Abstract
Community-associated methicillin-resistant Staphylococcus aureus (CA-MRSA) strains have emerged as serious health threats in the last 15 years. They are associated with large numbers of atopic dermatitis skin and soft tissue infections, but also originating from skin and mucous membranes, have the capacity to produce sepsis and highly fatal pulmonary infections characterized as necrotizing pneumonia, purpura fulminans, and post-viral TSS. This review is a discussion of the emergence of three major CA-MRSA organisms, designated CA-MRSA USA400, followed by USA300, and most recently USA200. CA-MRSA USA300 and USA400 isolates and their methicillin-sensitive counterparts (CA-MSSA) typically produce highly inflammatory cytolysins α-toxin, γ-toxin, δ-toxin (as representative of the phenol soluble modulin family of cytolysins), and Panton Valentine leukocidin. USA300 isolates produce the superantigens enterotoxin-like Q and a highly pyrogenic deletion variant of toxic shock syndrome toxin-1 (TSST-1), whereas USA400 isolates produce the superantigens staphylococcal enterotoxin (SE) B or SEC. USA200 CA-MRSA isolates produce small amounts of cytolysins but produce high levels of TSST-1. In contrast, their MSSA counterparts produce various cytolysins, apparently in part dependent on niche occupied in the host, and levels of TSST-1 expressed. Significant differences seen in production of secreted virulence factors by CA-MRSA versus hospital-associated MRSA and CA-MSSA strains appear to be due to the need to specialize as the result of energy drains from both virulence factor production and methicillin-resistance.
Keywords: Atopic Dermatitis, Staphylococcus aureus, Superantigens, Cytolysins, Methicillin-Resistance
Introduction
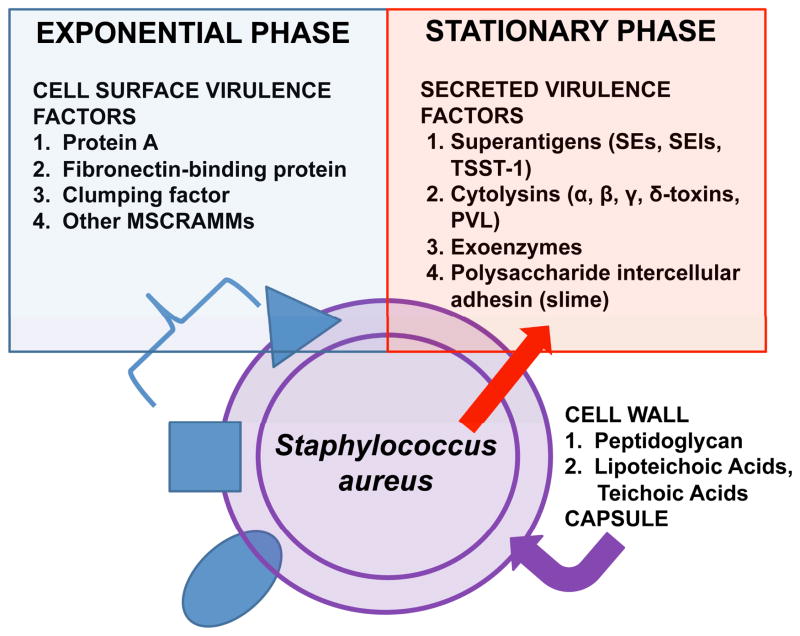
S. aureus is a gram-positive bacterium that produces a remarkable array of cell-surface and secreted virulence factors (Figure 1) to facilitate disease causation, and rapidly develops antimicrobial resistance almost as quickly as new therapeutic agents are developed1. The cell-surface virulence factors include microbial surface components recognizing adhesive matrix molecules (MSCRAMMs) as receptors in the human host, other surface proteins such as iron-regulated proteins, polysaccharide intercellular adhesin, and capsular polysaccharides2–4. The cell-surface MSCRAMMs typically are produced during exponential phase of growth. The function of these various factors is to provide nutrients (such as iron-uptake proteins) required for survival in the host, and microbial cell protection from the host immune system during lesion formation (such as protein A, fibronectin-binding proteins, and capsules). The secreted virulence factors (Figure 1), typically produced during post-exponential and stationary phase, include a large group of exoenzymes, such as proteases, glycerol ester hydrolase (lipase), and nucleases that make nutrients available to the microorganism, but also detoxify various innate immune mechanisms (for example, protease cleavage of host cytokines). Importantly, the secreted virulence factors also include a large group of true exotoxins: such as highly inflammatory cytolysins (mainly α, β, γ, and δ toxins and Panton-Valentine leukocidin [PVL]); superantigens (SAgs), including enterotoxins ([SEs]; SEA, SEB, SECn, SED, SEE, and SEI), SE-like proteins ([SEls]; SEl-G, SEl-H, and SEl-J to SEl-U), and toxic shock syndrome toxin-1 (TSST-1); and exfoliative toxins A and B5–7.
Figure 1. Virulence factor production by Staphylococcus aureus.
The organism produces cell surface virulence factors (microbial surface components recognizing adhesive matrix molecules, MSCRAMMs) during exponential phase, while producing exoproteins and exopolysaccharides during post-exponential/stationary phase.
The Centers for Disease Control and Prevention (CDC) and affiliated State Health Departments in 2007 reported from data obtained in 2005 that S. aureus is the most significant cause of serious infections in the United States8. This is certainly the result of the ubiquity of the organism (as many as 40% of humans may be colonized on mucosal surfaces, such as the anterior nares or vagina, or nearly all atopic dermatitis (AD) skin by various strains of the organism), production of a myriad of virulence factors, and ease of development of antibiotic resistance1. A great concern in clinical management of serious staphylococcal infections is the development of methicillin-resistance, predictive of global resistance to β-lactam antibiotics. For many years it has been known that more than 50% of hospital-associated S. aureus isolates are methicillin-resistant (referred to as HA-MRSA; methicillin-sensitive counterparts will be referred to as HA-MSSA). Recently, even a small number of vancomycin-resistant strains have been isolated in Michigan and Pennsylvania from patients in hospital settings9. In addition, as many as 30% of community-associated strains of S. aureus today are also methicillin-resistant (CA-MRSA). Methicillin-resistance is not a direct factor that increases virulence of S. aureus, but rather functions indirectly to influence production of secreted virulence factors, and clearly makes clinical management of staphylococcal infections more difficult.
This review is a discussion of secreted virulence factor production by MRSA strains compared to MSSA strains, with respect to emergence of CA-MRSA isolates, noting the influence of methicillin-resistance on selective production of certain secreted virulence factors. Key clinical concepts for AD, relative to CA-MRSA versus HA-MRSA and MSSA, are summarized in Table 1.
Table 1.
Clinical implications for atopic dermatitis of CA-MRSA versus HA-MRSA and CA- and HA-MSSA.
| One-third of S. aureus from AD patients are CA-MRSA; these are sometimes resistant to clindamycin. HA-MRSA are resistant to additional antibiotics. MSSA produce β-lactamases and are resistant to penicillin, ampicillin, and possibly some other β-lactam antibiotics. |
| Production of inflammatory cytolysins and high-level SAgs by CA-MRSA increases their abilities to cause extensive AD infections. SAgs (particularly TSST-1, SEB, and SEC) prevent healing of skin lesions in many patients. These patients respond to: 1) inclusion of antibiotics such as vancomycin, rifampin (due to great penetrating ability), and clindamycin (potential to shut of exotoxin production), 2) in difficult cases use of intravenous immunoglobulin (IVIG), and 3) antimicrobial scrubs should be considered. |
| Most AD patients respond to topical corticosteroids. However, there are patients with steroid-resistant AD; their S. aureus strains have been selected for great diversity in SAg types and likelihood of producing high-level SAgs. Patients with steroid-resistant AD respond to other T cell immunosuppressive agents, such as calcineurin inhibitors. |
| Skin sites with CA-MRSA, as well as consequent nasal carriage, can be associated with fatal pulmonary infections, including necrotizing pneumonia, purpura fulminans, and post-influenza TSS. These patients should be treated with: 1) antibiotics such as vancomycin, rifampin, and clindamycin; 2) fluid and electrolyte supportive care including use of vasopressors; and 3) possibly IVIG and activated protein C (drotrecogen alfa). |
Superantigens
SAgs were originally defined by their capacities to induce fever, amplify the lethal effects of other toxic molecules, including lipopolysaccharide10, 11, and in the case of SEs to cause the emesis seen in staphylococcal food poisoning12. Their abilities to stimulate T cell proliferation has been known for a long time13, 14, but in 1990, Marrack and Kappler described the novel mechanism by which SAgs stimulate T cells and macrophages to produce massive amounts of cytokines, leading to TSS15. It is now well-established that SAgs stimulate T cell proliferation by forming a cross-bridge between certain variable parts of the β-chains of T cell receptors (Vβ-TCRs), and invariant regions on either or both of the α- and β-chains of MHC II molecules on antigen-presenting cells, including macrophages7, 16, 17. Each SAg has a unique set of Vβ-TCRs with which it interacts. However, the consequence of the interaction of SAgs with T cells and macrophages is the same: massive cytokine production that includes IL-2, TNF-β, and Interferon-γ from CD4+ T cells, and IL-1β and TNF-α from macrophages. Collectively, these cytokines cause most of the clinical features of TSS. It has been shown that the ability of SEs to cause food poisoning is separable from SAg activity and appears to depend on amino acid structural elements within and adjacent to a cystine loop in the molecules18, 19. Additionally, SAgs stimulate epithelial and endothelial cells to produce IL-8 and MIP-3α (as well as other cytokines)20, 21. These two chemokines are attractants for PMNs and dendritic cells, and likely participate early in S. aureus infections through induction of inflammation, particularly on skin and mucosal surfaces. A model for SAg stimulation of human T cells is presented in Figure 2.
