Abstract
Allyl isothiocyanate (AITC), which occurs in many common cruciferous vegetables, is widely and often frequently consumed by humans. Besides antimicrobial activity against a wide spectrum of pathogens, it showed anticancer activity in both cultured cancer cells and animal models, although the underlining mechanisms remain largely undefined. Bioavailability of AITC is extremely high, as nearly 90% of orally administered AITC is absorbed. AITC absorbed in vivo is metabolized mainly through the mercapturic acid pathway and excreted in urine. Available data suggest that urinary concentrations of AITC equivalent are at least 10 times higher than in the plasma, and tissue levels of AITC equivalent in the urinary bladder were 14-79 times higher than in other organs after oral AITC administration to rats. These findings suggest that AITC may be most effective in the bladder as a cancer chemopreventive compound. AITC at high dose levels also exhibit a low degree of cytotoxicity and genotoxicity in animal studies, but such adverse effects are unlikely in humans exposed to dietary levels of AITC. Overall, AITC exhibits many desirable attributes of a cancer chemopreventive agent, and further studies are warranted in order to elucidate its mechanism of action and to assess its protective activity in humans.
Keywords: allyl isothiocyanate, chemoprevention, cruciferous vegetable, sinigrin
1 Introduction
Allyl isothiocyanate (AITC), also known as mustard oil, is one of the most common naturally occurring isothiocyanates (ITCs) [1, 2]. ITCs occur primarily in cruciferous vegetables, many of which show significant cancer chemopreventive activities, and therefore are widely suspected to account in part for the cancer preventive activities of these vegetables in humans [3]. Sulforaphane is perhaps the most widely known crucifer-derived cancer chemopreventive ITC [4]. ITCs are synthesized and stored in cruciferous vegetables as glucosinolates (β-thioglucoside N-hydroxysulfate), which are believed to be chemically and biologically inert, and formed from the latter when plant tissues are damaged. The conversion is catalyzed by myrosinase (a thioglucoside glucohydrolase), first forming thiohydroximate-O-sulfonates, which rapidly and spontaneously rearrange to give rise to ITCs. Myrosinase coexists with but is physically separated from glucosinolates under normal conditions. Conversion (up to 40%) to ITCs of ingested glucosinolates that escape plant myrosinase may take place in vivo, as the intestinal microflora of both humans and animals also possess myrosinase activity [5-7].
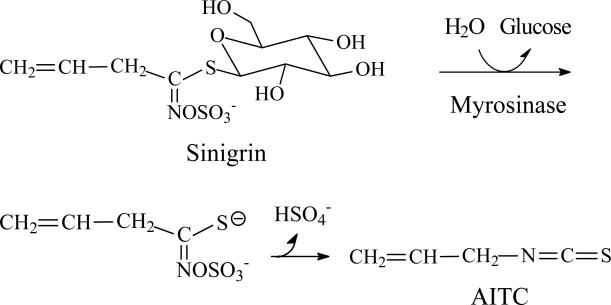
AITC is derived from sinigrin, as shown in Fig. 1, which is the predominant glucosinolate in many commonly consumed cruciferous vegetables, such as Brussels sprouts, cabbage, cauliflower and kale [1, 8], and are particularly abundant in mustard, horseradish and wasabi [9, 10]. For example, each gram of fresh wasabi yields as much as 34 μmol sinigrin/AITC [10]. Conversion of sinigrin to AITC by human microflora myrosinase has been well documented [11, 12]. However, the yield of AITC in certain vegetables such as cabbage may vary significantly, due to the presence of an epithiospecifier protein, which promotes formation of 1-cyano-2,3-epithiopropane, at the cost of AITC [8]. Interestingly, a recent study has found that 1-cyano-2,3-epithiopropane induces Phase 2 genes and affords cytoprotection [13]. AITC is a liquid at ambient temperature (melting point of -80°C) and has a very pungent taste, apparently due to its activation of the transient receptor potential A1 channel (TRPA1) in sensory neurons [14, 15]. Indeed, AITC is responsible for the pungent taste of the above-mentioned vegetables, and synthetic AITC is sometimes deliberately added to some vegetable products such as a prepared horseradish meal to enhance the flavor. AITC appears to serve the plant as a defense against herbivores, as chewing the plant by the herbivores generates AITC that presumably repels them.
Figure 1.
Myrosinase-catalyzed conversion from sinigrin to AITC
Human exposure to AITC is undoubtedly widespread and frequent, as many common cruciferous vegetables are a rich source of AITC, but the exposure levels have not been well documented. A large number of studies on the biological response to AITC have been published, many of which suggest that AITC is a highly attractive cancer chemopreventive agent. But a few other studies also raised the concern of potential toxicity. In this review, the evidence that argues for and against AITC as a cancer chemopreventive agent is presented and discussed: it is divided into five sections, including bioavailability and metabolic disposition of AITC, cellular uptake and tissue distribution of AITC, antimicrobial activity of AITC, anticancer activity of AITC, and dichotomy of cytoprotective activity and toxicity of AITC. To the best of my knowledge, a similar review on AITC has not been published. Hence, this article may be a useful reference on the biological response to AITC, as most if not all of the relevant data are cited and discussed herein.
2 Bioavailability and metabolic disposition of AITC
More than 90% of a single oral dose of [14C]AITC (25 or 250 μmol/kg body weight) was absorbed in mice and rats, and in both instances nearly 80% of the administered doses was recovered in the urine [16, 17]. These results indicate extremely high bioavailability of AITC and that absorbed AITC is primarily eliminated in the urine. Our recent study showed that urinary elimination of AITC was very rapid, as approximately 75% and 0.6% of a single oral dose of AITC were detected in the urine collected in the first and second 24-h periods after dosing [18]. No apparent sex-related differences were observed in the ability of these animals to absorb and dispose AITC. Human absorption and disposition of AITC appear to closely resemble that of animals, as studies showed that at least 42-54% of the dose was recovered in the urine as a metabolite (see the next paragraph for detail) within 10-12 h after each human volunteer was given 45-90 μmol of AITC supplied as either a horseradish paste or a mustard paste [5, 19].
