Abstract
Long-term central venous catheters (CVC) facilitate care for patients with chronic illnesses, but catheter occlusions and catheter-related thrombosis (CRT) are common complications. This review summarizes management of CVC and CRT.
Mechanical CVC occlusions require cause-specific therapy; whereas, thrombotic occlusions usually resolve with thrombolytic therapy, such as alteplase. Prophylaxis with thrombolytic flushes may decrease CVC infections and CRT, but confirmatory studies and cost-effectiveness analysis are needed.
Risk factors for CRT include previous catheter infections, malposition of the catheter tip, and prothrombotic states. CRT can lead to catheter infection, pulmonary embolism, and post-thrombotic syndrome. CRT is diagnosed primarily using Doppler ultrasound or venography and treated with anticoagulation for 6 weeks to a year, depending on the extent of the thrombus, response to initial therapy, and whether thrombophilic factors persist. Prevention of CRT includes proper positioning of the CVC and prevention of infections; anticoagulation prophylaxis is not recommended at present.
Introduction
Long-term central venous catheters (CVCs) facilitate care for patients with chronic illness by providing easy venous access for laboratory tests, administration of medication, and parenteral nutrition. However, several complications resulting from the use of CVCs, including sepsis, extravasation of infusions, and venous thrombosis, can increase associated morbidity and mortality. These complications can also interrupt and delay treatment for the underlying disease and thereby affect outcome. The most common CVC complications are occlusion and catheter-related thrombosis (CRT), which are the subject of this review.
CVC occlusion occurs in 14% to 36% of patients within 1–2 years of catheter placement.1–7 A CVC occlusion can be partial, such that blood cannot be aspirated but infusion through the catheter is possible, or complete, such that neither aspiration nor infusion is possible. A CVC occlusion can arise from mechanical obstruction, precipitation of medications or parenteral nutrition, or thrombotic causes. CRT occurs in up to 50% of children and 66% of adults with a long-term CVC, and can cause long-term vascular complications.8–20 In a survey of Children’s Cancer Study Group centers in the United Kingdom, CVC occlusion and CRT were considered clinically important by 80% and 70% of the respondents, respectively. However, the study found substantial variation in the diagnosis, management, and prevention of CVC occlusions and associated thrombotic complications.21
Search strategy and selection criteria
For this narrative review of the diagnosis, management, and prevention of long-term CVC occlusions and CRT, we searched Medline and PubMed for articles published from 1965 to January 2009, with the keywords “central venous catheter”, “central venous access device”, “central venous line” associated with “occlusion”, “obstruction”, and “catheter-related thrombosis.” Long-term CVCs were defined as those that included subcutaneously tunneled catheters or implanted ports, and did not include those placed in the intensive care or peri-operative setting and intended for short-term use. Short-term CVCs, including hemodialysis catheters, are not included in this review, since the incidence, risk factors, and management of occlusion and CRT differ between long- and short-term CVCs. However, the reader is referred to the recently-published Kids with Catheter-Associated Thrombosis (KIDCAT) study for important information about thrombosis of short-term CVCs.22 In some instances, review articles were selected over original articles because of space constraints.
Central Venous Catheter Occlusion
Causes of central venous catheter occlusion
Accurate diagnosis of the etiology of catheter occlusion is essential to effectively treat the problem. Table 1 lists etiologies and recommendations for management of catheter occlusions. An obstruction can occur secondary to a variety of mechanical problems, including an uncommon, but potentially life-threatening, pinch off syndrome, illustrated in Figure 1 (Table 1).23–25 Medication or parenteral nutrition can also cause obstruction, which can be acute or gradual, with increasingly sluggish flow through the catheter. Inappropriate concentrations or incompatible mixtures can cause medications to precipitate within the catheter lumen. An occlusion can result from the precipitation of calcium phosphate crystals when calcium and phosphorus are co-administered at inappropriate concentrations. If the pH of an infusion is too alkaline or acidotic, precipitation can occur. Parenteral nutrition preparations can leave a lipid residue that can obstruct a CVC. 26–28
Table 1.
Etiology, Evaluation, and Treatment of Central Venous Catheter Occlusions
| Etiologya | Diagnosis | Treatment | Level of Evidence** |
|---|---|---|---|
| Mechanical | |||
| Kink in catheter or tubing, tight suture, or clamp closed on external catheter26,27 | Inspect catheter | Correct mechanical dysfunction | 5 |
| Huber needle dislodged or occluded in port26,27 | Evaluate Huber needle placement | Replace needle if necessary | 5 |
| Catheter tip blocked by vessel wall26,27 | Reposition patient | ––– | 5 |
| Pinch-off syndrome23–25 | Fluoroscopy | Remove catheter if at risk for fracture | 3b |
| Medication or Parenteral nutrition-related | |||
| Medication | Review medications and parenteral nutrition preparations | ––– | |
| Low pH (acidic)26–28, 31,32 | ––– | Hydrochloric acid 0.1%* | 4 |
| High pH (basic)26–28, 31,32 | ––– | Sodium hydroxide or sodium bicarbonate | 4 |
| Calcium phosphate precipitate26–28, 31,32 | ––– | Hydrochloric acid 0.1%* | 4 |
| Lipid emulsion27,34 | ––– | Ethanol 70% | 4 |
| Thrombotic | |||
| Fibrin sheath or intraluminal clot46 | Radiography after instillation of contrast into the catheter (linogram) | Intraluminal thrombolytic | 1b |
| Mural thrombus or venous thrombosis46,78 | Ultrasound or venography* | Anticoagulant therapy (rarely resolves with intraluminal thrombolytics) | 2c |
Information taken from Stephens LC, Haire WD, Kotulak GD. J Parent Enteral Nutr 1995; 19:75–79.
No longer used in some institutions because of concern about damage to the catheter wall
Levels of evidence as defined in Oxford Centre for Evidence Based Medicine (http://www.cebm.net/index.aspx?o=1025 accessed 9/14/08)
Figure 1.
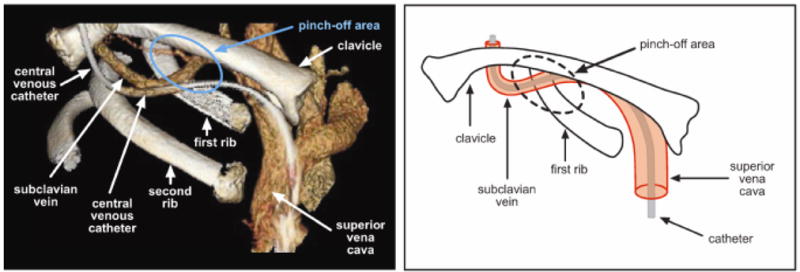
The pinch-off syndrome. The catheter passes through the narrow angle between the first rib and the lateral portion of the clavicle (the pinch-off area), placing it at risk for compression or transection. In the left panel, a 3-dimensional computed tomography image, the catheter passes through the pinch-off area parallel to the subclavian vein then inserts into the superior vena cava. In the right panel, the catheter inserts into the subclavian vein and is intravenous when it passes through the pinch-off area. Catheters that are external to the subclavian vein when they pass through the pinch-off area, as in the left panel, have a greater risk of pinch-off syndrome and fracture.
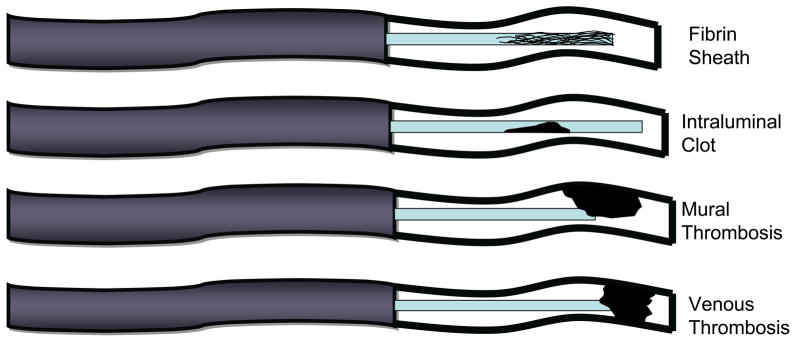
Catheters can also become occluded secondary to a thrombotic process, such as a fibrin sheath around the catheter tip, an intraluminal blood clot, or a venous thrombosis, which can occur separately or in combination (Figure 2). A fibrin sheath is one of the most common causes of thrombotic obstruction. It can occur within 24 hours after CVC placement and usually develops within 2 weeks.8,12,29 One autopsy study of patients with a long-term CVC found that a fibrin sheath encased the catheter tip in every case.29 The fibrin sheath does not usually affect catheter function, but may cause a partial obstruction by creating a one-way valve over the catheter tip. The negative pressure created when attempting to aspirate blood creates suction, which pulls the fibrin sheath over the CVC tip and prevents withdrawal of blood. The obstruction resolves when the negative pressure is relieved (e.g., during infusion or flushing the catheter), allowing for easy passage of fluids into the CVC.30 Although a fibrin sheath does not usually cause any clinical manifestations, there is a small risk of embolization of the fibrin material.17
Figure 2.
