Abstract
Background:
Vascular occlusion during liver resection results in ischaemia-reperfusion (IR) injury, which can lead to liver dysfunction. We performed a systematic review and meta-analysis to assess the benefits and harms of using various pharmacological agents to decrease IR injury during liver resection with vascular occlusion.
Methods:
Randomized clinical trials (RCTs) evaluating pharmacological agents in liver resections conducted under vascular occlusion were identified. Two independent reviewers extracted data on population characteristics and risk of bias in the trials, and on outcomes such as postoperative morbidity, hospital stay and liver function.
Results:
A total of 18 RCTs evaluating 17 different pharmacological interventions were identified. There was no significant difference in perioperative mortality, liver failure or postoperative morbidity between the intervention and control groups in any of the comparisons. A significant improvement in liver function was seen with methylprednisolone use. Hospital and intensive therapy unit stay were significantly shortened with trimetazidine and vitamin E use, respectively. Markers of liver parenchymal injury were significantly lower in the methylprednisolone, trimetazidine, dextrose and ulinastatin groups compared with their respective controls (placebo or no intervention).
Discussion:
Methylprednisolone, trimetazidine, dextrose and ulinastatin may have protective roles against IR injury in liver resection. However, based on the current evidence, they cannot be recommended for routine use and their application should be restricted to RCTs.
Keywords: ischaemia-reperfusion injury, liver resection, pharmacological drugs, vascular occlusion
Introduction
Elective liver resection is performed mainly for benign and malignant liver tumours.1 The malignant tumours may arise primarily within the liver (hepatocellular carcinoma and cholangiocarcinoma) or represent metastases from malignancies of other organs.1,2 More than 1000 elective liver resections are performed annually in the UK alone.3
Blood loss during liver resection is one of the important factors affecting perioperative outcomes.4–6 One of the methods used to reduce blood loss during liver resection involves occluding the blood flow to the liver. Various methods of vascular occlusion may be used.7 Although the incidence of liver failure is not increased by vascular occlusion, the enzymes indicative of liver parenchymal injury are elevated after vascular occlusion.8
Ischaemia-reperfusion (IR) injury of the liver is a complex multi-path process leading to the activation of inflammatory pathways.9 Cellular injury results from events occurring during both the ischaemic and reperfusion phases.9 Various methods have been attempted to decrease the reperfusion injury associated with prolonged duration of vascular occlusion, including the use of ischaemic preconditioning,10,11in situ cooling12,13 and pharmacological agents.
Many pharmacological agents have been shown in experimental models to ameliorate liver IR injury.14,15 Examples include anti-inflammatory agents such as methylprednisolone,16 antioxidants such as α-tocopherol (vitamin E),17 and various vasoactive agents such as dopamine and dopexamine.18,19 There are no systematic reviews or meta-analyses of randomized controlled trials (RCTs) to assess the benefits and harms of these agents.
Materials and methods
Identification of studies and data extraction
Randomized controlled trials (irrespective of blinding, language or publication status) comparing one or more pharmacological interventions vs. another pharmacological intervention or no pharmacological intervention (irrespective of the time, dose or pharmacological class of the administered drug) were included. Quasi-randomized studies (in which the methods of allocating participants to a treatment are not strictly random, but instead use, for example, date of birth, hospital record number or alternation as a method of allocation) were excluded from the review regarding benefits, but included for side-effects resulting directly from the pharmacological intervention. The Cochrane Hepato-Biliary Group Controlled Trials Register (Issue 4, 2008), the Cochrane Central Register of Controlled Trials (CENTRAL) in the Cochrane Library (Issue 4, 2008), MEDLINE (1951–January 2009), EMBASE (1974–January 2009) and the Science Citation Index Expanded (1945–January 2009) were searched.20 The references of the identified trials were searched to identify further relevant trials. The following medical subject heading (MeSH) terms were used in the search: ‘ischaemia’; ‘reperfusion’; ‘injury’; ‘liver’; ‘hepatectomy’; ‘reperfusion injury’; ‘gabexate’; ‘steroids’; ‘glucocorticoid’; ‘allopurinol’; ‘prostaglandin’; ‘amrinone’; ‘dopexamine’; ‘dopamine’; ‘antioxidant’; ‘bucillamine’, and ‘acetylcysteine’. Equivalent free-text search terms were used in the search strategy. A filter for identifying the RCTs recommended by the Cochrane Collaboration21 was used to filter out non-randomized trials in MEDLINE and EMBASE.
Two reviewers (MA-A and KG) identified the trials for inclusion and extracted population characteristics, details of the liver resection and vascular occlusion, and data on the liver background, outcome measures and risk of bias in the trials.
Outcome measures
The primary outcomes of interest were: mortality and liver failure/decompensation (however, defined by the authors). Secondary outcomes of interest were: perioperative morbidity (postoperative bleeding, bile leak, intra-abdominal infections, wound infections, ascites); intensive therapy unit (ITU) stay; hospital stay; blood transfusion requirements; blood loss; markers of liver function (bilirubin, prothrombin time), and biochemical markers of liver parenchymal injury (aspartate aminotransferase [AST], alanine aminotransferase [ALT]).
Assessment of risk of bias
High risk of bias in RCTs results in an overestimation of intervention effects.22 The risk of bias was assessed by the Cochrane methodology.21,23,24 Briefly, RCTs with adequate generation of the allocation sequence, adequate allocation concealment, adequate blinding, freedom from incomplete outcomes, and freedom from selective outcome reporting were considered to be at low risk of bias.
Statistical analysis
The meta-analyses were performed according to the recommendations of the Cochrane Collaboration21 and the Cochrane Hepato-Biliary Group Module23 using the software package RevMan 5.25 Whenever there were two or more trials in each comparison, the risk ratio (RR) with 95% confidence interval (CI) was calculated for dichotomous outcomes. For continuous outcomes, mean difference (MD) or standardized mean difference (SMD) (for outcomes such as prothrombin time, for which different authors used either the international normalized ratio [INR] or prothrombin time as a percentage of normal) with 95% CI were calculated. When there was only one trial in each comparison, the RR or MD with 95% CIs were calculated from the data available from the reports using RevMan 5. The random-effects model26 and the fixed-effects model were used in the presence of two or more trials for each comparison.27 In cases of discrepancy between the two models, both results were reported; otherwise only the results from the fixed-effects model were reported. Heterogeneity was explored by chi-squared test with significance set at a P-value of 0.10, and the quantity of heterogeneity was measured by I2.28 Standard deviation was calculated from the standard error or from P-values according to the guidelines of the Cochrane Collaboration.21 The analysis was performed on an intention-to-treat basis29 whenever possible. Otherwise, we adopted the ‘available case’ analysis.
Results
Description of studies
The reference flow is shown in Fig. 1. A total of 636 references were identified through the electronic searches of the Cochrane Hepato-Biliary Group Controlled Trials Register and CENTRAL in the Cochrane Library (n= 75), MEDLINE (n= 283), EMBASE (n= 171) and the Science Citation Index Expanded (n= 107). We excluded 161 duplicates and 427 clearly irrelevant references through reading abstracts. Forty-eight references were retrieved for further assessment. No references were identified through scanning reference lists of the identified RCTs. Of the 48 references, 24 were excluded as they represented non-randomized studies,30–43 a quasi-randomized study which did not report adverse outcomes related to the pharmacological intervention,44 a protocol of a study45 or studies that did not use vascular flow occlusion during liver resection.46–53 A total of 24 references describing 18 RCTs fulfilled the inclusion criteria.54–77 All the trials assessed the different pharmacological agents in open liver resections. The important characteristics of the included trials are summarized in Table 1. In the trials that reported follow-up, patients were followed either until discharge or until 30 days after surgery. The assessment of risk of bias in the included trials showed that none of the trials were at low risk of bias. The individual domains of bias risk assessment in each trial are shown in Fig. 2.
