Abstract
Successful proliferation and differentiation of hematopoietic progenitor cells in bone marrow (BM) is essential to generate all mature blood cell types, including those involved in the immune response. Although vaccinia virus (VV) is known to induce a strong immune response, the effect of VV infection on hematopoiesis remains largely unknown. Here, we showed that in vivo VV infection results in the expansion of c-KithiSca-1+Lin− (KSL) hematopoietic stem cells. The in vivo expansion of the KSL population requires MyD88 that is a critical adaptor for Toll-like receptor-mediated signaling. Moreover, in BM of VV-infected mice, common myeloid progenitors (CMP) was decreased because of the rapid differentiation of CMP to more mature cells. However, the CMP compartment was not affected by VV infection in the absence of MyD88. The common lymphoid progenitor (CLP) cell population was increased regardless of MyD88 status, suggesting the independent regulation of CMP and CLP compartments by VV infection. VV infection also enhanced the potential of progenitors that preferentially induce the programming of dendritic cell (DC) development toward plasmacytoid DC. Therefore, the host immune response is gearing toward antiviral responses as early as at the precursor level upon VV infection.
Keywords: Dendritic cells, Bone marrow, Committed progenitors, Differentiation
INTRODUCTION
All cells in blood originate from pluripotent hematopoietic stem cells (HSC) in bone marrow (BM). HSC undergo a series of cell divisions and develop into lymphoid- or myeloid-committed progenitors [1]. Lymphoid-restricted progenitors called common lymphoid progenitors (CLPs) give rise to T, B, and natural killer (NK) cells [2]. Myeloid-committed progenitors include common myeloid progenitors (CMPs), granulocyte-monocyte progenitors, and megakaryocyte-erythrocyte progenitors [3]. HSC and progenitor cells can be defined phenotypically by combinations of cell surface markers [2, 4, 5]. Hematopoiesis is a dynamic process and is modulated by environmental factors, including virus infection. For example, cytomegalovirus causes immune complications by affecting the differentiation of myeloid-committed progenitors [6], and changes in these progenitors can be modulated by NKT cells [7]. Also, lympholytic choriomeningitis virus (LCMV) impairs development of dendritic cells (DC) [8].
Vaccinia virus (VV) belongs to the Poxviridae family of DNA viruses and has been successfully used as an attenuated vaccine to eradicate human smallpox [9, 10]. However, VV infection also alters host immune response, and vaccination has been associated with significant complications, particularly in immunocompromised individuals [11]. VV inhibits DC functions and blocks cytokine signals by expressing decoy receptors for interleukin (IL)-1β, tumor necrosis factor-α, and interferon (IFN) [12, 13]. VV-infected antigen-presenting cells (APC) are impaired in their antigen presentation function [14, 15]. However, the effect of VV infection on hematopoiesis remains unknown. Here, we show that VV infection results in the expansion of phenotypically defined hematopoietic stem cells in a MyD88-dependent manner. This was associated with a drastic decrease in CMP and increased numbers of more restricted myeloid precursors and differentiated myeloid cells. Lastly, VV infection preferentially directed DC differentiation toward the plasmacytoid, not myeloid, DC lineage.
MATERIALS AND METHODS
Mice and Reagents
C57BL/6 and MyD88−/− mice were used at 6–10 weeks of age. C57BL/6 mice were purchased from Harlan (Indianapolis, http://www.harlan.com). MyD88−/− mice were maintained in the laboratory animal resource facility at the Indiana University School of Medicine.
Granulocyte macrophage-colony-stimulating factor (GM-CSF) was purchased from R&D Systems (Minneapolis, http://www.rndsystems.com), and Flt3-L was purchased from Peprotech (Rocky Hill, NJ, http://www.peprotech.com). Methocult media and lineage depletion biotin selection kit were purchased from StemCell Technologies (Vancouver, BC, Canada, http://www.stemcell.com). To exclude dead cells during flow cytometry analysis, LIVE/DEAD Fixable Violet Dead Cell Stain Kit from Invitrogen (Carlsbad, CA, http://www.invitrogen.com) was used. The following antibodies for flow cytometry were purchased from eBioscience Inc. (San Diego, http://www.ebioscience.com): fluorescein isothiocyanate-conjugated anti-CD34 (RAM34), phycoerythrin (PE)-conjugated anti-IL7Rα (A7R34), PE-conjugated anti-Flt3 (A2F10.1), PE-Cy7-conjugated anti-c-kit (2B8), allophycocyanin-conjugated anti-Sca-1(D7), biotinylated anti-CD11b (M1/70), biotinylated anti-TER 119 (TER-119), biotinylated anti-Gr-1 (RB6–8C5), biotinylated anti-CD11c (p150/90), and biotinylated anti-B220 (RA3–6B2). APC-Cy7-conjugated streptavidin was purchased from BD Pharmingen (San Diego, http://www.bdbiosciences.com/index_us.shtml). Flow cytometry data were acquired by LSR II (Becton, Dickinson and Company, San Jose, CA, http://www.bd.com), and data were analyzed using the CellQuest program (Becton Dickinson).
Vaccinia Virus Preparation and Infection
The Western Reserve strain of VV was propagated and titrated by using the TK cell line. Virus was harvested from infected cells by three cycles of freezing/thawing, sonication, and clarification by centrifugation. This VV preparation was further purified on a 36% sucrose gradient by ultracentrifugation. C57BL/6 and MyD88−/− mice were infected with 1 × 106 plaque-forming unit virus particles by intraperitoneal injection. Animals were sacrificed at the indicated times. BM cells were harvested from femur and tibia by repeated flushing with Hanks’ balanced saline solution containing 2% bovine serum albumin.
