Function
Our results indicate that transplantation of CPCs exerts important salutary effects on post-MI LV dysfunction even after the healing process is completed. Global LV systolic function was significantly improved, as documented by two independent methods (echocardiography and hemodynamic studies) and by a variety of parameters, both load-dependent and load-independent (Fig. 2). Regional function in the infarcted region was also ameliorated (Fig. 1), presumably as a result of the greater proportion of viable tissue in this region (Fig. 3) and/or of favorable metabolic adaptations in the surviving myocardium.20 Although LV dimensions did not differ significantly between vehicle-treated and CPC-treated hearts (Tables 1 and supplemental Table 4), the scarred region was thicker in the treated group (Fig. 3), which may reflect, again, increased viable myocardium content (Fig. 3). The LV expansion index, which quantitates both the degree of LV dilation and the degree of infarct wall thinning,21 was significantly reduced by CPCs (Fig. 3). Another salutary effect of CPC infusion was a reduction in collagen content in the noninfarcted region (Fig. 4), which constitutes not only a marker of reduced cardiac injury but also a potential mechanism for improved cardiac performance. The myocyte cross-sectional area in the noninfarcted region was not affected by CPC transplantation (supplemental Fig. 12); this, together with the similarity in LV weight between the two groups (Table 1), indicates no effect on LV hypertrophy. The smaller myocyte cross-sectional area in the infarcted region of CPC-treated hearts (supplemental Fig. 12) may reflect either an attenuation of the hypertrophic response in this region or the formation of new myocytes (presumably smaller than mature cells).
Previous studies of CPCs in models of acute MI4–7 have shown a more robust engraftment and differentiation of the transplanted cells compared with the present study. The reason(s) for this difference is unclear, but (in addition to the considerations outlined below for the chronic MI model) may relate to the difference in models (acute vs. old MI) and the scarcity of proinflammatory substances that facilitate homing of transplanted CPCs in the present model (old MI with scar). In a recent study in rats,6 intramyocardial injection of CPCs on the border of the infarct at 20 d after MI resulted in regeneration of cardiac tissue (~40% of the scar was replaced with new myocardium), attenuation of LV dilation, and alleviation of LV dysfunction. The present results are consistent with these observations in the sense that CPCs attenuated LV dysfunction. However, in contrast to that study,6 evidence of extensive regeneration of cardiac tissue by exogenous CPCs was not observed here: EGFPpos cells (i.e., transplanted CPCs or their progeny) were found only in 7 of 17 treated rats and accounted for only 2.6% of the tissue in the risk region (Fig. 5); furthermore, mature EGFPpos myocytes were observed only rarely (supplemental Figs. 14 and 15).
The reason(s) for this apparent discrepancy is unclear. The major differences between the two studies are intramyocardial injection vs. intracoronary infusion of CPCs, permanent coronary occlusion6 vs. transient occlusion followed by reperfusion, and longer follow-up in the present investigation (35 days vs. 20 days). One possible reason for the low yield of EGFPpos cells in the present study is the immunogenic potential of this foreign protein, which may potentially induce a T cell immune response against the labeled cells,22 such that cells labeled with EGFP may actively engraft but may be subsequently cleared by the immune system.22–23 The magnitude of immunological rejection of EGFP-carrying cells remains controversial, and is most likely context-dependent.22–24 Whether this phenomenon is operative in our system remains to be determined. An additional variable that may have influenced the assessment of myocardial regeneration by EGFP-tagged CPCs involves the insertion of the lentiviral integrant in repressive regions of the genome 25; transgene silencing would interfere with the recognition of labeled cells by immunocytochemistry, although the transgene should still be detectable by PCR.
Mechanism of the Beneficial Effects of CPCs
The mechanism(s) for the beneficial effects of CPCs in this study remains unclear. Coronary vessel density was not affected by CPCs (supplemental Fig. 23). The percentage of TUNELpos nuclei did not differ between the two groups at 35 days after treatment (supplemental Fig. 24), although we did not assess apoptosis at earlier time-points. Differentiation of transplanted CPCs into cardiac cells, resulting in regeneration of dead myocardium, is unlikely to have been a major factor. Despite a detailed pathologic analysis of 28–40 sections per heart, we were unable to find any EGFPpos cells in 10 of the 17 CPC-treated hearts, and this was supported by real time PCR (supplemental Fig. 13). In the remaining seven hearts, EGFPpos cells accounted for only ~2.6 % of the risk region and ~1.1 % of the noninfarcted region (Fig. 5), values that were insufficient to account for the functional improvement. Although we did observe colocalization of EGFP with cardiac-specific proteins (Fig. 5 and supplemental Figs. 14 and 15), these cells were usually small and did not exhibit the characteristic morphology of adult cardiac myocytes (e.g., shape, sarcomeres, etc.) (Fig. 5). Moreover, in the CPC-treated group, the increase in viable tissue within the infarcted region and the improvement in systolic LV performance were observed not only in the subset of seven hearts that had EGFPpos cells but also in the subset of 10 hearts that did not (supplemental Fig. 19). Taken together, these findings indicate that direct formation of new parenchyma from the transplanted CPCs was not the major reason for the benefits observed, implying the existence of other mechanisms.
