Abstract
The lymphatic system plays a critical role in melanoma metastasis, and yet, virtually no information exists regarding the cellular and molecular mechanisms that take place between melanoma cells and the lymphatic vasculature. Here, we generated B16-F1 melanoma cells that expressed high (B16α4+) and negligible (B16α4-) levels of α4 integrin to determine how the expression of α4 integrins affects tumor cell interactions with lymphatic endothelial cells in vitro and how it impacts lymphatic metastasis in vivo. We found a direct correlation between α4 integrin expression on B16-F1 melanoma cells and their ability to form adhesive interactions with monolayers of lymphatic endothelial cells. Adhesion of B16-F1 melanoma cells to lymphatic endothelial cells was mediated by the melanoma cell α4 integrin binding to its counterreceptor, vascular cell adhesion molecule 1 (VCAM-1), that was constitutively expressed on the lymphatic endothelial cells. VCAM-1 was also expressed on the tumor-associated lymphatic vessels of B16-F1 and B16α4+ tumors growing in the subcutaneous space of C57BL/6J mice. B16-F1 tumors metastasized to lymph nodes in 30% of mice, whereas B16α4+ tumors generated lymph node metastases in 80% of mice. B16-F1 melanoma cells that were deficient in α4 integrins (B16α4-) were nontumorigenic. Collectively, these data show that the α4 integrin expressed by melanoma cells contributes to tumorigenesis and may also facilitate metastasis to regional lymph nodes by promoting stable adhesion of melanoma cells to the lymphatic vasculature.
Introduction
Lymph nodes are the most common site of metastasis in patients with melanoma, and assessment of the lymphatic compartment is a critical component of the melanoma staging process [1,2]. Melanoma that has spread to lymph nodes, but not to other tissues, is classified as a stage III disease according to the American Joint Committee on Cancer tumor/node/metastasis system [3]. Studies have shown that one of the most powerful predictors of patient survival is the status of regional lymph nodes [4]. More metastatic nodes correlate with decreased survival, and the 5-year survival for melanoma patients with four or more positive nodes is approximately 25% [5]. Recent evidence also suggests that some cutaneous melanomas may actually stimulate the formation of new lymphatic vessels (i.e., lymphangiogenesis) that encourage tumor cell metastasis to regional lymph nodes [6]. However, despite the importance of the lymphatic circulation in promoting the dissemination of melanoma, virtually no information exists concerning the cellular and molecular interactions that take place between melanoma cells and the lymphatic vasculature.
During recent years, much investigative effort has focused on the dramatic alterations that take place on the tumor cell surface during the multistep progression of melanoma. For example, the expression of receptor complexes that participate in immune regulation becomes downregulated as the disease progresses [7,8], whereas there is a concomitant increase in the receptor density of proteins that regulate invasion and metastasis [9,10]. One of the most extensively studied proteins that mediate melanoma cell trafficking processes is the α4β1 integrin. Reports examining the expression of α4β1 integrin in melanoma have determined that the integrin is absent in primary and dysplastic nevi, but that α4β1 expression increases during the transition from radial growth phase to vertical growth phase [11,12]. Results generated from clinical studies of melanoma demonstrated a direct correlation between α4β1 expression and occurrence of metastasis, reduced disease-free survival, and decreased overall survival [12,13]. Several lines of evidence suggest that melanoma cells may use their α4β1 integrin to promote tumor cell retention in distal tissues. Examinations of the adhesive interactions that take place between melanoma cells and blood vascular endothelial cells indicate that tumor cell adhesion to activated endothelial monolayers is mediated by the melanoma cell α4β1 integrin binding to the inducible endothelial cell glycoprotein, vascular cell adhesion molecule 1 (VCAM-1) [14,15]. In experimental models of melanoma metastasis, incubation of melanoma cells with antibodies directed against α4β1 can significantly reduce the frequency of lung metastases in mice that have been pretreated with proinflammatory cytokines [16]. During the course of melanoma progression in spontaneous melanoma models, VCAM-1 becomes upregulated on the microvascular endothelium of organs that are considered preferred sites of metastases [17].
Whereas the previously mentioned reports demonstrated that tumor cell expression of α4β1 is critical for the hematogenous spread of melanoma cells, the contribution of α4β1 to lymphatic metastasis remains unknown. Recently, we generated a conditionally immortalized lymphatic endothelial cell line from afferent vessels of the mouse mesentery [18]. The lymphatic endothelial cell line enabled us to identify redundant pathways that signal for lymphangiogenesis and to define the molecular basis of therapy in a preclinical colon cancer model [18]. In this report, we used the cell line to determine how the expression of α4β1 integrin by B16-F1 melanoma cells affects their ability to interact with lymphatic endothelial cells in vitro. To study the effect of α4β1 on lymphatic metastasis in vivo, we established B16-F1 melanoma cell lines expressing high (B16α4+) and negligible (B16α4-) levels of α4 and implanted the cells into the subcutaneous space of syngeneic C57BL/6J mice. Our findings suggest that the ability of melanoma cells to form stable adhesive interactions with lymphatic endothelial cells may be more important for lymphatic metastasis than previously considered.
Materials and Methods
Reagents
The following antibodies were used in our study: anti-CD49d (integrin α4) (553154), anti-CD29 (integrin β1) (553715), anti-vascular endothelial growth factor receptor 3 (VEGFR-3) (552857), anti-CD16/CD32 (553142), anti-VCAM-1 (550547) (BD PharMingen, San Diego, CA); anti-VCAM-1 (sc-31048) and anti-CXCR-3 (sc-133121) from Santa Cruz Biotechnology (Santa Cruz, CA); anti-CXCR-4 (54007; AnaSpec, Fremont, CA); anti-CC-chemokine receptor-7 (CCR-7, 2059-1; Epitomics, Inc, Burlingame, CA); anti-CCR-10 (GTX21661; GeneTex, Inc, Irvine, CA); anti-Prox1 (11-002; AngioBio, Del Mar, CA); anti-β-actin (A5441; Sigma-Aldrich, St. Louis, MO); anti-lymphatic vessel endothelial hyaluronic acid receptor 1 (LYVE-1, 10350PA50S; Fitzgerald Industries, Concord, MA); and anti-VCAM-1 (MCA2297; Serotec, Raleigh, NC). The following secondary antibodies were used for colorimetric immunohistochemical analyses: goat antirabbit Alexa 488 (A-11034) and goat antirat Alexa 594 (A-11007; Molecular Probes, Inc, Eugene, OR).
For fluorescent-labeled cell sorting procedures, we used a goat antirat immunoglobulin G (IgG) Fab2 fluorescein isothiocianate (FITC)-conjugated secondary antibody (112-096-143; Jackson ImmunoResearch, West Grove, PA). The following secondary antibodies were used for immunoblot analysis: peroxidase-conjugated goat antirabbit IgG (111-036-045), goat antirat horseradish peroxidase (HRP) IgG (112-035-167), rabbit antigoat HRP IgG (305-036-003), and goat antimouse HRP IgG (115-036-003; Jackson ImmunoResearch Laboratories).
Cell Lines and Culture Conditions
B16-F1 mouse melanoma cells were maintained in Eagle's minimum essential medium (EMEM) supplemented with 10% fetal bovine serum (FBS), sodium pyruvate, nonessential amino acids, l-glutamine, a vitamin solution (all from Life Technologies, Grand Island, NY), and a penicillin/streptomycin mixture (Flow Laboratories, Rockville, MD). The cells were maintained at 37°C in a mixture of 5% CO2 and 95% air. The lymphatic endothelial cells [18] and brain microvascular endothelial cells [19] derived from H-2kb-tsA58 mice have been described previously. Both of these cell lines were maintained in Dulbecco's modified Eagle's medium (DMEM) containing 10% FBS and the previously mentioned supplements. All cell lines were determined to be free of mycoplasma and pathogenic murine viruses (assayed by Science Applications International Co, Frederick, MD).