Figure 2. Model for the activation of CD4+ T cells and macrophages by the SAg SEB, compared to antigenic peptide activation of the same cells.
Ribbon diagrams of TCRs, MHC II molecules, and SEB adapted from previously published studies95–97.
Three SAgs are most associated with production of TSS: 1) menstrual TSS caused nearly exclusively by TSST-1; and 2) non-menstrual TSS caused by TSST-1, SEB, and SEC14, 22–25. These three SAgs typically are produced in the highest concentrations (of the SAgs) by S. aureus strains as tested in vitro, with amounts being produced in broth (planktonic) cultures often in the 5–50 μg/ml range. Other SAgs typically are produced in vitro in amounts 104 to 106 lower than these three SAgs. These differences are important since it has been shown that SAg amounts as low as 0.1μg/human are able to cause TSS symptoms26. Like cytolysins, SAgs are usually redundantly produced and made by the majority of S. aureus strains, with some strains producing as many as 15 SAg types27. Although it is not entirely clear why strains produce so many SAgs, it is known that certain host niches appear to favor disease causation by SAgs; for example menstrual TSS is nearly exclusively caused by TSST-114, 22. Other SAgs do not appear to be able to penetrate mucosal barriers as effectively as TSST-1, and this may prevent their association with menstrual illness18.
Cytolysins
The cytolysins, as originally described, are hemolytic for red blood cells, but variability occurs in susceptibility dependent on species source of the red cells. However, it is more likely that the major function of cytolysins is to kill immune cells that are drawn into local areas of infection. It is useful to think of these factors as the second line of defense against the host acting locally, the first line being systemic action of SAgs to interfere with the immune system, and the third line of defense being MSCRAMMs and other bacterial surface factors that function at the individual bacterial cell level. Additionally, the cytolysins are exceptionally pro-inflammatory at sub-cytolytic doses21, 28–30, with pro-inflammatory activities up to 106 more than cell surface factors, including cell wall-associated peptidoglycan and lipoteichoic acids, and up to 103 more inflammatory than SAgs.
Since the initial description of PVL’s association with CA-MRSA USA400, and later USA300 strains, investigators have undertaken studies to determine if PVL has a causal role in skin and severe pulmonary diseases, or is simply a marker for the strains. Through use of isogenic PVL+ and PVL− CA-MRSA strains, the DeLeo, Otto, and Schneewind laboratories collaboratively determined in mice, and later in rabbits, that PVL is not necessary for fatal pulmonary diseases31–33. Both PVL+ and PVL− strains killed the mice comparably. In contrast, their studies strongly implicated α-toxin, and later also PSMs, such as δ-toxin, in disease causation34–36. Vaccination against α-toxin effectively protected mice from fatal pulmonary diseases35. At the same time, a series of contradictory studies were published by another collaborative research group from France and the United States, suggesting that PVL is critical to severe pulmonary diseases in mice as caused by these same CA-MRSA strains37. Additionally, vaccination against PVL provided protection to the mice38. To date there has not been a complete resolution of these apparently contradictory findings. However, studies suggest that PVL has minimal toxicity to mouse PMNs, compared to high toxicity to human PMNs, bringing into question how PVL can be critical in causing mouse pulmonary diseases39, 40. Our unpublished studies performed in rabbits, with use of the same isogenic CA-MRSA USA400 strains as the DeLeo, Otto, and Schneewind collaborative group used, suggest that PVL is not required for lethal pulmonary diseases; both PVL+ and PVL− strains were comparably lethal when administered intra-bronchially.
It appears to the present investigators that the cytolysins are likely to contribute variably to serious human pulmonary infections when they are made by the causative organisms. There are likely to be two important pathways to fatal pulmonary diseases: 1) hemorrhagic and inflammatory pneumonias where cytolysins, particularly α-toxin, play major roles; and 2) highly fatal SAg-induced TSS-like pulmonary illnesses, with activity potentially amplified by cytolysins. Because the cytolysins also have exceptionally strong pro-inflammatory effects on epithelial and endothelial cells, they are likely to be required for or contribute to skin and soft tissue infections5, other systemic infections5, and menstrual TSS (through disruption of the vaginal epithelial barrier and facilitating TSST-1 penetration)20, 21, 41. Their abilities to be produced by S. aureus strains, the concentrations made by causative bacteria, and host susceptibility to their various activities likely determine the relative contributions of each. Importantly, multiple cytolysins typically are produced by individual S. aureus strains, making it difficult to assign a disease-causing role to any single toxin5.
Atopic Dermatitis and S. aureus
S. aureus is a common commensal organism that colonizes up to 40% of humans mainly on mucous membranes42. The organism often colonizes AD damaged skin and anterior nares, and from these sites may spread to infect any other body sites. Nearly all patients with AD may be colonized with S. aureus43, 44. This is likely the result of a combination of host factors including skin barrier dysfunction as well as impaired host immune responses in AD45, 46. As well, there are many regulatory mechanisms within S. aureus that lead to the organism’s ability to colonize or infect almost any niche within the host. These mechanisms include both global transcriptional control systems and very recently identified post-transcriptional mechanisms. The global transcriptional regulatory systems have been extensively reviewed recently47–49, and thus will not be highly discussed in this review; one example will be given later, as applied to clinical disease.
Of importance to this review is that we are now coming to recognize that S. aureus strains appear often to be selected for host niches based on staphylococcal non-transcriptional controls, including: 1) “read-through” mechanisms for protein translation through stop codons and 2) altered protein secretion based on virulence factor selection for bacterial survival in various niches. Examples of these mechanisms will be given in a subsequent section, but briefly, menstrual TSS S. aureus isolates nearly always have stop codons in the structural genes for the highly inflammatory α-toxin. However, the organisms produce small amounts of α-toxin by “reading through” the stop codons in the messenger RNAs. This allows α-toxin to facilitate TSST-1 penetration of the vaginal mucosa without completely destroying the mucosa21. In contrast, S. aureus strains require wild-type α-toxin production for skin survival.
It is also likely that multiple host factors will participate in regulation of niche selection of S. aureus strains, but to date these have not been well-studied. One example that has been clarified, however, is that TSS S. aureus isolates do not produce the SAgs and cytolysins in blood. This is the result of α- and β-globin chains of hemoglobin altering the activity of staphylococcal two-component systems that are normally required to up-regulate exotoxin production50. Counterintuitively, this suggests that TSST-1 and cytolysins are not produced vaginally in tampon regions containing menstrual blood, but rather are produced in tampon regions that lack menstrual blood. Additionally, the data also suggest that, although S. aureus is a major cause of bloodstream infections51, the organism may not produce SAgs and cytolysins in blood. It has been proposed that this host action to suppress exotoxin production induced S. aureus strains to adapt to host niches in which the organism is walled-off from blood through the actions of coagulase and clumping factors, even when present in the bloodstream, allowing production of its critical secreted virulence factors50. It has also been suggested that positively-charged stretches of amino acid residues in the α- and β-globin chains may be responsible for the exotoxin-suppressive activity through their interaction with bacterial two-component systems50. Chitosan is another highly positively charged molecule that has similar capacity to interfere with exotoxin production with S. aureus through its effects on two-component systems52. These studies collectively suggest that other positively charged peptides, such as skin and mucous membrane defensins and cathelicidins53, 54, may inhibit S. aureus exotoxin production at sub-bacterial growth inhibiting concentrations. However, this remains to be determined.
Another example of S. aureus selection by the host occurs in AD. AD is a T cell mediated skin disease that can significantly compromise quality of life as patients suffer from sleep disturbances, social embarrassment, and emotional distress. S. aureus infection contributes to the worsening of skin inflammation in AD55–57. These organisms have been shown to produce SAgs, including SEs A, B and C, and TSST-1. Additionally, Leung and Colleagues have shown that these SAgs can serve as allergens, stimulating specific IgE responses in AD patients58. However, until recently the full spectrum of SAgs produced by S. aureus infecting AD patients had not been thoroughly examined27. Furthermore, SAgs have been demonstrated to induce corticosteroid resistance of T cells in vitro59. This could contribute to difficulty in management of AD because topical corticosteroids are the most common medication used for treatment of AD.