Lineage Depletion and Analysis
Bone marrow cells were treated with FcR blocker (StemCell Technologies) and enriched for lineage-negative cells by incubation with anti-CD11b, anti-Ly6G, anti-TCR, anti-CD45R, and anti-TER119, followed by negative selection with a biotin selection system (Stem-Cell Technologies). Lineage-negative cells were stained with Sca-1, c-kit, CD34, IL-7Rα, and Flt3 for detection of phenotypically defined hematopoietic stem and progenitor cells. Cells were then fixed with 1% paraformaldehyde and analyzed.
Virus Detection
Lineage-depleted BM cells were harvested at indicated time points; stained with a cocktail of antibodies of c-Kit, Sca-1, and CD34 (all from eBioscience); fixed with paraformaldehyde; and then stained by the TW2.3 antibody recognizing the VV E3L protein [16]. To detect the viral genome, the same cells were used to prepare RNA to do polymerase chain reaction (PCR). Primer sequences for E3L have been published [16].
Ex Vivo Culture
Lineage-negative BM cells were harvested from infected mice at 24 hours after infection and cultured with Stem Cells Pro medium supplemented with nutrients (Invitrogen) for 2 days. At the end of culture, cells were washed twice with phosphate-buffered saline (PBS); stained with Sca-1, c-Kit, and CD34 antibodies; and analyzed by flow cytometry. Dead cells were excluded by LIVE/DEAD Fixable Violet Dead Cell Stain Kit (Invitrogen).
In Vitro c-kithiSca-1+Lin− Culture
Feeder layers were prepared from total BM of C57BL/6 mice. BM cells were plated at 2× 106 cells per milliliter and expanded for 2 weeks. Cells were fed weekly with α-minimal essential medium supplemented with 2 mM l-glutamine, 0.16 mM i-Inositol, 16 µM folic acid, and 10−6 M hydrocortisone (StemCell Technologies). At the end of culture, feeder cells were inactivated by irradiating with 1,500 cGy from a gamma radiation source. Fluorescence-activated cell sorting-sorted c-kithiSca-1+Lin− (KSL) cells were plated on irradiated feeder layers in StemPro serum-free medium (Invitrogen), supplemented with 100 ng/ml lipopolysaccharide (LPS) or 10 µg/ml peptidoglycan, and incubated under hypoxic 5% O2/5% CO2 humidified conditions.
Cell Cycle Analysis
Cell cycle status of KSL cells was determined as previously described [17], with a minor modification. Briefly, lineage-negative BM cells were stained with antibodies recognizing surface molecules Sca-1 and c-Kit and then fixed with 4% paraformaldehyde on ice for 30 minutes. After being washed with PBS twice, cells were treated with 500 U/ml RNase A (Sigma-Aldrich, St. Louis, http://www.sigmaaldrich.com) at 37°C for 30 minutes. Cells were washed with PBS and stained with 40 µg/ml propidium iodide (Sigma). Data were acquired on a LSR II flow cytometer and analyzed with Cell Quest software (Becton Dickinson).
Dendritic Cell Generation from Bone Marrow Progenitor Cells
For generation of DC from progenitor cells, bone marrow cells were harvested, and red blood cells were depleted by lysis. Single-cell suspensions were seeded at 1 × 106 cells per milliliter in RPMI 1640 medium supplemented with 10% fetal calf serum, 2 mM l-glutamine, 100 U/ml penicillin and streptomycin, and either GM-CSF (10 ng/ml) or Flt3-L (100 ng/ml). GM-CSF cultured cells were harvested at day 5, and Flt3-L cultured cells were harvested at day 12. Every 3 days, medium was replenished with fresh medium. At the end of each experiment, viable cell recovery was determined by trypan blue staining. To determine DC differentiation from BM cells, cells were stained with a mixture of antibodies recognizing CD11c, NK1.1, CD3, CD11b, and B220. CD11c+NK1.1−CD3− cells were considered DC.
Statistical Analysis
The two-tailed Student’s t test was used to calculate statistical significance. p values less than .05 were considered statistically significant.
RESULTS
VV Infection Induces the Expansion of the Lin−Sca-1+c-Kit+ HSC Population in the Bone Marrow
Both in vivo infection and in vitro infection by VV are known to modulate the host immune response. Because BM is the organ that generates precursors for all hematopoietic cells, we investigated the effect of VV infection on the hematopoietic precursor population in BM. We infected C57/BL6 mice with VV and examined BM cells at different days postinfection. To enrich HSC and multipotent progenitor cells (MPP), we first eliminated lineage+ cells that were differentiated cells, as described in Materials and Methods. Lineage-depleted cells were then used for further analysis. Cells from mock-infected mice showed a typical profile of KSL cells that comprise a minor population of HSC and MPP (Fig. 1A). However, upon VV infection, both the proportion and the number of KSL cells were dramatically increased as early as 1 day after infection. The KSL population returned to close to normal at day 7 postinfection (Fig. 1A). A similar increase was observed in Sca-1+c-Kit− cells in VV-infected mice, whereas the Sca-1−c-Kit+ cell population was decreased dramatically during the early period of infection (Fig. 1A).
Figure 1.