Western Blot Analysis
It is well established that endothelial cells from different regional circulations are structurally and functionally distinct [20,21]. To confirm that the lymphatic endothelial cells used in our study maintained characteristic feature of lymphatic endothelial cells in vivo and that these cells could be distinguished from blood vascular endothelial cells, we compared the expression of VEGFR-3 and Prox1 in monolayers of lymphatic endothelial cells and brain microvascular endothelial cells. The brain endothelial cells were derived from the same mouse line (H-2Kb-tsA58 mice) and were selected for comparative analysis because the brain does not possess lymphatic vessels. We were also interested in determining expression of the inducible endothelial cell adhesion molecule, VCAM-1, given that this glycoprotein seems to play a prominent role in the blood-borne spread of melanoma [14–17]. Identical passage (passage 5) lymphatic endothelial cells and brain endothelial cells were seeded onto 10-cm dishes at a density of 4 x 105 cells per dish in DMEM containing 10% FBS. The cells were maintained in a 37°C incubator for a period 96 hours, at which time the medium was aspirated, and the cells were washed twice with PBS and then lysed with buffer (50 mM Tris-HCl [pH 7.5], 50 mM NaCl, 1% Triton X-100, 1 mM Na3VO4, and protease inhibitors).
Protein concentrations were determined using the Bradford method (Bio-Rad Laboratories, Hercules, CA). Total protein (50 µg) was resolved in 8% SDS-polyacrylamide gel electrophoresis under reducing conditions and transferred to polyvinylidene disulfide membranes. Membranes were blocked with 5% (wt./vol.) nonfat dried milk in 0.1% Tween 20 (Sigma) in PBS for 1 hour and incubated overnight with antibody directed against VEGFR-3 (BD PharMingen; 1:200), Prox1 (1:1000), or VCAM-1 (Santa Cruz Biotechnology; 1:500). β-Actin (Sigma-Aldrich)was used as an internal loading control. Immunodetection was performed using the corresponding secondary HRP-conjugated antibody, and activity was detected using enhanced chemiluminescence (Amersham Biosciences, Piscataway, NJ).
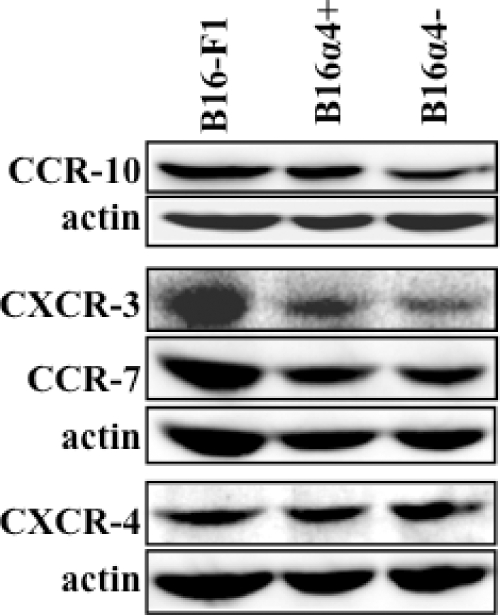
To evaluate the expression levels of chemokine receptors on the B16-F1 cell lines, protein lysates from B16-F1, B16α4+, and B16α4- melanoma cells were separated on SDS-polyacrylamide gel electrophoresis (10%) under reducing conditions and transferred to polyvinylidene disulfide membranes for immunoblot analysis with antibodies specific for CXCR-3 (1:100), CXCR-4 (1:500), CCR-7 (1:3000), and CCR-10 (1:1000).
Generation of B16-F1 Melanoma Cell Lines Based on Expression Levels of the α4 Integrin
Monolayers of B16-F1 cells (70–80% confluent) were harvested by briefly exposing the cells to a solution containing 0.25% trypsin and 0.02% EDTA. The cells were resuspended in EMEM containing 5% FBS, centrifuged for 10 minutes at 200g, and then prepared for cell sorting. We incubated 2 x 105 cells with 20 µl of an antibody directed against the N-terminus of the α4 integrin (BD PharMingen) for 45 minutes at 4°C, washed the cells twice with serum-free medium, and then resuspended them in EMEM containing 5% FBS. Cells were then incubated with an FITC-conjugated antirat secondary antibody for 45 minutes at 4°C, washed twice, and resuspended in 1 ml of EMEM containing 5% FBS. Cell staining was evaluated with a Beckman Epics Elite flow cytometer (Beckman Coulter, Miami, FL) equipped with an air-cooled argon ion laser. The emission wavelength used for the recognition of FITC was 520 nm, and gating parameters were adjusted based on the fluorescence histograms for the negative controls. B16-F1 melanoma cells expressing high (B16α4+) and negligible (B16α4-) levels of α4 were collected in sterile tubes containing 5% EMEM and transferred to T-25 flasks. The cells were expanded and subjected to an enrichment sort using the methodology described previously.
Adhesion of B16-F1 Cell Lines to Lymphatic Endothelial Cell Monolayers
We studied the ability of B16-F1, B16α4+, and B16α4- cells to stably adhere to monolayers of lymphatic endothelial cells. Lymphatic endothelial cells were seeded onto 96-well plates at a density of 3 x 103 cells per well in DMEM containing 10% FBS and grown to confluence (6 days). Cultures of tumor cells that were 70% to 80% confluent were labeled with Vybrant DiI cell-labeling solution (Molecular Probes) according to the manufacturer's instructions. After a 1-hour incubation period, we confirmed fluorescent labeling of the tumor cells and then aspirated the medium from the 96-well plates containing lymphatic endothelial cells. B16-F1, B16α4+, and B16α4- cells were prepared at a concentration of 2.5 x 104 cells/ml in EMEMcontaining 5% FBS, and 100 µl of this tumor cell-containing medium was added into individual wells that contained lymphatic endothelial cells. The tumor cells were incubated with the lymphatic endothelial cells for 30 minutes at 37°C. After this period, the medium was aspirated, and the wells were washed twice with EMEM to remove any unbound tumor cells. The number of adherent tumor cells in each well was determined by counting the number of fluorescently labeled cells in each well under magnification (100x) using an Axioplan II fluorescence microscope (Carl Zeiss, Inc, Thornwood, NY).
Contribution of α4β1-VCAM-1 to Adhesion of B16-F1 Melanoma Cells to Lymphatic Endothelial Cells and Brain Microvascular Endothelial Cells
To study the contribution of α4β1-VCAM-1 to tumor cell adhesion, we first blocked any Fc receptor present on both lymphatic endothelial cells and brain-derived endothelial cells by incubating each cell type with 1 µg/ml of antimouse CD16/CD32 antibody (PharMingen) in EMEM containing 5% FBS for 1 hour at 37°C. After this period, the medium was aspirated and replaced with EMEM containing either 10 or 15 µg/ml of anti-VCAM-1 monoclonal antibody (PharMingen). The lymphatic endothelial cells and brain endothelial cells were incubated in the antibody-containing medium for 1 hour. After this period, the medium from both brain endothelial cells and lymphatic endothelial cells was aspirated and replaced with medium containing tumor cells as described previously. In similar experiments, we incubated B16-F1, B16α4+, and B16α4- melanoma cells with 1 or 5 µg/ml of an anti-α4 antibody for 1 hour before their coincubation with lymphatic or brain endothelial cells. In both series of studies, the number of adherent cells was compared with control. In other experiments, both VCAM-1 and α4 integrins were blocked simultaneously before the addition of tumor cells. For the experimental control in the VCAM-1 experiments, the lymphatic endothelial cells were incubated with 15 µg/ml of an isotype control antibody, whereas in the α4 experiments, the B16-F1 cell lines were incubated with 5 µg/ml of an isotype standard antibody. All of the tumor cell adhesion experiments were repeated at least four times.