The recent large study mentioned above was conducted to examine SAg production by S. aureus strains from patients with AD, both steroid-resistant and non-selected for steroid-resistance, compared to normal vaginal mucosal isolates27. Several highly important findings were reported: 1) isolates of S. aureus from patients with steroid-resistant AD produced significantly more SAg types per organism than did non-selected AD and vaginal isolates (these latter two groups generally were not significantly different from each other in production of SAg types); 2) SAg production by isolates from patients with steroid-resistant AD appeared dysregulated in many respects. For example, SEA typically is produced in only picogram amounts and is thought to be produced during exponential growth of S. aureus60. However, isolates from steroid-resistant AD patients produced microgram amounts of SEA (>10,000-fold increase), and the toxin was post-exponential phase regulated (typical of staphylococcal exotoxins that are produced in high amounts)47. Additionally, some isolates from steroid-resistant patients showed mutations in a global regulator of exotoxin production, designated staphylococcal respiratory response A-B, whose function appears to include acting transcriptionally to prevent exotoxin production when S. aureus is grown under anaerobic conditions61–63. These isolates could produce exotoxins, even under anaerobic conditions, for example as would be expected to occur vaginally in the absence of tampons. This predicts that in the future menstrual TSS could be caused by TSST-1 production by such S. aureus strains in the absence of tampon use; the major role of tampons in menstrual TSS appears to be providing oxygen necessary for TSST-1 production64; and 3) the major SAgs associated with non-menstrual TSS were produced by large numbers of isolates from patients with steroid-resistant AD, and the isolates produced these three SAgs in unusual combinations. For example, until this study, where 10% of S. aureus isolates were shown to produce both SEB and TSST-1, these two SAgs were not co-produced in tests of over 5000 isolates from TSS patients. Collectively, this study suggested that use of topical steroids to manage AD is selecting for S. aureus strains that are adapting to their “new” skin surface niche by increasing their virulence capabilities. It is also likely that use of additional T cell immunosuppressive agents, such as tacrolimus and cyclosporine A, to manage steroid-resistant AD will ultimately select for S. aureus strains with even greater ability to produce diverse types and amounts of SAgs to continue causation of AD. Additionally, it is likely that the skin of such patients will serve as a reservoir for S. aureus isolates that can further adapt to new host niches, carrying along their newly-acquired virulence capabilities.
We have recently tested a small group of AD isolates for methicillin resistance, and have found that 25% are MRSA. MRSA may have either SCC mecII or SCC mecIV DNA elements. As expected, all skin strains, whether MRSA or MSSA, from patients produced comparable amounts of α-toxin, a cytolysin that appears critical for enhanced survival on skin. We have only recently initiated studies to examine differences in SAg production by these strains. However, evidence is presented in the next section that shows that S. aureus strains, not only differ in production of secreted virulence factors based on host niche, but also dependent on the presence or absence of SCC mec DNA.
CA-MRSA
Although previously described by Robert Daum and his Colleagues65, the emergence of CA-MRSA primarily came to the attention of the medical and scientific communities as a result of the description by the Minnesota Department of Health, CDC, and Colleagues of four infant deaths in the Upper Midwest in 199966. These infants developed pulmonary infections characterized as necrotizing pneumonia with septicemia. The four isolated CA-MRSA were reported as USA400, as determined by pulsed-field gel electrophoresis (PFGE) analysis of isolate DNAs (PFGE designations as described by the CDC have been adopted for use by many investigators to categorize CA-MRSA strains and will be used throughout this article). Since the original description of the disease association with these strains, additional articles also noted the association of CA-MRSA USA400 strains with a variety of severe pulmonary diseases, including necrotizing pneumonia, purpura fulminans, and post-viral TSS67, 68. At the same time, it was noted that an even larger number of the same strains were associated with highly significant skin and soft tissue infections69. The data suggest that these strains predominantly cause skin and soft tissue infections, but can gain access to the lungs to cause necrotizing pneumonia, purpura fulminans, or TSS following upper respiratory viral infections or in patients who are predisposed to lung infections as a result of asthma or other respiratory conditions.
The S. aureus strains from the four infants were immediately analyzed for a variety of factors that possibly could explain the virulence of the strains, the first being identification of the DNA element encoding methicillin-resistance. Multiple DNA elements have been described that account for methicillin-resistance, the major gene (mecA) encoding an altered penicillin-binding protein referred to as PBP2a70. PBP2a is able to mediate peptidoglycan cross-bridging in staphylococcal cell division in the presence of β-lactam antibiotics. PBP2a is encoded on a DNA element referred to as staphylococcal chromosomal cassette (SCC) mec. There are multiple SCC mec DNAs, but the major HA-MRSA DNA element is designated SCC mecII71, and is encoded on a large (>50 kb) heterologous DNA insert into the staphylococcal chromosome. SCC mecII appears uncommonly to transfer from one S. aureus strain to another because of its large size. The isolates from the four infants infected with CA-MRSA USA400 did not contain SCC mecII, but rather had a much smaller, previously unrecognized SCC mec, now referred to as SCC mecIV71; this SCC mec DNA is more likely to be easily transferrable from one S. aureus strain to another because of its small size. For a time, the presence of SCC mecIV could be used to distinguish CA-MRSA from HA-MRSA (SCC mecII+), but as might be expected based on the versatility of S. aureus, we are now seeing some blending of SCC mec DNAs in MRSA, with both SCC mecII and mecIV (and other SCC mecs) being progressively distributed between both CA-MRSA and HA-MRSA.
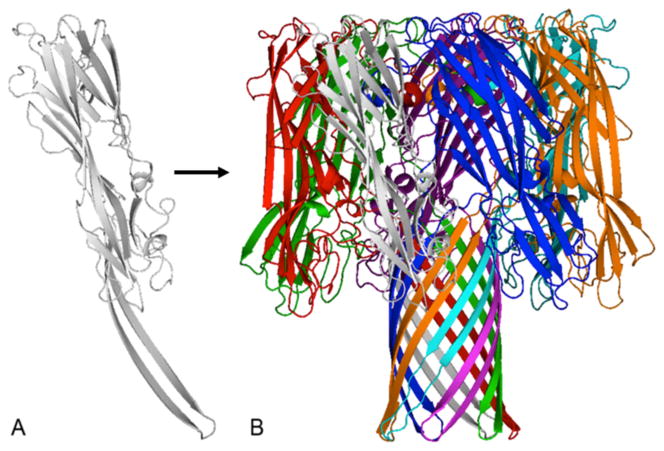
Additional analyses of CA-MRSA USA400 strains noted the production of a cytolysin that is uncommonly seen in human S. aureus strains67. This cytolysin, PVL, is a hetero-heptamer pore-forming toxin that belongs to the larger γ-toxin family72. PVL is composed of two peptide chains, designated F (Fast) and S (Slow) for elution from a chromatographic column, that polymerize into hetero-chain heptamers and insert into host plasma membranes to exert their cytolytic activity. PVL and the other γ-toxin hetero-heptamers were originally defined by their hemolytic activity, but PVL differs from other γ-toxins in that it has greater cytotoxic activity for polymorphonuclear leukocytes (PMNs) than other family members. PVL is produced by large numbers, but not all, of both CA-MRSA and CA-MSSA USA400 strains, compared to other S. aureus strains, giving rise to the hypothesis that the toxin has a causative role in serious pulmonary diseases 67. Other cytolysins are also produced by CA-MRSA (and CA-MSSA) USA400 strains and include α-toxin, γ-toxins, and δ-toxin (a member of the phenol soluble modulin [PSM] family)73; the staphylococcal β-toxin, a sphingomyelinase74, may also occasionally be produced, but the toxin gene is most often disrupted by bacteriophage integration, and is thus non-functional. α-toxin, like PVL and other members of the γ-toxin family, is a heptamer pore-forming toxin, except that α-toxin forms a homo-chain heptamer (Figure 3)75. δ-toxin and other PSMs are small peptides that form α-helical chains and appear to disrupt host cell membranes through formation of small pores, combined with membrane solubilization5, 73. The possible role of these cytolysins in S. aureus diseases will be discussed in more detail below.
Figure 3. Three dimensional cartoon diagram of the staphylococcal α-toxin homo-chain heptamer adapted from Gouaux et al.75.
γ-toxins and PVL structures are similar except the heptamers are composed of combinations of F and S peptides72. Gray is α-toxin monomer, multicolored is α-toxin heptamer.
In the initial description of the four CA-MRSA USA400 strains associated with infant pulmonary diseases, it was reported that two of the S. aureus strains produce the SAg SEB, and the remaining two isolates produce the SAg SEC66. SAgs, importantly TSST-1 and SEs B and C, cause staphylococcal TSS, characterized by high fever, hypotension (that may progress to shock and death), a scarlet fever-like rash, peeling of the skin if the patient recovers, and a variable multiorgan component, most often seen as vomiting and diarrhea, and confusion or combativeness14, 22, 25, 76–79. Soon after the initial description of TSS, it became clear that many TSS patients do not exhibit all of the defining clinical features, with rash and visible skin peeling often being missing. Thus, categories of probable TSS and toxin-mediated disease have been established to describe patients who fail to meet all of the defining criteria80, 81. Two major categories of TSS have been described based on body sites of infection by S. aureus, including menstrual TSS that is highly associated with vaginal S. aureus colonization and use of certain tampons77, 78, and non-menstrual TSS which may occur in association with any other type of staphylococcal infection or colonization81. SAgs cause TSS through combinations of their abilities to induce massive cytokine production from both CD4+ T cells and macrophages7, 15 and directly stimulate epithelial and endothelial cells (most likely also through cytokine production by these cells)20, 21, 82, 83. The SAgs SEB and SEC were later shown to be highly associated with both CA-MRSA USA400 and their CA-MSSA USA400 counterparts, whether from skin and soft tissue infections, or from serious pulmonary diseases25, 66, 69, 71.