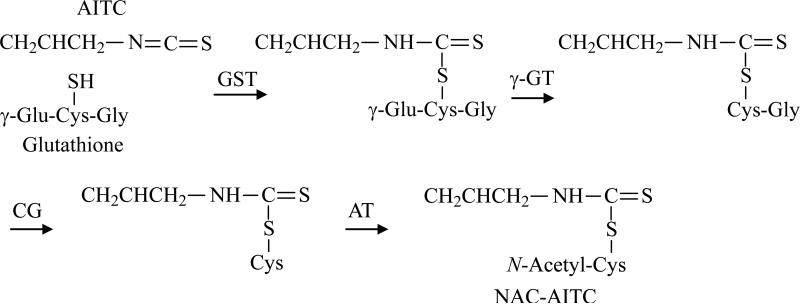
Although covalent modification of lysine residues (through the NH2 group) of protein by AITC can take place in physiological conditions [20], it predominantly undergoes conjugation with cysteine residues (through the SH group). AITC is primarily metabolized through the mercapturic acid pathway in vivo (Fig. 2). An initial conjugation through its –N=C=S group with glutathione (GSH) gives rise to the corresponding conjugate, which then undergoes further enzymatic modifications to finally form NAC conjugate, which is excreted in the urine. In rats dosed orally with [14C]AITC, approximately 80% of the 14C in the urine was present as the NAC conjugate, with the majority of the remaining radioactivity detected as thiocyanate [16, 17]. It is not clear if thiocyanate was generated directly from AITC or its NAC conjugate, nor is it known to possess any cancer chemopreventive activity. In contrast, in mice dosed orally with [14C]AITC, less than 20% of the urinary radioactivity was related to the NAC conjugate, and the level in female mice appeared to be only half of that in male mice, whereas the majority of the remaining radioactivity was associated with thiocyanate [16, 17]. The NAC conjugate was also the major metabolite in humans, as 42-54% of the dose was recovered in the urine as NAC-AITC within 10-12 h in each volunteer who consumed AITC [5, 19], although it is not known if AITC gives rise to thiocyanate in humans. Thus, the rat appears to resemble human more than mice in AITC metabolism.
Figure 2.
AITC metabolism through the mercapturic acid pathway. GST, glutathione S-transferase; γ-GT, γ-glutamyltranspeptidase; CG, cysteinylglycinase; AT, N-acetyltransferase.
3 Cellular uptake and tissue distribution of AITC
Studies in our laboratory have shown that AITC as well as other ITCs rapidly accumulate in cells. ITCs appear to enter cells by diffusion, but once in the cell, ITCs are rapidly conjugated with intracellular thiols [21-23]. GSH, which is the most abundant intracellular thiol, was found to be the major driving force for ITC accumulation [22], and cellular GSTs enhance ITC accumulation by promoting the conjugation reactions [24]. Not surprisingly, ITCs that are already conjugated with thiols, such as GSH, cysteine, and NAC, were unable to accumulate in cells [22]. Indeed, addition of excess GSH to culture medium was shown to completely block the cytotoxicity of AITC and benzyl ITC [25]. The peak intracellular ITC accumulation was achieved within 0.5-3 h of exposure, reaching 100-200-fold over the extracellular ITC concentration, and the total intracellular ITC accumulation can reach millimolar levels [21, 22].
However, intracellularly accumulated GSH conjugates of ITCs, perhaps other thiol conjugates as well, were exported out of cells rapidly. For example, the half-time stay of the accumulated sulforaphane equivalent in human prostate cancer LNCaP cells was only about 1 h [26]. The export of ITC conjugates appears to be mediated, at least partly, by membrane drug transporters, e.g., multidrug resistance associated protein-1 (MRP-1) [26, 27]. Thus, continuous intracellular accumulation may only be possible when ITCs persist in the extracellular space at a level that allows cellular uptake of ITC to offset the rapid export of the accumulated conjugates. Total intracellular accumulation levels of ITC (area under time-concentration curve) may be critical for their biological activity, as we previously showed that the total intracellular accumulation levels of ITCs determined their activity to induce Phase 2 cytoprotective enzymes [21, 28].
Bollard et al reported that the peak levels of AITC equivalents in the blood of mice and rats, following a single oral dose of [14C]AITC at 25 and 250 μmol/kg, were approximately 0.04 mM and 0.5 mM, respectively [17]. Our recent study showed that the average 24-h urinary concentrations of AITC equivalent were 0.36 and 4.2 mM, respectively, following a single oral dose of AITC at 25 and 250 μmol/kg [18]. These results show that the average urinary concentrations of AITC equivalent are nearly 10 times higher than the peak levels of AITC equivalent in the blood, following AITC consumption. In fact, the difference may be much greater, because the blood levels of AITC equivalent were determined based on an all-inclusive radioactivity measurement, whereas the urinary levels of AITC equivalent were measured using the cyclocondensation assay which detects only free AITC and AITC metabolites formed in the mercapturic acid pathway [18], excluding other metabolites such as thiocyanate. Consistent with this analysis, urinary concentrations of ITC equivalent were 2-3 orders of magnitude higher than that in the plasma of rats fed orally with ITCs contained in broccoli sprout extracts (mainly sulforaphane), where all samples were measured by the cyclocondensation assay [29]. Not surprisingly, Bollard et al found that tissue levels of radioactivity in the bladder were 14-79 times higher than in other organs after a single oral dose of [14C]AITC at 250 μmol/kg (Table 1) [17]. Thus, urinary bladder is by far the most exposed organ in vivo to orally ingested ITCs, including AITC, apparently resulting from selective urinary disposition of its metabolites, mainly the NAC conjugate. The NAC conjugates of ITCs as well as other ITC metabolites formed in the mercapturic acid pathway serve as carriers of ITCs, as they are unstable and dissociate to the parent ITCs [25, 30].
Table 1.
Amounts of radioactivity remaining within the organs of rats at various time intervals following oral administration of [14C]AITCa)
| Time after oral doing (h) | μg equivalent of [14C]AITC/g wet tissue weight | ||||
|---|---|---|---|---|---|
| Urinary bladder | Brain | Kidney | Liver | Spleen | |
| 0.33 | 235.3 ± 48.7 | 3.6 ± 3.0 | 18.1 ± 8.0 | 12.4 ± 1.7 | 10.5 ± 7.2 |
| 1 | 248.5 ± 27.4 | 2.8 ± 0.6 | 14.4 ± 3.6 | 14.5 ± 2.6 | 10.4 ± 3.7 |
| 2 | 248.3 ± 22.8 | 3.5 ± 1.6 | 19.3 ± 2.4 | 14.5 ± 3.6 | 9.6 ± 0.4 |
| 6 | 215.0 ± 39.9 | 3.0 ± 3.0 | 19.7 ± 6.7 | 16.0 ± 4.8 | 8.8± 3.7 |
A single oral dose of [14C]AITC at 250 μmol/kg was administered to male F344 rats and the organs were then collected at the indicate time points and measured for radioactivity (ref 17). The results in female rat were similar.
4 The antimicrobial activity of AITC
Whereas sinigrin itself is not known to possess anti-microbial properties, AITC displays bactericidal activity against a variety of pathogenic bacteria, including Helicobacter pylori, Escherichia coli, Salmonella typhimurium, Staphylococcus aureus, Streptococcus mutans, Penicillium notatum, Bacillus cereus, and Vibrio parahaemolyticus, with the minimum bactericidal concentrations of AITC (the lowest concentration needed for complete inhibition of growth) ranging from 3.8 μM to 16.7 mM [31-33]. It has not been clearly understood why the minimum bactericidal concentrations of AITC varied so widely, but it was reported that change in pH in the culture medium from 4.5 to 8.5 elevated the minimum bactericidal concentration against Escherichia coli by 20 fold [32]. The anti-microbial activity is a property shared by many ITCs, and the activity of AITC appears to be relatively weak compared with several other ITCs. For example, the bactericidal activities of phenethyl ITC against 3 strains of Helicobacter pylori were 7.8-20.5 times more potent than AITC [31]. The implication of the bactericidal activity of AITC in cancer and infection in humans is unclear, although Helicobacter pylori is known to cause gastritis, gastric ulcer and gastric cancer in humans.
