Abstract
Physicochemical properties of gene transfer vectors play an important role in both transduction efficiency and biodistribution following airway delivery. Adeno-associated virus (AAV) vectors are currently used in many gene transfer applications; however, the respiratory epithelium remains a challenging target. We synthesized two cationic sterol-based lipids, dexamethasone-spermine (DS) and disubstituted spermine (D2S) for pulmonary gene targeting. Scanning and transmission electron micrographs (TEM) confirmed that AAV/lipid formulations produced submicron-sized clusters. When AAV2/9 or AAV2/6.2 were formulated with these cationic lipids, the complexes had positive zeta potential (ζ) and the transduction efficiency in cultured A549 cells increased by sevenfold and sixfold, respectively. Transduction of cultured human airway epithelium with AAV2/6.2-lipid formulations also showed approximately twofold increase in green fluorescence protein (GFP) positive cells as quantified by flow cytometry. Intranasal administration of 1011 genome copies (GC) of AAV2/9 and AAV2/6.2 coformulated with lipid formulations resulted in an average fourfold increase in transgene expression for both vectors. Formulation of AAV2/9 with DS changed the tropism of this vector for the alveolar epithelium, resulting in successful transduction of conducting airway epithelium. Our results suggest that formulating AAV2/9 and AAV2/6.2 with DS and D2S can lead to improved physicochemical characteristics for in vivo gene delivery to lung.
Introduction
Gene transfer studies for the treatment of airway diseases such as cystic fibrosis (CF) and α-1-antitrypsin deficiency1,2,3,4 have demonstrated that the respiratory epithelium remains a challenging target due to a number of natural extracellular barriers including mucociliary clearance, the glycocalyx, and a slow rate of luminal endocytosis.5 Viral-mediated transduction offers favorable characteristics for pulmonary gene delivery based on the high efficiency, specificity, and potential for long-term transgene expression when compared to nonviral methods. One challenge related to viral-mediated gene transfer is targeting viral vectors to the intended cell population. Although improvements in tropism have been achieved by several technological advances, including pseudotyping6 and capsid modifications,7,8 many viral vectors are still characterized by low gene transfer efficiency in the target cell population. In addition, viral delivery vectors may induce an inflammatory response leading to upregulation of cytokines and chemokines that have been linked to low gene transfer in CF tracheal gland cells9 and generation of neutralizing antibodies (NABs), which can inhibit repeated administration.10 In addition to these obstacles pre-existing NABs to viral vectors may also limit vector transduction.
Adeno-associated viruses (AAVs) are icosahedral nonenveloped single-stranded DNA viruses that belong to the parvovirus family with surface capsids of ~22 nm in diameter.11 AAV vectors are among the most commonly used viral vectors in gene therapy applications due to their nonpathogenic nature, nonintegrating episomal expression, potential for long-term transgene expression, and low-inflammatory response.12 Initial observations of AAV2 infectivity across a wide range of cell types indicated the receptor heparan sulfate proteoglycan plays an important part in determining transduction efficiency although coreceptors, such as fibroblast growth factor 1 and αvβ5 integrin, as well as laminin receptor, have also been shown to be involved.13 Despite recent reports describing distinct tropisms and cellular receptors for several AAV capsids, specific receptors have not been defined for all serotypes and optimization of gene expression in the target cell population for the treatment of airway disease is still necessary.14,15
In an effort to obviate the need for modification of the virus capsid, several approaches have been tested to optimize the efficiency of viral transduction in vivo. Prior studies using adenovirus complexes with both cationic polymers and lipids demonstrated increased efficiency in vitro and in vivo and provided evidence that noncovalent capsid association can dramatically alter the vector properties.16,17,18,19 Additional studies have shown improvements in transduction by incorporating adenovirus in calcium phosphate precipitates20 and targeting adenovirus and AAV to the airway epithelium by coformulating with the cationic lipid DS.20,21 Surface modification of AAV2 with polyethylene glycol has also been attempted to prevent NABs,22 but to our knowledge, no studies reporting transduction efficiency and effects on tropism have been reported for other AAV serotypes by noncovalent surface association.
In the present study, we assessed the interaction of two cationic sterol-based lipids, dexamethasone-spermine (DS) and disubstituted spermine (D2S), with two AAV vectors (Supplementary Figure S1; AAV2/6.2 and AAV2/9) for in vitro and in vivo airway gene transfer. Interaction of these cationic molecules with the viral capsid surfaces of the vectors were expected to change the cellular attachment and internalization of vector particles. Helper lipids were also used to assess the impact of liposomal formulations. The unique tropisms of these two viral vectors, AAV2/9 to the alveolar epithelium only15 and AAV2/6.2 to the conducting airway/alveolar epithelium,23 allowed us to assess changes in both tropism and transduction efficiency in vivo. Based on the previously demonstrated anti-inflammatory effect of DS coformulations in vivo21 and the potent antibacterial activity of D2S (D.E. Fein, R. Bucki, F. Byfield, P.A. Janmey and S.L. Diamond, unpublished results), these two lipids were compared since both would be beneficial in reducing the pre-existing inflammation of CF lungs.
Results
Biophysical characterization of AAV2/9 and AAV2/6.2 formulated with cationic lipids
The zeta potential (ζ), a measure of net surface charge density, for AAV2 capsid has been reported to be −9.2 mV (ref. 24). In order to determine the relative binding strength of AAV2/9 and AAV2/6.2 capsids for cationic lipids, we determined the electrophoretic mobility in solution and calculated ζ for both vectors. The ζ for AAV particles were found to be −20 mV for AAV2/9 and −13 mV for AAV2/6.2. The size of vector particles were also measured concurrently with the ζ to ensure that the shifts in electrophoretic mobility were for individual viral particles, and was found to be within the expected range for both AAV vectors of ~22–25 nm. Addition of the cationic lipids DS and D2S to the AAV vector preparations initially resulted in a transition to less negative charge densities and continued addition led to the formation of larger particles with positive ζ up to +20 mV (Supplementary Figure S2). This response of the AAV particles to cationic lipid addition is consistent with an electrostatic interaction between the lipids and the viral capsids.
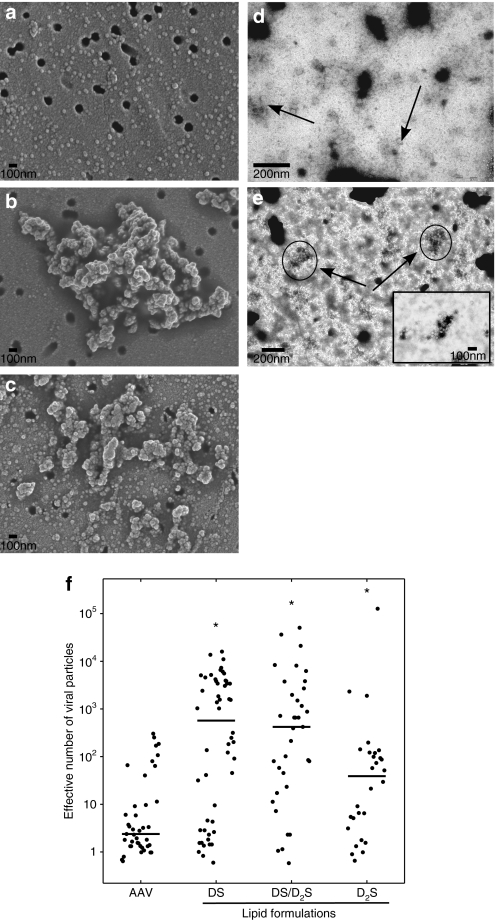
Electron microscopy was used to visualize the effects of cationic lipid coformulations with AAV vectors. AAV vector clusters were observed in the presence of DS, D2S, and mixture formulations using both scanning electron microscopy (SEM) with AAV2/9 and transmission electron micrographs (TEM) with AAV2/6.2 (Figure 1); however, individual particles were also observed in all samples of both vectors analyzed. Both SEM and TEM showed similar effects on AAV vectors with changes in lipid composition suggesting that the interaction of cationic lipids with AAV vectors is not serotype specific. Projected areas were calculated and used to assess relative size of cluster formation. Addition of DS to AAV2/9 (Figure 1b) yielded clusters with an average of ~1,000 effective AAV vectors per cluster. Formulation of D2S with AAV2/9 resulted in smaller clusters (Figure 1c) with an average of ~100 effective AAV vectors per cluster, whereas a mixture of DS/D2S with AAV2/9 (data not shown) and AAV2/6.2 (Figure 1a) resulted in clusters of intermediate size to the individual lipids.
