Abstract
Hepatocellular carcinoma (HCC) is one of the most common malignant tumors worldwide. Hepatitis B virus X protein (HBx) contributes to the development of HCC, whereas HBx with COOH-terminal deletion is a frequent event in the HCC tissues. Previously, we identified a natural mutant of HBx-truncated 27 amino acids at the COOH-terminal (termed HBxΔ127), which strongly enhanced cell growth. In the present study, we focused on investigating the mechanism. Accordingly, fatty acid synthase (FAS) plays a crucial role in cancer cell survival and proliferation; thus, we examined the signaling pathways involving FAS. Our data showed that HBxΔ127 strongly increased the transcriptional activities of FAS in human hepatoma HepG2 and H7402 cells. Moreover, we found that 5-lipoxygenase (5-LOX) was responsible for the up-regulation of FAS by using MK886 (an inhibitor of 5-LOX) and 5-LOX small interfering RNA. We observed that HBxΔ127 could upregulate 5-LOX through phosphorylated extracellular signal-regulated protein kinases 1/2 and thus resulted in the increase of released leukotriene B4 (LTB4, a metabolite of 5-LOX) by ELISA. The additional LTB4 could upregulate the expression of FAS in the cells as well. Interestingly, we found that FAS was able to upregulate the expression of 5-LOX in a feedback manner by using cerulenin (an inhibitor of FAS). Collectively, HBxΔ127 promotes cell growth through a positive feedback loop involving 5-LOX and FAS, in which released LTB4 is involved in the up-regulation of FAS. Thus, our finding provides a new insight into the mechanism involving the promotion of cell growth mediated by HBxΔ127.
Introduction
Hepatitis B virus (HBV) infection is a major global health problem, and more than 350 million people in the world are infected by HBV [1]. The small 3.2-kb DNA genome of HBV has four partially overlapping open reading frames: preC/C, P, preS/S, and X gene. Hepatitis B virus X protein (HBx) is an important regulator that regulates many kinds of host processes by interaction with the virus and host factors [2]. HBx can disrupt normal expression of proteins involved in transcriptional regulation, cell cycle control, protein degradation pathways, apoptosis, genetic stability metabolism, immune response, and cell adhesion [3]. However, studies have demonstrated that mutations and deletions of HBx, especially the COOH-terminal deletion of HBx, are frequently events in the HBV-associated hepatocellular carcinoma tissues [4–6]. HBxΔ127 is a natural mutant of the HBx gene with deletion from 382 to 401 bp [7], which is similar to the report of Liu et al. [4]. Our data demonstrated that HBxΔ127 was able to upregulate transcriptional activities of nuclear factor κB, survivin, and human telomerase reverse transcriptase, as well as the expression levels of c-Myc and proliferating cell nuclear antigen in the hepatoma cells [7]. In addition, it has been reported that mutations of the HBx gene might produce uncontrolled growth and contribute to multistep hepatocarcinogenesis [8]. Therefore, the mutations of HBx gene are very important in hepatocarcinogenesis [4,9–12]. However, the mechanism of HBx mutant in hepatocarcinogenesis remains unclear. Hence, it is necessary to demonstrate its molecular mechanism.
HBx plays critical roles in the induction of various liver failures including up-regulation of lipid synthesis-related genes and dysregulation of hepatic metabolism [13]. However, the mechanism that HBx accumulates fatty acid is largely unknown because no proper system is available to study the pathogenesis. Fatty acid synthase (FAS) is an important enzyme involved in de novo lipid synthesis and triglyceride accumulation in hepatocytes by catalyzing the reaction of acetyl-CoA and malonyl-CoA into palmitate that is esterified into triglyceride [14]. Although constitutively expressed at a very low or undetectable level, the transcription of FAS gene is highly upregulated in various human malignancies [15]. Inhibition of FAS showed strong antitumor effects, including cell cycle arrest and apoptosis in various human cancer cells, such as breast, prostate, colon, and ovarian cell lines [16–19]. Treatment of human breast and prostate cancer xenografts in athymic mice with FAS inhibitors has shown a significant antitumor effect without toxicity to proliferating normal tissues such as bone marrow, skin, liver, and gastrointestinal tract [18,19]. Importantly, the treatment of tumor cells with pharmacologic inhibitors of FAS leads to cell cycle arrest followed by apoptosis of the tumor cells [20–22]. Therefore, the highly expressed FAS is considered as a molecular marker in cancer cells [23]. Several studies suggested that FAS expression is primarily regulated at the transcriptional level by sterol regulatory element binding protein 1 (SREBP-1) [24]. Shimano et al. [25] found that SREBPs mediated FAS transcriptional regulation in the liver and adipose tissue by overexpressing the constitutively active mature forms of SREBP-1. Another study also showed that the HBx protein could induce the up-regulation of SREBP-1 and FAS by interacting with LXRα and could enhance the binding of LXRα to the LXR response element [26]. Thus, we suppose that HBxΔ127 may regulate cell proliferation through fatty acid metabolisms.
Recent studies demonstrated that arachidonic acid metabolites are associated with cancer development. Three key enzymes, including cyclooxygenase (COX), lipoxygenase (LOX), and epoxygenase (cytochrome P450, CYP4), can metabolize arachidonic acid to biologically active eicosanoids, such as leukotrienes (LTs), hydroperoxyeicosatetraenoic acids, hydroxy-eicosatetraenoic acid, and prostaglandins, which play an important role in cancer cell proliferation [27,28]. For instance, 5-lipoxygenase (5-LOX) converts arachidonic acid to hydroxyeicosatetraenoic acids or LTs, which are able to enhance proliferation, increase survival, and suppress the apoptosis of human cells. Our laboratory previously found that COX-2, 5-LOX, and phosphorylated extracellular signal-regulated protein kinases 1/2 (p-ERK1/2) were involved in the proliferation and migration of breast cancer LM-MCF-7 cells with high metastatic potential [29]. Therefore, we hypothesize that the promotion of cell growth mediated by HBxΔ127 maybe involved in arachidonic acid metabolism as well as FAS.
In the present study, we investigated the underlying mechanism involving the promotion of cell growth mediated by HBxΔ127. Our finding showed that HBxΔ127 could upregulate the expression of 5-LOX through p-ERK1/2. The released LTB4, a metabolite of 5-LOX, was able to upregulate the expression of FAS. Thus, we conclude that HBxΔ127 promotes cell growth through a positive feedback loop involving 5-LOX and FAS.
Materials and Methods
Cell Culture
Human hepatoma HepG2 and H7402 cells were maintained in Dulbecco's modified Eagle medium (Gibco, Santa Clara, CA) supplemented with heat-inactivated 10% fetal calf serum, 100 U/ml penicillin, and 100 mg/ml streptomycin in a humidified atmosphere of 5% CO2 and 95% air at 37°C.
Reagents, Plasmids, and Transfection
MK886, indomethacin (Indo), PD98059 (an inhibitor of mitogen-activated protein kinase kinase), and LTB4 were purchased from Sigma-Aldrich (St. Louis, MO). Cerulenin was purchased from Fermentek Ltd (Jerusalem, Israel). The enzyme immunoassay kit for measuring LTB4 was purchased from Adlitteram Diagnostic Laboratories (San Diego, CA). The plasmids of pCMV-X, pCMV-XΔ127, and pSilencer3.0-X were described previously [7,30]. The plasmids of pFAS-WT-Luc, pFAS-ΔSRE-Luc, SREBP-1c-571-Luc-WT, and pcDNA3-DN-SREBP-1 were obtained from Dr. Q. Liu (University of Saskatchewan, Canada) [31]. The small interfering RNA (siRNA) targeting messenger RNA (mRNA) of 5-LOX and the negative control siRNA were designed and synthesized by RiboBio (Guangzhou, China). The engineered cells of HepG2-X/H7402-X (stably transfected with the pCMV-X plasmid), HepG2-XΔ127/H7402-XΔ127 (stably transfected with the pCMV-XΔ127 plasmid), and HepG2-pCMV/H7402-pCMV (stably transfected with the empty pCMV-Tag2B vector plasmid) were generated in hepatoma HepG2 and H7402 cells, respectively, using the Lipofectamine method according to the manufacturer's protocol (Invitrogen, Carlsbad, CA). The successful stable transfection of HBx and HBxΔ127 genes were confirmed by reverse transcription-polymerase chain reaction (RT-PCR) and Western blot analyses.