Types of thrombotic occlusion.
Intraluminal clots account for 5–25% of all catheter occlusions and may cause complete catheter obstruction. Catheter-related venous thrombosis refers to a thrombus that develops in proximity to a CVC. A mural thrombus is a blood clot that adheres to the vessel wall and can occlude the tip of the catheter, but does not completely occlude the vein in which the catheter is positioned (Figure 2). A deep vein thrombosis refers to a CRT that occludes the vein.17
Management
Mechanical obstruction of catheter
A standard procedure should be followed to diagnose and manage CVC obstruction (Figure 3). First, any obvious mechanical obstruction (e.g., a kink in the catheter tubing, a suture that is too tight, a clamp inadvertently left closed, a catheter tip blocked by the blood vessel wall, or a malpositioned subcutaneous port access [also termed “Huber”] needle) should be ruled out by carefully inspecting the CVC and repositioning the patient. Repositioning maneuvers include raising the ipsilateral arm, having the patient sit or stand, or rolling the patient onto one side. A dye study can also be used to diagnose an internal kink in the catheter.
Figure 3.
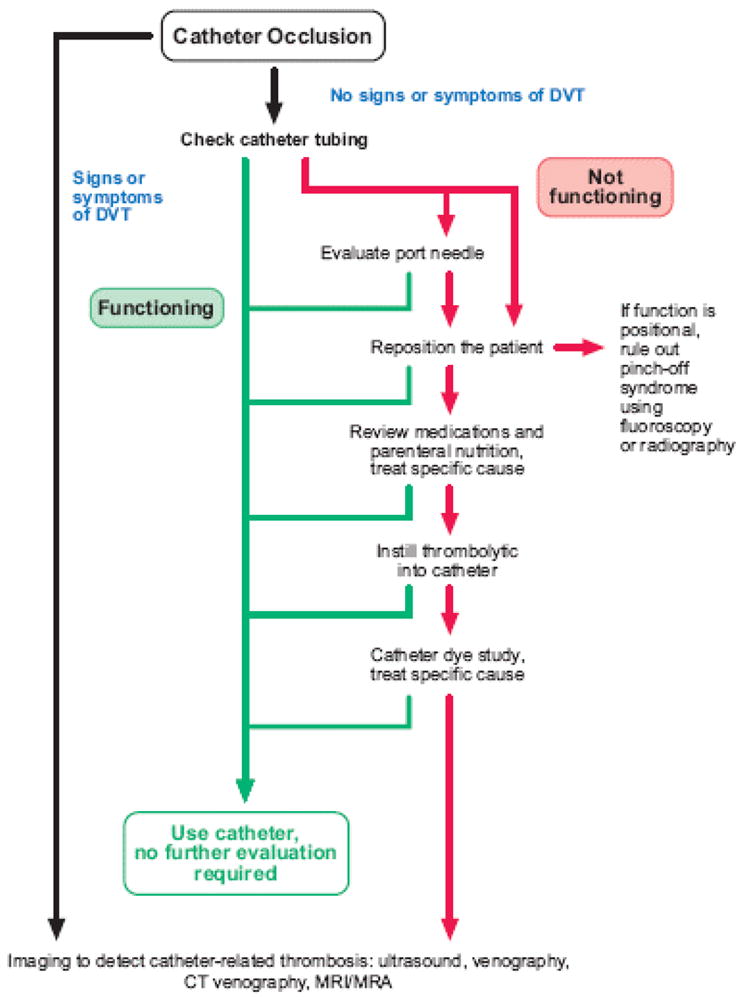
Algorithm for management of a central venous catheter obstruction. CVC: central venous catheter; DVT: deep-vein thrombosis; MRI: magnetic resonance imaging; MRA: magnetic resonance angiography.
Catheter obstruction related to medication or parenteral nutrition
If a mechanical obstruction is not found, obstruction by medication or parenteral nutrition should be excluded (Figure 3). The medications and parenteral nutrition preparations administered through the catheter should be carefully reviewed to identify any incompatibility that could have led to the obstruction. Appropriate treatment depends on the suspected cause of the occlusion. Obstructions thought to be caused by precipitation of low-pH medications or calcium phosphate crystals that become insoluble in basic solutions can be treated with 0.1% hydrochloric acid.26–28,31,32 Although our center still uses this method, the practice has been discontinued in some institutions because of concern about damaging the wall of the catheter. Obstructions caused by high-pH medications that precipitate in an acidic environment (e.g. phenytoin), are treated with sodium bicarbonate or sodium hydroxide.26–28, 33,34 A lipid residue from parenteral nutrition can be successfully cleared with a 70% ethanol solution. However, no large studies of this practice have been conducted and side effects include dizziness, fatigue, and light headedness.26–28,34,35
Thrombotic catheter obstruction
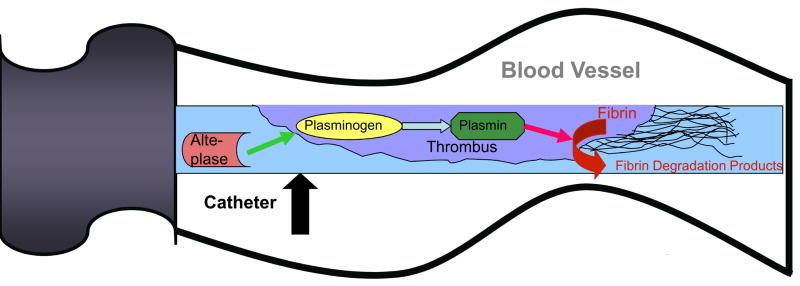
After ruling out mechanical dysfunction and medication- or parenteral nutrition-related etiologies, the next step is to exclude thrombotic obstruction (Table 1). A contrast study of the catheter (sometimes referred to as a “linogram”), can be used to detect an intraluminal clot or fibrin sheath. However, a common practice is to treat suspected thrombotic occlusions empirically with thrombolytics. The current standard treatment for CVC occlusions in the United States is instillation of alteplase with a concentration of 2 mg/2mL. A dose of 2 mL, or 110% of the volume of the catheter lumen if less than 2 mL (maximum dose 2 mg), is placed in the catheter lumen. Alteplase catalyzes the conversion of clot-bound plasminogen to plasmin and initiates fibrinolysis (Figure 4). Haire et al.36 showed that a 2 mg dose of alteplase was more effective than urokinase (5000 IU) for treating radiographically proven thrombotic occlusion of a CVC after a dwell time of 120 min.
Figure 4.
Mechanism of action of tissue plasminogen activator. Tissue plasminogen activator catalyzes the conversion of plasminogen to plasmin, which then cleaves fibrin into fibrin degradation products to dissolve the thrombus.
In the COOL trial (Cardiovascular thrombolytic used to Open Occluded Lines), one 2 mg dose of alteplase cleared the catheter occlusion after 120 min in 74% of patients, compared to only 17% of patients who received placebo. The overall catheter clearance rate for alteplase was 90% after up to 2 doses with no reports of major hemorrhage.37 Larger studies subsequently confirmed the safety and efficacy of alteplase administered at various time intervals in different long-term catheters, including peripherally inserted central catheters, with major hemorrhage reported in 0.3% of patients.38,39
After demonstrating the success of alteplase to treat CVC occlusions in adults, it was proven safe and effective in children as well, with catheter clearance rates of 85–95% and no major hemorrhage.40–43 A subsequent subset analysis of pediatric patients in the COOL trial44 and an open-label, single-arm multicenter trial45 with the same dosing regimen and dwell times as those of the COOL trial confirmed that alteplase was as effective in children as in adults, with catheter clearance rates of 83–87%, and no evidence of major hemorrhage.44,45
Current recommendations include administration of a thrombolytic agent into the catheter lumen with a dwell time of at least 30 minutes and a repeated dose if needed.46 If catheter patency is not restored, a low dose of alteplase can be infused over 6 to 8 hours. An ultrasound, venogram, or other diagnostic study is warranted if venous thrombosis is suspected (Table 1; Figure 3).
If thrombolytic therapy fails to clear the catheter, a guide wire can be inserted through the catheter lumen to dislodge a thrombus at the tip of the CVC. Fibrin sheath stripping has also been used for CVC occlusion that is resistant to medical management. The procedure uses femoral venous access to pass a vascular snare device to dislodge and remove the fibrin sheath.47 Although effective, these procedures are more invasive and are only used as a last resort.
