Abstract
Many axonal growth inhibitors that contribute to the usual failure of axon regeneration in the central nervous system (CNS) exert their effects via the RhoA-Rho kinase (ROCK) signal pathway. In this study, we investigated whether lentiviral vector (LV)–mediated neuron-specific expression of a dominant negative mutant of ROCK (DNROCK) could promote axon outgrowth in vitro and in vivo. Dissociated adult rat dorsal root ganglion (DRG) neurons were seeded on solubilized myelin proteins and transduced with either LV/DNROCK or LV/green fluorescent protein (GFP). DNROCK-expressing neurons were shown to have a greater chance of generating neurites and a longer mean length of neurite than GFP-expressing neurons. In the in vivo studies, lentiviruses were injected into the adult rat red nucleus followed by unilateral rubrospinal tract (RST) transection at the fourth cervical level. Rats in the DNROCK group showed better functional recovery in the affected hindlimbs and forelimbs than those in the GFP group. Examination of the spinal cord sections revealed more rubrospinal axonal profiles growing to the spinal cord caudal to the lesion in the DNROCK group than in the GFP group. These results indicate that blocking the RhoA-ROCK signal pathway by expressing DNROCK can enhance regenerative axonal sprouting and lead to partial recovery of limb function.
Introduction
Most neurons in adult mammalian central nervous system (CNS) fail to regenerate their axons after injury. One major contributing factor is the presence of axonal outgrowth inhibitors in the CNS, such as Nogo, myelin-associated glycoprotein, oligodendrocyte myelin glycoprotein, chondroitin sulfate proteoglycans, semaphorins, and ephrins.1,2,3,4 Strategies to block these inhibitors have been explored previously,5,6 including the use of degrading enzymes,7 neutralizing antibodies,8 a Nogo-66 receptor antagonist peptide,9 and a soluble Nogo receptor.10 However, as many inhibitory molecules contribute to the failure of CNS axonal regeneration, blocking or neutralizing individual molecules may not be sufficient to achieve satisfactory axonal regeneration.
Many inhibitors of neurite growth exert their effects by activating RhoA1,11,12,13 and its downstream effector, Rho kinase (ROCK).14 RhoA acts as a molecular switch to control a signal transduction pathway that links membrane receptors to the cytoskeleton.15 RhoA and ROCK have been implicated in signaling to the growth cone cytoskeleton and in regulating growth cone collapse and the retraction of neurites.1,11,16 Therefore, targeting the RhoA-ROCK signal pathway has been seen as an attractive strategy to promote axonal regeneration.12,17,18 Previous studies have shown that blocking the activation of RhoA with C3 transferase or ROCK with Y27632 could stimulate neurite growth over inhibitory substrates in vitro and promotes the regeneration of optic nerves and corticospinal axons in vivo.19,20,21 However, the clinical use of these antagonists may be limited by factors such as side effects and pharmacokinetics. To achieve sustained inhibition of the RhoA-ROCK pathway in vivo, genetic manipulation of these molecules may be an effective alternative.
A dominant negative mutant of ROCK (DNROCK), Rho binding (RB)/pleckstrin-homology (PH)(TT), composed of the RB and PH domains with two point mutations of Asn-1036 and Lys-1037 to Thr in the RB domain that abolishes its Rho-binding activity, was generated by Amano et al.22 This mutant interacts with the catalytic domain of ROCK and specifically inhibits ROCK activity without titrating out RhoA in vitro23,24 and serves as a powerful dominant negative form.22 This construct was shown to enhance neurite outgrowth on myelin substrates.25 We postulated that this DNROCK, if expressed in injured CNS neurons in vivo, would also be able to overcome neurite growth inhibitors in the CNS and promote axonal regeneration. In this study, we transduced neurons with the RB/PH(TT) mutant (referred as DNROCK thereafter) using lentiviral vectors (LVs) to study its effects in promoting neurite outgrowth both in vitro and in vivo.
Results
Effect of DNROCK on the stress fiber formation in NIH 3T3 cells
The formation of stress fibers in NIH 3T3 cells following serum starvation and serum challenge is a process mediated by the RhoA-ROCK signal pathway.26 To test whether the Flag-tagged DNROCK construct is able to block the RhoA-ROCK signal pathways, NIH 3T3 cells were transfected with either pRRL/green fluorescent protein (GFP) or pRRL/Flag-DNROCK. The cells were stained with rhodamine-conjugated phalloidin to detect F-actin following serum starvation and serum challenge. In the untransfected cells and cells transfected with pRRL/GFP, serum-induced formation of stress fibers was clearly visible (Figure 1a–c). No obvious difference in morphology and stress fiber densities was demonstrable between the GFP transfected and untransfected cells. However, cells expressing DNROCK (indicated by the immunoreactivity of Flag tag) displayed elongated processes, with very few visible stress fibers (Figure 1d–f), indicating that this Flag-tagged DNROCK construct is effective in inhibiting the RhoA-ROCK signal pathway as demonstrated by the myc-tagged DNROCK construct reported previously.22
Figure 1.
Flag-tagged DNROCK blocks the formation of stress fibers in NIH 3T3 cells. NIH 3T3 cells were transfected with either (a–c) pRRL/GFP or (d–f) pRRL/DNROCK and starved of serum followed by serum challenge. F-actin was stained with rhodamine-conjugated phalloidin (red in b,c,e,f). Flag-tagged DNROCK was detected by immunostaining with an anti-Flag antibody (green in d,f). Bar = 50 µm. DNROCK, dominant negative Rho kinase; GFP, green fluorescent protein.
LV-mediated DNROCK expression in DRG neurons
LVs carrying the Flag-tagged DNROCK construct and the IRES-GFP sequence under the control of a neuron-specific human synapsin (hSyn) I promoter (LV/DNROCK) were produced. LVs carrying GFP complementary DNA (LV/GFP) were also produced as control. Both vectors were used to infect dissociated adult rat dorsal root ganglion (DRG) neurons. GFP expression in LV/DNROCK transduced neurons was too weak to be visualized directly, whereas DNROCK expression can be identified by immunostaining with anti-Flag antibody in neurons (marked by pan-neuronal marker protein gene product 9.5) 40 hours after infection (Figure 2a–c). DNROCK molecules were present in all parts of the neurons, from the somata to the growth cones. Flag-tagged DNROCK protein was also detected in LV/DNROCK transduced DRG neurons with immunoblotting. A band of 55 kd protein, corresponding to the predicted molecular mass of the Flag-tagged DNROCK protein, was identified (Figure 2d). GFP fluorescence was easily visible in LV/GFP transduced neurons (Figure 3a,b).
Figure 2.
Expression of DNROCK in DRG neurons. Expression of Flag-tagged DNROCK was detected with anti-Flag antibody (green in a and c) in LV-transduced DRG neurons. DRG neurons were labeled with anti-PGP9.5 (red in b and c). (d) Immunoblotting using anti-Flag antibody detected the expression of Flag-tagged DNROCK (band is indicated by an arrow) in LV/DNROCK transduced DRG neurons; while the band for Flag-DNROCK was absent in LV/GFP transduced neurons. Bar = 50 µm. DNROCK, dominant negative Rho kinase; DRG, dorsal root ganglion; LV, lentiviral vector; PGP9.5, protein gene product 9.5.
Figure 3.