Animals and B16-F1 Tumor Models
C57BL/6J mice and athymic nude mice (NCI-nu) were purchased from the Animal Production Area of the National Cancer Institute Frederick Cancer Research and Development Center (Frederick, MD). The mice were housed and maintained under specific pathogen-free conditions in facilities approved by the American Association for Accreditation of Laboratory Animal Care and in accordance with current regulations and standards of the US Department of Agriculture, the US Department of Health and Human Services, and the National Institutes of Health. The mice were used, in accordance with institutional guidelines, when they were 6 to 8 weeks old.
To produce melanoma tumors, we harvested B16-F1, B16α4+, and B16α4- melanoma cells from subconfluent cultures by briefly exposing the cells to a solution containing 0.25% trypsin and 0.02% EDTA. The cells were washed twice and then resuspended in Hanks' buffered saline solution. The cell concentration was adjusted to ensure that a volume of 100 µl was delivered into the subcutaneous space of each mouse. Melanoma cell injection into the pinna was performed as previously described [22]. For surgical resection of primary melanoma tumors, mice were anesthetized with an intraperitoneal injection of pentobarbital sodium (Abbott Laboratories, North Chicago, IL). Mice were prepared with a betadine solution, and hemostasis was controlled using a portable electrocautery device (Braintree Scientific, Braintree, MA). Stainless steel wound clips were used for surgical closure.
Necropsy Procedures and Histologic Studies
Mice were killed with pentobarbital sodium, and their body weights were recorded. After necropsy, tumors were excised, and the tumor weight was recorded. For immunohistochemical and hematoxylin and eosin staining procedures, a portion of the tumor was fixed in formalin and then embedded in paraffin. Another portion of the tumor was embedded in ornithine carbamyl transferase compound (Miles Laboratories, Elkhart, IN), rapidly frozen in liquid nitrogen, and stored at -80°C. All of the ipsilateral axillary lymph nodes, inguinal lymph nodes, and lungs were harvested and processed in a similar fashion. The presence of metastatic disease was determined by histologic examination.
Immunofluorescent Staining of B16-F1 Tumors for Lymphatic Vessels (LYVE-1), VCAM-1, and α4 Integrin
We used frozen tissues to identify lymphatic vessels, VCAM-1, and the α4 integrin. Frozen sections of B16-F1 tumors that were harvested from C57BL/6J mice were mounted on slides and fixed using a protocol consisting of three sequential immersions in ice-cold solutions containing acetone, 50:50 (vol./vol.) acetone-chloroform, and acetone (5 minutes each). Samples were incubated in a blocking solution containing 5% normal horse serum and 1% normal goat serum in PBS for 20 minutes at room temperature and were then incubated with antibody directed against LYVE-1, VCAM-1, or α4 integrin (each antibody used at 1:100 dilution) at 4°C overnight. The samples were washed three times in PBS, incubated with a blocking solution, and then incubated for 1 hour with either a 1:1500 dilution of Alexa 488 antibody (for LYVE-1) or a 1:1200 dilution of Alexa 594 antibody (for VCAM-1 and α4). Control samples were incubated with goat antirabbit IgG and goat antirat IgG isotype primary antibodies and with Alexa 488 and Alexa 594 fluorescent secondary antibodies. All samples were rinsed and incubated with Hoechst stain (Polysciences, Inc, Warrington, PA) to visualize the cell nuclei. The slides were mounted with a glycerol/PBS solution containing 0.1 M propyl gallate to minimize fluorescent bleaching. Immunofluorescent microscopy was performed using a Zeiss Axiplan fluorescent microscope (Carl Zeiss, Inc) equipped with a 100-W Hg lamp and narrow band-pass excitation filters. Images were obtained using a cooled charge-coupled device Hamamatsu C5810 camera (Hamamatsu Photonics, Bridgewater, NJ) and ImagePro software (Media Cybernetics, Silver Spring, MD). Composite images were created using Photoshop software (Adobe Systems, Inc, Mountain View, CA).
Determination of Lymphatic Vascular Density
Lymphatic vascular density (LVD) in the B16-F1 and B16α4+ tumors was determined as previously described [23]. In brief, tumors were examined microscopically to identify regions that stained intensely for LYVE-1 by low-power (original magnification, x40) scanning of the section. The mean LVD was quantified by counting the number of lymphatic vessels at high magnification (original magnification, x100) in a minimum of five microscopic fields for each tumor sample.
Tumor Cell Proliferation In Vitro
B16 melanoma cell lines were plated into 96-well plates in 100 µl of EMEM containing 5% FBS at a density of 7.0 x 103 cells per well. After a 24-hour incubation at 37°C, the medium was aspirated and replaced with 200 µl of EMEM containing 5% FBS, and the plates were placed in a 37°C incubator. After 72 hours, metabolically active cells were determined by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenoltetrazolium bromide (MTT) assay [24]. MTT conversion to formazan by metabolically active cells was measured at 570 nm using an MR-5000 96-well microtiter plate reader (Dynatech, Inc, Chantilly, VA).
Statistics Analysis
Statistical analyses were performed using GraphPad Prism software (San Diego, CA). P values for adhesion assays and MTT assays were calculated using one-way analysis of variance. Means determined to be significantly different (P < .05) were then subjected to post hoc analysis using Tukey's multiple comparison testing. The sizes of primary melanoma tumors were compared using Student's t test. Comparison between the incidences of lymph node metastases was determined using Fisher's exact probability testing.
Results
In Vitro Expression of VEGFR-3, Prox1, and VCAM-1 in Lymphatic Endothelial Cells
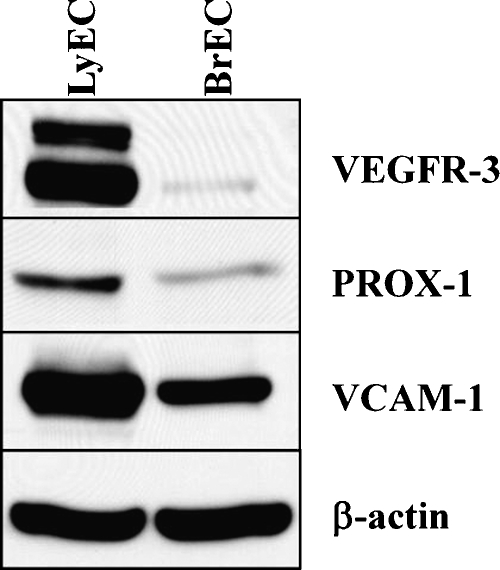
Western blot analysis was performed to determine expression of VEGFR-3, Prox1, and VCAM-1 in cultures of lymphatic endothelial cells and identical passage brain microvascular endothelial cells. VEGFR-3 and Prox1 are considered markers of lymphatic endothelia in vivo but not of most blood vascular endothelial cells [25]. Lymphatic endothelial cells expressed marked amounts of VEGFR-3 and Prox1, whereas expression of these two proteins in brain microvascular endothelial cells was markedly reduced (Figure 1). We also measured expression of VCAM-1 in each of the endothelial cell lines because this protein has been implicated in the hematogenous spread of melanoma. We noted that expression of VCAM-1 was significantly more pronounced in lymphatic endothelial cells when compared with that in brain endothelial cells (Figure 1).
Figure 1.
Expression of VEGFR-3, Prox1, and VCAM-1 by lymphatic endothelial cells and brain-derived microvascular endothelial cells. Immunoblot analysis results show that lymphatic endothelial cells retain their characteristic features despite transfer to cell culture. Lymphatic endothelial cells (LyEC) express high levels of VEGFR-3, Prox1, and VCAM-1 compared with brain-derived vascular endothelial cells (BrEC). β-Actin is shown as an internal loading control.