AITC also showed fungicidal activity against a variety of fungi and yeasts, including Aspergillus flavus, Endomyces fibuliger, Penicillium commune, Penicillium corylophilum, Penicillium discolor, Penicillium palitans, Penicillium polonicum, Penicillium roqueforti, Penicillium solitum, and Pichia anomala [34], and the mustard oil, of which 99% was AITC, was one of the strongest antifungal substances among the various natural oils examined [35].
The mechanism by which AITC kills bacteria or fungi is largely unknown, but its action appears to resemble polymyxin B [36], which is known to bind to cell membrane and to increase its permeability. AITC was also shown to significantly inhibit both thioredoxin reductase and acetate kinase isolated from Escherichia coli at approximately 100 μM [32]. These enzymes play an important role in cell growth and proliferation. In addition, AITC was also shown to cause oxidative stress and DNA damage in Escherichia coli [37]. Furthermore, as described below, studies in mammalian cells have revealed other mechanisms by which AITC causes cell death, some of which may be relevant to its bactericidal activity. However, both glutathione and cysteine were shown to almost completely abolish the bactericidal effect of AITC [38], which likely resulted from inhibition of its cellular uptake, as these agents were shown to block ITC uptake by mammalian cells (see Section 3 for detail).
5 The anticancer activity of AITC
5.1 Inhibition of cell proliferation
Whereas sinigrin itself is not known to possess any antiproliferative activity, AITC inhibits proliferation of various types of human cancer cells, with the IC50 values at the low micromolar range, regardless of their tissue origins and p53 status, and even in drug resistant cells that over express drug transporter MRP-1 or Pgp-1 [39-43]. In fact, exposure of cells to AITC for only 3 h seems sufficient to achieve growth inhibition [39, 42]. More interestingly, AITC appears to be significantly less toxic to normal cells. For example, 83% of normal human prostate epithelial cells were viable following a 24-h exposure to 40 μM AITC, whereas only 36-38% of human prostate cancer cells (LNCaP cells and PC-3 cells) survived under similar conditions of AITC treatment [40]. Detransformation of human colorectal cancer HT29 cells also rendered them more resistant to the cytotoxic effect of AITC, elevating the maximal concentration at which no cell is killed from 3.2 μM in HT29 cells to 7.4 μM in detransformed counterparts (24 h treatment) [41]. The IC50 value of AITC in normal human bladder epithelial cells is approximately 10 times higher than that in human bladder cancer cells (our unpublished observation).
5.2 Cell cycle arrest and induction of apoptosis
Inhibition of cell proliferation by AITC was associated with cell cycle arrest and/or induction of apoptosis. AITC at concentrations near its IC50 value caused significant arrest of cells (up to 80%) in either G1 phase or G2/M phase. For example, it arrested human leukemia HL60 cells in G1 phase [39], but caused G2/M arrest in bladder cancer UM-UC-3 cells [42], human cervical cancer HeLa cells [44], human colorectal cancer HT29 cells [45], and human prostate cancer cells (PC-3 and LNCaP) [40]. Smith et al subsequently showed that approximately 25% of AITC-treated HT29 cells were arrest in M phase. The reason as to why AITC causes G1 arrest in some cells but G2/M arrest or M arrest in other cells is not known. In LNCaP cells, however, where AITC causes G2/M arrest, AITC was shown to modulate a number of important G2/M regulators, including down regulation of cyclin B1, cdk1, cdc25B and cdc25C, and to cause the disruption of tubulin [40, 45].
Treatment of HL60 cells with AITC at 10 μM for 24 h rendered nearly 30% cells apoptotic, which was associated with disruption of mitochondrial transmembrane potential, activation of several caspases (caspse-3, -8, -9 and -12), and activation of c-Jun N-terminal kinase (JNK) [39, 46]. AITC also significantly induced apoptosis in PC-3 cells and LNCaP cells, which was associated with down regulation of anti-apoptotic Bcl-2 and Bcl-xl and activation of extracellular signal-regulated kinase and JNK [40, 47]. However, AITC was a poor apoptosis inducer in other cell lines, such as HT29 cells and UM-UC-3 cells (no more than 5% cells became apoptotic after AITC treatment) [42, 45]. Interestingly, it is of note that AITC induces c-Jun, a key component of activator protein 1 (AP-1), increased the transactivation activity and/or DNA binding activity of AP-1 in both HT29 cells and UM-UC-3 cells [48, 49]. The pro-survival or apoptosis inhibitory function of AP-1 is well known.
5.3 Other anticancer activities
Matrix metalloproteinases (MMPs) play important roles in cancer metastasis. Both AITC and its NAC conjugate were reported to significantly inhibit the transcription of MMP-2/-9 in human hepatoma SK-Hep1 cells at 0.1-5 μM, which was associated with inhibition of cell adhesion, migration and invasion [50]. MMP-2 and MMP-9 degrade components of basement membrane and are strongly implicated in the invasion and metastasis of cancer cells [51, 52]. The extent of histone acetylation also influences the growth of cancer cells and increasing histone acetylation is a recognized strategy for cancer prevention and therapy [53, 54]. AITC at 20 μM was shown to stimulate histone acetylation in mouse erythroleukemia DS19 cells, but this does not appear to result from inhibition of histone deacetylase [55]. However, sulforaphane was shown to inhibit histone deacetylase in cancer cells [56]. AITC was also found to significantly inhibit the production of nitric oxide (NO) and the expression of inducible nitric oxide synthase (iNOS) in lipopolysaccharide-treated J774.1 macrophages at <10 μM [57], and to inhibit NF-κB activation in lipopolysaccharide-treated HT-29 cells at 25-100 μM [58]. NO, iNOS and NF-κB are important signaling molecules in inflammation and cancer.