In 2003, the CDC and Colleagues reported the emergence of a new clone of CA-MRSA, referred to as CA-MRSA USA30084, 85. These isolates were also highly associated with skin and soft tissue infections, and serious pulmonary diseases similar to USA 400 isolates. Numerous studies have shown that CA-MRSA USA300 strains, not only emerged, but began to replace CA-MRSA USA400 strains, such that the majority of skin and soft tissue infections (and consequently serious pulmonary infections) became associated with CA-MRSA USA300. Our laboratory tests S. aureus strains submitted by physicians, primarily from Minnesota, who suspect TSS in their patients, for production of PVL and SAgs. We also noted the relative reduction in non-menstrual TSS isolates submitted that were USA400 (both CA-MRSA and CA-MSSA), beginning in 2003, and replaced by CA-MRSA and CA-MSSA USA300 strains. However, beginning in June, 2008, we noted a resurgence of USA400 strains and a significant relative reduction of submitted USA300 strains. It is probable that variations in dominant S. aureus strains continue to occur as has happened many times in the past, with strains emerging and disappearing in roughly 10-year cycles86–88.
The USA300 strains have been thoroughly examined for production of factors that may account for their emergence. CA-MRSA USA300 strains most often contain the small SCC mecIV DNA element that explains their methicillin-resistance84, 85. The organisms, whether CA-MRSA or CA-MSSA, often produce PVL, and they make other cytolysins such as α-toxin, γ-toxins, and δ-toxin (and other PSMs); they occasionally produce β-toxin. Unlike USA400 strains, CA-MRSA and CA-MSSA USA300, do not produce the SAgs SEB and SEC, but instead produce SEl-Q (and other SAgs including occasionally SEA and often SEl-K) and apparently an N-terminal one-half deletion variant of TSST-184, 85, 89, 90. Although its activity is incompletely characterized, the deletion variant of TSST-1 has recently been associated with a newly described illness, extreme pyrexia syndrome caused by S. aureus, in which patients rapidly develop fevers in excess of 108 °F and quickly succumb; all described patients thus far have died89.
Finally, although not well-described in the literature CA-MRSA USA200 strains are emerging. These strains also cause skin and soft tissue infections, are associated with serious pulmonary diseases, but their CA-MSSA counterparts also are well-known to cause menstrual TSS. Based on our tests of the organisms, regional differences exist in SCC mec DNAs associated with the strains. In Minnesota, nearly all of the isolates have SCC mecII, reminiscent of HA-MRSA, but in isolates tested from New York, both SCC mecII and IV may be present. The isolates rarely produce PVL, the strains variably produce α-toxin, β-toxin, and γ-toxin (discussed in below), but the strains all produce δ-toxin, and presumably other PSMs. These isolates also produce the SAg TSST-1, as these isolates are the predominant causes of both menstrual and one-half of non-menstrual TSS.
Comparison of Exotoxin Production by USA200 CA-MRSA Versus CA-MSSA
In 1982 Schlievert et al. showed that menstrual, vaginal mucosal TSS S. aureus strains produce less α-toxin and more TSST-1 than skin S. aureus isolates91. It was hypothesized that high levels of cytolysins are required for producing inflammation to disrupt the intact skin barrier and facilitate S. aureus infection. Conversely, production of the same amounts of cytolysins on mucosal surfaces would lead to such strong host responses that the host would either quickly succumb to overwhelming infection or the microbe would quickly be eliminated by the host immune system. These studies have been confirmed by others who reported that most menstrual TSS S. aureus isolates have a defective α-toxin structural gene, containing a stop codon within the gene92.
The above studies suggest that S. aureus strains may be selected to occupy niches in the host based on secreted virulence factor production91, but the data highlighted an unresolved issue. Our prior studies suggested that menstrual, vaginal TSS isolates made reduced amounts of α-toxin, but not zero α-toxin91. In contrast, the presence of the stop codon within the α-toxin gene indicated there should be no α-toxin produced92. With use of hyperimmune antibodies to α-toxin, we have recently studied production of α-toxin (Table 2) in vitro by representative menstrual TSS CA-MSSA USA200 isolates (example TSST-1+ CDC587, containing a stop codon within the α-toxin gene), non-menstrual TSS CA-MSSA USA 200 (example TSST-1+ MNPE with wild-type α-toxin gene), and typical menstrual TSS CA-MRSA USA200 (example TSST-1+ isolate MNWH with a stop codon in the α-toxin gene). None of these isolates produce PVL and β-toxin. (It is important to note that the USA 200 strains used in the following discussion are typical of large numbers of organism analyzed.) Strain MNPE, with a wild-type α-toxin gene, as expected produced 55 μg/ml of α-toxin by red cell lysis bioassay. Surprisingly, CDC587 also produced α-toxin in spite of the stop codon in the α-toxin gene, but at approximately 10-fold lower concentration (5 μg/ml). Additionally, the CA-MRSA USA200 isolate MNWH produced 3.5 μg/ml α-toxin despite having the α-toxin gene stop codon. Thus, menstrual TSS S. aureus strains have developed “read through” mechanisms to allow some translation of α-toxin protein, even in the presence of a stop codon. Although not previously described for S. aureus, at least two mechanisms have been described for this phenomenon in gram-negative bacteria: 1) altered transfer RNAs to allow an amino acid to be inserted instead of recognition of the stop signal, and 2) altered ribosome structure. Both of these mechanisms can allow “read through” at approximately 1/10 wild-type level.
Table 2.
Production of virulence factors by TSST-1+ CA-MRSA and CA-MSSA USA200 S. aureus
| Strains | α-toxin (μg/ml) | TSST-1 (μg/ml) | Pigment (Yes/No) |
|---|---|---|---|
| Menstrual CA-MSSA TSS (CDC587) | 5.0 | 3 | Yes |
| Post-viral Respiratory TSS (MNPE) | 55 | 3 | Yes |
| Menstrual CA-MRSA TSS (MNWH) | 3.5 | 15 | No |
Total exoprotein production by the same strains was also examined, including the production of TSST-1 (Figure 4). CDC587, with the stop codon in the α-toxin gene, produced many exoproteins, but in low amounts, and produced approximately 3 μg/ml TSST-1. Strain MNPE, with wild-type α-toxin, produced fewer exoproteins than CDC587, but also produced approximately 3 μg/ml TSST-1. Finally, strain MNWH produced almost no exoproteins except TSST-1, with TSST-1 being produced in higher concentrations than in the CA-MSSA strains. These data suggest that total protein production burden as a function of host niche can post-transcriptionally control exotoxin production. Thus, MNPE is presumed to be a skin isolate of S. aureus due to its wild-type α-toxin gene; the strain unfortunately was isolated from a death case of post-influenza pulmonary TSS93 with the assumption, that like USA300 and USA400 CA-MRSA, the organisms are skin isolates that occasionally are transferred into the lungs to cause highly fatal TSS-like illnesses66, 69, 84. The production of high levels of α-toxin appears required for skin survival of S. aureus, and in producing high levels of α-toxin the organism, MNPE, reduces production of many other, less essential secreted virulence factors than seen from CDC587. When the additional burden of the large SCC mecII DNA element is added into the USA200 organism, as in MNWH, even fewer secreted virulence factors are produced, but TSST-1 amounts are increased. It appears high-level production of TSST-1 is required, more so than other secreted factors including cytolysins, for survival of the organism.
Figure 4. Secreted protein profiles of CA-MSSA USA200 strains CDC587 and MNPE and CA-MRSA USA200 strain MNWH.
All organisms were cultured to stationary phase, cells removed by centrifugation, sterile supernates concentrated 10-fold, SDS-PAGE performed, and gel stained with Coomassie brilliant blue R-250.
S. aureus strains also produce carotinoid pigments which in certain host niches appear to function as virulence factors94. Figure 5 shows carotinoid pigment production by strains CDC587, MNPE, and MNWH, as representative of the above groups of isolates. Both CDC587 and MNPE produce the typical gold pigment expected from S. aureus strains. In contrast, the CA-MRSA USA200 strain does not produce detectable pigment. As noted above, the presence of the large SCC mecII DNA may have required selection in the host of an organism that cannot produce pigment (as well as not producing other secreted virulence factors).
Figure 5. Carotinoid pigment production by CA-MSSA USA200 strains CDC587 and MNPE, and CA-MRSA USA200 strain MNWH.
Organisms were cultured to stationary phase, and cells were pelleted by centrifugation.
Our analysis of the CA-MRSA and CA-MSSA USA200 strains above suggest that production of secreted virulence factors among these highly related strains can vary considerable based on host niche and virulence factor gene load, and determine collective production of secreted virulence factors. One additional observation should be noted with respect to the typical menstrual USA200 CA-MSSA TSST-1+ organisms with the stop codon in the α-toxin gene. The organisms resemble in many ways normal flora coagulase-negative staphylococci except for low-level production of cytolysins and production of TSST-1. Our recent studies suggest that production of α-toxin in low amounts by these isolates facilitates penetration of TSST-1 across the mucosal barrier21. We hypothesize that the low production of α-toxin by these strains in the presence of a stop codon in the structural gene provides a novel, non-transcriptional regulatory mechanism for the organism. It is possible that these organisms have developed mechanisms to “read through” the stop codon. If strain USA200 CA-MSSA MNPE were to be found vaginally, the strain would either cause so much inflammation that the organism would be quickly eliminated by the host immune system, or the organism would quickly kill the host through increased TSST-1 penetration or sepsis.