Figure 1.
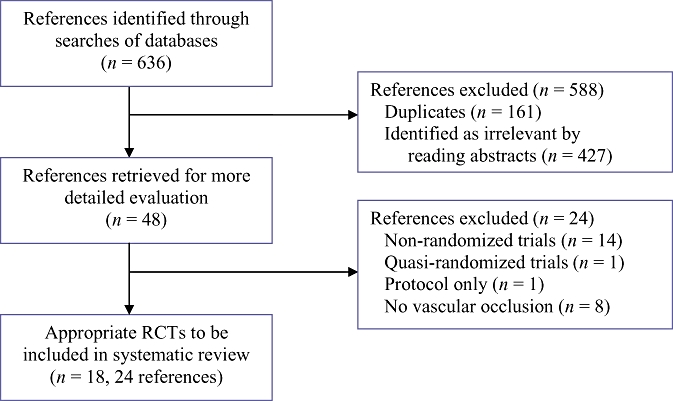
Flow chart of identified, included and excluded references. RCT, randomized controlled trial
Table 1.
Characteristics of included trials
| Trial reference(s) | Intervention (n) | Control (n) | Major resections, n (%) | Cirrhosis, n (%) | Steatosis, n (%) | Vascular occlusion method |
Mean ischaemic time, min |
|
|---|---|---|---|---|---|---|---|---|
| Intervention | Control | |||||||
| Vriens et al. 200276 | Allopurinol (8) | Control (8) | Not stated | 0 | Not stated | Not stated | 53a | 45a |
| Tang et al. 2002, 200774,75 | Dextrose (29) | Control (28) | 38 (66.7) | 50 (87.7) | Not stated | Intermittent PTC | 19a | 21a |
| Holtje et al. 199960Marx et al. 200064 | Dopexamine (9) | Dopamine (10) | 19 (100) | Not stated | Not stated | Continuous PTC | 26a | 27a |
| Kostopanagiotou et al. 200662 | Mannitol (15) | Placebo (15) | 28 (93.3) | Not stated | Not stated | Continuous PTC | 33a | 34a |
| Aldrighetti et al. 2006,54 Finnazi et al. 2005,59 Pulitano et al. 2005, 200767–69 | Methylprednisolone (36) | Control (37) | 53 (72.6) | 26 (34.2) | 8 (10.9) | Intermittent PTC | 52a | 43a |
| Muratore et al. 200365 | Methylprednisolone (25) | Control (28) | 28 (52.8) | 16 (30.2) | 14 (26.4) | Continuous PTC | 41a | 37a |
| Yamashita et al. 200177 | Methylprednisolone (17) | Control (16) | 11 (33.3) | Not stated | Not stated | Continuous PTC or HHVO | Not stated | Not stated |
| Cerwenka et al. 199858 | Multivitamin (32) | Control (26) | 19 (32.8) | 13 (26) | Not stated | Continuous PTC | 48a | 48a |
| Cerwenka et al. 199957 | Multivitamin (25) | Control (25) | 19 (32.8) | Not stated | Not stated | Continuous PTC | 54a | 52a |
| Shirabe et al. 199671 | OKY 046 (9) | Control (8) | 9 (52.9) | Not stated | Not stated | Continuous HHVO | 35a | 40a |
| Kawano et al. 200561 | Prostaglandin E1 (10) | Control (12) | Not stated | Not stated | Not stated | Segmental PTC | 74a | 50a |
| Orii et al. 200066 | Prostaglandin E1 (15) | Amrinone (15), Control (15) | 0 | 45 (100) | Not stated | Intermittent PTC | 69a | 70a |
| Stadheim et al. 200072 | Prostaglandin E1 (13) | Pentoxifylline (10), Control (7) | 30 (100) | Not stated | Not stated | Afferent and efferent vessels | Not stateda | Not stateda |
| Sugawara et al. 199873 | Prostaglandin E1 (12) | Placebo (12) | Not stated | 24 (100) | Not stated | Continuous PTC | 70a | 73a |
| Beck-Schimmer et al. 200856 | Sevoflurane (30) | Propofol (34) | 28 (43.8) | 0 | 30 (46.9) | Continuous PTC | 36a | 35a |
| Settaf et al. 200170 | Trimetazidine (38) | Placebo (38) | Not stated | Not stated | Not stated | Continuous PTC | 40a | 40a |
| Li & Liang 200463 | Ulinastatin (16) | Gantaile (15) | Not stated | 27 (87.1) | Not stated | Continuous PTC | 18a | 17a |
| Bartels et al. 200455 | Vitamin E (19) | Placebo (28) | 33 (70.2) | Not stated | Not stated | Continuous PTC | 29a | 33a |
No significant difference between intervention and control group mean ischaemic times (P > 0.05)
PTC, portal triad clamping; HHVO, hemihepatic vascular occlusion
Figure 2.
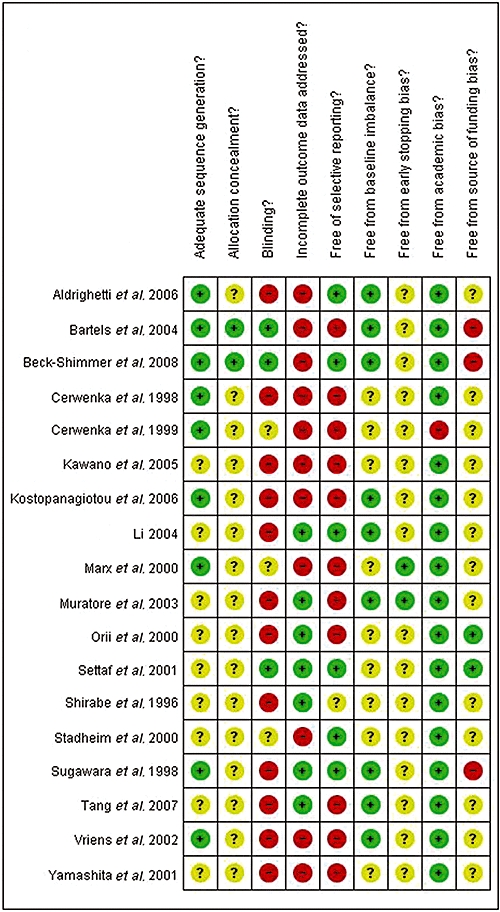
Individual methodological quality criteria for each trial included
Primary outcomes
Mortality
There was no difference in perioperative mortality between the groups in any of the comparisons in which mortality was reported (mannitol vs. placebo,62 RR 0.33, 95% CI 0.01–7.58; sevoflurane vs. propofol,56 RR 1.13, 95% CI 0.07–17.34; vitamin E vs. placebo,55 RR 0.74, 95% CI 0.07–7.56). None of the deaths reported were caused by the study medication or liver failure.