DNROCK enhances neurite outgrowth of DRG neurons on myelin substrate. DRG neurons transduced with (a) LV carrying GFP cDNA were plated on laminin (0.1 µg/well) or (b) laminin (0.1 µg/well) + myelin protein (40 µg/well) coated coverslips. DRG neurons transduced with (c) LV/DNROCK were plated on laminin or (d) laminin + myelin protein coated coverslips. Merged photomicrographs showing GFP expression (green) in LV/GFP transduced cells in a and b, and Flag-tagged DNROCK expression (green) in LV/DNROCK transduced cells in c and d. Neuronal marker PGP9.5 immunoreactivity (red) is used to define neurons and their neurites. (e) Quantification of neurite length and (f) percentage of neurite-bearing neurons in LV/GFP and LV/DNROCK groups. Values are presented as mean ± SEM. *P < 0.05; **P < 0.01; ***P < 0.001 [ANOVA with post hoc comparisons and Bonferroni correction on SPSS software] (Chicago, IL). Bar = 100 µm. ANOVA, analysis of variance; cDNA, complementary DNA; DNROCK, dominant negative Rho kinase; DRG, dorsal root ganglion; GFP, green fluorescent protein; LV, lentiviral vector.
DNROCK promotes neurite growth of DRG neurons on myelin proteins
To assess whether overexpression of DNROCK in neurons can overcome the inhibitory effects of CNS myelin, the dissociated DRG neurons were plated on laminin (0.1 µg/well) or laminin + CNS myelin protein (40 µg/well) coated eight-well chamber slides and infected with LV/DNROCK or LV/GFP. After being cultured for 40 hours, 45% of LV/GFP transduced DRG neurons grew neurites longer than the diameters of their cell bodies (Figure 3a,f) and the percentage dropped to 33% in the presence of myelin proteins (Figure 3b,f). More LV/DNROCK transduced neurons bore neurites than LV/GFP transduced neurons (Figure 3c,f). Myelin proteins also significantly reduced the neurite-bearing neurons in DNROCK group (Figure 3d,f), which is still significantly higher than that of LV/GFP transduced neurons on myelin proteins (Figure 3f).
In addition to reduce the percentage of neurite-bearing neurons, myelin proteins also significantly reduced the neurite lengths in both LV/GFP and LV/DNROCK groups (Figure 3e). However, LV/DNROCK transduced neurons had considerably longer neurites than LV/GFP transduced neurons on both the laminin only substrate and the laminin + myelin substrate (Figure 3e).
DNROCK inhibits phosphorylation of collapsin response mediator protein 2 at the growth cones of DRG neurons on myelin proteins
One of the main targets of ROCK that mediates growth cone collapse is collapsin response mediator protein 2 (CRMP2).27 Immunostaining of phosphor T514 CRMP2 (pCRMP2) was carried out to assess the activities of ROCK in LV-transduced DRG neurons. Immunoreactivities of pCRMP2 were observed in the somata and neurites of both LV/GFP and LV/DNROCK transduced DRG neurons (Figure 4). There is no significant difference in CRMP2 phosphorylation levels in the somata between the two groups of neurons on either laminin or laminin + myelin substrates. However, quantification of fluorescence intensities of pCRMP2 immunoreactivity at the growth cones revealed that myelin proteins caused a significant increase in pCRMP2 immunoreactivity (compare Figure 4b,d, also see Figure 4i) in LV/GFP transduced neurons, whereas myelin proteins did not elicit a significant change in pCRMP2 level in the growth cones of LV/DNROCK transduced neurons (compare Figure 4f,h, also see Figure 4i). LV/DNROCK transduced neurons also showed reduced level of pCRMP2 immunoreactivity on the laminin substrate (Figure 4i).
Figure 4.
DNROCK inhibits phosphorylation of CRMP2 in the growth cones of neurons. Dorsal root ganglion neurons were infected with (a,c) LV carrying GFP cDNA or (e,g) Flag-tagged DNROCK cDNA in the (a,e) absence or (c,g) presence of myelin proteins (40 µg/well). Merged micrographs showing the expression of GFP in (a,c) LV/GFP transduced cells and phosphorylated CRMP2 immunoreactivity (pCRMP2, red), or (e,g) the expression of Flag-DNROCK (green) and pCRMP2 immunoreactivity (red). The immunoreactivity of pCRMP2 in the associated axons and growth cones (framed areas in a,c,e,g) are presented in confocal micrographs (b,d,f,h). (i) Immunofluorescence of pCRMP2 at growth cones was quantified by densitometry. Data presented are mean ± SEM of arbitrary units of densities measured with ImageJ. *P < 0.05, **P < 0.01, ***P < 0.001. Bar = 50 µm for a,c,e,g; 10 µm for b,d,f,h. cDNA, complementary DNA; DNROCK, dominant negative Rho kinase; GFP, green fluorescent protein; LV, lentiviral vector; pCRMP2, phosphor collapsin response mediator protein 2.
Neuron-specific expression of GFP and DNROCK in red nucleus and RST axons
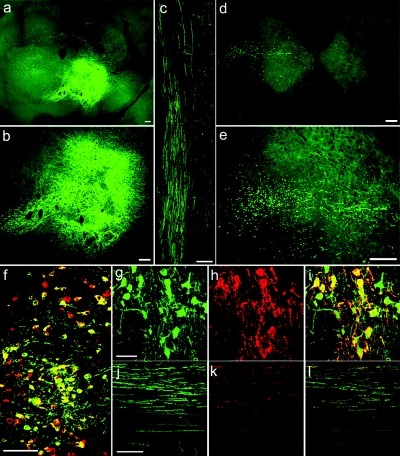
In preliminary experiments, we have examined the expression of the transgenes in red nucleus after LV injections at various time points from 3 days to 10 weeks. One-week after injection of LV/GFP, the GFP proteins were expressed to a level that could be visualized directly under a fluorescence microscope. Images presented in Figure 5 were from animals killed 18 days after injection of LVs into the right red nucleus. In LV/GFP-injected animals, a large number of cells in the red nucleus expressed GFP with their projections crossing to the left midbrain (Figure 5a,b) and descending in the brainstem to the lateral funiculus in the spinal cord (Figure 5c). While descending in the lateral funiculus, these axons sent projections that terminate in the intermediate zones of the gray matter where their normal target neurons are located (Figure 5c–e). To verify neuronal expression of the transgenes, a neuron-specific marker protein NeuN was immunostained in sections containing red nucleus. All GFP+ cells were colocalized with NeuN staining (Figure 5f). Neuron-specific expression of transgenes in the red nucleus was also achieved in LV/DNROCK + LV/GFP-injected animals (data not shown). To assess the co-transduction efficiency of LV/GFP and LV/DNROCK in neurons, immunostaining with anti-Flag antibody demonstrated the colocalization of GFP and Flag-tagged DNROCK (Figure 5g–i) with a co-transduction efficiency of 95.2 ± 0.5% (Flag+ versus GFP+ cells). Therefore, GFP fluorescence in rubrospinal tract (RST) axons in the DNROCK and GFP-co-injected group was used as an indicator of DNROCK expression in the same axons because the immunosignal for Flag tag was too weak in the axons in the spinal cord to be quantified accurately (Figure 5j–l). GFP-transduced axons were counted in horizontal or transverse sections of all the LV-injected animals at the end of the experiment (10 weeks after injury) to assess the transduction efficiency, which were 15.9 ± 2.3 and 13.6 ± 1.7% (GFP+ axons versus total number of neurons in red nucleus) in LV/GFP and LV/DNROCK + LV/GFP-injected animals, respectively. There is no significant difference in transduction efficiency between the two groups, indicating that the expression of DNROCK did not affect the expression of GFP in the co-injection group.
Figure 5.