Comparison of Exotoxin Production by USA400 CA-MRSA Versus CA-MSSA
As S. aureus strains colonize human mucous membranes and skin, as observed in AD patients, the organism is present primarily as thin biofilms of organisms growing on surfaces, rather than as planktonic (broth) cultures. As noted earlier, even in the bloodstream, it appears S. aureus quickly becomes walled off from the blood into biofilms through the actions of coagulase and clumping factors47. The data are consistent with the organism having difficulty producing secreted virulence factors when present in blood50; thus it may be selectively advantageous for the organisms to be sequestered from the blood to facilitate production of cytolysins and SAgs, both of which may be critical to protection from the host immune system.
We have recently performed studies to assess production of TSST-1 and SEC in vitro by CA-MRSA and CA-MSSA USA200 and USA400 strains, respectively in biofilm versus planktonic cultures, in the absence of blood (unpublished data). The studies confirmed the above studies that show that CA-MRSA USA200 strains produce more TSST-1 in planktonic cultures than their CA-MSSA counterparts, but the studies also showed that this difference is magnified in biofilm cultures. CA-MRSA USA200 strains produced approximately 4000 μg/ml of TSST-1 in tampon biofilms in vitro, compared to 1000 μg/ml produced by CA-MSSA USA200 isolates under the same conditions (unpublished). The data also emphasized that biofilm cultures may have 200 times more TSST-1 produced compared to planktonic cultures, presumably as a result of quorum-sensing and other global transcription regulation47. We have also compared CA-MRSA USA400 strains to CA-MSSA USA400 strains for production of SEC in biofilm versus planktonic cultures. Similar results as with USA200 strains were obtained, in that CA-MRSA USA400 strains made more SEC than CA-MSSA USA400 strains in planktonic cultures, that both organisms made up to 200 times more SEC in biofilm cultures than planktonic cultures, and that CA-MRSA USA400 strains made more SEC in biofilms than CA-MSSA USA400 strains.
Conclusions, Future Research Directions, and Clinical Implications
One purpose of this review was to emphasize that S. aureus strains have become selected for their various niches within humans as a function of secreted virulence factors produced. When they become stressed by the host, as in attempting to survive on the skin versus on mucous membranes, or when they must grow in the presence of antibiotic therapy, as in acquisition of SCC mec DNA, the organisms specialize in production of secreted virulence factors, progressively emphasizing production of “most necessary” factors, including cytolysins and SAgs. Thus in the example given, the mucosal, menstrual vaginal TSST-1+ CA-MSSA USA200 isolate is the least specialized of organisms this review has discussed. It appears the organism maintains its ability to colonize mucosal surfaces and resemble normal flora coagulase-negative staphylococci by production of only low levels of secreted virulence factors. In contrast, when the same organism produces wild-type α-toxin, needed for skin survival, and combined with TSST-1 production, the organism loses ability to produce large numbers of secreted factors. The same is the case when the organism acquires the SCC mec DNA. The organism up-regulates production of TSST-1, but produces almost no other secreted factors, as if gene expression from the SCC mec element places an excessive burden on the microbe.
There appear to be multiple mechanisms that regulate production of secreted virulence factors as a function of host niche. First, the niche itself regulates virulence factor production through influences of nutrient availability and the presence of the innate and adaptive immune systems. S. aureus also has at least two major types of regulatory systems that influence secreted virulence factor production: 1) global transcriptional regulators, and 2) previously unrecognized post-transcriptional regulators, examples of which were discussed in this review. Through combinations of these mechanisms, S. aureus has become the master commensal and ultimate pathogen, causing more total and more types of infections than any other organism. At the same time, the abundance of these mechanisms makes it difficult to sort out their relative contributions to virulence factor expression, and may have contributed to our lack of recognition of the importance of S. aureus in numerous human diseases. For example, our present studies are the first to explore the major influence of SCC mecII DNA on virulence factor secretion. It is our opinion that studies of the influence of post-transcriptional mechanisms provide cutting edge research for the next five or more years.
It is well-recognized that S. aureus strains colonize the anterior nares of humans. Indeed, it is commonly thought that this body site most often is the source of staphylococcal diseases occurring at other body site. This thought must now change. With the emergence of CA-MRSA USA300 and 400 isolates, we now recognize that the skin is often the source of potentially highly virulent S. aureus strains, such as those causing fatal pulmonary infections. AD patients provide an exceptionally rich source of S. aureus strains, and as shown in this review, required management of such patients can select for a variety of even more pathogenic strains. This makes it clear that we as a research community must develop as great an understanding of skin S. aureus strains in AD, as the research community is attempting to do with understanding mucosal isolates. This is necessary not only for management of AD, but also to understand and manage S. aureus contribution to non-cutaneous infections.
Finally, what are the potential clinical implications in AD of the emergence of CA-MRSA versus HA-MRSA and MSSA strains? First it is important to recognize that CA-MRSA generally differ from both HA-MRSA and MSSA strains in antibiotic susceptibility patterns8, 69. CA-MRSA strains are methicillin-resistant, and some have become clindamycin-resistant, but generally these organisms are susceptible to non-β-lactam antibiotics (Table 1). In contrast, HA-MRSA typically are resistant to larger numbers of antibiotics than CA-MRSA, and MSSA strains predictably produce β-lactamases but are generally susceptible to the majority of β-lactam antibiotics. This difference in antibiotic susceptibility patterns allows the clinician also to predict whether or not the patient is likely to be infected with strains that produce high-levels of the SAgs, TSST-1, SEB, and SEC, exotoxins that are most often associated with serious staphylococcal diseases (including TSS, purpura fulminans, and necrotizing pneumonia). These illnesses often occur as the result of spread from skin sources. As indicated above CA-MRSA USA200 strains produce TSST-1, CA-MRSA USA300 strains produce a toxic, deletion variant of TSST-1 (and SEl-Q), and CA-MRSA USA400 strains produce either SEB or SEC. HA-MRSA strains typically do not produce these SAgs69. Additionally, we are preparing a manuscript to describe a new clinical presentation associated with S. aureus SAgs that relate to skin infections: TSS associated with AD skin infections and impaired healing in patients. Some of these patients have had infections with CA-MRSA TSS S. aureus (producing TSST-1, SEB, or SEC) for more than a year and have exhibited accompanying weight losses in excess of 50% of their original body-weights. Resultant skin abscesses from such infections have been the size of footballs, and in many cases the infections simply do not heal. Our preliminary studies suggest that both IgE responses to SAgs and the presence of SAgs themselves contribute to the failure of skin lesion healing.
We have also demonstrated that CA-MRSA isolates typically produce more SAgs than their corresponding CA-MSSA counterparts. The clinical significance of this is incompletely understood. However, as mentioned in a previous section, microbial stress induced by clinical management of patients, such as patients who develop steroid-resistant AD and those whose organisms have acquired SCC mec DNA, increases production of SAgs by the associated S. aureus strains. Since we do not have a full understanding of all SAg-induced staphylococcal illnesses, we likewise do not completely understand the significance of the increased SAg production by the strains; presumably, increased amounts of SAg improves their survival in the host but at the same time may lead to more serious illnesses.
Acknowledgments
National Institutes of Health grants R01s AI74283, AI73366, and AR41256; U54-AI57153 (Great Lakes Regional Center of Excellence in Biodefense and Emerging Infectious Diseases where PMS is a member); and contract N01 AI40029 to DYML.