Figure 1.
Coformulation of AAV with cationic lipids results in formation of virion clusters. (a) Scanning electron micrographs of AAV2/9, (b) AAV2/9 coformulated with DS, and (c) AAV2/9 coformulated with D2S. (d) Transmission electron micrographs of AAV2/6.2, and (e) AAV2/6.2 coformulated with 60/40 mol% DS/D2S with DOPE. All samples with cationic lipids contain ~7 × 10−14 µmol/GC and were incubated with AAV particles for 15 minutes followed by the preparation procedure for microscopy described in the materials and methods. (f) Surface areas of clusters were calculated from multiple micrographs for AAV2/9. The bar represents the mean value from at least 28 measurements for each condition. All coformulations resulted in statistical increases in effective particle size with respect to AAV2/9 control calculated using the Mann–Whitney U-test (*P < 0.01). AAV, adeno-associated virus; DS, dexamethasone-spermine; D2S, disubstituted spermine; GC, genome copy.
Transduction efficiency of AAV vectors coformulated with cationic lipids in cultured epithelial cells
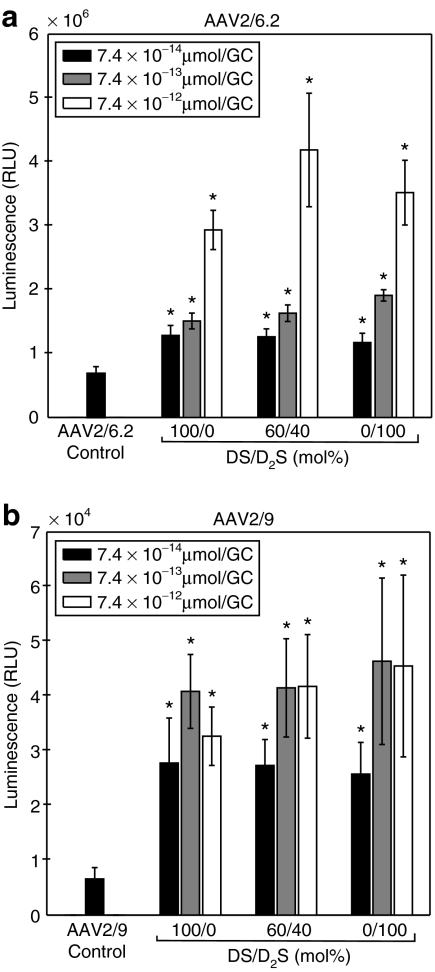
Transduction efficiency of AAV2/9 and AAV2/6.2 in A549 cells was improved when vectors were coformulated with DS and D2S compared to control as shown in Figure 2. Coformulations were prepared with and without 1,2 dioleoylphosphatidylethanolamine (DOPE) and no significant differences were observed for any conditions. For AAV2/9, we did not observe a dose-dependent transgene expression response to lipid concentration, as no significant differences were observed for coformulation with >7.4 × 10−13 µmol/genome copy (GC). The transduction efficiency of AAV2/9 did not vary with the coformulation lipid composition. However, all cationic lipid coformulations with AAV2/9 did result in significant increases in transgene expression with a maximum sevenfold improvement for D2S at the highest lipid concentration (P < 0.01, Mann–Whitney U-test). A dose-dependent transgene expression response to lipid concentration was observed for AAV2/6.2 at all lipid compositions tested and all cationic lipid coformulations resulted in significant increases in transgene expression (P < 0.01, Mann–Whitney U-test for all experimental conditions). The peak transgene expression for AAV2/6.2 was observed with DS liposomes alone (twofold) at the lowest lipid dose; however, the maximum signal shifted to include formulation with D2S for higher total lipid concentrations (sixfold increase with 60/40 mol% DS/D2S: DOPE at 7.4 × 10−12 µmol/GC).
Figure 2.
Transduction efficiency in A549 cells with DS and D2S liposomes (1:1 molar ratio with DOPE) at three charge ratios with ffluc2 transgene at 48 hours after exposure to AAV. (a) Coformulations with AAV2/6.2, (b) Coformulations with AAV2/9. Results presented as the mean and error bars represent SD from 48 replicates for control and 8 replicates of each experimental condition. All coformulations resulted in statistical increases in luminescence with respect to the positive control (AAV alone) as calculated using the Mann–Whitney U-test (*P < 0.01). AAV, adeno-associated virus; DS, dexamethasone-spermine; D2S, disubstituted spermine; ffluc2, firefly luciferase; RLU, relative light unit.
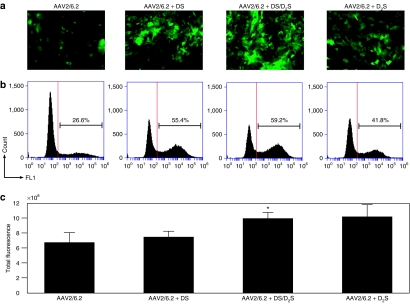
Normal human airway epithelial cells grown on plastic were used to assess the potential for increased transduction. Representative fluorescent images were taken of each replicate (Figure 3a). We found that transduction efficiency was improved with both DS and a 60/40 mol% mixture of DS/D2S coformulated with AAV2/6.2 resulting in greater than twofold increases in gated positive cells compared to AAV2/6.2 alone (Figure 3b). Coformulation with D2S also increased transduction efficiency by 50% over control, indicating that DS and D2S liposomes generate differences in vector surface interaction in cultured cells. A greater number of cells express GFP when treated with AAV2/6.2 coformulated with both cationic lipids, which is important considering the effectiveness of AAV2/6.2 in transducing normal airway epithelial cells in this system.
Figure 3.
Transduction efficiency of AAV2/6.2 expressing GFP transgene coformulated with DS and D2S in cultures of human airway measured 72-hours after exposure. (a) All coformulations were prepared at 1.86 × 10−13 µmol/GC. Representative fluorescent images of each condition are shown. (b) Flow cytometric analysis for each condition with positive gate shown as vertical line shows an increase in positive population with coformulation. AAV, adeno-associated virus; DS, dexamethasone-spermine; D2S, disubstituted spermine.