New Thrombolytic Medications
Reteplase
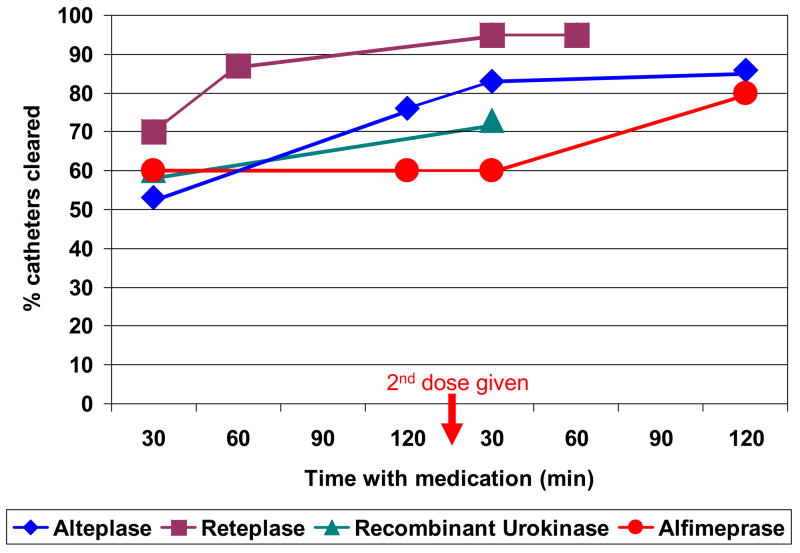
New thrombolytic medications with potentially higher efficacy and shorter dwell times than alteplase are being studied, although the trials published to date have been non-randomized and performed in relatively small patient cohorts (Table 2). One promising new thrombolytic is reteplase, a variant of alteplase whose structural differences increase its half-life and penetration of a thrombus. In adults, reteplase effectively restored patency to occluded CVCs, with catheter clearance rates of 67–74% after a dwell time of 30–40 min, as compared to an average 53% with a dwell time of 30 min for alteplase (Table 2; Figure 5).38,39,44,45,48,49 Reteplase was also effective after longer dwell times, with overall catheter clearance rates approaching 96% and no reports of major hemorrhage (Table 2; Figure 5).48–50 Although reteplase appears to be more effective in a shorter dwell time than alteplase, prospective randomized trials with larger numbers of patients are required to confirm superior efficacy and adequate safety of this medication.
Table 2.
Thrombolytics
| Thrombolytic | Definition | Mechanism of action | Maximum Dwell Time (each dose) | Average catheter clearance at 30 minutes with 1st dose | Overall Catheter clearance with 2 doses |
|---|---|---|---|---|---|
| Alteplase37–45, 55 | Tissue plasminogen activator (t-PA) made via recombinant DNA technology from vascular endothelial cells | Converts clot bound plasminogen to plasmin | 30–120 minutes | 52.1% ± 2.6% | 86.1% ± 1.7% |
| Reteplase48–50 | A variant of t-PA that lacks several structural domains. It does contain the kringle-2 and protease structures. | Converts clot bound plasminogen to plasmin | 30–60 minutes | 69.8% ± 5.8% | 95.2% ± 2.8% |
| Recombinant Urokinase51–53 | The DNA recombinant form of urokinase, a physiologic thrombolytic from renal parenchymal cells | Converts clot bound plasminogen to plasmin | 15–30 minutes | 59.7% ± 3.2% | 72.5% ± 2.7% |
| Alfimeprase54,55 | A truncated form of the metalloproteinase, Fibrolase. It is made via recombinant DNA technology. | Binds Aα chain of fibrin to directly degrade thrombus | 15–120 minutes | 60% ± 30% | 80% ± 24.8% |
Figure 5.
Cumulative incidence of catheter clearance after administering a thrombolytic agent. In the 16 studies represented in this figure, if the catheter did not clear within 120 min, a second dose of the agent was administered.37–45, 48–55
Recombinant Urokinase
Recombinant urokinase has been studied as a potential candidate for managing CVC occlusions in adults. Compared with the average clearance rate of 53% for alteplase, recombinant urokinase appears to have greater efficacy within the first 30 min, with an average clearance rate of 60% (Table 2; Figure 5).38,39,44,45,51–53 Whether recombinant urokinase would be more effective than alteplase at time points beyond 30 min remains to be studied. Recombinant urokinase is less effective than reteplase at all time points (Figure 5),48–53 and the incidence of major hemorrhage was 0.6% within 72 hours and 1.8% within 30 days following administration.53 In one study, a higher dose of recombinant urokinase increased the risk of bleeding but did not improve catheter clearance rates.51 Although only a trial that directly compares the 3 medications can unequivocally determine the most effective and safe treatment, existing data suggest that recombinant urokinase is more effective than alteplase at early time points but less so after 2 doses, and is less effective than reteplase at all time points in treating occluded catheters.
Alfimeprase
Alfimeprase is a new thrombolytic under investigation for its ability to clear catheter occlusions. Its site of action is different from that of other thrombolytics and independent of the plasminogen activation system. Alfimeprase is a recombinant form of the metalloproteinase fibrolase that binds to the Aα-chain of fibrin to directly degrade a thrombus (Table 2).54,55 These characteristics are likely responsible for the rapid onset of action, and rapid inactivation of alfimeprase by plasma α 2-macroglobulin may account for its lack of systemic side effects.
A phase II randomized, double-blind, multicenter, dose-ranging study compared the safety and efficacy of 3 doses of alfimeprase (0.3, 1.0, and 3.0 mg) with that of the standard 2 mg dose of alteplase in 55 adult patients.55 A 3 mg dose of alfimeprase was more effective than alteplase within the first 30 min and cleared 40% of catheters in 5 min and 60% at 30 min versus 0% and 23%, respectively, for alteplase. However, after the initial dose, no additional catheters were cleared after 30 min until a second dose was administered (Table 2; Figure 5).55 This implies that despite its rapid onset of action, a second dose of alfimeprase may be required if patency is not restored shortly after administration of the initial dose. There were no episodes of major hemorrhage in the 55 patients, but a larger study is needed to further document the incidence of major side effects.
Prophylaxis
To prevent thrombotic CVC occlusions, most institutions that use long term CVCs have standard protocols regarding the method and frequency of flushing the catheter. However, there is insufficient medical evidence on which to base universal guidelines for these practices, specifically with regard to the type of solution used (10 U/mL heparin vs. 100 U/mL heparin vs. normal saline) and frequency of flushing the catheter. For peripheral intravenous catheters, studies in adults have shown no difference between a saline or heparin lock, although these results have not been corroborated uniformly in children.56–58 For CVCs, some studies have suggested that there is no difference between a saline and heparin lock.59–61 One study in children showed no difference in catheter occlusion or clot formation when flushing the external catheter twice a day with a heparin solution (10 U/mL) versus once a week with a saline solution.60 Although there is general consensus that subcutaneous ports should be flushed monthly, one retrospective study in adults demonstrated that ports flushed less frequently than once a month did not have an increased frequency of catheter complications.62 Although the literature suggests that the current practice of frequent heparin locks for CVC may not be necessary, randomized studies are required to determine the ideal flush solution, its concentration, and its administration schedule for each type of long term CVC.
Investigators have also studied methods such as anticoagulation prophylaxis to prevent thrombotic catheter occlusions.1,63 Two recent studies including one large prospective randomized phase III multicenter trial, demonstrated a significantly lower incidence of catheter occlusions in children who received urokinase prophylaxis (19–23%) than that for the control group (31–68%).1,63 However, these studies required administration of urokinase every 1 to 2 weeks in all patients over a period of many months to achieve this modest decrease in the incidence of CVC occlusion. Since urokinase is costly and has potential side effects, administration of many doses solely to reduce catheter occlusions may not be justified, since catheter clearance rates are 80–90% with 1 to 2 doses of thrombolytic therapy.
However, prevention of CVC occlusion may not be the only benefit to thrombolytic prophylaxis. Interestingly, both studies1,63 found that prophylaxis decreased the rate of catheter-related infections, which concords with a meta analysis looking at routine urokinase use in patients with a CVC.64 In several small studies, monthly alteplase was found to decrease infections in long term CVCs placed in patients with hemophilia, supporting the concept that clot lysis may decrease the frequency of catheter related infections.65 However, whether monthly alteplase decreases thrombosis is not known. Studies also suggest that urokinase prophylaxis may decrease the rate of catheter-related thrombosis as well.63 Therefore, all potential risks and benefits of prophylaxis, including impact on complications associated with catheter occlusions (catheter infections, catheter-related thrombosis, and the need for catheter removal) and the costs and side effects of prophylactic thrombolytic treatment must be considered to define the optimal strategy.