Nitro Blue Tertrazolium Assay
To examine the cytotoxicity mediated by the tested inhibitors, the HepG2 and the HepG2-XΔ127 (or H7402 and H7402-XΔ127) cells were cultured in a 96-well plate for 24 hours as previously mentioned; after that, the cells were recultured in serum-free medium for 12 hours. In brief, HepG2 and HepG2-XΔ127 (or H7402 and H7402-XΔ127) cells were treated with MK886 (5, 10, or 20 µM) for 6 hours, with Indo (10, 20, or 50 µM) for 6 hours, and with cerulenin (2.5, 5, or 10 µg/ml) for 12 hours, respectively. The cell cytotoxicity was analyzed by the reduction of nitro blue tertrazolium (NBT, Sigma-Aldrich). Briefly, 0.75 µM NBT was added into the previously mentioned treated cells, which were then incubated at 37°C for 1 hour. Then, the cells were observed under a light microscope to examine at an absorbance of 560 nm. The conditions of the NBT assay were adapted from Wei et al. [32].
Treatments of Tumor Cells
HepG2 and HepG2-XΔ127 cells (or H7402 and H7402-XΔ127 cells) were cultured in serum-free medium for 12 hours. Briefly, the engineered cells were treated with MK886 (5, 10, or 20 µM) for 6 hours and with Indo (10, 20, or 50 µM) for 6 hours, respectively. The engineered cells were treated with PD98059 (20, 35, or 50 µM) for 4 hours. In a separate experiment, the supernatants derived from the HepG2-XΔ127 (or H7402-XΔ127) cell cultures, termed HepG2-XΔ127 CM (or H7402-XΔ127 CM) and the boiled supernatants HepG2-XΔ127 BCM (or H7402-XΔ127 BCM), were harvested and stored at -80°C until use as a conditioned medium for cell treatment. HepG2-XΔ127 (or H7402-XΔ127) cells were pretreated by the inhibitors, such as MK886 or Indo, for 6 hours and were subsequently recultured in fresh medium for 48 hours after three washes with the fresh medium; the supernatants were harvested and stored at -80°C until use as a conditioned medium. HepG2 (or H7402) cells were cultured for 48 hours, followed by treatment with 50% or 100% HepG2-XΔ127 CM (or H7402-XΔ127 CM) and HepG2-XΔ127 BCM (or H7402-XΔ127 BCM) for 24 hours, as controls HepG2 (or H7402) cells were also treated with 100% HepG2-XΔ127 CM (or H7402-XΔ127 CM), which were pretreated with 20 µM MK886 for 6 hours. In this study, HepG2 (or H7402) cells were treated with LTB4 (0.1, 1, 10, or 100 nM) for 3, 6, and 12 hours. HepG2 (or H7402) cells were treated with 100 nM LTB4, followed by the treatment with MK886 (5, 10, or 20 µM) for 6 hours. HepG2 and HepG2-XΔ127 (or H7402 and H7402-XΔ127) cells were treated with cerulenin (2.5, 5, or 10 µg/ml) for 12 hours. The treated cells were subjected to luciferase reporter gene assays, real-time PCR, immunoblot analysis, and enzyme-linked immunosorbent assay (ELISA), respectively.
Luciferase Reporter Gene Assays
The treated cells were harvested after 48 hours and lysed in 1x passive lysis buffer. The luciferase activity was determined by using Dual-Luciferase Reporter Assay System (Promega, Madison, WI) on a Luminometer (TD-20/20; Turner Designs, Sunnyvale, CA) according to the manufacturer's instructions. The pCMV-Tag2B empty vector, pGL3-basic plasmid, and mock were used as controls. Luciferase activity was normalized for transfection efficiency using the corresponding Renilla luciferase activity. All experiments were performed at least three times. The data of luciferase reporter gene assays were analyzed by Student's t test for statistical differences. P < .05 was considered statistically significant.
RNA Extraction and Real-time PCR
Extraction of total RNA of the cells and reverse transcription were carried out as described previously [33]. Real-time PCR was performed using the SYBR Premix Ex Taq II PCR kit (Takara, China). One microliter of each RT reaction was PCR-amplified in a 25-µl aliquot containing 2.5 mM MgCl2, 0.4 µM gene-specific primers, and 1x SYBR Green Master Mix. Amplification was performed on an iQ5 Real-time PCR System (Bio-Rad, Hercules, CA) according to the manufacturer's instructions. The relative amounts of mRNA were calculated using the ΔΔCt method [34] with β-actin as the endogenous reference gene amplified from the samples. The sequences of the primers are given in Table W1.
RNA Interference Experiment
HepG2-XΔ127 (or H7402-XΔ127) cells were transfected with a pSilencer-X vector that produces siRNA that targets the HBxΔ127 mRNA or control siRNA [30]. Duplex siRNA targeting bases 315 to 335 (5′-GCGCAAGTACTGGCTGAATGA-3′) of the human 5-LOX mRNA (NM_000698) were introduced into HepG2-XΔ127 (or H7402-XΔ127) cells according to the manufacturer's protocol. Each experiment included controls containing the transfection reagent with control siRNA. Luciferase reporter gene assays and real-time PCR were performed 48 hours after the transfection.
Western Blot Analysis
The treated cells were washed three times with ice-cold PBS and extracted directly in the lysis buffer (62.5 mM Tris-HCl, pH 6.8, 2% SDS, 5% 2-mercaptoethanol, 10% glycerol), respectively. The equal amounts of protein (30 µg) were separated by 12% SDS-polyacrylamide gel electrophoresis and transferred onto a poly vinilidene difluoride membranes for 90 minutes. The membrane was blocked in blocking buffer (PBS, 5% skim milk, 0.1% Tween 20) at room temperature for 2 hours and then incubated with the appropriate primary antibody (diluted in blocking buffer). The primary antibodies included anti-Flag and anti-actin (Sigma-Aldrich), anti-HBx (Abcam, Cambridge, UK), anti-5-LOX (Santa Cruz Biotechnology, Santa Cruz, CA), and anti-p-ERK1/2 (Cell Signaling Technology, Danvers, MA). The membranes were washed thee times in PBS (0.1% Tween 20) and incubated for 1 hour with the secondary antibody (peroxidase-conjugated antirabbit or antimouse immunoglobulin G). The membranes were then washed thee times, and the bands were visualized by an enhanced chemiluminescence reagent (Amersham Pharmacia Biotech, Piscataway, NJ).
Enzyme-Linked Immunosorbent Assay
The amount of LTB4 (a metabolite of 5-LOX) was determined by ELISA according to the manufacturer's instructions. The concentration of LTB4 was normalized to the total protein. The protein concentrations in these extracts were determined using a standard protein assay method (Bio-Rad Laboratories, Inc, Hercules, CA).
Flow Cytometry Analysis
The detailed procedures of flow cytometry analysis were performed accordingly [7]. Briefly, HepG2-XΔ127 (or H7402-XΔ127) cells were grown in serum-free Dulbecco's modified Eagle medium for 12 hours and then treated with 10 µg/ml cerulenin for 12 hours and 20 µM MK886 for 6 hours, respectively. At the end of incubation, cells were harvested and washed twice in PBS and resuspended in 200 µl of PBS followed by the addition of 2 ml of 70% ice-cold ethanol and fixed overnight at 4°C. Then, 100 µl of RNaseA (1 mg/ml) and 100 µl of propidium iodide (100 mg/ml) were added into the cell suspensions and incubated at 37°C for 30 minutes, followed by examination of cell proliferation by FACScan flow cytometer (Becton Dickinson, San Jose, CA). Cell proliferative index (PI) is the sum of the S and G2/M phase activities of the cell cycle expressed as a fraction of the total cell population, i.e., PI = [(S + G2 / M) / (G0 / G1 + S + G2 /M)] x 100 [7]. Data are representative of three independent experiments.
Statistical Analysis
Statistical analyses were performed using SigmaPlot 2001 (Systat Software, Inc, Richmond, CA; http://www.systat.com). Statistical significance was assessed by comparing the mean values (±SD) using a Student's t test or χ2 test.
Results
HBxΔ127 Upregulates Expression of FAS
Our previous study showed that HBxΔ127 could significantly promote the proliferation of hepatoma cells [7]. Here, we try to identify the molecular mechanism. Recently, it has been reported that FAS is associated with the progression of many kinds of tumor and that FAS is primarily regulated at the transcriptional level. To investigate whether FAS is involved in promoting cell proliferation mediated by HBxΔ127, we examined the effect of HBxΔ127 on the transcription of FAS. First, we established the engineered cell lines including HepG2-X (or H7402-X) and HepG2-XΔ127 (or H7402-XΔ127). The expressions of HBx and HBxΔ127 at the mRNA and protein levels in the engineered cells were determined by RT-PCR (Figure 1A) and Western blot analysis (Figure 1B), respectively.