Transduction efficiency of AAV vectors coformulated with cationic lipids in mouse airway in vivo
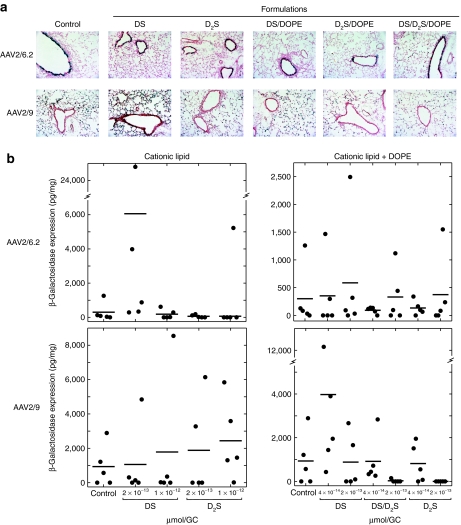
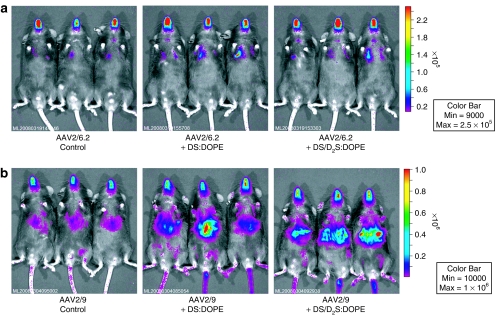
In order to determine the impact on in vivo transduction efficiency and tropism, both AAV2/9 and AAV2/6.2 expressing β-galactosidase (β-gal) were coformulated with different cationic lipid mixtures and delivered by intranasal instillation to C57BL/6 mice (n = 5). Analysis of lung homogenates for β-gal expression showed an approximately fourfold increase when AAV2/9 was coformulated with DS/DOPE liposomes compared to AAV2/9 formulated with vehicle control (Figure 4b). Although coformulations of AAV2/9 with DS/DOPE liposomes exhibited the highest transduction efficiencies compared to control (AAV2/9 only), without the helper lipid DOPE, greater β-gal expression was demonstrated by cohorts treated with AAV2/9 coformulated with D2S only. Similarly, AAV2/6.2 coformulated with DS resulted in an approximately fourfold increase in β-gal expression compared to AAV2/6.2 formulated with vehicle control (Figure 4b), even without inclusion of an outlier which exhibited >80-fold higher transgene expression. AAV2/6.2 displayed the highest level of transgene expression for cohorts treated with AAV2/6.2 coformulated with DS; however, increases over control (AAV2/6.2 only) were also observed for AAV2/6.2 coformulations containing D2S. Interestingly for AAV2/6.2, coformulations of cationic lipids without inclusion of the helper DOPE exhibited the highest transduction efficiencies compared to similar coformulations with liposomes containing DOPE.
Figure 4.
AAV2/6.2 and AAV2/9 gene expression with lipid coformulations in mouse lung. Vectors were delivered to mice by intranasal instillation. (a) Lungs were analyzed for β-gal expression after 35–41 days. Representative histological cross-sections from each group (n = 5) stained for β-gal expression (blue) and counterstained with nuclear fast red are shown. (b) Lung homogenates from all animals were analyzed for total expression of β-gal normalized to total protein (solid circles) with groups means shown as horizontal solid bars. Increases in total expression for both viral vectors were observed in some lipid-formulated groups with increased expression of transgene in the conducting airway compared to control. Scales for each subset of conditions were set independently to show all data points clearly due to the variability in maximum transgene expression. AAV, adeno-associated virus; β-gal, β-galactosidase; DOPE, 1,2 dioleoylphosphatidylethanolamine; DS, dexamethasone-spermine; D2S, disubstituted spermine; GC, genome copy.
Analogous experiments were conducted with the luciferase (luc2) transgene to determine the effect on transduction efficiency and biodistribution of vectors following coformulation with both DS and D2S. Bioluminescent imaging was performed and used for quantitative comparison (Supplementary Figure S3), which demonstrated similar trends to the β-gal data presented earlier (n = 5/group, Figure 5). Bioluminescent imaging also demonstrated high systemic transduction for AAV2/9 vectors coformulated with lipids compared to AAV2/9 alone (Figure 5b) indicating the potential for greater transcytosis of AAV2/9 through the alveolar epithelium.
Figure 5.
Bioluminescent imaging showing AAV2/6.2 and AAV2/9 gene expression with lipid coformulations in mouse lung. Vectors were delivered to mice by intranasal instillation. (a) AAV2/6.2 imaged in vivo after 29 days. (b) AAV2/9 imaged in vivo after 14 days. Scales for each vector were set independently to show full range of expression. Representative images for each group (n = 5) are shown. Increases in total expression for both viral vectors were observed in all lipid-formulated groups compared to control. Higher systemic transduction was noted for AAV2/9 coformulated with both lipids. AAV, adeno-associated virus; DOPE, 1,2 dioleoylphosphatidylethanolamine; DS, dexamethasone-spermine; D2S, disubstituted spermine.
Histological analysis of lung cross-sections stained for β-gal expression demonstrated that mice treated with AAV2/9 coformulated with DS or DS/D2S resulted in increased conducting airway transduction relative to AAV2/9 control (Figure 4a), which transduced only alveolar epithelium as previously reported.23 Lipid coformulations with AAV2/9 appear to have altered the cellular targets for this serotype. Significant transduction of the alveolar epithelium, however, was still evident in all groups suggesting that lipid coformulation does not ablate typical AAV2/9 tropism, but may lead to increased transduction by targeting different cell types. For AAV2/6.2, coformulation with DS, and to a lesser extent the mixture of DS/D2S, showed increased transduction in the conducting airway, with little expression in the alveolar epithelium. Because AAV2/6.2 is a variant of AAV6,15 which exhibits natural tropism for conducting airway, AAV2/6.2 vector preparations coformulated with these cationic lipids did not appear to have significantly altered its tropism for conducting airway.
Immune responses to gene delivery vectors can be critical due to the requirement to readminister vectors to maintain transgene expression that diminishes with cell turnover.25,26 AAV2/9-specific NABs were measured in bronchoalveolar lavage fluid at day 35 (Supplementary Table S1). No NAB was detected for the AAV2/9-treated animals at this time point, which was not unexpected.23 Although coformulation of AAV2/9 with DS at 2 × 10−13 µmol/GC resulted in only one animal with detectable NAB (1:20; limit of the detection assay), none was detected as lipid concentration was increased. Detectable NAB was observed in animals treated with AAV2/9 coformulated with DS/DOPE at 2 × 10−13 µmol/GC (1:20), D2S at 1 × 10−12 µmol/GC (1:40), and the liposomal mixture of DS/D2S/DOPE (1:20 to 1:160). We did not observe NAB in all other AAV2/9 coformulation conditions. Analysis of bronchoalveolar lavage fluid at day 41 for immunoglobulin A response to AAV2/6.2 (Supplementary Table S1) revealed that coformulation with DS at 2 × 10−13 µmol/GC increased twofold (1:400 to 1:6,400) compared to AAV2/6.2 control (1:200 to 1:3,200); however, increasing the lipid concentration of DS resulted in decrease in capsid immunoglobulin A levels by fourfold (1:200 to 1:800). Coformulation of AAV2/6.2 with D2S as well as all liposomal formulations decreased capsid immunoglobulin A compared to control.