Neuron-specific expression of GFP and DNROCK in red nucleus and rubrospinal tract. (a) Many cells in the right red nucleus were transduced by a LV carrying GFP cDNA 18 days after injection of viruses. (b) A higher magnification of a. (c) GFP-labeled rubrospinal tract axons in left lateral funiculus were shown in a horizontal section of cervical spinal cord and some axons projected to the gray matter. (d) Transverse section of cervical spinal cord demonstrates the discrete location of GFP-labeled rubrospinal tract axons in the superficial dorsolateral quadrant of cervical spinal cord and projection to the intermediate zone of the gray matter. (e) A higher magnification of d. (f) Merged photomicrograph demonstrates that GFP+ cells (green) were colocalized (yellow) with neuronal marker NeuN (red). (g,h) Confocal photomicrographs demonstrating the co-transduction of neurons by LV/GFP (green in g) and LV/DNROCK (shown as immunoreactivity of Flag tag, red in h). (i) Merged image of g and h shows that most neurons (yellow) were co-transduced by both viral vectors. (j–l) Confocal photomicrographs showing that both GFP (green in j) and Flag-DNROCK proteins (red in k, shown as immunoreactivity of Flag tag) were transported along the same axons of rubrospinal tract after the co-transduction of neurons in red nucleus by both LV/GFP and LV/DNROCK. GFAP staining was shown as blue in l. Bar = 200 µm for a–e; 50 µm for g–i; 100 µm for f and j–l. cDNA, complementary DNA; GFP, green fluorescent protein; GFAP, glial fibrillary acidic protein; LV, lentiviral vector.
DNROCK promotes axonal outgrowth of axotomized RST
To investigate whether expression of DNROCK can promote regeneration of RST axons, unilateral C4 spinal cord dorsolateral funiculotomy was performed. In order to achieve a high-level expression of transgenes before the onset of axon regeneration,28,29 LV was injected into the right red nucleus 1 week before the RST transection. After RST transection, a lesion cavity formed, surrounded by glial scar as was demonstrated with hematoxylin–eosin staining of spinal sections in preliminary experiments. Animals were killed 10 weeks after spinal cord injury. The lesion size in each animal was measured by tracing the glial fibrillary acidic protein-labeled borders using ImageJ from six horizontal sections at the level between the dorsal column and the central canal. The lesion areas are 1.46 ± 0.28 mm2 for DNROCK group and 1.37 ± 0.22 mm2 for GFP group (mean ± SEM, n = 6 in each group). Statistic analysis using Student's t-test showed no difference in lesion sizes between the two groups (P > 0.05).
GFP-labeled axons were clearly visible in the spinal sections containing the lesion site in both groups (Figure 6) and those in the white matters in five regions around the lesion site were counted for statistic analysis (Figure 7a,d). On the rostral part (4–5 mm to the lesion center), no appreciable difference in the numbers of GFP-labeled axons was seen between GFP and DNROCK groups (Figure 7d). However, at the rostral border of the lesion site (within 1.5 mm rostral to the lesion center), significantly more GFP-labeled axons were counted in the DNROCK group than in the GFP group (Figure 7d). Many axons in the DNROCK group were seen projecting laterally to the gray matter (Figure 6c). On horizontal sections, GFP-labeled axons were seen growing around the internal wall of lesion cavity to the white matter at the caudal end, or grew through gray matter and back to white matter (Figures 6 and 7c). Compared with the normal linear rostro-caudal alignment of the RST axons located in the dorsolateral funiculus, most of the GFP-labeled axons were randomly aligned, although generally orientated in a rostral–caudal direction. In the GFP group, on average, 27 GFP-labeled axons per animal were seen within 5 mm of white matter caudal to the lesion center on horizontal sections and 75 in the DNROCK group, which accounts for 5.6% of the total GFP-labeled axons in the rostral side in the GFP group and 16.3% in the DNROCK group, respectively (Figure 7d). Few GFP-labeled axons were seen growing into the lesion cavity in the GFP group (Figure 7d), whereas many more were observed in the DNROCK group (Figure 7d). On transverse sections at 5 mm caudal to the lesion epicenter, significantly more GFP-labeled axons were counted in the DNROCK group than in the GFP group (Figure 7d). Statistic analysis using one-way analysis of variance (ANOVA) for individual distance indexes demonstrates that in the DNROCK group there were significantly more axons growing toward the rostral lesion border, into lesion cavity, and beyond the lesion site to the white matter caudal to the lesion (Figure 7d).
Figure 6.
GFP-labeled rubrospinal tract axons in cervical spinal cord 10 weeks after left rubrospinal tract transection. (a) A horizontal section from an animal injected with a LV carrying GFP cDNA shows that most of GFP-labeled axons forming end bulbs stopped before they reached the injury site. Lesion border is demonstrated by the immunoreactivity of GFAP (blue). (b) An image from a LV/DNROCK-injected animal shows a panoramic view of the rostral side, the rostral border of the lesion cavity, the lesion cavity, and the caudal side. Letter-labeled white boxes indicate the areas enlarged in c–k. (c) Enlarged image from b shows GFP-labeled rubrospinal axons growing toward the rostral border and inside the lesion cavity (arrowheads). (d,e) Axons in the rostral border of the lesion cavity, with some growing beyond the border of glial scar (arrowheads). (f) Axons growing inside the lesion cavity along the glial scar. (g,h) Axons around the internal wall of the lesion cavity. (i) Axons near the caudal border of the lesion cavity. (j,k) Axons caudal to the lesion site. a, c and d–k are images from a confocal microscope. (l,m) GFP-labeled rubrospinal axons 5 mm rostral to the lesion site (l) and 5 mm caudal to the lesion centre (m) in transverse sections of spinal cord in GFP group. (n,o) GFP-labeled rubrospinal axons 5 mm rostral to the lesion site (n) and 5 mm caudal to the lesion centre (o) in transverse sections of spinal cord in DNROCK group. Arrow heads in m and o show the representative regenerating axons in left lateral funiculus of white matter. Arrow in o shows a regenerating axon growing toward its original target in the intermediate zone of gray matter. Blue in a–k represents immunoreactivity of GFAP. Bar = 100 µm for a–c; 50 µm for d–k as shown in f; 100 µm for l–o as shown in l. DNROCK, dominant negative Rho kinase; GFP, green fluorescent protein; GFAP, glial fibrillary acidic protein; LV, lentiviral vector.
Figure 7.
DNROCK promotes axonal sprouting rostral and causal to the site of rubrospinal tract transection. (a) A drawing illustrates the injury site of the spinal cord and the regions of spinal sections used for quantification of GFP-labeled axons. (b) Projection of camera lucida drawings based on three sections from one typical animal from GFP group and (c) DNROCK group schematically show the lesion cavity and the distribution of regenerating axons 10 weeks after injury. (d) Quantification of GFP-labeled rubrospinal axon numbers at both rostral and caudal sides of the lesion cavity. The numbers of axons presented were mean ± SEM from six animals in each group. *P < 0.05, **P < 0.01, ***P < 0.001 via one-way ANOVA for each of the distance index. ANOVA, analysis of variance; DNROCK, dominant negative Rho kinase; GFP, green fluorescent protein.