Expression of α4 Integrin in B16-F1, B16α4-, and B16α4+ Melanoma Cells
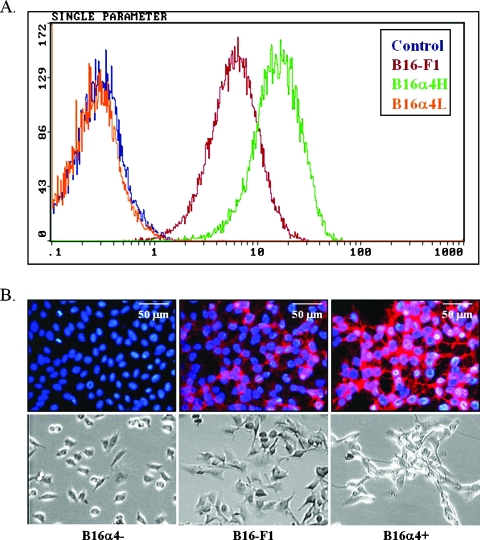
Consistent with reports from other laboratories [26], we noted that B16-F1 murine melanoma cells express elevated levels of the α4 integrin in vitro (Figure 2, A and B). However, we were able to identify and isolate a subpopulation of cells that did not express measurable levels of α4 and designated these cells B16α4-. We also isolated a population of cells that expressed very high levels of the α4 integrin on their surface and termed these cells B16α4+. Measurements of the amount of α4 integrin expressed by B16α4+ cells indicated that expression on these cells was three-fold higher than expression on the B16-F1 cell line. B16α4+ and B16α4- cell lines were expanded and subjected to an enrichment sort.
Figure 2.
Expression of α4 by B16 melanoma cell lines. (A) Selection of variant sublines from the parental B16-F1 melanoma cells was performed as described within the text. Successive selection from the B16-F1 cells yielded a subline that expressed high levels of α4 (B16α4+) and a subline that was deficient in α4 integrin (B16α4-). (B) Immunohistochemical staining of α4 and representative examples of morphologic differences as observed with phase-contrast microscopy. When compared with B16-F1 melanoma cells, B16α4- cells display decreased homotypic adhesion in vitro, whereas B16α4+ cells are more inclined to form cell-cell aggregates.
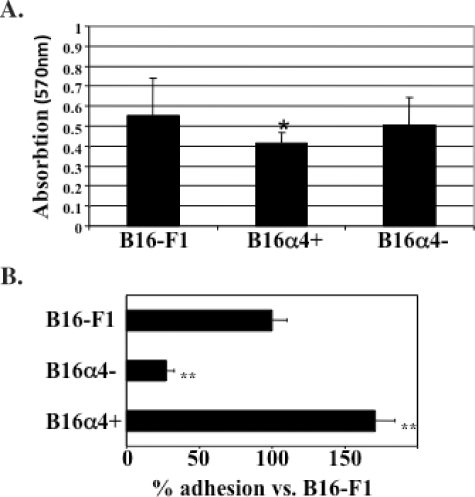
An examination of the B16-F1, B16α4-, and B16α4+ cell lines growing in cell culture conditions revealed marked differences with respect to cell morphology. For example, the B16α4- cells have a more rounded morphology and tend to grow as single entities, whereas the B16 melanoma cells that are enriched in α4 (B16α4+) were much more likely to form homotypic cell interactions (Figure 2B). Both of these growth patterns could be observed in cultures of the parental B16-F1 melanoma cell line. The expression levels of α4 integrins and individual growth patterns observed in the different B16 melanoma cell lines remained stable for a period of at least 6 weeks in cell culture (data not shown). The turnover time of B16α4+ cells growing in cell culture was slightly longer than that of B16-F1 and B16α4- tumor cells (Figure 3A). However, the adhesion of B16α4+ cells to lymphatic endothelial cells was significantly greater than the B16-F1 melanoma cells and the α4-deficient B16α4- melanoma cells (Figure 3B).
Figure 3.
Analysis of B16 melanoma cell proliferation and melanoma cell adhesion to lymphatic endothelial cells. (A) Cell proliferation of B16-F1 melanoma cell lines in vitro was assessed by MTT. Cell division of both B16-F1 and B16α4- tumor cells was significantly greater than the growth of B16α4+ melanoma cells. (B) The adhesion of B16α4+ to lymphatic endothelial cells is seven times greater than the adhesion of B16α4- melanoma cells. Data are expressed as mean ± SEM and repeated at least four times with comparable results.
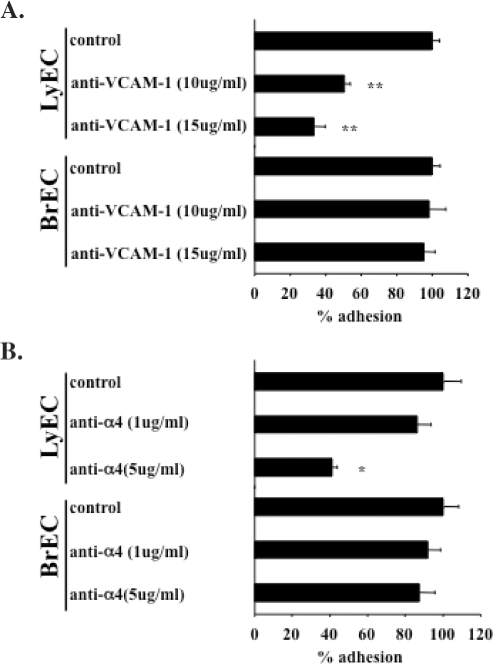
B16-F1 Melanoma Cell Adhesion to Lymphatic Endothelial Cells, but not Brain Microvascular Endothelial Cells, Is Mediated by VCAM-1
To more directly examine the contribution of the melanoma cell α4 integrin to lymphatic endothelial cell adhesion, we performed a series of antibody-blocking experiments with B16-F1 melanoma cells. We also compared B16-F1 tumor cell adhesion to brain microvascular endothelial cells to determine whether the tumor cells used a common receptor to adhere to endothelial cells from different tissues. Tumor cell adhesion to lymphatic endothelial cells was significantly attenuated by blocking VCAM-1 on lymphatic endothelial cells (Figure 4A) and also by blocking the α4 integrin on melanoma cells (Figure 4B). Simultaneous blockade of both VCAM-1 on endothelial cells and α4 integrins on melanoma cells did not result in any additional reduction in tumor cell adhesion. This antibody-blocking strategy had no effect on B16-F1 melanoma cell adhesion to brain endothelial cells (Figure 4, A and B). The results of these experiments suggest that the ability of melanoma cells to stably adhere to lymphatic endothelial cells is largely dependent on α4β1-VCAM-1 interactions. The results also indicate that melanoma cells may rely on different receptor-ligand pairs to facilitate their adhesion to vascular endothelium of different tissues.
Figure 4.
B16-F1 melanoma cell adhesion to lymphatic endothelial cells and brain microvascular endothelial cells. (A) Blockade of VCAM-1 expressed on lymphatic endothelial cells results in a significant reduction in number of adherent tumors cells to lymphatic endothelial cells, whereas the same treatment has no effect on tumor cell adhesion to brain endothelial cells. (B) Blockade of the melanoma cell α4 integrin also results in dose-dependent reduction in tumor cell adhesion to lymphatic endothelial cells. Identical treatment has no effect on ability of melanoma cells to adhere to brain endothelial cells. Data are expressed as mean ± SEM. *P < .05, **P < .001.