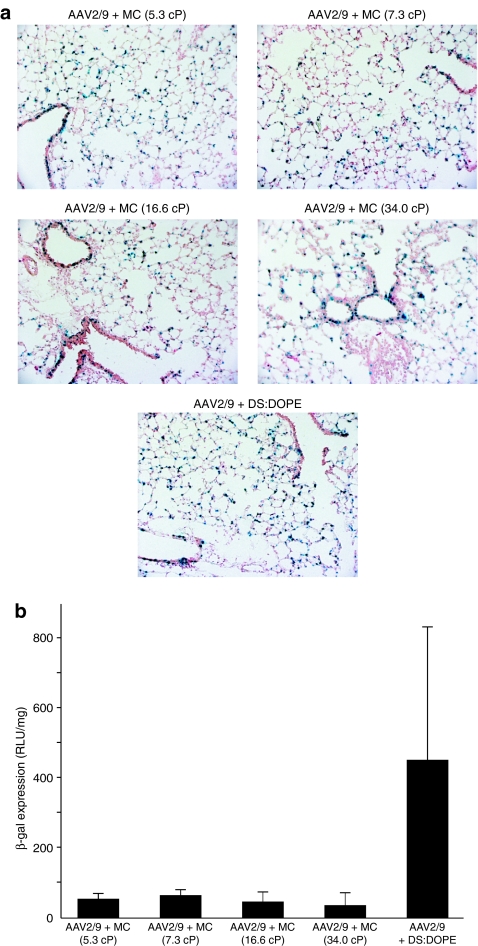
Increased mouse conducting airway transduction efficiency of AAV vectors coformulated with methylcellulose (MC) gels and cationic lipids
Based on the observation of AAV virion clusters following coformulation with DS and D2S via TEM/SEM (Figure 1a), we hypothesized that the change in tropism noted for AAV2/9 in vivo could be the result of an increase in residence time for larger particles in the conducting airway. To test this hypothesis, we generated four viscoelastic MC gels with increasing intrinsic viscosities and mixed with AAV2/9 expressing β-gal prior to instillation. Transgene expression in lung cross-sections (Figure 6a) were compared to lipid coformulation of AAV2/9 with DS/DOPE, which had previously been shown to result in increased transduction of conducting airway (Figure 6a). We found that increasing the viscosity of the delivery vehicle can have a dramatic effect on altering the tropism of the AAV2/9 vector. All formulations of AAV2/9 with MC displayed transduction of conducting airway not typically observed with AAV2/9 alone, and additionally we observed a higher transduction of the conducting airway relative to the AAV2/9 coformulation with DS/DOPE. However, analysis of lung homogenates for β-gal expression indicated that formulation of AAV2/9 with DS/DOPE resulted in 7–12-fold increases in total transgene expression relative to all MC formulations with AAV2/9 (Figure 6b), but also displayed transduction of the conducting airway.
Figure 6.
Gene transfer with AAV2/9 after formulation with lipid and MC gels in mouse lung. Mice were treated by intranasal instillation and analyzed for β-gal expression after 30 days. (a) Representative histological cross-sections from each group (n = 5) stained for β-gal expression (blue) and counterstained with nuclear fast red are shown. (b) Lung homogenates from all animals were analyzed for total expression of β-gal normalized to total protein. Transgene expression in the conducting airway was observed for all groups indicating a change in tropism for this serotype; however, coformulation with DS/DOPE liposomes resulted in greater total β-gal expression in lung homogenate. Results presented as the mean and error bars represent standard deviations from two replicates for each condition (n = 5). AAV, adeno-associated virus; β-gal, β-galactosidase; DOPE, 1,2 dioleoylphosphatidylethanolamine; DS, dexamethasone-spermine; D2S, disubstituted spermine; MC, methylcellulose.
Discussion
Successful treatment of airway genetic disorders by gene therapy will depend on the availability of highly efficient and low-immunogenic gene delivery vectors that can target the correct airway cell populations. For certain airway diseases, such as CF, correction of the electrophysiological defect throughout the airway epithelium is considered vital to the success of the gene therapy.27,28,29 Although certain AAV vectors transduce the airway effectively, increasing the efficiency and/or retargeting the tropism of the vector is an important advantage. We studied two AAV vectors in order to determine whether noncovalent surface modification can improve on their existing transduction efficiency in lung and/or change their tropism. In addition, we included cationic lipid formulations alone and with the neutral helper lipid DOPE, which is typically included in cationic liposomes to facilitate escape from endosomes.30
Transduction efficiency was improved for both AAV2/9 and AAV2/6.2 vectors coformulated with DS and D2S in vitro and in vivo. The in vitro transduction experiments were used to determine optimal lipid composition and concentrations. Despite the relatively minor variations in efficiency, all lipid and liposome formulations significantly increased in transgene expression over control for both AAV vectors in vitro. This is important because AAV vectors were tested at optimized multiplicity of infections both in vitro and in vivo, which maximized transgene expression in the absence of cationic lipids, and no toxicity or adverse events were observed for any lipid formulations in culture or in mice. AAV vectors enter cells through a receptor-mediated process,13 therefore, the increased transgene expression observed for AAV vectors coformulated with DS and D2S is suggestive of an uptake through nonreceptor-mediated endocytosis of the lipid structures. Clusters of AAV vectors formed following coformulation with both cationic lipids may also increase internalization of complexes containing multiple viral particles. It is also important to note that although some facially amphiphilic cationic lipids have been shown to promote membrane destabilization (D.E. Fein, R. Bucki, F. Byfield, P.A. Janmey and S.L. Diamond, unpublished results and ref. 31), no significant cellular toxicity was observed for any of the AAV vector coformulations indicating that membrane disruption and epithelial shedding/denudation were not important factors related to the increases in transgene expression.
Because the efficiency of delivery vectors in vivo can depend on a number of factors not relevant in vitro,5 representative formulations containing each lipid, liposome, and AAV vector were tested for effectiveness in mouse lung. For both AAV2/9 and AAV2/6.2 vectors, DS and DS/DOPE liposomes were found to increase gene expression more effectively than most D2S coformulation conditions. Although AAV2/9 coformulations with both DS and D2S without inclusion of DOPE improved transgene expression, only coformulation with lipid containing DS and DS/DOPE liposomes resulted in β-gal expression in the conducting airway. This finding is consistent with previous reports that DS/DOPE liposomes can improve targeting of viral-mediated airway transduction,21 but this is the first report of increased AAV vector transduction and retargeting of AAV vectors to the conducting airway. The combination of enhanced transgene expression and retargeting to include ciliated conducting airway epithelial cells is an important finding considering AAV2/9 vectors can be effectively readministered in the presence of high levels of NABs.23 No considerable increases in NAB were observed for AAV2/9 coformulated with DS or DS/DOPE, which increased both transduction efficiency and retargeting to the conducting airway for AAV2/9. Therefore, modification of AAV2/9 vectors with DS formulations may potentially allow promising low-immunogenic lung-specific gene transfer vectors to effectively target the conducting airway epithelium and be readministered. AAV2/6.2 vector transduction was increased when compared to DS formulated without DOPE, possibly indicating that increased endocytosis associated with lipid formulation is more important than intracellular processing of the lipid-formulated AAV vectors. For AAV2/6.2, coformulation with cationic liposomes was found to decrease the immune response to the vector, whereas transduction efficiency was increased with lipid concentration indicating potential for improved readministration for coformulated vectors.