VV infection results in increased KSL cells in bone marrow (BM). (A): Differential expansion of BM cells upon VV infection. BM cells were isolated from M- and VV-infected mice at 1, 2, and 7 D postinfection, and lineage+ cells were depleted followed by staining with antibodies recognizing Sca-1 and c-Kit. The numbers in dot plots are the percentages of lin− cells. The bar graph shows the mean ± SD of KSL cells from a total of three independent experiments. In each experiment, BM cells (two femurs and two tibias) were pooled from four mice per group. Asterisks denote a statistically significant increase in KSL cells of the VV-infected group compared with the M-infected group (p < .01). (B): Comparison of different subpopulations of BM cells. Sca-1+c-Kit+, Sca-1+c-Kit−, and Sca-1−c-Kit+ cells in (A) were used to determine CD34 and Flt3 expression. The frequencies of CD34+Flt3− and CD34+Flt+ cells in these subpopulations in M- and VV-infected mice at indicated time point are shown. (C): Increase in the cell number of CD34+Flt3− cells. The bar graph shows the average cell number of CD34+Flt3− and CD34+Flt+ populations shown in (B). Asterisks denote a statistically significant increase in CD34+Flt3− KSL cells of VV-infected mice compared with M-infected mice (* and ** indicate p < .05 and p < .01, respectively). (D): Detection of the VV gene product. Polymerase chain reaction was performed with RNA from Lin− BM cells that were prepared from VV-infected mice 24 hours postinfection. Houskeeping gene, HPRT, was used as control for RT-PCR reaction. (E): Determination of cell proliferation. Lin− cells were isolated from BM of M- and VV-infected mice for 1 D. Cells were then cultured in vitro for 1 and 2 D and analyzed as described in Materials and Methods. Abbreviations: D, days; KSL, c-KithiSca-1+Lin−; M, mock; HPRT, hypoxanthine-guanine phosphoribosyl transferase; VV, vaccinia virus.
Lack of Flt3 expression is used to distinguish the HSC population (Flt3− KSL), which possesses extensive and sustained long-and short-term self-renewal potential, from the MPP population (Flt3+ KSL), which has the ability to efficiently reconstitute lymphoid but not myeloid compartment [18]. When we examined KSL cells using Flt3 and CD34 markers to compare HSC and MPP, Flt3− but not Flt3+ KSL cells were primarily expanded in VV-infected BM (Fig. 1B, 1C).
The alteration in the KSL population can be explained by at least two possibilities: cell intrinsic or extrinsic factors. It is possible that VV infects hematopoietic cells and that infected cells proliferate. Alternatively, the expansion of KSL could be caused by an indirect effect, such as environmental changes. To distinguish these two possibilities, we first investigated whether Lin− cells are infected by VV or not. We prepared RNA from Lin− BM cells from mock- and VV-infected mice and performed PCR to detect E3L, which is the early gene product of VV. As shown in Figure 1D, VV-infected BM cells expressed E3L, suggesting that VV infected Lin− BM cells in vivo.
We next asked whether VV-infected cells indeed expand or not. If the observed changes in KSL and Lin−Sca-1−Kit+ populations upon VV infection were due to intrinsic changes in these cells, similar increase and decrease of the two populations could emerge during the 2 days of culture. To get this answer, Lin− BM cells were prepared 24 hours postinfection and cultured for 2 days. Cells were then subjected to a cell viability analysis using LIVE/DEAD Fixable Violet Dead Cell Stain Kit (Invitrogen). We found that lineage-negative cells did not proliferate in vitro and that Lin+ cells were not present (Fig. 1E). These data suggest that the KSL expansion in vivo in VV-infected mice is likely not due to the direct effect of VV infection of precursor cells.
In Vivo Expansion of KSL Cells by VV Infection Is MyD88-Dependent
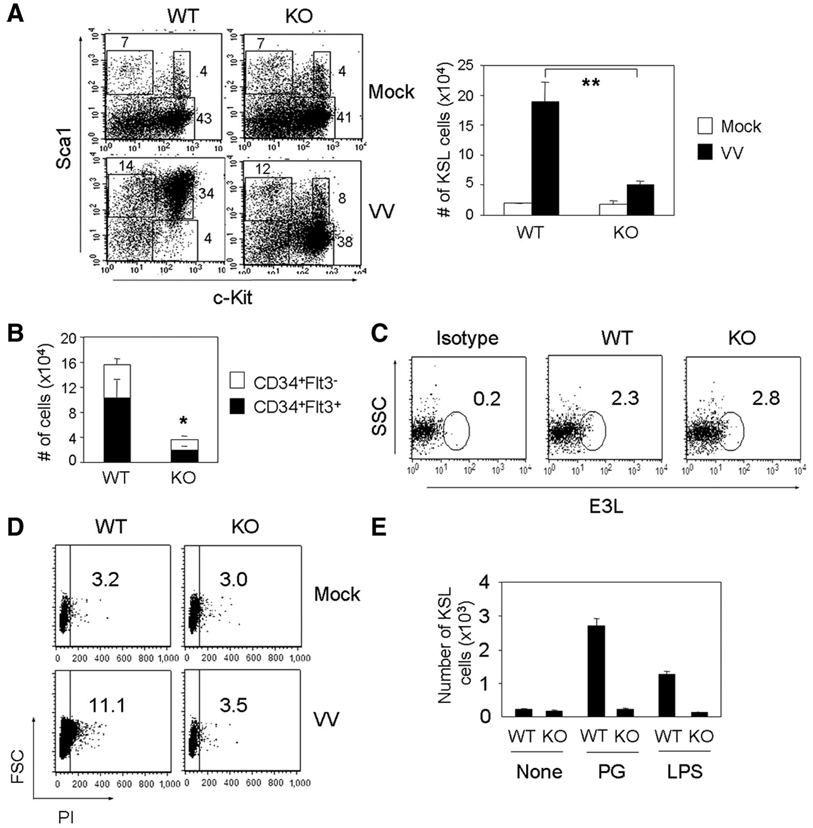
A recent study reported the presence of toll-like receptors (TLRs) on KSL and progenitor cells [19]. In addition, the VV protein A52R disrupts the formation of TLR complexes that play an important role in delivering signals in many immune cells [20]. Therefore, we evaluated whether expansion of the KSL population in VV-infected BM requires TLR-mediated signals. For this purpose, we analyzed mice deficient in MyD88 (MyD88−/−), a critical adaptor for TLR-mediated signal transduction [21]. MyD88−/− mice and wild-type (WT) littermates were infected, and the KSL populations were examined 2 days after infection, at which time the maximum expansion of progenitor cells was observed in normal mice. In vivo VV infection of WT mice resulted in dramatic increases in both percentages and the cell numbers of the KSL population, similar to the pattern observed in C57BL/6 mice (Fig. 2A). In contrast, a marginal induction of KSL was observed in BM from MyD88−/− mice (Fig. 2A). Moreover, the KSL population in MyD88−/− BM was composed of comparable proportions of CD34+Flt3− and CD34+Flt3+ cells, whereas WT BM cells have a preferential expansion of CD34+Flt3− cells after infection (Fig. 2B).