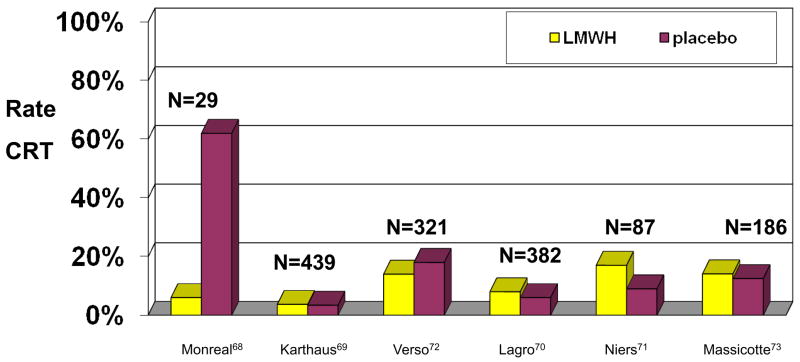
Prevention of a catheter related thrombosis is an issue that has also been addressed. Early studies indicated that prophylactic low doses of warfarin significantly reduced the rate of asymptomatic CRT in adults66,67, with one study reporting that 37.5% of patients in the control group had CRT compared with only 9.5% in the treatment group.66 However, subsequent trials showed no benefit of low-dose warfarin prophylaxis.68,69 Similar studies were performed to determine whether low molecular weight heparin prophylaxis reduced the incidence of CRTs. Although an early study documented lower rates of asymptomatic CRT in adults 70 these results were refuted by subsequent randomized, placebo-controlled trials that evaluated symptomatic and asymptomatic CRT in adults71–74 and children75 (Figure 6). Although the study in children had to be closed early because of slow patient accrual, at the time of closure there was no decrease in the incidence of asymptomatic CRT in those who had received low molecular weight heparin prophylaxis.75
Figure 6.
Incidence of catheter-related thrombosis in patients who received prophylaxis with low-molecular-weight heparin or placebo in 7 randomized trials.70–75 Only the study by Lagro et al included solely symptomatic patients.
Potential side effects of heparin therapy must be considered when contemplating thromboprophylaxis. Heparin-induced thrombocytopenia may complicate heparin therapy by causing a decrease in the platelet count associated with thrombosis. Although this occurs less frequently with low molecular weight heparins, it remains a potential risk. Osteoporosis and osteopenia have been documented after long-term heparin therapy, and patients on low molecular weight heparin with renal dysfunction are at increased risk for bleeding.76
A meta analysis of thromboprophylaxis demonstrated that although there was no harm, there was also no additional benefit, supporting the current recommendation that patients with a long-term CVC should not receive anticoagulation prophylaxis. 46,77,78 One of the limitations of this meta analysis was the heterogeneity with regard to the definition and the method of diagnosis for a CRT. Larger studies with more homogeneous patient cohorts and comparable outcomes are required to definitely prove whether or not there is a benefit to antithrombotic prophylaxis. Most importantly, such studies must be conducted in patients at very high risk for CVC occlusion and CRT, since the potential benefits could outweigh risks and costs in this group.
Catheter-related thrombosis
Patients with long-term CVCs are prone to CRT because of the direct effects of the catheter on the adjacent veins and blood flow, underlying disease and its treatment, nature of substances being infused, and location and time of placement of the catheter (Figure 2).79 A CRT occurs most often in the upper extremity where most long-term catheters are located.80 Symptoms include pain, tenderness to palpation, swelling, edema, warmth, erythema, and development of collateral vessels in the surrounding area.30 Symptomatic CRT occurs in up to 28% of adults and 12% of children with a CVC; however, most CRTs are asymptomatic, with incidences as high as 66% in adults and 50% in children.8–10,12,14,16–19 Reported incidences vary considerably depending upon criteria for studying patients and imaging used for diagnosis.
Some studies have shown that the risk of CRT may be related to the position of the catheter with increased risk associated with catheters located in the left subclavian vein or with malposition of the catheter tip.8,17, 81–83 Prior catheter infections are also a risk factor for the development of CRT.16,84 Some bacteria, particularly those responsible for most catheter infections, are highly thrombogenic, creating an environment favorable for the development of a thrombus. Indirect comparisons have implied that upper extremity DVT occur less frequently with subcutaneous ports than with external catheters; however this has not been confirmed. What has been shown is that increase in lumen size and number does increase the risk of CRT, likely secondary to the increased disruption of the vascular endothelium.19,83,85
Complications
CRTs can lead to further complications such as increased risk of subsequent catheter infections, pulmonary embolism, post-thrombotic syndrome, and persistent vascular compromise.9,17,20,30,79 Microbiological studies have shown that proteins within a clot, such as fibrinogen and fibronectin, attract staphylococcal species and increase their adherence to the catheter surface, thereby increasing the risk of catheter infection.85,86 A study of children with Hickman catheters found that 18% of patients who developed a clot also had a catheter-related bloodstream infection, whereas patients without clots had no infections.87
Pulmonary embolism is another significant complication of CRT. In adult patients with an upper-extremity deep-vein thrombosis (DVT), pulmonary embolism occurs symptomatically in 5–14% and asymptomatically in 15–36% of them.16,88–90 According to a Canadian Registry, pulmonary embolism developed in 16% of children with a CRT (13% non-fatal and 3% fatal).91,92
Post-thrombotic syndrome is a long-term complication of a DVT that presents with edema, skin hyperpigmentation, and pain, with more severe cases associated with skin ulceration.7 Post thrombotic syndrome occurs in 7–46% of patients following an upper extremity DVT with a weighted average of 15%, but occurs in 27–88% of patients with any type of DVT, with a higher percentage occurring after lower extremity DVT.13,94 This may explain the decreased frequency of post thrombotic syndrome in patients with CRT, since most CVCs are located in the upper extremities; whereas DVTs not associated with a CVC occur more frequently in lower extremities. Factors that increase the risk for post-thrombotic syndrome include elevated factor VIII and D-dimer levels at the time of thrombosis and persistent elevation thereafter, lack of clot resolution, more vessels affected by the original DVT, delay in initiating DVT treatment, recurrence of the DVT, and longer duration of follow up.7,11,13,15,93,94 Post-thrombotic syndrome can be difficult to treat and may not develop until many years after the original venous insult; thus, long-term follow-up after DVT is required.
Patients with CRT can also have persistent vascular occlusion years after the catheter had been removed, increasing their risk for post-thrombotic syndrome and recurrent thrombosis.20 Hence, CRT is a serious complication that can have significant consequences if not diagnosed and treated appropriately.
Diagnosis
If a CRT is suspected, venography or ultrasound can help evaluate the venous system. Although venography is the current gold standard, it is often not performed because it is invasive and involves exposure to intravenous contrast and radiation. Ultrasonography is a suitable alternative that is noninvasive, readily available, and accurate.95 In adults, ultrasonography has a sensitivity of 78–100% and a specificity of 86–100% in diagnosing symptomatic upper-extremity DVT.96–98 However, these excellent results have not been replicated in children. One study comparing these two modalities found that ultrasonography had a sensitivity of only 37% for diagnosis of an asymptomatic upper-extremity DVT, compared to 79% with venography.99 The study found that ultrasonography reliably evaluated the jugular veins, but not vessels within the thorax, such as the subclavian veins.99 These limitations are thought to be secondary to the shadows caused by the clavicle, sternum, and lungs surrounding the central vessels as well as the inability to compress areas of the subclavian veins secondary to the overlying clavicles.100 In the case of symptomatic upper extremity DVT in children, further studies are required to determine the sensitivity and specificity of ultrasonography.
Other methods of diagnosis, such as computed tomographic venography and magnetic resonance imaging and angiography are also being explored for use in the diagnosis of an upper-extremity DVT. Computed tomographic venography exposes the patient to radiation, but the addition of 3-dimensional reconstruction may aid the diagnosis of upper-extremity DVT. Magnetic resonance imaging and angiography does not require contrast or exposure to radiation, but the presence of motion artifact makes accurate diagnosis of an intrathoracic DVT difficult.98 Additional studies are required to fully evaluate the optimal diagnostic approach and the role of each radiographic method to diagnose an upper-extremity DVT.
We recommend performing an ultrasound first when a CRT is suspected in any patient. If the ultrasound is negative and an upper-extremity DVT is suspected based on convincing clinical evidence, venography should be performed for a complete evaluation.96–98 Additional studies on using these modalities in children for detecting upper-extremity DVT are warranted.