Liver failure
None of the trials that reported on liver failure showed statistically significant differences between the groups (trimetazidine vs. placebo,70 RR 0.2, 95% CI 0.01–4.03; multivitamin antioxidant infusion vs. control,57 RR 4.0, 95% CI 0.48–33.33; methylprednisolone vs. control,54,59,67–69,77 RR 0.5, 95% CI 0.10–2.63; ulinastatin vs. gantaile,63 RR 0.31, 95% CI 0.04–2.68). None of the participants in the comparisons (prostaglandin E1 vs. placebo,73 pentoxifylline vs. control,72 prostaglandin E1 vs. control,72 sevoflurane vs. propofol56) developed liver failure.
Secondary outcomes
Perioperative mortality
There was no statistically significant difference in perioperative morbidity in any of the comparisons. Reported perioperative morbidities are summarized in Table 2.
Table 2.
Perioperative morbidity
| Comparison | Postoperative bleeding RR (95% CI) | Bile leak RR (95% CI) | Intra-abdominal infections RR (95% CI) | Wound infections RR (95% CI) | Ascites RR (95% CI) |
|---|---|---|---|---|---|
| Multivitamins vs. control57 | 0.14 (0.01–2.63) | Not reported | Not reported | Not reported | Not reported |
| Methylprednisolonea vs. control54,59,67–69,77 | 0.34b (0.01–8.14) | 0.34 (0.01–8.14) | 0.94c (0.06–13.82) | 0.25 (0.03–2.15) | 2.83c (0.12–64.89) |
| Sevoflurane vs. propofol56 | 0.23 (0.01–4.52) | 1.13 (0.07–17.34) | 0.16 (0.01–3.00) | Not reported | Not reported |
| Prostaglandin E1 vs. placebo73 | Not reported | 0.5 (0.05–4.81) | Not reported | 3.00 (0.13–67.06) | Not reported |
| Trimetazidine vs. placebo70 | Not reported | 0.5 (0.05–5.28) | 0.14 (0.01–2.67) | 7.00 (0.37–131.06) | Not reported |
| Ulinastatin vs. gantaile63 | Not reported | Not reported | 0.94 (0.06–13.68) | 0.63 (0.12–3.24) | Not reported |
ITU and hospital stay
The postoperative ITU stay was reported to be significantly shorter in the vitamin E compared with placebo groups, although the exact length of stay was not provided for each group.55 None of the remaining comparisons reported any significant difference in postoperative ITU stay.
Postoperative hospital stay was significantly shorter in the trimetazidine compared with placebo groups70 (MD −3.0 days, 95% CI −3.57 to −2.43) and the methylprednisolone compared with control groups54,59,65,67–69,77 using the fixed-effects model (MD −1.69 days, 95% CI −2.90 to −0.47), but not the random-effects model (MD −1.07 days, 95% CI −3.36 to −1.21). None of the remaining comparisons reported any significant difference in length of postoperative hospital stay.
Blood transfusion requirements and blood loss
Blood transfusion requirements were significantly lower in the methylprednisolone compared with control groups54,59,65,67–69 (SMD −0.68, 95% CI −1.06 to −0.31). None of the remaining comparisons reported any significant difference in blood transfusion requirements.
None of the comparisons showed any significant difference in the rate of blood loss.
Markers of function
The bilirubin level was significantly lower in the methylprednisolone compared with control groups on the first postoperative day (POD)54,59,67–69,77 (MD −15.66, 95% CI −20.77 to −10.55), second POD54,59,67–69 (MD −18.64, 95% CI −27.78 to −9.50) and fifth POD54,59,67–69 (MD −8.72, 95% CI −15.65 to −1.79). The bilirubin level was reported to be significantly lower in the ulinastatin compared with gantaile groups on the third POD, although exact levels were not provided for each group.63 None of the remaining comparisons reported any significant differences in postoperative bilirubin level.
Prothrombin time was significantly improved in the methylprednisolone compared with control groups on the first POD54,59,67–69,77 (SMD −0.58, 95% CI −0.97 to −0.19) and second POD54,59,67–69 (SMD −1.07, 95% CI −1.56 to −0.58). None of the remaining comparisons reported any significant differences in postoperative prothrombin times.
Enzyme markers of liver injury
Postoperative AST and ALT levels that were reported to show significant differences between groups are summarized in Table 3. None of the remaining comparisons reported any significant differences in postoperative AST or ALT levels.
Table 3.
Postoperative enzyme markers of liver injurya
| Comparison | Postoperative day | AST, MD (95% CI) | ALT MD (95% CI) |
|---|---|---|---|
| Dextrose vs. control74,75 | 1 | −107.20 (−1.53 to −0.83) | −123.3 (−182.47 to −64.13) |
| 5 | −89.7 (−133.04 to −6.36) | −191.0 (−243.15 to −138.85) | |
| Methylprednisolone vs. control54,59,67–69 | 2 | −117.0 (−221.03 to −12.97) | −125.0 (−239.48 to −10.52) |
| Trimetazidine vs. placebo70 | 1 | −29.0 (−56.92 to −1.08) | −30.0 (−59.50 to −0.50) |
| 3 | −20.0 (−39.69 to −0.31) | −19.0 (−37.71 to −0.29) | |
| 5 | −7.0 (−13.56 to −0.44) | −15.0 (−29.75 to −0.25) | |
| 7 | −8.0 (−15.37 to −0.63) | ||
| 10 | −5.0 (−9.90 to −0.10) | ||
| Sevoflurane vs. propofol56 | Peak levels | −254.18 (−450.59 to −57.77) | |
| Ulinastatin vs. gantaileb63 | AST on 3 ALT on 1, 3, 7 | Exact value not reported | Exact values not reported |
| Vitamin E vs. placeboc55 | 1 to 6 | Area under curve. Exact value not reported |
Only significant results are shown
The trial authors reported a significant difference between the groups but did not provide the exact numerical values
The trial authors reported a significant reduction in AST levels on days 1–6 postoperatively as measured by the area under the curve
AST, aspartate aminotransferase; ALT, alanine aminotransferase; CI, confidence interval; MD, mean difference
Side-effects
Two of the pharmacological interventions resulted in side-effects. Pentoxifylline caused nausea and vomiting in 46% of participants, who consequently failed to receive all the doses of study medication. One patient in the vitamin E group complained of a headache.
Discussion
Effectiveness
None of the interventions resulted in a decrease in mortality, liver failure or perioperative morbidity. However, some of the interventions resulted in improvement in secondary outcomes such as length of hospital stay, markers of liver function and enzyme markers of liver injury. These interventions were methylprednisolone, trimetazidine, dextrose and ulinastatin. These are discussed in further detail.
Methylprednisolone
Methylprednisolone is a glucocorticoid steroid which acts as an anti-inflammatory agent, reducing inflammatory markers and apoptotic cell count in experimental liver IR injury.16 Three trials evaluated methylprednisolone.54,59,65,67–69,77Methylprednisolone decreased hospital stay and blood transfusion requirements. It also improved liver function and showed a trend favouring a decreased postoperative complication rate. However, there was considerable heterogeneity among the trials. The trial by Aldrighetti et al.54,59,67–69 generally showed beneficial effects of the steroid, but the other two trials (Muratore et al.,65 Yamashita et al.77) did not demonstrate any such benefits.