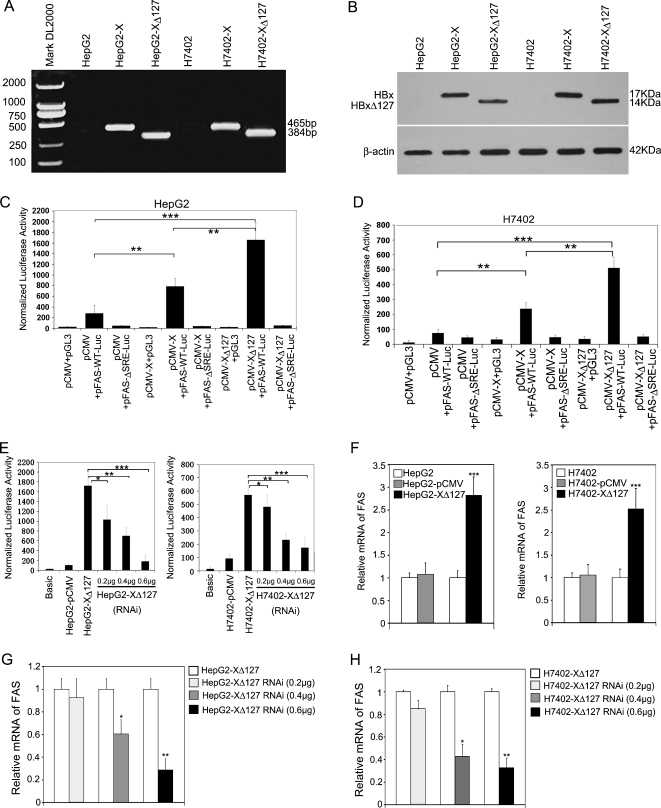
Figure 1.
HBxΔ127 upregulates the expression of FAS in hepatoma HepG2 (or H7402) cells. (A) HBx and HBxΔ127 genes were identified in HepG2-X and HepG2-XΔ127 (or H7402-X and H7402-XΔ127) cells by RT-PCR. (B) The expression of HBx and HBxΔ127 was detected by immunoblot analysis. (C) Luciferase reporter gene assay showed that the transcriptional activities of FAS were higher in HepG2 cells when cotransfecting the plasmids of pFAS-WT-Luc and pCMV-X or pCMV-XΔ127 than controls by transient transfection, suggesting that HBxΔ127 has stronger ability to upregulate FAS than HBx. However, pCMV-X or pCMV-XΔ127 failed to stimulate pFAS-ΔSRE-Luc (a mutant of FAS promoter) as control. The data are representative of three independent experiments. Values represent mean ± SD (n = 3). *P < .05, **P < .01, ***P < .001 (Student's t test). (D) The previously mentioned experiments were repeatable in another hepatoma H7402 cells. (E) In stable transfection system, pSilencer-X plasmid (RNAi targeting mRNA of HBxΔ127) could abolish the enhancement of transcriptional activities of FAS in HepG2-XΔ127 (or H7402-XΔ127) cells by luciferase reporter gene assay in a dose-dependent manner. The data are representative of three independent experiments. Values represent mean ± SD (n = 3). *P < .05, **P < .01, ***P < .001 (Student's t test). (F) The expression levels of FAS mRNA in HepG2-XΔ127 (or H7402-XΔ127) cells were examined by real-time PCR. Plotted are the means ± SD of three samples normalized to β-actin. Statistically significant differences relative to FAS mRNA levels in HepG2 (or H7402) cells, arbitrarily set to 1.0, are indicated: *P < .05, **P < .01, ***P < .001 (Student's t test). (G, H) The expression levels of FAS mRNA in HepG2-XΔ127 (or H7402-XΔ127) cells transfected with a pSilencer-X plasmid were examined by real-time PCR. Plotted are the means ± SD of three samples normalized to β-actin. Statistically significant differences relative to FAS mRNA levels in HepG2-XΔ127 (or H7402-XΔ127) cells, arbitrarily set to 1.0, are indicated: *P < .05, **P < .01, ***P < .001 (Student's t test).
Then, we investigated whether HBxΔ127 could influence the transcriptional activity of FAS using pFAS-WT-Luc, a FAS promoter-luciferase reporter. The finding showed that HBxΔ127 could significantly increase the transcriptional activity of FAS relative to the controls (P < .01, Student's t test; Figure 1, C and D). Meanwhile, RNA interference (RNAi) targeting HBx mRNA was able to abolish the upregulation of FAS (P < .001, Student's t test; Figure 1E). In addition, we found that HBxΔ127 was able to upregulate FAS at the mRNA level in HepG2-XΔ127 (or H7402-XΔ127) cells (P < .001, Student's t test; Figure 1F). The down-regulation of HBxΔ127 mediated by RNAi targeting HBx mRNA could abolish the up-regulation of FAS at the mRNA level (P < .001, Student's t test; Figure 1, G and H).
HBxΔ127 Upregulates Transcriptional Activity of FAS through SREBP-1
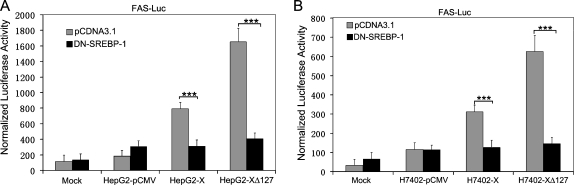
Because SREBP-1 is a major transcriptional factor for the transcriptional regulation of FAS by binding to the SRE site, we examined the transcriptional activity of FAS by using FAS-ΔSRE-Luc, a truncated FAS promoter-luciferase reporter without the SREBP binding site (ΔSRE). Our data showed that the luciferase activity of FAS was barely detected by pFAS-ΔSRE-Luc promoter reporter (Figure 1, C and D). Furthermore, to verify the involvement of SREBP-1 in HBxΔ127-induced FAS up-regulation, the DN-SREBP-1, a plasmid encoding a dominant-negative (DN) mutant SREBP-1 protein was transfected into HepG2-XΔ127 (or H7402-XΔ127) stable cells, together with the wild-type FAS promoter reporter. The results revealed that the luciferase activity of FAS was significantly decreased in DN-SREBP-1-transfected cells while having no effect in control vector-transfected cells (P < .001, Student's t test; Figure 2, A and B). Thus, these data suggest that the up-regulation of FAS mediated by HBxΔ127 is involved in the SREBP-1-FAS pathway.
Figure 2.
HBxΔ127 upregulates FAS promoter activity through SREBP-1. (A) The transcriptional activity of FAS was examined by luciferase reporter gene assay in HepG2-XΔ127 cells when cotransfection was performed using plasmids of pCDNA3.1 (empty vector) or DN-SREBP-1 (a mutant of SREBP-1). The data showed that DN-SREBP-1 was able to inhibit the transcriptional activity of FAS by competition with wild type SREBP-1 in HepG2-XΔ127 cells, suggesting that HBxΔ127 upregulates FAS promoter activity through SREBP-1. Data are representative of three independent experiments. Values represent mean ± SD (n = 3). ***P < .001 (Student's t test). (B) The previously mentioned experiments were repeatable in H7402-XΔ127 cells. Data are representative of three independent experiments. Values represent mean ± SD (n = 3). ***P < .001 (Student's t test).