Tumorigenicity and Lymph Node Metastasis of B16-F1 Melanoma Cells Expressing Different Levels of α4 Integrin
To determine whether the differential expression of α4 integrin in the B16 melanoma cell lines had any effect on tumor growth and metastasis in mice, we injected B16-F1, B16α4-, and B16α4+ tumor cells into the subcutaneous space of C57BL/6J mice. Whereas injection of B16-F1 and B16α4+ into mice resulted in a 100% tumor take within 3 weeks of injection, B16α4- cells were unable to generate a tumor even after 8 weeks after tumor cell injection. On the basis of these results, additional experiments were performed to determine the minimal number of tumor cells required to produce a tumor. We found that subcutaneous injection of 5 x 103 cells was the minimum number of B16-F1 and B16α4+ tumor cells needed for tumor formation. In contrast, even as many as 4 x 105 tumor cells from the B16α4- cell line were insufficient to produce a tumor (data not shown). B16α4- tumor cells were also unable to form tumors in athymic nude mice, suggesting that the lack of tumor formation was not due to immune rejection. These data suggest that melanoma cell expression of α4 integrins may be a prerequisite for tumorigenesis.
Experiments were performed to determine the correlation between expression of the α4 integrin and regional lymph node metastasis. Mice were injected with B16-F1 or B16α4+ melanoma cells (5 x 104 cells) into the mouse flank, and 21 days later, the primary tumors were surgically resected, and the wounds closed with stainless steel wound clips. All mice were killed 3 weeks later, and the lymph nodes were harvested for histologic evaluation. We found that 30% of mice with B16-F1 tumors developed lymph node metastases, whereas 80% of mice with B16α4+ tumors formed lymph node metastases (Table 1). The frequency of pulmonary metastases in B16-F1 and B16α4+ tumors was low (10% and 20%, respectively), and there were no significant differences between the two groups. Whereas the rate of cell division of B16α4+ tumor cells growing in cell culture was significantly slower than B16-F1 and B16α4- tumor cells (Figure 3A), B16α4+ tumors growing in mice were significantly larger than B16-F1 tumors.
Table 1.
Tumor Size and Incidence of Spontaneous Lymph Node and Lung Metastasis.
| Melanoma Cell Line | Primary Tumor Incidence | Tumor Volume* (mm3) | Lymph Node Metastases | Lung Metastases |
| B16-F1 | 10/10 | 877† (463–1183) | 3/10‡ | 1/10 |
| B16α4+ | 10/10 | 1852† (958–2912) | 8/10‡ | 2/10 |
| B16α4- | 0/10† | — | — | — |
B16 melanoma cell lines (5 x 104/100 µl) were injected subcutaneously into the flank of C57BL/6J mice. Three weeks later, tumors were surgically resected and mice were allowed to recover. Two weeks after resection, mice were euthanized and evidence of metastasis was determined histologically.
Tumor volume was given as mean (range).
P ≤ .01.
P ≤ .05.
Expression of Chemokine Receptors on B16-F1, B16α4-, and B16α4+ Melanoma Cells
Recent studies have shown that chemokines and their receptors play an important role in melanoma tumorigenesis and metastasis [27], so we next examined expression levels of four chemokine receptors that have been associated with melanoma progression. Each of the B16 cell lines expressed similar amounts of CXCR-4, CCR-7, and CCR-10 as determined by Western blot analysis, whereas expression of CXCR-3 was slightly decreased on B16α4- tumor cells when compared with either parental or B16α4+ cells (Figure 5). The patterns of chemokine receptors expressed by the different B16 melanoma cells do not satisfactorily explain the enhanced metastatic potential of the B16α4+ melanoma cells or explain the diminished capacity of the B16α4- cells to form tumors.
Figure 5.
Chemokine receptor expression byB16 melanoma cell lines. Immunoblot analysis results demonstrate that B16-F1, B16α4+, and B16α4- express similar amounts of the chemokine receptors CCR-10, CCR-7, and CXCR-4. CXCR-3 expression was slightly greater in B16-F1 cells in comparison to B16α4+ and B16α4- melanoma cells. β-Actin is shown as an internal loading control.
Lymphatic Vascular Density and Expression of LYVE-1, α4 Integrin, and VCAM-1 in B16-F1 and B16α4+ Tumors
To determine whether the increase in lymphatic metastasis observed in B16α4+ tumors was the result of an increase in LVD, we quantified the number of lymphatic vessels associated with B16-F1 tumors and B16α4+ tumors. We observed lymphatic vessels in the peritumoral region of both tumors (Figure 6B). However, there were no significant differences between the mean number of lymphatic vessels in B16-F1 and B16α4+ tumors (data not shown). Hence, the increase in lymph node metastasis observed in B16α4+ tumors was not the result of an increase in lymphatic vascular surface area.
Figure 6.
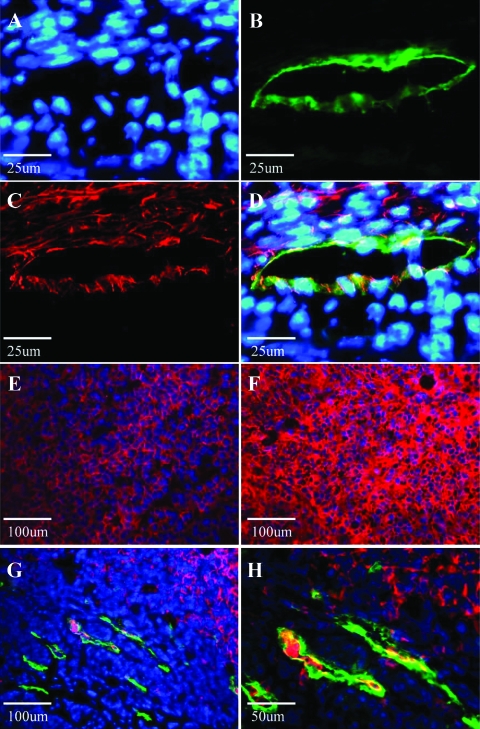
VCAM-1 is expressed on B16 tumor-associated lymphatic endothelial cells. (A–D) Double immunostaining for the lymphatic specific marker LYVE-1 (green) and VCAM-1 (red) reveals colocalization (yellow) of VCAM-1 and LYVE-1 on B16 tumor-associated lymphatic vessels. Nuclei are stained with Hoescht (blue). (E) The intensity of α4 integrin expression (red) in parental B16-F1 tumors is significantly reduced in comparison to expression found in (F) B16α4+ tumors. (G, H) LYVE-1 (green) and α4 (red) expressions at the peritumoral region of B16α4+ tumors growing subcutaneously. B16α4+ tumor cells (confirmed with hematoxylin and eosin staining) can readily be identified around and within peritumoral lymphatic vessels.
We then used immunofluorescent staining to evaluate expression of VCAM-1 and the α4 integrin in the B16 melanoma tumors. VCAM-1 was present in both B16-F1 and B16α4+ tumors (Figure 6C), and moreover, the glycoprotein was localized to lymphatic vessels (Figure 6D). Similarly, the pattern of α4 integrin expression that we observed in vitro was conserved in vivo. That is, B16-F1 parental tumors expressed the α4 integrin (Figure 6E), but the expression was considerably more pronounced in B16α4+ tumors (Figure 6F). In the B16α4+ tumors, we could also observe tumor cells interacting with the peritumoral lymphatic vessels, and in several instances, the tumor cells had gained access to the lymphatic vessels (Figure 6, G and H).
Discussion
Lymphatic vessels facilitate the spread of melanoma cells to regional lymph nodes and distal tissues, and melanoma patients are required to undergo a comprehensive clinical inspection of the lymphatic compartment to determine tumor stage, prognosis, and appropriate therapeutic intervention. However, there is a paucity of data regarding the cellular and molecular interactions that take place between melanoma cells and lymphatic endothelial cells. In the present report, we examined how expression of α4 integrins on melanoma cells affects their ability to interact with lymphatic endothelial cells in vitro and how it impacts metastasis to regional lymph nodes in vivo. The results of these studies provide new insight into lymphatic metastasis including the following: 1) melanoma cell adhesion to lymphatic endothelial cells is mediated by the α4 integrin receptor on melanoma cells binding to its counterreceptor, VCAM-1, which is constitutively expressed on lymphatic endothelial cells; 2) VCAM-1 is expressed on melanoma-associated lymphatic vessels in vivo; 3) there is a direct correlation between levels of α4 integrin expression on melanoma cells and lymphatic metastasis; and 4) melanoma cell adhesion to brain endothelial cells occurs through a VCAM-1-independent process. During our investigation, we also determined that melanoma tumorigenesis is also dependent on the α4 integrin.