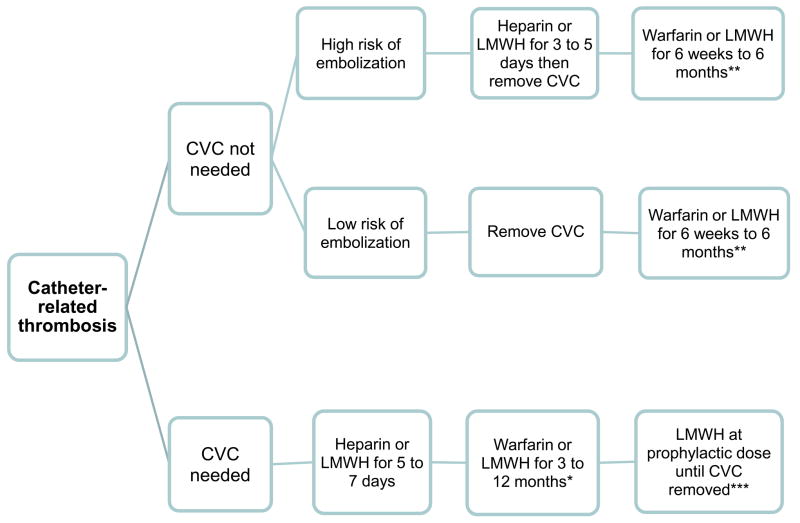
Management
Due to the lack of prospective studies, controversy continues regarding optimal management of a CRT. Recent guidelines suggest to divide patients with CRT into 2 categories based on whether or not they continue to need central venous access (Figure 7).46 For patients who have developed a CRT but no longer need a CVC or in whom it is no longer functioning, guidelines from the American College of Chest Physicians (ACCP) recommend to remove the catheter after 3–5 days of anticoagulation therapy. However, some believe that the CVC can be removed once a patient has been appropriately anticoagulated, as documented by an appropriate partial thromboplastin time (if unfractionated heparin is used) or anti-Xa level (if low molecular weight heparin is used). It should be noted that in adults an adequate anti-Xa level has not been associated with improved clinical outcomes with low molecular weight heparin, and that some clinicians do not advocate routine monitoring of the anti-Xa level in uncomplicated patients. In pediatrics, the response to low molecular weight heparin is less predictable and most clinicians advocate measurement of anti-Xa levels until therapeutic and then periodically to assure that they remain within a therapeutic range. The length of time a patient should be anticoagulated following removal of the CVC is controversial. Although some physicians advocate anticoagulation for 3 months after the CVC has been removed, others may shorten the course depending on the patient and the severity of the clot.46,78
Figure 7.
Recommended algorithm for the management of catheter related thrombosis. CVC: central venous catheter, LMWH: low molecular weight heparin.
*The duration of anticoagulation therapy required depends on a variety of clinical factors. In patients with no prothrombotic risk factors who’s CVC has been removed, most consider 3 months of anticoagulation sufficient. In patients with cancer, low molecular weight heparin is preferred over warfarin since the rate of recurrent thrombosis is twice as high with warfarin, and the duration of anticoagulant therapy should be at least 6 months, and possibly one year or longer.
** If the CVC is removed after development of catheter-related thrombosis, no prothrombotic risk factors remain, and the clot is small and does not completely obstruct the vein, 6 weeks of anticoagulation may be sufficient. A longer duration of anticoagulation is recommended for large, obstructing clots and when prothrombotic risk factors are present after CVC removal.
*** In a patient with catheter-related thrombosis whose CVC is left in place after 3 to 12 months of anticoagulation therapy, prophylactic doses of anticoagulant therapy are recommended, especially if other prothrombotic risk factors are present, as is the case for most patients with cancer.
For the majority of patients who continue to require central venous access, the catheter can be left in place and anticoagulation therapy initiated. There are some patients that develop a thrombosis that may threaten life or limb or in whom anticoagulation may be contraindicated, in which case the CVC would likely require removal regardless of the patient’s need for central venous access. For patient’s that retain their catheter, current recommendations include initial anticoagulation for several days, with unfractionated heparin or low molecular weight heparin, followed by at least 3 months of anticoagulation with a vitamin K antagonist or low-molecular-weight heparin.16,46,78 Low-molecular-weight heparin is preferred for cancer patients since it more effectively prevents recurrent thrombosis, and because warfarin interferes with some chemotherapy regimens and is more difficult to adjust when thrombocytopenia occurs. Thrombolytic treatment for an upper extremity DVT is not recommended for initial therapy of a CRT. Additionally, if the catheter remains in place once the course of full-dose anticoagulation is complete, the American College of Chest Physicians recommends continued anticoagulation therapy at a prophylactic dose until the catheter is removed.46,78
However, some pediatric patients require an indwelling catheter for a long period of time secondary to their treatment regimens and long-term anticoagulation prophylaxis may be difficult to continue. Therefore, clinicians sometimes individualize the duration of anticoagulation for documented CRT based on the size and location of the clot, perceived length of time the patient has had the thrombosis, persistence of risk factors such as continued use of thrombophilic medications such as glucocorticoids and L-asparaginase, and the time span for which the catheter is required. Clinicians who treat adults should refer to the recently updated ACCP guidelines and those who treat children can get useful advice from the guidelines and by calling 1-800-NO-CLOTS.78, 101
Conclusion
Long-term CVCs are important for the medical care of children and adults with chronic illness, but can lead to various complications such as CVC occlusions and CRT. The etiology of a catheter occlusion determines the appropriate treatment, but most occlusions are thrombotic and should be treated with thrombolytic therapy. Alteplase is most commonly used in North America but new agents have shown promising improvements in efficacy and onset of action. Further studies are required to compare new thrombolytics to those currently available.
Thrombotic CVC occlusions can cause CRT, which can lead to post-thrombotic syndrome, pulmonary embolism, and an increased risk for catheter infections. Although prevention of CRT is the key to decreasing the incidence of subsequent complications, effective prophylactic measures have not been established. Studies in adults have shown sufficient accuracy of ultrasound in diagnosing symptomatic upper extremity DVT, a result that has yet to be confirmed in children. Despite the consensus that CRT mandates immediate anticoagulation therapy, there is considerable variation in the treatment of patients who require central venous access for long periods of time. Future research should focus on optimal strategies for prevention, diagnosis, treatment of CVC occlusions and catheter related thrombosis, and the role of new thrombolytic agents in clinical practice.
Acknowledgments
This work was supported in part by grant CA21765 from the National Institutes of Health and by the American Lebanese Syrian Associated Charities (ALSAC)..
We thank Carolyn Chesney, MD, Marianne van de Wetering, MD, Menno Huisman, MD, and Janna Journeycake, MD for critical review of the manuscript and Betsy Williford for graphic design.
References
- 1.Dillon PW, Jones GR, Bagnall-Reeb HA, et al. Prophylactic urokinase in the management of long-term venous access devices in children: a Children’s Oncology Group study. J Clin Oncol. 2004;22:2718–2723. doi: 10.1200/JCO.2004.07.019. [DOI] [PubMed] [Google Scholar]
- 2.Fratino G, Molinari AC, Parodi S, et al. Central venous catheter-related complications in children with oncological/hematological diseases: an observational study of 418 devices. Ann Oncol. 2005;16:648–654. doi: 10.1093/annonc/mdi111. [DOI] [PubMed] [Google Scholar]
- 3.Lokich JJ, Bothe A, Jr, Benotti P, Moore C. Complications and management of implanted venous access catheters. J Clin Oncol. 1985;3:710–717. doi: 10.1200/JCO.1985.3.5.710. [DOI] [PubMed] [Google Scholar]
- 4.Rubin RN. Local installation of small doses of streptokinase for treatment of thrombotic occlusions of long-term access catheters. J Clin Oncol. 1983;1:572–573. doi: 10.1200/JCO.1983.1.9.572. [DOI] [PubMed] [Google Scholar]
- 5.Stephens LC, Haire WD, Kotulak GD. Are clinical signs accurate indicators of the cause of central venous catheter occlusion? JPEN J Parenter Enteral Nutr. 1995;19:75–79. doi: 10.1177/014860719501900175. [DOI] [PubMed] [Google Scholar]