List of Abbreviations
- AD
atopic dermatitis
- CA-MRSA
community-associated methicillin-resistant Staphylococcus aureus
- CA-MSSA
community-associated methicillin-sensitive Staphylococcus aureus
- CDC
Centers for Disease Control and Prevention
- HA-MRSA
hospital-associated methicillin-resistant Staphylococcus aureus
- HA-MSSA
hospital-associated methicillin-sensitive Staphylococcus aureus
- MSCRAMMs
microbial surface components recognizing adhesive matrix molecules
- PVL
Panton Valentine leukocidin
- SAgs
superantigens
- SE
staphylococcal enterotoxin
- SEl
staphylococcal enterotoxin-like
- Vβ-TCR
variable region of the β-chain of the T cell receptor
- PSMs
phenol soluble modulins
- SCC mec
staphylococcal chromosome cassette methicillin resistance gene
- PFGE
pulsed-field gel electrophoresis
- SDS-PAGE
sodium dodecyl sulfate polyacrylamide gel electrophoresis
- TSS
toxic shock syndrome
- TSST-1
toxic shock syndrome toxin-1
References
- 1.Lowy FD. Staphylococcus aureus infections. N Engl J Med. 1998;339:520–32. doi: 10.1056/NEJM199808203390806. [DOI] [PubMed] [Google Scholar]
- 2.O’Riordan K, Lee JC. Staphylococcus aureus capsular polysaccharides. Clin Microbiol Rev. 2004;17:218–34. doi: 10.1128/CMR.17.1.218-234.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.O’Gara JP. ica and beyond: biofilm mechanisms and regulation in Staphylococcus epidermidis and Staphylococcus aureus. FEMS Microbiol Lett. 2007;270:179–88. doi: 10.1111/j.1574-6968.2007.00688.x. [DOI] [PubMed] [Google Scholar]
- 4.Foster TJ, Hook M. Surface protein adhesins of Staphylococcus aureus. Trends Microbiol. 1998;6:484–8. doi: 10.1016/s0966-842x(98)01400-0. [DOI] [PubMed] [Google Scholar]
- 5.Dinges MM, Orwin PM, Schlievert PM. Exotoxins of Staphylococcus aureus. Clin Microbiol Rev. 2000;13:16–34. doi: 10.1128/cmr.13.1.16-34.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ladhani S. Recent developments in staphylococcal scalded skin syndrome. Clin Microbiol Infect. 2001;7:301–7. doi: 10.1046/j.1198-743x.2001.00258.x. [DOI] [PubMed] [Google Scholar]
- 7.McCormick JK, Yarwood JM, Schlievert PM. Toxic shock syndrome and bacterial superantigens: an update. Annu Rev Microbiol. 2001;55:77–104. doi: 10.1146/annurev.micro.55.1.77. [DOI] [PubMed] [Google Scholar]
- 8.Klevens RM, Morrison MA, Nadle J, Petit S, Gershman K, Ray S, et al. Invasive methicillin-resistant Staphylococcus aureus infections in the United States. Jama. 2007;298:1763–71. doi: 10.1001/jama.298.15.1763. [DOI] [PubMed] [Google Scholar]
- 9.Chang S, Sievert DM, Hageman JC, Boulton ML, Tenover FC, Downes FP, et al. Infection with vancomycin-resistant Staphylococcus aureus containing the vanA resistance gene. N Engl J Med. 2003;348:1342–7. doi: 10.1056/NEJMoa025025. [DOI] [PubMed] [Google Scholar]
- 10.Schlievert PM. Enhancement of host susceptibility to lethal endotoxin shock by staphylococcal pyrogenic exotoxin type C. Infect Immun. 1982;36:123–8. doi: 10.1128/iai.36.1.123-128.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Watson DW. Host-parasite factors in group A streptococcal infections. Pyrogenic and other effects of immunologic distinct exotoxins related to scarlet fever toxins. J Exp Med. 1960;111:255–84. doi: 10.1084/jem.111.2.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bergdoll MS. Monkey feeding test for staphylococcal enterotoxin. Methods Enzymol. 1988;165:324–33. doi: 10.1016/s0076-6879(88)65048-8. [DOI] [PubMed] [Google Scholar]
- 13.Barsumian EL, Schlievert PM, Watson DW. Nonspecific and specific immunological mitogenicity by group A streptococcal pyrogenic exotoxins. Infect Immun. 1978;22:681–8. doi: 10.1128/iai.22.3.681-688.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schlievert PM, Shands KN, Dan BB, Schmid GP, Nishimura RD. Identification and characterization of an exotoxin from Staphylococcus aureus associated with toxic-shock syndrome. J Infect Dis. 1981;143:509–16. doi: 10.1093/infdis/143.4.509. [DOI] [PubMed] [Google Scholar]
- 15.Marrack P, Kappler J. The staphylococcal enterotoxins and their relatives. Science. 1990;248:705–11. doi: 10.1126/science.2185544. [DOI] [PubMed] [Google Scholar]
- 16.Kotzin BL, Leung DY, Kappler J, Marrack P. Superantigens and their potential role in human disease. Adv Immunol. 1993;54:99–166. doi: 10.1016/s0065-2776(08)60534-9. [DOI] [PubMed] [Google Scholar]
- 17.Li H, Llera A, Malchiodi EL, Mariuzza RA. The structural basis of T cell activation by superantigens. Annu Rev Immunol. 1999;17:435–66. doi: 10.1146/annurev.immunol.17.1.435. [DOI] [PubMed] [Google Scholar]
- 18.Schlievert PM, Jablonski LM, Roggiani M, Sadler I, Callantine S, Mitchell DT, et al. Pyrogenic toxin superantigen site specificity in toxic shock syndrome and food poisoning in animals. Infect Immun. 2000 doi: 10.1128/iai.68.6.3630-3634.2000. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hovde CJ, Marr JC, Hoffmann ML, Hackett SP, Chi YI, Crum KK, et al. Investigation of the role of the disulphide bond in the activity and structure of staphylococcal enterotoxin C1. Mol Microbiol. 1994;13:897–909. doi: 10.1111/j.1365-2958.1994.tb00481.x. [DOI] [PubMed] [Google Scholar]
- 20.Peterson M, Ault K, Kremer MJ, Klingelhutz AJ, Davis CC, Squier CA, Schlievert PM. Innate immune system is activated by stimulation of vaginal epithelial cells with Staphylococcus aureus and toxic shock syndrome toxin-1. Infect Immun. 2005;73:2164–74. doi: 10.1128/IAI.73.4.2164-2174.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brosnahan AJ, Mantz MJ, Squier CA, Peterson ML, Schlievert PM. Cytolysins augment superantigen penetration of stratified mucosa. J Immunol. 2009;182:2364–73. doi: 10.4049/jimmunol.0803283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bergdoll MS, Crass BA, Reiser RF, Robbins RN, Davis JP. A new staphylococcal enterotoxin, enterotoxin F, associated with toxic- shock-syndrome Staphylococcus aureus isolates. Lancet. 1981;1:1017–21. doi: 10.1016/s0140-6736(81)92186-3. [DOI] [PubMed] [Google Scholar]
- 23.Crass BA, Bergdoll MS. Involvement of staphylococcal enterotoxins in nonmenstrual toxic shock syndrome. J Clin Microbiol. 1986;23:1138–9. doi: 10.1128/jcm.23.6.1138-1139.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schlievert PM. Staphylococcal enterotoxin B and toxic-shock syndrome toxin-1 are significantly associated with non-menstrual TSS. Lancet. 1986;1:1149–50. doi: 10.1016/s0140-6736(86)91859-3. [DOI] [PubMed] [Google Scholar]
- 25.Schlievert PM, Tripp TJ, Peterson ML. Reemergence of staphylococcal toxic shock syndrome in Minneapolis-St. Paul, Minnesota, during the 2000–2003 surveillance period. J Clin Microbiol. 2004;42:2875–6. doi: 10.1128/JCM.42.6.2875-2876.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Giantonio BJ, Alpaugh RK, Schultz J, McAleer C, Newton DW, Shannon B, et al. Superantigen-based immunotherapy: a phase I trial of PNU-214565, a monoclonal antibody-staphylococcal enterotoxin A recombinant fusion protein, in advanced pancreatic and colorectal cancer. J Clin Oncol. 1997;15:1994–2007. doi: 10.1200/JCO.1997.15.5.1994. [DOI] [PubMed] [Google Scholar]
- 27.Schlievert PM, Case LC, Strandberg KL, Abrams BB, Leung DY. Superantigen profile of Staphylococcus aureus isolates from patients with steroid-resistant atopic dermatitis. Clin Infect Dis. 2008;46:1562–7. doi: 10.1086/586746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bartlett AH, Foster TJ, Hayashida A, Park PW. Alpha-toxin facilitates the generation of CXC chemokine gradients and stimulates neutrophil homing in Staphylococcus aureus pneumonia. J Infect Dis. 2008;198:1529–35. doi: 10.1086/592758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Konig B, Prevost G, Piemont Y, Konig W. Effects of Staphylococcus aureus leukocidins on inflammatory mediator release from human granulocytes. J Infect Dis. 1995;171:607–13. doi: 10.1093/infdis/171.3.607. [DOI] [PubMed] [Google Scholar]