5-LOX Is Responsible for the Up-regulation of FAS
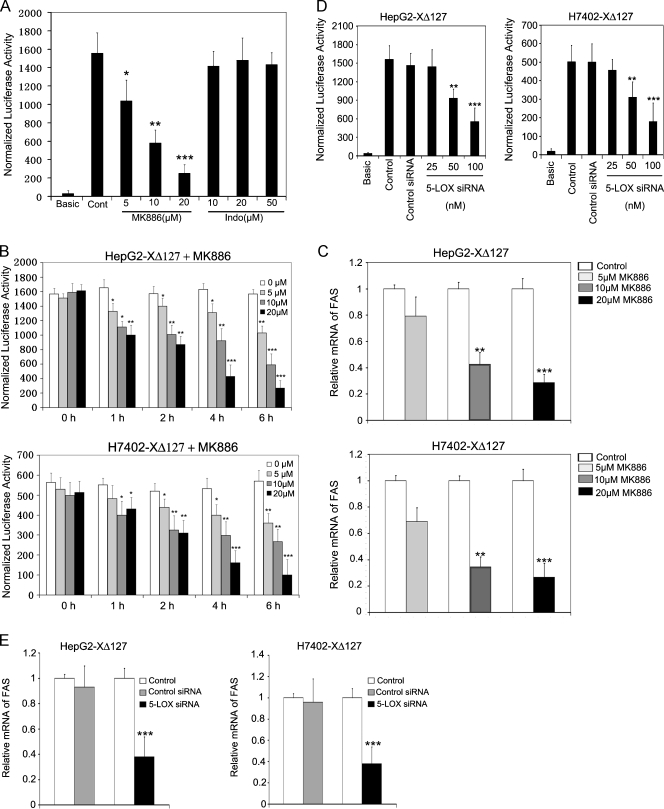
Arachidonic acid is an important intermediate in lipid metabolism, whose catalyzing enzymes, COX-2 and 5-LOX, are overexpressed during multistage tumor progression in many neoplastic disorders including lung, breast, and pancreatic cancers and hepatocellular carcinoma integrated HBx gene in host hepatocytes [35,36]. Therefore, we suppose that the arachidonic acid metabolism may be correlated with the up-regulation of FAS mediated by HBxΔ127. First, we examined the cytotoxicity of MK886 (a specific 5-LOX inhibitor), Indo (a COX-2 inhibitor), and cerulenin (a FAS inhibitor) in HepG2 and HepG2-XΔ127 (or H7402 and H7402-XΔ127) cells, respectively, by NBT assay. The data showed that the dose of inhibitors used in this experiment has no cytotoxicity to HepG2 and HepG2-XΔ127 (or H7402 and H7402-XΔ127) cells (Figure W1). In addition, we also detected the effect of these inhibitors on the expression of HBxΔ127 in HepG2-XΔ127 (or H7402-XΔ127) cells by immunoblot analysis. The result showed that MK886, Indo, and cerulenin could not affect the expression of HBxΔ127 (Figure W2), respectively. Furthermore, we treated the engineered cells with MK886 and Indo for 6 hours. We found that MK886 could abolish the up-regulation of FAS in HepG2-XΔ127 (or H7402-XΔ127) cells in a dose-dependent manner by luciferase reporter gene assays (P < .05, vs control, Student's t test; Figure 3, A and B) and real-time PCR (P < .05, vs control, Student's t test; Figure 3C). However, treatment with Indo failed to affect the expression level of FAS (Figure 3, A–C). In addition, we found that siRNA targeting of the mRNA of 5-LOX could significantly attenuate the mRNA levels of FAS in HepG2-XΔ127 (or H7402-XΔ127) as well (P < .05, vs control, Student's t test; Figure 3, D and E), which is consistent with the previously mentioned observation. As controls, we treated HepG2 (or H7402) cells with MK886 and Indo for 6 hours. The results showed that MK886 was able to decrease the intrinsic FAS in HepG2 (or H7402) cells rather than Indo (Figures W3 and W4). Thus, our finding suggests that 5-LOX is responsible for the up-regulation of FAS mediated by HBxΔ127.
Figure 3.
5-LOX is responsible for the up-regulation of FAS mediated by HBxΔ127. (A) The transcriptional activities of FAS were examined by luciferase reporter gene assay in HepG2-XΔ127 cells when the MK886 (an inhibitor of 5-LOX) and Indo (an inhibitor of COX-2) were used, respectively. Data are representative of three independent experiments. Values represent mean ±S D (n = 3). *P < .05 versus control, **P < .01 versus control, ***P < .001 versus control (Student's t test). (B) The experiments were repeated in HepG2-XΔ127 (or H7402-XΔ127) cells in a dose-dependent and time course manner. Data are representative of three independent experiments. Values represent mean ± SD (n = 3). *P < .05 versus control, **P < .01 versus control, ***P < .001 versus control (Student's t test). (C) HepG2-XΔ127 (or H7402-XΔ127) cells (2 x 105) were incubated with or without MK886 (5, 10, and 20 µM) for 6 hours. The mRNA levels of FAS were examined by real-time PCR. Plotted are the means ± SD of three samples normalized to β-actin. Statistically significant differences relative to FAS mRNA levels in HepG2-XΔ127 (or H7402-XΔ127) cells, arbitrarily set to 1.0, are indicated: *P < .05, **P < .01, ***P < .001 (Student's t test). (D) HepG2-XΔ127 cells were transfected with 100 nM 5-LOX siRNA or control siRNA for 48 hours. Then, the transcriptional activities of FAS were examined by luciferase reporter gene assay. Data are representative of three independent experiments. Values represent mean ± SD (n = 3). **P < .01 versus control (Student's t test). (E) Real-time PCR showed the expression levels of FAS mRNA in control cells and 5-LOX siRNA-transfected HepG2-XΔ127 (or H7402-XΔ127) cells. Plotted are the means ± SD of three samples normalized to β-actin. Statistically significant differences relative to FAS mRNA levels in HepG2-XΔ127 (or H7402-XΔ127) cells, arbitrarily set to 1.0, are indicated: *P < .05, **P < .01, ***P < .001 (Student's t test).
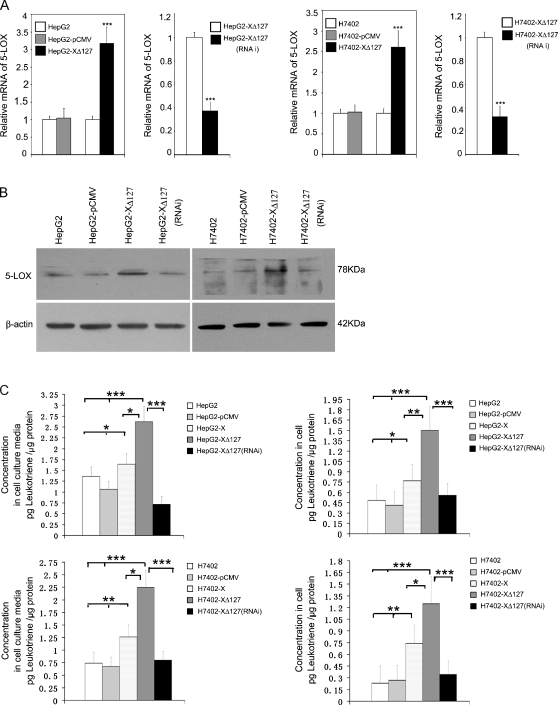
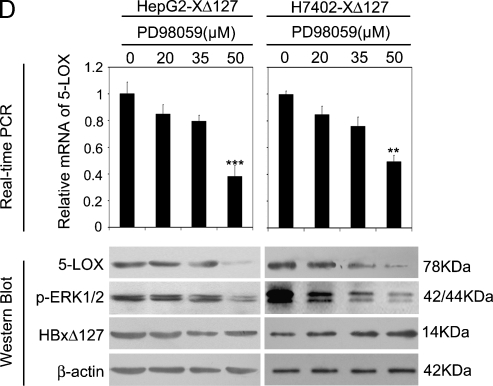
HBxΔ127 Upregulates the Expression of 5-LOX through p-ERK1/2
Accordingly, HBxΔ127 upregulates the expression of FAS, meanwhile 5-LOX is responsible for the up-regulation of FAS mediated by HBxΔ127, so we examined whether HBxΔ127 upregulated the expression of 5-LOX. Our data showed that, relative to controls, the expression level of 5-LOX was upregulated at the mRNA and protein levels in HepG2-XΔ127 (or H7402-XΔ127) cells by real-time PCR (P < .05, vs control, Student's t test; Figure 4A) and immunoblot analysis, respectively (Figure 4B). Interestingly, RNAi targeting mRNA of HBxΔ127 could abolish the up-regulation of 5-LOX (Figure 4, A and B), suggesting that HBxΔ127 is responsible for the up-regulation of 5-LOX. The up-regulation of 5-LOX should increase the amount of the metabolites of 5-LOX, so we determined the amount of LTB4, a metabolite of 5-LOX, in both of the conditioned media of HepG2/HepG2-XΔ127 (or H7402/H7402-XΔ127) cells and the cell lysates by ELISA. We found that the amount of LTB4 was higher in the conditioned medium of HepG2-XΔ127 (or H7402-XΔ127) cells relative to the conditioned medium of HepG2 (or H7402 cells), which could be abolished by RNAi targeting mRNA of HBxΔ127 (P < .05, Student's t test; Figure 4C). Our previous work found that p-ERK1/2 could upregulate cPLA2, COX-2, 5-LOX, and 12R-LOX expression in LM-MCF-7 cells or MDA-MB-231 cells [29]. Therefore, we supposed that p-ERK1/2 may be involved in the up-regulation of 5-LOX in HepG2-XΔ127 (or H7402-XΔ127) cells. Then, we examined the effect of p-ERK1/2 on the expression level of 5-LOX in HepG2-XΔ127 (or H7402-XΔ127) cells. We found that the treatment with 50 µM PD98059 (a specific inhibitor of mitogen-activated protein kinase kinase) could abolish the up-regulation of 5-LOX in HepG2-XΔ127 (or H7402-XΔ127) cells at the mRNA and protein levels by real-time PCR and Western blot analysis (Figure 4D). Thus, we conclude that HBxΔ127 is able to upregulate the expression of 5-LOX through p-ERK1/2.
Figure 4.