DNROCK enhances functional recovery
The forelimb use of the rats during spontaneous vertical exploration in a glass cylinder was used to assess the functional recovery of forelimbs after RST transection. Before RST transection, rats in both LV/GFP and LV/DNROCK + LV/GFP-injected groups spontaneously reared and explored the wall of the cylinder using their right (contralateral to lesion site) or left (ipsilateral to lesion site) forelimbs alone, or both forelimbs together (Figure 8a–c). There is no significant difference in their forelimb use before and after injection of LV into red nucleus. At 1 week after left C4 spinal cord dorsolateral funiculotomy, rats in both groups mainly used their right forelimbs (Figure 8a), and the left forelimb (ipsilateral to lesion site) usage was reduced close to nil in both groups (Figure 8b). The usage of both forelimbs was also reduced significantly (Figure 8c). There is no significant difference in forelimb use between the two groups at this time point (Figure 8a–c). Functional recovery in both groups was recorded by the end of the second week after injury and the animals in the DNROCK group showed significantly increased use of both forelimbs and reduced use of right forelimbs than the GFP group at this time point (Figure 8a,c). Five weeks after injury, the usage of left forelimb alone in the DNROCK group became significantly higher than the GFP group (Figure 8b). Using two-way ANOVA to analyze all the time points after injury shows that the animals in the DNROCK group had significantly better recovery of left forelimb use than those in the GFP group (P < 0.0001). The use of both forelimbs in the DNROCK group remained higher than the GFP group from 2 to 10 weeks after injury. Although only the 2 and 4 week points were statistically significant between the two groups when analyzed using Student's t-test, analysis of all time points using two-way ANOVA demonstrates that animals in DNROCK group had significantly more use of both forelimbs than those in GFP group (P < 0.0001). As the use of left forelimbs and both forelimbs gradually increased, the use of right forelimbs alone was gradually reduced in both groups (Figure 8a). Two-way ANOVA of all the time points also shows that animals in the DNROCK group had significantly reduced use of right forelimb alone than those in the GFP group (P < 0.0001). Statistical analysis of all the time points after injury and all the three indexes combined using two-way ANOVA reveals that animals in DNROCK group had significantly better recovery in their forelimb function than those in GFP group (P < 0.0001).
Figure 8.
Expression of DNROCK promotes the recovery of limb functions after rubrospinal tract transection. Percentage of usage of (a) contralateral (right) forelimbs, (b) ipsilateral (left) forelimbs, and (c) both forelimbs in cylinder test. (d) Hindlimb functional recovery was assessed using error index (total slip/step ratio) of horizontal rope crossing test. Two-way ANOVA tests of all the time points after injury combined for each of the four indexes demonstrate significant difference between the DNROCK and GFP groups (P < 0.0001). Student's t-test was used to compare the difference at each time point between the two groups. *P < 0.05, **P < 0.01. Data are expressed as mean ± SEM, n = 6 for each group. ANOVA, analysis of variance; DNROCK, dominant negative Rho kinase; GFP, green fluorescent protein.
Horizontal rope crossing was used to assess the hindlimb function of the rats. After training for 3 weeks, animals crossed the horizontal rope with an occasional minor slip but no falls. The animals were then trained daily after LV injection. Injection itself did not alter the performance of rats on crossing the rope (Figure 8d). From 4 weeks after injury, DNROCK group showed significantly improved hindlimb performance judged by the total error/step ratio, followed by a further gradual improvement until the end of the experiment at 10 weeks after injury. The GFP group recovered at a slower rate and also to a lesser degree compared with the DNROCK group (Figure 8d). Two-way ANOVA reveals that animals in the DNROCK group had a significantly better recovery in their hindlimb function than those in the GFP group (P < 0.0001).
Discussion
Manipulating the RhoA-ROCK signal pathway has been viewed as an effective means to promote axon regeneration after CNS injury. In this study, we targeted the RhoA-ROCK signal pathway by lentiviral delivery of DNROCK to neurons. DNROCK enhanced the neurite outgrowth of DRG neurons on a substrate of inhibitory myelin proteins in vitro. When expressed in neurons in the red nucleus of adult rats, DNROCK promoted growth from the severed RST axons into and around the lesion cavity to the distal spinal cord. This regrowth of RST axons was accompanied by significant functional recovery of the ipsilateral limbs. In the animals receiving LV/GFP injection in the red nucleus, fewer RST axons were observed caudal to the lesion site and the functional recovery was slower and to a lesser degree compared with the DNROCK group.
Because RhoA-ROCK pathway may play a crucial role in mediating growth cone collapse, many attempts have been made to promote axonal regeneration by inhibiting this pathway. The effects of C3 transferase and Y27632 in promoting axonal regeneration have been tested in several in vivo models including optic nerve crush,19 dorsal spinal cord transection of adult mice,20 corticospinal tract injury of adult rats,21 and C4/5 dorsal column transection of adult rats.30 Although C3 treatment failed to promote neurite outgrowth after corticospinal tract lesions in the adult rats,21 all the other experiments showed enhanced axonal sprouting in vivo. The evidence above indicates that blocking the RhoA-ROCK signal pathway promotes regenerative axonal sprouting in vivo to a certain degree. However, long-distance regeneration of these axons was not observed with ROCK inhibition by Y27632 and low doses of Y27632 were detrimental to functional recovery due to its effects on other types of cells in the spinal cord.30 Y27632 treatment was found to activate astrocytes and increase the expression of chondroitin sulfate proteoglycans in the injury site, which counteracts the growth-promoting effects of Y27632.31 The shortcomings of conventional agents, such as low cell-permeability, diffusion, short-term effects, nonspecific effects, and toxicity, can be overcome by gene targeting strategies that can achieve long-term, stable, and cell-specific effects.
The DNROCK construct RB/PH(TT) has been shown to be one of the most potent dominant negative mutants in blocking the RhoA-ROCK pathway.22 This mutant inhibits neurite retraction induced by lysophosphatidic acid in N1E-115 neuroblastoma cells and phosphorylation of myosin light chain by serum in NIH 3T3 cells.22 In this study, we also found that expression of Flag-tagged DNROCK in NIH 3T3 cells significantly reduced the serum-induced formation of stress fibers, which is in agreement with the result obtained from direct injection of maltose-binding protein fused RB/PH(TT) into Madin–Darby canine kidney epithelial cells.23,32
DRG neurons expressed high-level Flag-tagged DNROCK after infection with LV/DNROCK. The expressed DNROCK was distributed in both the somata and neurites of the DRG neurons and promoted neurite growth on laminin substrate. In the presence of myelin proteins, the effects of DNROCK in overcoming the inhibitory effect of myelin proteins were quite significant, which was demonstrated by both longer neurites and higher percentage of neurite-bearing cells in comparison with GFP-expressing neurons. The fact that these results are in agreement with those obtained from the study on embryonic DRG neurons transduced with herpes simplex viral vector carrying myc-tagged DNROCK,25 indicates that the Flag-tagged DNROCK is as effective as the original myc-tagged form in blocking the myelin-associated inhibitors. Further evidence to support the effectiveness of Flag-tagged DNROCK comes from the observation that exposure of DRG neuron growth cones to myelin proteins induced the phosphorylation of CRMP2, a target molecule of ROCK that mediates growth cone collapse, and Flag-DNROCK significantly reduced the myelin-induced pCRMP2 at growth cones.
In the in vivo experiment, we observed much more GFP-labeled RST axons that were close to the rostral border of the lesion cavity in the DNROCK group than in the GFP group, which indicates either the promotion of regrowth or the prevention of retraction of transected axons. Although it has been well documented that inhibition of RhoA-ROCK signal pathway can prevent axonal retraction in vitro,22,33,34 such effect has not been studied in vivo. In the DNROCK group, many GFP-labeled RST axons on the rostral side of the lesion sprouted to the gray matter. Some GFP-labeled axons were seen inside the lesion cavity along the glial scar, indicating that this conventionally prohibitive, debris-filled structure can be overcome to some extent by suppression of the RhoA-ROCK pathway with DNROCK. We did not observe axons that directly grew out of the lesion cavity to the caudal end; instead, the RST axons grew around lesion cavity and extended into the caudal spinal cord. Similarly, Chan et al. observed that no regenerating corticospinal tract and dorsal column tract axons crossed the glial scar in Y27632-treated animals.30 Some axons were seen growing inside the gray matter, which might grow back into white matter, bypassing the lesion site. Recently it was demonstrated that regenerating corticospinal axons could bypass the lesion site by growing through gray matter and descending in ventral columns in mice.35 To this end, we surmise that the axonal sprouts inside the lesion cavity were still impeded by the physical and biochemical barriers, and therefore, speculate that the combined treatments of DNROCK expression in injured neurons with modification of glial scar by a permissive molecule such as polysialic acid36,37 or introduction of genetically modified Schwann cells38 would have a greater effect in promoting the regeneration of CNS axons across the lesion site. Another potential strategy is to coexpress DNROCK with other growth-promoting molecules in injured neurons to synergistically promote regeneration.