- 6.Tschirhart JM, Rao MK. Mechanism and management of persistent withdrawal occlusion. Am Surg. 1988;54:326–328. [PubMed] [Google Scholar]
- 7.Kuhle S, Koloshuk B, Marzinotto V, et al. A cross-sectional study evaluating post-thrombotic syndrome in children. Thromb Res. 2003;111:227–233. doi: 10.1016/j.thromres.2003.09.008. [DOI] [PubMed] [Google Scholar]
- 8.Balestreri L, De CM, Matovic M, Coran F, Morassut S. Central venous catheter-related thrombosis in clinically asymptomatic oncologic patients: a phlebographic study. Eur J Radiol. 1995;20:108–111. doi: 10.1016/0720-048x(95)00633-2. [DOI] [PubMed] [Google Scholar]
- 9.Boersma RS, Jie KS, Verbon A, van Pampus EC, Schouten HC. Thrombotic and infectious complications of central venous catheters in patients with hematological malignancies. Ann Oncol. 2008;19:433–442. doi: 10.1093/annonc/mdm350. [DOI] [PubMed] [Google Scholar]
- 10.Glaser DW, Medeiros D, Rollins N, Buchanan GR. Catheter-related thrombosis in children with cancer. J Pediatr. 2001;138:255–259. doi: 10.1067/mpd.2001.111272. [DOI] [PubMed] [Google Scholar]
- 11.Goldenberg NA. Long-term outcomes of venous thrombosis in children. Curr Opin Hematol. 2005;12:370–376. doi: 10.1097/01.moh.0000160754.55131.14. [DOI] [PubMed] [Google Scholar]
- 12.Journeycake JM, Buchanan GR. Thrombotic complications of central venous catheters in children. Curr Opin Hematol. 2003;10:369–374. doi: 10.1097/00062752-200309000-00008. [DOI] [PubMed] [Google Scholar]
- 13.Kahn SR, Ginsberg JS. The post-thrombotic syndrome: current knowledge, controversies, and directions for future research. Blood Rev. 2002;16:155–165. doi: 10.1016/s0268-960x(02)00008-5. [DOI] [PubMed] [Google Scholar]
- 14.Luciani A, Clement O, Halimi P, et al. Catheter-related upper extremity deep venous thrombosis in cancer patients: a prospective study based on Doppler US. Radiology. 2001;220:655–660. doi: 10.1148/radiol.2203001181. [DOI] [PubMed] [Google Scholar]
- 15.Manco-Johnson MJ. Postthrombotic syndrome in children. Acta Haematol. 2006;115:207–213. doi: 10.1159/000090937. [DOI] [PubMed] [Google Scholar]
- 16.Rooden CJ, Tesselaar ME, Osanto S, Rosendaal FR, Huisman MV. Deep vein thrombosis associated with central venous catheters - a review. J Thromb Haemost. 2005;3:2409–2419. doi: 10.1111/j.1538-7836.2005.01398.x. [DOI] [PubMed] [Google Scholar]
- 17.Rosovsky RP, Kuter DJ. Catheter-related thrombosis in cancer patients: pathophysiology, diagnosis, and management. Hematol Oncol Clin North Am. 2005;19:183–202. vii. doi: 10.1016/j.hoc.2004.09.007. [DOI] [PubMed] [Google Scholar]
- 18.van Rooden CJ, Rosendaal FR, Barge RM, et al. Central venous catheter related thrombosis in haematology patients and prediction of risk by screening with Doppler-ultrasound. Br J Haematol. 2003;123:507–512. doi: 10.1046/j.1365-2141.2003.04638.x. [DOI] [PubMed] [Google Scholar]
- 19.Verso M, Agnelli G. Venous thromboembolism associated with long-term use of central venous catheters in cancer patients. J Clin Oncol. 2003;21:3665–3675. doi: 10.1200/JCO.2003.08.008. [DOI] [PubMed] [Google Scholar]
- 20.Wilimas JA, Hudson M, Rao B, et al. Late vascular occlusion of central lines in pediatric malignancies. Pediatrics. 1998;101:E7. doi: 10.1542/peds.101.2.e7. [DOI] [PubMed] [Google Scholar]
- 21.Skinner R, Koller K, McIntosh N, McCarthy A, Pizer B. Prevention and management of central venous catheter occlusion and thrombosis in children with cancer. Pediatr Blood Cancer. 2008;50:826–830. doi: 10.1002/pbc.21332. [DOI] [PubMed] [Google Scholar]
- 22.Hanslik A, Thom K, Haumer M, et al. Incidence and diagnosis of thrombosis in children with short-term central venous lines of the upper venous system. Pediatrics. 2008;122:1284–1291. doi: 10.1542/peds.2007-3852. [DOI] [PubMed] [Google Scholar]
- 23.Bagnall-Reeb HA, Ruccione K. Management of cutaneous reactions and mechanical complications of central venous access devices in pediatric patients with cancer: algorithms for decision making. Oncol Nurs Forum. 1990;17:677–681. [PubMed] [Google Scholar]
- 24.Fazeny-Dorner B, Wenzel C, Berzlanovich A, et al. Central venous catheter pinch-off and fracture: recognition, prevention and management. Bone Marrow Transplant. 2003;31:927–930. doi: 10.1038/sj.bmt.1704022. [DOI] [PubMed] [Google Scholar]
- 25.Andris DA, Krzywda E, Schulte W, Ausman R, Quebbeman EJ. Pinch-off syndrome: a rare etiology for central venous catheter occlusion. J Parenter Enter Nutr. 1994;18(6):531–3. doi: 10.1177/0148607194018006531. [DOI] [PubMed] [Google Scholar]
- 26.Hooke C. Recombinant tissue plasminogen activator for central venous access device occlusion. J Pediatr Oncol Nurs. 2000;17:174–178. doi: 10.1053/jpon.2000.8065. [DOI] [PubMed] [Google Scholar]
- 27.Kerner JA, Jr, Garcia-Careaga MG, Fisher AA, Poole RL. Treatment of catheter occlusion in pediatric patients. JPEN J Parenter Enteral Nutr. 2006;30:S73–S81. doi: 10.1177/01486071060300S1S73. [DOI] [PubMed] [Google Scholar]
- 28.Werlin SL, Lausten T, Jessen S, et al. Treatment of central venous catheter occlusions with ethanol and hydrochloric acid. JPEN J Parenter Enteral Nutr. 1995;19:416–418. doi: 10.1177/0148607195019005416. [DOI] [PubMed] [Google Scholar]
- 29.Hoshal VL, Jr, Ause RG, Hoskins PA. Fibrin sleeve formation on indwelling subclavian central venous catheters. Arch Surg. 1971;102:353–358. doi: 10.1001/archsurg.1971.01350040115023. [DOI] [PubMed] [Google Scholar]
- 30.McCloskey DJ. Catheter-related thrombosis in pediatrics. Pediatr Nurs. 2002;28:97–6. [PubMed] [Google Scholar]
- 31.Shulman RJ, Reed T, Pitre D, Laine L. Use of hydrochloric acid to clear obstructed central venous catheters. J Parenter Enteral Nutr. 1988 Sep-Oct;12(5):509–510. doi: 10.1177/0148607188012005509. [DOI] [PubMed] [Google Scholar]
- 32.Breaux CW, Jr, Duke D, Georgeson KE, et al. Calcium phosphate crystal occlusion of central venous catheters used for total parenteral nutrition in infants and children: prevention and treatment. J Pediatr Surg. 1987;22:829–32. doi: 10.1016/s0022-3468(87)80648-6. [DOI] [PubMed] [Google Scholar]
- 33.Akinwande KI, Keehn DM. Dissolution of phenytoin precipitate with sodium bicarbonate is an occluded central venous access device. Ann Pharmacother. 1995;29:707–9. doi: 10.1177/106002809502907-811. [DOI] [PubMed] [Google Scholar]
- 34.Holcombe BJ, Forloines-Lynn S, Garmhausen LW. Restoring patency of long-term central venous access devices. J Intraven Nurs. 1992;15(1):36–41. [PubMed] [Google Scholar]
- 35.Pennington CR, Pithie AD. Ethanol lock in the management of catheter occlusion. J Parenter Enteral Nutr. 1987;11:507–8. doi: 10.1177/0148607187011005507. [DOI] [PubMed] [Google Scholar]
- 36.Haire WD, Atkinson JB, Stephens LC, Kotulak GD. Urokinase versus recombinant tissue plasminogen activator in thrombosed central venous catheters: a double-blinded, randomized trial. Thromb Haemost. 1994;72:543–547. [PubMed] [Google Scholar]
- 37.Ponec D, Irwin D, Haire WD, et al. Recombinant tissue plasminogen activator (alteplase) for restoration of flow in occluded central venous access devices: a double-blind placebo-controlled trial--the Cardiovascular Thrombolytic to Open Occluded Lines (COOL) efficacy trial. J Vasc Interv Radiol. 2001;12:951–955. doi: 10.1016/s1051-0443(07)61575-9. [DOI] [PubMed] [Google Scholar]
- 38.Deitcher SR, Fesen MR, Kiproff PM, et al. Safety and efficacy of alteplase for restoring function in occluded central venous catheters: results of the cardiovascular thrombolytic to open occluded lines trial. J Clin Oncol. 2002;20:317–324. doi: 10.1200/JCO.2002.20.1.317. [DOI] [PubMed] [Google Scholar]
- 39.Ng R, Li X, Tu T, Semba CP. Alteplase for treatment of occluded peripherally inserted central catheters: safety and efficacy in 240 patients. J Vasc Interv Radiol. 2004;15:45–49. doi: 10.1097/01.rvi.000099538.29957.f7. [DOI] [PubMed] [Google Scholar]