5.4 Inhibition of tumor growth
Intraperitoneal injection of 10 μmole AITC (approximately 333 μmol/kg body weight) three times per week for three weeks, beginning the day of tumor cell inoculation, inhibited PC-3 human prostate cancer xenografts in athymic mice by approximately 45%, with no apparent toxicity [59]. In another study, male Wistar rats were given dimethylhydrazine (DMH) subcutaneously twice (separated by 5 days) to induce aberrant crypt foci in the colonic mucosa, and AITC or sinigrin was given to the rats in the diet for 5 weeks, starting the next day after the second dose of DMH. Both sinigrin and AITC reduced the number of DMH-induced aberrance crypt foci in the colonic mucosa by approximately 40% [60]. Interestingly, in this study, sinigrin was more potent than AITC, as sinigrin at 1 μmol/kg diet was as effective as AITC at 4 μmol/kg diet. Since sinigrin itself is not known to possess cancer preventive activity, its inhibition of DMH-induced colonic aberrant crypt foci formation most likely resulted from its myrosinase-catalyzed conversion to AITC in vivo. In another study where hepatocarcinogenesis in ACI/N rats was induced by adding diethylnitrosamine in drinking water for 5 week, dietary supplementation with sinigrin at 1200 ppm (3 μmol sinigrin/g diet) during the carcinogen treatment period also reduced tumor incidence by 50% and reduced tumor multiplicity by more than 90% [61]. However, in 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK)-induced lung tumorigenesis in A/J mice, where a single oral dose of AITC at 1 or 5 μmol/mouse was given to the animal 2 h prior to a single intraperitoneal injection of NNK and lung tumorigenesis assessed 16 weeks later, AITC was ineffective, while a number of synthetic ITCs, especially 1-dodecyl ITC and 1,2-diphenylelthyl ITC were highly effective under the same experimental conditions [62]. The last animal model differs from other three models in that it is designed to evaluate acute inhibition of carcinogen activation (inhibition of carcinogen-activating enzymes) by a test agent.
6 The dichotomy of cytoprotective activity and toxicity of AITC
6.1 Stimulation of cytoprotective mechanisms
AITC has been shown to induce several Phase 2 enzymes, including NAD[P]H:quinone oxidoreductase-1, glutathione S-transferase, glutamate cysteine ligase and/or heme oxygenase 1 in both cultured cells in vitro and animal tissues in vivo [18, 21, 28, 42, 63-65]. Induction of the Phase 2 proteins by AITC must have resulted at least in part from the activation of nuclear factor erythroid 2-related factor 2 (Nrf2), a key transcription activator of the above-mentioned Phase 2 genes and many other genes, as AITC at 25 μM rapidly and markedly elevated Nrf2 level and Nrf2 transactivation activity in human hepatoma HepG2 cells [28, 63]. Nrf2 activates Phase 2 gene transcription by binding to the upstream regulatory element, namely the antioxidant response element (ARE). Indeed, McWalter et al showed that AITC was unable to stimulate the transcription of the downstream gene linked to a mutated ARE [65]. Given that Nrf2 is known to regulate a variety of Phase 2 genes and other genes [66], AITC probably stimulates many such genes. Because many Phase 2 proteins are major cellular antioxidant and carcinogen detoxification enzymes, it seems reasonable to assume that AITC would prevent oxidant- and carcinogen-induced damage. Indeed, AITC was found to significantly inhibit in a dose-dependent manner the formation of gastric lesions induced by ethanol, hydrochloric acid, ammonia, aspirin, and indomethacin in Sprague-Dawley rats at the oral dose levels of 1.25-10 mg/kg body weight (12.5-100 μmol/kg) [67].
However, to what extent stimulation of cytoprotective proteins by AITC contributes to its cancer chemopreventive activity is not clear. Nor is it clear whether activation of Nrf2 signaling attenuates its anticancer activities such as induction of cell cycle arrest and apoptosis of cancer cells.
6.2 Cytotoxicity and genotoxicity of AITC
Pretreatment of HepG2 cells with AITC at up to 6 μM for 24 h enhanced benzo(a)pyrene (BP)-induced DNA damage by almost 2 fold, as measured by the single cell gel electrophoresis assay [68]. The reason why AITC increased BP genotoxicity is not known, as its effect on Nrf2 and carcinogen-detoxifying enzymes was not measured in these cells. Treatment of HL60 cells with AITC at 2-5 μM for only 3 h was shown to cause DNA damage and the formation of 8-oxo-7,8-dihydro-2’-deoxyguanosine, which was thought to result from increased formation of reactive oxygen species [69]. Intracellular generation of reactive oxygen species and DNA damage were also detected in bacterial cells treated with AITC [70].
However, DNA damage by AITC in HepG2 cells (formation of micronucleus) was negligible in HeLa cells (unscheduled DNA synthesis), and its mutagenicity in bacterial cells (Ames test) occurred only at relatively high concentrations (>50 μM) [37, 71]. Nor did AITC cause significant chromosome aberrations or sister chromatic exchanges in a SV40-transformed Indian muntjac cell line and a Chinese hamster ovary cell line even at highly cytotoxic doses [72, 73]. Likewise, unscheduled DNA synthesis was not detected in the livers of Sprague-Dawley rats receiving a single oral dose of AITC up to 125 mg/kg body weight (1.25 mmol/kg) [74].
Rats receiving a single oral dose of AITC at approximately 13 mg/kg showed reduced uptake of iodine by the thyroid gland [75], suggesting a weak goitrogenic activity of AITC. However, another study showed that rats (Shoe: WIST) given AITC at oral doses up to 40 mg/kg 5 days/week for 4 weeks did not show any changes in thyroid weight, even though the highest AITC dose caused a significant decrease in body weight [76]. F344 rats and B6C3F1 mice given oral AITC at 50 mg/kg body weight (500 μmol/kg) 5 days per week for 2 weeks showed a thickened mucosal surface of the stomach in both rats and mice and a thickened urinary bladder wall in male mice, but no gross or microscopic lesions were detected in the animals given oral AITC at 25 mg/kg (250 μmol/kg) 5 days per week for 13 week [77]. In a further experiment where F344 rats and B6C3F1 mice of either sex were administered orally with 12 or 25 mg/kg (120 or 250 μmol/kg) AITC 5 times per week for 103 weeks [77], urinary bladder cancer was detected in 4% and 8% male rats treated with the low and high doses of AITC respectively, whereas no bladder tumor was detected in any other groups. Subcutaneous fibrosarcoma was detected in 6% of female rats receiving the high dose of AITC, but not in any other groups. Human relevance of these findings is likely to be very limited, if any, because average human consumption of AITC has been estimated to be less than 1 mg/day (approximately 10 μg/kg body weight) [78]. The sex-, species-, and organ-specific susceptibilities of tumorigenesis to AITC have not been well understood. A single instillation of AITC into the urinary bladder of female F344 rats at 2.8 mg/ml/kg body weight for 2 h via the urethra using a catheter caused acute toxic damage to the bladder, including hemorrhage, inflammatory cell infiltration, vacuolar degeneration and apoptosis/necrosis of the mucosal/submucosal tissues, and delayed increase in BrdU labeling index [79]. But interpretation of this data needs caution, because AITC was given at very high concentration (28 mM), and its NAC conjugate (the principal urinary metabolite) was not examined.
7 Concluding remarks
AITC, a common dietary phytochemical, presents many desirable attributes of a cancer chemopreventive agent, including extremely high bioavailability after oral administration, rapid uptake by cells, microbicidal activity against a wide spectrum of pathogens, significantly higher toxicity in malignant cell than in normal cells, its ability to rapidly induce cancer cell death regardless of its tissue origin or p53 status and even in drug resistant cells, activation of Nrf2 signaling, and inhibition of cancer development in vivo. However, the AITC dose levels used in the preclinical studies are far greater than what humans are normally exposed to, raising the question of whether the preclinical data are relevant to humans and whether dietary consumption of AITC significantly contributes to cancer prevention in humans. The observation that bladder is the tissue which is by far the most exposed to orally administered AITC, apparently resulting from its almost exclusive elimination through the urine, suggests that AITC may most be useful for bladder cancer prevention.