Among these, the most plausible would appear to be the secretion of cytokines/growth factors by CPCs, resulting in paracrine actions on endogenous cells that may have persisted even after the transplanted CPCs had disappeared – a phenomenon that has been reported to occur with various stem/progenitor cells.26–27 CPCs have been shown to express HGF and IGF-1, which may exert important actions on both the surrounding myocardium and the resident endogenous CPCs.28 The mitigation of myocardial fibrosis in the noninfarcted region (Fig. 4) could be one manifestation of such a paracrine action of transplanted CPCs. Another could be the activation of endogenous (EGFPneg) cells, such as c-kitpos CPCs, which in turn would generate new cardiac cells. This possibility is supported by several observations: i) the content of BrdUpos and Ki67pos cells was increased in both the infarcted and the noninfarcted regions of CPC-treated hearts (Fig. 6 and supplemental Fig. 20), indicating that the infusion of CPCs was associated with cellular proliferation; ii) although in the CPC-treated hearts exhibiting EGFPpos cells most of this proliferative activity was accounted for by the transplanted (EGFPpos) cells or their progeny (Fig. 6D), BrdU incorporation was also increased in CPC-treated EGFPneg hearts (Fig. 6C), indicating that the exogenous CPCs induced proliferation of endogenous cells; iii) more directly, CPC-treated hearts contained more c-kitpos cells than vehicle-treated hearts, independently of the presence of EGFPpos cells (Fig. 7) (the increased content of c-kitpos cells in EGFPpos hearts shown in Fig. 7 cannot be accounted for by the transplanted EGFPpos cells because in these hearts c-kitpos cells were deliberately counted in areas devoid of EGFPpos cells); iv) the percentage of total c-kitpos cells that were new (i.e., formed in the last two weeks of life), as detected by BrdU incorporation, was significantly increased in CPC-treated hearts, even in those with no EGFPpos cells (Fig. 7D); v) the amount of viable tissue in the risk region was augmented by CPC infusion even in hearts devoid of EGFPpos cells (supplemental Fig. 19A), suggesting formation of new myocytes from endogenous CPCs; vi) the amount of viable tissue in the risk region correlated significantly with the number of c-kitpos cells (Fig. 7E); vii) more directly, the number of c-kitpos/EGFPneg cells that expressed markers of cardiomyogenic differentiation (Nkx2.5 and MHC) was increased in CPC-treated hearts, both in the risk and in the noninfarcted regions (Figs. 8A and B), suggesting an increase in the number of endogenous CPCs committed to cardiac lineage; and viii) the number of new (BrdU labeled) α–sarcomeric actinpos cells was also increased in CPC-treated hearts (Fig. 8C and D), suggesting increased formation of new myocytes.
The most straightforward and plausible interpretation of all of these facts is that exogenous CPCs promoted proliferation of endogenous CPCs resulting in formation of new cardiac cells. Robust quantification of this phenomenon, however, is limited by the inability to track endogenous CPCs effectively through commitment and loss of c-kit expression after CPC treatment. Although our quantitative analysis revealed that the absolute number of new (BrdUpos) α–sarcomeric actinpos cells was small (20–206 cells/mm2)(Fig. 8D), and although we rarely observed BrdU labeling in cells with an adult myocyte phenotype, it should be kept in mind that BrdU was administered only in the last 2 weeks of life; thus, the magnitude of total new myocyte formation cannot be ascertained from the present data.
Conclusions
This is the first study to demonstrate that intracoronary delivery of CPCs improves LV structure and function even when implemented as late as 1 month after MI, when the infarcted tissue has been replaced by a scar. This is also the first study to provide evidence that infusion of exogenous CPCs promotes the recruitment of endogenous CPCs, and that this phenomenon persists for at least five weeks after CPC transplantation. The present findings suggest new potential therapeutic applications for the use of autologous CPCs in the large population of patients with old MI and chronic ischemic cardiomyopathy.