Figure 2.
Expansion of KSL cells in VV-infected mice is MyD88-dependent. (A): Expansion of KSL cells from WT and MyD88−/− mice. WT and MyD88−/− mice were infected for 2 days, and lineage-depleted bone marrow (BM) cells were used to compare the KSL populations. Numbers in dot plots represent the percentages of lineage-depleted cells. The bar graph illustrates the average number of KSL cells in mock- and VV-infected mouse BM from three independent experiments. In each experiment, BM cells were pooled from three mice per group. Asterisks denote a statistically significant decrease in KSL cells of the MyD88−/− group compared with the WT group (p < .01). (B): The bar graph shows mean ± SD of CD34+Flt3− and CD34+Flt+ KSL cells in BM of VV-infected WT and MyD88−/− mice 2 days postinfection. Results are from three independent experiments. In each experiment, BM cells were pooled from three mice per group. An asterisk denotes a statistically significant difference in CD34+Flt− KSL cells of the MyD88−/− group compared with the WT group (p < .05). (C): Detection of viral E3L protein expression in KSL cells. BM cells were harvested from VV-infected WT and MyD88−/− mice 2 days postinfection, and lineage-negative cells were stained with the TW3.2 antibody. (D): Cell cycle analysis. Lineage-depleted BM cells were prepared from WT or MyD88−/− mice 2 days postinfection and used for cell-cycle analysis as described in Materials and Methods. Data shown are electronically gated Lin−Sca-1+Kit+ subsets. Similar results were obtained from two independent experiments. (E): Differential responses to toll-like receptor ligands. Sorted KSL cells from WT and MyD88−/− mice were cultured on irradiated feeder cells for 7 days with PG or LPS. KSL expansion was determined by flow cytometry by gating on Lin−Sca-1+c-Kit+ cell population. Abbreviations: FSC, forward scatter; KO, knockout; KSL, c-Kit+Sca-1+Lin−; LPS, lipopolysaccharide; PG, peptidoglycan; PI, propidium iodide; SSC, side scatter; VV, vaccinia virus; WT, wild-type.
We next asked whether the lack of KSL expansion in MyD88−/− mice was due to a defect in virus infection or replication. When we examined the expression of E3L in the lineage-depleted cell population, the percentage of E3L+ cells was comparable between WT and MyD88−/− cells (Fig. 2C). The data suggest that infection and replication of VV were not compromised in the absence of MyD88. Instead, MyD88−/− cells may not proliferate as much as wild-type cells. To test this, we performed cell cycle analysis. As reported previously [22], only a small fraction of KSL cells was found in the S-G2-M phase of cell cycle in both WT and MyD88−/− mice without VV infection (Fig. 2D). After infection, however, we observed that a higher proportion of WT cells than MyD88−/− cells were cycling (Fig. 2D). In agreement with this observation, the proliferative potential of WT KSL in response to TLR ligands such as LPS or peptidoglycan was greater than that of MyD88−/− KSL (Fig. 2E). Together, VV infection induces expansion of CD34+Flt− KSL cells through a mechanism involving MyD88-dependent signaling.
Changes in the Common Myeloid and Lymphoid Progenitor Cells by VV Infection
Having observed increases in the KSL population upon VV infection, we then investigated the myeloid versus lymphoid progenitor cell compartments. Using Sca-1, c-Kit, CD34, and IL-7Rα, we assessed effects on the CMP that are Lin− IL-7Rα− CD34+ c-kithi Sca-1− and the CLP with the phenotype Lin− IL-7Rα+c-Kitlo Sca-1lo [23]. As shown in Figure 3A, the CMP and CLP populations were decreased and increased, respectively, during 1–2 days of infection (Fig. 3A). Similar to changes in KSL cells, the CMP and CLP compartments returned to normal 7 days postinfection.
Figure 3.