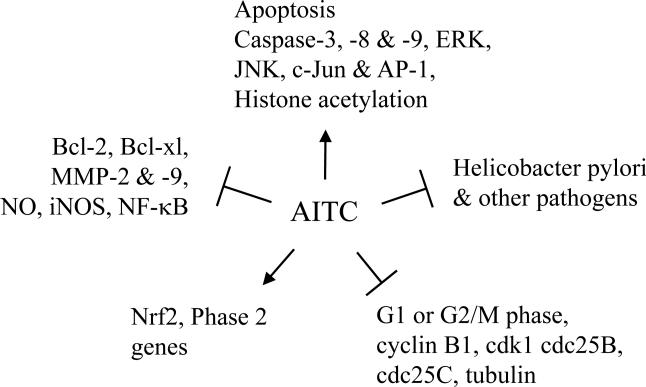
The molecular mechanisms by which AITC attacks bacteria, fungi, and cancer cells remain poorly defined, but the putative chemopreventive mechanisms are summarized in Fig. 3. Further studies are needed to verify and extend these findings. Chronic administration of AITC to rodents at high doses levels caused low incidence of urinary bladder transitional cell carcinoma and subcutaneous fibrosarcoma among other toxicities. But it is highly unlikely that such toxicities would occur in humans, because dietary consumption levels of AITC appear to be several orders of magnitude lower than the doses used in the animal studies.
Figure 3.
Putative cancer chemopreventive mechanisms of AITC. The arrows indicate activation, and the Ts indicate inhibition. The information is compiled from a collection of published studies in different cell lines, which are discussed in this review. AP-1, activator protein 1; ERK, extracellular signal-regulated kinase; iNOS, inducible nitric oxide synthase; JNK, c-Jun N-terminal kinase; MMP-2 &-9;matrix metalloproteinase-2 & -9; NO, nitric oxide; Nrf2, nuclear factor erythroid 2-relarted factor 2.
Acknowledgments
The author would like to thank Dr. Arup Bhattacharya of Roswell Park Cancer Institute for critical reading of the manuscript. The work was supported in part by National Cancer Institute R01 grant CA124627.
Abbreviations
- AITC
allyl isothiocyanate
- DMH
dimethylhydrazine
- GSH
glutathione
- ITC
isothiocyanate
- NAC
N-acetylcysteine
- Nrf2
nuclear factor erythroid 2-relarted factor 2
Footnotes
The author has declared no conflict of interest.
8 References
- 1.Kushad MM, Brown AF, Kurilich AC, Juvik JA, et al. Variation of glucosinolates in vegetable crops of Brassica oleracea. J Agric Food Chem. 1999;47:1541–1548. doi: 10.1021/jf980985s. [DOI] [PubMed] [Google Scholar]
- 2.Fahey JW, Zalcmann AT, Talalay P. The chemical diversity and distribution of glucosinolates and isothiocyanates among plants. Phytochemistry. 2001;56:5–51. doi: 10.1016/s0031-9422(00)00316-2. [DOI] [PubMed] [Google Scholar]
- 3.Organization, I. A. f. C. R. o. C. W. H. Cruciferous Vegetables, Isothiocyanates and Indoles. IARC Press; Lyon: 2004. [Google Scholar]
- 4.Zhang Y, Tang L. Discovery and development of sulforaphane as a cancer chemopreventive phytochemical. Acta Pharmacol Sin. 2007;28:1343–1354. doi: 10.1111/j.1745-7254.2007.00679.x. [DOI] [PubMed] [Google Scholar]
- 5.Shapiro TA, Fahey JW, Wade KL, Stephenson KK, Talalay P. Human metabolism and excretion of cancer chemoprotective glucosinolates and isothiocyanates of cruciferous vegetables. Cancer Epidemiol Biomarkers Prev. 1998;7:1091–1100. [PubMed] [Google Scholar]
- 6.Getahun SM, Chung FL. Conversion of glucosinolates to isothiocyanates in humans after ingestion of cooked watercress. Cancer Epidemiol Biomarkers Prev. 1999;8:447–451. [PubMed] [Google Scholar]
- 7.Kensler TW, Chen JG, Egner PA, Fahey JW, et al. Effects of glucosinolate-rich broccoli sprouts on urinary levels of aflatoxin-DNA adducts and phenanthrene tetraols in a randomized clinical trial in He Zuo township, Qidong, People's Republic of China. Cancer Epidemiol Biomarkers Prev. 2005;14:2605–2613. doi: 10.1158/1055-9965.EPI-05-0368. [DOI] [PubMed] [Google Scholar]
- 8.Rungapamestry V, Duncan AJ, Fuller Z, Ratcliffe B. Changes in glucosinolate concentrations, myrosinase activity, and production of metabolites of glucosinolates in cabbage (Brassica oleracea Var. capitata) cooked for different durations. J Agric Food Chem. 2006;54:7628–7634. doi: 10.1021/jf0607314. [DOI] [PubMed] [Google Scholar]
- 9.Uematsu Y, Hirata K, Suzuki K, Iida K, et al. Determination of isothiocyanates and related compounds in mustard extract and horseradish extract used as natural food additives. Shokuhin Eiseigaku Zasshi. 2002;43:10–17. doi: 10.3358/shokueishi.43.10. [DOI] [PubMed] [Google Scholar]
- 10.Sultana T, Savage GP, McNeil DL, Porter NG, et al. Effects of fertilisation on the allyl isothiocyanate profile of above-ground tissue of New Zealand-grown wasabi. Journal of the Science of Food and Agriculture. 2002;82:1477–1482. [Google Scholar]
- 11.Krul C, Humblot C, Philippe C, Vermeulen M, et al. Metabolism of sinigrin (2-propenyl glucosinolate) by the human colonic microflora in a dynamic in vitro large-intestinal model. Carcinogenesis. 2002;23:1009–1016. doi: 10.1093/carcin/23.6.1009. [DOI] [PubMed] [Google Scholar]
- 12.Elfoul L, Rabot S, Khelifa N, Quinsac A, et al. Formation of allyl isothiocyanate from sinigrin in the digestive tract of rats monoassociated with a human colonic strain of Bacteroides thetaiotaomicron. FEMS Microbiol Lett. 2001;197:99–103. doi: 10.1111/j.1574-6968.2001.tb10589.x. [DOI] [PubMed] [Google Scholar]
- 13.Kelleher MO, McMahon M, Eggleston IM, Dixon MJ, et al. 1-Cyano-2,3-epithiopropane is a novel plant-derived chemopreventive agent which induces cytoprotective genes that afford resistance against the genotoxic α,β-unsaturated aldehyde acrolein. Carcinogenesis. 2009 doi: 10.1093/carcin/bgp182. advanced access. [DOI] [PubMed] [Google Scholar]
- 14.Eid SR, Crown ED, Moore EL, Liang HA, et al. HC-030031, a TRPA1 selective antagonist, attenuates inflammatory- and neuropathy-induced mechanical hypersensitivity. Mol Pain. 2008;4:48. doi: 10.1186/1744-8069-4-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cavanaugh EJ, Simkin D, Kim D. Activation of transient receptor potential A1 channels by mustard oil, tetrahydrocannabinol and Ca2+ reveals different functional channel states. Neuroscience. 2008;154:1467–1476. doi: 10.1016/j.neuroscience.2008.04.048. [DOI] [PubMed] [Google Scholar]