The adhesive interactions that take place between tumor cells and endothelial cells of the blood vasculature are widely regarded as a key, rate-limiting step in hematogenous metastasis [28]. To date, however, it remains unclear whether tumor cells exploit endothelial cell adhesion molecules to form stable adhesive interactions with lymphatic vessels. Recent results from investigations of leukocyte trafficking would argue that cell navigation through lymphatic channels is not so dissimilar from the cell migration that occurs in the blood vascular system and that endothelial cell adhesion molecules, including VCAM-1, play a critical role in this process [29,30]. Nevertheless, previous reports have emphasized that the size of the primary melanoma is the principal factor that determines whether tumor cells will metastasize to regional lymph nodes. For example, Nathanson et al. [31] reported that there was a direct correlation between the size of B16 melanoma tumors and the occurrence of both lymphatic and pulmonary metastasis. In our study, we did not observe any correlation between pulmonary metastasis and tumor size. We did, however, observe a correlation between tumor size and metastasis to lymph nodes. In addition to the size of the primary tumor, we propose a mechanism for melanoma lymph node metastasis that is related to the ability of tumor cells to adhere to the lymphatic vasculature. We base this assertion on our data that show a direct correlation between the ability of B16-F1 melanoma cells to adhere to lymphatic endothelial cells and the ability of these cells to generate lymphatic metastases.
VCAM-1 is a cytokine-inducible endothelial cell adhesion molecule that belongs to the immunoglobulin superfamily of receptors [32]. During an inflammatory response, VCAM-1 becomes upregulated on the surface of vascular endothelial cells, where it mediates the firm adhesion and transmigration of blood leukocytes [32]. Aberrant expression of VCAM-1 has been implicated in the initiation and perpetuation of several pathological disorders such as atherosclerosis [33], colitis [34], and arthritis [35]. Studies performed on human umbilical vein endothelial cells that were stimulated with proinflammatory cytokines were the first to show that VCAM-1 could support the adhesion of melanoma cells [36]. Experimental models of melanoma metastasis provided support for the in vitro data in that antibodies directed against VCAM-1 or the α4 integrin significantly attenuated the number of pulmonary metastases in mice that had been pretreated with inflammatory cytokines [16]. The results of the present report are unique in that we found that the constitutive expression of VCAM-1 by lymphatic endothelial cells is sufficient to support melanoma cell adhesion. We also noted that the lymphatic vessels associated with B16 melanomas growing in mice express constitutive levels of VCAM-1. Whereas the in vivo data lend some credibility to the lymphatic endothelial cells used in our study, the results also add to the growing evidence that suggests VCAM-1 is an important cofactor in the tissue-specific spread of melanoma. Gene expression profiles comparing endothelial cells derived from lymphatic vessels and from blood vessels suggest that each cell type possesses a distinct molecular signature [37]. Studies of two circulations in the whole animal have determined that one of the key features that distinguish lymphatic endothelium from blood vascular endothelium is that lymphatic endothelial cells constitutively express the transcription factor nuclear factor κB (NF-κB) [38]. NF-κB has been shown to be a central regulator of VCAM-1 expression [39] and, hence, may be responsible for the constitutive expression of VCAM-1 observed in the lymphatic vessels of melanoma tumors. Collectively, these data may help to explain the proclivity of melanoma to spread through the lymphatic system. Studies are ongoing to compare VCAM-1 expression levels in nonpathologic tissues with expression in other tumor models.
The results of our studies demonstrate that, as the level of α4 integrin expressed on melanoma cells increases, there is a greater likelihood that these cells will spread to regional lymph nodes. Our findings are in stark contrast to a previously published report that found that expression of α4β1 on melanoma cells inhibits the invasive stage of metastasis formation [40]. The authors of that article reported that melanoma cells that express α4β1 integrins have a greater tendency to form homotypic cell-cell aggregates and, therefore, cannot assume the phenotype necessary for invasion. We also observed that the B16α4+ cells that overexpressed the α4 integrin had a greater tendency to form homotypic interactions in cell culture; however, this pattern of growth did not preclude tumor cells from invading adjacent lymphatic vessels and metastasizing to regional lymph nodes. The discrepancy in the results of the two studies may be related to fundamental differences in experimental design in that we directed our attention to lymphatic spread, whereas the previous report focused on lung metastases. In several tumor sections taken from B16α4+ tumors, we noted α4-positive tumor cells in contact with the wall of LYVE-1-positive lymphatic vessels and, in some instances, inside the lymphatic vessel lumen. The results of the present work are consistent with clinical data demonstrating that α4 is absent in benign nevi and becomes increasingly expressed during tumor progression.
Investigations of the mechanisms that regulate the patterns of metastasis of malignant tumors have determined that tissue-specific gradients of chemoattractant cytokines, called chemokines, play an important role in the recruitment of tumor cells to distinct anatomic sites [41]. One of the primary biologic functions of the chemokine proteins is to provide the directional information necessary for leukocyte trafficking processes [42]. For example, the chemokine CCL21 is constitutively expressed in secondary lymphoid organs and is responsible for the homing of CCR-7 expressing lymphocytes and dendritic cells to lymph nodes [43]. We were interested in determining the expression of chemokine receptors on our cells because some chemokines, such as CXCL12, have been shown to enhance the affinity of α4 integrins for VCAM-1 [44]. In addition, recent studies have shown that melanoma cells that overexpress CCR-7 are much more likely to metastasize to lymph nodes than melanoma cells that are deficient in the receptor [45]. However, we found no differences in the amount of CCR-7 expressed on the different B16 cell lines, and hence, we concluded that the expression of this receptor alone does not account for the differences that we observed in the metastatic potential. The chemokine receptors CXCR-3 and CXCR-4 have also been implicated in lymphatic metastasis [46,47], but their expression did not correlate with lymphatic metastasis in our study. In all likelihood, the dissemination of tumor cells to lymph nodes is more complex than previously considered and may require successful completion of a coordinated series of interactions similar to those that take place during hematogenous metastasis.
Whereas the primary objective of our study was to examine melanoma cell interactions with lymphatic endothelial cells, our results also demonstrated that the α4 integrin may be essential for tumorigenesis of B16 cells. Although B16-F1 and B16α4- cells grew more rapidly in culture than B16α4+ cells, the α4-deficient B16α4- cells were unable to form tumors in syngeneic C57BL mice. The B16α4- cells were also unable to establish tumors in athymic nude mice, suggesting that the lack of tumorigenicity was not related to enhanced immune clearance of the B16α4- cells. Studies of integrin expression on neural crest cells, the population of cells from which melanoma cells are derived, have determined that α4β1 plays an essential role in cell survival by protecting these cells from programmed cell death [48]. Other studies have reported that the expression of α4β1 promotes the survival of retinal ganglion cells [49] and CD34+ bone marrow progenitor cells [50]. Whether the α4-deficient B16α4- cells are unable to divide when implanted into the animal and/or activate programs that regulate their destruction requires further investigation. Nevertheless, identifying the molecular phenotype of the melanoma cell population with tumorigenic potential is critical for the development of novel anticancer therapies. Our data suggest that melanoma cells with both tumorigenic and metastatic properties express the α4 integrin.
Acknowledgments
The authors thank Arminda Martinez for assistance with the preparation of the manuscript.
Footnotes
This work was supported in part by the Specialized Program of Research Excellence in Prostate Cancer grant CA902701 from the National Cancer Institute, National Institutes of Health.