VV infection alters the CMP and CLP compartments. (A): Lineage-depleted bone marrow (BM) cells from mock- or VV-infected mice at the indicated time points after infection were used to assess CMP and CLP populations. The numbers in dot plots are the percentages of lineage-depleted cells. The bar graph shows the cell numbers of CMP and CLP in BM of mock- and VV-infected mice from three independent experiments. In each experiment, BM cells (two femurs and two tibias) were pooled from four mice per group. Asterisks denote a statistically significant difference in CMP and CLP populations of the VV-infected group compared with the mock-infected group (* and ** indicate p < .05 and p < .01, respectively). (B): More differentiated myeloid cells in VV-infected BM. BM cells were isolated from mock- and VV-infected mice at the indicated times followed by staining. Fluorescence-activated cell sorting profiles shown are representative examples from a single experiment, and the number indicates the percentage of CD11intGr-1int. Similar results were obtained from a total of three experiments. (C): Peripheral blood cells were prepared from mock- and VV-infected mice at the indicated time points and stained with antibodies to identify myeloid (CD11b+, F4/80+, or Gr-1+) and lymphoid (B220+, CD19+, CD3+, or NK1.1+) cells. Numbers shown in the bottom graph are the number of myeloid cells (neutrophils and monocytes) (mean ± SD; n = 4 mice per group) and lymphoid cells (B and T cells) (mean ± SD; n = 4 mice per group). Asterisks denote statistically significant differences in myeloid and lymphoid populations of the VV-infected group compared with the mock-infected group (* and ** indicate p < .05 and p < .01, respectively). (D): Lineage-depleted BM cells were prepared from WT or MyD88−/− mice after 2 days of infection and analyzed for CMP and CLP compartments. The numbers shown in dot plots are the percentages of CMP and CLP of the lineage-depleted cell population. Dot plots are representative of three independent experiments, and the average cell numbers shown in bar graphs are from three independent experiments. In each experiment, BM cells (two femurs and two tibias) were pooled from three mice per group. ** indicates p < .01. Abbreviations: CLP, common lymphoid progenitor; CMP, common myeloid progenitor; FSC, forward scatter; IL, interleukin; KO, knockout; VV, vaccinia virus; WT, wild-type.
Decreases in CMP could be due to either a decline in generation of progenitor cells or enhanced differentiation of more mature cells. Because KSL numbers were increased, we hypothesized that loss of CMP is likely due to the rapid differentiation to mature cells. In this context, we examined cells expressing CD11bintGr-1int because these makers are known to be associated with more differentiated myeloid precursors. As shown in Figure 3B, VV-infected BM showed the increase in CD11bintGr-1int cells. Consistent with this observation, more myeloid cells were present in blood after VV infection (Fig. 3C). Therefore, the reduction of CMP is likely due at least in part to enhanced differentiation.
We then compared the CLP and CMP compartments between WT and MyD88−/− mice 2 days after VV infection. Unlike WT mice, the CMP population was not decreased in MyD88−/− BM, whereas CLP cells were comparable between WT and MyD88−/− mice (Fig. 3D). Thus, differentiation of CMP requires a signal mediated by MyD88, and CLP and CMP compartments appear to be regulated independently by VV infection.
VV Infection Preferentially Increases Development of Plasmacytoid DC
DC are the most potent professional APC and play essential roles in both innate and adaptive immune responses. Among different DC subsets, plasmacytoid dendritic cells (pDC) are important for antiviral immunity by virtue of their ability to produce type I IFN [24]. Moreover, LCMV infection alters DC subsets from pDC to the myeloid dendritic cell (mDC) subset [25]. We questioned whether VV infection also influenced DC differentiation from its progenitors. To address this issue, we first compared the differentiation potential of BM cells with that of the mDC versus pDC subset ex vivo.
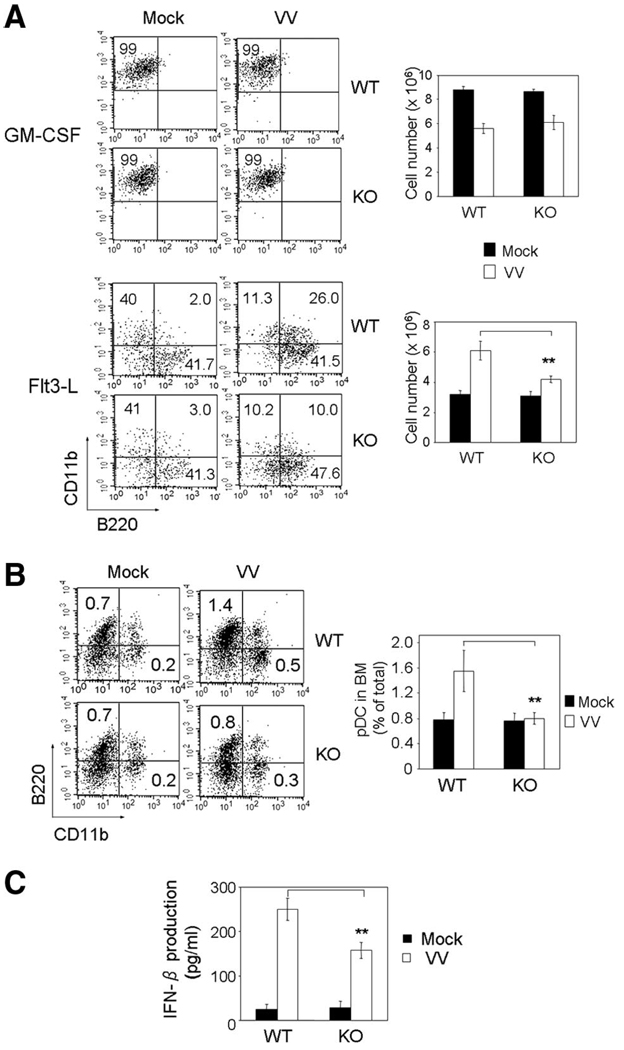
BM cells were harvested from mock or VV-infected WT and MyD88−/− mice and cultured in the presence of GM-CSF or Flt3-L. GM-CSF preferentially induces differentiation of mDC from CMP, whereas Flt3-L promotes the generation of DC from both CLP and CMP [26]. In the presence of GM-CSF, DC were primarily CD11c+CD11b+B220− with or without VV infection (Fig. 4A, top left). However, the numbers of recovered DC at the end of the culture were reduced, if BM cells were from mice infected with VV for 2 days, regardless of the type of mice (Fig. 4A, top right). In contrast, a majority of CD11c+ DC in the Flt-3 culture expressed B220, a marker of pDC, and DC generation was more efficient when precursors were prepared from VV-infected mice (Fig. 4A, bottom group). Moreover, BM cells from VV-infected MyD88−/− mice did not expand as much as from WT mice, which were associated with decreased CD11b+B220+ cells. We next examined whether the similar expansion of pDC occurs during in vivo infection. When freshly isolated BM cells were examined, the pDC population in BM of WT mice was increased upon VV infection (Fig. 4B). However, this increase was not observed in the absence of MyD88 (Fig. 4B). The hallmark of pDC is the ability to produce type I IFN upon virus infection [25]. Our data also showed that pDC generated from VV-infected BM cells in vitro produced IFN-β and that the amount of IFN-β was higher in the culture of WT than MyD88−/− pDC (Fig. 4C).