Supplementary Material
Acknowledgments
We gratefully acknowledge Qiuli Bi for expert technical assistance during the course of these studies and Maiying Kong for expert consultation on statistical analysis.
FUNDING SOURCES
This study was supported in part by NIH Grants R01-HL-68088, HL-70897, HL-76794, HL-78825, HL-55757, HL- 74351, and HL-91202.
Footnotes
DISCLOSURES
None
CLINICAL PERSPECTIVE
Heart failure after myocardial infarction (MI) is one of the most prevalent causes of morbidity and mortality worldwide. While pharmaceutical and interventional therapies have greatly ameliorated this problem, new more effective approaches are urgently needed. The discovery of resident cardiac progenitor cells (CPCs) in the adult mammalian heart has sparked hope for development of an “ideal” cell type for cardiac reparative/regenerative therapy. These resident CPCs have been shown to commit to a myocardial lineage and can be harvested from the heart and expanded in culture. Previous in vivo studies have shown that administration of CPCs to rats with acute MI results in tissue regeneration and LV functional improvement. We sought to extend these findings by determining whether intracoronary infusion of CPCs (the most clinically relevant strategy) can improve function in the setting of an old MI (scar). We found that intracoronary infusion of CPCs into a scar produces beneficial structural and functional effects and that, although exogenous CPCs can differentiate into new cardiac cells, this mechanism is not sufficient to explain all of the observed benefits, suggesting paracrine effects. Infusion of exogenous CPCs activated endogenous CPCs, suggesting that this may be a major mechanism for the benefit. These data support the potential therapeutic utility of CPCs in patients with old MI.
References
- 1.Dimmeler S, Zeiher AM, Schneider MD. Unchain my heart: the scientific foundations of cardiac repair. J Clin Invest. 2005;115:572–583. doi: 10.1172/JCI24283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wollert KC, Drexler H. Clinical applications of stem cells for the heart. Circ Res. 2005;96:151–163. doi: 10.1161/01.RES.0000155333.69009.63. [DOI] [PubMed] [Google Scholar]
- 3.Abdel-Latif A, Bolli R, Tleyjeh IM, Montori VM, Perin EC, Hornung CA, Zuba-Surma EK, Al-Mallah M, Dawn B. Adult bone marrow-derived cells for cardiac repair: a systematic review and meta-analysis. Arch Intern Med. 2007;167:989–997. doi: 10.1001/archinte.167.10.989. [DOI] [PubMed] [Google Scholar]
- 4.Bearzi C, Rota M, Hosoda T, Tillmanns J, Nascimbene A, De Angelis A, Yasuzawa-Amano S, Trofimova I, Siggins RW, Lecapitaine N, Cascapera S, Beltrami AP, D’Alessandro DA, Zias E, Quaini F, Urbanek K, Michler RE, Bolli R, Kajstura J, Leri A, Anversa P. Human cardiac stem cells. Proc Natl Acad Sci U S A. 2007;104:14068–14073. doi: 10.1073/pnas.0706760104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Beltrami AP, Barlucchi L, Torella D, Baker M, Limana F, Chimenti S, Kasahara H, Rota M, Musso E, Urbanek K, Leri A, Kajstura J, Nadal-Ginard B, Anversa P. Adult cardiac stem cells are multipotent and support myocardial regeneration. Cell. 2003;114:763–776. doi: 10.1016/s0092-8674(03)00687-1. [DOI] [PubMed] [Google Scholar]