Functional recovery of limbs was evident in the LV/DNROCK-injected animals, whereas the recovery from the LV/GFP-injected animals was much slower and to a much lesser degree: these observations indicate that expression of DNROCK in the red nucleus enhanced functional recovery. The functional recovery in the DNROCK group is conceivably partially due to the regenerative sprouting of RST axons as significantly more GFP-labeled axons sprouted toward the gray matter in the rostral side and more GFP-labeled axons were observed in the caudal end of the injury site. In addition to bypass the lesion site via gray matter to reach the distal spinal cord as mentioned above, the sprouts from the transected RST axons may form new circuits with other descending pathways that may contribute to the functional recovery.39 Previous studies of incomplete injury of the spinal cord have shown remarkable spontaneous recovery in rats40,41 and the formation of new intraspinal circuits.42 After corticospinal tract lesions, RST fibers were found to sprout laterally to motor neurons in ventral horns that are normally innervated by the corticospinal tract fibers.39,43 In this study, functional recovery of affected forelimbs and hindlimbs was seen in the LV/GFP-injected group 2 weeks after injury. The spontaneous functional recovery in the control group may be due to the collateral sprouting from other motor tracts to the original target neurons of RST in the lamina V, VI, and dorsal part of lamina VII and IX. The functional recovery of LV/DNROCK-injected group is significantly faster and greater.
The results from this study demonstrate that suppression of the RhoA-ROCK signal pathway in CNS neurons using viral vector delivery of DNROCK can promote the regenerative sprouting of injured axons and facilitate functional recovery. Being one component of future treatment of CNS injury, more detailed studies on other descending motor pathways and other injury models are required to confirm the effectiveness of such a strategy.
Materials and Methods
Production of LVs. The DNROCK construct, RB/PH(TT), of cow ROCK α (also called ROCK-II, p160ROCK) was a gift from Kaibuchi.22,44 The RB/PH(TT) domain was tagged with a 3×Flag sequence at its N-terminal and subcloned into the lentiviral transfer vector, pRRL, with the IRES-GFP sequence to generate pRRL/3Flag-RB/PH(TT)-IRES-GFP (pRRL/3Flag-DNROCK). For neuron-specific expression of DNROCK, the CMV promoter in the original pRRL vector was replaced with a 564 bp hSyn I promoter (from pDrive-synapsin of Invivogen, San Diego, CA). A similar construct was also made for neuron-specific expression of GFP (pRRL/hSyn_GFP). The production of the self-inactivating LVs was based on the protocol of Dull et al.45 The viral particles were concentrated by ultracentrifugation. The titers of the viral vectors were determined by infecting nerve growth factor–induced PC12 cells. LV/hSyn_3Flag-RB/PH(TT)-IRES-GEP is referred as LV/DNROCK and LV/hSyn-GFP as LV/GFP.
Effect of DNROCK on stress fiber formation in NIH 3T3 fibroblast cells. To test whether the 3×Flag tagged DNROCK was effective at inhibiting ROCK function, stress fiber formation in NIH 3T3 fibroblast cells following serum challenge after serum starvation was used—a known ROCK-dependent process.26 NIH 3T3 cells were transfected with either pRRL/3Flag-DNROCK (without IRES-GFP sequence) or pRRL/GFP using Lipofectamine 2000 (Invitrogen, Paisley, UK). Twenty-four hours after transfection, the cells were starved from serum for 48 hours and then exposed to medium containing 10% of fetal bovine serum for 1 hour. To detect F-actin, the cells were fixed and stained with rhodamine conjugated phalloidin (Molecular Probes, Invitrogen) according to the manufacturer's instruction. The expression of Flag-tagged DNROCK was detected by immunostaining using a Sigma monoclonal anti-Flag M2 antibody diluted 1:500 for 2 hours at 37 °C, followed by application of fluorescein isothiocyanate -conjugated anti-mouse immunoglobin G antibody at 1:1,000 for 45 minutes at 37 °C.
Preparation of solubilized CNS myelin proteins. To challenge outgrowing axons with a more complex inhibitory environment, dissociated myelin proteins were used as growth substrates for adult rat DRG neurons. Myelin was prepared from the corpus callosum of a piglet brain based on a standard protocol.46 The myelin pellets were resuspended in solubilization buffer [0.2 mol/l sodium phosphate buffer (pH 6.8), 0.1 mol/l Na2SO4, 1 mmol/l EDTA, 1 mmol/l dithiothreitol, Complete protease inhibitors (Roche Diagnostics, Burgess Hill, UK), and 1% octyl β–D-glucoside (Sigma-Aldrich, Poole, UK)]. The sample was rotated at 4 °C overnight and centrifuged in a Sorvall TH641 rotor at 32,000 g for 1 hour at 4 °C. The supernatant was dialyzed against phosphate buffered saline for 4 hours. The protein concentration was measured using DC protein assay kit (Bio-Rad Laboratories, Hemel Hempstead, UK) with bovine serum albumin (BSA) as standard.
Neurite outgrowth assay. All the culture surfaces for the neurite outgrowth assays were precoated with poly-L-lysine (0.01%; Sigma-Aldrich) before other coating was applied. To prepare dissociated DRG neurons, DRGs from adult Wister rats (~250 g) were digested with 0.125% collagenase (type XI; Sigma-Aldrich) and triturated in 1 ml BSF2 medium [F-12/DMEM, 1× N2 supplement (Invitrogen), 0.3% BSA (Sigma-Aldrich), and 1× penicillin/streptomycin mixture (Invitrogen)] and centrifuged through a cushion of 2 ml 15% BSA at 100g for 5 minutes. The cell pellet was resuspended in BSF2 and the dissociated neurons were plated at 1,000 cells/well in eight well-chamber slides precoated with a mixture of myelin proteins (40 µg/well) and laminin (0.1 µg/well; Invitrogen) or laminin (0.1 µg/well) only as control and cultured at 37 °C with 95% air and 5% CO2. After the cells had been allowed to settle down for 1 hour, LV/DNROCK or LV/GFP was added at a final concentration of 106 transduction units/ml.
Neurons were fixed with 4% paraformaldehyde 40 hours later and immunostained with either polyclonal anti-protein gene product 9.5 antibody (1:400; Serotec, Oxford, UK) for LV/GFP transduced cells, or antibodies against protein gene product 9.5 and Flag tag (monoclonal anti-Flag M2, 1:100; Sigma-Aldrich) for LV/DNROCK transduced cells at 4 °C overnight. Alexa Fluor 488- or 568-conjugated goat anti-rabbit or anti-mouse immunoglobin G (1:400; Invitrogen) were then applied for 2 hours at room temperature. The diameter of soma and the length of the longest neurite of each neuron were measured for LV/GFP transduced neurons (GFP+) or LV/DNROCK transduced neurons (Flag+) with ImageJ (Rasband, WS, http://rsbweb.nih.gov/ij/). Neurons with neurites longer than the diameter of their own cell body were recorded as neurite-bearing neurons and the percentage of neurite-bearing neurons were counted. Neurite lengths of about 200 viral vector transduced cells (both with and without neurites) from each group were measured and the experiments were repeated four times.