References
- 1.Balch CM. The role of elective lymph node dissection in melanoma: rationale, results, and controversies. J Clin Oncol. 1988;6:163–172. doi: 10.1200/JCO.1988.6.1.163. [DOI] [PubMed] [Google Scholar]
- 2.Balch CM, Buzaid AC, Soong SJ, Atkins MB, Cascinelli N, Coit DG, Fleming ID, Gershenwald JE, Houghton A Jr, Kirkwood JM, et al. New TNM melanoma staging system: linking biology and natural history to clinical outcomes. Semin Surg Oncol. 2003;21:43–52. doi: 10.1002/ssu.10020. [DOI] [PubMed] [Google Scholar]
- 3.Balch CM, Buzaid AC, Soong SJ, Atkins MB, Cascinelli N, Coit DG, Fleming ID, Gershenwald JE, Houghton A Jr, Kirkwood JM, et al. Final version of the American Joint Committee on Cancer staging system for cutaneous melanoma. J Clin Oncol. 2001;19:3635–3648. doi: 10.1200/JCO.2001.19.16.3635. [DOI] [PubMed] [Google Scholar]
- 4.Balch CM, Soong SJ, Gershenwald JE, Thompson JF, Reintgen DS, Cascinelli N, Urist M, McMasters KM, Ross MI, Kirkwood JM, et al. Prognostic factors analysis of 17,600 melanoma patients: validation of the American Joint Committee on Cancer melanoma staging system. J Clin Oncol. 2001;19:3622–3634. doi: 10.1200/JCO.2001.19.16.3622. [DOI] [PubMed] [Google Scholar]
- 5.Callery C, Cochran AJ, Roe DJ, Rees W, Nathanson SD, Benedetti JK, Elashoff RM, Morton DL. Factors prognostic for survival in patients with malignant melanoma spread to the regional lymph nodes. Ann Surg. 1982;196:69–75. doi: 10.1097/00000658-198207000-00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rinderknecht M, Detmar M. Tumor lymphangiogenesis and melanoma metastasis. J Cell Physiol. 2008;216:347–354. doi: 10.1002/jcp.21494. [DOI] [PubMed] [Google Scholar]
- 7.Chang CC, Campoli M, Restifo NP, Wang X, Ferrone S. Immune selection of hot-spot β2-microglobulin gene mutations, HLA-A2 allospecificity loss, and antigen-processing machinery component down-regulation in melanoma cells derived from recurrent metastases following immunotherapy. J Immunol. 2005;174:1462–1471. doi: 10.4049/jimmunol.174.3.1462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chang CC, Ogino T, Mullins DW, Oliver JL, Yamshchikov GV, Bandoh N, Slingluff CL, Ferrone S. Defective human leukocyte antigen class I-associated antigen presentation caused by a novel β2-microglobulin loss-of-function in melanoma cells. J Biol Chem. 2006;281:18763–18773. doi: 10.1074/jbc.M511525200. [DOI] [PubMed] [Google Scholar]
- 9.Johnson JP. Cell adhesion molecules in the development and progression of malignant melanoma. Cancer Metastasis Rev. 1994;18:345–357. doi: 10.1023/a:1006304806799. [DOI] [PubMed] [Google Scholar]
- 10.Nesbit M, Herlyn M. Adhesion receptors in human melanoma progression. Invasion Metastasis. 1994;14:131–146. [PubMed] [Google Scholar]
- 11.Albelda SM, Mette SA, Elder DE, Stewart R, Damjanovich L, Herlyn M, Buck CA. Integrin distribution in malignant melanoma: association of the β3 subunit with tumor progression. Cancer Res. 1990;50:6757–6764. [PubMed] [Google Scholar]
- 12.Schadendorf D, Gawlik C, Haney U, Ostmeier H, Suter L, Czarnetzki BM. Tumour progression and metastatic behaviour in vivo correlates with integrin expression on melanocytic tumours. J Pathol. 1993;170:429–434. doi: 10.1002/path.1711700405. [DOI] [PubMed] [Google Scholar]
- 13.Schadendorf D, Heidel J, Gawlik C, Suter L, Czarnetzki BM. Association with clinical outcome of expression of VLA-4 in primary cutaneous malignant melanoma as well as P-selectin and E-selectin on intratumoral vessels. J Natl Cancer Inst. 1995;87:366–371. doi: 10.1093/jnci/87.5.366. [DOI] [PubMed] [Google Scholar]
- 14.Klemke M, Weschenfelder T, Konstandin MH, Samstag Y. High affinity interaction of integrin α4β1 (VLA-4) and vascular cell adhesion molecule 1 (VCAM-1) enhances migration of human melanoma cells across activated endothelial cell layers. J Cell Physiol. 2007;212:368–374. doi: 10.1002/jcp.21029. [DOI] [PubMed] [Google Scholar]
- 15.Martin-Padura I, Mortarini R, Lauri D, Bernasconi S, Sanchez-Madrid F, Parmiani G, Mantovani A, Anichini A, Dejana E. Heterogeneity in human melanoma cell adhesion to cytokine activated endothelial cells correlates with VLA-4 expression. Cancer Res. 1991;51:2239–2241. [PubMed] [Google Scholar]
- 16.Garofalo A, Chirivi RG, Foglieni C, Pigott R, Mortarini R, Martin-Padura I, Anichini A, Gearing AJ, Sanchez-Madrid F, Dejana E, et al. Involvement of the very late antigen 4 integrin on melanoma in interleukin 1-augmented experimental metastases. Cancer Res. 1995;55:414–419. [PubMed] [Google Scholar]
- 17.Langley RR, Carlisle R, Ma L, Specian RD, Gerritsen ME, Granger DN. Endothelial expression of vascular cell adhesion molecule-1 correlates with metastatic pattern in spontaneous melanoma. Microcirculation. 2001;8:335–345. doi: 10.1038/sj/mn/7800098. [DOI] [PubMed] [Google Scholar]
- 18.Rebhun RB, Langley RR, Yokoi K, Fan D, Gershenwald JE, Fidler IJ. Targeting receptor tyrosine kinase on lymphatic endothelial cells for the therapy of colon cancer lymph node metastasis. Neoplasia. 2006;8:747–757. doi: 10.1593/neo.06322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Langley RR, Ramirez KM, Tsan RZ, Van Arsdall M, Nilsson MB, Fidler IJ. Tissue-specific microvascular endothelial cell lines from H-2K(b)-tsA58 mice for studies of angiogenesis and metastasis. Cancer Res. 2003;63:2971–2976. [PubMed] [Google Scholar]
- 20.Garlanda C, Dejana E. Heterogeneity of endothelial cells. Specific markers. Arterioscler Thromb Vasc Biol. 1997;17:1193–1202. doi: 10.1161/01.atv.17.7.1193. [DOI] [PubMed] [Google Scholar]
- 21.Gerritsen ME. Functional heterogeneity of vascular endothelial cells. Biochem Pharmacol. 1987;36:2701–2711. doi: 10.1016/0006-2952(87)90252-8. [DOI] [PubMed] [Google Scholar]
- 22.Rebhun RB, Lazar AJ, Fidler IJ, Gershenwald JE. Impact of sentinel lymphadenectomy on survival in a murine model of melanoma. Clin Exp Metastasis. 2008;25:191–199. doi: 10.1007/s10585-008-9141-y. [DOI] [PubMed] [Google Scholar]
- 23.Sasaki T, Nakamura T, Rebhun RB, Cheng H, Hale KS, Tsan RZ, Fidler IJ, Langley RR. Modification of the primary tumor microenvironment by transforming growth factor α-epidermal growth factor receptor signaling promotes metastasis in an orthotopic colon cancer model. Am J Pathol. 2008;173:205–216. doi: 10.2353/ajpath.2008.071147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Carmichael J, DeGraff WG, Gazdar AF, Minna JD, Mitchell JB. Evaluation of a tetrazolium-based semiautomated colorimetric assay: assessment of chemosensitivity testing. Cancer Res. 1987;47:936–942. [PubMed] [Google Scholar]