The size of AAV vector particles can affect residence time in the conducting airway. Analysis of the TEM and SEM images revealed that the lipids increased the aggregation of viral particles in solution. This result was correlated to measure decreases in the net surface charge of the viral particles following addition of the cationic lipids. Differences in cluster size for DS and D2S could be related to lipid structure, because D2S is larger and has more hydrophobic character, potentially limiting the number of molecules that can associate with the viral capsid surface. We used viscoelastic MC gels, previously shown to enhance the efficiency of AAV5-mediated gene transfer to mouse airway,32 to determine whether increased residence time for AAV coformulated with DS and D2S was responsible for the improved transduction efficiency and retargeting of the AAV vectors. Although formulation of AAV2/9 with MC gels resulted in significant conducting airway transduction not seen in AAV2/9 preparations without MC, total transgene expression was 7–12-fold lower compared to AAV2/9 coformulated with DS/DOPE liposomes. The lower total surface area of the conducting airway relative to the alveolar epithelium noted in several species33 likely caused total transgene expression in lung homogenate to be lower for MC formulated AAV2/9 vectors compared to control; however, AAV2/9 coformulated with DS and DS/DOPE resulted in a fourfold increase in transduction efficiency for AAV2/9 vectors and transduction of the conducting airway. Therefore, retargeting gene transfer to the conducting airway does not explain the differences in total transgene expression between the MC gels and the DS formulations. We hypothesize that the larger particle size following coformulation of AAV vectors with DS/DOPE liposomes contributed to the increase in transduction of the conducting airway, but that nonspecific liposomal endocytosis also played an important role in improving the transduction efficiency of this vector.
Our results show that coformulation of AAV vectors with the cationic lipids DS and D2S can alter the physicochemical properties of the vectors resulting in improved transduction efficiency and the potential for modification of vector tropism. DS and D2S have been extensively characterized and represent unique coformulation components for viral-mediated airway gene delivery. DS has retained its glucocorticoid properties and has been previously shown to improve airway targeting, attenuate vector-induced inflammation, and facilitate readministration in vivo when formulated with adenovirus vectors.21,34,35 D2S has potent antibacterial activity against both Gram-positive and Gram-negative bacteria and can inactivate lipopolysaccharide, effectively suppressing bacterial-mediated inflammation (D.E. Fein, R. Bucki, F. Byfield, P.A. Janmey and S.L. Diamond, unpublished results). Therefore, use of these two lipids as coformulation agents with AAV vectors to improve transduction or alter tropism may have added therapeutic potential in diseases requiring correction of large cell populations and which are characterized by bacterial lung infections and inflammation, such as CF.
Materials and Methods
Synthesis of cationic glucocorticoids. DS and D2S were prepared as previously described.34 Briefly, dexamethasone-mesylate (Steraloids, Newport, RI), Traut's reagent (Sigma-Aldrich, St Louis, MO), and spermine (Sigma-Aldrich) were reacted in a 1:1:1 molar ratio in a one-step reaction in ethanol at 40 °C and was quenched with trifluoroacetic acid (Sigma-Aldrich). Ethanol was evaporated under vacuum and the reaction products were resuspended in water prior to separation.
Instrumentation for semipreparative purification and analytical characterization. The liquid chromatography-mass spectrometry system consisted of a Shimadzu (Columbia, MD) LC-20AB solvent delivery system and Shimadzu SIL-20A autosampler coupled to Shimadzu SPD-20A dual wavelength ultraviolet-visible detector and Shimadzu LCMS 2010EV mass spectrometer. Purification was adapted from the method described previously.34 The semipreparative separation system consisted of the Shimadzu instrument coupled to a Hamilton (Reno, NV) PRP-1 column (150 mm × 10 mm internal diameter, 10-µm particle size). The mobile phase flow rate was 4 ml/minute with a starting ratio of 90% mobile phase A (water) and 10% mobile phase B (acetonitrile). The elution profile consisted of: (i) an isocratic step to 16% B for 30 minutes and (ii) 30% B for 30 minutes to separate the reaction products. Fractions were collected as either trifluoroacetic acid or formate salts followed by complete solvent removal by lyophilization. Final products were dissolved in either nuclease free water or methanol/chloroform (50/50 vol%) at 5–10 mg/ml. Analytical characterization was performed with the Shimadzu instrument coupled to a Hamilton PRP-1 column (150 mm × 2.1 mm internal diameter, 5-µm particle size). The mobile phase flow rate was 0.25 ml/minute with a starting ratio of 90% mobile phase A (water) and 10% mobile phase B (acetonitrile). The elution profile consisted of: (i) an isocratic step to 16% B for 60 minutes and (ii) 30% B for 60 minutes to quantify purity with mass spectrometry performed on the eluent.
AAV preparation. The AAV vectors flanked with AAV2 inverted terminal repeats contained either a LacZ or firefly luciferase (ffluc2) gene fused to a nucleus localization sequence at the N-terminus or an enhanced green fluorescence protein (GFP) gene under the transcriptional control of the cytomegalovirus (CMV)–enhanced chicken-β-actin or the CMV promoter, respectively and produced as previously described.15,23 AAV vectors with CMV promoters were used for all in vitro experiments and chicken-β-actin promoters were used for in vivo experiment.
Preparation of liposomes and lipid-AAV coformulations. To form the liposomes, DOPE (Avanti Polar Lipids, Alabaster, AL) was added to a glass tube in chloroform, and the solvent was removed under vacuum to generate a lipid film. Cationic lipids were added to the lipid film in a 1:1 molar ratio in either sterile water or reduced serum medium (Optimem; Gibco, Grand Island, NY), to achieve the desired cationic lipid concentrations. Following hydration, the lipid mixtures were probe sonicated for 30 seconds and briefly vortexed prior to use. Lipids or liposomes (with DOPE) were added in equal volume to AAV suspensions in phosphate buffered saline (PBS) to achieve the concentrations described in the figures and were incubated for 15 minutes prior to use in all experiments.
Size distribution and ζ measurements. Particle sizes were determined by dynamic light scattering with a ZetaPlus (Brookhaven Instruments, Holtsville, NY) with particle sizing option equivalent to the Brookhaven 90Plus. The measured autocorrelation function (90Plus) is analyzed using a cumulant analysis, the first cumulant yielding an effective diameter, a type of average hydrodynamic diameter. The ζ was calculated from the electrophoretic mobility using the ZetaPlus and the Smoluchowski equation. The Doppler shifted frequency spectrum at 15° scattering angle and 25 °C yielded an average Doppler shift that was measured 10 times and averaged to determine an electrophoretic velocity. The mobility was calculated by dividing the velocity by the electric field strength. An optimal concentration of AAV particles of 1010 GC with a solution ionic strength of <8,000 µS were prepared for each condition.
Electron micrographs. For SEM sample preparation, samples were placed on track-etched polycarbonate membranes (black 0.1 µm pore size; SPI, West Chester, PA) for 10 minutes then washed four times in 0.2 mol/l sodium cacodylate (Sigma-Aldrich), fixed in 2.5% glutaraldehyde solution (Sigma-Aldrich), then overnight in 0.2 mol/l sodium cacodylate at 4 °C. The following day, samples were dehydrated for 15 minutes in graded ethanol solutions (ThermoFisher Scientific, Pittsburg, PA) of 50, 70, 80, 90, and twice in 100% then dried for 15 minutes in 50% hexamethyldisiloxane (United Chemical Technologies, Bristol, PA) in ethanol followed by 100% hexamethyldisiloxane. All samples were sputtered with platinum/palladium then imaged on a field emission scanning electron microscope (JOEL 7500F; JOEL, Tokyo, Japan) in gentle beam secondary electron imaging mode (GB) with a 2 kV accelerating voltage (4-kV gun voltage, 2 kV sample bias). Electron micrographs were analyzed using ImageJ software (U.S. National Institutes of Health, Bethesda, MD).