- 30.Dragneva Y, Anuradha CD, Valeva A, Hoffmann A, Bhakdi S, Husmann M. Subcytocidal attack by staphylococcal alpha-toxin activates NF-kappaB and induces interleukin-8 production. Infect Immun. 2001;69:2630–5. doi: 10.1128/IAI.69.4.2630-2635.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Diep BA, Palazzolo-Ballance AM, Tattevin P, Basuino L, Braughton KR, Whitney AR, et al. Contribution of Panton-Valentine leukocidin in community-associated methicillin-resistant Staphylococcus aureus pathogenesis. PLoS One. 2008;3:e3198. doi: 10.1371/journal.pone.0003198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bubeck Wardenburg J, Palazzolo-Ballance AM, Otto M, Schneewind O, DeLeo FR. Panton-Valentine leukocidin is not a virulence determinant in murine models of community-associated methicillin-resistant Staphylococcus aureus disease. J Infect Dis. 2008;198:1166–70. doi: 10.1086/592053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Voyich JM, Otto M, Mathema B, Braughton KR, Whitney AR, Welty D, et al. Is Panton-Valentine leukocidin the major virulence determinant in community-associated methicillin-resistant Staphylococcus aureus disease? J Infect Dis. 2006;194:1761–70. doi: 10.1086/509506. [DOI] [PubMed] [Google Scholar]
- 34.Bubeck Wardenburg J, Bae T, Otto M, Deleo FR, Schneewind O. Poring over pores: alpha-hemolysin and Panton-Valentine leukocidin in Staphylococcus aureus pneumonia. Nat Med. 2007;13:1405–6. doi: 10.1038/nm1207-1405. [DOI] [PubMed] [Google Scholar]
- 35.Bubeck Wardenburg J, Schneewind O. Vaccine protection against Staphylococcus aureus pneumonia. J Exp Med. 2008;205:287–94. doi: 10.1084/jem.20072208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang R, Braughton KR, Kretschmer D, Bach TH, Queck SY, Li M, et al. Identification of novel cytolytic peptides as key virulence determinants for community-associated MRSA. Nat Med. 2007;13:1510–4. doi: 10.1038/nm1656. [DOI] [PubMed] [Google Scholar]
- 37.Labandeira-Rey M, Couzon F, Boisset S, Brown EL, Bes M, Benito Y, et al. Staphylococcus aureus Panton-Valentine leukocidin causes necrotizing pneumonia. Science. 2007;315:1130–3. doi: 10.1126/science.1137165. [DOI] [PubMed] [Google Scholar]
- 38.Brown EL, Dumitrescu O, Thomas D, Badiou C, Koers EM, Choudhury P, et al. The Panton-Valentine leukocidin vaccine protects mice against lung and skin infections caused by Staphylococcus aureus USA300. Clin Microbiol Infect. 2009;15:156–64. doi: 10.1111/j.1469-0691.2008.02648.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hongo I, Baba T, Oishi K, Morimoto Y, Ito T, Hiramatsu K. Phenol-soluble modulin alpha3 enhances the human neutrophil lysis mediated by Panton-Valentine leukocidin. J Infect Dis. 2009;200:715–23. doi: 10.1086/605332. [DOI] [PubMed] [Google Scholar]
- 40.Schlievert PM. Cytolysins, superantigens, and pneumonia due to community-associated methicillin-resistant Staphylococcus aureus. J Infect Dis. 2009;200:676–8. doi: 10.1086/605333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Davis CC, Kremer MJ, Schlievert PM, Squier CA. Penetration of toxic shock syndrome toxin-1 across porcine vaginal mucosa ex vivo: permeability characteristics, toxin distribution, and tissue damage. Am J Obstet Gynecol. 2003;189:1785–91. doi: 10.1016/s0002-9378(03)00873-1. [DOI] [PubMed] [Google Scholar]
- 42.Kluytmans J, van Belkum A, Verbrugh H. Nasal carriage of Staphylococcus aureus: epidemiology, underlying mechanisms, and associated risks. Clin Microbiol Rev. 1997;10:505–20. doi: 10.1128/cmr.10.3.505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Monti G, Tonetto P, Mostert M, Oggero R. Staphylococcus aureus skin colonization in infants with atopic dermatitis. Dermatology. 1996;193:83–7. doi: 10.1159/000246218. [DOI] [PubMed] [Google Scholar]
- 44.Leyden JJ, Marples RR, Kligman AM. Staphylococcus aureus in the lesions of atopic dermatitis. Br J Dermatol. 1974;90:525–30. doi: 10.1111/j.1365-2133.1974.tb06447.x. [DOI] [PubMed] [Google Scholar]
- 45.Schauber J, Gallo RL. Antimicrobial peptides and the skin immune defense system. J Allergy Clin Immunol. 2008;122:261–6. doi: 10.1016/j.jaci.2008.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.O’Regan GM, Sandilands A, McLean WH, Irvine AD. Filaggrin in atopic dermatitis. J Allergy Clin Immunol. 2008;122:689–93. doi: 10.1016/j.jaci.2008.08.002. [DOI] [PubMed] [Google Scholar]
- 47.Novick RP. Autoinduction and signal transduction in the regulation of staphylococcal virulence. Mol Microbiol. 2003;48:1429–49. doi: 10.1046/j.1365-2958.2003.03526.x. [DOI] [PubMed] [Google Scholar]
- 48.Pragman AA, Schlievert PM. Virulence regulation in Staphylococcus aureus: the need for in vivo analysis of virulence factor regulation. FEMS Immunol Med Microbiol. 2004;42:147–54. doi: 10.1016/j.femsim.2004.05.005. [DOI] [PubMed] [Google Scholar]
- 49.Yarwood JM, Schlievert PM. Quorum sensing in Staphylococcus infections. J Clin Invest. 2003;112:1620–5. doi: 10.1172/JCI20442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Schlievert PM, Case LC, Nemeth KA, Davis CC, Sun Y, Qin W, et al. Alpha and beta chains of hemoglobin inhibit production of Staphylococcus aureus exotoxins. Biochemistry. 2007;46:14349–58. doi: 10.1021/bi701202w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Edmond MB, Wallace SE, McClish DK, Pfaller MA, Jones RN, Wenzel RP. Nosocomial bloodstream infections in United States hospitals: a three- year analysis. Clin Infect Dis. 1999;29:239–44. doi: 10.1086/520192. [DOI] [PubMed] [Google Scholar]
- 52.Schlievert PM. Chitosan malate inhibits growth and exotoxin production of toxic shock syndrome-inducing Staphylococcus aureus strains and group A streptococci. Antimicrob Agents Chemother. 2007;51:3056–62. doi: 10.1128/AAC.01295-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ganz T. Defensins: antimicrobial peptides of innate immunity. Nat Rev Immunol. 2003;3:710–20. doi: 10.1038/nri1180. [DOI] [PubMed] [Google Scholar]
- 54.Lehrer RI, Lichtenstein AK, Ganz T. Defensins: antimicrobial and cytotoxic peptides of mammalian cells. Annu Rev Immunol. 1993;11:105–28. doi: 10.1146/annurev.iy.11.040193.000541. [DOI] [PubMed] [Google Scholar]
- 55.Boguniewicz M, Schmid-Grendelmeier P, Leung DY. Atopic dermatitis. J Allergy Clin Immunol. 2006;118:40–3. doi: 10.1016/j.jaci.2006.04.044. [DOI] [PubMed] [Google Scholar]
- 56.Homey B, Steinhoff M, Ruzicka T, Leung DY. Cytokines and chemokines orchestrate atopic skin inflammation. J Allergy Clin Immunol. 2006;118:178–89. doi: 10.1016/j.jaci.2006.03.047. [DOI] [PubMed] [Google Scholar]
- 57.McGirt LY, Beck LA. Innate immune defects in atopic dermatitis. J Allergy Clin Immunol. 2006;118:202–8. doi: 10.1016/j.jaci.2006.04.033. [DOI] [PubMed] [Google Scholar]
- 58.Leung DY, Harbeck R, Bina P, Reiser RF, Yang E, Norris DA, et al. Presence of IgE antibodies to staphylococcal exotoxins on the skin of patients with atopic dermatitis. Evidence for a new group of allergens. J Clin Invest. 1993;92:1374–80. doi: 10.1172/JCI116711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hauk PJ, Hamid QA, Chrousos GP, Leung DY. Induction of corticosteroid insensitivity in human PBMCs by microbial superantigens. J Allergy Clin Immunol. 2000;105:782–7. doi: 10.1067/mai.2000.105807. [DOI] [PubMed] [Google Scholar]
- 60.Betley MJ, Lofdahl S, Kreiswirth BN, Bergdoll MS, Novick RP. Staphylococcal enterotoxin A gene is associated with a variable genetic element. Proc Natl Acad Sci U S A. 1984;81:5179–83. doi: 10.1073/pnas.81.16.5179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Pragman AA, Herron-Olson L, Case LC, Vetter SM, Henke EE, Kapur V, et al. Sequence analysis of the Staphylococcus aureus srrAB loci reveals that truncation of srrA affects growth and virulence factor expression. J Bacteriol. 2007;189:7515–9. doi: 10.1128/JB.00547-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Pragman AA, Yarwood JM, Tripp TJ, Schlievert PM. Characterization of virulence factor regulation by SrrAB, a two-component system in Staphylococcus aureus. J Bacteriol. 2004;186:2430–8. doi: 10.1128/JB.186.8.2430-2438.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yarwood JM, McCormick JK, Schlievert PM. Identification of a novel two-component regulatory system that acts in global regulation of virulence factors of Staphylococcus aureus. J Bacteriol. 2001;183:1113–23. doi: 10.1128/JB.183.4.1113-1123.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Schlievert PM, Blomster DA. Production of staphylococcal pyrogenic exotoxin type C: influence of physical and chemical factors. J Infect Dis. 1983;147:236–42. doi: 10.1093/infdis/147.2.236. [DOI] [PubMed] [Google Scholar]