To investigate the effect of DNROCK on the downstream target of ROCK in viral vector transduced DRG neurons, another set of cells was immunostained with antibodies against phosphorylated CRMP2 [polyclonal phosphoCRMP2 (Thr514) antibody, 1:200; Abcam, Cambridge, UK] and Flag tag. Images were captured using a Zeiss confocal microscope (Carl Zeiss, Gena, Germany) at the same exposure and scanning parameters. Mean fluorescence intensities at the growth cones of ~30 neurons from each group were measured with ImageJ and the corresponding background signals were subtracted.
Immunoblotting assay. Transduced DRG neurons were lysed in buffer A [20 mmol/l Tris–HCl, 150 mmol/l NaCl, 2 mmol/l EDTA, 0.1 mmol/l ethylene glycol tetraacetic acid, 1% Triton X-100, 0.5% deoxyocholine with complete protease inhibitors (Roche Diagnostics)]. Samples were centrifuged at 13,000 g for 15 minutes and the protein concentration in supernatants was determined using DC Protein Assay Kit (Bio-Rad). Twenty micrograms of proteins was subjected to 10% sodium dodecyl sulfate polyacrylamide gel electrophoresis using dual color protein standards (Bio-Rad) as markers. After protein transfer, the polyvinylidene difluoride membrane was blocked with 5% nonfat milk in Tris-buffered saline + 0.1% Tween-20 for 1 hour. The membranes were probed with monoclonal anti-Flag antibody (1:1,000, Sigma-Aldrich) overnight at 4 °C. Immunosignals were detected using the ECL Plus kit (GE Healthcare, Chalfont St Giles, United Kingdom).
LV injection to red nucleus and transection of RST. All animal experiments were conducted in accordance with the Animals (Scientific Procedures) Act 1986 of the UK government. Female Wister rats (200–250 g) were anesthetized with isoflurane and oxygen, positioned on a stereotaxic apparatus, and a burr hole was made according to the coordinates from the rat brain atlas of Paxinos and Watson.47 The coordinates for the injection were 6.0 mm caudal from the bregma, 0.7 mm from the midline, and 7.0 mm from the dura matter of the brain. One microliter of LV was injected into the right red nucleus using a 33 gauge needle at a speed of 200 nl/minute controlled by UltraMicroPump II (World Precision Instruments, Sarasota, FL). The needle was left in place for 5 minutes and gradually withdrawn over 2–3 minutes to allow diffusion of viral vector from the injection site and to prevent leakage from the needle track. One group of rats (n = 10) was injected with LV/GFP (1 × 107 transduction units) as control while the other group (n = 10) was injected with a mixture of LV/DNROCK and LV/GFP (1 × 107 transduction units for each vector) for easy identification of transduced neurons and their axons by GFP fluorescence.
One-week later, unilateral C4 cervical spinal cord dorsolateral funiculotomy, involving the RST, was performed as previously described.48,49 In brief, rats were anesthetized as described above. The spinal cord was exposed via dorsal laminectomy of the fourth cervical vertebra and the dura was opened. Subsequently, the left dorsolateral funiculus of the spinal cord was transected with a pair of microscissors to a depth of 1 mm from the dorsal surface, and a 25 gauge needle was used to plough the lesion site to assure the complete transection of RST. Muscles and skin were then sutured in separate layers.
Behavioral test. Examination of forelimb use during spontaneous vertical exploration is a sensitive test to monitor limb use asymmetries.48 The rats were placed in a clear glass cylinder (20 cm in diameter and 30 cm in depth) for 5 minutes and the testing session was videotaped. The usage of the right forelimb (unimpaired, contralateral), left forelimb (impaired, ipsilateral), and both forelimbs for contacting the wall of the cylinder during vertical exploration was scored blindly by another researcher at a later date. The results were expressed as a percentage of usage of the left, right, or both forelimbs relative to the total number of forelimb usage.
Function of hindlimbs was assayed by crossing a 1.25-m-long rope (4 cm in diameter) in accordance with previous reports.49,50 The number of slips during rope crossing was counted and the ratio of slips versus total steps in two runs at each time point for each animal was calculated and presented as an error index. We defined the ratio as 1 whenever the rat fell.
For both forelimb and hindlimb function assays, the rats were trained 3 weeks before surgery and baseline behavior measurements were obtained. The rats were tested 3 days after the RST transection; two rats from each group were excluded from further studies as they showed no significant loss of left limb function signifying incomplete transection of the RST in these animals. This was confirmed on histological examination. All the remaining rats were tested weekly from 1 to 10 weeks after left RST transection. The animals were subsequently killed and the lesion sites in the spinal cords were examined. Two rats from each group with lesions larger than a quarter of the dorsolateral spinal cord were excluded from the final statistical analysis for the limb functions as other descending tracts were affected in these animals. The calculated percentage use of right forelimbs alone, left forelimbs alone, and both forelimbs, and error index for hindlimbs were analyzed for differences between the LV/DNROCK and the LV/GFP groups using two-way ANOVA. Differences at individual time points between the two groups were analyzed using Student's t-test.
Immunohistochemistry and quantification of RST axon numbers. After being deeply anesthetized, animals were perfused with 4% paraformaldehyde. The midbrain and spinal cord segments containing the lesion site were dissected, postfixed in 4% paraformaldehyde, and soaked in 30% sucrose solution. Each spinal cord was divided into three blocks, a 10 mm lesion block centered at the lesion site (“lesion block”), and one 5 mm block at each end of the lesion block (rostral and caudal blocks). Horizontal sections of 15 µm in thickness were cut for the lesion block and mounted in series, while transverse sections were cut for both the rostral and caudal blocks of the spinal cord as well as the midbrains (see Figure 7a).
In LV/GFP-injected group, the expression of GFP in the red nucleus and in RST could be viewed directly under a fluorescence microscope. For confirmation of the neuron-specific expression of the transgenes, sections of midbrain were immunostained with mouse anti-NeuN antibody (1:500; Millipore, Watford, United Kingdom). To assess the coexpression of DNROCK and GFP in the neurons in the red nucleus and their axons in the spinal cord, sections of midbrain and cervical spinal cord from animals injected with LV/DNROCK and LV/GFP were incubated with mouse anti-Flag M2 antibody (1:250; Sigma-Aldrich) for two nights at 4 °C, followed by incubation with biotinylated horse anti-mouse immunoglobin G (1:400) in 3% BSA/Tris-buffered saline + 0.1% Triton X-100 for 2 hours at room temperature. Sections were then incubated in avidin–biotin peroxidase complex from an ABC kit (Vector Laboratories, Burlingame, CA) for 1.5 hours at room temperature, followed by incubation in cyanine 3 tyramide solution (1:75; PerkinElmer Life and Analytical Sciences, Waltham, MA) for 10 minutes. For identification of astrocytes and glial scar, spinal sections were incubated with mouse anti-glial fibrillary acidic protein (1:1,000; Millipore) overnight at 4 °C, followed by Alexa Fluor 350 conjugated goat anti-mouse immunoglobin G (1:400; Invitrogen) in 3% BSA/Tris-buffered saline + 0.1% Triton X-100 for one night at 4 °C.