For negative staining of AAV vector preparations for TEM sample preparation, 5 µl of each vector preparation was placed on 400-mesh copper grids coated with a formvar/thin carbon film (EMS, Hatfield, PA). Vector particles were allowed to adsorb to the film for 1 minute. Excessive fluid was removed from the side with blotting paper and the grids were washed with several drops of 5 mmol/l Tris/Cl (pH 7.5) and water, blotted dry again and stained for 30 seconds with 1% uranyl acetate. After removal of the staining solution and drying the specimens were viewed using a Philips EM100 TEM (FEI, Hillsboro, OR).
MC-gel preparations. Two molecular weight MC powders (MW = 17,000 and 41,000; Sigma-Aldrich) were prepared each in two different weight ratios to yield four viscoelastic gels. MC gels using the MW = 17,000 powder were made as 1 and 2% solutions and the MW = 41,000 powder was used as 0.5 and 1% solutions. All solutions were prepared by adding MC to hot water with agitation until complete dispersion was observed followed by addition of cold water to achieve the desired volume. Stirring was continued for ~1 hour until the gels reached room temperature and appeared as clear homogenous solutions and were filtered prior to use. Kinematic viscosities were measured prior to formulation with AAV with an Ubbelohde viscometer (ASTM2; ThermoFisher Scientific).
In vitro transduction. Transduction experiments were performed with A549 cells and normal human airway cells. Both cell lines were cultured at 37 °C and 5% CO2. For A549 cells, Dulbecco's modified Eagle's medium (Invitrogen, Carlsbad, CA) supplemented with 10% fetal bovine serum (Invitrogen), 2% penicillin/streptomycin (Mediatech, Manassas, VA), and 1% L-glutamine (Mediatech) was used. All experiments with A549 cells were executed with cells seeded 24 hours prior to transfection at 60–70% confluence. Human bronchial epithelial cells were purchased from Lonza (Walkersville, MD) and initially grown in a T-75 flask for 4–5 days in bronchial epithelial cell growth medium supplemented with bronchial epithelial growth medium singlequots (BPE, hydrocortisone, hEGF, epinephrine, transferrin, insulin, retinoic acid, and GA-1000; Lonza). Cells were then seeded on 96-well plates (BD Bioscience, Franklin Lakes, NJ) at 105/well and maintained in bronchial epithelial basal media mixed with Dulbecco's modified Eagle's medium (Invitrogen) at a 1:1 ratio. Cells reached confluence within 4 days and remained submerged for 2 weeks at that time they were used in gene transfer experiments. Untreated AAV2/9 and AAV2/6.2 at multiplicity of infection = 10,000 were used as a positive controls for all experiments because this level maximized transfection efficiency with no observed toxicity. Lipid composition, dose, and inclusion of the helper lipid DOPE were tested in order to assess how excess lipid/cationic charge affected transgene expression. After coformulation, all experimental solutions were incubated with the plated cells for 24 hours at that time the solutions were removed and replaced with fresh medium. AAV2/6.2.CMV.EGFP was used to transduce cells seeded 96-well plates with four replicates of each condition. Transduced cells expressing GFP were analyzed by fluorescence microscopy and flow cytometry. Specifically, cells were imaged 72–96 hours after transduction on an inverted microscope (Olympus IX81, Center Valley, PA) and then harvested in 500 µl PBS and kept on ice until analysis. An Accuri C6 (Accuri Cytometers, Ann Arbor, MI) flow cytometer was used to obtain fluorescence data with 20,000 counts recorded per condition. For the luminescence assays, either AAV2/6.2.CMV.ffluc2 or AAV2/9.CMV.ffluc2 was used to transduce cells seeded in 96-well plates with eight replicates of each condition. To measure transgene expression, BrightGlo (Promega, Madison, WI) was added and luminescence was measured 30 minutes following addition of the reagent and measured with an EnVision Multilabel Plate Reader (Perkin Elmer, Wellesley, MA).
In vivo transduction. Cationic lipids and liposomes (1:1 mole ratio with DOPE) at concentrations listed in the figure legends were mixed with AAV (1011 GC/dose) in equal volume at room temperature for 15 minutes prior to instillation. All MC gels were mixed with AAV2/9 as 75% solutions to achieve the desired concentration of AAV for dosing. C57BL/6 mice (6–8 weeks of age) were anesthetized by an intraperitoneal injection of a 3:2 mixture of xylazil:ketamine. For dosing, mice were suspended from their dorsal incisors (hind quarters supported) and a dose of 1011 GC of AAV vector was delivered as a 51-µl bolus (delivered as three 17-µl aliquots) into the nostrils. All animal experiments were reviewed and approved by the University of Pennsylvania Institutional Animal Care and Use Committee.
To evaluate ffluc2 expression in mice, 250 µl of a 15-mg/ml luciferin solution (Caliper Life Sciences, Hopkinton, MA) was injected per 25-g body mass by intraperitoneal injection. Five minutes after luciferin injection the mice were anesthetized by an intraperitoneal injection of a 3:2 mixture of xylazil:ketamine. Animals were imaged 10 minutes later using the Xenogen IVIS bioluminescent imaging system (Caliper Life Sciences).
For β-gal transgene expression evaluation, lungs were harvested and inflated with a 1:1 mixture of PBS and optimum cutting temperature embedding compound. One lobe was then submerged in optimum cutting temperature, frozen in isopentane cooled with liquid nitrogen, and cryosectioned (8 µm). Sections were fixed in 0.5% glutaraldehyde in PBS (Electron Microscopy Sciences, Hatfield, PA) for 10 minutes (4 °C) and then washed twice in PBS/1 mmol/l MgCl2 (4 °C) and stained with X-gal substrate (5-bromo-4-chloro-3-indoyl-β-D-galactopyranoside) for 16 hours at 37 °C to reveal β-gal-positive cells. After being washed in PBS, sections were counterstained with nuclear fast red, dehydrated through a graded ethanol series and xylene, coverslipped, and imaged. The other lobe was placed in 2 ml of lysis buffer (Roche, Indianapolis, IN) and placed on ice. Directly after all samples were collected, each sample was homogenized for ~10 seconds. The instrument was washed between each sample using 70% ethanol and PBS. Samples were then immediately centrifuged at 3,000 r.p.m. (Sorvall; Thermo Scientific, Waltham, MA) for 10 minutes. The supernatant was removed and stored at −80 °C prior to analysis of transgene expression. To quantitatively measure β-gal gene expression a Galacto-Light Plus System (Applied Biosystems, Foster City, CA) assay was used on lung homogenates according to the manufacturer's instructions. All values were normalized to total protein content in the sample using the bicinchoninic acid protein assay (Pierce Biotechnololgy, Rockford, IL).