These differences may reflect the much higher dose of the steroid administered in the trial by Muratore et al.65 In all three trials, methylprednisolone was administered as a single intravenous dose preoperatively because the biological actions of this steroid last for 36 hours78 and therefore cover both the early and delayed phases of IR injury. Two of the trials used a dose of 500 mg (Yamashita et al.,77 Aldrighetti et al.54,59,67–69), which results in blood and liver tissue levels that significantly attenuate postoperative inflammatory pathways,79 whereas the third trial (Muratore et al.65) used a dose of 30 mg/kg based on previous work showing a decrease of inflammatory markers in patients who were administered methylprednisolone at this dose.41 This translates to a dose approximately four times that used in the other trials for an average 70-kg man.
Other possible explanations for the differences observed between the trials may reflect the type of liver ischaemia, the length of liver ischaemia, and the proportion of trial participants undergoing major liver resection. In the trial by Aldrighetti et al.,54,59,67–69 intermittent portal triad clamping (PTC) was used. The other two trials65,77 used continuous PTC. In a recent Cochrane review7 intermittent PTC tended to produce better outcomes than continuous PTC. Aldrighetti et al.54,59,67–69 also performed a multivariate analysis in which the methylprednisolone group had significantly lower ALT and bilirubin levels compared with the control group when the total liver ischaemia time was >30 min or when the liver volume resected amounted to >60% of liver volume. This multivariate analysis supports the results of the three trials under this comparison. This is because the trial (Aldrighetti et al.54,59,67–69) with the longest mean ischaemic time (47.6 min) and highest proportion of participants undergoing major liver resection (72%) reported the most significant improvements in ALT and bilirubin in the postoperative period compared with the other two trials (Yamashita et al.,77 Muratore et al.65). The Muratore et al.65 trial had a mean ischaemic time of 39.2 min and 53% of all trial participants underwent a major resection, whereas the Yamashita et al.77 trial did not report ischaemic time and only 33% of its participants underwent a major resection.
Recognized side-effects of steroid use, such as infection, poor wound healing and glucose intolerance, were not reported in these trials to any greater extent in the methylprednisolone groups. This is most probably the result of the single-dose treatment method adopted in these trials as the aforementioned side-effects are usually related to longer periods of treatment with steroids. Methylprednisolone is an immunomodulator which affects various immunological pathways, many of which are involved in the pathophysiology of tumour development, progression and recurrence.80 Theoretically, it is possible that methylprednisolone use could increase cancer progression and recurrence. Therefore, future trials evaluating methylprednisolone in cancer patients should ensure longterm follow-up to monitor disease recurrence and survival.
Given the possibility that the protective effects of methylprednisolone become more apparent as the extent of liver resection increases (methylprednisolone has a greater protective effect when >60% of liver volume is resected), as well as in the presence of chronic liver disease (the protective effect increases in patients with chronic liver disease or cirrhosis), according to the method of vascular occlusion (the protective effect increases in intermittent vascular occlusion), and depending on the duration of ischaemia (the protective effect increases when total ischaemic time is >30 min), further research is warranted to clarify the benefits, or otherwise, of methylprednisolone in these subgroups.
Trimetazidine
Trimetazidine is an antianginal drug that works by shifting cellular energy metabolism from fatty acid oxidation to glucose oxidation, leading to increased adenosine triphosphate (ATP) production and reduced oxygen consumption.81 Trimetazidine has been shown to decrease liver IR injury in experimental models.82 In the one trial that evaluated trimetazidine (Settaf et al.70), there were no mortalities and no significant differences in rates of liver decompensation or perioperative morbidity. However, postoperative AST and ALT levels were significantly lower in the trimetazidine group compared with the placebo group. Furthermore, hospital stay was significantly shorter in the trimetazidine group. Trimetazidine was administered at 40 mg twice per day starting 5 days before the procedure until the day of surgery. The authors of this trial admit that their dosing regimen may not have been optimal and further trials evaluating the optimal dose–response relationship will be needed. There were no side-effects reported. Based on the results of this trial, trimetazidine may have a role in protecting the liver during resection under vascular occlusion. However, further investigations including trials to evaluate the optimal time and dose of administration, as well as liver function, are required.
Dextrose
Dextrose elevates liver glycogen stores and ATP content, thereby maintaining hepatocyte and mitochondrial membrane integrity and leading to a reduction in hepatocyte necrosis.74,75 One trial evaluated dextrose (Tang et al.74,75). Dextrose decreased the AST and ALT levels. A dose of 250 ml of 25% dextrose with 10 units of insulin and 10 ml of 10% potassium chloride was administered four times daily on the preoperative day. The timing and dose of dextrose were chosen to significantly elevate the hepatic glycogen content immediately prior to ischaemia. There were no reported side-effects of the study medication. Based on these results, dextrose may play a role in liver protection during resection under vascular control. However, this trial failed to report on many outcomes of relevance, including mortality and liver decompensation. Therefore, further trials of high methodological quality to assess these and the other outcomes of interest are needed.
Ulinastatin
Ulinastatin is a protease inhibitor that acts by reducing the activation of white blood cells and the release of inflammatory cytokines in liver IR injury.83 One trial (Li & Liang63) evaluated ulinastatin and gantaile. There was no reported mortality in this clinical trial. Ulinastatin lowered the AST, ALT and bilirubin levels without affecting rates of liver decompensation, perioperative morbidity or length of hospital stay. Ulinastatin was commenced at a dose of 10 000 IU intraoperatively, followed by twice daily administration at the same dose combined with vitamin K1 and glucose for 5 consecutive days. The dosage at which gantaile was administered was not reported by the investigators, but vitamin K1 and glucose were administered as for ulinastatin. The authors did not explain why they had chosen this dosing regime. There were no reported side-effects of the study medication. Based on these results, it seems that ulinastatin may offer a protective role in elective liver resections under vascular occlusion. Further trials of good methodological quality are required.
Other interventions
None of the remaining interventions showed any consistent benefit in any of the outcome measures. Furthermore, two of these interventions, utilizing prostaglandin E1 and multivitamin antioxidants, were reported on by more than one RCT each. The results of the outcome measures in each of these interventions were consistent in the different trials.
Relative effectiveness of the pharmacological agents
The number of trials under each comparison was so few that an indirect comparison is unlikely to yield any meaningful inferences. Therefore, we did not attempt to infer the relative effectiveness of the pharmacological agents from the available data.
Subgroup analysis
Patients with liver cirrhosis, steatosis or undergoing major liver resections are known to be at high risk for developing IR injury. For this reason, we intended to perform a subgroup analysis on each of these. However, the lack of numerical reporting of outcome measures in each of these subgroups and the few trials included within each comparison made us unable to do so.
Safety
The pharmacological agents used in the RCTs cited in this review can be divided into those that are clinically licensed, for which the side-effects have been well profiled, and those that are not clinically licensed. The clinically licensed drugs include trimetazidine, vitamin E, multivitamin infusion, pentoxifylline, mannitol, amrinone, methylprednisolone, allopurinol, dextrose, dopexamine, dopamine, sevoflurane and propofol. The side-effects reported for these interventions were nausea and vomiting in 46% of patients in the pentoxifylline group, who failed to receive all the doses of study medication as a consequence. In addition, one case of headache was reported in the vitamin E group and four cases of liver decompensation were reported in the multivitamin infusion group. Although this level of incidence does not attain statistical significance compared with the control group, these liver decompensations raise concerns about the safety of the multivitamin antioxidant infusion in liver resections. This is because liver decompensation or failure is considered a direct indicator of liver injury. Thus, using the multivitamin antioxidant mixture may actually cause harm in the clinical setting of this review. There has been no previous work showing a causal relationship between multivitamin intake and liver failure. However, a comprehensive meta-analysis of 47 RCTs at low risk of bias investigating antioxidant consumption, including multivitamin combinations, showed a significant increase in mortality.84 Therefore, it may be possible that multivitamin usage in the setting of liver resections with vascular occlusion is not without serious consequences. Further basic animal research is needed to confirm or reject this theory.