- 65.Herold BC, Immergluck LC, Maranan MC, Lauderdale DS, Gaskin RE, Boyle-Vavra S, et al. Community-acquired methicillin-resistant Staphylococcus aureus in children with no identified predisposing risk. Jama. 1998;279:593–8. doi: 10.1001/jama.279.8.593. [DOI] [PubMed] [Google Scholar]
- 66.From the Centers for Disease Control and Prevention. Four pediatric deaths from community-acquired methicillin-resistant Staphylococcus aureus--Minnesota and North Dakota, 1997–1999. Jama. 1999;282:1123–5. [PubMed] [Google Scholar]
- 67.Dufour P, Gillet Y, Bes M, Lina G, Vandenesch F, Floret D, et al. Community-acquired methicillin-resistant Staphylococcus aureus infections in France: emergence of a single clone that produces Panton-Valentine leukocidin. Clin Infect Dis. 2002;35:819–24. doi: 10.1086/342576. [DOI] [PubMed] [Google Scholar]
- 68.Vandenesch F, Naimi T, Enright MC, Lina G, Nimmo GR, Heffernan H, et al. Community-acquired methicillin-resistant Staphylococcus aureus carrying Panton-Valentine leukocidin genes: worldwide emergence. Emerg Infect Dis. 2003;9:978–84. doi: 10.3201/eid0908.030089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Fey PD, Said-Salim B, Rupp ME, Hinrichs SH, Boxrud DJ, Davis CC, et al. Comparative molecular analysis of community- or hospital-acquired methicillin-resistant Staphylococcus aureus. Antimicrob Agents Chemother. 2003;47:196–203. doi: 10.1128/AAC.47.1.196-203.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ito T, Kuwahara K, Hiramatsu K. Staphylococcal cassette chromosome mec(SCC mec) analysis of MRSA. Methods Mol Biol. 2007;391:87–102. doi: 10.1007/978-1-59745-468-1_7. [DOI] [PubMed] [Google Scholar]
- 71.Naimi TS, LeDell KH, Como-Sabetti K, Borchardt SM, Boxrud DJ, Etienne J, et al. Comparison of community- and health care-associated methicillin-resistant Staphylococcus aureus infection. Jama. 2003;290:2976–84. doi: 10.1001/jama.290.22.2976. [DOI] [PubMed] [Google Scholar]
- 72.Kaneko J, Kamio Y. Bacterial two-component and hetero-heptameric pore-forming cytolytic toxins: structures, pore-forming mechanism, and organization of the genes. Biosci Biotechnol Biochem. 2004;68:981–1003. doi: 10.1271/bbb.68.981. [DOI] [PubMed] [Google Scholar]
- 73.Diep BA, Otto M. The role of virulence determinants in community-associated MRSA pathogenesis. Trends Microbiol. 2008;16:361–9. doi: 10.1016/j.tim.2008.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Huseby M, Shi K, Brown CK, Digre J, Mengistu F, Seo KS, et al. Structure and biological activities of beta toxin from Staphylococcus aureus. J Bacteriol. 2007;189:8719–26. doi: 10.1128/JB.00741-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Gouaux JE, Braha O, Hobaugh MR, Song L, Cheley S, Shustak C, et al. Subunit stoichiometry of staphylococcal alpha-hemolysin in crystals and on membranes: a heptameric transmembrane pore. Proc Natl Acad Sci U S A. 1994;91:12828–31. doi: 10.1073/pnas.91.26.12828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Bergdoll MS, Schlievert PM. Toxic-shock syndrome toxin. Lancet. 1984;ii:691. [Google Scholar]
- 77.Davis JP, Chesney PJ, Wand PJ, LaVenture M. Toxic-shock syndrome: epidemiologic features, recurrence, risk factors, and prevention. N Engl J Med. 1980;303:1429–35. doi: 10.1056/NEJM198012183032501. [DOI] [PubMed] [Google Scholar]
- 78.Shands KN, Schmid GP, Dan BB, Blum D, Guidotti RJ, Hargrett NT, et al. Toxic-shock syndrome in menstruating women: association with tampon use and Staphylococcus aureus and clinical features in 52 cases. N Engl J Med. 1980;303:1436–42. doi: 10.1056/NEJM198012183032502. [DOI] [PubMed] [Google Scholar]
- 79.Todd JK, Kapral FA, Fishaut M, Welch TR. Toxic shock syndrome associated with phage group 1 staphylococci. Lancet. 1978;2:1116–8. doi: 10.1016/s0140-6736(78)92274-2. [DOI] [PubMed] [Google Scholar]
- 80.Parsonnet J. Case definition of staphylococcal TSS: a proposed revision incorporating laboratory findings. International Congress and Symposium Series. 1998;229:15. [Google Scholar]
- 81.Reingold AL, Hargrett NT, Dan BB, Shands KN, Strickland BY, Broome CV. Nonmenstrual toxic shock syndrome: a review of 130 cases. Ann Intern Med. 1982;96:871–4. doi: 10.7326/0003-4819-96-6-871. [DOI] [PubMed] [Google Scholar]
- 82.Brosnahan AJ, Schaefers MM, Amundson WH, Mantz MJ, Squier CA, Peterson ML, et al. Novel toxic shock syndrome toxin-1 amino acids required for biological activity. Biochemistry. 2008;47:12995–3003. doi: 10.1021/bi801468w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Lee PK, Vercellotti GM, Deringer JR, Schlievert PM. Effects of staphylococcal toxic shock syndrome toxin 1 on aortic endothelial cells. J Infect Dis. 1991;164:711–9. doi: 10.1093/infdis/164.4.711. [DOI] [PubMed] [Google Scholar]
- 84.Methicillin-resistant Staphylococcus aureus infections in correctional facilities---Georgia, California, and Texas, 2001–2003. MMWR Morb Mortal Wkly Rep. 2003;52:992–6. [PubMed] [Google Scholar]
- 85.Diep BA, Chambers HF, Graber CJ, Szumowski JD, Miller LG, Han LL, et al. Emergence of multidrug-resistant, community-associated, methicillin-resistant Staphylococcus aureus clone USA300 in men who have sex with men. Ann Intern Med. 2008;148:249–57. doi: 10.7326/0003-4819-148-4-200802190-00204. [DOI] [PubMed] [Google Scholar]
- 86.Altemeier WA, Lewis S, Schlievert PM, Bjornson HS. Studies of the staphylococcal causation of toxic shock syndrome. Surg Gynecol Obstet. 1981;153:481–5. [PubMed] [Google Scholar]
- 87.Altemeier WA, Lewis SA, Bjornson HS, Staneck JL, Schlievert PM. Staphylococcus in toxic shock syndrome and other surgical infections. Development of new bacteriophages. Arch Surg. 1983;118:281–4. doi: 10.1001/archsurg.1983.01390030013002. [DOI] [PubMed] [Google Scholar]
- 88.Altemeier WA, Lewis SA, Schlievert PM, Bergdoll MS, Bjornson HS, Staneck JL, et al. Staphylococcus aureus associated with toxic shock syndrome: phage typing and toxin capability testing. Ann Intern Med. 1982;96:978–82. doi: 10.7326/0003-4819-96-6-978. [DOI] [PubMed] [Google Scholar]
- 89.Assimacopoulos AP, Strandberg KL, Rotschafer JH, Schlievert PM. Extreme pyrexia and rapid death due to Staphylococcus aureus infection: analysis of 2 cases. Clin Infect Dis. 2009;48:612–4. doi: 10.1086/597009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Diep BA, Gill SR, Chang RF, Phan TH, Chen JH, Davidson MG, et al. Complete genome sequence of USA300, an epidemic clone of community-acquired meticillin-resistant Staphylococcus aureus. Lancet. 2006;367:731–9. doi: 10.1016/S0140-6736(06)68231-7. [DOI] [PubMed] [Google Scholar]
- 91.Schlievert PM, Osterholm MT, Kelly JA, Nishimura RD. Toxin and enzyme characterization of Staphylococcus aureus isolates from patients with and without toxic shock syndrome. Ann Intern Med. 1982;96:937–40. doi: 10.7326/0003-4819-96-6-937. [DOI] [PubMed] [Google Scholar]
- 92.O’Reilly M, Kreiswirth B, Foster TJ. Cryptic alpha-toxin gene in toxic shock syndrome and septicaemia strains of Staphylococcus aureus. Mol Microbiol. 1990;4:1947–55. doi: 10.1111/j.1365-2958.1990.tb02044.x. [DOI] [PubMed] [Google Scholar]
- 93.MacDonald KL, Osterholm MT, Hedberg CW, Schrock CG, Peterson GF, Jentzen JM, et al. Toxic shock syndrome. A newly recognized complication of influenza and influenzalike illness. Jama. 1987;257:1053–8. doi: 10.1001/jama.257.8.1053. [DOI] [PubMed] [Google Scholar]
- 94.Liu GY, Essex A, Buchanan JT, Datta V, Hoffman HM, Bastian JF, et al. Staphylococcus aureus golden pigment impairs neutrophil killing and promotes virulence through its antioxidant activity. J Exp Med. 2005;202:209–15. doi: 10.1084/jem.20050846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Fields BA, Malchiodi EL, Li H, Ysern X, Stauffacher CV, Schlievert PM, et al. Crystal structure of a T-cell receptor beta-chain complexed with a superantigen. Nature. 1996;384:188–92. doi: 10.1038/384188a0. [DOI] [PubMed] [Google Scholar]
- 96.Jardetzky TS, Brown JH, Gorga JC, Stern LJ, Urban RG, Chi YI, et al. Three-dimensional structure of a human class II histocompatibility molecule complexed with superantigen. Nature. 1994;368:711–8. doi: 10.1038/368711a0. [DOI] [PubMed] [Google Scholar]
- 97.Li H, Llera A, Tsuchiya D, Leder L, Ysern X, Schlievert PM, et al. Three-dimensional structure of the complex between a T cell receptor beta chain and the superantigen staphylococcal enterotoxin B. Immunity. 1998;9:807–16. doi: 10.1016/s1074-7613(00)80646-9. [DOI] [PubMed] [Google Scholar]