- 16.Ioannou YM, Burka LT, Matthews HB. Allyl isothiocyanate: comparative disposition in rats and mice. Toxicol Appl Pharmacol. 1984;75:173–181. doi: 10.1016/0041-008x(84)90199-6. [DOI] [PubMed] [Google Scholar]
- 17.Bollard M, Stribbling S, Mitchell S, Caldwell J. The disposition of allyl isothiocyanate in the rat and mouse. Food Chem Toxicol. 1997;35:933–943. doi: 10.1016/s0278-6915(97)00103-8. [DOI] [PubMed] [Google Scholar]
- 18.Munday R, Zhang Y, Fahey JW, Jobson HE, et al. Evaluation of isothiocyanates as potent inducers of carcinogen-detoxifying enzymes in the urinary bladder: critical nature of in vivo bioassay. Nutr Cancer. 2006;54:223–231. doi: 10.1207/s15327914nc5402_9. [DOI] [PubMed] [Google Scholar]
- 19.Jiao D, Ho CT, Foiles P, Chung FL. Identification and quantification of the N-acetylcysteine conjugate of allyl isothiocyanate in human urine after ingestion of mustard. Cancer Epidemiol Biomarkers Prev. 1994;3:487–492. [PubMed] [Google Scholar]
- 20.Nakamura T, Kawai Y, Kitamoto N, Osawa T, Kato Y. Covalent modification of lysine residues by allyl isothiocyanate in physiological conditions: pausible transformation of isothiocyanate from thiol to amine. Chemical Research in Toxicology. 2009;22:536–542. doi: 10.1021/tx8003906. [DOI] [PubMed] [Google Scholar]
- 21.Zhang Y, Talalay P. Mechanism of differential potencies of isothiocyanates as inducers of anticarcinogenic Phase 2 enzymes. Cancer Res. 1998;58:4632–4639. [PubMed] [Google Scholar]
- 22.Zhang Y. Role of glutathione in the accumulation of anticarcinogenic isothiocyanates and their glutathione conjugates by murine hepatoma cells. Carcinogenesis. 2000;21:1175–1182. [PubMed] [Google Scholar]
- 23.Tang L, Li G, Song L, Zhang Y. The principal urinary metabolites of dietary isothiocyanates, N-acetylcysteine conjugates, elicit the same anti-proliferative response as their parent compounds in human bladder cancer cells. Anticancer Drugs. 2006;17:297–305. doi: 10.1097/00001813-200603000-00008. [DOI] [PubMed] [Google Scholar]
- 24.Zhang Y. Molecular mechanism of rapid cellular accumulation of anticarcinogenic isothiocyanates. Carcinogenesis. 2001;22:425–431. doi: 10.1093/carcin/22.3.425. [DOI] [PubMed] [Google Scholar]
- 25.Bruggeman IM, Temmink JH, van Bladeren PJ. Glutathione- and cysteine-mediated cytotoxicity of allyl and benzyl isothiocyanate. Toxicol Appl Pharmacol. 1986;83:349–359. doi: 10.1016/0041-008x(86)90312-1. [DOI] [PubMed] [Google Scholar]
- 26.Zhang Y, Callaway EC. High cellular accumulation of sulphoraphane, a dietary anticarcinogen, is followed by rapid transporter-mediated export as a glutathione conjugate. Biochem J. 2002;364:301–307. doi: 10.1042/bj3640301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Callaway EC, Zhang Y, Chew W, Chow HH. Cellular accumulation of dietary anticarcinogenic isothiocyanates is followed by transporter-mediated export as dithiocarbamates. Cancer Lett. 2004;204:23–31. doi: 10.1016/j.canlet.2003.09.021. [DOI] [PubMed] [Google Scholar]
- 28.Ye L, Zhang Y. Total intracellular accumulation levels of dietary isothiocyanates determine their activity in elevation of cellular glutathione and induction of Phase 2 detoxification enzymes. Carcinogenesis. 2001;22:1987–1992. doi: 10.1093/carcin/22.12.1987. [DOI] [PubMed] [Google Scholar]
- 29.Munday R, Mhawech-Fauceglia P, Munday CM, Paonessa JD, et al. Inhibition of urinary bladder carcinogenesis by broccoli sprouts. Cancer Res. 2008;68:1593–1600. doi: 10.1158/0008-5472.CAN-07-5009. [DOI] [PubMed] [Google Scholar]
- 30.Conaway CC, Krzeminski J, Amin S, Chung FL. Decomposition rates of isothiocyanate conjugates determine their activity as inhibitors of cytochrome p450 enzymes. Chem Res Toxicol. 2001;14:1170–1176. doi: 10.1021/tx010029w. [DOI] [PubMed] [Google Scholar]
- 31.Shin IS, Masuda H, Naohide K. Bactericidal activity of wasabi (Wasabia japonica) against Helicobacter pylori. Int J Food Microbiol. 2004;94:255–261. doi: 10.1016/S0168-1605(03)00297-6. [DOI] [PubMed] [Google Scholar]
- 32.Luciano FB, Holley RA. Enzymatic inhibition by allyl isothiocyanate and factors affecting its antimicrobial action against Escherichia coli O157:H7. Int J Food Microbiol. 2009;131:240–245. doi: 10.1016/j.ijfoodmicro.2009.03.005. [DOI] [PubMed] [Google Scholar]
- 33.Tunc S, Chollet E, Chalier P, Preziosi-Belloy L, Gontard N. Combined effect of volatile antimicrobial agents on the growth of Penicillium notatum. Int J Food Microbiol. 2007;113:263–270. doi: 10.1016/j.ijfoodmicro.2006.07.004. [DOI] [PubMed] [Google Scholar]
- 34.Nielsen PV, Rios R. Inhibition of fungal growth on bread by volatile components from spices and herbs, and the possible application in active packaging, with special emphasis on mustard essential oil. Int J Food Microbiol. 2000;60:219–229. doi: 10.1016/s0168-1605(00)00343-3. [DOI] [PubMed] [Google Scholar]
- 35.Suhr KI, Nielsen PV. Antifungal activity of essential oils evaluated by two different application techniques against rye bread spoilage fungi. J Appl Microbiol. 2003;94:665–674. doi: 10.1046/j.1365-2672.2003.01896.x. [DOI] [PubMed] [Google Scholar]
- 36.Lin CM, Preston JF, 3rd, Wei CI. Antibacterial mechanism of allyl isothiocyanate. J Food Prot. 2000;63:727–734. doi: 10.4315/0362-028x-63.6.727. [DOI] [PubMed] [Google Scholar]