HBxΔ127 upregulates the expression levels of 5-LOX and increases the release of LTB4 (a metabolite of 5-LOX). (A) The expression level of 5-LOX was increased in HepG2-XΔ127 (or H7402-XΔ127) cells by real-time PCR, which was abolished by RNAi targeting mRNA of HBx gene using pSilencer3.0-X plasmid. Plotted are the means ± SD of three samples normalized to β-actin. Statistically significant differences relative to control, arbitrarily set to 1.0, are indicated: *P < .05, **P < .01, ***P < .001 (Student's t test). (B) The expression level of 5-LOX was examined by Western blot analysis as well. (C) The level of LTB4 was determined by ELISA in the conditioned medium or in the cell lysates from HepG2-X/HepG2-XΔ127 (or H7402-X/H7402-XΔ127) cells. The amount of LTB4 in HepG2/HepG2-pCMV (or H7402/H7402-pCMV) cells was used as control. Data are representative of three independent experiments. Values represent mean ± SD (n = 3). * P < .05, ** P < .01, ***P < .001 (Student's t test). (D) HepG2-XΔ127 (or H7402-XΔ127) cells were treated with or without PD98059 (20, 35, and 50 µM) for 4 hours. Then, we examined the mRNA levels of 5-LOX by real-time PCR. Plotted are the means ± SD of three samples normalized to β-actin. Statistically significant differences relative to FAS mRNA levels in HepG2-XΔ127 (or H7402-XΔ127) cells, arbitrarily set to 1.0, are indicated: *P < .05, **P < .01, ***P < .001 (Student's t test). The protein expression levels of 5-LOX were examined by Western blot analysis. The protein levels of HBxΔ127 and p-ERK1/2 were also determined as controls.
LTB4 Activates the Expression of FAS
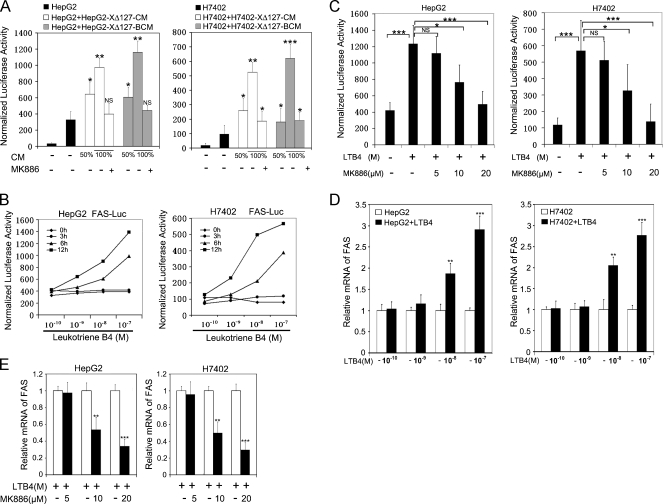
Accordingly, we identified whether LTB4 was involved in the upregulation of FAS. We examined the effect of conditioned medium from HepG2-XΔ127 (or H7402-XΔ127) cells on the expression of FAS in HepG2 (or H7402) cells. Luciferase reporter gene assay showed that the treatment with conditioned medium (boiled or not) from HepG2-XΔ127 (or H7402-XΔ127) cells led to a strong up-regulation of FAS in HepG2 (or H7402) cells in a dose-dependent manner. However, when HepG2 (or H7402) cells were treated with 100% HepG2-XΔ127 CM (or H7402-XΔ127 CM), which were pretreated with 20 µM MK886 for 6 hours, the up-regulation of FAS was abolished (P < .05, Student's t test; Figure 5A), suggesting that the released metabolites in the conditioned medium of HepG2-XΔ127 (or H7402-XΔ127) cells contribute to the up-regulation of FAS.
Figure 5.
LTB4 is involved in the up-regulation of FAS. (A) HepG2 (or H7402) cells were transfected with 0.3 µg of plasmid of FAS promoter-luciferase reporter gene, respectively. We treated the transfected cells with conditioned medium (CM) from HepG2-XΔ127 (or H7402-XΔ127) cells or boiled conditioned medium (BCM) from HepG2-XΔ127 (or H7402-XΔ127) cells at indicated the different concentration for 12 hours before the cell lysates were obtained and luciferase activity was measured. In addition, HepG2 (or H7402) cells were also treated with 100% HepG2-XΔ127 CM (or H7402-XΔ127 CM), which were pretreated with 20 µM MK886 for 6 hours. Data are representative of three independent experiments. Values represent mean ± SD (n = 3). *P < .05 versus control, **P < .01 versus control, ***P < .001 versus control (Student's t test). (B) HepG2 (or H7402) cells were transfected with 0.3 µg of plasmid of FAS promoter-luciferase reporter gene, respectively. We treated the transfected cells with the addition of 0.1, 1, 10, or 100 nM LTB4 or without LTB4 at different time points (3, 6, and 12 hours) before cell lysates were obtained and luciferase activity was measured. Data are representative of three independent experiments. P < .01 (Student's t test) (C) HepG2 (or H7402) cells were incubated with the addition of 100 nM LTB4 together with 5, 10, or 20 µM MK886 for 6 hours before cell lysates were obtained and luciferase activity was measured. Data are representative of three independent experiments. Values represent mean ± SD (n = 3). *P < .05 versus control, **P < .01 versus control, ***P < .001 versus control (Student's t test). (D) HepG2 (or H7402) cells were treated with the addition of LTB4 (0.1, 1, 10, or 100 nM) for 12 hours. Then, the FAS mRNA was examined by real-time PCR, respectively. Plotted are the means ± SD of three samples normalized to β-actin. Statistically significant differences relative to FAS mRNA levels in HepG2 (or H7402) cells, arbitrarily set to 1.0, are indicated: *P < .05, **P < .01, ***P < .001 (Student's t test). (E) HepG2 (or H7402) cells were incubated with the addition of 100 nM LTB4 together with 5, 10, or 20 µM MK886 for 6 hours. The mRNA levels of FAS were examined by real-time PCR, respectively. Plotted are the means ± SD of three samples normalized to β-actin. Statistically significant differences relative to FAS mRNA levels in HepG2 (or H7402) cells without MK886, arbitrarily set to 1.0, are indicated: *P < .05, **P < .01, ***P < .001 (Student's t test).
On cell activation, 5-LOX is translocated to the perinuclear membrane where it converts arachidonic acid into many metabolites, for example, bioactive lipid intermediate LTB4 [37]. To further confirm the role of LTB4 in the up-regulation of FAS, we pretreated HepG2 (or H7402) cells with exogenous LTB4. The data showed that exogenous LTB4 could upregulate the expression level of FAS in HepG2 (or H7402) cells in a dose-dependent manner by luciferase reporter gene assays (P < .01, Student's t test; Figure 5B) and real-time PCR (P < .05, Student's t test; Figure 5D), respectively. Notably, the exogeneous LTB4-mediated up-regulation of FAS could be completely abolished by pretreatment with 20 µM MK886 by luciferase reporter gene assays (P < .05, Student's t test; Figure 5C) and real-time PCR (P < .05, Student's t test; Figure 5E), suggesting that LTB4 is responsible for the upregulation of FAS.
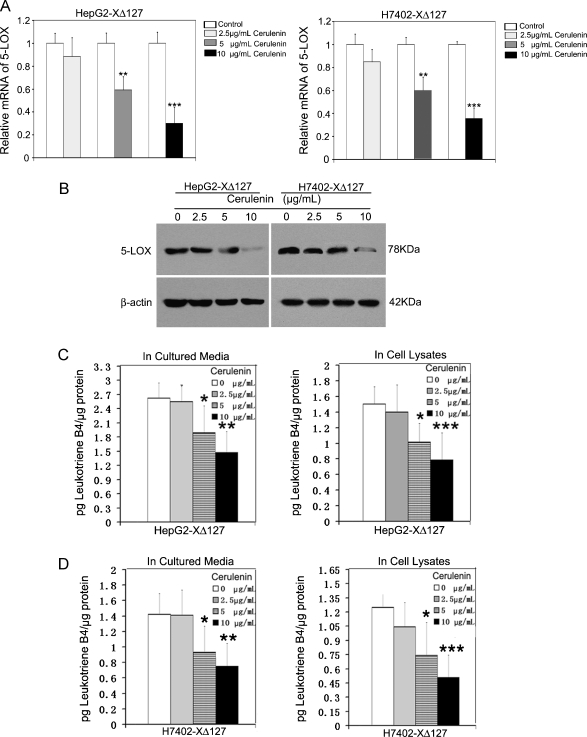
FAS Upregulates the Expression Level of 5-LOX in a Positive Feedback Manner
To further examine the signal transduction pathways involving 5-LOX and FAS, we suppose that FAS could activate 5-LOX in a positive feedback manner mediated by HBxΔ127 in HepG2-XΔ127 (or H7402-XΔ127) cells. Interestingly, the treatment with 10 µg/ml cerulenin, an inhibitor of FAS, could greatly downregulate the expression of 5-LOX at the mRNA and protein levels in HepG2-XΔ127 (or H7402-XΔ127) cells (P < .05, Student's t test; Figure 6, A and B). As controls, we treated HepG2 (or H7402) cells with 10 µg/ml cerulenin. The results revealed that the cerulenin could decrease the intrinsic 5-LOX in HepG2 (or H7402) cells (Figures W5 and W6). In addition, we examined the effect of cerulenin on LTB4 by ELISA. We found that 10 µg/ml cerulenin could largely decrease the amount of released LTB4 in the conditioned medium of HepG2-XΔ127 (or H7402-XΔ127) cells and cell lysates in a dose-dependent manner (P < .05, Student's t test; Figure 6C), suggesting that HBxΔ127 promotes and keeps the proliferation through a positive feedback loop involving FAS and 5-LOX.