For quantification of regenerating axons, six animals from each group, which were used for analysis of functional recovery, were included. In these animals GFP-labeled axons in the white matters were counted in 6–7 horizontal sections containing all RST axons at 45 µm intervals and in one transverse section 5 mm caudal to the lesion site. The number of axons was presented into five distance indexes as illustrated in Figure 7a: rostral part (4–5 mm to the lesion center), rostral border of the lesion site (within 1.5 mm rostral to the lesion center), the lesion site, and caudal part (within 5 mm caudal to the lesion centre) on horizontal sections, and at 5 mm caudal to the lesion centre on transverse sections. The number of axons in the rostral part on horizontal sections or in transverse sections 5 mm rostral to the lesion center was used to evaluate the transduction efficiency on the assumption that the total number of neurons in the red nucleus is 3,000.48 The data are presented as mean ± SEM. One-way ANOVA was performed to analyze the differences between LV/DNROCK and LV/GFP-injected groups.
Acknowledgments
This work was supported by a grant from the Barts and the London Charity to X.B. and Y.Z. Y. Z. was supported by the Wellcome Trust and Stryker Corporation. We thank Kozo Kaibuchi (Nagoya University, Japan) for providing the dominant negative mutant of Rho kinase.
REFERENCES
- Filbin MT. Myelin-associated inhibitors of axonal regeneration in the adult mammalian CNS. Nat Rev Neurosci. 2003;4:703–713. doi: 10.1038/nrn1195. [DOI] [PubMed] [Google Scholar]
- Sandvig A, Berry M, Barrett LB, Butt A., and , Logan A. Myelin-, reactive glia-, and scar-derived CNS axon growth inhibitors: expression, receptor signaling, and correlation with axon regeneration. Glia. 2004;46:225–251. doi: 10.1002/glia.10315. [DOI] [PubMed] [Google Scholar]
- Yiu G., and , He Z. Glial inhibition of CNS axon regeneration. Nat Rev Neurosci. 2006;7:617–627. doi: 10.1038/nrn1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie F., and , Zheng B. White matter inhibitors in CNS axon regeneration failure. Exp Neurol. 2008;209:302–312. doi: 10.1016/j.expneurol.2007.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon BK, Fisher CG, Dvorak MF., and , Tetzlaff W.2005Strategies to promote neural repair and regeneration after spinal cord injury Spine 3017 suppl.): S3–S13. [DOI] [PubMed] [Google Scholar]
- Thuret S, Moon LD., and , Gage FH. Therapeutic interventions after spinal cord injury. Nat Rev Neurosci. 2006;7:628–643. doi: 10.1038/nrn1955. [DOI] [PubMed] [Google Scholar]
- Bradbury EJ, Moon LD, Popat RJ, King VR, Bennett GS, Patel PN, et al. Chondroitinase ABC promotes functional recovery after spinal cord injury. Nature. 2002;416:636–640. doi: 10.1038/416636a. [DOI] [PubMed] [Google Scholar]
- Liebscher T, Schnell L, Schnell D, Scholl J, Schneider R, Gullo M, et al. Nogo-A antibody improves regeneration and locomotion of spinal cord-injured rats. Ann Neurol. 2005;58:706–719. doi: 10.1002/ana.20627. [DOI] [PubMed] [Google Scholar]
- GrandPré T, Li S., and , Strittmatter SM. Nogo-66 receptor antagonist peptide promotes axonal regeneration. Nature. 2002;417:547–551. doi: 10.1038/417547a. [DOI] [PubMed] [Google Scholar]
- Fournier AE, Gould GC, Liu BP., and , Strittmatter SM. Truncated soluble Nogo receptor binds Nogo-66 and blocks inhibition of axon growth by myelin. J Neurosci. 2002;22:8876–8883. doi: 10.1523/JNEUROSCI.22-20-08876.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikolic M. The role of Rho GTPases and associated kinases in regulating neurite outgrowth. Int J Biochem Cell Biol. 2002;34:731–745. doi: 10.1016/s1357-2725(01)00167-4. [DOI] [PubMed] [Google Scholar]
- McKerracher L., and , Higuchi H. Targeting Rho to stimulate repair after spinal cord injury. J Neurotrauma. 2006;23:309–317. doi: 10.1089/neu.2006.23.309. [DOI] [PubMed] [Google Scholar]
- Lingor P, Teusch N, Schwarz K, Mueller R, Mack H, Bähr M, et al. Inhibition of Rho kinase (ROCK) increases neurite outgrowth on chondroitin sulphate proteoglycan in vitro and axonal regeneration in the adult optic nerve in vivo. J Neurochem. 2007;103:181–189. doi: 10.1111/j.1471-4159.2007.04756.x. [DOI] [PubMed] [Google Scholar]
- Mueller BK, Mack H., and , Teusch N. Rho kinase, a promising drug target for neurological disorders. Nat Rev Drug Discov. 2005;4:387–398. doi: 10.1038/nrd1719. [DOI] [PubMed] [Google Scholar]
- Etienne-Manneville S., and , Hall A. Rho GTPases in cell biology. Nature. 2002;420:629–635. doi: 10.1038/nature01148. [DOI] [PubMed] [Google Scholar]
- Hu F., and , Strittmatter SM. Regulating axon growth within the postnatal central nervous system. Semin Perinatol. 2004;28:371–378. doi: 10.1053/j.semperi.2004.10.001. [DOI] [PubMed] [Google Scholar]
- Ellezam B, Dubreuil C, Winton M, Loy L, Dergham P, Sellés-Navarro I, et al. Inactivation of intracellular Rho to stimulate axon growth and regeneration. Prog Brain Res. 2002;137:371–380. doi: 10.1016/s0079-6123(02)37028-6. [DOI] [PubMed] [Google Scholar]
- Schwab JM, Brechtel K, Mueller CA, Failli V, Kaps HP, Tuli SK, et al. Experimental strategies to promote spinal cord regeneration--an integrative perspective. Prog Neurobiol. 2006;78:91–116. doi: 10.1016/j.pneurobio.2005.12.004. [DOI] [PubMed] [Google Scholar]
- Lehmann M, Fournier A, Selles-Navarro I, Dergham P, Sebok A, Leclerc N, et al. Inactivation of Rho signaling pathway promotes CNS axon regeneration. J Neurosci. 1999;19:7537–7547. doi: 10.1523/JNEUROSCI.19-17-07537.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dergham P, Ellezam B, Essagian C, Avedissian H, Lubell WD., and , McKerracher L. Rho signaling pathway targeted to promote spinal cord repair. J Neurosci. 2002;22:6570–6577. doi: 10.1523/JNEUROSCI.22-15-06570.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fournier AE, Takizawa BT., and , Strittmatter SM. Rho kinase inhibition enhances axonal regeneration in the injured CNS. J Neurosci. 2003;23:1416–1423. doi: 10.1523/JNEUROSCI.23-04-01416.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amano M, Chihara K, Nakamura N, Fukata Y, Yano T, Shibata M, et al. Myosin II activation promotes neurite retraction during the action of Rho and Rho-kinase. Genes Cells. 1998;3:177–188. doi: 10.1046/j.1365-2443.1998.00181.x. [DOI] [PubMed] [Google Scholar]
- Amano M, Chihara K, Nakamura N, Kaneko T, Matsuura Y., and , Kaibuchi K. The COOH terminus of Rho-kinase negatively regulates rho-kinase activity. J Biol Chem. 1999;274:32418–32424. doi: 10.1074/jbc.274.45.32418. [DOI] [PubMed] [Google Scholar]
- Amano M, Fukata Y., and , Kaibuchi K. Regulation and functions of Rho-associated kinase. Exp Cell Res. 2000;261:44–51. doi: 10.1006/excr.2000.5046. [DOI] [PubMed] [Google Scholar]