- 37.Kassie F, Knasmuller S. Genotoxic effects of allyl isothiocyanate (AITC) and phenethyl isothiocyanate (PEITC). Chem Biol Interact. 2000;127:163–180. doi: 10.1016/s0009-2797(00)00178-2. [DOI] [PubMed] [Google Scholar]
- 38.Luciano FB, Hosseinian FS, Beta T, Holley RA. Effect of free-SH containing compounds on allyl isothiocyanate antimicrobial activity against Escherichia coli O157:H7. J Food Sci. 2008;73:M214–220. doi: 10.1111/j.1750-3841.2008.00762.x. [DOI] [PubMed] [Google Scholar]
- 39.Zhang Y, Tang L, Gonzalez V. Selected isothiocyanates rapidly induce growth inhibition of cancer cells. Mol Cancer Ther. 2003;2:1045–1052. [PubMed] [Google Scholar]
- 40.Xiao D, Srivastava SK, Lew KL, Zeng Y, et al. Allyl isothiocyanate, a constituent of cruciferous vegetables, inhibits proliferation of human prostate cancer cells by causing G2/M arrest and inducing apoptosis. Carcinogenesis. 2003;24:891–897. doi: 10.1093/carcin/bgg023. [DOI] [PubMed] [Google Scholar]
- 41.Musk SR, Johnson IT. Allyl isothiocyanate is selectively toxic to transformed cells of the human colorectal tumour line HT29. Carcinogenesis. 1993;14:2079–2083. doi: 10.1093/carcin/14.10.2079. [DOI] [PubMed] [Google Scholar]
- 42.Tang L, Zhang Y. Dietary isothiocyanates inhibit the growth of human bladder carcinoma cells. J Nutr. 2004;134:2004–2010. doi: 10.1093/jn/134.8.2004. [DOI] [PubMed] [Google Scholar]
- 43.Xu K, Thornalley PJ. Studies on the mechanism of the inhibition of human leukaemia cell growth by dietary isothiocyanates and their cysteine adducts in vitro. Biochem Pharmacol. 2000;60:221–231. doi: 10.1016/s0006-2952(00)00319-1. [DOI] [PubMed] [Google Scholar]
- 44.Hasegawa T, Nishino H, Iwashima A. Isothiocyanates inhibit cell cycle progression of HeLa cells at G2/M phase. Anticancer Drugs. 1993;4:273–279. doi: 10.1097/00001813-199304000-00021. [DOI] [PubMed] [Google Scholar]
- 45.Smith TK, Lund EK, Parker ML, Clarke RG, Johnson IT. Allyl-isothiocyanate causes mitotic block, loss of cell adhesion and disrupted cytoskeletal structure in HT29 cells. Carcinogenesis. 2004;25:1409–1415. doi: 10.1093/carcin/bgh149. [DOI] [PubMed] [Google Scholar]
- 46.Xu K, Thornalley PJ. Signal transduction activated by the cancer chemopreventive isothiocyanates: cleavage of BID protein, tyrosine phosphorylation and activation of JNK. Br J Cancer. 2001;84:670–673. doi: 10.1054/bjoc.2000.1636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Xu C, Shen G, Yuan X, Kim JH, et al. ERK and JNK signaling pathways are involved in the regulation of activator protein 1 and cell death elicited by three isothiocyanates in human prostate cancer PC-3 cells. Carcinogenesis. 2006;27:437–445. doi: 10.1093/carcin/bgi251. [DOI] [PubMed] [Google Scholar]
- 48.Jeong WS, Kim IW, Hu R, Kong AN. Modulation of AP-1 by natural chemopreventive compounds in human colon HT-29 cancer cell line. Pharm Res. 2004;21:649–660. doi: 10.1023/b:pham.0000022412.69380.d7. [DOI] [PubMed] [Google Scholar]
- 49.Li J, Yao S, Zhang Y. The role of c-Jun in the AP-1 activation induced by naturally occurring isothiocyanates. Food Chem Toxicol. 2005;43:1373–1380. doi: 10.1016/j.fct.2005.03.011. [DOI] [PubMed] [Google Scholar]
- 50.Hwang ES, Lee HJ. Allyl isothiocyanate and its N-acetylcysteine conjugate suppress metastasis via inhibition of invasion, migration, and matrix metalloproteinase-2/-9 activities in SK-Hep 1 human hepatoma cells. Exp Biol Med (Maywood) 2006;231:421–430. doi: 10.1177/153537020623100408. [DOI] [PubMed] [Google Scholar]
- 51.Zucker S, Vacirca J. Role of matrix metalloproteinases (MMPs) in colorectal cancer. Cancer Metastasis Rev. 2004;23:101–117. doi: 10.1023/a:1025867130437. [DOI] [PubMed] [Google Scholar]
- 52.Vincenti MP. The matrix metalloproteinase (MMP) and tissue inhibitor of metalloproteinase (TIMP) genes. Transcriptional and posttranscriptional regulation, signal transduction and cell-type-specific expression. Methods Mol Biol. 2001;151:121–148. doi: 10.1385/1-59259-046-2:121. [DOI] [PubMed] [Google Scholar]
- 53.Mahlknecht U, Hoelzer D. Histone acetylation modifiers in the pathogenesis of malignant disease. Mol Med. 2000;6:623–644. [PMC free article] [PubMed] [Google Scholar]
- 54.Marks PA, Richon VM, Rifkind RA. Histone deacetylase inhibitors: inducers of differentiation or apoptosis of transformed cells. J Natl Cancer Inst. 2000;92:1210–1216. doi: 10.1093/jnci/92.15.1210. [DOI] [PubMed] [Google Scholar]
- 55.Lea MA, Randolph VM, Lee JE, desBordes C. Induction of histone acetylation in mouse erythroleukemia cells by some organosulfur compounds including allyl isothiocyanate. Int J Cancer. 2001;92:784–789. doi: 10.1002/ijc.1277. [DOI] [PubMed] [Google Scholar]
- 56.Myzak MC, Hardin K, Wang R, Dashwood RH, Ho E. Sulforaphane inhibits histone deacetylase activity in BPH-1, LnCaP and PC-3 prostate epithelial cells. Carcinogenesis. 2006;27:811–819. doi: 10.1093/carcin/bgi265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ippoushi K, Itou H, Azuma K, Higashio H. Effect of naturally occurring organosulfur compounds on nitric oxide production in lipopolysaccharide-activated macrophages. Life Sci. 2002;71:411–419. doi: 10.1016/s0024-3205(02)01685-5. [DOI] [PubMed] [Google Scholar]
- 58.Jeong WS, Kim IW, Hu R, Kong AN. Modulatory properties of various natural chemopreventive agents on the activation of NF-κB signaling pathway. Pharm Res. 2004;21:661–670. doi: 10.1023/b:pham.0000022413.43212.cf. [DOI] [PubMed] [Google Scholar]