Immunological response assays. NABs to AAV2/9 in bronchoalveolar lavage fluid was assayed as previously described.36,37 Bronchoalveolar lavage fluid was analyzed for immunoglobulin A against AAV2/6.2 and AAV2/9 capsids and β-gal transgene as previously described.38
SUPPLEMENTARY MATERIALFigure S1. Chemical structures of DS (1) and D2S (2).Figure S2. Coformulation of AAV2/9 vectors with cationic lipids results in decrease in zeta potential. Error bars represent SE from 10 replicates of each condition.Figure S3. Average cohort luminescent flux in lung from bioluminescent imaging of AAV2/6.2-mediated gene ffluc2 expression with lipid coformulations in mouse lung after 29 days (a) and following AAV2/9-mediated gene ffluc2 expression with lipid coformulations in mouse lung after 14 days (b). Increases in expression were observed in lipid formulated groups.Table S1. Immune response to AAV2/6.2 and AAV2/9 vector capsids following gene transfer to the lung in C57BL/6 mice in bronchoalveolar lavage fluid (BALF). The values reported represent dilution of BALF required to achieve absorbance equal to the negative control (naïve). ND = not detected which was <1:20 for both assays.
Supplementary Material
Chemical structures of DS (1) and D2S (2).
Coformulation of AAV2/9 vectors with cationic lipids results in decrease in zeta potential. Error bars represent SE from 10 replicates of each condition.
Average cohort luminescent flux in lung from bioluminescent imaging of AAV2/6.2-mediated gene ffluc2 expression with lipid coformulations in mouse lung after 29 days (a) and following AAV2/9-mediated gene ffluc2 expression with lipid coformulations in mouse lung after 14 days (b). Increases in expression were observed in lipid formulated groups.
Immune response to AAV2/6.2 and AAV2/9 vector capsids following gene transfer to the lung in C57BL/6 mice in bronchoalveolar lavage fluid (BALF). The values reported represent dilution of BALF required to achieve absorbance equal to the negative control (naïve). ND = not detected which was <1:20 for both assays.
Acknowledgments
We gratefully acknowledge Regina Munden and Deirdre McMenamin for assistance with the animal studies, Peter Bell for TEM preparation and imaging, Roberto Calcedo for NAB assays, Penn Vector for supplying the AAV vector preparations and the Penn Regional Nanotechnology Facility. This work was supported by a predoctoral fellowship from Merck & Co., Inc. (D.E.F.), NIH S10RR022442 (S.L.D.), CFF R881 (J.M.W.), P01-HL051746 (J.M.W), and CFF08G (M.P.L). J.M.W. is an inventor on patents licensed to various biopharmaceutical companies.
REFERENCES
- Buckley SM, Howe SJ, Sheard V, Ward NJ, Coutelle C, Thrasher AJ, et al. Lentiviral transduction of the murine lung provides efficient pseudotype and developmental stage-dependent cell-specific transgene expression. Gene Ther. 2008;15:1167–1175. doi: 10.1038/gt.2008.74. [DOI] [PubMed] [Google Scholar]
- Davies LA, Varathalingam A, Painter H, Lawton AE, Sumner-Jones SG, Nunez-Alonso GA, et al. Adenovirus-mediated in utero expression of CFTR does not improve survival of CFTR knockout mice. Mol Ther. 2008;16:812–818. doi: 10.1038/mt.2008.25. [DOI] [PubMed] [Google Scholar]
- Flotte TR. Recombinant adeno-associated virus gene therapy for cystic fibrosis and alpha(1)-antitrypsin deficiency. Chest. 2002;121:98S–102S. doi: 10.1378/chest.121.3_suppl.98s. [DOI] [PubMed] [Google Scholar]
- Tagalakis AD, McAnulty RJ, Devaney J, Bottoms SE, Wong JB, Elbs M, et al. A receptor-targeted nanocomplex vector system optimized for respiratory gene transfer. Mol Ther. 2008;16:907–915. doi: 10.1038/mt.2008.38. [DOI] [PubMed] [Google Scholar]
- Pickles RJ. Physical and biological barriers to viral vector-mediated delivery of genes to the airway epithelium. Proc Am Thorac Soc. 2004;1:302–308. doi: 10.1513/pats.200403-024MS. [DOI] [PubMed] [Google Scholar]
- Rabinowitz JE, Rolling F, Li C, Conrath H, Xiao W, Xiao X, et al. Cross-packaging of a single adeno-associated virus (AAV) type 2 vector genome into multiple AAV serotypes enables transduction with broad specificity. J Virol. 2002;76:791–801. doi: 10.1128/JVI.76.2.791-801.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Z, Asokan A., and , Samulski RJ. Adeno-associated virus serotypes: vector toolkit for human gene therapy. Mol Ther. 2006;14:316–327. doi: 10.1016/j.ymthe.2006.05.009. [DOI] [PubMed] [Google Scholar]
- Buning H, Perabo L, Coutelle O, Quadt-Humme S., and , Hallek M. Recent developments in adeno-associated virus vector technology. J Gene Med. 2008;10:717–733. doi: 10.1002/jgm.1205. [DOI] [PubMed] [Google Scholar]
- Bastonero S, Gargouri M, Ortiou S, Gueant JL., and , Merten MD. Inhibition by TNF-alpha and IL-4 of cationic lipid mediated gene transfer in cystic fibrosis tracheal gland cells. J Gene Med. 2005;7:1439–1449. doi: 10.1002/jgm.789. [DOI] [PubMed] [Google Scholar]
- Halbert CL, Rutledge EA, Allen JM, Russell DW., and , Miller AD. Repeat transduction in the mouse lung by using adeno-associated virus vectors with different serotypes. J Virol. 2000;74:1524–1532. doi: 10.1128/jvi.74.3.1524-1532.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coura Rdos S., and , Nardi NB. The state of the art of adeno-associated virus-based vectors in gene therapy. Virol J. 2007;4:99. doi: 10.1186/1743-422X-4-99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daya S., and , Berns KI. Gene therapy using adeno-associated virus vectors. Clin Microbiol Rev. 2008;21:583–593. doi: 10.1128/CMR.00008-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akache B, Grimm D, Pandey K, Yant SR, Xu H., and , Kay MA. The 37/67-kilodalton laminin receptor is a receptor for adeno-associated virus serotypes 8, 2, 3, and 9. J Virol. 2006;80:9831–9836. doi: 10.1128/JVI.00878-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seiler MP, Miller AD, Zabner J., and , Halbert CL. Adeno-associated virus types 5 and 6 use distinct receptors for cell entry. Hum Gene Ther. 2006;17:10–19. doi: 10.1089/hum.2006.17.10. [DOI] [PubMed] [Google Scholar]
- Limberis MP, Vandenberghe LH, Zhang L, Pickles RJ., and , Wilson JM. Transduction efficiencies of novel AAV vectors in mouse airway epithelium in vivo and human ciliated airway epithelium in vitro. Mol Ther. 2009;17:294–301. doi: 10.1038/mt.2008.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byk T, Haddada H, Vainchenker W., and , Louache F. Lipofectamine and related cationic lipids strongly improve adenoviral infection efficiency of primitive human hematopoietic cells. Hum Gene Ther. 1998;9:2493–2502. doi: 10.1089/hum.1998.9.17-2493. [DOI] [PubMed] [Google Scholar]