Figure 4.
VV infection influences DC development. (A): Effect of VV infection on DC differentiation during ex vivo culture. BM cells were prepared from WT and MyD88−/− mice 2 days postinfection and cultured in the presence of GM-CSF (top) or Flt3-L (bottom) for 5 and 12 days, respectively, as described in the Materials and Methods. Numbers in dot plots represent the percentages of NK1.1−CD3−CD11c+ gated cells. The bar graph illustrates the average number of live cells at the end of the culture determined by trypan blue staining. Data are the average of three independent experiments using four mice per group. Asterisks denote statistically significant differences in the cell number between VV-infected WT and MyD88−/− mice (p < .01). (B): Effect of in vivo infection of VV on DC subsets. BM cells harvested from WT or MyD88−/− mice 2 days postinfection were used for DC subset analysis. The cells in the dot plot were gated on NK1.1−CD3−CD11c+ cells and the numbers indicate the percentage of total BM populations. Bar graph shows the average in the percentage of CD11c+ cells from three independent experiments by using four mice per group. (C): IFN-β production by BM DC. BM cells were harvested from WT or MyD88−/− mice 2 days postinfection and cultured for 12 days in the presence of Flt3-L. Supernatants were collected to measure IFN-β by enzyme-linked immunosorbent assay. Data are the average of three independent experiments. ** indicates p < .01. Abbreviations: BM, bone marrow; GM-CSF, granulocyte macrophage-colony-stimulating factor; IFN, interferon; KO, knockout; pDC, plasmacytoid dendritic cells; VV, vaccinia virus; WT, wild-type.
DISCUSSION
Microbial infection stimulates rapid emigration of peripheral leukocytes to the infected area, a necessity to initiate an innate immune response [27, 28]. Thus, large numbers of cells are generated to replenish the pool upon infection to maintain homeostasis and provide sufficient cells to fight against the microbial pathogens. Our current study supports this concept because VV infection induces expansion of hematopoietic stem cells and progenitor cells at early stages of infection. In addition, VV infection preferentially promotes rapid differentiation of CMP in vivo and efficient generation of plasmacytoid dendritic cells from BM precursors during the ex vivo culture. We also observed a dramatic increase in neutrophils and monocytes in peripheral blood shortly after VV infection (data not shown), which may reflect rapid differentiation and expansion of precursors upon infection. Increased blood neutrophils and monocytes may also reflect enhanced release of these cells to the blood from marrow.
Rapid increase and decrease in KSL cells during VV infection appear to control KSL generation to maintain the homeostasis of hematopoietic cells. Expansion of KSL cells immediately after VV infection would accommodate the demand of cells to clear the virus. However, this increase subsides, and the hematopoietic cell compartment returns to normal within 1 week. Our results suggest that this controlling mechanism is likely operated by an indirect effect. Evidence supporting this includes death of BM cells when they were infected in vitro, very low representation of VV-infected Lin− cells in BM of VV-infected mice, and the decrease in the viability and the number of KSL cells from VV-infected BM during ex vivo culture.
Hematopoietic progenitor cells express several TLRs, which are responsible for rapid replenishment of the innate immune system during infection [19, 21, 29]. Our study suggests physiological significance of TLR expression in hematopoietic cells during in vivo VV infection. Absence of MyD88 prevented differentiation of CMP to more committed monocytes and granulocytes precursors, but not CLP expansion upon VV infection. This implicates a greater contribution of MyD88-mediated signaling to CMP than CLP cell differentiation and supports a study by others that showed that TLR ligands selectively stimulate differentiation of CMP [15]. However, it is not clear whether MyD88 expression in HSC progenitors plays a direct role for VV-induced proliferation or whether expansion is mediated indirectly by other factors, such as cytokines, adhesion molecules, or signaling molecules, that are regulated by MyD88. Our results showed that VV-infected cells themselves do not survive and thus cannot contribute to the expansion and differentiation. Perhaps VV infection induces an environmental change caused by VV products or death of VV-infected cells. Uninfected neighboring cells then may sense the virus products released by dying cells via TLRs and activate the MyD88 signaling pathway. It is possible that CLP and CMP express different members of the TLR family, and thus the response of the two against the environmental change could be different. In addition, it is not clear how MyD88 delivers the signal to cell cycle machinery in KSL cells. Further investigation is necessary to delineate signaling molecules that control expansion of KSL cell upon VV infection. Whatever the mechanisms involved, our data clearly demonstrate the essential role of MyD88 in hematopoiesis during VV infection.