- 6.Rota M, Padin-Iruegas ME, Misao Y, De Angelis A, Maestroni S, Ferreira-Martins J, Fiumana E, Rastaldo R, Arcarese ML, Mitchell TS, Boni A, Bolli R, Urbanek K, Hosoda T, Anversa P, Leri A, Kajstura J. Local activation or implantation of cardiac progenitor cells rescues scarred infarcted myocardium improving cardiac function. Circ Res. 2008;103:107–116. doi: 10.1161/CIRCRESAHA.108.178525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dawn B, Stein AB, Urbanek K, Rota M, Whang B, Rastaldo R, Torella D, Tang XL, Rezazadeh A, Kajstura J, Leri A, Hunt G, Varma J, Prabhu SD, Anversa P, Bolli R. Cardiac stem cells delivered intravascularly traverse the vessel barrier, regenerate infarcted myocardium, and improve cardiac function. Proc Natl Acad Sci U S A. 2005;102:3766–3771. doi: 10.1073/pnas.0405957102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Askari AT, Unzek S, Popovic ZB, Goldman CK, Forudi F, Kiedrowski M, Rovner A, Ellis SG, Thomas JD, DiCorleto PE, Topol EJ, Penn MS. Effect of stromal-cell-derived factor 1 on stem-cell homing and tissue regeneration in ischaemic cardiomyopathy. Lancet. 2003;362:697–703. doi: 10.1016/S0140-6736(03)14232-8. [DOI] [PubMed] [Google Scholar]
- 9.Yamaguchi J, Kusano KF, Masuo O, Kawamoto A, Silver M, Murasawa S, Bosch-Marce M, Masuda H, Losordo DW, Isner JM, Asahara T. Stromal cell-derived factor-1 effects on ex vivo expanded endothelial progenitor cell recruitment for ischemic neovascularization. Circulation. 2003;107:1322–1328. doi: 10.1161/01.cir.0000055313.77510.22. [DOI] [PubMed] [Google Scholar]
- 10.Abbott JD, Huang Y, Liu D, Hickey R, Krause DS, Giordano FJ. Stromal cell-derived factor-1alpha plays a critical role in stem cell recruitment to the heart after myocardial infarction but is not sufficient to induce homing in the absence of injury. Circulation. 2004;110:3300–3305. doi: 10.1161/01.CIR.0000147780.30124.CF. [DOI] [PubMed] [Google Scholar]
- 11.Zhang M, Mal N, Kiedrowski M, Chacko M, Askari AT, Popovic ZB, Koc ON, Penn MS. SDF-1 expression by mesenchymal stem cells results in trophic support of cardiac myocytes after myocardial infarction. FASEB J. 2007;21:3197–3207. doi: 10.1096/fj.06-6558com. [DOI] [PubMed] [Google Scholar]
- 12.Linke A, Muller P, Nurzynska D, Casarsa C, Torella D, Nascimbene A, Castaldo C, Cascapera S, Bohm M, Quaini F, Urbanek K, Leri A, Hintze TH, Kajstura J, Anversa P. Stem cells in the dog heart are self-renewing, clonogenic, and multipotent and regenerate infarcted myocardium, improving cardiac function. Proc Natl Acad Sci U S A. 2005;102:8966–8971. doi: 10.1073/pnas.0502678102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Urbanek K, Torella D, Sheikh F, De Angelis A, Nurzynska D, Silvestri F, Beltrami CA, Bussani R, Beltrami AP, Quaini F, Bolli R, Leri A, Kajstura J, Anversa P. Myocardial regeneration by activation of multipotent cardiac stem cells in ischemic heart failure. Proc Natl Acad Sci U S A. 2005;102:8692–8697. doi: 10.1073/pnas.0500169102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cheng W, Reiss K, Li P, Chun MJ, Kajstura J, Olivetti G, Anversa P. Aging does not affect the activation of the myocyte insulin-like growth factor-1 autocrine system after infarction and ventricular failure in Fischer 344 rats. Circ Res. 1996;78:536–546. doi: 10.1161/01.res.78.4.536. [DOI] [PubMed] [Google Scholar]
- 15.Ono K, Matsumori A, Shioi T, Furukawa Y, Sasayama S. Enhanced Expression of Hepatocyte Growth Factor/c-Met by Myocardial Ischemia and Reperfusion in a Rat Model. Circulation. 1997;95:2552–2558. doi: 10.1161/01.cir.95.11.2552. [DOI] [PubMed] [Google Scholar]
- 16.King SB, 3rd, Smith SC, Jr, Hirshfeld JW, Jr, Jacobs AK, Morrison DA, Williams DO, Feldman TE, Kern MJ, O’Neill WW, Schaff HV, Whitlow PL, Adams CD, Anderson JL, Buller CE, Creager MA, Ettinger SM, Halperin JL, Hunt SA, Krumholz HM, Kushner FG, Lytle BW, Nishimura R, Page RL, Riegel B, Tarkington LG, Yancy CW. 2007 Focused Update of the ACC/AHA/SCAI 2005 Guideline Update for Percutaneous Coronary Intervention: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines: 2007 Writing Group to Review New Evidence and Update the ACC/AHA/SCAI 2005 Guideline Update for Percutaneous Coronary Intervention, Writing on Behalf of the 2005 Writing Committee. Circulation. 2008;117:261–295. doi: 10.1161/CIRCULATIONAHA.107.188208. [DOI] [PubMed] [Google Scholar]