- Fasbender A, Zabner J, Chillon M, Moninger TO, Puga AP, Davidson BL, et al. Complexes of adenovirus with polycationic polymers and cationic lipids increase the efficiency of gene transfer in vitro and in vivo. J Biol Chem. 1997;272:6479–6489. doi: 10.1074/jbc.272.10.6479. [DOI] [PubMed] [Google Scholar]
- Fouletier-Dilling CM, Bosch P, Davis AR, Shafer JA, Stice SL, Gugala Z, et al. Novel compound enables high-level adenovirus transduction in the absence of an adenovirus-specific receptor. Hum Gene Ther. 2005;16:1287–1297. doi: 10.1089/hum.2005.16.1287. [DOI] [PubMed] [Google Scholar]
- Han SY, Lee YJ, Jung HI, Lee SW, Lim SJ, Hong SH, et al. Gene transfer using liposome-complexed adenovirus seems to overcome limitations due to coxsackievirus and adenovirus receptor-deficiency of cancer cells, both in vitro and in vivo. Exp Mol Med. 2008;40:427–434. doi: 10.3858/emm.2008.40.4.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fasbender A, Lee JH, Walters RW, Moninger TO, Zabner J., and , Welsh MJ. Incorporation of adenovirus in calcium phosphate precipitates enhances gene transfer to airway epithelia in vitro and in vivo. J Clin Invest. 1998;102:184–193. doi: 10.1172/JCI2732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price A, Limberis M, Gruneich JA, Wilson JM., and , Diamond SL. Targeting viral-mediated transduction to the lung airway epithelium with the anti-inflammatory cationic lipid dexamethasone-spermine. Mol Ther. 2005;12:502–509. doi: 10.1016/j.ymthe.2005.03.033. [DOI] [PubMed] [Google Scholar]
- Lee GK, Maheshri N, Kaspar B., and , Schaffer DV. PEG conjugation moderately protects adeno-associated viral vectors against antibody neutralization. Biotechnol Bioeng. 2005;92:24–34. doi: 10.1002/bit.20562. [DOI] [PubMed] [Google Scholar]
- Limberis MP., and , Wilson JM. Adeno-associated virus serotype 9 vectors transduce murine alveolar and nasal epithelia and can be readministered. Proc Natl Acad Sci USA. 2006;103:12993–12998. doi: 10.1073/pnas.0601433103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le HT, Yu QC, Wilson JM., and , Croyle MA. Utility of PEGylated recombinant adeno-associated viruses for gene transfer. J Control Release. 2005;108:161–177. doi: 10.1016/j.jconrel.2005.07.019. [DOI] [PubMed] [Google Scholar]
- Sumner-Jones SG, Davies LA, Varathalingam A, Gill DR., and , Hyde SC. Long-term persistence of gene expression from adeno-associated virus serotype 5 in the mouse airways. Gene Ther. 2006;13:1703–1713. doi: 10.1038/sj.gt.3302815. [DOI] [PubMed] [Google Scholar]
- Sumner-Jones SG, Gill DR., and , Hyde SC. Lack of repeat transduction by recombinant adeno-associated virus type 5/5 vectors in the mouse airway. J Virol. 2007;81:12360–12367. doi: 10.1128/JVI.01010-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engelhardt JF, Yankaskas JR, Ernst SA, Yang Y, Marino CR, Boucher RC, et al. Submucosal glands are the predominant site of CFTR expression in the human bronchus. Nat Genet. 1992;2:240–248. doi: 10.1038/ng1192-240. [DOI] [PubMed] [Google Scholar]
- Fang X, Fukuda N, Barbry P, Sartori C, Verkman AS., and , Matthay MA. Novel role for CFTR in fluid absorption from the distal airspaces of the lung. J Gen Physiol. 2002;119:199–207. doi: 10.1085/jgp.119.2.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rochelle LG, Li DC, Ye H, Lee E, Talbot CR., and , Boucher RC. Distribution of ion transport mRNAs throughout murine nose and lung. Am J Physiol Lung Cell Mol Physiol. 2000;279:L14–L24. doi: 10.1152/ajplung.2000.279.1.L14. [DOI] [PubMed] [Google Scholar]
- Karanth H., and , Murthy RS. pH-Sensitive liposomes–principle and application in cancer therapy. J Pharm Pharmacol. 2007;59:469–483. doi: 10.1211/jpp.59.4.0001. [DOI] [PubMed] [Google Scholar]
- Chin JN, Rybak MJ, Cheung CM., and , Savage PB. Antimicrobial activities of ceragenins against clinical isolates of resistant Staphylococcus aureus. Antimicrob Agents Chemother. 2007;51:1268–1273. doi: 10.1128/AAC.01325-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinn PL, Shah AJ, Donovan MD., and , McCray PB., Jr Viscoelastic gel formulations enhance airway epithelial gene transfer with viral vectors. Am J Respir Cell Mol Biol. 2005;32:404–410. doi: 10.1165/rcmb.2004-0410OC. [DOI] [PubMed] [Google Scholar]
- Mercer RR, Russell ML, Roggli VL., and , Crapo JD. Cell number and distribution in human and rat airways. Am J Respir Cell Mol Biol. 1994;10:613–624. doi: 10.1165/ajrcmb.10.6.8003339. [DOI] [PubMed] [Google Scholar]
- Gruneich JA, Price A, Zhu J., and , Diamond SL. Cationic corticosteroid for nonviral gene delivery. Gene Ther. 2004;11:668–674. doi: 10.1038/sj.gt.3302214. [DOI] [PubMed] [Google Scholar]
- Price AR, Limberis MP, Wilson JM., and , Diamond SL. Pulmonary delivery of adenovirus vector formulated with dexamethasone-spermine facilitates homologous vector re-administration. Gene Ther. 2007;14:1594–1604. doi: 10.1038/sj.gt.3303031. [DOI] [PubMed] [Google Scholar]
- Gao GP, Alvira MR, Wang L, Calcedo R, Johnston J., and , Wilson JM. Novel adeno-associated viruses from rhesus monkeys as vectors for human gene therapy. Proc Natl Acad Sci USA. 2002;99:11854–11859. doi: 10.1073/pnas.182412299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Calcedo R, Nichols TC, Bellinger DA, Dillow A, Verma IM, et al. Sustained correction of disease in naive and AAV2-pretreated hemophilia B dogs: AAV2/8-mediated, liver-directed gene therapy. Blood. 2005;105:3079–3086. doi: 10.1182/blood-2004-10-3867. [DOI] [PubMed] [Google Scholar]
- Chirmule N, Xiao W, Truneh A, Schnell MA, Hughes JV, Zoltick P, et al. Humoral immunity to adeno-associated virus type 2 vectors following administration to murine and nonhuman primate muscle. J Virol. 2000;74:2420–2425. doi: 10.1128/jvi.74.5.2420-2425.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Chemical structures of DS (1) and D2S (2).
Coformulation of AAV2/9 vectors with cationic lipids results in decrease in zeta potential. Error bars represent SE from 10 replicates of each condition.
Average cohort luminescent flux in lung from bioluminescent imaging of AAV2/6.2-mediated gene ffluc2 expression with lipid coformulations in mouse lung after 29 days (a) and following AAV2/9-mediated gene ffluc2 expression with lipid coformulations in mouse lung after 14 days (b). Increases in expression were observed in lipid formulated groups.
Immune response to AAV2/6.2 and AAV2/9 vector capsids following gene transfer to the lung in C57BL/6 mice in bronchoalveolar lavage fluid (BALF). The values reported represent dilution of BALF required to achieve absorbance equal to the negative control (naïve). ND = not detected which was <1:20 for both assays.