Viral infection has been reported to change the differentiation potential and the function of DC [8, 25]. In contrast to LCMV and measles virus, which cause immunosuppression by interfering with DC differentiation from BM precursor [25, 30], VV infection increased the generation of pDC precursors in a MyD88-dependent manner. Although the precise mechanisms responsible for the different potential of viruses are less clear, different viruses would produce a distinct set of gene products that likely modulate the host immune response differently. In VV-infected mice, we showed increases in CLP that correlate with a higher yield of pDC that can be generated from BM cells prepared from VV-infected mice compared with that generated from mock-infected mice. pDC are considered the first line of defense against virus infection because of their ability to produce type I IFN [25]. Type I IFN plays a critical role in virus clearance, as evidenced by studies that showed high susceptibility of mice deficient in type I IFN receptors to VV infection [31] and that supplying IFN-β to the DC culture in vitro prevents the inhibitory effect mediated by VV [16]. Thus, the production of IFN-β by VV-infected DC in the presence of Flt3-L may facilitate better cell survival.
Previously, we have shown that DC from VV-infected mice are impaired in their antigen presentation by MHC class II-mediated pathway [16]. However, the same DC expressed increased levels of MHC I and costimulatory molecules, and the ability to produce IFN-β and inflammatory cytokines was not compromised. Therefore, VV specifically target MHC class II antigen presentation by DC, which in turn prevents the activation of CD4 T cells. These data together with the current study demonstrating VV-mediated alteration in hematopoietic precursors provide evidence that VV infection results in multiple changes in host immune responses. Healthy individuals can recover from VV infection, and they seem to able to mount an appropriate immune response against subsequent infection [9, 10, 32, 33]. However, immunocompromised individuals suffer from clinical complications associated with VV infection, suggesting that the ability to mount the initial immune response is critical to protecting the host.
CONCLUSION
The current study demonstrated that VV infection induces rapid and extensive expansion and differentiation followed by the constriction of hematopoietic stem and progenitor cells in BM. This change in BM that requires MyD88-mediated signaling is likely to facilitate the demand of immune cells to clear the viruses. Therefore, the immune response against VV infection seems to be controlled as early as the hematopoietic progenitor level to mount appropriate protective immunity.
ACKNOWLEDGMENTS
We are grateful to Dr Renukaradhya Gourapura, Kristin Gillett, and Beau Champ for conducting vaccinia infection and providing organs from infected mice and Drs. Janice Blum, Randy Brutkiewicz, and Mark Kaplan for helpful discussions. This study was supported by PO1 A1056097 (to C.-H.C. and S.-C.H.) and in part by NIH R01-DE-13988 (to S.-C.H.). C.-H.C. is currently affiliated with the Department of Microbiology and Immunology, University of Michigan, Ann Arbor, MI.
Footnotes
DISCLOSURE OF POTENTIAL CONFLICTS OF INTEREST
The authors indicate no potential conflicts of interest.
REFERENCES
- 1.Weissman IL. Stem cells: Units of development, units of regeneration, and units in evolution. Cell. 2000;100:157–168. doi: 10.1016/s0092-8674(00)81692-x. [DOI] [PubMed] [Google Scholar]
- 2.Kondo M, Weissman IL, Akashi K. Identification of clonogenic common lymphoid progenitors in mouse bone marrow. Cell. 1997;91:661–672. doi: 10.1016/s0092-8674(00)80453-5. [DOI] [PubMed] [Google Scholar]
- 3.Akashi K, Traver D, Miyamoto T, et al. A clonogenic common myeloid progenitor that gives rise to all myeloid lineages. Nature. 2000;404:193–197. doi: 10.1038/35004599. [DOI] [PubMed] [Google Scholar]
- 4.Okada S, Nakauchi H, Nagayoshi K, et al. Enrichment and characterization of murine hematopoietic stem cells that express c-kit molecule. Blood. 1991;78:1706–1712. [PubMed] [Google Scholar]
- 5.Spangrude GJ, Heimfeld S, Weissman IL. Purification and characterization of mouse hematopoietic stem cells. Science. 1988;241:58–62. doi: 10.1126/science.2898810. [DOI] [PubMed] [Google Scholar]
- 6.Gibbons AE, Price P, Shellam GR. Analysis of hematopoietic stem and progenitor cell populations in cytomegalovirus-infected mice. Blood. 1995;86:473–481. [PubMed] [Google Scholar]
- 7.Broxmeyer HE, Dent A, Cooper S, et al. A role for natural killer T cells and CD1d molecules in counteracting suppression of hematopoiesis in mice induced by infection with murine cytomegalovirus. Exp Hematol. 2007;35:87–93. doi: 10.1016/j.exphem.2007.01.015. [DOI] [PubMed] [Google Scholar]
- 8.Sevilla N, McGavern DB, Teng C, et al. Viral targeting of hematopoietic progenitors and inhibition of DC maturation as a dual strategy for immune subversion. J Clin Invest. 2004;113:737–745. doi: 10.1172/JCI20243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Crotty S, Felgner P, Davies H, et al. Cutting edge: Long-term B cell memory in humans after smallpox vaccination. J Immunol. 2003;171:4969–4973. doi: 10.4049/jimmunol.171.10.4969. [DOI] [PubMed] [Google Scholar]
- 10.Cherry JD, McIntosh K, Connor JD, et al. Clinical and serologic study of four smallpox vaccines comparing variations of dose and route of administration. Primary percutaneous vaccination. J Infect Dis. 1977;135:145–154. doi: 10.1093/infdis/135.1.145. [DOI] [PubMed] [Google Scholar]