- 40.Chesler L, Feusner JH. Use of tissue plasminogen activator (rt-PA) in young children with cancer and dysfunctional central venous catheters. J Pediatr Hematol Oncol. 2002;24:653–656. doi: 10.1097/00043426-200211000-00010. [DOI] [PubMed] [Google Scholar]
- 41.Choi M, Massicotte MP, Marzinotto V, et al. The use of alteplase to restore patency of central venous lines in pediatric patients: a cohort study. J Pediatr. 2001;139:152–156. doi: 10.1067/mpd.2001.115019. [DOI] [PubMed] [Google Scholar]
- 42.Fisher AA, Deffenbaugh C, Poole RL, Garcia M, Kerner JA., Jr The use of alteplase for restoring patency to occluded central venous access devices in infants and children. J Infus Nurs. 2004;27:171–174. doi: 10.1097/00129804-200405000-00006. [DOI] [PubMed] [Google Scholar]
- 43.Jacobs BR, Haygood M, Hingl J. Recombinant tissue plasminogen activator in the treatment of central venous catheter occlusion in children. J Pediatr. 2001;139:593–596. doi: 10.1067/mpd.2001.118195. [DOI] [PubMed] [Google Scholar]
- 44.Blaney M, Shen V, Kerner JA, et al. Alteplase for the treatment of central venous catheter occlusion in children: results of a prospective, open-label, single-arm study (The Cathflo Activase Pediatric Study) J Vasc Interv Radiol. 2006;17:1745–1751. doi: 10.1097/01.RVI.0000241542.71063.83. [DOI] [PubMed] [Google Scholar]
- 45.Shen V, Li X, Murdock M, et al. Recombinant tissue plasminogen activator (alteplase) for restoration of function to occluded central venous catheters in pediatric patients. J Pediatr Hematol Oncol. 2003;25:38–45. doi: 10.1097/00043426-200301000-00009. [DOI] [PubMed] [Google Scholar]
- 46.Monagle P, Chalmers E, Chan A, et al. Antithrombotic therapy in neonates and children: American College of Chest Physicians evidence-based clinical practice guidelines (8th edition) Chest. 2008;133:887–968. doi: 10.1378/chest.08-0762. [DOI] [PubMed] [Google Scholar]
- 47.Barnacle A, Arthurs OJ, Roebuck D, Hioms MP. Malfuncitoning central venous catheters in children: a diagnostic approach. Pediatr Radiol. 2008 Apr;38(4):363–78. doi: 10.1007/s00247-007-0610-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Liu CY, Jain V, Shields AF, Heilbrun LK. Efficacy and safety of reteplase for central venous catheter occlusion in patients with cancer. J Vasc Interv Radiol. 2004;15:39–44. doi: 10.1097/01.rvi.0000106385.63463.ec. [DOI] [PubMed] [Google Scholar]
- 49.Owens L. Reteplase for clearance of occluded venous catheters. Am J Health Syst Pharm. 2002;59:1638–1640. doi: 10.1093/ajhp/59.17.1638. [DOI] [PubMed] [Google Scholar]
- 50.Terrill KR, Lemons RS, Goldsby RE. Safety, dose, and timing of reteplase in treating occluded central venous catheters in children with cancer. J Pediatr Hematol Oncol. 2003;25:864–867. doi: 10.1097/00043426-200311000-00008. [DOI] [PubMed] [Google Scholar]
- 51.Deitcher SR, Fraschini G, Himmelfarb J, et al. Dose-ranging trial with a recombinant urokinase (urokinase alfa) for occluded central venous catheters in oncology patients. J Vasc Interv Radiol. 2004;15:575–580. doi: 10.1097/01.rvi.0000124950.24134.19. [DOI] [PubMed] [Google Scholar]
- 52.Haire WD, Deitcher SR, Mullane KM, et al. Recombinant urokinase for restoration of patency in occluded central venous access devices. A double-blind, placebo-controlled trial. Thromb Haemost. 2004;92:575–582. doi: 10.1160/TH03-11-0686. [DOI] [PubMed] [Google Scholar]
- 53.Svoboda P, Barton RP, Barbarash OL, et al. Recombinant urokinase is safe and effective in restoring patency to occluded central venous access devices: a multiple-center, international trial. Crit Care Med. 2004;32:1990–1996. doi: 10.1097/01.ccm.0000142706.01717.eb. [DOI] [PubMed] [Google Scholar]
- 54.Deitcher SR, Toombs CF. Non-clinical and clinical characterization of a novel acting thrombolytic: alfimeprase. Pathophysiol Haemost Thromb. 2005;34:215–220. doi: 10.1159/000092427. [DOI] [PubMed] [Google Scholar]
- 55.Moll S, Kenyon P, Bertoli L, et al. Phase II trial of alfimeprase, a novel-acting fibrin degradation agent, for occluded central venous access devices. J Clin Oncol. 2006;24:3056–3060. doi: 10.1200/JCO.2006.05.8438. [DOI] [PubMed] [Google Scholar]
- 56.Danek GD, Noris EM. Pediatric i.v. catheters: efficacy of saline flush. Pediatr Nurs. 1992;18:111–113. [PubMed] [Google Scholar]
- 57.Goode CJ, Titler M, Rakel B, et al. A meta-analysis of effects of heparin flush and saline flush: quality and cost implications. Nurs Res. 1991;40:324–330. [PubMed] [Google Scholar]
- 58.LeDuc K. Efficacy of normal saline solution versus heparin solution for maintaining patency of peripheral intravenous catheters in children. J Emerg Nurs. 1997;23:306–309. doi: 10.1016/s0099-1767(97)90216-6. [DOI] [PubMed] [Google Scholar]
- 59.Gillies H, Rogers HJ, Johnston J, Harper PG, Rudge CJ. Is repeated flushing of Hickman catheters necessary? Br Med J(Clin Res Ed) 1985;290:1708. doi: 10.1136/bmj.290.6483.1708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Smith S, Dawson S, Hennessey R, Andrew M. Maintenance of the patency of indwelling central venous catheters: is heparin necessary? Am J Pediatr Hematol Oncol. 1991;13:141–143. doi: 10.1097/00043426-199122000-00005. [DOI] [PubMed] [Google Scholar]
- 61.Stephens LC, Haire WD, Tarantolo S, et al. Normal saline versus heparin flush for maintaining central venous catheter patency during apheresis collection of peripheral blood stem cells (PBSC) Transfus Sci. 1997;18:187–193. doi: 10.1016/s0955-3886(97)00008-8. [DOI] [PubMed] [Google Scholar]
- 62.Kuo YS, Schwartz B, Santiago J, et al. How often should a port-A-cath be flushed? Cancer Invest. 2005;23:582–585. doi: 10.1080/07357900500276923. [DOI] [PubMed] [Google Scholar]
- 63.Kalmanti M, Germanakis J, Stiakaki E, et al. Prophylaxis with urokinase in pediatric oncology patients with central venous catheters. Pediatr Hematol Oncol. 2002;19:173–179. doi: 10.1080/088800102753541323. [DOI] [PubMed] [Google Scholar]
- 64.Kethireddy S, Safdar N. Urokinase lock or flush solution for prevention of bloodstream infections associated with central venous catheters for chemotherapy: a meta-analysis of prospective randomized trials. J Vasc Access. 2008 Jan-Mar;9(1):51–57. [PubMed] [Google Scholar]
- 65.Ragni MV, Journeycake JM, Brambilla DJ. Tissue plasminogen activator to prevent central venous access device infections: a systematic review of central venous access catheter thrombosis, infection, and thromboprophylaxis. Haemophilia. 2008;14(1):30–38. doi: 10.1111/j.1365-2516.2007.01599.x. [DOI] [PubMed] [Google Scholar]
- 66.Bern MM, Lokich JJ, Wallach SR, et al. Very low doses of warfarin can prevent thrombosis in central venous catheters. A randomized prospective trial. Ann Intern Med. 1990;112:423–428. doi: 10.7326/0003-4819-76-3-112-6-423. [DOI] [PubMed] [Google Scholar]
- 67.Boraks P, Seale J, Price J, et al. Prevention of central venous catheter associated thrombosis using minidose warfarin in patients with haematological malignancies. Br J Haematol. 1998;101:483–486. doi: 10.1046/j.1365-2141.1998.00732.x. [DOI] [PubMed] [Google Scholar]
- 68.Couban S, Goodyear M, Burnell M, et al. Randomized placebo-controlled study of low-dose warfarin for the prevention of central venous catheter-associated thrombosis in patients with cancer. J Clin Oncol. 2005;23:4063–4069. doi: 10.1200/JCO.2005.10.192. [DOI] [PubMed] [Google Scholar]
- 69.Ruud E, Holmstrom H, De Lange C, Hogstad EM, Wesenberg F. Low-dose warfarin for the prevention of central line-associated thromboses in children with malignancies--a randomized, controlled study. Acta Paediatr. 2006;95:1053–1059. doi: 10.1080/08035250600729092. [DOI] [PubMed] [Google Scholar]
- 70.Monreal M, Alastrue A, Rull M, et al. Upper extremity deep venous thrombosis in cancer patients with venous access devices--prophylaxis with a low molecular weight heparin (Fragmin) Thromb Haemost. 1996;75:251–253. [PubMed] [Google Scholar]