Quality of evidence and future trials
None of the included RCTs were at low risk of bias. This reflects poor trial design. This is one of the few instances in the field of hepatopancreatobiliary surgery in which trials with adequate randomization and blinding can be conducted. Poor trial design can lead to erroneous conclusions (systematic errors).22 Only two trials (Marx et al.,64 Muratore et al.65) provided sample size calculations, but even these trials were not powered to measure any differences in the clinically relevant outcomes. The number of trials included under each comparison was few. Thus, there is a high risk of type I (false positive) and type II (false negative) errors (random errors). Therefore, the risk of both random and systematic errors in the trials assessed in this review is high. Aldrighetti et al.54 observed that methylprednisolone was more beneficial in a subgroup of patients. Stratification of patients based on the background liver (cirrhosis, steatosis, normal liver) and the extent of liver resection (major or minor liver resection) will allow the identification of specific subgroups for which the pharmacological interventions are beneficial.
The length of follow-up in the trials should be appropriate. Most of the trials included in this review followed patients only until their discharge. Recently, there have been concerns about using vascular occlusion in liver resections carried out for malignancy as it is hypothesized that IR injury can increase the rate of recurrence.85 Therefore, interventions that influence IR injury may also influence disease recurrence and patient survival. The length of follow-up in the trials should be long enough to assess patient survival and disease recurrence rates.
Future trials evaluating pharmacological interventions in liver resections under vascular occlusion should include patient-oriented outcomes. Direct markers of liver function or dysfunction, such as postoperative morbidity, should be given more priority than surrogate markers of liver function, such as enzyme markers of liver injury. This is supported by the use of validated systems for classifying postoperative surgical complications.86 The measurement of clinically oriented outcomes and potential surrogate markers will allow the simultaneous assessment of interventions and the validation of surrogate markers so that future randomized clinical trials can be powered to measure any validated surrogate outcome.
Conclusions
Methylprednisolone, trimetazidine, dextrose and ulinastatin may have protective roles against IR injury in liver resections performed under vascular occlusion. However, based on the current evidence, they cannot be recommended for routine use and their application should be restricted to RCTs.
Acknowledgments
We would like to acknowledge the Cochrane Hepato-Biliary Group for its support.
Conflicts of interest
This paper is a substantially shortened version of two Cochrane reviews submitted to the Cochrane Hepato-Biliary Group. Cochrane reviews are regularly updated as new evidence emerges and in response to comments and criticisms. The Cochrane Library should be consulted for the most recent version of the reviews. The results of Cochrane reviews can be interpreted differently, depending on the reader's perspectives and circumstances. Please consider the conclusions presented carefully. They are the opinions of the review authors and are not necessarily shared by the Cochrane Collaboration.
References
- 1.Belghiti J, Kabbej M, Sauvanet A, Vilgrain V, Panis Y, Fekete F. Drainage after elective hepatic resection. A randomized trial. Ann Surg. 1993;218:748–753. doi: 10.1097/00000658-199312000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chouker A, Martignoni A, Schauer R, Dugas M, Rau HG, Jauch KW, et al. Beneficial effects of ischaemic preconditioning in patients undergoing hepatectomy: the role of neutrophils. Arch Surg. 2005;140:129–136. doi: 10.1001/archsurg.140.2.129. [DOI] [PubMed] [Google Scholar]
- 3.Hospital Episode Statistics. Main operations. 3 character: 2004–05. http://www.hesonline.nhs.uk/Ease/servlet/ContentServer?siteID=1937&categoryID=205. [Accessed 17 April 2007.
- 4.Ibrahim S, Chen CL, Lin CC, Yang CH, Wang CC, Wang SH, et al. Intraoperative blood loss is a risk factor for complications in donors after living donor hepatectomy. Liver Transplant. 2006;12:950–957. doi: 10.1002/lt.20746. [DOI] [PubMed] [Google Scholar]
- 5.Shimada M, Takenaka K, Fujiwara Y, Gion T, Shirabe K, Yanaga K, et al. Risk factors linked to postoperative morbidity in patients with hepatocellular carcinoma. Br J Surg. 1998;85:195–198. doi: 10.1046/j.1365-2168.1998.00567.x. [DOI] [PubMed] [Google Scholar]
- 6.Yoshimura Y, Kubo S, Shirata K, Hirohashi K, Tanaka H, Shuto T, et al. Risk factors for postoperative delirium after liver resection for hepatocellular carcinoma. World J Surg. 2004;28:982–986. doi: 10.1007/s00268-004-7344-1. [DOI] [PubMed] [Google Scholar]
- 7.Gurusamy KS, Sheth H, Kumar Y, Sharma D, Davidson BR. Methods of vascular occlusion for elective liver resections. Cochrane Database Syst Rev. 2009;21:CD007632. doi: 10.1002/14651858.CD007632. [DOI] [PubMed] [Google Scholar]
- 8.Gurusamy KS, Kumar Y, Ramamoorthy R, Sharma D, Davidson BR. Vascular occlusion for elective liver resections. Cochrane Database Syst Rev. 2009;21:CD007530. doi: 10.1002/14651858.CD007530. [DOI] [PubMed] [Google Scholar]
- 9.Kupiec-Weglinski JW, Busuttil RW. Ischaemia and reperfusion injury in liver transplantation. Transplant Proceed. 2005;37:1653–1656. doi: 10.1016/j.transproceed.2005.03.134. [DOI] [PubMed] [Google Scholar]
- 10.Azoulay D, Lucidi V, Andreani P, Maggi U, Sebagh M, Ichai P, et al. Ischaemic preconditioning for major liver resection under vascular exclusion of the liver preserving the caval flow: a randomized prospective study. J Am Coll Surg. 2006;202:203–211. doi: 10.1016/j.jamcollsurg.2005.10.021. [DOI] [PubMed] [Google Scholar]
- 11.Smyrniotis V, Theodoraki K, Arkadopoulos N, Fragulidis G, Condi-Pafiti A, Plemenou-Fragou M, et al. Ischaemic preconditioning versus intermittent vascular occlusion in liver resections performed under selective vascular exclusion: a prospective randomized study. Am J Surg. 2006;192:669–674. doi: 10.1016/j.amjsurg.2006.02.019. [DOI] [PubMed] [Google Scholar]