- 25.Baluk P, McDonald DM. Markers for microscopic imaging of lymphangiogenesis and angiogenesis. Ann N Y Acad Sci. 2008;1131:1–12. doi: 10.1196/annals.1413.001. [DOI] [PubMed] [Google Scholar]
- 26.Wang HH, McIntosh AR, Hasinoff BB, Rector ES, Ahmed N, Nance DM, Orr FW. B16 melanoma cell arrest in the mouse liver induces nitric oxide release and sinusoidal cytotoxicity: a natural hepatic defense against metastasis. Cancer Res. 2000;60:5862–5869. [PubMed] [Google Scholar]
- 27.Kakinuma T, Hwang ST. Chemokines, chemokine receptors, and cancer metastasis. J Leuk Biol. 2006;79:639–651. doi: 10.1189/jlb.1105633. [DOI] [PubMed] [Google Scholar]
- 28.Langley RR, Fidler IJ. Tumor cell-organ microenvironment interactions in the pathogenesis of cancer metastasis. Endocr Rev. 2007;28:297–321. doi: 10.1210/er.2006-0027. [DOI] [PubMed] [Google Scholar]
- 29.Alvarez D, Vollmann EH, von Andrian UH. Mechanisms and consequences of dendritic cell migration. Immunity. 2008;29:325–342. doi: 10.1016/j.immuni.2008.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Johnson LA, Jackson DG. Cell traffic and the lymphatic endothelium. Ann N Y Acad Sci. 2008;1131:119–133. doi: 10.1196/annals.1413.011. [DOI] [PubMed] [Google Scholar]
- 31.Nathanson SD, Haas GP, Mead MJ, Lee M. Spontaneous regional lymph node metastases of three variants of the B16 melanoma: relationship to primary tumor size and pulmonary metastases. J Surg Oncol. 1986;33:41–45. doi: 10.1002/jso.2930330112. [DOI] [PubMed] [Google Scholar]
- 32.Bevilacqua MP. Endothelial-leukocyte adhesion molecules. Annu Rev Immunol. 1993;11:767–804. doi: 10.1146/annurev.iy.11.040193.004003. [DOI] [PubMed] [Google Scholar]
- 33.Ley K, Huo Y. VCAM-1 is critical in atherosclerosis. J Clin Invest. 2001;107:1209–1210. doi: 10.1172/JCI13005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Soriano A, Salas A, Sans M, Gironella M, Elena M, Anderson DC, Pique JM, Panes J. VCAM-1, but not ICAM-1 or MAdCAM-1, immunoblockade ameliorates DSS-induced colitis in mice. Lab Invest. 2000;80:1541–1551. doi: 10.1038/labinvest.3780164. [DOI] [PubMed] [Google Scholar]
- 35.Carter RA, Wicks IP. Vascular cell adhesion molecule 1 (CD106): a multifaceted regulator of joint inflammation. Arthritis Rheum. 2001;44:985–994. doi: 10.1002/1529-0131(200105)44:5<985::AID-ANR176>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 36.Rice GE, Bevilacqua MP. An inducible endothelial cell surface glycoprotein mediates melanoma adhesion. Science. 1989;246:1303–1306. doi: 10.1126/science.2588007. [DOI] [PubMed] [Google Scholar]
- 37.Nelson GM, Padera TP, Garkavtsev I, Shioda T, Jain RK. Differential gene expression of primary cultured lymphatic and blood vascular endothelial cells. Neoplasia. 2007;9:1038–1045. doi: 10.1593/neo.07643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Saban MR, Memet S, Jackson DG, Ash J, Roig AA, Israel A, Saban R. Visualization of lymphatic vessels through NF-κB activity. Blood. 2004;104:3228–3230. doi: 10.1182/blood-2004-04-1428. [DOI] [PubMed] [Google Scholar]
- 39.Collins T, Read MA, Neish AS, Whitley MZ, Thanos D, Maniatis T. Transcriptional regulation of endothelial cell adhesion molecules: NF-κB and cytokine-inducible enhancers. FASEB J. 1995;9:899–909. [PubMed] [Google Scholar]
- 40.Qian F, Vaux DL, Weissman IL. Expression of the integrin α4β1 on melanoma cells can inhibit the invasive stage of metastasis formation. Cell. 1994;77:335–347. doi: 10.1016/0092-8674(94)90149-x. [DOI] [PubMed] [Google Scholar]
- 41.Muller A, Homey B, Soto H, Ge N, Catron D, Buchanan ME, McClanahan T, Murphy E, Yuan W, Wagner SN, et al. Involvement of chemokine receptors in breast cancer metastasis. Nature. 2001;410:50–56. doi: 10.1038/35065016. [DOI] [PubMed] [Google Scholar]
- 42.Murphy WJ, Tian ZG, Asai O, Funakoshi S, Rotter P, Henry M, Strieter RM, Kunkel SL, Longo DL, Taub DD. Chemokines and T lymphocyte activation: II. Facilitation of human T cell trafficking in severe combined immunodeficiency mice. J Immunol. 1996;156:2104–2111. [PubMed] [Google Scholar]
- 43.Forster R, Davalos-Misslitz AC, Rot A. CCR7 and its ligands: balancing immunity and tolerance. Nat Rev Immunol. 2008;8:362–371. doi: 10.1038/nri2297. [DOI] [PubMed] [Google Scholar]
- 44.Chan JR, Hyduk SJ, Cybulsky MI. Chemoattractants induce a rapid and transient upregulation of monocyte α4 integrin affinity for vascular cell adhesion molecule 1 which mediates arrest: an early step in the process of emigration. J Exp Med. 2001;193:1149–1158. doi: 10.1084/jem.193.10.1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wiley HE, Gonzalez EB, Maki W, Wu M, Hwang ST. Expression of CC chemokine receptor-7 and regional lymph node metastasis of B16 murine melanoma. J Natl Cancer Inst. 2001;93:1638–1643. doi: 10.1093/jnci/93.21.1638. [DOI] [PubMed] [Google Scholar]
- 46.Uchida D, Begum NM, Tomizuka Y, Bando T, Almofti A, Yoshida H, Sato M. Acquisition of lymph node, but not distant metastatic potentials, by the overexpression of CXCR4 in human oral squamous cell carcinoma. Lab Invest. 2004;84:1538–1546. doi: 10.1038/labinvest.3700190. [DOI] [PubMed] [Google Scholar]
- 47.Kawada K, Sonoshita M, Sakashita H, Takabayashi A, Yamaoka Y, Manabe T, Inaba K, Minato N, Oshima M, Taketo MM. Pivotal role of CXCR3 in melanoma cell metastasis to lymph nodes. Can Res. 2004;64:4010–4017. doi: 10.1158/0008-5472.CAN-03-1757. [DOI] [PubMed] [Google Scholar]
- 48.Testaz S, Duband JL. Central role of the α4β1 integrin in the coordination of avian truncal neural crest cell adhesion, migration, and survival. Dev Dyn. 2001;222:127–140. doi: 10.1002/dvdy.1181. [DOI] [PubMed] [Google Scholar]
- 49.Leu ST, Jacques SA, Wingerd KL, Hikita ST, Tolhurst EC, Pring JL, Wiswell D, Kinney L, Goodman NL, Jackson DY, et al. Integrin α4β1 function is required for cell survival in developing retina. Dev Biol. 2004;276:416–430. doi: 10.1016/j.ydbio.2004.09.003. [DOI] [PubMed] [Google Scholar]
- 50.Delforge M, Raets V, Van Duppen V, Vandenberghe P, Boogaerts M. CD34+ marrow progenitors from MDS patients with high levels of intramedullary apoptosis have reduced expression of α4β1 and α5β1 integrins. Leukemia. 2005;19:57–63. doi: 10.1038/sj.leu.2403551. [DOI] [PubMed] [Google Scholar]