- 17.Johnston PV, Sasano T, Mills K, Evers R, Lee ST, Smith RR, Lardo AC, Lai S, Steenbergen C, Gerstenblith G, Lange R, Marban E. Engraftment, differentiation, and functional benefits of autologous cardiosphere-derived cells in porcine ischemic cardiomyopathy. Circulation. 2009;120:1075–1083. 1077. doi: 10.1161/CIRCULATIONAHA.108.816058. p following 1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fishbein MC, Maclean D, Maroko PR. Experimental myocardial infarction in the rat: qualitative and quantitative changes during pathologic evolution. Am J Pathol. 1978;90:57–70. [PMC free article] [PubMed] [Google Scholar]
- 19.Shabbir A, Zisa D, Suzuki G, Lee T. Heart failure therapy mediated by the trophic activities of bone marrow mesenchymal stem cells: a noninvasive therapeutic regimen. Am J Physiol Heart Circ Physiol. 2009;296:H1888–1897. doi: 10.1152/ajpheart.00186.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Heusch G, Schulz R, Rahimtoola SH. Myocardial hibernation: a delicate balance. Am J Physiol Heart Circ Physiol. 2005;288:H984–999. doi: 10.1152/ajpheart.01109.2004. [DOI] [PubMed] [Google Scholar]
- 21.Hochman JS, Choo H. Limitation of myocardial infarct expansion by reperfusion independent of myocardial salvage. Circulation. 1987;75:299–306. doi: 10.1161/01.cir.75.1.299. [DOI] [PubMed] [Google Scholar]
- 22.Stripecke R, Carmen Villacres M, Skelton D, Satake N, Halene S, Kohn D. Immune response to green fluorescent protein: implications for gene therapy. Gene Ther. 1999;6:1305–1312. doi: 10.1038/sj.gt.3300951. [DOI] [PubMed] [Google Scholar]
- 23.Kung SK, An DS, Bonifacino A, Metzger ME, Ringpis GE, Mao SH, Chen IS, Donahue RE. Induction of transgene-specific immunological tolerance in myeloablated nonhuman primates using lentivirally transduced CD34+ progenitor cells. Mol Ther. 2003;8:981–991. doi: 10.1016/j.ymthe.2003.08.020. [DOI] [PubMed] [Google Scholar]
- 24.Kang E, Giri N, Wu T, Sellers S, Kirby M, Hanazono Y, Tisdale J, Dunbar CE. In vivo persistence of retrovirally transduced murine long-term repopulating cells is not limited by expression of foreign gene products in the fully or minimally myeloablated setting. Hum Gene Ther. 2001;12:1663–1672. doi: 10.1089/10430340152528156. [DOI] [PubMed] [Google Scholar]
- 25.Stewart R, Yang C, Anyfantis G, Przyborski S, Hole N, Strachan T, Stojkovic M, Keith WN, Armstrong L, Lako M. Silencing of the expression of pluripotent driven-reporter genes stably transfected into human pluripotent cells. Regen Med. 2008;3:505–522. doi: 10.2217/17460751.3.4.505. [DOI] [PubMed] [Google Scholar]
- 26.Gnecchi M, He H, Noiseux N, Liang OD, Zhang L, Morello F, Mu H, Melo LG, Pratt RE, Ingwall JS, Dzau VJ. Evidence supporting paracrine hypothesis for Akt-modified mesenchymal stem cell-mediated cardiac protection and functional improvement. FASEB J. 2006;20:661–669. doi: 10.1096/fj.05-5211com. [DOI] [PubMed] [Google Scholar]
- 27.Kinnaird T, Stabile E, Burnett MS, Shou M, Lee CW, Barr S, Fuchs S, Epstein SE. Local delivery of marrow-derived stromal cells augments collateral perfusion through paracrine mechanisms. Circulation. 2004;109:1543–1549. doi: 10.1161/01.CIR.0000124062.31102.57. [DOI] [PubMed] [Google Scholar]
- 28.Gonzalez A, Rota M, Nurzynska D, Misao Y, Tillmanns J, Ojaimi C, Padin-Iruegas ME, Muller P, Esposito G, Bearzi C, Vitale S, Dawn B, Sanganalmath SK, Baker M, Hintze TH, Bolli R, Urbanek K, Hosoda T, Anversa P, Kajstura J, Leri A. Activation of cardiac progenitor cells reverses the failing heart senescent phenotype and prolongs lifespan. Circ Res. 2008;102:597–606. doi: 10.1161/CIRCRESAHA.107.165464. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.