- Alabed YZ, Grados-Munro E, Ferraro GB, Hsieh SH., and , Fournier AE. Neuronal responses to myelin are mediated by rho kinase. J Neurochem. 2006;96:1616–1625. doi: 10.1111/j.1471-4159.2006.03670.x. [DOI] [PubMed] [Google Scholar]
- Ridley AJ., and , Hall A. Signal transduction pathways regulating Rho-mediated stress fibre formation: requirement for a tyrosine kinase. EMBO J. 1994;13:2600–2610. doi: 10.1002/j.1460-2075.1994.tb06550.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riento K., and , Ridley AJ. Rocks: multifunctional kinases in cell behaviour. Nat Rev Mol Cell Biol. 2003;4:446–456. doi: 10.1038/nrm1128. [DOI] [PubMed] [Google Scholar]
- Fischer D, He Z., and , Benowitz LI. Counteracting the Nogo receptor enhances optic nerve regeneration if retinal ganglion cells are in an active growth state. J Neurosci. 2004;24:1646–1651. doi: 10.1523/JNEUROSCI.5119-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yip PK, Wong LF, Pattinson D, Battaglia A, Grist J, Bradbury EJ, et al. Lentiviral vector expressing retinoic acid receptor beta2 promotes recovery of function after corticospinal tract injury in the adult rat spinal cord. Hum Mol Genet. 2006;15:3107–3118. doi: 10.1093/hmg/ddl251. [DOI] [PubMed] [Google Scholar]
- Chan CC, Khodarahmi K, Liu J, Sutherland D, Oschipok LW, Steeves JD, et al. Dose-dependent beneficial and detrimental effects of ROCK inhibitor Y27632 on axonal sprouting and functional recovery after rat spinal cord injury. Exp Neurol. 2005;196:352–364. doi: 10.1016/j.expneurol.2005.08.011. [DOI] [PubMed] [Google Scholar]
- Chan CC, Wong AK, Liu J, Steeves JD., and , Tetzlaff W. ROCK inhibition with Y27632 activates astrocytes and increases their expression of neurite growth-inhibitory chondroitin sulfate proteoglycans. Glia. 2007;55:369–384. doi: 10.1002/glia.20466. [DOI] [PubMed] [Google Scholar]
- Kawano Y, Fukata Y, Oshiro N, Amano M, Nakamura T, Ito M, et al. Phosphorylation of myosin-binding subunit (MBS) of myosin phosphatase by Rho-kinase in vivo. J Cell Biol. 1999;147:1023–1038. doi: 10.1083/jcb.147.5.1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katoh H, Aoki J, Ichikawa A., and , Negishi M. p160 RhoA-binding kinase ROKalpha induces neurite retraction. J Biol Chem. 1998;273:2489–2492. doi: 10.1074/jbc.273.5.2489. [DOI] [PubMed] [Google Scholar]
- Thies E., and , Davenport RW. Independent roles of Rho-GTPases in growth cone and axonal behavior. J Neurobiol. 2003;54:358–369. doi: 10.1002/neu.10135. [DOI] [PubMed] [Google Scholar]
- Steward O, Zheng B, Tessier-Lavigne M, Hofstadter M, Sharp K., and , Yee KM. Regenerative growth of corticospinal tract axons via the ventral column after spinal cord injury in mice. J Neurosci. 2008;28:6836–6847. doi: 10.1523/JNEUROSCI.5372-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Ghadiri-Sani M, Zhang X, Richardson PM, Yeh J., and , Bo X. Induced expression of polysialic acid in the spinal cord promotes regeneration of sensory axons. Mol Cell Neurosci. 2007;35:109–119. doi: 10.1016/j.mcn.2007.02.011. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Zhang X, Wu D, Verhaagen J, Richardson PM, Yeh J, et al. Lentiviral-mediated expression of polysialic acid in spinal cord and conditioning lesion promote regeneration of sensory axons into spinal cord. Mol Ther. 2007;15:1796–1804. doi: 10.1038/sj.mt.6300220. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Zhang X, Yeh J, Richardson P., and , Bo X. Engineered expression of polysialic acid enhances Purkinje cell axonal regeneration in L1/GAP-43 double transgenic mice. Eur J Neurosci. 2007;25:351–361. doi: 10.1111/j.1460-9568.2007.05311.x. [DOI] [PubMed] [Google Scholar]
- Raineteau O., and , Schwab ME. Plasticity of motor systems after incomplete spinal cord injury. Nat Rev Neurosci. 2001;2:263–273. doi: 10.1038/35067570. [DOI] [PubMed] [Google Scholar]
- Noble LJ., and , Wrathall JR. Correlative analyses of lesion development and functional status after graded spinal cord contusive injuries in the rat. Exp Neurol. 1989;103:34–40. doi: 10.1016/0014-4886(89)90182-9. [DOI] [PubMed] [Google Scholar]
- Basso DM, Beattie MS., and , Bresnahan JC. Graded histological and locomotor outcomes after spinal cord contusion using the NYU weight-drop device versus transection. Exp Neurol. 1996;139:244–256. doi: 10.1006/exnr.1996.0098. [DOI] [PubMed] [Google Scholar]
- Bareyre FM, Kerschensteiner M, Raineteau O, Mettenleiter TC, Weinmann O., and , Schwab ME. The injured spinal cord spontaneously forms a new intraspinal circuit in adult rats. Nat Neurosci. 2004;7:269–277. doi: 10.1038/nn1195. [DOI] [PubMed] [Google Scholar]
- Raineteau O, Fouad K, Bareyre FM., and , Schwab ME. Reorganization of descending motor tracts in the rat spinal cord. Eur J Neurosci. 2002;16:1761–1771. doi: 10.1046/j.1460-9568.2002.02243.x. [DOI] [PubMed] [Google Scholar]
- Amano M, Chihara K, Kimura K, Fukata Y, Nakamura N, Matsuura Y, et al. Formation of actin stress fibers and focal adhesions enhanced by Rho-kinase. Science. 1997;275:1308–1311. doi: 10.1126/science.275.5304.1308. [DOI] [PubMed] [Google Scholar]
- Dull T, Zufferey R, Kelly M, Mandel RJ, Nguyen M, Trono D, et al. A third-generation lentivirus vector with a conditional packaging system. J Virol. 1998;72:8463–8471. doi: 10.1128/jvi.72.11.8463-8471.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norton WT., and , Poduslo SE. Myelination in rat brain: method of myelin isolation. J Neurochem. 1973;21:749–757. doi: 10.1111/j.1471-4159.1973.tb07519.x. [DOI] [PubMed] [Google Scholar]
- Paxinos G., and , Watson C.1998The Rat Brain in Stereotaxic Coordinates4th edn., Academic Press: New York [Google Scholar]
- Liu Y, Kim D, Himes BT, Chow SY, Schallert T, Murray M, et al. Transplants of fibroblasts genetically modified to express BDNF promote regeneration of adult rat rubrospinal axons and recovery of forelimb function. J Neurosci. 1999;19:4370–4387. doi: 10.1523/JNEUROSCI.19-11-04370.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruitenberg MJ, Plant GW, Hamers FP, Wortel J, Blits B, Dijkhuizen PA, et al. Ex vivo adenoviral vector-mediated neurotrophin gene transfer to olfactory ensheathing glia: effects on rubrospinal tract regeneration, lesion size, and functional recovery after implantation in the injured rat spinal cord. J Neurosci. 2003;23:7045–7058. doi: 10.1523/JNEUROSCI.23-18-07045.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim D, Schallert T, Liu Y, Browarak T, Nayeri N, Tessler A, et al. Transplantation of genetically modified fibroblasts expressing BDNF in adult rats with a subtotal hemisection improves specific motor and sensory functions. Neurorehabil Neural Repair. 2001;15:141–150. doi: 10.1177/154596830101500207. [DOI] [PubMed] [Google Scholar]