- 12.Azoulay D, Eshkenazy R, Andreani P, Castaing D, Adam R, Ichai P, et al. In situ hypothermic perfusion of the liver versus standard total vascular exclusion for complex liver resection. Ann Surg. 2005;241:277–285. doi: 10.1097/01.sla.0000152017.62778.2f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kim YI, Hiratsuka K, Kitano S, Joo DH, Kamada N, Sugimachi K. Simple in situ hypothermia reduced ischaemic injury to human liver during hepatectomy. Eur J Surg. 1996;162:717–721. [PubMed] [Google Scholar]
- 14.Galaris D, Barbouti A, Korantzopoulos P. Oxidative stress in hepatic ischaemia-reperfusion injury: the role of antioxidants and iron chelating compounds. Curr Pharm Des. 2006;12:2875–2890. doi: 10.2174/138161206777947614. [DOI] [PubMed] [Google Scholar]
- 15.Georgiev P, Dahm F, Graf R, Clavien PA. Blocking the path to death: anti-apoptotic molecules in ischaemia/reperfusion injury of the liver. Curr Pharm Des. 2006;12:2911–2921. doi: 10.2174/138161206777947588. [DOI] [PubMed] [Google Scholar]
- 16.Saidi RF, Chang J, Verb S, Brooks I, Nalbantoglu I, Adsay V, et al. The effect of methylprednisolone on warm ischaemia-reperfusion injury in the liver. Am J Surg. 2007;193:345–347. doi: 10.1016/j.amjsurg.2006.09.017. [DOI] [PubMed] [Google Scholar]
- 17.Soltys K, Dikdan G, Koneru B. Oxidative stress in fatty livers of obese Zucker rats: rapid amelioration and improved tolerance to warm ischaemia with tocopherol. Hepatology. 2001;34:13–18. doi: 10.1053/jhep.2001.25452. [DOI] [PubMed] [Google Scholar]
- 18.Kullmann R, Breull WR, Reinsberg J, Wassermann K, Konopatzki A. Dopamine produces vasodilation in specific regions and layers of the rabbit gastrointestinal tract. Life Sci. 1983;32:2115–2122. doi: 10.1016/0024-3205(83)90100-5. [DOI] [PubMed] [Google Scholar]
- 19.Lokhandwala MF, Jandhyala BS. Effects of dopaminergic agonists on organ blood flow and function. Clin Intensive Care. 1992;3(Suppl. 1):12–16. [Google Scholar]
- 20.Royle P, Milne R. Literature searching for randomized controlled trials used in Cochrane reviews: rapid versus exhaustive searches. Int J Technol Assess Health Care. 2003;19:591–603. doi: 10.1017/s0266462303000552. [DOI] [PubMed] [Google Scholar]
- 21.Higgins JPT, Green S. Cochrane Handbook for Systematic Reviews of Interventions Version 5.0.0. Cochrane Collaboration.
- 22.Wood L, Egger M, Gluud LL, Schulz K, Jüni P, Altman DG, et al. Empirical evidence of bias in treatment effect estimates in controlled trials with different interventions and outcomes: meta-epidemiological study. BMJ (Clinical research ed.) 2008;336:601–605. doi: 10.1136/bmj.39465.451748.AD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gluud C, Nikolova D, Klingenberg SL, Alexakis N, Als-Nielsen B, D’Amico G, et al. Cochrane Hepato-Biliary Group About the Cochrane Collaboration (Cochrane Review Groups (CRGs)), Issue 1. Art. No.: LIVER.
- 24.Gurusamy KS, Gluud C, Nikolova D, Davidson BR. Assessment of risk of bias in randomized clinical trials in surgery. Br J Surg. 2009;96:342–349. doi: 10.1002/bjs.6558. [DOI] [PubMed] [Google Scholar]
- 25.Cochrane Collaboration. Copenhagen: Nordic Cochrane Centre, Cochrane Collaboration; Review Manager (RevMan) (computer programme) [Google Scholar]
- 26.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 27.DeMets DL. Methods for combining randomized clinical trials: strengths and limitations. Stat Med. 1987;6:341–350. doi: 10.1002/sim.4780060325. [DOI] [PubMed] [Google Scholar]
- 28.Higgins JPT, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21:1539–1558. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 29.Newell DJ. Intention-to-treat analysis: implications for quantitative and qualitative research. Int J Epidemiol. 1992;21:837–841. doi: 10.1093/ije/21.5.837. [DOI] [PubMed] [Google Scholar]
- 30.Baek Y, Nakano H, Kumada K, Nagasaki H, Kigawa G, Sasaki J, et al. Administration of prostaglandin E1 reduces postoperative hepatocellular damage and restores hepatic integrity in patients undergoing hepatectomy. Hepatogastroenterology. 1999;46:1836–1841. [PubMed] [Google Scholar]
- 31.Garcia-Valdecasas JC, Rull R, Rimola E. The role of systemic prostaglandins during human liver transplantation. Ann N Y Acad Sci. 1994;723:473–475. [PubMed] [Google Scholar]
- 32.Hanazaki K, Kajikawa S, Fujimori Y, Nakata S, Shimozawa N, Koide N, et al. Effects of prostaglandin E1 administration during hepatectomy for cirrhotic hepatocellular carcinoma. Hepatogastroenterology. 2000;47:461–464. [PubMed] [Google Scholar]
- 33.Hayakawa J, Yoshida G, Usuda Y. Effect of prostaglandin E1 on arterial ketone body ratio in hepatectomy. J Anesth. 1994;8:167–171. doi: 10.1007/BF02514707. [DOI] [PubMed] [Google Scholar]
- 34.Inagaki H, Kurokawa T, Nonami T, Miwa T, Nakao A, Takagi H. The effect of intraportal administration of prostaglandin E1 on liver blood flow and liver function. Hepatogastroenterology. 1999;46:2909–2913. [PubMed] [Google Scholar]
- 35.Kaiho T, Tsuchiya S, Yanagisawa S, Takeuchi O, Togawa A, Okamoto R, et al. Effect of the herbal medicine Inchin-Ko-To for serum bilirubin in hepatectomized patients. Hepatogastroenterology. 2008;55:150–154. [PubMed] [Google Scholar]
- 36.Katsuramaki T, Mukaiya M, Yamashiro K, Kimura H, Denno R, Hirata K. Beneficial effects of administering intraportal prostaglandin E1 postoperatively to hepatectomy patients with massive intraoperative blood loss. Surg Today. 1996;26:895–899. doi: 10.1007/BF00311791. [DOI] [PubMed] [Google Scholar]
- 37.Kim YI, Hwang YJ, Song KE, Yun YK, Lee JW, Chun BY. Hepatocyte protection by a protease inhibitor against ischaemia/reperfusion injury of human liver. J Am Coll Surg. 2002;195:41–50. doi: 10.1016/s1072-7515(01)01118-8. [DOI] [PubMed] [Google Scholar]
- 38.Kim YI, Chung HJ, Song KE, Hwang YJ, Lee JW, Lee Y. Evaluation of a protease inhibitor in the prevention of ischaemia and reperfusion injury in hepatectomy under intermittent Pringle manoeuvre. Am J Surg. 2006;191:72–76. doi: 10.1016/j.amjsurg.2005.04.018. [DOI] [PubMed] [Google Scholar]