- 71.Karthaus M, Kretzschmar A, Kroning H, et al. Dalteparin for prevention of catheter-related complications in cancer patients with central venous catheters: final results of a double-blind, placebo-controlled phase III trial. Ann Oncol. 2006;17:289–296. doi: 10.1093/annonc/mdj059. [DOI] [PubMed] [Google Scholar]
- 72.Lagro SW, Verdonck LF, Borel RI, Dekker AW. No effect of nadroparin prophylaxis in the prevention of central venous catheter (CVC)-associated thrombosis in bone marrow transplant recipients. Bone Marrow Transplant. 2000;26:1103–1106. doi: 10.1038/sj.bmt.1702675. [DOI] [PubMed] [Google Scholar]
- 73.Niers TM, Di NM, Klerk CP, et al. Prevention of catheter-related venous thrombosis with nadroparin in patients receiving chemotherapy for hematologic malignancies: a randomized, placebo-controlled study. J Thromb Haemost. 2007;5:1878–1882. doi: 10.1111/j.1538-7836.2007.02660.x. [DOI] [PubMed] [Google Scholar]
- 74.Verso M, Agnelli G, Bertoglio S, et al. Enoxaparin for the prevention of venous thromboembolism associated with central vein catheter: a double-blind, placebo-controlled, randomized study in cancer patients. J Clin Oncol. 2005;23:4057–4062. doi: 10.1200/JCO.2005.06.084. [DOI] [PubMed] [Google Scholar]
- 75.Massicotte P, Julian JA, Gent M, et al. An open-label randomized controlled trial of low molecular weight heparin for the prevention of central venous line-related thrombotic complications in children: the PROTEKT trial. Thromb Res. 2003;109:101–108. doi: 10.1016/s0049-3848(03)00099-9. [DOI] [PubMed] [Google Scholar]
- 76.Lichtman MA, Beutler E, Kipps TJ, et al., editors. Williams Hematology. 7. pp. 286–287. copyright 2006. [Google Scholar]
- 77.Chaukiyal P, Nautiyal A, Radhakrishnan S, et al. Thromboprophylaxis in cancer patients with central venous catheters. A systematic review and meta-analysis. Thromb Haemost. 2008;99(1):38–43. doi: 10.1160/TH07-07-0446. [DOI] [PubMed] [Google Scholar]
- 78.Kearon C, Kahn S, Agnelli G, et al. Antithrombotic therapy for venous thromboembolic disease: American College of Chest Physicians evidence-based clinical practice guidelines (8th edition) Chest. 2008;133:454–545. doi: 10.1378/chest.08-0658. [DOI] [PubMed] [Google Scholar]
- 79.Raad II, Luna M, Khalil SA, et al. The relationship between the thrombotic and infectious complications of central venous catheters. JAMA. 1994;271:1014–1016. [PubMed] [Google Scholar]
- 80.Joffe HV, Kucher N, Tapson VF, Goldhaber SZ. Upper-extremity deep vein thrombosis: a prospective registry of 592 patients. Circulation. 2004;110:1605–1611. doi: 10.1161/01.CIR.0000142289.94369.D7. [DOI] [PubMed] [Google Scholar]
- 81.Male C, Chait P, Andrew M, et al. Central venous line-related thrombosis in children: association with central venous line location and insertion technique. Blood. 2003;101:4273–4278. doi: 10.1182/blood-2002-09-2731. [DOI] [PubMed] [Google Scholar]
- 82.Male C, Julian JA, Massicotte P, Gent M, Mitchell L. Significant association with location of central venous line placement and risk of venous thrombosis in children. Thromb Haemost. 2005;94:516–521. doi: 10.1160/TH03-02-0091. [DOI] [PubMed] [Google Scholar]
- 83.Tesselaar ME, Ouwerkerk J, Nooy MA, Rosendaal FR, Osanto S. Risk factors for catheter-related thrombosis in cancer patients. Eur J Cancer. 2004;40:2253–2259. doi: 10.1016/j.ejca.2004.06.023. [DOI] [PubMed] [Google Scholar]
- 84.van Rooden CJ, Schippers EF, Barge RM, et al. Infectious complications of central venous catheters increase the risk of catheter-related thrombosis in hematology patients: a prospective study. J Clin Oncol. 2005;23:2655–2660. doi: 10.1200/JCO.2005.05.002. [DOI] [PubMed] [Google Scholar]
- 85.Bona RD. Thrombotic complications of central venous catheters in cancer patients. Semin Thromb Hemost. 1999;25:147–155. doi: 10.1055/s-2007-994916. [DOI] [PubMed] [Google Scholar]
- 86.Mehall JR, Saltzman DA, Jackson RJ, Smith SD. Fibrin sheath enhances central venous catheter infection. Crit Care Med. 2002;30:908–912. doi: 10.1097/00003246-200204000-00033. [DOI] [PubMed] [Google Scholar]
- 87.Barzaghi A, Dell’Orto M, Rovelli A, et al. Central venous catheter clots: incidence, clinical significance and catheter care in patients with hematologic malignancies. Pediatr Hematol Oncol. 1995;12:243–250. doi: 10.3109/08880019509029565. [DOI] [PubMed] [Google Scholar]
- 88.Monreal M, Lafoz E, Ruiz J, Valls R, Alastrue A. Upper-extremity deep venous thrombosis and pulmonary embolism. A prospective study Chest. 1991;99:280–283. doi: 10.1378/chest.99.2.280. [DOI] [PubMed] [Google Scholar]
- 89.Monreal M, Raventos A, Lerma R, et al. Pulmonary embolism in patients with upper extremity DVT associated to venous central lines--a prospective study. Thromb Haemost. 1994;72:548–550. [PubMed] [Google Scholar]
- 90.Prandoni P, Polistena P, Bernardi E, et al. Upper-extremity deep vein thrombosis. Risk factors, diagnosis, and complications. Arch Intern Med. 1997;157:57–62. [PubMed] [Google Scholar]
- 91.Massicotte MP, Dix D, Monagle P, Adams M, Andrew M. Central venous catheter related thrombosis in children: analysis of the Canadian Registry of Venous Thromboembolic Complications. J Pediatr. 1998;133:770–776. doi: 10.1016/s0022-3476(98)70149-0. [DOI] [PubMed] [Google Scholar]
- 92.Monagle P, Adams M, Mahoney M, et al. Outcome of pediatric thromboembolic disease: a report from the Canadian Childhood Thrombophilia Registry. Pediatr Res. 2000;47:763–766. doi: 10.1203/00006450-200006000-00013. [DOI] [PubMed] [Google Scholar]
- 93.Elman EE, Kahn SR. The post-thrombotic syndrome after upper extremity deep vein thrombosis in adults: a systematic review. Thromb Res. 2006;117(6):609–14. doi: 10.1016/j.thromres.2005.05.029. [DOI] [PubMed] [Google Scholar]
- 94.Sharathkumar AA, Pipe SW. Post-thrombotic syndrome in children: a single center experience. J Pediatr Hematol Oncol. 2008;30:261–266. doi: 10.1097/MPH.0b013e318162bcf5. [DOI] [PubMed] [Google Scholar]
- 95.Manco-Johnson MJ. How I treat venous thrombosis in children. Blood. 2006;107:21–29. doi: 10.1182/blood-2004-11-4211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Sajid MS, Ahmed N, Desai M, Baker D, Hamilton G. Upper limb deep vein thrombosis: a literature review to streamline the protocol for management. Acta Haematol. 2007;118:10–18. doi: 10.1159/000101700. [DOI] [PubMed] [Google Scholar]
- 97.Mustafa BO, Rathbun SW, Whitsett TL, Raskob GE. Sensitivity and specificity of ultrasonography in the diagnosis of upper extremity deep vein thrombosis: a systematic review. Arch Intern Med. 2002;162:401–404. doi: 10.1001/archinte.162.4.401. [DOI] [PubMed] [Google Scholar]
- 98.Baarslag HJ, van Beek EJ, Koopman MM, Reekers JA. Prospective study of color duplex ultrasonography compared with contrast venography in patients suspected of having deep venous thrombosis of the upper extremities. Ann Intern Med. 2002;136:865–872. doi: 10.7326/0003-4819-136-12-200206180-00007. [DOI] [PubMed] [Google Scholar]
- 99.Male C, Chait P, Ginsberg JS, et al. Comparison of venography and ultrasound for the diagnosis of asymptomatic deep vein thrombosis in the upper body in children: results of the PARKAA study. Prophylactic Antithrombin Replacement in Kids with ALL treated with Asparaginase. Thromb Haemost. 2002;87:593–598. [PubMed] [Google Scholar]
- 100.Gupta H, Araki Y, Davidoff AM, et al. Evaluation of pediatric oncology patients with previous multiple central catheters for vascular access: is Doppler ultrasound needed? Pediatr Blood Cancer. 2007;48:527–531. doi: 10.1002/pbc.20875. [DOI] [PubMed] [Google Scholar]
- 101.Kuhle S, Massicotte P, Chan A, et al. Systemic thromboembolism in children. Data from the 1-800-NO-CLOTS Consultation Service. Thromb Haemost. 2004;92:722–728. doi: 10.1160/TH04-04-0207. [DOI] [PubMed] [Google Scholar]