Figure 6.
FAS contributes to the up-regulation of 5-LOX in a positive feedback loop manner. HepG2-XΔ127 (or H7402-XΔ127) cells (2 x 105) were incubated with or without cerulenin (2.5, 5, and 10 µg/ml) for 12 hours. (A) The mRNA level of 5-LOX was detected by real-time PCR. Plotted are the means ± SD of three samples normalized to β-actin. Statistically significant differences relative to 5-LOX mRNA levels in HepG2-XΔ127 (or H7402-XΔ127) cells without the action of cerulenin, arbitrarily set to 1.0, are indicated: *P < .05, **P < .01, ***P < .001 (Student's t test). (B) The protein expression level of 5-LOX was examined by Western blot analysis. (C, D) The amount of LTB4 was examined by ELISA in the conditioned medium or in the cell lysates from the previously mentioned treated HepG2-XΔ127 (or H7402-XΔ127) cells. Data are representative of three independent experiments. Values represent mean ±S D (n = 3). *P < .05 versus control, **P < .01 versus control, ***P < .001 versus control (Student's t test).
HBxΔ127 Enhances Cell Growth through FAS and 5-LOX
Furthermore, we investigated the effect of FAS and 5-LOX on the proliferation in the HepG2-XΔ127 (or H7402-XΔ127) cells by flow cytometry analysis. The results showed that 10 µg/ml cerulenin and 20 µM MK886 could significantly decrease the cell proliferation of HepG2-XΔ127 (or H7402-XΔ127) cells according to the percentage of cells in S phase and PI (P < .05 vs control, Student's t test; Figure W7), suggesting that HBxΔ127 enhanced cell proliferation by FAS and 5-LOX.
Discussion
FAS is a key enzyme capable of the reductive de novo synthesis of long-chain fatty acids from acetyl-CoA, malonyl-CoA, and nicotinamide adenine dinucleotide phosphate [38]. Its expression and activity are extremely low in nearly all nonmalignant adult tissues, whereas it is highly expressed in most human cancers as well as in precancerous lesions with a poor prognosis [39–43], with levels increasing as tumor grade and severity increase [39,40]. The up-regulation of FAS is associated with poor prognosis. Therefore, the enzyme has been recognized in recent years as a target of antitumor therapy [41]. Jensen et al. showed that the high expression of FAS together with a high PI of breast cancer cells (>17%) were associated with a nine-fold increased risk of patient mortality [42,44].
Increasing studies have suggested that the X gene contains a frequent deletion in COOH-terminal region [4–6]. In addition, it has been showed that the COOH-terminal-truncated HBx rather than the full-length HBx could effectively transform immortalized liver cell line MIHA in vitro and in vivo, and the expression profiling revealed differential expression of key genes that were involved in the control of cell cycle and apoptosis [12], which strongly suggest that the COOH-terminal-truncated HBx plays a key role in hepatocarcinogenesis through activating cell proliferation. In our present study, we used a C-terminal deletion of hepatitis B virus X protein (HBxΔ127) [7], which is similar to the report of Liu et al. [4], to demonstrate the molecular mechanism as a model. Interestingly, we found that HBxΔ127 upregulated the expression of FAS at the levels of transcriptional activity and mRNA, in which the effect of HBxΔ127 on up-regulating FAS was stronger relative to the wild-type HBx (Figure 1). Then, we demonstrated that the up-regulation of FAS was involved in the FAS-SREBP-1 pathway (Figure 2), which is consistent with the finding [26]. However, the mechanism is unclear. So we try to identify the activators involving the up-regulation of FAS mediated by HBxΔ127. It has been reported that arachidonic acid or its metabolites are able to stimulate tumor cell proliferation. Several studies have confirmed that the metabolites of 5-LOX were able to enhance cell proliferation and increase cell survival [12]. The expression of 5-LOX is increased in different human cancers, such as breast and prostate cancers [45]. Arachidonic acid can be converted to 5-hydroperoxyeicosatetraenoic acid by 5-LOX, and then to LTA4, all of which show certain levels of biologic activity in humans [46,47]. However, LTA4 can be further hydrolased to LTB4 by LTA4 hydrolase [48,49]. Several studies have demonstrated that LTB4 can enhance cell proliferation and suppress apoptosis in human cancer cells and that it is an important chemoattractant in the fields of immunology and hematology [46,48]. A recent study found that the expression levels of FAS, COX-2, and 5-LOX played important roles in mediating breast cancer formation [50]. Therefore, we present a hypothesis that arachidonic acid metabolism and FAS expression may contribute to sustaining a high proliferation rate mediated by the HBxΔ127.
Interestingly, we found that HBxΔ127 upregulated the expression of FAS in HepG2-XΔ127 (or H7402-XΔ127) cells through 5-LOX by using MK886 (a specific inhibitor of 5-LOX) rather than Indo (an inhibitor of COX-2) (Figure 3). Moreover, we confirmed that HBxΔ127 could increase the expression of 5-LOX through p-ERK1/2 in HepG2-XΔ127 (or H7402-XΔ127) cells (Figure 4). We also examined the amount of LTB4 (a metabolite of 5-LOX) by ELISA. The data showed that the amount of LTB4 was significantly increased in both of the conditioned medium of HepG2-XΔ127 (or H7402-XΔ127) cells and cell lysates relative to the controls (Figure 4C). So we used the conditioned medium of HepG2-XΔ127 (or H7402-XΔ127) cells to treat the HepG2 (or H7402) cells. The data showed that the conditioned media were able to upregulate the expression of FAS (Figure 5A), suggesting that the high level of LTB4 released in the conditioned medium was responsible for the up-regulation of FAS. To further confirm the role of LTB4 in the up-regulation of FAS, we used 100 nM exogenesis LTB4 to treat HepG2 (or H7402) cells. The results further demonstrated that LTB4 could upregulate the expression of FAS in HepG2 (or H7402) cells (Figure 5B), which could be abolished by the pretreatment of MK886 (Figure 5C). Thus, we conclude that HBxΔ127 enhances the expression of FAS through 5-LOX and released LTB4.
Previously, we reported that breast cancer cells grow faster in a positive feedback manner involving activated ERK and COX/LOX [29]. In this study, we suppose that HBxΔ127 may promote cell growth in a similar way, such as a positive feedback loop involving FAS and 5-LOX. Therefore, we used 10 µg/ml cerulenin (an inhibitor of FAS) to treat HepG2-XΔ127 (or H7402-XΔ127) cells. Interestingly, we found that the inactivation of FAS could downregulate the expression level of 5-LOX in HepG2-XΔ127 (or H7402-XΔ127) cells and decrease the amount of LTB4 (a metabolite of 5-LOX) in both of the conditioned media of HepG2-XΔ127 (or H7402-XΔ127) cells and cell lysates (Figure 6), suggesting that HBxΔ127 promotes cell growth through a positive feedback loop involving FAS and 5-LOX in hepatoma cells.
Taken together, our data reveal a novel signal transduction pathway involving a positive feedback loop between FAS and 5-LOX, which contributes to cell growth mediated by HBxΔ127. This finding provides a new insight into a mechanism of promotion of cell growth mediated by HBx mutants.
Supplementary Material
Abbreviations
- 5-LOX
5-lipoxygenase
- COX
cyclooxygenase
- FAS
fatty acid synthase
- HBx
hepatitis B virus X protein
- HCC
hepatocellular carcinoma
- Indo
indomethacin
- LTB4
leukotriene B4
- p-ERK1/2
phosphorylated extracellular signal-regulated protein kinases 1/2
- SREBP-1
sterol regulatory element binding protein 1
Footnotes
This work was supported by grants from the National Basic Research Program of China (973 Program; nos. 2007CB914804, 2007CB914802, and 2009CB521702) and the National Natural Science Foundation of China (no. 30670959).
This article refers to supplementary materials, which are designated by Table W1 and Figures W1 to W7 and are available online at www.neoplasia.com.