- 11.Bray M. Pathogenesis and potential antiviral therapy of complications of smallpox vaccination. Antiviral research. 2003;58:101–114. doi: 10.1016/s0166-3542(03)00008-1. [DOI] [PubMed] [Google Scholar]
- 12.Smith GL. Vaccinia virus glycoproteins and immune evasion. The sixteenth Fleming Lecture. J Gen Virol. 1993;74:1725–1740. doi: 10.1099/0022-1317-74-9-1725. [DOI] [PubMed] [Google Scholar]
- 13.Alcami A, Smith GL. A soluble receptor for interleukin-1 beta encoded by vaccinia virus: A novel mechanism of virus modulation of the host response to infection. Cell. 1992;71:153–167. doi: 10.1016/0092-8674(92)90274-g. [DOI] [PubMed] [Google Scholar]
- 14.Deng L, Dai P, Ding W, et al. Vaccinia virus infection attenuates innate immune responses and antigen presentation by epidermal dendritic cells. J Virol. 2006;80:9977–9987. doi: 10.1128/JVI.00354-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li P, Wang N, Zhou D, et al. Disruption of MHC class II-restricted antigen presentation by vaccinia virus. J Immunol. 2005;175:6481–6488. doi: 10.4049/jimmunol.175.10.6481. [DOI] [PubMed] [Google Scholar]
- 16.Yao Y, Li P, Singh P, et al. Vaccinia virus infection induces dendritic cell maturation but inhibits antigen presentation by MHC class II. Cell Immunol. 2007;246:92–102. doi: 10.1016/j.cellimm.2007.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Takemoto S, Mulloy JC, Cereseto A, et al. Proliferation of adult T cell leukemia/lymphoma cells is associated with the constitutive activation of JAK/STAT proteins. Proc Natl Acad Sci U S A. 1997;94:13897–13902. doi: 10.1073/pnas.94.25.13897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Adolfsson J, Borge OJ, Bryder D, et al. Upregulation of Flt3 expression within the bone marrow Lin(−)Sca1(+)c-kit(+) stem cell compartment is accompanied by loss of self-renewal capacity. Immunity. 2001;15:659–669. doi: 10.1016/s1074-7613(01)00220-5. [DOI] [PubMed] [Google Scholar]
- 19.Nagai Y, Garrett KP, Ohta S, et al. Toll-like receptors on hematopoietic progenitor cells stimulate innate immune system replenishment. Immunity. 2006;24:801–812. doi: 10.1016/j.immuni.2006.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stack J, Haga IR, Schroder M, et al. Vaccinia virus protein A46R targets multiple Toll-like-interleukin-1 receptor adaptors and contributes to virulence. J Exp Med. 2005;201:1007–1018. doi: 10.1084/jem.20041442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Akira S. TLR signaling. Curr Top Microbiol Immunol. 2006;311:1–16. doi: 10.1007/3-540-32636-7_1. [DOI] [PubMed] [Google Scholar]
- 22.Uchida N, He D, Friera AM, et al. The unexpected G0/G1 cell cycle status of mobilized hematopoietic stem cells from peripheral blood. Blood. 1997;89:465–472. [PubMed] [Google Scholar]
- 23.Kondo M, Wagers AJ, Manz MG, et al. Biology of hematopoietic stem cells and progenitors: Implications for clinical application. Ann Rev Immunol. 2003;21:759–806. doi: 10.1146/annurev.immunol.21.120601.141007. [DOI] [PubMed] [Google Scholar]
- 24.Krug A, Veeraswamy R, Pekosz A, et al. Interferon-producing cells fail to induce proliferation of naive T cells but can promote expansion and T helper 1 differentiation of antigen-experienced unpolarized T cells. J Exp Med. 2003;197:899–906. doi: 10.1084/jem.20021091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zuniga EI, McGavern DB, Pruneda JL-Paz, et al. Bone marrow plasmacytoid dendritic cells can differentiate into myeloid dendritic cells upon virus infection. Nat Immunol. 2004;5:1227–1234. doi: 10.1038/ni1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McKenna HJ. Role of hematopoietic growth factors/flt3 ligand in expansion and regulation of dendritic cells. Curr Opin Hematol. 2001;8:149–154. doi: 10.1097/00062752-200105000-00004. [DOI] [PubMed] [Google Scholar]
- 27.Prlic M, Gibbs J, Jameson SC. Characteristics of NK cell migration early after vaccinia infection. J Immunol. 2005;175:2152–2157. doi: 10.4049/jimmunol.175.4.2152. [DOI] [PubMed] [Google Scholar]
- 28.Selin LK, Santolucito PA, Pinto AK, et al. Innate immunity to viruses: Control of vaccinia virus infection by gamma delta T cells. J Immunol. 2001;166:6784–6794. doi: 10.4049/jimmunol.166.11.6784. [DOI] [PubMed] [Google Scholar]
- 29.Hemmi H, Akira S. TLR signalling and the function of dendritic cells. Chem Immunol Allergy. 2005;86:120–135. doi: 10.1159/000086657. [DOI] [PubMed] [Google Scholar]
- 30.Hahm B, Trifilo MJ, Zuniga EI, et al. Viruses evade the immune system through type I interferon-mediated STAT2-dependent, but STAT1-independent, signaling. Immunity. 2005;22:247–257. doi: 10.1016/j.immuni.2005.01.005. [DOI] [PubMed] [Google Scholar]
- 31.Luker KE, Hutchens M, Schultz T, et al. Bioluminescence imaging of vaccinia virus: Effects of interferon on viral replication and spread. Virology. 2005;341:284–300. doi: 10.1016/j.virol.2005.06.049. [DOI] [PubMed] [Google Scholar]
- 32.Precopio ML, Betts MR, Parrino J, et al. Immunization with vaccinia virus induces polyfunctional and phenotypically distinctive CD8(+) T cell responses. J Exp Med. 2007;204:1405–1416. doi: 10.1084/jem.20062363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jacobs N, Chen RA, Gubser C, et al. Intradermal immune response after infection with Vaccinia virus. J Gen Virol. 2006;87:1157–1161. doi: 10.1099/vir.0.81556-0. [DOI] [PubMed] [Google Scholar]