- 39.Kim YI, Fujita S, Hwang YJ, Chun JM, Song KE, Chun BY. Successful intermittent application of the Pringle manoeuvre for 30 minutes during human hepatectomy: a clinical randomized study with use of a protease inhibitor. Hepatogastroenterology. 2007;54:2055–2060. [PubMed] [Google Scholar]
- 40.Nakagawa I, Izumi H, Fujii K, Kurokawa H, Kusunoki K, Yuge O, et al. Comparison of the effect of PGE1 and dopamine on arterial blood ketone body ratio in hepatectomized patients. Hiroshima J Anesth. 1991;27:367–371. [Google Scholar]
- 41.Shimada M, Saitoh A, Kano T, Takenaka K, Sugimachi K. The effect of a perioperative steroid pulse on surgical stress in hepatic resection. Int Surg. 1996;81:49–51. [PubMed] [Google Scholar]
- 42.Suehiro T, Shimura T, Okamura K, Okada T, Okada K, Hashimoto S, et al. The effect of hyperbaric oxygen treatment on postoperative morbidity of left lobe donor in living donor adult liver transplantation. Hepatogastroenterology. 2008;55:1014–1019. [PubMed] [Google Scholar]
- 43.Une Y, Shimamura T, Uchino J. Effect of prostaglandin E(1) on hepatic function after hepatectomy in patients with chronic liver disease. Clin Ther. 1995;17:1118–1125. doi: 10.1016/0149-2918(95)80090-5. [DOI] [PubMed] [Google Scholar]
- 44.Dunschede F, Erbes K, Kircher A, Westermann S, Seifert J, Schad A, et al. Reduction of ischaemia-reperfusion injury after liver resection and hepatic inflow occlusion by (alpha)-lipoic acid in humans. World J Gastroenterol. 2006;12:6812–6817. doi: 10.3748/wjg.v12.i42.6812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schemmer P, Nickkholgh A, Schneider H, Sobirey M, Weigand M, Koch M, et al. Pilot study on the safety and tolerance of preoperative melatonin application in patients undergoing major liver resection: a double-blind randomized placebo-controlled trial. BMC Surg. 2008;8:2. doi: 10.1186/1471-2482-8-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hassanain M, Schricker T, Metrakos P, Carvalho G, Vrochides D, Lettermann R. Hepatic protection by perioperative metabolic support? Nutrition. 2008;24:1217–1219. doi: 10.1016/j.nut.2008.05.019. [DOI] [PubMed] [Google Scholar]
- 47.Inagaki H, Nonami T, Kurokawa T, Takeuchi Y, Okuda N, Nakao A. Effects of nafamostat mesilate, a synthetic protease inhibitor, on immunity and coagulation after hepatic resection. Hepatogastroenterology. 1999;46:3223–3228. [PubMed] [Google Scholar]
- 48.Nakayama M, Kanaya N, Fujita S, Kawana S, Namiki A. Effect of prostaglandin E1 on arterial ketone body ratio during and after hepatectomy. Masui. 1995;44:1224–1227. [PubMed] [Google Scholar]
- 49.Schmidt SC, Hamann S, Langrehr JM, Hoflich C, Mittler J, Jacob D, et al. Preoperative high-dose steroid administration attenuates the surgical stress response following liver resection: results of a prospective randomized study. J Hepato-Biliary-Pancreatic Surg. 2007;14:484–492. doi: 10.1007/s00534-006-1200-7. [DOI] [PubMed] [Google Scholar]
- 50.Sheth H, Glantzounis G, Hafez T, Quaglia A, Duncan J, Davidson BR. Does perioperative N-aceylcysteine prevent ischaemia-reperfusion injury during liver resection? A prospectively randomized double-blind clinical trial. Gut. 2005;54:A39. [Google Scholar]
- 51.Sheth H, Glantzounis G, Hafez T, Quaglia A, Duncan J, Davidson BR. A randomized double-blind controlled clinical trial to assess the effects of prophylactic N-acetylcysteine on liver injury during liver resection. Br J Surg. 2005;92:1307. [Google Scholar]
- 52.Shimada M, Matsumata T, Taketomi A, Shirabe K, Yamamoto K, Sugimachi K. The role of prostaglandins in hepatic resection. Prostaglandins Leukot Essent Fatty Acids. 1994;50:65–68. doi: 10.1016/0952-3278(94)90149-x. [DOI] [PubMed] [Google Scholar]
- 53.Wada Y, Zaima M, Mori K, Egawa H, Higashiyama H, Iwata S, et al. Effect of gabexate mesilate on thrombin and plasmin generation after hepatic resection in cirrhotic patients. Eur Surg Res. 1995;27:57–62. doi: 10.1159/000129373. [DOI] [PubMed] [Google Scholar]
- 54.Aldrighetti L, Pulitano C, Arru M, Finazzi R, Catena M, Soldini L. Impact of preoperative steroids administration on ischaemia-reperfusion injury and systemic responses in liver surgery: a prospective randomized study. Liver Transplant. 2006;12:941–949. doi: 10.1002/lt.20745. [DOI] [PubMed] [Google Scholar]
- 55.Bartels M, Biesatski HK, Engelhart K, Sendlhofer G, Rehak P, Nagel E. Pilot study on the effect of parenteral vitamin E on ischaemia and reperfusion-induced liver injury: a double-blind, randomized, placebo-controlled trial. Clin Nutr. 2004;23:1360–1370. doi: 10.1016/j.clnu.2004.05.003. [DOI] [PubMed] [Google Scholar]
- 56.Beck-Schimmer B, Breitenstein S, Urech S, De Conno E, Wittlinger M, Puhan M, et al. A randomized controlled trial on pharmacological preconditioning in liver surgery using a volatile anaesthetic. Ann Surg. 2008;248:909–918. doi: 10.1097/SLA.0b013e31818f3dda. [DOI] [PubMed] [Google Scholar]
- 57.Cerwenka H, Khoschsorur G, Bacher H, Werkgartner G, El-Shabrawi A, Quehenberger F, et al. Normothermic liver ischaemia and antioxidant treatment during hepatic resections. Free Radic Res. 1999;30:463–469. doi: 10.1080/10715769900300501. [DOI] [PubMed] [Google Scholar]
- 58.Cerwenka H, Bacher H, Werkgartner G, El-Shabrawi A, Quehenberger F, Hauser H. Antioxidant treatment during liver resection for alleviation of ischaemia-reperfusion injury. Hepatogastroenterology. 1998;45:777–782. [PubMed] [Google Scholar]
- 59.Finnazi R, Pulitano C, Aldrighetti L, Arru M, Catena M, Milani F. Benefit of preoperative corticosteroid therapy on the hepatic ischaemia-reperfusion injury and cytokine response in patients undergoing hepatic resection: a prospective randomized trial. J Hepatolol. 2005;42:49. [Google Scholar]
- 60.Holtje M, Mahr KH, Bornscheuer A, Marx G, Stamme C, Rueckoldt H. Circulatory function and oxygenation during hemihepatectomy. Dopamine versus dopexamine. Anaesthesist. 1999;48:224–230. doi: 10.1007/s001010050694. [DOI] [PubMed] [Google Scholar]
- 61.Kawano T, Hosokawa N, Maruta T, Maruta N, Takasaki M. Re-evaluation of protective effects of alprostadil on hepatic function in patients undergoing hepatectomy. Masui. 2005;54:982–991. [PubMed] [Google Scholar]
- 62.Kostopanagiotou G, Pandazi AK, Andreadou I, Markantonis SL, Niokou D, Teloudis A. Effects of mannitol in the prevention of lipid peroxidation during liver resection with hepatic vascular exclusion. J Clin Anesth. 2006;18:570–574. doi: 10.1016/j.jclinane.2006.03.014. [DOI] [PubMed] [Google Scholar]