References
- 1.Lok AS. Chronic hepatitis B. N Engl J Med. 2002;346:1682–1683. doi: 10.1056/NEJM200205303462202. [DOI] [PubMed] [Google Scholar]
- 2.Murakami S. Hepatitis B virus X protein: a multifunctional viral regulator. J Gastroenterol. 2001;36:651–660. doi: 10.1007/s005350170027. [DOI] [PubMed] [Google Scholar]
- 3.Tang H, Oishi N, Kaneko S, Murakami S. Molecular functions and biological roles of hepatitis B virus X protein. Cancer Sci. 2006;97:977–983. doi: 10.1111/j.1349-7006.2006.00299.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liu XH, Wang L, Zhang SH, Lin J, Zhang SM, Feitelson MA, Gao HJ, Zhu MH. Mutations in the carboxyl terminus of the X protein of Hepatitis B Virus regulate Wnt-5a expression in hepatoma Huh7 cells: cDNA microarray and proteomic analyses. Carcinogenesis. 2008;29:1207–1214. doi: 10.1093/carcin/bgn111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liu XH, Lin J, Zhang SH, Zhang SM, Feitelson MA, Gao HJ, Zhu MH. COOH-terminal deletion of HBx gene is a frequent event in HBV-associated hepatocellular carcinoma. World J Gastroenterol. 2008;14:1346–1352. doi: 10.3748/wjg.14.1346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Iavarone M, Trabut JB, Delpuech O, Carnot F, Colombo M, Kremsdorf D, Brechot C, Thiers V. Characterisation of hepatitis B virus X protein mutants in tumour and non-tumour liver cells using laser capture microdissection. J Hepatol. 2003;39:253–261. doi: 10.1016/s0168-8278(03)00217-4. [DOI] [PubMed] [Google Scholar]
- 7.Zhang H, Shan CL, Li N, Zhang X, Zhang XZ, Xu FQ, Zhang S, Qiu LY, Ye LH, Zhang XD. Identification of a natural mutant of HBV X protein truncated 27 amino acids at the COOH terminal and its effect on liver cell proliferation. Acta Pharmacol Sin. 2008;29:473–480. doi: 10.1111/j.1745-7254.2008.00764.x. [DOI] [PubMed] [Google Scholar]
- 8.Sirma H, Giannini C, Poussin K, Paterlini P, Kremsdorf D, Brechot C. Hepatitis B Virus X mutants, present in hepatocellular carcinoma tissue abrogate both the antiproliferative and transactivation effects of HBx. Oncogene. 1999;26:4848–4859. doi: 10.1038/sj.onc.1202867. [DOI] [PubMed] [Google Scholar]
- 9.Liu X, Zhang S, Lin J, Zhang S, Feitelson MA, Gao H, Zhu MH. Hepatitis B virus X protein mutants exhibit distinct biological activities in hepatoma Huh7 cells. Biochem Biophys Res Commun. 2008;373:643–647. doi: 10.1016/j.bbrc.2008.06.087. [DOI] [PubMed] [Google Scholar]
- 10.Liu J, Lian Z, Han S, Waye MM, Wang H, Wu MC, Wu K, Ding J, Arbuthnot P, Kew M, et al. Down-regulation of E-cadherin by hepatitis B virus X antigen in hepatocellullar carcinoma. Oncogene. 2006;25:1008–1017. doi: 10.1038/sj.onc.1209138. [DOI] [PubMed] [Google Scholar]
- 11.Chen GG, Li MY, Ho RL, Chak EC, Lau WY, Lai PB. Identification of hepatitis B virus X gene mutation in Hong Kong patients with hepatocellular carcinoma. J Clin Virol. 2005;34:7–12. doi: 10.1016/j.jcv.2005.01.006. [DOI] [PubMed] [Google Scholar]
- 12.Ma NF, Lau SH, Hu L, Xie D, Wu J, Yang J, Wang Y, Wu MC, Fung J, Bai X, et al. COOH-terminal truncated HBV X protein plays key role in hepatocarcinogenesis. Clin Cancer Res. 2008;14:5061–5068. doi: 10.1158/1078-0432.CCR-07-5082. [DOI] [PubMed] [Google Scholar]
- 13.Kim KH, Shin HJ, Kim K, Choi HM, Rhee SH, Moon HB, Kim HH, Yang US, Yu DY, Cheong J. Hepatitis B virus X protein induces hepatic steatosis via transcriptional activation of SREBP1 and PPARγ. Gastroenterology. 2007;132:1955–1967. doi: 10.1053/j.gastro.2007.03.039. [DOI] [PubMed] [Google Scholar]
- 14.Semenkovich CF. Regulation of fatty acid synthase (FAS) Prog Lipid Res. 1997;36:43–53. doi: 10.1016/s0163-7827(97)00003-9. [DOI] [PubMed] [Google Scholar]
- 15.Little JL, Kridel SJ. Fatty acid synthase activity in tumor cells. Subcell Biochem. 2008;49:169–194. doi: 10.1007/978-1-4020-8831-5_7. [DOI] [PubMed] [Google Scholar]
- 16.Knowles LM, Axelrod F, Browne CD, Smith JW. A fatty acid synthase blockade induces tumor cell-cycle arrest by down-regulating Skp2. J Biol Chem. 2004;279:30540–30545. doi: 10.1074/jbc.M405061200. [DOI] [PubMed] [Google Scholar]
- 17.Menendez JA, Mehmi I, Atlas E, Colomer R, Lupu R. Novel signaling molecules implicated in tumor-associated fatty acid synthase-dependent breast cancer cell proliferation and survival: Role of exogenous dietary fatty acids, p53–p21WAF1/CIP1, ERK1/2 MAPK, p27KIP1, BRCA1, and NF-κB. Int J Oncol. 2004;24:591–608. [PubMed] [Google Scholar]
- 18.Pizer ES, Pflug BR, Bova GS, Han WF, Udan MS, Nelson JB. Increased fatty acid synthase as a therapeutic target in androgen-independent prostate cancer progression. Prostate. 2001;47:102–110. doi: 10.1002/pros.1052. [DOI] [PubMed] [Google Scholar]
- 19.Puig T, Vázquez-Martín A, Relat J, Pétriz J, Menéndez JA, Porta R, Casals G, Marrero PF, Haro D, Brunet J, et al. Fatty acid metabolism in breast cancer cells: differential inhibitory effects of epigallocatechin gallate (EGCG) and C75. Breast Cancer Res Treat. 2008;109:471–479. doi: 10.1007/s10549-007-9678-5. [DOI] [PubMed] [Google Scholar]
- 20.Jensen KC, Schaeffer DF, Cheang M, Montgomery K, West RB, Gilks CB, Ross D, Turashvili G, Schnitt S, van de Rijn M. Characterization of a novel anti-fatty acid synthase (FASN) antiserum in breast tissue. Mod Pathol. 2008;21:1413–1420. doi: 10.1038/modpathol.2008.163. [DOI] [PubMed] [Google Scholar]
- 21.Knowles LM, Yang C, Osterman A, Smith JW. Inhibition of fatty-acid synthase induces caspase-8-mediated tumor cell apoptosis by up-regulating DDIT4. J Biol Chem. 2008;283:31378–31384. doi: 10.1074/jbc.M803384200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bandyopadhyay S, Zhan R, Wang Y, Pai SK, Hirota S, Hosobe S, Takano Y, Saito K, Furuta E, Iiizumi M, et al. Mechanism of apoptosis induced by the inhibition of fatty acid synthase in breast cancer cells. Cancer Res. 2006;66:5934–5940. doi: 10.1158/0008-5472.CAN-05-3197. [DOI] [PubMed] [Google Scholar]
- 23.Kridel SJ, Lowther WT, Pemble CWIV. Fatty acid synthase inhibitors: new directions for oncology. Expert Opin Investig Drugs. 2007;16:1817–1829. doi: 10.1517/13543784.16.11.1817. [DOI] [PubMed] [Google Scholar]
- 24.McPherson S, Jonsson JR, Barrie HD, O'Rourke P, Clouston AD, Powell EE. Investigation of the role of SREBP-1c in the pathogenesis of HCV-related steatosis. J Hepatol. 2008;149:1046–1054. doi: 10.1016/j.jhep.2008.06.022. [DOI] [PubMed] [Google Scholar]
- 25.Shimano H, Horton JD, Shimomura I, Hammer RE, Brown MS, Goldstein JL. Isoform 1c of sterol regulatory element binding protein is less active than isoform 1a in livers of transgenic mice and in cultured cells. J Clin Invest. 1997;99:846–854. doi: 10.1172/JCI119248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kim K, Kim KH, Kim HH, Cheong J. Hepatitis B virus X protein induces lipogenic transcription factor SREBP1 and fatty acid synthase through the activation of nuclear receptor LXRa. Biochem J. 2008;416:219–230. doi: 10.1042/BJ20081336. [DOI] [PubMed] [Google Scholar]