- 59.Srivastava SK, Xiao D, Lew KL, Hershberger P, et al. Allyl isothiocyanate, a constituent of cruciferous vegetables, inhibits growth of PC-3 human prostate cancer xenografts in vivo. Carcinogenesis. 2003;24:1665–1670. doi: 10.1093/carcin/bgg123. [DOI] [PubMed] [Google Scholar]
- 60.Smith T, Musk SRR, Johnson IT. Allyl isothiocyanate seletively kills undifferentiated HT29 cells in vitro and suppresses aberrant crypt foci in the colonic mucosa of rats. Biochemical Society Transactions. 1996;24:381S. doi: 10.1042/bst024381s. [DOI] [PubMed] [Google Scholar]
- 61.Tanaka T, Mori Y, Morishita Y, Hara A, et al. Inhibitory effect of sinigrin and indole-3-carbinol on diethylnitrosamine-induced hepatocarcinogenesis in male ACI/N rats. Carcinogenesis. 1990;11:1403–1406. doi: 10.1093/carcin/11.8.1403. [DOI] [PubMed] [Google Scholar]
- 62.Jiao D, Eklind KI, Choi CI, Desai DH, et al. Structure-activity relationships of isothiocyanates as mechanism-based inhibitors of 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone-induced lung tumorigenesis in A/J mice. Cancer Res. 1994;54:4327–4333. [PubMed] [Google Scholar]
- 63.Jeong WS, Keum YS, Chen C, Jain MR, et al. Differential expression and stability of endogenous nuclear factor E2-related factor 2 (Nrf2) by natural chemopreventive compounds in HepG2 human hepatoma cells. J Biochem Mol Biol. 2005;38:167–176. doi: 10.5483/bmbrep.2005.38.2.167. [DOI] [PubMed] [Google Scholar]
- 64.Bogaards JJ, van Ommen B, Falke HE, Willems MI, van Bladeren PJ. Glutathione S-transferase subunit induction patterns of Brussels sprouts, allyl isothiocyanate and goitrin in rat liver and small intestinal mucosa: a new approach for the identification of inducing xenobiotics. Food Chem Toxicol. 1990;28:81–88. doi: 10.1016/0278-6915(90)90014-e. [DOI] [PubMed] [Google Scholar]
- 65.McWalter GK, Higgins LG, McLellan LI, Henderson CJ, et al. Transcription factor Nrf2 is essential for induction of NAD(P)H:quinone oxidoreductase 1, glutathione S-transferases, and glutamate cysteine ligase by broccoli seeds and isothiocyanates. J Nutr. 2004;134:3499S–3506S. doi: 10.1093/jn/134.12.3499S. [DOI] [PubMed] [Google Scholar]
- 66.Kwak MK, Wakabayashi N, Itoh K, Motohashi H, et al. Modulation of gene expression by cancer chemopreventive dithiolethiones through the Keap1-Nrf2 pathway. Identification of novel gene clusters for cell survival. J Biol Chem. 2003;278:8135–8145. doi: 10.1074/jbc.M211898200. [DOI] [PubMed] [Google Scholar]
- 67.Matsuda H, Ochi M, Nagatomo A, Yoshikawa M. Effects of allyl isothiocyanate from horseradish on several experimental gastric lesions in rats. Eur J Pharmacol. 2007;561:172–181. doi: 10.1016/j.ejphar.2006.12.040. [DOI] [PubMed] [Google Scholar]
- 68.Uhl M, Laky B, Lhoste E, Kassie F, et al. Effects of mustard sprouts and allylisothiocyanate on benzo(a)pyrene-induced DNA damage in human-derived cells: a model study with the single cell gel electrophoresis/Hep G2 assay. Teratog Carcinog Mutagen. 2003;(Suppl 1):273–282. doi: 10.1002/tcm.10051. [DOI] [PubMed] [Google Scholar]
- 69.Murata M, Yamashita N, Inoue S, Kawanishi S. Mechanism of oxidative DNA damage induced by carcinogenic allyl isothiocyanate. Free Radic Biol Med. 2000;28:797–805. doi: 10.1016/s0891-5849(00)00168-4. [DOI] [PubMed] [Google Scholar]
- 70.Yonezawa Y, Nishikawa K, Nishioka H. DNA damage and intracellular generation of reactive oxygen species by allyl isothiocyanate in Escherichia coli. Journal of Environmental Medicine. 1999;1:27–31. [Google Scholar]
- 71.Schiffmann D, Eder E, Neudecker T, Henschler D. Induction of unscheduled DNA synthesis in HeLa cells by allylic compounds. Cancer Lett. 1983;20:263–269. doi: 10.1016/0304-3835(83)90023-x. [DOI] [PubMed] [Google Scholar]
- 72.Musk SR, Johnson IT. The clastogenic effects of isothiocyanates. Mutat Res. 1993;300:111–117. doi: 10.1016/0165-1218(93)90128-z. [DOI] [PubMed] [Google Scholar]
- 73.Musk SR, Smith TK, Johnson IT. On the cytotoxicity and genotoxicity of allyl and phenethyl isothiocyanates and their parent glucosinolates sinigrin and gluconasturtiin. Mutat Res. 1995;348:19–23. doi: 10.1016/0165-7992(95)90016-0. [DOI] [PubMed] [Google Scholar]
- 74.Bechtel D, Henderson L, Proudlock R. Lack of UDS activity in the livers of rats exposed to allylisothiocyanate. Teratog Carcinog Mutagen. 1998;18:209–217. doi: 10.1002/(sici)1520-6866(1998)18:5<209::aid-tcm1>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 75.Langer P, Stolc V. Goitrogenic Activity of Allylisothiocyanate--a Widespread Natural Mustard Oil. Endocrinology. 1965;76:151–155. doi: 10.1210/endo-76-1-151. [DOI] [PubMed] [Google Scholar]
- 76.Lewerenz HJ, Plass R, Bleyl DW, Macholz R. Short-term toxicity study of allyl isothiocyanate in rats. Nahrung. 1988;32:723–728. doi: 10.1002/food.19880320802. [DOI] [PubMed] [Google Scholar]
- 77.Group, N. T. P. P. W. National Toxicology Program Technical Report Series. Washington D.C.: 1982. [Google Scholar]
- 78.NAS/NRC . National Academy of Sciences and the National Research Council; Washington, DC: 1982. [Google Scholar]
- 79.Masutomi N, Toyoda K, Shibutani M, Niho N, et al. Toxic effects of benzyl and allyl isothiocyanates and benzyl-isoform specific metabolites in the urinary bladder after a single intravesical application to rats. Toxicol Pathol. 2001;29:617–622. doi: 10.1080/019262301753385942. [DOI] [PubMed] [Google Scholar]