- 63.Li SQ, Liang LJ. Protection of liver function with protease inhibitor from ischaemia-reperfusion injury in hepatocellular carcinoma patients undergoing hepatectomy after hepatic inflow occlusion. Chin J Bases Clin Gen Surg. 2004;11:61–64. [Google Scholar]
- 64.Marx G, Leuwer M, Holtje M, Bornscheuer A, Herrmann H, Mahr K. Low-dose dopexamine in patients undergoing hemihepatectomy: an evaluation of effects on reduction of hepatic dysfunction and ischaemic liver injury. Acta Anaesthesiol Scand. 2000;44:410–416. doi: 10.1034/j.1399-6576.2000.440409.x. [DOI] [PubMed] [Google Scholar]
- 65.Muratore A, Ribero D, Ferrero A, Bergero R, Capussotti L. Prospective randomized study of steroids in the prevention of ischaemic injury during hepatic resection with pedicle clamping. Br J Surg. 2003;90:17–22. doi: 10.1002/bjs.4055. [DOI] [PubMed] [Google Scholar]
- 66.Orii R, Sugawara Y, Hayashida M, Yamada Y, Chang K, Takayama T, et al. Effects of amrinone on ischaemia-reperfusion injury in cirrhotic patients undergoing hepatectomy: a comparative study with prostaglandin E1. Br J Anaesth. 2000;85:389–395. doi: 10.1093/bja/85.3.389. [DOI] [PubMed] [Google Scholar]
- 67.Pulitano C, Aldrighetti L, Finazzi R, Arru M, Catena M, Ferla G, et al. Inhibition of cytokine response by methylprednisolone attenuates antithrombin reduction following hepatic resection (2) Thromb Haemost. 2005;93:1199–1200. doi: 10.1160/TH06-01-0058. [DOI] [PubMed] [Google Scholar]
- 68.Pulitano C, Aldrighetti L, Arru M, Finnazi R, Soldini L, Catena M, et al. Prospective randomized study of the benefits of preoperative corticosteroid administration on hepatic ischaemia-reperfusion injury and cytokine response in patients undergoing hepatic resection. HPB. 2007;9:183–189. doi: 10.1080/13651820701216984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Pulitano C, Aldrighetti L, Arru M, Finazzi R, Catena M, Guzzetti E, et al. Preoperative methylprednisolone administration maintains coagulation homeostasis in patients undergoing liver resection: importance of inflammatory cytokine modulation. Shock. 2007;28:401–405. doi: 10.1097/shk.0b013e318063ed11. [DOI] [PubMed] [Google Scholar]
- 70.Settaf A, Zaim N, Bellouch M, Tillement JP, Morin D. Trimetazidine alleviates ischaemia-reperfusion injury induced by vascular clamping of the liver. Therapie. 2001;56:569–574. [PubMed] [Google Scholar]
- 71.Shirabe K, Takenaka K, Yamamoto K, Kitamura M, Itasaka H, Matsumata T. The role of prostanoid in hepatic damage during hepatectomy. Hepatogastroenterology. 1996;43:596–601. [PubMed] [Google Scholar]
- 72.Stadheim LM, Gores GJ, Lee TC, Nagorney DM. Normothermic ischaemia-reperfusion injury during hepatic resection: a prospective randomized placebo-controlled study of pretreatment with misoprostol or pentoxifylline. Hepatology. 2000;32:250A. [Google Scholar]
- 73.Sugawara Y, Kubota K, Ogura T, Esumi H, Inoue K, Takayama T. Protective effect of prostaglandin E1 against ischaemia/reperfusion-induced liver injury: results of a prospective, randomized study in cirrhotic patients undergoing subsegmentectomy. J Hepatol. 1998;29:969–976. doi: 10.1016/s0168-8278(98)80125-6. [DOI] [PubMed] [Google Scholar]
- 74.Tang L, Tian F, Tao W, Cui J. Hepatocellular glycogen in alleviation of liver ischaemia-reperfusion injury during partial hepatectomy. World J Surg. 2007;31:2039–2043. doi: 10.1007/s00268-007-9186-0. [DOI] [PubMed] [Google Scholar]
- 75.Tang LJ, Tian FZ, Gao XM. Hepatocellular glycogen in alleviation of liver ischaemia-reperfusion injury. Hepatobiliary Pancreat Dis Int. 2002;1:532–535. [PubMed] [Google Scholar]
- 76.Vriens MR, Marinelli A, Harinck HIJ, Zwinderman KH, van de Velde CJH. The role of allopurinol in human liver ischaemia/reperfusion injury: a prospective randomized clinical trial. Hepatogastroenterology. 2002;49:1069–1073. [PubMed] [Google Scholar]
- 77.Yamashita Y, Shimada M, Hamatsu T, Rikimaru T, Tanaka S, Shirabe K. Effects of preoperative steroid administration on surgical stress in hepatic resection – prospective randomized trial. Arch Surg. 2001;136:328–333. doi: 10.1001/archsurg.136.3.328. [DOI] [PubMed] [Google Scholar]
- 78.Staubbs SS. Corticosteroids and bioavailability. Transplant Proc. 1975;7:11–19. [PubMed] [Google Scholar]
- 79.Webel ML, Ritts RE, Jr, Taswell HF, Danadio JV, Jr, Woods JE. Cellular immunity after intravenous administration of methylprednisolone. J Lab Clin Med. 1974;83:383–392. [PubMed] [Google Scholar]
- 80.Waldmann TA. Effective cancer therapy through immunomodulation. Annu Rev Med. 2006;57:65–81. doi: 10.1146/annurev.med.56.082103.104549. [DOI] [PubMed] [Google Scholar]
- 81.Lopasch GD, Marzilli M. Mode of action of trimetazidine and other new metabolic agents in the treatment of ischaemic heart disease. Semin Cardiothorac Vasc Anesth. 2003;7:91–96. [Google Scholar]
- 82.Settaf A, Morin D, Lamchouri F, Elimadi A, Cherrah Y, Tillement JP. Trimetazidine ameliorates the hepatic injury associated with ischaemia-reperfusion in rats. Pharmacol Res. 1999;39:211–216. doi: 10.1006/phrs.1998.0427. [DOI] [PubMed] [Google Scholar]
- 83.Okuhama Y, Shiraishi M, Higa T, Tomori H, Taira K, Mamadi T, et al. Protective effects of ulinastatin against ischaemia-reperfusion injury. J Surg Res. 1999;82:34–42. doi: 10.1006/jsre.1998.5496. [DOI] [PubMed] [Google Scholar]
- 84.Bjelakovic G, Nikolova D, Gluud LL, Simonetti RG, Gluud C. Mortality in randomized trials of antioxidant supplements for primary and secondary prevention: systematic review and meta-analysis. J Am Med Assoc. 2007;297:842–857. doi: 10.1001/jama.297.8.842. [DOI] [PubMed] [Google Scholar]
- 85.Xiaobin F, Zipei L, Shuguo Z, Jiahong D, Xiaowu L. The Pringle manoeuvre should be avoided in hepatectomy for cancer patients due to its side-effects on tumour recurrence and worse prognosis. Med Hypotheses. 2009;72:398–401. doi: 10.1016/j.mehy.2008.11.029. [DOI] [PubMed] [Google Scholar]
- 86.Dindo D, Demartines N, Clavien PA. Classification of surgical complications. A new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240:205–213. doi: 10.1097/01.sla.0000133083.54934.ae. [DOI] [PMC free article] [PubMed] [Google Scholar]