- 27.Pidgeon GP, Lysaght J, Krishnamoorthy S, Reynolds JV, O'Byrne K, Nie D, Honn KV. Lipoxygenase metabolism: roles in tumor progression and survival. Cancer Metastasis Rev. 2007;26:503–524. doi: 10.1007/s10555-007-9098-3. [DOI] [PubMed] [Google Scholar]
- 28.Hammamieh R, Sumaida D, Zhang X, Das R, Jett M. Control of the growth of human breast cancer cells in culture by manipulation of arachidonate metabolism. BMC Cancer. 2007;7:138–147. doi: 10.1186/1471-2407-7-138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.You J, Mi D, Zhou X, Qiao L, Zhang H, Zhang X, Ye LH. A positive feedback between activated ERK and COX/LOX maintains proliferation and migration of breast cancer cells. Endocrinology. 2009;150:1607–1617. doi: 10.1210/en.2008-0616. [DOI] [PubMed] [Google Scholar]
- 30.Zhang XD, Dong N, Yin L, Cai N, Ma HT, You JC, Zhang H, Wang HH, He R, Ye LH. Hepatitis B virus X protein up-regulates survivin expression in hepatoma tissues. J Med Virol. 2005;77:374–381. doi: 10.1002/jmv.20466. [DOI] [PubMed] [Google Scholar]
- 31.Jackel-Cram C, Babiuk LA, Liu Q. Up-regulation of fatty acid synthase promoter by hepatitis C virus core protein: genotype-3a core has a stronger effect than genotype-1b core. J Hepatol. 2007;46:999–1008. doi: 10.1016/j.jhep.2006.10.019. [DOI] [PubMed] [Google Scholar]
- 32.Wei Y, Chen L, Chen J, Ge L, He R. Rapid glycation with d-ribose induces globular amyloid-like aggregations of BSA with high cytotoxicity to SH-SY5Y cells. BMC Cell Biol. 2009;10:10. doi: 10.1186/1471-2121-10-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Qin XR, Zhang H, Zhou XL, Wang CY, Zhang H, Zhang XD, Ye LH. Proliferation and migration mediated by Dkk-1/Wnt/β-catenin cascade in a model of hepatocellular carcinoma cells. Transl Res. 2007;150:281–294. doi: 10.1016/j.trsl.2007.06.005. [DOI] [PubMed] [Google Scholar]
- 34.Winer JC, Jung K, Shackel I, Williams PM. Development and validation of real-time quantitative reverse transcriptase-polymerase chain reaction for monitoring gene expression in cardiac myocytes in vitro. Anal Biochem. 1999;270:41–49. doi: 10.1006/abio.1999.4085. [DOI] [PubMed] [Google Scholar]
- 35.Covey TM, Edes K, Fitzpatrick FA. Akt activation by arachidonic acid metabolism occurs via oxidation and inactivation of PTEN tumor suppressor. Oncogene. 2007;26:5784–5792. doi: 10.1038/sj.onc.1210391. [DOI] [PubMed] [Google Scholar]
- 36.Cheng AS, Chan HL, Leung WK, To KF, Go MY, Chan JY, Liew CT, Sung JJ. Expression of HBx and COX-2 in chronic hepatitis B, cirrhosis and hepatocellular carcinoma: implication of HBx in up-regulation of COX-2. Mod Pathol. 2004;17:1169–1179. doi: 10.1038/modpathol.3800196. [DOI] [PubMed] [Google Scholar]
- 37.Montuschi P. Leukotrienes, antileukotrienes and asthma. Mini Rev Med Chem. 2008;8:647–656. doi: 10.2174/138955708784567395. [DOI] [PubMed] [Google Scholar]
- 38.Baron A, Migita T, Tang D, Loda M. Fatty acid synthase: a metabolic oncogene in prostate cancer? J Cell Biochem. 2004;91:47–53. doi: 10.1002/jcb.10708. [DOI] [PubMed] [Google Scholar]
- 39.Kuhajda FP. Fatty acid synthase and cancer: new application of an old pathway. Cancer Res. 2006;66:5977–5980. doi: 10.1158/0008-5472.CAN-05-4673. [DOI] [PubMed] [Google Scholar]
- 40.Menendez JA, Decker JP, Lupu R. In support of fatty acid synthase (FAS) as a metabolic oncogene: extracellular acidosis acts in an epigenetic fashion activating FAS gene expression in cancer cells. J Cell Biochem. 2005;94:1–4. doi: 10.1002/jcb.20310. [DOI] [PubMed] [Google Scholar]
- 41.Zhou W, Han WF, Landree LE, Thupari JN, Pinn ML, Bililign T, Kim EK, Vadlamudi A, Medghalchi SM, El Meskini R, et al. Fatty acid synthase inhibition activates AMP-activated protein kinase in SKOV3 human ovarian cancer cells. Cancer Res. 2007;67:2964–2971. doi: 10.1158/0008-5472.CAN-06-3439. [DOI] [PubMed] [Google Scholar]
- 42.Lupu R, Menendez JA. Targeting fatty acid synthase in breast and endometrial cancer: an alternative to selective estrogen receptormodulators? Endocrinology. 2006;147:4056–4066. doi: 10.1210/en.2006-0486. [DOI] [PubMed] [Google Scholar]
- 43.Jensen V, Ladekarl M, Holm-Nielsen P, Melsen F, Soerensen FB. The prognostic value of oncogenic antigen 519 (OA-519) expression and proliferative activity detected by antibody MIB-1 in node-negative mammary tumor. J Pathol. 1995;176:343–352. doi: 10.1002/path.1711760405. [DOI] [PubMed] [Google Scholar]
- 44.Swinnen JV, Van Veldhoven PP, Timmermans L, De Schrijver E, Brusselmans K, Vanderhoydonc F, Van De Sande T, Heemers H, Heyns W, Verhoeven G. Fatty acid synthase drives the synthesis of phospholipids partitioning into detergent-resistant membrane microdomains. Biochem Biophys Res Commun. 2003;302:898–903. doi: 10.1016/s0006-291x(03)00265-1. [DOI] [PubMed] [Google Scholar]
- 45.Li N, Sood S, Wang S, Fang M, Wang P, Sun Z, Yang CS, Chen X. Overexpression of 5-lipoxygenase and cyclooxygenase 2 in hamster and human oral cancer and chemopreventive effects of zileuton and celecoxib. Clin Cancer Res. 2005;11:2089–2096. doi: 10.1158/1078-0432.CCR-04-1684. [DOI] [PubMed] [Google Scholar]
- 46.Melstrom LG, Bentrem DJ, Salabat MR, Kennedy TJ, Ding XZ, Strouch M, Rao SM, Witt RC, Ternent CA, Talamonti MS, et al. Overexpression of 5-lipoxygenase in colon polyps and cancer and the effect of 5-LOX inhibitors in vitro and in a murine model. Clin Cancer Res. 2008;14:6525–6530. doi: 10.1158/1078-0432.CCR-07-4631. [DOI] [PubMed] [Google Scholar]
- 47.Matsuyama M, Yoshimura R, Mitsuhashi M, Tsuchida K, Takemoto Y, Kawahito Y, Sano H, Nakatani T. 5-Lipoxygenase inhibitors attenuate growth of human renal cell carcinoma and induce apoptosis through arachidonic acid pathway. Oncol Rep. 2005;14:73–79. [PubMed] [Google Scholar]
- 48.Baker N, O'Meara SJ, Scannell M, Maderna P, Godson C, DuBois RN. Lipoxin A4: anti-inflammatory and anti-angiogenic impact on endothelial cells. J Immunol. 2009;182:3819–3826. doi: 10.4049/jimmunol.0803175. [DOI] [PubMed] [Google Scholar]
- 49.Sveinbjörnsson B, Rasmuson A, Baryawno N, Wan M, Pettersen I, Ponthan F, Orrego A, Haeggström JZ, Johnsen JI, Kogner P. Expression of enzymes and receptors of the leukotriene pathway in human neuroblastoma promotes tumor survival and provides a target for therapy. FASEB J. 2008;22:3525–3536. doi: 10.1096/fj.07-103457. [DOI] [PubMed] [Google Scholar]
- 50.Wei N, Wang B, Zhang QY, Mi MT, Zhu JD, Yu XP, Yuan JL, Chen K, Wang J, Chang H. Effects of different dietary fatty acids on the fatty acid compositions and the expression of lipid metabolic-related genes in mammary tumor tissues of rats. Nutr Cancer. 2008;60:810–825. doi: 10.1080/01635580802192858. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.